Abstract
Four long-chain, linear fatty acid dopamides (N-acyldopamines) have been identified in nervous bovine and rat tissues. Two unsaturated members of this family of lipids, N-arachidonoyl-dopamine (NADA) and N-oleoyl-dopamine, were shown to potently activate the transient receptor potential channel type V1 (TRPV1), also known as the vanilloid receptor type 1 for capsaicin. However, the other two congeners, N-palmitoyl- and N-stearoyl-dopamine (PALDA and STEARDA), are inactive on TRPV1. We have investigated here the possibility that the two compounds act by enhancing the effect of NADA on TRPV1 (‘entourage' effect).
When pre-incubated for 5 min with cells, both compounds dose-dependently enhanced NADA's TRPV1-mediated effect on intracellular Ca2+ in human embryonic kidney cells overexpressing the human TRPV1. In the presence of either PALDA or STEARDA (0.1–10 μM), the EC50 of NADA was lowered from ∼90 to ∼30 nM.
The effect on intracellular Ca2+ by another endovanilloid, N-arachidonoyl-ethanolamine (anandamide, 50 nM), was also enhanced dose-dependently by both PALDA and STEARDA. PALDA and STEARDA also acted in synergy with low pH (6.0–6.7) to enhance intracellular Ca2+ via TRPV1.
When co-injected with NADA (0.5 μg) in rat hind paws, STEARDA (5 μg) potentiated NADA's TRPV1-mediated nociceptive effect by significantly shortening the withdrawal latencies from a radiant heat source. STEARDA (1 and 10 μg) also enhanced the nocifensive behavior induced by carrageenan in a typical test of inflammatory pain.
These data indicate that, despite their inactivity per se on TRPV1, PALDA and STEARDA may play a role as ‘entourage' compounds on chemicophysical agents that interact with these receptors, with possible implications in inflammatory and neuropathic pain.
Keywords: Anandamide, cannabinoid, endocannabinoid, VR1, receptor, channel, calcium, pain, hyperalgesia, inflammation
Introduction
The transient receptor potential channel of type V1 (TRPV1), originally known as vanilloid receptor type 1 (VR1), was identified and cloned in the attempt to find a molecular target for capsaicin, the pungent principle of hot chili peppers (Caterina et al., 1997; Szallasi & Blumberg, 1999). Indeed, as shown by experiments carried out with transgenic mice lacking the gene encoding for TRPV1, this channel is involved in the transmission of inflammatory and thermal nociception, in agreement with its abundant expression in peripheral sensory efferents, as well as in trigeminal and dorsal root ganglia, particularly at the level of small, unmyelinated C-fibers (see Di Marzo et al. (2002) for a review). It is now accepted that TRPV1 receptors play a role as molecular transducers of both physical (heat, low pH) and chemical (capsaicin, resiniferatoxin) nociceptive stimuli (Tominaga et al., 1998). Of the large family of the TRPV channels, TRPV1 is the only one that is activated by both capsaicin and endogenous arachidonic acid derivatives, such as two members of the endocannabinoid family, anandamide (Devane et al., 1992; Zygmunt et al., 1999; Smart et al., 2000) and N-arachidonoyl-dopamine (NADA) (Bisogno et al., 2000; Huang et al., 2002), and some lypoxygenase derivatives (Hwang et al., 2000). These compounds have been named ‘endovanilloids' (Di Marzo et al., 2002), and may play an important role in TRPV1 gating, particularly in the CNS, where high (>42°C) temperature and low (<7) pH are not likely to occur. It is now known that also TRPV4 can act as a ligand-activated channel using both phorboids and cytochrome p450-derived arachidonate metabolites as ligands (Watanabe et al., 2003).
We have recently shown that, along with NADA, the bovine CNS also contains some congeners of this compound, namely N-oleoyl-, N-palmitoyl- and N-stearoyl-dopamine (OLDA, PALDA and STEARDA) (Chu et al., 2003). This is not surprising, since the other ‘endovanilloid' anandamide is also accompanied in nervous tissues by its oleoyl, palmitoyl and stearoyl congeners. Interestingly, potent pharmacological activities have been described both in vitro and in vivo for each of these anandamide congeners (Lambert & Di Marzo, 1999; Maccarrone et al., 2002a, 2002b; Fu et al., 2003), although only in the case of N-oleoyl-ethanolamine has its high-affinity molecular target been identified as PPAR-α only very recently (Fu et al., 2003). More relevant to research on TRPV1, many anandamide congeners that are inactive on TRPV1 have been shown to potentiate anandamide effects on this channel (De Petrocellis et al., 2001; Smart et al., 2002). Apart from enhancing anandamide TRPV1-mediated actions, N-oleoyl-ethanolamine was also shown to directly gate TRPV1 under certain conditions (Ahern, 2003); much in the same way, OLDA is as active on this receptor as NADA (Chu et al., 2003). However, no pharmacological effect, be it on TRPV1 or other targets, has yet to be reported for PALDA and STEARDA.
In the present study, we have investigated whether PALDA and STEARDA can enhance the actions of NADA and anandamide on TRPV1, and whether they can act in synergy with low pH to gate TRPV1. Furthermore, we have tested the hypothesis that the most abundant of these compounds in tissues, STEARDA, enhances the TRPV1-mediated effects of NADA also in vivo, in a typical test of hyperalgesia in rats. Finally, we have examined the possibility that STEARDA also enhances thermal hyperalgesia under conditions of inflammatory pain.
Methods
Intracellular Ca2+ assays
Human embryonic kidney (HEK-293) cells overexpressing the human TRPV1 receptor were a kind gift from John Davis (GlaxoSmithKline, Harlow, U.K.). Cells were grown as monolayers in minimum essential medium supplemented with nonessential amino acids, 10% fetal calf serum and 0.2 mM glutamine, and maintained under 95%/5% O2/CO2 at 37°C. The effect of substances on [Ca2+]i was determined by using Fluo-3, a selective intracellular fluorescent probe for Ca2+. At 1 day prior to experiments, cells were transferred into six-well dishes coated with poly-L-lysine (Sigma) and grown in the culture medium mentioned above. On the day of the experiment, the cells (50–60,000 per well) were loaded for 2 h at 25°C with 4 μM Fluo-3 methylester (Molecular Probes) in DMSO containing 0.04% pluoronic. After loading, cells were washed with Tyrode's pH=7.4, trypsinized, resuspended in Tyrode's and transferred to the cuvette of the fluorescence detector (Perkin-Elmer LS50B) under continuous stirring. Experiments were carried out by measuring cell fluorescence at 25°C (λEX=488 nm, λEM=540 nm) before and after the addition of the test compounds at various concentrations. PALDA and STEARDA (0.1, 1.0 and 10 μM) were added 5 min prior to addition of increasing concentrations (0.02–0.5 μM) of NADA or of 0.05 μM anandamide. In a separate set of experiments, 5 min prior to the addition of either PALDA (1 and 10 μM), STEARDA (10 μM) or capsaicin (0.01 μM), increasing volumes of a diluted solution (1%) of acetic acid were added to the medium to lower its pH (down to 6.7, 6.4 and 6.0). In all experiments, data are expressed as percent of the maximal effect on intracellular Ca2+ obtained with 4 μM ionomycin. As a negative control, experiments were also carried out in wild-type HEK-293 cells, which do not express TRPV1 receptors.
In vivo experiments
Male Sprague–Dawley rats weighing 200–250 g served as subjects. They were housed in metal cages in a temperature-regulated (22–23°C) room with free access to food and water for at least 24 h prior to testing. Artificial lighting was provided from 06:00 to 18:00 h. On the day of the experiment, each animal was placed in plastic chambers and allowed to acclimate for a 25–30 min period. Following the determination of baseline withdrawal latencies of the hind paw from a radiant heat source, NADA (0.5 μg), STEARDA (5 μg), NADA (0.5 μg) with STEARDA (5 μg), or 50 μl vehicle (2 : 3 : 5 : 90 DMSO/ethanol/emulphor/saline) was injected subcutaneously into the plantar (i.pl.) surface of the left paw of each animal (n=6–15 per dose). Doses of NADA and STEARDA chosen were based on their inability to induce thermal hyperalgesia when administered alone. Care was taken to deliver each injection to the center of the paw superficially under the skin so as to minimize tissue injury. Withdrawal latencies were observed at 10 min after injection and every 20 min thereafter for 140 min. The paw was tested once per time point and a 20 s cutoff was imposed to prevent tissue damage. Data are expressed as the mean deviation from baseline±s.e.m. at 50 and 70 min after injection. The effect of test compounds on hyperalgesia across time was analyzed using two-way repeated-measures analysis of variance (BMDP Statistical Software, Los Angeles, CA, U.S.A.), followed by post hoc analysis of means (the t-test). P<0.05 was considered statistically significant.
For examination of the effect of STEARDA on carrageenan-induced hyperalgesia, baseline withdrawal latencies from a radiant heat source were determined and 50 μl of STEARDA (0.5, 1, 5, or 10 μg) or vehicle (2 : 3 : 5 : 90 DMSO/ethanol/emulphor/saline) was injected subcutaneously into the plantar (i.pl.) surface of the left paw of each animal (n=6–9 per dose) 10 min prior to injection of 100 μl 1% carrageenan or vehicle (saline). Withdrawal latencies were observed every hour after injection for 4 h. Data are expressed as the mean deviation from baseline ±s.e.m. at 3 and 4 h after injection. The effects of test compounds on hyperalgesia across time were analyzed using three-way repeated-measures analysis of variance (BMDP Statistical Software, Los Angeles, CA, U.S.A.), followed by post hoc analysis of means (the t-test). P<0.05 was considered statistically significant.
Results
Effects on NADA-induced Ca2+ influx
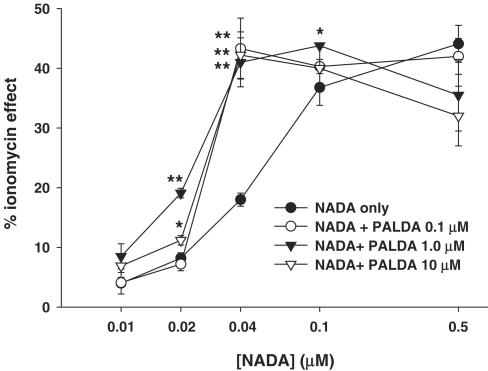
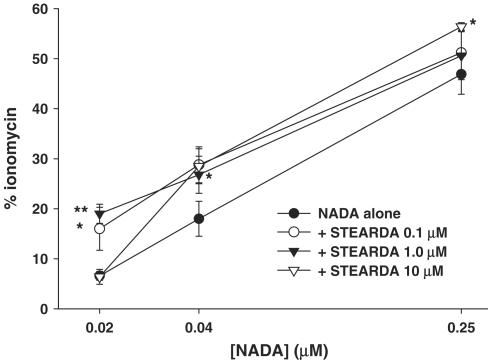
Figure 1 shows the dose-related effect of NADA on intracellular Ca2+ in HEK-293 cells overexpressing the human TRPV1, in the presence or absence of increasing concentrations of PALDA. The EC50 for the effect of NADA was shifted to significantly lower values with all the three concentrations of PALDA, although the intermediate concentration of 1 μM appeared to be more efficacious with lower concentrations of NADA. Likewise, as shown in Figure 2, STEARDA also enhanced the effect of NADA on intracellular Ca2+, with a higher efficacy on the two lower concentrations of NADA used, and a statistically significant effect already at the concentration of STEARDA of 0.1 μM. No effect of NADA, and no potentiation by PALDA and STEARDA, was observed in wild-type HEK-293 cells, which do not express TRPV1 receptors (not shown).
Figure 1.
‘Entourage' effect of increasing concentrations of PALDA on the TRPV1-mediated, dose-dependent action of NADA on human TRPV1 in HEK-293 cells. Data are expressed as % of the effect on intracellular Ca2+ of ionomycin (4 μM) and are means±s.e.m. of n=3 determinations. *P<0.05 and **P<0.02 vs NADA only, as assessed by ANOVA followed by Bonferroni's test.
Figure 2.
‘Entourage' effect of increasing concentrations of STEARDA on TRPV1-mediated, dose-dependent action of NADA on human TRPV1 in HEK-293 cells. Data are expressed as % of the effect on intracellular Ca2+ of ionomycin (4 μM) and are means±s.e.m. of n=3 determinations. *P<0.05 and **P<0.02 vs NADA only, as assessed by ANOVA followed by Bonferroni's test.
Effects on anandamide-induced Ca2+ influx
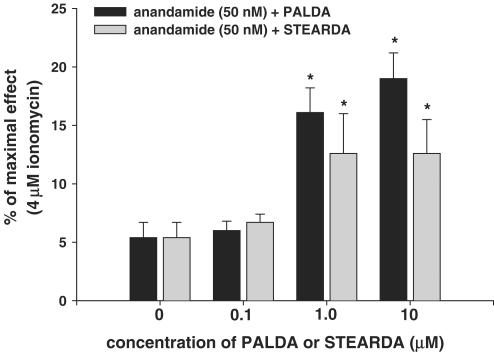
As depicted in Figure 3, both PALDA and STEARDA also significantly enhanced the effect of 50 nM anandamide on intracellular Ca2+ in HEK-293 cells overexpressing human TRPV1. Like with NADA, the effect of the two compounds was already maximal at their intermediate (1 μM) concentration. No synergic effect of PALDA or STEARDA on anandamide was observed in wild-type HEK-293 cells (not shown).
Figure 3.
‘Entourage' effect of increasing concentrations of STEARDA and PALDA on the TRPV1-mediated action of anandamide (50 nM) on human TRPV1 in HEK-293 cells. Data are expressed as % of the effect on intracellular Ca2+ of ionomycin (4 μM), and are means±s.e.m. of n=3 determinations. *P<0.05 vs anandamide only, as assessed by ANOVA followed by Bonferroni's test.
Synergic effects of PALDA or STEARDA and low pH on Ca2+ influx
Both PALDA and STEARDA, at concentrations normally inactive on TRPV1 when using the intracellular Ca2+ concentration assay, became significantly active in enhancing intracellular Ca2+ in HEK-293 cells overexpressing human TRPV1 when tested at pH values between 6.4 and 7.2 (Table 1). Low pH also significantly enhanced the effect of a weakly active concentration (10 μM) of PALDA. No synergic effect of low pH and PALDA or STEARDA was observed in wild-type HEK-293 cells (not shown).
Table 1.
Synergic effect of STEARDA and PALDA with low pH on the gating of human TRPV1 channels in HEK-293 cells
| pH=7.4 | pH=6.7 | pH=6.4 | pH=6.0 | |
|---|---|---|---|---|
| Vehicle | 0 | −0.15±0.04 | −0.27±0.06 | −0.5±0.3 |
| PALDA 1 μM | 3.8±1.4 | 6.7±0.1a,b | 11.5±3.0a,b | 6.9±0.9a |
| PALDA 10 μM | 17.3±4.8a | 23.2±5.6a | 24.4±0.9a,b | 26.5±1.2a,b |
| STEARDA 1 μM | 4.1±0.5 | 11.1±2.5a,c | 10.6±2.0a,c | 12.9±1.5a,c |
| Capsaicin 0.01 μM | 12.7±1.8a | 18.8±1.0a,d | 35.3±3.7a,d | ND |
Data are expressed as the % of the maximal obtainable effect on intracellular Ca2+ (i.e. effect of 4 μM ionomycin) and are means±s.e.m. of n=3 determinations. The synergic effect of low pH and a low concentration of capsaicin is shown for a comparison.
P<0.05 vs corresponding vehicle at the same pH.
P<0.05 vs PALDA at pH=7.4.
P<0.05 vs STEARDA at pH=7.4.
P<0.05 vs capsaicin at pH=7.4; statistically significant differences were assessed by ANOVA followed by Bonferroni's test. ND, not determined.
Effects on NADA-induced nociception
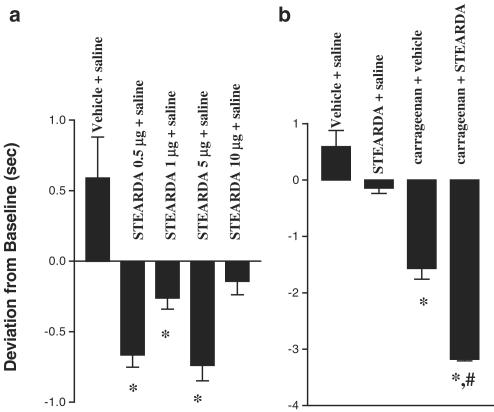
The effect of subcutaneous injections of NADA, STEARDA and NADA with STEARDA in the rat hind paw on withdrawal latencies from a radiant heat source was tested (Figure 4). Analysis of variance revealed that groups treated with STEARDA and drug were significantly more hyperalgesic than groups treated without STEARDA (F5, 47=57.4, P<0.001). Post hoc analysis of means revealed that withdrawal latencies after administration of vehicle were not significantly different from baseline, and withdrawal latencies after administration of NADA or STEARDA were not significantly different from vehicle group latencies. Administration of NADA with STEARDA induced thermal hyperalgesia that lasted up to 150 min post-injection and was significantly greater than hyperalgesia induced by NADA (t=4.365, df=10, P<0.005) or STEARDA (t=3.189, df=19, P<0.005) alone.
Figure 4.
NADA (0.5 μg), STEARDA (5 μg), NADA (0.5 μg) with STEARDA (5 μg), or 50 μl vehicle was injected into the rat hind paw (i.pl.) and withdrawal latency from a radiant heat source was determined at 10 min after injection and every 20 min thereafter for 140 min (n=6–15 per dose). Data are expressed as mean deviation from baseline±s.e.m. at 50 and 70 min after injection. Groups treated with STEARDA and NADA were significantly more hyperalgesic than groups treated without STEARDA (F5, 47=57.4, P<0.001; analysis of variance). Post hoc analysis of means revealed that withdrawal latencies after administration of vehicle were not significantly different from baseline, and withdrawal latencies after administration of NADA or STEARDA were not significantly different from vehicle group latencies. Administration of NADA with STEARDA induced thermal hyperalgesia that was significantly greater than that induced by NADA (t=4.365, df=10, P<0.005) or STEARDA (t=3.189, df=19, P<0.005) alone, and also greater than that induced by vehicle (P<0.005). NS, not significantly different from vehicle alone.
Effects on inflammatory hyperalgesia in the carrageenan test
The effect of subcutaneous injections of STEARDA with carrageenan was also examined. All groups treated with vehicle and carrageenan or STEARDA and carrageenan exhibited significantly greater thermal hyperalgesia than those treated with vehicle and saline or STEARDA and saline (F1, 62=24.94, P<0.00005). Post hoc analysis of means revealed that the group treated with 10 μg STEARDA and carrageenan (t=6.719, df=13, P<0.0001) and the group treated with 1 μg STEARDA and carrageenan (t=2.906, df=16, P<0.05) were significantly more hyperalgesic than the group treated with vehicle and carrageenan. Some doses of STEARDA (0.5, 1 and 5 μg) alone induced a slight hyperalgesia under these conditions (Figure 5a). Treatment groups injected with both STEARDA and carrageenan exhibited an additive hyperalgesia in all groups, except for those treated with 0.5 and 5 μg STEARDA and carrageenan. The ‘entourage' effect of STEARDA was most noticeable with the 10 μg dose of the compound, which was inactive per se (Figure 5b).
Figure 5.
STEARDA enhances the thermal hyperalgesia induced by carrageenan. (a) Effect of increasing doses of STEARDA on average withdrawal latencies from a radiant heat source induced by vehicle. (b) Effect of STEARDA (10 μg) on average withdrawal latencies from a radiant heat source induced by carrageenan. In all, 50 μl of STEARDA (0.5, 1, 5, or 10 μg) or vehicle was injected into the rat hind paw (intraplantar) (n=6–9 per dose) 10 min prior to injection of 100 μl 1% carrageenan or vehicle (saline). Withdrawal latencies from a radiant heat source were determined every hour after injection for 4 h. Data are expressed as mean deviation from baseline±s.e.m. at 3 and 4 h after injection. Groups treated with vehicle and carrageenan or STEARDA plus carrageenan exhibited significantly greater thermal hyperalgesia than groups treated with vehicle plus saline or STEARDA plus saline (F1, 62=24.94, P<0.00005; ANOVA, not shown). Post hoc analysis of means revealed that the group treated with 10 μg STEARDA and carrageenan (t=6.719, df=13, #P<0.0001) and the group treated with 1 μg STEARDA and carrageenan (t=2.906, df=16, P<0.05, not shown) were significantly more hyperalgesic than the group treated with vehicle and carrageenan. In the figure, * represents a statistically significant difference (P<0.05) from vehicle+saline.
Discussion
In the present study, we have shown that the two naturally occurring N-acyldopamines, PALDA and STEARDA, which are inactive or weakly active per se, significantly enhance the TRPV1-mediated effects of NADA, anandamide and low pH on intracellular Ca2+ in HEK-293 cells overexpressing the human TRPV1. Regarding the potentiation of NADA and anandamide effects, we found that a concentration (0.1 μM) not far from the possible tissue concentration of the two compounds in the bovine CNS is already sufficient to elicit strong facilitatory actions. The extent of these ‘entourage' effects appears to be higher when using concentrations of NADA (20–40 nM) near to those previously detected in the brain (Huang et al., 2002). By contrast, no effect was observed when using nonphysiological concentrations of either PALDA/STEARDA (10 μM) or NADA (>100 nM). With both PALDA and STEARDA, the EC50 of NADA in our intracellular Ca2+ assay was decreased by approximately three-fold.
Since STEARDA is likely to be the most abundant saturated N-acyldopamine found so far in tissues (Chu et al., 2003), we decided to assess here its action on a typical TRPV1-mediated effect of NADA, that is, the nocifensive action following exposure of rats to a radiant heat source (Huang et al., 2002). We found that STEARDA significantly enhanced the pharmacological action of NADA in this assay. It must be pointed out that both STEARDA (Figure 4) and PALDA (Chu et al., 2003), when assessed in this assay of thermal pain, where withdrawal latencies are measured starting 10 min and up to 70–90 min after administration, are completely inactive. Therefore, these data strongly suggest that at least STEARDA can exert an ‘entourage' effect on endogenous TRPV1 agonists also in vivo. Apart from ‘central' and ‘peripheral' pain, it is tempting to speculate that PALDA and STEARDA also actively participate in those central and peripheral physiopathological conditions where endovanilloids and TRPV1 seem to play a major role, that is, synaptic plasticity, sensory vasodilation and airway hyperactivity. Regarding pain, possibly the finding of this study that is most relevant to inflammatory hyperalgesia is the observation that PALDA and STEARDA also synergize with low pH to gate TRPV1-mediated Ca2+ influx in transfected HEK-293 cells (Table 1). Low pH is likely to concur to TRPV1-mediated inflammatory hyperalgesia (Szallasi & Blumberg, 1999), and hence our finding is suggestive of a role of PALDA and STEARDA in those nocifensive responses occurring during inflammation. Indeed, we found that, in a typical assay of inflammatory pain, the carrageenan-induced thermal hyperalgesia, where activation of TRPV1 plays a crucial role in determining nociception (Davis et al., 2000), STEARDA could significantly enhance the nocifensive behavior induced by carrageenan. Interestingly, when using the more long-term (up to 4 h) assessment of withdrawal latencies typical of the protocol utilized for this latter assay, STEARDA was also found to exert a slight hyperalgesia per se, that is, in the absence of carrageenan, although not in a dose-related manner (and not at the dose where the maximal ‘entourage' effect on carrageenan was observed). This may suggest that this compound, under certain conditions, that is, at longer intervals of time after administration, is also capable of lowering the threshold of the TRPV1-mediated nociception induced by high temperature, that is, to act as an ‘entourage' compound not only for endovanilloids and protons but also for heat. Unfortunately, we could not test this possibility in our in vitro, isolated cell assay, since this assay does not allow measurement of the effect of drugs on intracellular Ca2+ at temperatures higher than room temperature.
In conclusion, our findings suggest a possible biological function as ‘entourage' compounds for two natural congeners of the proposed endovanilloids NADA and OLDA. Furthermore, our data extend previous findings (De Petrocellis et al., 2001; Smart et al., 2002; Ahern, 2003) showing that even fatty acid derivatives that appear to be inactive or weakly active per se on TRPV1 can modulate the activity of this channel under particular conditions, such as those leading to the sensitization (via lowering of pH, protein phosphorylation, etc.) of the receptor to the action of ligands, or enhance its gating by submaximal concentrations of more potent agonists. Based on these and previous observations, long-chain fatty acid ethanolamides and dopamides should be regarded as endogenous modulators of TRPV1 activity, together with other lipids, such as phosphatidyl-inositol-bis-phosphates (Chuang et al., 2001) and lipoxygenase derivatives (Hwang et al., 2000). Due to the presence of TRPV1 not only in peripheral sensory efferents, but also in epithelial cells, keratinocytes and central neurons (Caterina, 2003), these findings expand the range of possible biological actions that such fatty acid derivatives can exert under both physiological and pathological conditions.
Acknowledgments
This work was partly funded by a grant from the Volkswagen Stiftung (to V.DM.) and grants K02DA00375, NS33247, and DA13012 (to J.M.W.) and DA016827 (to C.J.C.).
Abbreviations
- HEK
human embryonic kidney
- NADA
N-arachidonoyl-dopamine
- OLDA, PALDA, STEARDA
N-oleoyl-, N-palmitoyl-, N-stearoyl-dopamines
- TRPV1
transient receptor potential channel of type V1
References
- AHERN G.P. Activation of TRPV1 by the satiety factor oleoylethanolamide. J. Biol. Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., MELCK D., BOBROV M.YU., GRETSKAYA N.M., BEZUGLOV V.V., DE PETROCELLIS L., DI MARZO V. N-acyl-dopamines: novel synthetic CB(1) cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem. J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- CATERINA M.J. Vanilloid receptors take a TRP beyond the sensory afferent. Pain. 2003;105:5–9. doi: 10.1016/s0304-3959(03)00259-8. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CHU C.J., HUANG S.M., DE PETROCELLIS L., BISOGNO T., EWING S.A., MILLER J.D., ZIPKIN R.E., DADDARIO N., APPENDINO G., DI MARZO V., WALKER J.M. N-oleoyldopamine, a novel endogenous capsaicin-like lipid that produces hyperalgesia. J. Biol. Chem. 2003;278:13633–13639. doi: 10.1074/jbc.M211231200. [DOI] [PubMed] [Google Scholar]
- CHUANG H.H., PRESCOTT E.D., KONG H., SHIELDS S., JORDT S.E., BASBAUM A.I., CHAO M.V., JULIUS D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- DAVIS J.B., GRAY J., GUNTHORPE M.J., HATCHER J.P., DAVEY P.T., OVEREND P., HARRIES M.H., LATCHAM J., CLAPHAM C., ATKINSON K., HUGHES S.A., RANCE K., GRAU E., HARPER A.J., PUGH P.L., ROGERS D.C., BINGHAM S., RANDALL A., SHEARDOWN S.A. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., DAVIS J.B., DI MARZO V. Palmitoylethanolamide enhances anandamide stimulation of human vanilloid VR1 receptors. FEBS Lett. 2001;506:253–256. doi: 10.1016/s0014-5793(01)02934-9. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BLUMBERG P.M., SZALLASI A. Endovanilloid signaling in pain. Curr. Opin. Neurobiol. 2002;12:372–379. doi: 10.1016/s0959-4388(02)00340-9. [DOI] [PubMed] [Google Scholar]
- FU J., GAETANI S., OVEISI F., LO VERME J., SERRANO A., RODRIGUEZ DE FONSECA F., ROSENGARTH A., LUECKE H., DI GIACOMO B., TARZIA G., PIOMELLI D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., BISOGNO T., TREVISANI M., AL-HAYANI A., DE PETROCELLIS L., FEZZA F TOGNETTO M., PETROS T.J., KREY J.F., CHU C.J., MILLER J.D., DAVIES S.N., GEPPETTI P., WALKER J.M., DI MARZO V. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8400–8405. doi: 10.1073/pnas.122196999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HWANG S.W., CHO H., KWAK J., LEE S.Y., KANG C.J., JUNG J., CHO S., MIN K.H., SUH Y.G., KIM D., OH U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAMBERT D.M., DI MARZO V. The palmitoylethanolamide and oleamide enigmas: are these two fatty acid amides cannabimimetic. Curr. Med. Chem. 1999;6:757–773. [PubMed] [Google Scholar]
- MACCARRONE M., CARTONI A., PAROLARO D., MARGONELLI A., MASSI P., BARI M., BATTISTA N., FINAZZI-AGRO' A. Cannabimimetic activity, binding, and degradation of stearoylethanolamide within the mouse central nervous system. Mol. Cell Neurosci. 2002a;21:126–140. doi: 10.1006/mcne.2002.1164. [DOI] [PubMed] [Google Scholar]
- MACCARRONE M., PAUSELLI R., DI RIENZO M., FINAZZI-AGRO' A. Binding, degradation and apoptotic activity of stearoylethanolamide in rat C6 glioma cells. Biochem. J. 2002b;366:137–144. doi: 10.1042/BJ20020438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., GUNTHORPE M.J., JERMAN J.C., NASIR S., GRAY J., MUIR A.I., CHAMBERS J.K., RANDALL A.D., DAVIS J.B. The endogenous lipid anandamide is a full agonist at the human vanilloid receptor (hVR1) Br. J. Pharmacol. 2000;129:227–230. doi: 10.1038/sj.bjp.0703050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMART D., JONSSON K.O., VANDEVOORDE S., LAMBERT D.M., FOWLER C.J. ‘Entourage' effects of N-acyl ethanolamines at human vanilloid receptors. Comparison of effects upon anandamide-induced vanilloid receptor activation and upon anandamide metabolism. Br. J. Pharmacol. 2002;136:452–458. doi: 10.1038/sj.bjp.0704732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- TOMINAGA M., CATERINA M.J., MALMBERG A.B., ROSEN T.A., GILBERT H., SKINNER K., RAUMANN B.E., BASBAUM A.I., JULIUS D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- WATANABE H., VRIENS J., PRENEN J., DROOGMANS G., VOETS T., NILIUS B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature. 2003;424:434–438. doi: 10.1038/nature01807. [DOI] [PubMed] [Google Scholar]
- ZYGMUNT P.M., PETERSSON J., ANDERSSON D.A., CHUANG H., SORGARD M., DI MARZO V., JULIUS D., HOGESTATT E.D. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]