Abstract
We investigated the generation of reactive oxygen species (ROS) from bronchoalveolar lavage (BAL) cells of either control or LPS-exposed rats and the effects of PDE4 inhibitors on ROS production.
The PDE4 inhibitors, rolipram and Ariflo (cilomilast, SB 207499) dose-dependently (0.1–10 μM) inhibited fMLP-induced superoxide anion (O2•−) production (IC50s: 0.03 and 0.55 μM, respectively) in BAL cells of Wistar rats collected 3 h after an LPS-aerosol (200 μg ml−1, 1 h). These BAL contained 85–95% neutrophils (BAL cells enriched in neutrophils).
In contrast, BAL cells collected at the end of the challenge contained only macrophages and in these conditions, rolipram and Ariflo (0.1–10 μM) could only inhibit 25 and 45% of fMLP-induced O2•− release, respectively.
We also observed that the inhibition of p44/42MAPK by PD98059 (1–10 μM) increased O2•− release by BAL cells enriched in neutrophils, but not by macrophages, and prevented the inhibition of O2•− production induced by PDE4 inhibitors.
Western blot analysis showed that PDE4 inhibitors strongly activated p44/42MAPK in BAL cells enriched in neutrophils but not in macrophages. And in these cells, PDE4 and p44/42MAPK were co-immunoprecipitated by a polyclonal anti-PDE4 antibody.
The following cell permeable-cAMP analogues, dbcAMP (10 μM–1 mM), 8-CPT-cAMP (1 mM) and 8-pMeOPT-2′-O-Me-cAMP (0.5 mM), could not reduce fMLP-induced O2•− production and both PKA inhibitors, PKA inhibitor 14–22 amide myristoylated (50 nM–1 μM) and H-89 (100 nM–1 μM), did not affect the decrease of O2•− release induced by PDE4 inhibitors in BAL cells enriched in neutrophils.
These data suggest that PDE4 inhibitors decreased fMLP-induced O2•− release in BAL cells enriched in neutrophils but not in macrophages, through p44/42MAPK activation by a cAMP- and a PKA-independent mechanism.
Keywords: Rodent, neutrophils, macrophages, type 4 phosphodiesterases, MAP kinases, superoxide anion, inflammation, signal transduction
Introduction
The selective targeting of phosphodiesterases type 4 (PDE4) has been actively pursued as a novel therapeutic approach in the treatment of respiratory diseases associated with inflammatory processes, such as asthma and chronic obstructive pulmonary disease (COPD). The rationale for their use in respiratory disease stems from the clinical efficacy of non-selective PDE inhibitors such as theophylline, the detection of PDE4 in many of the cells involved in these diseases and the emergence of positive results from a number of pharmacological studies and clinical trials currently evaluating the efficacy of selective PDE4 inhibitors in respiratory diseases (Barnette, 1999; Spina, 2000).
PDE4 represent the major class of PDE expressed in human inflammatory cells (Wang et al., 1999) and in particular in macrophages and neutrophils, the main cell types present in the lungs of COPD patients (Franchini et al., 1998).
PDE4 are members of the phosphodiesterase (PDE) superfamily of enzymes, which comprises at least 11 members hydrolyzing cyclic AMP (adenosine 3′,5′-cyclic monophosphate) and/or cyclic GMP (guanosine 3′,5′-cyclic monophosphate) (Conti & Jin, 1999; Francis et al., 2000; Giembycz, 2000; Houslay, 2001). In the case of PDE4, there are four gene products and multiple splice variants resulting in a variety of PDE4 isoforms. These enzymes are distributed widely throughout the body, differentially expressed in cells and localized to different compartments within cells (Engels et al., 1994; Conti & Jin, 1999; Houslay, 2001). However, the functional significance of these PDE isoforms and subtypes is not completely understood.
The ability of compounds to inhibit PDE4 catalytic activity correlated with the anti-inflammatory potency of these agents (Barnette et al., 1996; Souness et al., 1996). However, while some of the anti-inflammatory mechanisms of PDE4 inhibitors are clearly mediated by cAMP (Barnette et al., 1996; Yamane et al., 2000), a cAMP-independent pathway would trigger some others, such as the regulation of IL-10 controlling TNFα and IL-6 release (Kambayashi et al., 1995; Escofier et al., 1999).
In this study, we have investigated the effects of PDE4 inhibitors on fMLP-induced O2•− release from macrophages and neutrophils collected in the bronchoalveolar lavages (BAL) of a rat model of lung neutrophilia, used as an experimental model of COPD. It has already been demonstrated that PDE4 inhibitors could decrease O2•− release in different inflammatory cells by a cAMP-dependent mechanism (Wang et al., 1998; Germain et al., 2001). In the present study, we observed that PDE4 inhibitors could trigger a cAMP-independent inhibition of fMLP-induced O2•− release.
PDE4 and mitogen-activated protein kinases (MAPK) are both involved in O2•− generation, but little is known about the influence of PDE4 on MAPK activation. Rolipram was reported to be able to inhibit IFNγ-stimulated phosphorylation of p38 MAPK in U937 cells (Mackenzie & Houslay, 2000). PDE4 have been shown to be activated by IL-3, IL-4, GM-CSF and PMA by a MEK1 or ERK1/2-dependent mechanism in FDCP2 myeloid cells (Ahmad et al., 1999). Other studies reported that PDE4 could provide substrates for ERK2: MacKenzie et al. (2000) have observed a direct interaction between ERK2 and PDE4D3, the activation of ERK2 inducing the inhibition of PDE4D3 by phosphorylation at Ser(579). Baillie et al. (2000) have pursued this study showing that PDE4B, PDE4C and PDE4D, but not PDE4A, can act as a substrate for ERK2, which inhibits long PDE4 isoforms and activates short ones. However, so far nothing has been related concerning the effect of PDE4 on ERK activation. Therefore, we have also investigated the effects of two MAPK, p38MAPK and p44/42MAPK (or ERK1/2), on fMLP-induced O2•− release.
Methods
Chemicals
Dimethylsulfoxide (DMSO), lipopolysaccharide (LPS), N-Formyl-Methionine-Leucine-Phenylalanine (fMLP), dibutyryl cyclic AMP (dbcAMP), forskolin, okadaic acid, wortmannin, chelerythrine chloride and anisomycin were purchased from Sigma (St Quentin-Fallavier, France). SB203580 ([4-(4-Fluorophenyl)-2-(4-methylsulfinylphenyl)-5-(4-pyridyl)1H-imidazole], p38MAPK inhibitor), PD98059 (2′-Amino-3′-methoxyflavone, MEK1 inhibitor), Protein kinase A inhibitor 14–22 amide myristoylated and H-89 were from Calbiochem (France Biochem, Meudon, France). 8-CPT-cAMP (8-(4-Chlorophenylthio) adenosine-3′,5′-cyclic monophosphate) and 8-pMeOPT-2′-O-Me-cAMP (8-(4-Methoxyphenylthio)-2′-O-methyladenosine-3′,5′-cyclic monophosphate) were purchased by BIOLOG Life Science Institute (Bremen, Germany). 6% Pentobarbital sodium salt solution was from Sanofi (Libourne, France). Hank's balanced salt solution (HBSS) was purchased from Gibco/BRL (Life Technologies, Cergy Pontoise, France). All antibodies were from New England Biolabs (Ozyme, St Quentin, France). Diogenes kit was from National Diagnostics (Prolabo, Nogent-sur-Marne, France). Protease inhibitor cocktail tablets Complete was purchased from Roche Diagnostics (Meylan, France). Protein assay dye reagent was from Bio-Rad (Ivry-sur-Seine, France). ECL Plus Western Blotting Detection System was from Amersham Pharmacia Biotech (Orsay, France). The selective PDE4 inhibitors Rolipram and Ariflo (cilomilast) were synthesized in our Department of Chemistry (Pfizer Global R&D, Fresnes Laboratories, France).
Treatment of Wistar rats and collection of bronchoalveolar lavages
Male Wistar rats (Janvier, Le Genest St-Isle, France) were subjected to an aerosol (Nebulizer air flow: eight LPM Air, Drying air flow: five LPM Air, SPAG-2 6000 Series Small Particle Aerosol Generator, ICN Pharmaceuticals, Inc., Costa Mesa, CA, U.S.A.) of 200 μg ml−1 LPS dissolved in saline solution (0.9% sodium chloride, Cooper, Rhône-Poulenc-Rorer, Melun, France), for 60 min. BAL were collected immediately or 3 h after the end of the aerosol by three successive 5 ml-lavages of saline solution containing 1 mg ml−1 EDTA (Gibco) in anaesthetized (6% pentobarbital, 10 mg kg−1) rats. Bronchoalveolar lavage cells were washed with HBSS and resuspended at the concentration of 5 × 106 cells ml−1 in HBSS. They were kept at 4°C until use. The cellular composition of BAL was determined using May-Grunwald Giemsa staining (Sigma) and differential counting on 200 cells using standard morphological criteria. For each experiment, the BAL cells of six (Figure 2b) to 12 (Figure 2a) animals have been pooled, allowing to perform on the same cellular population the dosage of reactive oxygen species (ROS) in triplicate in the presence of different compounds by chemiluminescence and the detection of MAP kinases activation by Western blot (see below).
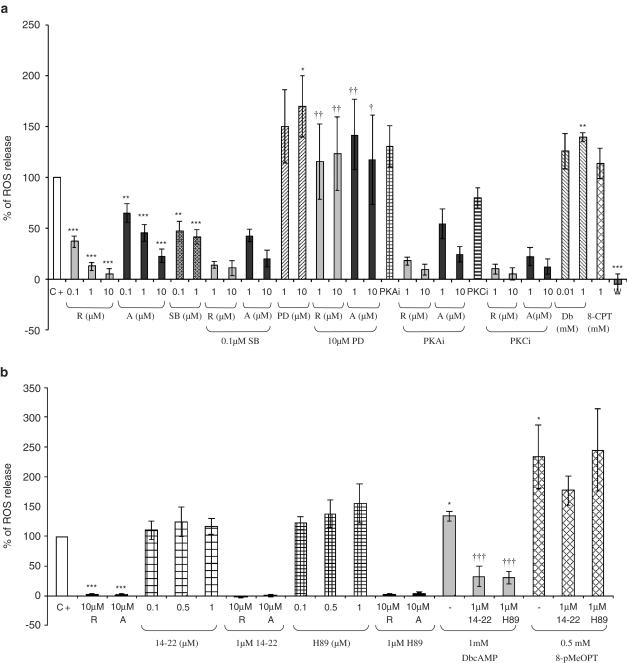
Figure 2.
(a) Percentage of reactive oxygen species (ROS) production in bronchoalveolar lavages (containing 90% neutrophils) of LPS-treated rats 10 min after fMLP (2 μM) addition in the presence of MAPK inhibitors, PKA inhibitor or PKC inhibitor and/or PDE4 inhibitors (C+=positive control, R=rolipram, A=Ariflo, SB=SB203580, PD=PD98059, PKAi=0.05 μM protein kinase A inhibitor 14–22 amide myristoylated, PKCi=0.4 μM protein kinase C inhibitor, W=0.05 μM wortmannin). This figure represents the results of 15 experiments. (b) Percentage of reactive oxygen species (ROS) production in bronchoalveolar lavages (containing 90% neutrophils) of LPS-treated rats 10 min after fMLP (2 μM) addition in the presence of cAMP analogs (DbcAMP=1 mM, 8-pMeOPT=1 mM 8-pMeOPT-2′-O-Me-cAMP), PKA inhibitors (14–22=0.1–1 μM protein kinase A inhibitor 14–22 amide myristoylated, H-89=0.1–1 μM) and/or PDE4 inhibitors (R=10 μM rolipram, A=10 μM Ariflo). This figure represents the results of five experiments. Statistical analysis for (a) and (b): the effects of each compound were compared to a theoretical mean of 100% representing the positive control (*P<0.01, **P<0.001, ***P<0.0001), and the effects of rolipram or Ariflo in the presence of MAPK, PKA, PKC inhibitors or cAMP analogs were compared to the effects of rolipram or Ariflo alone (†P<0.01, ††P<0.001, †††P<0.0001), using the Holm's multiple test procedure.
Additionally to the fact that cells collected in the BAL of animals submitted to a LPS aerosol were used as a model of COPD cells, the need to work on these cells was justified by the fact that either circulating neutrophils or monocytes and lung resident macrophages were not reactive to fMLP unless they were pretreated with LPS.
Detection of ROS release
This experiment was performed in a white 96-well plate (96 MicroWell™ plate, Nunc, Fisher Bioblock Scientific, Illkirch, France) suitable for luminescence. BAL cells (2.5 × 105 well−1) were incubated with compounds or their vehicle (controls) for 30 min at 4°C. The cells were incubated with compounds at 4°C to avoid spontaneous activation and to slow down the activation induced by fMLP, which occurs 1 min after fMLP addition when cells are incubated with compounds at 37°C. Diogenes kit mixed with fMLP (2 μM final) or its vehicle (negative controls) was then added to each well and the plate was immediately placed in the 37°C thermostated chamber of a luminometer (Victor 2, Wallac Berthold, EG&G Instruments, Evry, France). Chemiluminescence was registered continuously for 30 min. The compounds used in these experiments were (1) PDE4 inhibitors: rolipram (0.1–10 μM), Ariflo (0.1–10 μM); (2) MAPK inhibitors: SB203580 (0.1–1 μM,), PD98059 (1–10 μM); (3) Phosphatidylinositol-3 kinase (PI3K) inhibitor: wortmannin (0.05 μM); (4) PKA inhibitor: protein kinase A inhibitor 14–22 amide cell-permeable myristoylated (0.05–1 μM), H-89 (0.1–1 μM), (5) PKC inhibitor: chelerythrine choride (0.4 μM); and (6) dbcAMP (10 μM and 1 mM), 8-CPT-cAMP (1 mM), 8-pMeOPT-2′-O-Me-cAMP (0.5 mM). Accordingly with their IC50s in cells and with our preliminary experiments, we used the lowest effective concentrations of above compounds to avoid unspecific effects. Bronchoalveolar lavage cells were incubated with MAPK inhibitors, the PKA inhibitor, the PKC inhibitor, dbcAMP or 8-pMeOPT-2′-O-Me-cAMP alone or in combination with PDE4 inhibitors or PKA inhibitors. In this latter case, MAPK inhibitors, PKA inhibitors, the PKC inhibitor, dbcAMP or 8-pMeOPT-2′-O-Me-cAMP were added 15 min before PDE4 inhibitors or PKA inhibitors. All the compounds were dissolved in DMSO and diluted in HBSS ([DMSO]final=0.1%; which had no effect on O2•− release by the cells). Diogenes kit is constituted of luminol and enhancer of chemiluminescence, and is able to detect ROS. We used superoxide dismutase (SOD) to evaluate the percentage of O2•− release among ROS. As the amount of O2•− release was variable depending on the experiment, all the data are expressed in percentage of the control (positive control–negative control), which represents 100% of O2•− release.
The results are expressed as mean±standard error of the mean (s.e.m.). The effects of each compound were compared to a theoretical mean of 100% representing the positive control. Statistical analyses were carried out by our Department of Biostatistics (Pfizer, Fresnes, France) using Holm's multiple test procedure (Holm, 1979), which consists in an ANOVA test corrected by a factor α, dedicated to eliminate false positive results. Holm's multiple test procedure is employed to assess whether or not a compound has a significant effect, when used at different doses.
Western blot analysis
Bronchoalveolar lavage cells (5 × 106 ml−1 in HBSS) were preincubated with 2 μM okadaic acid, inhibiting protein phosphatases 1 (PP1) and 2A (PP2A), for 30 min at 4°C to avoid MEK1/2 dephosphorylation. We previously performed experiments without adding okadaic acid to cells and we observed the same results, but the use of okadaic acid allowed us to have more reproducible results in term of signal intensity. Cells were incubated with compounds or their vehicle for 30 min at 4°C and then stimulated by fMLP (2 μM) for 10 min at 37°C or by forskolin (10 μM) for 30 min at 37°C or by DbcAMP (1 mM) for 30 min at 37°C. All the compounds were dissolved in DMSO and diluted in HBSS ([DMSO]final=0.1%). The reaction was stopped by centrifugation at 500 × g for 5 min, pellets were frozen and stored at −80°C or resuspended immediately in lysis buffer containing antiproteases.
The protein concentration of each tube was determined by the Coomassie Bradford method using Bio-Rad protein assay dye reagent (Bradford, 1976).
The same amount of proteins (50 μg lane−1) from each sample was submitted to a SDS–PAGE in a 10% SDS/12% polyacrylamide gel. After migration, proteins were transferred onto a nitrocellulose membrane (pore diameter=0.45 μM) for Western blot analysis, as previously described (Towbin et al., 1992). Briefly, unspecific binding sites were blocked with 5% non-fat dry milk in TBS, pH 7.4/0.1% Tween 20, for 1 h, at room temperature. The membrane was then incubated for 3 h at room temperature or overnight at 4°C with the primary antibody, directed against the protein of interest, diluted at the appropriate concentration in blocking buffer. The membrane was washed with TBS/0.1% Tween 20 for 30 min and incubated for 1 h at room temperature with the HRP (horseradish peroxidase)-conjugated anti-rabbit secondary antibody at a 1 : 5000 dilution in blocking buffer. After last steps of washing, the membrane was incubated for 5 min with ECL Plus Western Blotting Detection System to reveal the protein of interest.
The primary antibodies used were antiphospho-p44/42MAPK (Thr202/Tyr204) at a 1 : 2000 dilution, antiphospho-p38MAPK (Thr180/Tyr182), antiphospho-Raf (Ser259), antiphospho-MEK1/2 (Ser217/221), antiphospho-SEK1/MKK4 (Thr261), antiphospho-MKK3/MKK6 (Ser189/207) at a 1 : 1000 dilution and antiphospho-CREB (ser133) at a 1 : 1000 dilution.
The results were analyzed by densitometry (OD mm−2, calculated against the background by the Gel Doc 1000 from BioRad, Ivry-sur-Seine, France) and are expressed as the percentage of activation compared to the positive control (BAL cells incubated with fMLP (2 μM) for 10 min in the presence of compound vehicle), representing 100% activation. For each kinase and for CREB, we stripped and reblotted membranes with a selective antibody detecting phosphorylated and non-phosphorylated kinase or CREB, allowing to check that we loaded the same amount of protein in every lanes. Each phospho-protein study has been carried out 3 to 10 times.
Co-immunoprecipitation of PDE4 & p44/42MAPK
After treatment, cells were lysed in 1 ml RIPA buffer containing protease inhibitors Complete tablet (one tablet for 10 ml buffer) and were incubated at 4°C for 10 min. They were centrifuged for 20 min at 500 × g and the supernatant was transferred to a new microcentrifuge tube to be incubated with 2 μg of antibodies (K116, K118 or AC55) on a rotator overnight at 4°C. Then 30 μl of Protein A/G Plus (Roche Diagnostics, Indianapolis, IN, U.S.A.) were added and incubated for 2 h to immobilize the antibody. The tubes were centrifuged for 5 min at 500 × g to precipitate the beads, the supernatant was discarded and the beads were washed three times with 1 ml RIPA buffer. The beads were re-suspended in 30μl of sample loading buffer and boiled for 10 min to release the proteins, which were then submitted to a SDS–PAGE in a 10% SDS/10% polyacrylamide gel to be analysed by Western blot (as previously described). Both p44/42MAPK and phosphorylated-p44/42MAPK were detected by an anti-p44/42MAPK antibody and an antiphospho-p44/42MAPK (Thr202/Tyr204) antibody, respectively. Both antibodies were used at a 1 : 2000 dilution. The membranes were first blotted with the antiphospho-p44/42MAPK (Thr202/Tyr204) antibody, then with the anti-p44/42MAPK antibody, then stripped (incubation 20 min at 50°C with 2% SDS/0.7% β-mercaptoethanol) and re-blotted with K116 antibody. K116, a polyclonal anti-rat PDE4 antibody detecting the catalytic domain of PDE4, K118, a polyclonal anti-rat PDE4B antibody and AC55, a polyclonal anti-rat PDE4A antibody were produced as described (Iona et al., 1998) in Marco Conti's lab (Department of Gynecology and Obstetrics, Stanford University Medical Center, CA, U.S.A.).
Results
Cellular composition of bronchoalveolar lavages
BAL were constituted of 90±1.4% neutrophils and 10±1.4% macrophages 3 h after the end of the LPS aerosol (BAL Neutrophils), whereas they were composed by 98±2% macrophages (BAL Macrophages) when collected just at the end of the LPS aerosol.
Detection of ROS release in the presence of PDE4 inhibitors or MAPK inhibitors
When SOD was added to cells prior fMLP, we did not detect any ROS production, meaning that chemiluminescent signals were entirely due to O2•− release.
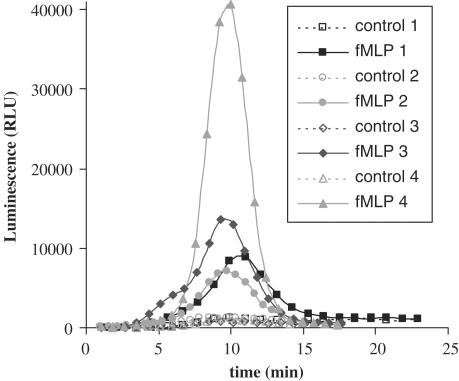
For both BAL neutrophils and macrophages, the intensities of O2•− production varied between different experiments, but this activation invariably reached a maximal level 10 min after 2 μM fMLP addition as presented in Figure 1. We did not notice any significant difference between neutrophils and macrophages in the amount of O2•− produced. BAL fluids collected after LPS aerosols contained red blood cells (RBC) in variable amounts depending on the experiment. We did not use RBC lysis buffer to avoid spontaneous activation of cells. We have observed that the intensity of O2•− production was weak in BAL fluids containing a lot of RBC and we hypothesized that the variability of O2•− production intensity could be due to erythrocyte anti-oxidant activity in BAL fluids.
Figure 1.
Reactive oxygen species generation in bronchoalveolar lavages (containing 90% neutrophils) of LPS-treated rats after 2 μM fMLP addition. This figure represents the results of four experiments.
BAL neutrophils
Therefore, the effects of PDE4 and MAPK inhibitors on O2•− generation were determined at the peak of activation (Figure 2). In these conditions, rolipram and Ariflo dose-dependently inhibited fMLP-induced O2•− production with IC50 values of 0.03±0.01 and 0.55±0.22 μM, respectively. The p38MAPK inhibitor, SB203580, inhibited 60% of O2•− generation, at both concentrations tested, whereas the MEK inhibitor, PD98059, significantly activated O2•− release when used at 10 μM. The PI3K inhibitor, wortmannin, was used as an internal control of the experiment, since a complete extinction of fMLP-induced activation by PI3K inhibition allowed us to validate the experiment.
Preincubation of cells with SB203580 (0.1 μM) prior to PDE4 inhibition did not affect the ability of rolipram or Ariflo to inhibit fMLP-induced ROS release. In contrast, pretreatment of cells with PD98059 (10 μM) was able to prevent PDE4 inhibitors from inhibiting O2•− release. Dibutyryl cyclic AMP (1 mM) significantly activated O2•− release, whereas 8-CPT-cAMP (1 mM), PKA (0.05 μM) or PKC (0.4 μM) inhibitors had no significant effect on fMLP-induced O2•− production and they did not modify the inhibitory effect of rolipram and Ariflo when incubated with cells prior to PDE4 inhibition (Figure 2a). The Figure 2b shows that both PKA inhibitors, 14–22 amide myristoylated and H-89 (0.1–1 μM) did not have any significant effect on fMLP-induced O2•− production and that they did not affect the inhibitory effect of PDE4 inhibitors. Additionally, we observed that both PKA inhibitors were able to significantly inhibit the effect of dbcAMP (which activates PKA) on fMLP-induced O2•− release, whereas they were ineffective to impair the significant increase of O2•− release mediated by the EPAC agonist 8-pMeOPT-2′-O-Me-cAMP (which does not activate PKA).
BAL macrophages
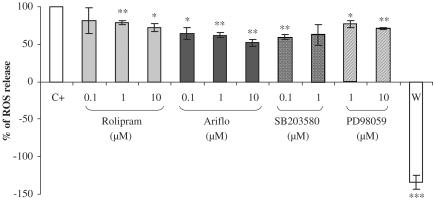
The effects of PDE4 inhibitors and MAPK inhibitors were determined at the activation peak of fMLP-induced O2•− production (Figure 3). Rolipram and Ariflo slightly decreased O2•− release. Indeed, at their maximal effect rolipram and Ariflo could inhibit O2•− release by 30 and 50%, respectively. The p38MAPK inhibitors SB203580 (0.1 μM) and the MEK inhibitor PD98059 (10 μM) inhibited O2•− production by 40 and 25%, respectively. In contrast, the selective PI3K inhibitor wortmannin was able to completely abolish O2•− production, as it even inhibited the basal O2•− production (Figure 3).
Figure 3.
Percentage of reactive oxygen species (ROS) production in bronchoalveolar lavages (containing 98% macrophages) of LPS-treated rats at the activation peak induced by 2 μM fMLP in the presence of PDE4 inhibitors or MAPK inhibitors (C+=positive control, W=0.05 nM wortmannin). Statistical analysis: the effects of each compound were compared to a theoretical mean of 100% representing the positive control, using the Holm's multiple test procedure (*P<0.01, **P<0.001, ***P<0.0001).
Western blot analysis
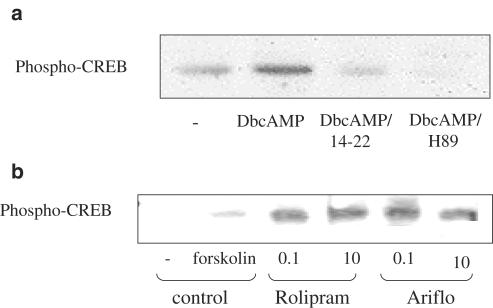
Phospho-CREB
The anti-phospho-CREB antibody revealed an expected 43 KDa band (Figures 4a and b). CREB was phosphorylated by either 10 μM forskolin or 1 mM Db-cAMP. Both PKA inhibitors, 14–22 amide myristoylated (1 μM) and H-89 (1 μM) were able to decrease Db-cAMP-induced CREB phosphorylation and both PDE4 inhibitors, rolipram and Ariflo enhanced CREB phosphorylation.
Figure 4.
(a) Western blot of phospho-CREB in BAL neutrophils of LPS-treated rats 30 min after 1 mM DbcAMP addition in the presence of PKA inhibitors (14–22=1 μM protein kinase A inhibitor 14–22 amide myristoylated, H-89=1 μM). (b) Western blot of phospho-CREB in BAL neutrophils of LPS-treated rats 30 min after 10 μM forskolin addition in the presence of PDE4 inhibitors (rolipram=0.1–10 μM, Ariflo=0.1–10 μM).
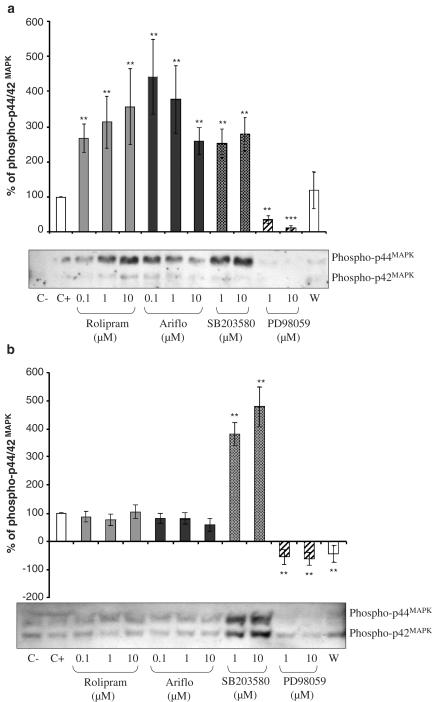
Phosphorylated p44/42MAPK
As presented in Figure 5a for neutrophils, two bands were detected at the expected sizes: 42 and 44 kDa. When cells were at their resting state (C−=negative control), phosphorylated p44/42MAPK (or phospho-ERK1/2) was undetectable, showing that okadaic acid did not induce ERK1/2 activation. A fMLP (2 μM)-treatment induced ERK1/2 phosphorylation. As compared to the positive control (C+=cells activated with fMLP), both PDE4 inhibitors strongly increased (three- to four-fold) ERK1/2 phosphorylation induced by fMLP. SB203580 increased ERK1/2 phosphorylation and wortmannin inhibited it. PD98059, the MEK inhibitor, inhibited ERK1/2 activation, thus validating the experiment.
Figure 5.
(a) Western blot of phosphorylated p44/42MAPK and percentage of phosphorylated p44/42MAPK evaluated by densitometry (OD mm−2) of the blots in BAL neutrophils of LPS-treated rats 10 min after 2 μM fMLP addition in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, W=0.05 nM wortmannin). (b) Western blot of phosphorylated p44/42MAPK and percentage of phosphorylated p44/42MAPK evaluated by densitometry (OD mm−2) of the blots in BAL macrophages of LPS-treated rats at the activation peak induced by 2 μM fMLP in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, W=0.05 nM wortmannin).
The results obtained from macrophages are presented in Figure 5b. Neither rolipram nor Ariflo were able to phosphorylate p44/42MAPK in these cells. The selective p38MAPK inhibitor SB203580 could strongly activate p44/42MAPK.
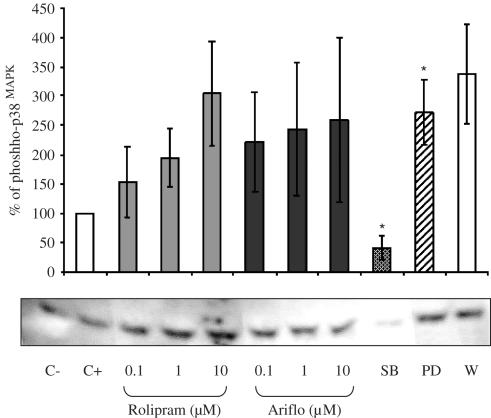
Phosphorylated p38 MAPK
As observed in Figure 6, the expected 43 kDa band for p38MAPK was detected. This experiment showed that a 2 μM fMLP-treatment could not increase p38MAPK activation and that p38MAPK was already activated in these cells without fMLP. Rolipram and Ariflo failed to significantly activate p38MAPK phosphorylation. PD98059 induced a significant increase of phosphorylated p38MAPK levels. The specific p38MAPK inhibitor SB203580 (1 μM) inhibited p38MAPK activation and its basal level of phosphorylation.
Figure 6.
Western blot of phosphorylated p38MAPK and percentage of phosphorylated p38MAPK evaluated by densitometry (OD mm−2) of the blots in bronchoalveolar lavages of LPS-treated rats 10 min after 2 μM fMLP addition in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, SB=SB203580, PD=PD98059, W=0.05 nM wortmannin).
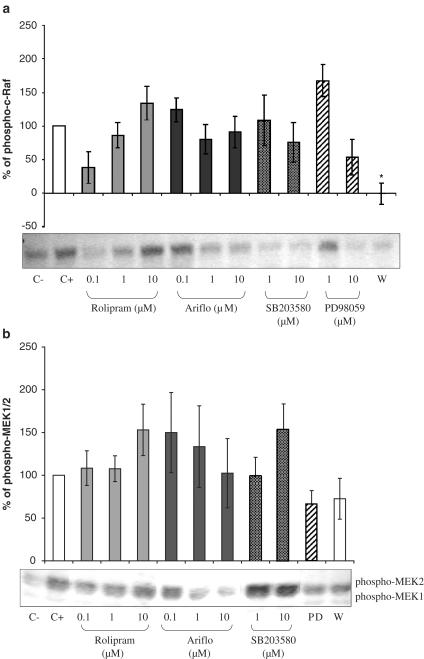
Phosphorylated c-Raf
As presented in Figure 7a, the antiphospho-c-Raf antibody allowed us to detect the expected 74 kDa band. FMLP (2 μM) induced c-Raf phosphorylation, whereas rolipram, Ariflo, SB203580 and PD98059 had no effect. In contrast, the PI3K inhibitor, wortmannin, significantly inhibited c-Raf phosphorylation.
Figure 7.
(a) Western blot of phosphorylated c-Raf and Percentage of phosphorylated c-Raf evaluated by densitometry (OD mm−2) of the blots in bronchoalveolar lavages of LPS-treated rats 10 min after 2 μM fMLP addition in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, W=0.05 nM wortmannin). (b) Western blot of phosphorylated MEK1/2 and percentage of phosphorylated MEK1/2 evaluated by densitometry (OD mm−2) of the blots in bronchoalveolar lavages of LPS-treated rats 10 min after 2 μM fMLP addition in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, PD=PD98059, W=0.05 nM wortmannin).
Phosphorylated MEK1/2
In Figure 7b, two bands at the expected sizes, 45 and 46 kDa, were detected. MEK2 (46 Kda) was highly activated whereas phospho-MEK1 (45 kDa) appeared very weak. Phosphorylated MEK2 levels were increased under the fMLP-treatment. PDE4 inhibitors were ineffective in activating MEK2. SB203580, wortmannin and the MEK inhibitor, PD98059, did not have any significant effect on MEK phosphorylation.
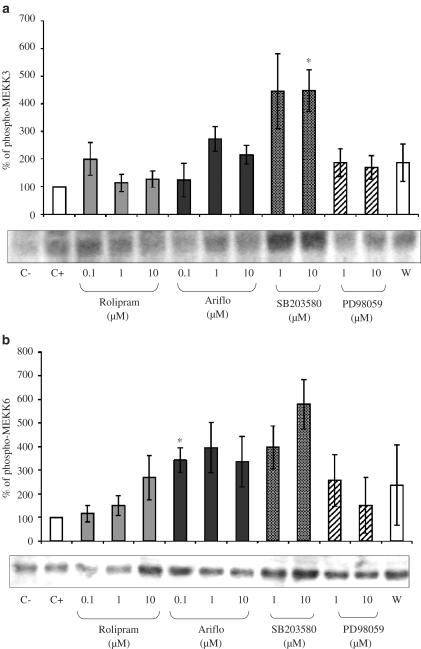
Phosphorylated MKK3/MKK6
The anti-phospho-MKK3/MKK6 antibody was able to detect two bands at their expected sizes: 40 and 41 kDa (Figures 8a and b). The fMLP (2 μM) treatment of cells could slightly phosphorylate MKK3 (Figure 8a) but not MKK6 (Figure 8b). PDE4 inhibitors did not activate MKK3 but Ariflo could slightly activate MKK6. SB203580 significantly activated MKK6. PD98059 and wortmannin had no significant effect on these kinases.
Figure 8.
(a) Western blot of phosphorylated MKK3 and percentage of phosphorylated MKK3 evaluated by densitometry (OD mm−2) of the blots in bronchoalveolar lavages of LPS-treated rats 10 min after 2 μM fMLP addition in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, W=0.05 nM wortmannin). (b) Western blot of phosphorylated MKK6 and percentage of phosphorylated MKK6 evaluated by densitometry (OD mm−2) of the blots in bronchoalveolar lavages of LPS-treated rats 10 min after 2 μM fMLP addition in the presence of PDE4 inhibitors or MAPK inhibitors (C−=negative control, C+=positive control, W=0.05 nM wortmannin).
Co-immunoprecipitation of PDE4 and p44/42MAPK
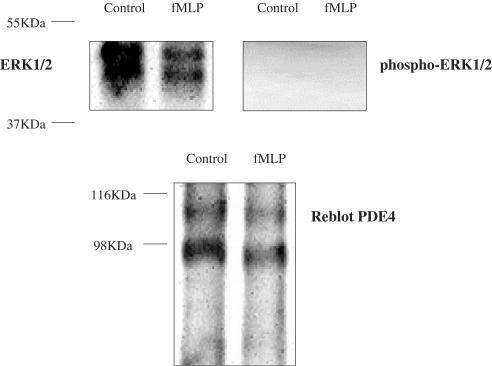
Figure 9 shows that high amounts of ERK1/2 were co-immunoprecipitated with PDE4 using K116 antibody whereas no phosphorylated-ERK1/2 was co-immunoprecipitated with PDE4 (Figure 9). We did not detect any co-immunoprecipitated ERK1/2 or phospho-ERK1/2 with PDE4A or PDE4B using AC55 or K118 antibodies, respectively.
Figure 9.
Western blot of p44/42MAPK and phospho-p44/42MAPK co-immunoprecipitated with PDE4 by the polyclonal anti-rat PDE4 antibody K116 in lysates of neutrophils from LPS-treated rat bronchoalveolar lavages (C−=control cells, C+=cells activated with fMLP (2 μM)) and re-blot of the membrane after stripping with K116.
Discussion
In the present study, we have observed that PDE4 inhibitors triggered a cAMP-independent inhibition of fMLP-induced O2•− release. We have also demonstrated the existence of a novel pathway involving MAPK and connecting PDE4 to fMLP-induced O2•− release. While cells collected in the BAL fluids from control rats were mainly composed of alveolar macrophages, LPS challenge induced a massive recruitment of neutrophils reaching 90% of the cell population after 3 h. Lung neutrophilia is a major feature in COPD and is mainly responsible for the release of different inflammatory mediators including ROS. Previous studies have shown that PDE4 inhibitors were able to decrease O2•− generation from various cell types (Dent et al., 1998; Wang et al., 1998; Germain et al., 2001). Using BAL neutrophils, we presently showed that rolipram and Ariflo, a first and a second generation PDE4 selective inhibitors, respectively, were able to dose-dependently inhibit fMLP-induced O2•− production. Rolipram appeared more potent (IC50: 0.03±0.01 μM) to reduce O2•− generation than Ariflo (IC50: 0.55±0.22 μM). It has been currently described that PDE4 possesses a low- and a high-affinity binding site for rolipram (LARBS and HARBS, respectively) (Barnette et al., 1996; Souness & Rao, 1997; Torphy, 1998), which have been reported to be two different conformations of the same protein (Alvarez et al., 1995; Owens et al., 1997; Rocque et al., 1997; McPhee et al., 1999). Ariflo has been described to be more potent (IC50: 95 nM) than rolipram (IC50: 300 nM) to inhibit PDE4 LARBS, whereas Ariflo has been shown to be less potent (IC50: 120 nM) than rolipram (IC50: 5 nM) to bind to HARBS (Christensen et al., 1998). Regarding the binding to HARBS, the potency ratio between rolipram and Ariflo (0.04) is very close to the potency ratio between rolipram and Ariflo to inhibit ROS release (0.05). These data would suggest that the inhibition of fMLP-induced O2•− release by PDE4 inhibitors can be due to their binding to HARBS or to the high affinity conformation of PDE4. In contrast to what was observed in human mononuclear cells (Germain et al., 2001), the three cell-permeable analogues of cAMP, dbcAMP, 8-CPT-cAMP (both PKA agonists) and 8-pMeOPT-2′-O-Me-cAMP (EPAC agonist) did not inhibit fMLP-induced O2•− release but increased it. In addition, pretreatment with PKA inhibitors did not affect the ability of PDE4 inhibitors to reduce fMLP-induced O2•− generation. These results indicate that the effect of PDE4 inhibitors on O2•− generation is independent of both cAMP/PKA and cAMP/EPAC pathways. The inhibitory effect of PDE4 inhibitors was not mediated by PKC either, since a preincubation with the PKC inhibitor had no impact on the inhibition of O2•− production triggered by PDE4 inhibitors.
Additionally, we explored the involvement of both p38MAPK and p44/42MAPK (ERK1/2) using the selective p38MAPK inhibitor SB203580 and the selective MEK inhibitor PD98059 on fMLP-induced O2•− release. The inhibition of p38MAPK induced a decrease of O2•− generation from BAL cells enriched in neutrophils, whereas the inhibition of p44/42MAPK increased O2•− formation. Our data showing that p38MAPK activates fMLP-induced O2•− release are consistent with already published studies (Zervos et al., 1995; Zu et al., 1996; Nick et al., 1997; Partrick et al., 2000). Regarding the role of p44/42MAPK in fMLP-induced O2•− production, some previous studies have reported that its inhibition decreased fMLP-induced O2•− release in circulating neutrophils from healthy donors (Downey et al., 1998; Dewas et al., 2000). In contrast, Zu et al. (1998) showed that the inhibition of this MAPK did not have any effect on fMLP-induced O2•− release. Our results, showing that the inhibition of p44/42MAPK increased fMLP-induced O2•− generation in BAL cells enriched in neutrophils of LPS-treated rats, suggest that the activation of this MAPK could prevent from fMLP-induced O2•− production in these cells. This hypothesis is supported by our results showing that p38MAPK inhibition induces the activation of p44/42MAPK (consistently with Zhang et al., 2001) and the inhibition of fMLP-induced O2•− release.
PDE4 inhibitors having the potency to inhibit fMLP-induced O2•− release, we hypothesized that PDE4 inhibitors and p44/42MAPK could act through a common pathway. This hypothesis was supported by the fact that the MEK inhibitor PD98059 could prevent PDE4 inhibitors from decreasing fMLP-induced O2•− release, whereas a pretreatment with the p38MAPK inhibitor SB203580 did not affect the ability of PDE4 inhibitors to decrease ROS release. To assess a potential effect of PDE4 inhibitors on MAPK activation pathways, we explored the phosphorylation of p38MAPK and p44/42MAPK by Western blot in lysates of BAL cells enriched in neutrophils treated with fMLP in the presence of rolipram or Ariflo. These experiments showed that PDE4 inhibitors could activate p44/42MAPK but could not significantly activate p38MAPK. Thus, the facts that p44/42MAPK inhibition leads to an increase of fMLP-induced O2•− production and that PDE4 inhibitors activate p44/42MAPK suggest that the inhibitory effect of PDE4 inhibitors on O2•− release could be attributed to p44/42MAPK activation.
Interestingly, PDE4 inhibitors did not elicit the same activity on fMLP-induced O2•− production in BAL macrophages. Indeed, in these cells rolipram and Ariflo triggered a very moderate inhibitory effect on ROS production. Moreover, PDE4 inhibitors were unable to activate p44/42MAPK in fMLP-treated macrophages. These results could explain the fact that rolipram and Ariflo did not inhibit fMLP-induced O2•− release in these cells, as they could in BAL cells enriched in neutrophils.
In order to clarify the connection between PDE4 and MAPK in BAL cells enriched in neutrophils, we explored the activation of kinases leading to MAPK phosphorylation. PDE4 inhibitors activated p44/42MAPK, but neither c-Raf nor MEK1/2. They were unable to significantly activate MKK3 and Ariflo could only slightly increase MKK6 phosphorylation. The phosphorylated form of SEK1, also called MKK4 or Jun kinase kinase (JNKK), was undetectable, whatever treatment applied.
The inhibition of MEK1/2 prevented PDE4 inhibitors from reducing fMLP-induced O2•− release, suggesting that PDE4 inhibitors could activate p44/42MAPK through MEK1/2. Nevertheless, the fact that we did not detect any activation of MEK1/2 by PDE4 inhibitors appears in contradiction with this hypothesis. The absence of MEK1/2 activation by PDE4 inhibitors could be due to its dephosphorylation. In our experiments, the incubation with fMLP was stopped 10 min after its addition to the cells, this time allowing a maximal activation of O2•− generation, but possibly being too long to properly detect MEK1/2 phosphorylation. Thus, we checked the effects of rolipram and Ariflo 2, 5, 7 and 10 min after fMLP addition and we did not detect any activation of MEK1/2 by PDE4 inhibitors, at any time (data not shown). We also investigated the effects of rolipram and Ariflo on p44/42MAPK in the absence of any activator and we observed that PDE4 inhibitors had no effect on p44/42MAPK in these conditions (data not shown). These results indicate that PDE4 inhibitors alone are unable to activate p44/42MAPK and require its preactivation by fMLP through the cascade c-Raf/MEK1/2. To assess whether or not PDE4 directly interact with p44/42MAPK, we immunoprecipitated PDE4 and we observed that PDE4 directly interacts with unphosphorylated p44/42MAPK. No phosphorylated p44/42MAPK was co-immunoprecipitated with PDE4. We observed a co-immunoprecipitation of p44/42MAPK using an anti-PDE4 antibody recognizing the four PDE4 isotypes, but not when using anti-PDE4B or anti-PDE4A antibodies, suggesting that p44/42MAPK was co-immunoprecipitated with PDE4D (PDE4C being not expressed in these cells). These data suggest that PDE4D could inhibit the phosphorylation of p44/42MAPK by a direct interaction. These results are consistent with previous studies (MacKenzie et al., 2000), which have shown a direct interaction between ERK2 (p42MAPK) and PDE4D3 and they are also supported by two papers, the first showing the interaction between beta-arrestins and MAP kinases (Defea et al., 2000) and the second between beta-arrestins and PDE4D (Perry et al., 2002), suggesting a likely interaction between MAPK and PDE4D.
In contrast to previous studies performed in different inflammatory cells (Wang et al., 1998; Germain et al., 2001), we showed that PDE4 inhibitors decreased O2•− release by a cAMP-independent pathway, since cAMP analogs increased O2•− release, whereas PDE4 inhibitors decreased it. Cyclic AMP activates PKA and PKA activates B-Raf and inhibits C-Raf, B-Raf and C-Raf both activating MEK1/2, then ERK1/2. We detected B-Raf and C-Raf by Western blot in rat BAL neutrophils and we observed that C-Raf was highly expressed in these cells, whereas B-Raf was weakly detected (data not shown), suggesting that the effects of cAMP analogs and PKA on ERK1/2 were more likely due to inhibition of C-Raf than activation of B-Raf. Thus, the resulting effect of an increase of cAMP levels would be an inhibition of ERK1/2 and we showed that the inhibition of ERK1/2 induced an activation of O2•− release. PDE4 inhibitors did increase cAMP levels, as they induced CREB phosphorylation (Figure 4b), which is mediated by a cAMP-induced PKA activation. Consequently, the effects of PDE4 inhibitors on fMLP-induced O2•− release must be due to both cAMP increase and ERK1/2 phosphorylation, this activation of ERK1/2 having a stronger effect on O2•− release than cAMP. The effects of PDE4 inhibitors on ERK1/2 could also be due to a side effect of the compounds, but this appears highly unlikely, as both PDE4 inhibitors tested have different chemical structures and their effects on ERK1/2 were observed in BAL cells enriched in neutrophils but not in BAL macrophages. However, the precise mechanism by which PDE4 inhibitors facilitate ERK1/2 activation remains unclear and needs to be further analysed.
In conclusion, this study allowed us to identify a novel mechanism involving MAPK in the pathway connecting PDE4 to fMLP-induced O2•− generation in neutrophils. We showed that the inhibitory effect of PDE4 inhibitors on O2•− production is mediated by the activation of p44/42MAPK and that PDE4 inhibit p44/42MAPK by a direct interaction. This study contributes to a better understanding of PDE4 inhibitors mechanisms of action and to a better use of these potent anti-inflammatory compounds.
Acknowledgments
To Professor Marco Conti (Department of Gynecology and Obstetrics, Stanford University Medical Center, CA, U.S.A.) to have provided the anti-PDE4 antibodies, for the use of his laboratory and equipment to achieve the last experiments of this study and for his helpful expertise.
Abbreviations
- A
Ariflo
- BAL
bronchoalveolar lavage
- cAMP
cyclic adenosine 3′,5′-cyclic monophosphate
- COPD
chronic obstructive pulmonary disease
- 8-CPT or 8-CPT cAMP
8-(4-Chlorophenylthio) adenosine 3′,5′-cyclic monophosphate
- dbcAMP
dibutyryl cyclic AMP
- DMSO
dimethylsulfoxide
- ERK
extracellular signal-regulated protein kinase
- fMLP
N-Formyl-Methionine-Leucine-Phenylalanine
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GMP
guanosine 3′,5′-cyclic monophosphate
- HBSS
Hank's balanced salt solution
- IFNγ
interferon gamma
- 1L-3 (-4, -6, -10)
interleukin-3 (-4, -6, -10)
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- O2•−
superoxide anion
- PD
PD98059
- PDE4
phosphodiesterases type 4
- PI3K
phosphatidylinositol-3 kinase
- PKA
protein kinase A
- PKAi
protein kinase A inhibitor
- PKC
protein kinase C
- PKCi
protein kinase C inhibitor
- PMA
phorbol 12-myristate 13-acetate
- 8-p MeOPT or 8-p MeOPT-2′-O-Me-cAMP
8-(4-Methoxyphenylthio) 2′-O-methyladenosine 3′,5′-cyclic monophosphate
- PP1
protein phosphatase 1
- PP2A
protein phosphatase 2A
- R
rolipram
- RBC
red blood cells
- ROS
reactive oxygen species
- SB
SB203580
- SOD
superoxide dismutase
- TNFα
tumor necrosis factor alpha
- W
wortmannin
References
- AHMAD F., GAO G., WANG L.M., LANDSTROM T.R., DEGERMAN E., PIERCE J.H., MANGANIELLO V.C. IL-3 and IL-4 activate cyclic nucleotide phosphodiesterases 3 (PDE3) and 4 (PDE4) by different mechanisms in FDCP2 myeloid cells. J. Immunol. 1999;162:4864–4875. [PubMed] [Google Scholar]
- ALVAREZ R., SETTE C., YANG D., EGLEN R.M., WILHELM R., SHELTON E.R., CONTI M. Activation and selective inhibition of a cyclic AMP-specific phosphodiesterase, PDE-4D3. Mol. Pharmacol. 1995;48:616–622. [PubMed] [Google Scholar]
- BAILLIE G.S., MACKENZIE S.J., MCPHEE I., HOUSLAY M.D. Sub-family selective actions in the ability of erk2 MAP kinase to phosphorylate and regulate the activity of PDE4 cyclic AMP-specific phosphodiesterases. Br. J. Pharmacol. 2000;131:811–891. doi: 10.1038/sj.bjp.0703636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNETTE M.S. Phosphodiesterase 4 (PDE4) inhibitors in asthma and chronic obstructive pulmonary disease (COPD) Prog. Drug Res. 1999;53:193–229. doi: 10.1007/978-3-0348-8735-9_5. [DOI] [PubMed] [Google Scholar]
- BARNETTE M.S., BARTUS J.O., BURMAN M., CHRISTENSEN S.B., CIESLINSKI L.B., ESSER K.M., PRABHAKAR U.S., RUSH J.A., TORPHY T.J. Association of the anti-inflammatory activity of phosphodiesterase 4 (PDE4) inhibitors with either inhibition of PDE4 catalytic activity or competition for [3H]rolipram binding. Biochem. Pharmacol. 1996;51:949–956. doi: 10.1016/0006-2952(96)00053-6. [DOI] [PubMed] [Google Scholar]
- BRADFORD M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN S.B., GUIDER A., FORSTER C.J., GLEASON J.G., BENDER P.E., KARPINSKI J.M., DEWOLF W.E., JR, BARNETTE M.S., UNDERWOOD D.C., GRISWOLD D.E., CIESLINSKI L.B., BURMAN M., BOCHNOWICZ S., OSBORN R.R., MANNING C.D., GROUS M., HILLEGAS L.M., BARTUS J.O., RYAN M.D., EGGLESTON D.S., HALTIWANGER R.C., TORPHY T.J. 1,4-Cyclohexanecarboxylates: potent and selective inhibitors of phosophodiesterase 4 for the treatment of asthma. J. Med. Chem. 1998;41:821–835. doi: 10.1021/jm970090r. [DOI] [PubMed] [Google Scholar]
- CONTI M., JIN S.L. The molecular biology of cyclic nucleotide phosphodiesterases. Prog. Nucleic Acid Res. Mol. Biol. 1999;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- DEFEA K.A., ZALEVSKY J., THOMA M.S., DERY O., MULLINS R.D., BUNNETT N.W. Beta-arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DENT G., POPPE H., EGERLAND J., MARX D., SZELENYI I., BRANSCHEID D., MAGNUSSEN H., RABE K.F. Effects of a selective PDE4 inhibitor, D-22888, on human airways and eosinophils in vitro and late phase allergic pulmonary eosinophilia in guinea pigs. Pulm. Pharmacol. Ther. 1998;11:13–21. doi: 10.1006/pupt.1998.0111. [DOI] [PubMed] [Google Scholar]
- DEWAS C., FAY M., GOUGEROT-POCIDALO M.A., EL-BENNA J. The mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway is involved in formyl-methionyl-leucyl-phenylalanine-induced p47(phox) phosphorylation in human neutrophils. J. Immunol. 2000;165:5238–5244. doi: 10.4049/jimmunol.165.9.5238. [DOI] [PubMed] [Google Scholar]
- DOWNEY G.P., BUTLER J.R., TAPPER H., FIALKOW L., SALTIEL A.R., RUBIN B.B., GRINSTEIN S. Importance of MEK in neutrophil microbicidal responsiveness. J. Immunol. 1998;160:434–443. [PubMed] [Google Scholar]
- ENGELS P., FICHTEL K., LUBBERT H. Expression and regulation of human and rat phosphodiesterase type IV isogenes. FEBS Lett. 1994;350:291–295. doi: 10.1016/0014-5793(94)00788-8. [DOI] [PubMed] [Google Scholar]
- ESCOFIER N., BOICHOT E., GERMAIN N., E SILVA P.M., MARTINS M.A., LAGENTE V. Effects of interleukin-10 and modulators of cyclic AMP formation on endotoxin-induced inflammation in rat lung. Fundam. Clin. Pharmacol. 1999;13:96–101. doi: 10.1111/j.1472-8206.1999.tb00326.x. [DOI] [PubMed] [Google Scholar]
- FRANCHINI M., GILLI U., AKENS M.K., FELLENBERG R.V., BRACHER V. The role of neutrophil chemotactic cytokines in the pathogenesis of equine chronic obstructive pulmonary disease (COPD) Vet. Immunol. Immunopathol. 1998;66:53–65. doi: 10.1016/s0165-2427(98)00178-0. [DOI] [PubMed] [Google Scholar]
- FRANCIS S.H., TURKO I.V., CORBIN J.D. Cyclic nucleotide phosphodiesterases: relating structure and function. Prog. Nucleic Acid Res. Mol. Biol. 2000;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- GERMAIN N., CORBEL M., BELLEGUIC C., BOICHOT E., LAGENTE V. Effects of PDE4 inhibitors on lipopolysaccharide-induced priming of superoxide anion production from human mononuclear cells. Mediators Inflamm. 2001;10:117–123. doi: 10.1080/09629350123856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIEMBYCZ M.A. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here. Drugs. 2000;59:193–212. doi: 10.2165/00003495-200059020-00004. [DOI] [PubMed] [Google Scholar]
- HOLM S. A simple sequentially rejective multiple test procedure. Scand. J. Stat. 1979;6:65. [Google Scholar]
- HOUSLAY MD. PDE4 cAMP-specific phosphodiesterases. Prog. Nucleic Acid Res. Mol. Biol. 2001;9:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- IONA S., CUOMO M., BUSHNIK T., NARO F., SETTE C., HESS M., SHELTON E.R., CONTI M. Characterization of the rolipram-sensitive, cyclic AMP-specific phosphodiesterases: identification and differential expression of immunologically distinct forms in the rat brain. Mol. Pharmacol. 1998;53:23–32. doi: 10.1124/mol.53.1.23. [DOI] [PubMed] [Google Scholar]
- KAMBAYASHI T., JACOB C.O., ZHOU D., MAZUREK N., FONG M., STRASSMANN G. Cyclic nucleotide phosphodiesterase type IV participates in the regulation of IL-10 and in the subsequent inhibition of TNF-alpha and IL-6 release by endotoxin-stimulated macrophages. J. Immunol. 1995;155:4909–4916. [PubMed] [Google Scholar]
- MACKENZIE S.J., BAILLIE G.S., MCPHEE I., BOLGER G.B., HOUSLAY M.D. ERK2 mitogen-activated protein kinase binding, phosphorylation, and regulation of the PDE4D cAMP-specific phosphodiesterases. The involvement of COOH-terminal docking sites and NH2-terminal UCR regions. J. Biol. Chem. 2000;275:16609–16617. doi: 10.1074/jbc.275.22.16609. [DOI] [PubMed] [Google Scholar]
- MACKENZIE S.J., HOUSLAY M.D. Action of rolipram on specific PDE4 cAMP phosphodiesterase isoforms and on the phosphorylation of cAMP-response-element-binding protein (CREB) and p38 mitogen-activated protein (MAP) kinase in U937 monocytic cells. Biochem. J. 2000;347:571–578. doi: 10.1042/0264-6021:3470571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCPHEE I., YARWOOD S.J., SCOTLAND G., HUSTON E., BEARD M.B., ROSS A.H., HOUSLAY E.S., HOUSLAY M.D. Association with the SRC family tyrosyl kinase LYN triggers a conformational change in the catalytic region of human cAMP-specific phosphodiesterase HSPDE4A4B. Consequences for rolipram inhibition. J. Biol. Chem. 1999;274:11796–11810. doi: 10.1074/jbc.274.17.11796. [DOI] [PubMed] [Google Scholar]
- NICK J.A., AVDI N.J., YOUNG S.K., KNALL C., GERWINS P., JOHNSON G.L., WORTHEN G.S. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and FMLP. J. Clin. Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OWENS R.J., CATTERALL C., BATTY D., JAPPY J., RUSSELL A., SMITH B., O'CONNELL J., PERRY M.J. Human phosphodiesterase 4A: characterization of full-length and truncated enzymes expressed in COS cells. Biochem. J. 1997;326:53–60. doi: 10.1042/bj3260053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARTRICK D.A., MOORE E.E., OFFNER P.J., MELDRUM D.R., TAMURA D.Y., JOHNSON J.L., SILLIMAN C.C. Maximal human neutrophil priming for superoxide production and elastase release requires p38 mitogen-activated protein kinase activation. Arch. Surg. 2000;135:219–225. doi: 10.1001/archsurg.135.2.219. [DOI] [PubMed] [Google Scholar]
- PERRY S.J., BAILLIE G.S., KOHOUT T.A., MCPHEE I., MAGIERA M.M., ANG K.L., MILLER W.E., MCLEAN A.J., CONTI M., HOUSLAY M.D., LEFKOWITZ R.J. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- ROCQUE W.J., TIAN G., WISEMAN J.S., HOLMES W.D., ZAJAC-THOMPSON I., WILLARD D.H., PATEL I.R., WISELY G.B., CLAY W.C., KADWELL S.H., HOFFMAN C.R., LUTHER M.A. Human recombinant phosphodiesterase 4B2B binds (R)-rolipram at a single site with two affinities. Biochemistry. 1997;36:14250–14261. doi: 10.1021/bi971112e. [DOI] [PubMed] [Google Scholar]
- SOUNESS J.E., GRIFFIN M., MASLEN C., EBSWORTH K., SCOTT L.C., POLLOCK K., PALFREYMAN M.N., KARLSSON J.A. Evidence that cyclic AMP phosphodiesterase inhibitors suppress TNF alpha generation from human monocytes by interacting with a ‘low-affinity' phosphodiesterase 4 conformer. Br. J. Pharmacol. 1996;118:649–658. doi: 10.1111/j.1476-5381.1996.tb15450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOUNESS J.E., RAO S. Proposal for pharmacologically distinct conformers of PDE4 cyclic AMP phosphodiesterases. 1997;9:227–236. doi: 10.1016/s0898-6568(96)00173-8. [DOI] [PubMed] [Google Scholar]
- SPINA D. The potential of PDE4 inhibitors in asthma or COPD. Curr. Opin. Invest. Drugs. 2000;1:204–213. [PubMed] [Google Scholar]
- TORPHY T.J. Phosphodiesterase isozymes: molecular targets for novel antiasthma agents. Am. J. Respir. Crit. Care Med. 1998;157:351–370. doi: 10.1164/ajrccm.157.2.9708012. [DOI] [PubMed] [Google Scholar]
- TOWBIN H., STAEHELIN T., GORDON J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnology. 1992;24:145–149. [PubMed] [Google Scholar]
- WANG J.P., RAUNG S.L., HUANG L.J., KUO S.C. Involvement of cyclic AMP generation in the inhibition of respiratory burst by 2-phenyl-4-quinolone (YT-1) in rat neutrophils. Biochem. Pharmacol. 1998;56:1505–1514. doi: 10.1016/s0006-2952(98)00265-2. [DOI] [PubMed] [Google Scholar]
- WANG P., WU P., OHLETH K.M., EGAN R.W., BILLAH M.M. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol. Pharmacol. 1999;56:170–174. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- YAMANE H., SUGIMOTO Y., TANAKA S., ICHIKAWA A. Prostaglandin E(2) receptors, EP2 and EP4, differentially modulate TNF-alpha and IL-6 production induced by lipopolysaccharide in mouse peritoneal neutrophils. Biochem. Biophys. Res. Commun. 2000;278:224–228. doi: 10.1006/bbrc.2000.3779. [DOI] [PubMed] [Google Scholar]
- ZERVOS A.S., FACCIO L., GATTO J.P., KYRIAKIS J.M., BRENT R. Mxi2, a mitogen-activated protein kinase that recognizes and phosphorylates Max protein. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10531–10534. doi: 10.1073/pnas.92.23.10531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H., SHI X., HAMPONG M., BLANIS L., PELECH S. Stress-induced Inhibition of ERK1 and ERK2 by Direct Interaction with p38 MAP Kinase. J. Biol. Chem. 2001;276:6905–6908. doi: 10.1074/jbc.C000917200. [DOI] [PubMed] [Google Scholar]
- ZU Y.L., AI Y., GILCHRIST A., LABADIA M.E., SHA'AFI R.I., HUANG C.K. Activation of MAP kinase-activated protein kinase 2 in human neutrophils after phorbol ester or fMLP peptide stimulation. Blood. 1996;87:5287–5296. [PubMed] [Google Scholar]
- ZU Y.L., QI J., GILCHRIST A., FERNANDEZ G.A., VAZQUEZ-ABAD D., KREUTZER D.L., HUANG C.K., SHA'AFI R.I. p38 mitogen-activated protein kinase activation is required for human neutrophil function triggered by TNF- alpha or FMLP stimulation. J. Immunol. 1998;160:1982–1989. [PubMed] [Google Scholar]