Abstract
The many components of G-protein-coupled receptor (GPCR) signal transduction provide cells with numerous combinations with which to customize their responses to hormones, neurotransmitters, and pharmacologic agonists. GPCRs function as guanine nucleotide exchange factors for heterotrimeric (α, β, γ) G proteins, thereby promoting exchange of GTP for GDP and, in turn, the activation of ‘downstream' signaling components. Recent data indicate that individual cells express mRNA for perhaps over 100 different GPCRs (out of a total of nearly a thousand GPCR genes), several different combinations of G-protein subunits, multiple regulators of G-protein signaling proteins (which function as GTPase activating proteins), and various isoforms of downstream effector molecules. The differential expression of such protein combinations allows for modulation of signals that are customized for a specific cell type, perhaps at different states of maturation or differentiation. In addition, in the linear arrangement of molecular interactions involved in a given GPCR–G-protein–effector pathway, one needs to consider the localization of receptors and post-receptor components in subcellular compartments, microdomains, and molecular complexes, and to understand the movement of proteins between these compartments. Co-localization of signaling components, many of which are expressed at low overall concentrations, allows cells to tailor their responses by arranging, or spatially organizing in unique and kinetically favorable ways, the molecules involved in GPCR signal transduction. This review focuses on the role of lipid rafts and a subpopulation of such rafts, caveolae, as a key spatial compartment enriched in components of GPCR signal transduction. Recent data suggest cell-specific patterns for expression of those components in lipid rafts and caveolae. Such domains likely define functionally important, cell-specific regions of signaling by GPCRs and drugs active at those GPCRs.
Keywords: Caveolae, lipid rafts, β-adrenergic receptors, adenylyl cyclase, compartmentation, G protein-coupled receptors, G proteins
Introduction
Ever since ideas developed by Ehrlich, Langley, Dale, and others in the early 20th century, pharmacologists have recognized the key role of receptors as entities for extracellular agonists to regulate target cells. The emergence of new data led to the general concept of signal transduction, in particular across the lipid bilayer of the plasma membrane that separates the predominantly hydrophilic extracellular and intracellular environments. With the initial completion of the human genome project and the deciphering of the genomes of other species, it has become clear that signal transduction molecules are highly represented in numerous genomes. For example, membrane-bound G-protein-coupled receptors (GPCRs) are one of the largest superfamilies, comprising approximately 3% of human genes. Recent studies suggest that there are 750–800 human GPCRs (Fredriksson et al., 2003; Vassilatis et al., 2003) and that individual cells express >100 different GPCR, a substantial number of different subunits for heterotrimeric (α, β, γ) G proteins and multiple G-protein-linked effectors (Hakak et al., 2003; Ostrom et al., 2003; Tang & Insel, 2004). Expression of such large numbers of the critical components involved in GPCR signaling leads to many questions, some of which we will address in this review: What are the absolute and relative levels of protein expression of the different components? How do cells organize such components in a kinetically favorable way so as to promote rapid changes in second messenger formation? Do development, differentiation, aging, and disease states alter such organization? Does such organization play an important role in influencing pharmacologic responses?
Overview of GPCR signaling and questions
Although individual GPCRs can couple to multiple heterotrimeric G proteins, many GPCRs appear to couple preferentially to particular G proteins. This interaction, primarily driven by intrinsic affinity between the GPCR and G protein, is favored by agonist occupancy of the GPCR. Certain GPCRs can couple to more than one G protein. Examples of such ‘promiscuous' receptors include P2Y11, β2AR, β3AR, 5HT2C, and several dopamine receptor subtypes (Hermans, 2003). In addition, different agonists that bind to the same receptor appear able to direct the G-protein pathway that is activated by the receptor, a process termed ligand-selective agonism; such patterns of response differ among different cell types that express the same receptor (Kenakin, 2001; 2003). What are the critical cellular and molecular determinants of such patterns of GPCR–G protein coupling? Might the coupling, which was termed in early work, ‘collision coupling' (Levitzki & Bar-Sinai, 1991), of these receptors to one of several potential pathways be governed by compartmentation (i.e., co-localization of components)? Although it is tempting to speculate that the answer is ‘yes', no unequivocal evidence has thus far been shown to support this view.
Each of the four principal subtypes of heterotrimeric G proteins regulate particular effector systems: Gs stimulates adenylyl cyclase (AC) activity and regulates certain Ca2+ channels; Gi inhibits AC activity and regulates K+ and Ca2+ channels, Gq/11 stimulates phospholipase C (PLC) activity, and G12/13 regulates GTP exchange factors (GEF) of rho, a low-molecular-weight G protein. Thus, α subunits of heterotrimeric G proteins likely possess intrinsic affinity for a particular effector; in addition, regulation of the activity of such effectors occurs via actions of beta–gamma subunits (Clapham & Neer, 1997). One can readily imagine how compartmentation could contribute to this step in the pathways, as the short activation cycle of Gα proteins likely limits their effective radius of activity. The kinetics of G protein activation is determined by the intrinsic GTPase activity of Gα and by enhancement of this activity by effector molecules and RGS proteins, which serve as GTPase-activating proteins, or GAPs (Dohlman & Thorner, 1997; Zheng et al., 1999; 2001). RGS proteins display inherent affinity for terminating the activation of particular Gα proteins, an action that has been termed ‘kinetic scaffolding' or ‘spatial focusing' (Ross & Wilkie, 2000; Zhong et al., 2003). The Gβγ heterodimer functions as a single entity and regulates effector molecules and other proteins involved in GPCR signaling, in particular when not interacting with Gα subunits in their GDP-bound (inactive) state.
Is the signaling by Gα subunits dependent upon co-localization with effectors? The G-protein-regulated enzymes AC and PLC generate key second messengers following activation of certain GPCRs. AC catalyzes the synthesis from ATP of cyclic adenosine 3′,5′ monophosphate (cAMP), which, in turn, regulates cell function primarily via activation of cyclic AMP-dependent protein kinase (PKA). PKA phosphorylates serine and threonine residues on targets to initiate cellular actions of cAMP; this phosphorylation is targeted to specific substrates via A kinase anchoring proteins (AKAPs) (Michel & Scott, 2002). Recent data indicate that cAMP can also act via PKA-independent mechanisms, including the GEF of Rap-1, a low-molecular-weight G protein, and certain cAMP-gated ion channels (Kawasaki et al., 1998; Yatani et al., 1999; Kopperud et al., 2003). PLC hydrolyzes phosphatidylinositol bisphosphate (PIP2) into inositol trisphosphate (IP3) and diacylglycerol (DAG). IP3 causes release of calcium from intracellular stores while DAG activates protein kinase C (PKC), second messengers that have rapid and delayed effects on cell metabolism and function.
In addition to the ability of RGS proteins to accelerate GTP hydrolysis and contribute to termination of GPCR signaling, other mechanisms contribute to homologous (receptor-specific) and heterologous (receptor-nonspecific) desensitization of GPCRs. A key mechanism for homologous desensitization involves G-protein receptor kinases (GRKs), which are recruited to active GPCR, in part, at least for some GRKs, via their affinity for free Gβγ subunits (Krupnick & Benovic, 1998; Lefkowitz, 1998). GRKs phosphorylate the associated GPCR, causing the recruitment of β-arrestin, which impairs receptor interaction with G proteins and can act as an adapter between the receptors and clathrin-coated pits (Luttrell & Lefkowitz, 2002; Kohout & Lefkowitz, 2003). In many cases, association of a GPCR with clathrin-coated pits leads to internalization of the GPCR in clathrin-coated vesicles and eventual degradation of the receptor via lysosomes (or proteasomes (von Zastrow, 2003)) or recycling of the receptor back to the plasma membrane (Ferguson, 2001). Clathrin-coated pits thus function as a membrane microdomain involved in signal termination, but, in addition, in the activation of certain intracellular events such as activation of mitogen-activated protein kinase cascades (Hall et al., 1999; Pierce et al., 2001).
A basic tenet of signal transduction by GPCRs is that high-affinity protein–protein interactions determine the G-protein heterotrimer and, in turn, the signal transduction pathway that a particular GPCR activates. However, different receptors that couple to the same G protein can elicit different biochemical, cellular or physiological responses (Steinberg & Brunton, 2001). A ‘classical' one-dimensional view of GPCR signal transduction (i.e., that a given GPCR activates a single G-protein and single effector) cannot readily account for such observations. Moreover, GPCRs, G proteins, and effector enzymes are expressed at relatively low concentrations in mammalian cells. This is particularly the case for GPCRs and effectors (Alousi et al, 1991; Milligan, 1996; Ostrom et al., 2000a), yet GPCR–G-protein–effector systems display rapid, high-fidelity signaling characteristics. Such observations make the idea that the components of GPCR signal transduction are ‘pre-arranged' or ‘selectively compartmentalized' quite attractive.
Signaling proteins must physically interact in order to transmit information. It is presumed that, once activated, most signaling molecules possess inherent high affinity for binding to their partners, but data on the affinity of such interactions are limited. However, even high-affinity interactions require effective concentrations of the reactants in order to thermodynamically favor rapid conformational changes (in the case of proteins) and information exchange between molecules. Therefore, mechanisms likely exist for rapid and efficient signal transduction, since bulk concentrations of the reactants are relatively low. Typical receptor concentrations are <∼10,000/cell while certain effectors, such as ACs, appear to be expressed at similar orders of magnitude (Alousi et al., 1991; Post et al., 1995; Milligan, 1996). In GPCR signaling, receptors, G proteins, effector enzymes, and key accessory proteins, the latter of which may be quite numerous (Bockaert et al., 2003), are generally thought to be membrane-associated. Given the overall low abundance of key signaling molecules in cells, uniform distribution within the plasma membrane seems unlikely to provide sufficient enrichment to achieve the rapid signaling responses characteristic of GPCR activation. Since target cells have relatively low concentrations of signaling components, one way to account for rapid response is that cells concentrate signaling molecules in membrane microdomains.
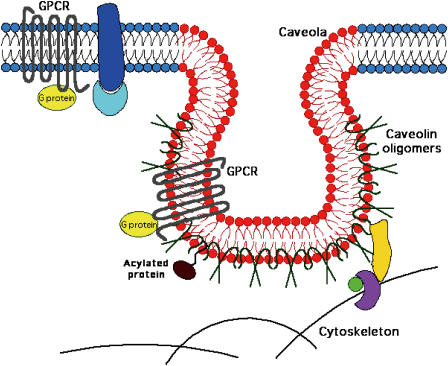
One such microdomain may be lipid rafts in the plasma membrane that are formed by the coalescence of sphingolipid and cholesterol. Caveolae are 50–100 nm flask-like indentations, or ‘little caves' of the plasma membrane that have a lipid composition similar to rafts but that also contain caveolin proteins localized on the inner leaflet of the membrane bilayer (Anderson, 1998) (Figure 1). Based on their similar lipid composition, caveolae are generally considered subsets of lipid rafts but these entities may have other differences (Sowa et al., 2001; Williams & Lisanti, 2004). While only cells expressing caveolins express caveolae, all mammalian cells express lipid rafts (Hooper, 1999); leukocytes, for example, have lipid rafts but not caveolae. There are three isoforms of caveolins: caveolin-1, caveolin-2, and caveolin-3. Caveolae generally form if cells express either caveolin-1, the predominant isoform, or caveolin-3, the striated muscle-specific isoform (Song et al., 1996a; Tang et al., 1996). Caveolin-2 is found in hetero-oligomers with caveolin-1 and caveolin-3; it is not clear if caveolin-2 can induce caveolae biosynthesis on its own (Scherer et al., 1996; 1997; Razani et al., 2002b; Lahtinen et al., 2003; Rybin et al., 2003). Thus, enrichment of GPCR signaling components in lipid rafts or caveolae may be a universal mechanism for increasing the effective concentration of these proteins by restricting their movement, thereby favoring interaction of components in the signal transduction pathway.
Figure 1.
Schematic representation of the lipid and protein organization of a caveola. Sphingolipid- and cholesterol-rich domain is shown in red and nonraft lipid domains are shown in blue. Caveolae contain a coat of oligomeric caveolin molecules inserted into the cytoplasmic leaflet of the membrane. Some proteins, including certain GPCR (shown as heptahelical structures with associated G protein), partition to caveolar domains due to either acylation, binding to caveolin or formation of a sphingolipid ‘shell' around the protein (or by a combination of these, and/or yet unknown, mechanisms). Also shown are undefined cytoskeletal interacting proteins (orange, green, purple) and noncaveolar membrane proteins (blue) and partners (light blue).
Methodologically, lipid rafts and caveolae are most often studied by disrupting cells, extracting these domains based upon their insolubility in certain detergent or nondetergent conditions, then isolating them by centrifugation based upon their differential buoyancy in a gradient (Anderson, 1998; Ostrom et al., 2000a; Pike, 2003). Such methodologies cannot distinguish between caveolae and lipid rafts since they rely upon properties common to both these domains. While lipid rafts were initially described as detergent-resistant membrane (DRM) fractions, nondetergent approaches are now generally preferred, based, at least in part, upon their ability to exclude nonmembrane markers and to include certain loosely associated membrane proteins (Smart et al., 1995; Shaul et al., 1996; Song et al., 1996b; Rybin et al., 1999). Caveolae can be preferentially isolated from lipid rafts using immunological approaches to trap caveolin-rich membrane domains (Oh & Schnitzer, 1999; Ostrom et al., 2001). Use of these approaches has led to the idea that lipid rafts and caveolae share common qualities but can differ in terms of the nature of the signaling proteins they contain (Oh & Schnitzer, 1999). The role of caveolins as scaffolding proteins has also been assessed using immunoprecipitation of caveolin or by expressing peptides that interfere with the caveolin-binding motif, a domain on caveolin-1 and caveolin-3 that preferentially serves as a docking site for binding (and inhibiting) signaling molecules (Okamoto et al., 1998). The function of lipid rafts and caveolae in signaling or other cellular processes can also be inferred from studies using cholesterol depletion. Methyl-β-cyclodextrin, a cholesterol-binding agent, can remove cholesterol from cells in culture and disrupt lipid rafts and caveolae. Filipin, a polyene antibiotic and sterol-binding agent, is generally more cytotoxic, but can also be used to disrupt lipid rafts and caveolae, albeit with less efficiency in certain cells (Schnitzer et al., 1994; Orlandi & Fishman, 1998; Awasthi-Kalia et al., 2001). Both methods of cholesterol depletion are associated with nonspecific effects that must be controlled for, usually by adding back cholesterol to cells in order to show reversibility and specificity of action of the agent.
Compartmentation of GPCR signaling
The concept of compartmentation (compartmentalization) as a means to achieve selective responses to certain GPCR agonists is not new, but the identification of caveolins has provided a molecular ‘tag' to assist in biochemically defining caveolae as a subset of lipid rafts, and to show that signal transduction proteins are enriched in lipid rafts and/or caveolae (Anderson, 1998; Razani et al., 2002c; Pike, 2003). Mitogen-activated protein kinases and receptor tyrosine kinases were first recognized as residing in caveolin-rich microdomains; certain GPCRs and associated molecules were subsequently shown to be enriched in these domains (Chun et al., 1994; de Weerd & Leeb-Lundberg, 1997; Feron et al., 1997; Schwencke et al., 1999b). The bulk of the work to date has relied upon biochemical isolation of lipid rafts and caveolae, typically using sucrose density centrifugation, an approach that cannot distinguish between these two domains. We will use this operational definition in our discussion.
Compartmentation of GPCR signaling proteins in caveolae or lipid rafts appears to be a major determinant of receptor–effector coupling. β1AR and β2AR are enriched with AC type 6 (AC6) in caveolae or lipid rafts (buoyant, caveolin-rich membrane fractions) of cardiac myocytes, together with a portion of the membrane Gs, and these receptors couple efficiently to the activation of AC6. (Schwencke et al., 1999a, 1999b; Ostrom et al., 2000b; 2001; Rybin et al., 2000; Xiang et al., 2002b). However, not all GPCRs (or G proteins) are found in these lipid raft fractions. For example, in cardiac myocytes, prostanoid EP2 receptors are excluded from lipid raft fractions and cannot activate AC6 despite their ability to activate Gs (Ostrom et al., 2001). Gs is found in both lipid rafts and nonraft fractions (Rybin et al., 2000; Ostrom et al., 2001). In addition, cardiac myocyte β2AR activate AC6 less efficiently than do β1AR, and this appears attributable to rapid agonist-promoted translocation of β2AR into clathrin-coated pits (Goodman et al., 1996; Gagnon et al., 1998) and out-of-lipid rafts and caveolae compared to β1AR, which, at least initially, remain localized with AC6 in caveolae upon agonist activation (Rybin et al., 2000; Ostrom et al., 2001). Therefore, the degree to which a given GPCR couples to a signaling pathway appears to depend upon a close physical association between an activated R–G complex and a suitable effector enzyme.
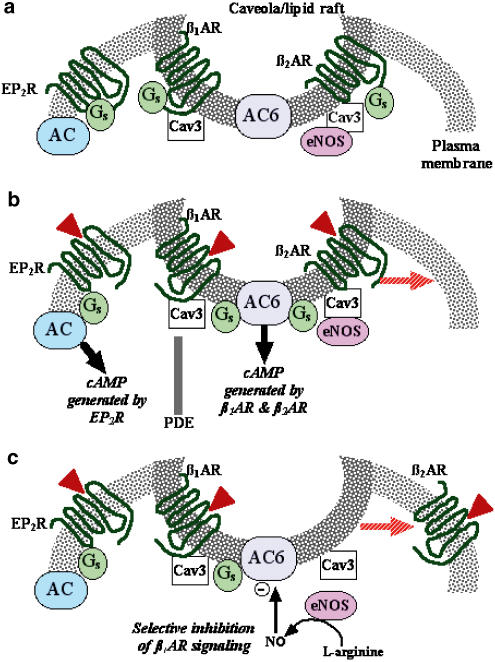
Such intimate association of signaling proteins in lipid rafts or caveolae also appears to be important for crosstalk between different signaling pathways. We have recently shown that, in cardiac myocytes, nitric oxide (NO) production by endothelial nitric oxide synthase (e.g., NOS3, eNOS) inhibits βAR-stimulated cAMP production, but has little effect on prostanoid-stimulated cAMP accumulation (Ostrom et al., 2004). This selective effect of eNOS activity appears attributable to two factors: (1) activity of AC6 (and AC5 but not other AC isoforms) is inhibited via direct nitrosylation by NO (McVey et al., 1999; Hill et al., 2000), and (2) βAR, but not prostanoid receptors, couple to AC6 due to βAR-AC6 localization in lipid rafts or caveolae. Disruption of lipid rafts with methyl-β-cyclodextrin treatment uncovers an NO-mediated inhibition of the prostanoid response in conditions of high eNOS activity, indicating that the organization provided by lipid rafts or caveolae normally compartmentalizes the NO signal with the components of the βAR signal transduction pathway. Figure 2 illustrates this concept.
Figure 2.
Schematic representation of GPCR-Gs-AC signaling in lipid rafts and caveolae of cardiac myocytes and the effects of eNOS. (a) Caveolar/lipid raft domains contain β1AR, β2AR, AC6, Gs, eNOS, and caveolin-3 (Cav3), but exclude prostanoid EP2R (Ostrom et al., 2000b). (b) Catecholamines stimulate βAR and the generation of cAMP in the caveolar/lipid raft domain. Prostanoids activate receptors located outside lipid rafts and, as a result of compartmentation by phosphodiesterases (PDE), a separate pool of cAMP is generated. β2AR translocate out of caveolae upon agonist activation, presumably to internalize via clathrin-coated pits, thus generating a more transient activation of the caveolar/lipid raft pool of cAMP (Ostrom et al., 2001). (c) Activation of eNOS activity leads to the generation of high NO concentrations in the caveolar/lipid raft domain. Due to the localization of eNOS in caveolae and the sensitivity of AC6 to NO-mediated nitrosylation, eNOS activity selectively inhibits the caveolar/lipid raft pool of cAMP (i.e., that stimulated by βAR) (Ostrom et al., 2004).
New data shed some light on the different behaviors of β1AR and β2AR with respect to association with lipid rafts. Using phosphorylation-deficient mutants of the β1AR, Rapacciuolo et al. (2003) have suggested that PKA-mediated phosphorylation directs internalization of the receptor via caveolae, while GRK-promoted phosphorylation directs internalization via clathrin-coated pits. Although such results are of interest, it is unclear whether β1AR are primarily phosphorylated by GRK in native cell settings and whether such results with the β1AR can be applied to β2AR or other GPCRs. β1AR and β2AR are known to signal differently in cardiac myocytes, where they are co-expressed, due to β2AR coupling sequentially to Gs and then Gi (Xiao et al., 1994; 1999). Intact lipid rafts are necessary for the β2AR to couple to Gi (Xiang et al., 2002b); both β1AR and β2AR require a carboxy-terminal PDZ-binding motif and interaction with PDZ domain-containing proteins for their signaling and trafficking behaviors (Xiang et al., 2002a; Xiang & Kobilka, 2003). Such data suggest that interaction of βAR with PDZ-containing proteins is related to their co-localization in lipid rafts; we speculate that this is the case, but further studies are needed to test this idea.
Studies of the oxytocin receptor in MDCK cells provide another example of localization determining receptor signaling. In those cells, this Gq-coupled receptor is predominantly expressed in nonraft domains and its activation inhibits cell growth (Guzzi et al., 2002). However, fusion of the oxytocin receptor with caveolin-2 localizes it to lipid rafts and ‘switches' receptor activation to stimulation of cell proliferation. While this latter effect might result from altered desensitization of the oxytocin receptor fused to caveolin (the chimeric receptor does not internalize upon agonist exposure), an alternative possibility is that localization of the receptor in lipid rafts leads to its coupling to effector molecules that elicit a different cellular response.
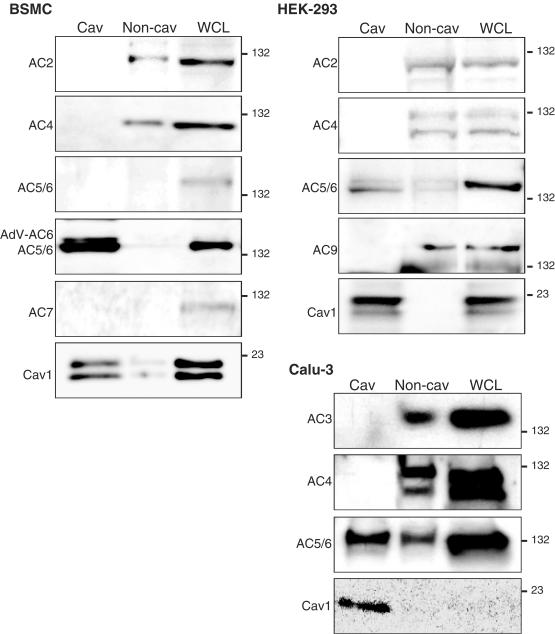
Recent evidence indicates that, of the nine isoforms of the transmembrane ACs, only certain isoforms localize to lipid rafts. Different tissues express different patterns of expression of these AC isoforms, which are subject to various types of regulation by intracellular factors (Hanoune & Defer, 2001; Ludwig & Seuwen, 2002). Using immunoblot analysis of lipid raft fractions, we have analyzed the AC isoform expression and localization in several cell types. Adult rat cardiac fibroblasts express AC2, AC3, AC4, AC5/6, and AC7, but only AC3 and AC 5/6 proteins (AC5 and AC6 are detected by a single antibody) are enriched in lipid raft fractions (Ostrom et al., 2003). Fractionation and immunoblot analysis of AC isoforms expressed in cultured human bronchial smooth muscle cells (BSMC) indicate that AC2 and AC4 are the most readily detected AC isoforms, but these isoforms are not detected in caveolin-rich, lipid raft fractions (Figure 3). Results from similar studies with HEK-293 cells, airway epithelial cells, and vascular smooth muscle cells are consistent with the idea that AC3, AC5 and AC6, but perhaps not all other AC isoforms, preferentially localize to lipid rafts (Figure 3; Ostrom et al., 2002). We have not detected endogenous AC8 expression in any of the cells we have examined, but data from others indicate that this isoform, too, localizes in lipid rafts (Fagan et al., 2000; Smith et al., 2002).
Figure 3.
Immunoblot analyses of AC isoform expression in caveolae/lipid raft fractions isolated from human BSMC, HEK-293 cells or human airway epithelial cells (Calu-3). Buoyant lipid raft fractions were isolated from the indicated cells using a nondetergent method followed by centrifugation on a sucrose density gradient, as described previously (Ostrom et al., 2004). The buoyant fractions (cav) and the nonbuoyant fractions (non-cav) were collected from the gradient and, along with whole-cell lysates (WCL), were subjected to SDS–PAGE and immunoblot analyses. The data and others discussed in the text are consistent with the idea that AC5, AC6, and AC3 (except in Calu-3 cells) localize to caveolae/lipid raft fractions, while other AC isoforms localize to nonraft fractions. AC8 has also been shown to localize to lipid raft fractions (Fagan et al., 2000; Smith et al., 2002).
The localization of AC isoforms in lipid raft or caveolar microdomains is likely to be important for the regulation of the enzyme. Overexpression of AC6, which is subject to inhibition by multiple regulators (Ostrom et al., 2000a, 2000b), does not increase basal cAMP production in cardiac myocytes. By contrast, expression of AC6 in RASMC can lead to nonraft localization of the enzyme and a concomitant increase in basal cAMP production (Ostrom et al., 2002). Thus, localization of AC6 (and perhaps other isoforms) in lipid rafts may serve to maintain a low basal activity of the enzyme, perhaps, in the case of AC6 which is inhibited by calcium, via co-localization with sites of calcium entry (Fagan et al., 2000; Lohn et al., 2000) and/or NO generation (Ostrom et al., 2004). Expression of AC8 in HEK-293 cells leads to localization of this calcium-stimulable isoform in lipid rafts and imparts an activation of cAMP production by capacitive calcium entry (Smith et al., 2002; Cooper, 2003), which is likely mediated by lipid raft-localized Trp1 channel (Lockwich et al., 2000; Clapham et al., 2001). Thus, the localization of calcium-sensitive AC isoforms appears critical for determining the type of calcium signal that regulates cAMP production.
Different types of G proteins appear to segregate differently with respect to lipid rafts versus caveolae, an observation suggesting that differences exist between the two domains. Although this issue has not been extensively studied, certain data have shown that Gq preferentially localizes in caveolae, while Gs and Gi localize in lipid rafts (Oh & Schnitzer, 2001). Consistent with the idea that expression of caveolin is a major distinguishing feature between lipid rafts and caveolae, Oh & Schnitzer (2001) found that Gq, but not Gs and Gi, could be immunoprecipitated with caveolin-1, but if a cell lacked caveolin expression Gq would localize in lipid rafts. Thus, while all three G proteins can be found in lipid rafts, Gq ‘prefers' caveolae in cells that express caveolin (and contain morphologic caveolae), presumably due to its ability to bind to the caveolin scaffolding domain. These data highlight a fundamental question: What causes a protein to localize to lipid rafts or caveolae? Some proteins require interaction with caveolin, implying that such proteins will preferentially localize in caveolae (relative to lipid rafts, such as Gs and Gi, in the study of Oh & Schnitzer, 2001), while other proteins do not interact with caveolin and thus would be found in the lipid environment common to both lipid rafts and caveolae. As will be discussed below, the cell type in which a given signaling protein is expressed may also be a critical determinant of lipid raft or caveolar localization.
Another G protein, transducin, has been shown to translocate to lipid rafts upon activation by its cognate GPCR, rhodopsin, and its agonist, light, as part of the activation pathway of cGMP phosphodiesterase, and in turn cGMP-gated cation channels (Nair et al., 2002). Interestingly, the translocation of transducin appears to occur in a complex with RGS9, Gβγ, and arrestin. Given that rhodopsin and the cGMP phosphodiesterase were found in both lipid raft and nonraft fractions but that guanylyl cyclase was found only in lipid rafts of vertebrate retina, translocation of the transducin complex appears to favor interaction between the activated G protein and the pools of effector (cGMP phosphodiesterase) that are localized with the site of second messenger synthesis (guanylyl cyclase in the lipid raft). It is not known whether the cGMP-gated cation channels are co-localized in lipid rafts of vertebrate photoreceptor cells. These data in the retinal system strongly suggest that large multi-protein complexes can translocate in a rapid manner to facilitate interaction of components of GPCR signaling.
Agonist activation of βAR recruits β-arrestin to the plasma membrane, where it interacts with the activated receptor and, in addition, can recruit cyclic nucleotide phosphodiesterase (in particular, the PDE4 isoform), which degrades cAMP (Perry et al., 2002; Baillie et al., 2003). Therefore, β-arrestin translocation to the plasma membrane enhances the local degradation of cAMP and terminates the stimulus by desensitizing the receptor. Furthermore, β-arrestin-PDE4 recruitment to activated β2AR appears critical for regulating a G protein switching (from Gs to Gi) of this receptor by limiting its phosphorylation by PKA (the key step in causing the G protein switch (Baillie et al., 2003)). Given that β2AR and AC are expressed in lipid rafts and/or caveolae (Rybin et al., 2000; Ostrom et al., 2001) and that pools of cAMP localize in cardiac T-tubules where the bulk of caveolae can be found (Zaccolo & Pozzan, 2002), it is evident that lipid rafts and caveolae are likely to be key sites for both cAMP generation and cAMP response (Insel, 2003). Thus, cAMP-PKA signaling is highly localized in and targeted to multiple subcellular compartments, increasing its specificity for certain biological effects (Tasken & Aandahl, 2004).
Numerous ion channel proteins or subunits have been described as associating with lipid rafts, including Kv1.4, Kv1.5, Kv4.2, L-type Ca2+ channel, the plasma membrane Ca2+ pump, voltage-gated Na+ channel, Aquaporin-1, Trp4, and Trp1 (Schnitzer & Oh, 1996; Page et al., 1998; Darby et al., 2000; Martens et al., 2000; 2004; Torihashi et al., 2002; Yarbrough et al., 2002; Bergdahl et al., 2003; Brady et al., 2004; Wong & Schlichter, 2004). Of these, however, only a few have thus far been shown to display direct regulation by GPCR in a lipid raft-dependent manner. Voltage-gated Na+ channels expressed in the heart associate with lipid rafts and can be activated by βAR-Gs in a cAMP-independent manner (Yarbrough et al., 2002). As the introduction of a caveolin-3 antibody inhibits the activation of voltage-gated Na+ channel activity by a βAR agonist, the pathway appears to depend upon interaction of a co-localized receptor, G protein, and the channel that is facilitated by the caveolin scaffold. Other data indicate that endothelin-induced contraction of endothelium-denuded arteries is partially dependent upon activation of Trp1 channels, which localize with ETA receptors in lipid rafts (Bergdahl et al., 2003). In that system, depletion of cellular cholesterol (a treatment that disrupts caveolae and lipid rafts) causes both the loss of Trp1-caveolin-1 co-localization and a diminution of the endothelin-mediated contractile response. Thus, regulation of vascular reactivity by endothelin appears dependent, at least in part, on the co-localization of ET receptors with key effector molecules, Trp1 channels. Cyclic nucleotide-gated ion channels, effector molecules of light and olfactory receptors, localize to lipid rafts in olfactory epithelium and, when heterologously expressed, in HEK-293 cells (Brady et al., 2004). Cholesterol depletion of HEK-293 cells expressing a cyclic nucleotide-gated ion channel abolishes the stimulation of channel activity by prostaglandin, but this effect is likely attributable to diminished affinity of the channel for cAMP rather than a loss of juxtaposition of the GPCR and the channel.
While much work on the compartmentation of cAMP generation focuses on differentiated responses (such as regulation of contractility), other data imply that cAMP regulation of certain metabolic pathways may also show compartmentation in lipid raft versus nonraft domains. Disruption of caveolae and lipid rafts by cholesterol depletion inhibits glycolysis but stimulates gluconeogenesis in vascular smooth muscle cells (Lloyd & Hardin, 2001), likely reflective of the fact that a major glycolytic enzyme, phosphofructokinase, is expressed in lipid rafts in those cells (Vallejo & Hardin, 2004). Thus, cAMP generated in lipid rafts in response to activation of raft-localized βAR (or other GPCRs) may selectively regulate metabolic enzymes in the same compartment.
Determinants of protein localization to lipid rafts and caveolae
Little is known about how proteins localize to different lipid domains. Three mechanisms for lipid raft targeting have been proposed or described: (1) Proteins may bind to caveolin via a scaffolding domain located near the N-terminus of caveolin-1 and caveolin-3 (Song et al., 1996b; Okamoto et al., 1998). Many proteins that bind to caveolin contain a putative caveolin-binding motif (a loosely defined pattern of aromatic and nonaromatic residues) (Couet et al., 1997). (2) N-linked myristoylation (of a glycine residue following the initial methionine) or thio-acylation with palmitate (palmitoylation of cysteine residues) causes partitioning into the lipid-ordered phase of lipid rafts (Milligan et al., 1995; Shaul et al., 1996; Mumby, 1997; Song et al., 1997; Galbiati et al., 2001; Zacharias et al., 2002). Caveolins, G proteins, and other proteins rely upon these types of post-translational modifications for their interaction with membranes and lipid rafts. Indeed, coupling of the 5HT1A receptor to Gi and the inhibition of AC activity depends upon the palmitoylation of two cysteine residues in the C-terminal region of the receptor, which presumably serves to retain the receptor in lipid rafts together with Gi and AC (Papoucheva et al., 2004). (3) Structural components of the transmembrane-spanning region of proteins, in particular hydrophobic residues, cause proteins to ‘prefer' the slightly thicker membrane of the lipid raft (Anderson & Jacobson, 2002; Yamabhai & Anderson, 2002). As this mechanism depends upon the protein containing at least one transmembrane-spanning domain, it cannot readily account for targeting of lipid raft-associated proteins that lack such domains. It is important to note that these three mechanisms are not mutually exclusive and that it is not currently possible to predict, based upon amino-acid sequence, the localization of a given membrane-associated protein. Localization of different proteins may rely upon different mechanisms, or a combination thereof.
Caveolins function not only as scaffolds that localize signaling proteins, but, in addition, can inhibit numerous enzymes, including AC, eNOS, and several kinases and serine/threonine phosphatases (García-Cardeña et al., 1997; Oka et al., 1997; Engelman et al., 1998; Feron et al., 1998; Toya et al., 1998; Carman et al., 1999; Razani et al., 1999; Razani & Lisanti, 2001; Hnasko & Lisanti, 2003). Consistent with the latter findings, data from studies with knockout animal models and from overexpression protocols suggest that caveolins play central roles in regulating signal transduction by various systems (Razani et al., 2002a, 2002b; Schubert et al., 2002; Woodman et al., 2002; Hnasko & Lisanti, 2003). Such roles imply that caveolins are not just organizers, but regulators of signal transduction. Therefore, approaches to increase or decrease caveolin expression, including expression of caveolin peptides corresponding to the scaffolding domain, cannot be viewed as pure ‘probes' of lipid raft localization or compartmentation.
It has also become apparent that some molecules localize to lipid rafts and caveolae in a cell-dependent manner. Low levels of AC6 overexpression in vascular smooth muscle cells lead to localization of AC6 in lipid rafts (its native location), but with higher levels of expression the enzyme is found in nonraft fractions, where the bulk of both β1AR and β2AR were detected in these cells (Ostrom et al., 2002). Such results imply that lipid raft domains in some cells may contain a limited, saturable pool of signaling molecules, such as AC. The results with vascular smooth muscle cells contrast with those from cardiac myocytes, where overexpression of a large range of levels of AC6 leads to co-localization with both βAR subtypes in caveolin-rich fractions (Ostrom et al., 2000b; 2001). Therefore, different cells can localize the same protein differently, implying that the mechanisms governing lipid raft localization are not wholly dependent upon protein sequence, but instead appear to be cell type-dependent. Perhaps this is the result of lipid modifications that occur to a greater extent in one cell versus another or from some cells containing limited quantities of lipid rafts, but clearly other explanations are also possible. Data from several cell types on the localization of various GPCR and AC isoforms are summarized in Table 1. Taken together, these results add uncertainty to the conclusions reached from studies with only a single cell type, especially data that involve heterologous expression. At a minimum, the findings add complexity to our understanding of GPCR signaling to include the idea that differentiated cells can tailor their arrangement of signaling proteins, perhaps to attain customized response characteristics.
Table 1.
Summary of cell-specific localization of GPCR and AC in caveolae/lipid rafts
| Cell type | Caveolae/lipid rafts | Non-cav/non-raft |
|---|---|---|
| Cardiac myocyte | β1AR, β2AR, Gs, AC5/6, PKA, AKAP | EP2R, EP4R, Gs, (H2R, A2R, Glucagon-R) |
| Cardiac fibroblast | β2AR, Gs, AC5/6, AC3, Gs (IPR) | EP2R, Gs, AC2, AC4, AC7 (A2R) |
| Aortic smooth muscle | AC5/6, AC3, Gs | β1AR, β2AR, AdV-AC6, EP2R, Gs |
| Airway epithelia | AC5/6, Gs | β1AR, β2AR, AC3, AC4, Gs |
| Airway smooth muscle | β1AR, β2AR, Gs, AC5/6 (CGRP-R) | EP2R, EP4R, Gs (IPR) |
Conclusions and future directions
The present techniques for studying caveolae and lipid rafts are rife with methodological pitfalls and limitations (Pike, 2003). As noted above, most approaches utilize cell fractionation procedures that break cells apart and destroy cell morphology before analysis by biochemical assays or immunological reagents. Yet compartmentation is, by nature, a morphological phenomenon best addressed with microscopic techniques. Unfortunately, light microscopy (including fluorescence) lacks the resolution required for detecting lipid rafts and caveolae, while electron microscopic approaches are limited by the effectiveness of antibodies in detecting low-abundance membrane-associated proteins. Newer approaches, such as fluorescent and bioluminescent resonance energy transfer (FRET and BRET, Zacharias et al., 2002), are powerful but largely limited to expression of exogenous proteins and the associated pitfalls of not studying native proteins. New methodologies for studying lipid rafts are needed. Such new approaches may emerge as the mechanisms for protein targeting to lipid rafts become clearer. In the meantime, the combined use of experimental methods that yield confirmatory results with one another appears to be the best approach for drawing conclusions regarding lipid rafts and caveolar microdomains.
Lipid rafts and caveolae have sparked great interest among investigators interested in signal transduction, especially because these entities define microdomains likely involved in compartmentation and scaffolding of signaling proteins. Current evidence implies that compartmentation plays an important role in cell signaling by creating intimate juxtaposition between and among signaling molecules, thereby facilitating efficient and rapid information flow in a given signal transduction pathway as well as contributing to crosstalk among pathways. A cell's ability to express different proteins with different patterns of localization allows it to tailor its signaling to match the needs of its differentiated state. Understanding the three-dimensional nature of signal transduction in the context of cell structure, be it lipid rafts, caveolae or other domains, is likely to be critical for building complete circuit maps of signaling.
From a pharmacological perspective, the recognition of signaling microdomains in the membrane – in lipid rafts/caveolae, clathrin-coated pits, or perhaps other specialized regions – provides an important advancement, albeit one that adds complexity. Such complexity mirrors that of other aspects of GPCR signaling: for example, identification of a large number of known and orphan GPCRs in genomes and individual cells, homo- and heterodimerization of receptors, combinatorial assembly of different subunits of Gα, Gβ, and Gγ, expression of multiple isoforms of key effector molecule regions, ligand-specific and inverse agonism, etc. The challenge will be to develop ways to integrate the evolving data from these newly recognized complexities to further our understanding of drug action and to lay the groundwork for the discovery of new types of drugs. Tissue-specific differences in expression of signaling components in microdomains (Table 1) may provide an opportunity to target microdomains and differentially influence regions of different receptors in different cell types. Thus, just as in life itself, adversity and complexity provide both a challenge and an opportunity.
Acknowledgments
Work on this topic from the authors' laboratories is supported by NIH grants HL71781 (R.S.O.), HL66941, HL53773 and HL63885 (P.A.I.).
Abbreviations
- AC
adenylyl cyclase
- AKAP
A kinase anchoring protein
- βAR
beta-adrenergic receptor
- cAMP
3′,5′-cyclic adenosine monophosphate
- cGMP
3′,5′-cyclic guanosine monophosphate
- eNOS
endothelial nitric oxide synthase
- GPCR
G-protein-coupled receptor
- GRK
G protein receptor kinase
- NO
nitric oxide
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
References
- ALOUSI A.A., JASPER J.R., INSEL P.A., MOTULSKY H.J. Stoichiometry of receptor-Gs-adenylate cyclase interactions. FASEB J. 1991;5:2300–2303. doi: 10.1096/fasebj.5.9.1650314. [DOI] [PubMed] [Google Scholar]
- ANDERSON R.G. The caveolae membrane system. Annu. Rev. Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- ANDERSON R.G., JACOBSON K. A role for lipid shells in targeting proteins to caveolae, rafts, and other lipid domains. Science. 2002;296:1821–1825. doi: 10.1126/science.1068886. [DOI] [PubMed] [Google Scholar]
- AWASTHI-KALIA M., SCHNETKAMP P.P., DEANS J.P. Differential effects of filipin and methyl-beta-cyclodextrin on B cell receptor signaling. Biochem. Biophys. Res. Commun. 2001;287:77–82. doi: 10.1006/bbrc.2001.5536. [DOI] [PubMed] [Google Scholar]
- BAILLIE G.S., SOOD A., MCPHEE I., GALL I., PERRY S.J., LEFKOWITZ R.J., HOUSLAY M.D. beta-Arrestin-mediated PDE4 cAMP phosphodiesterase recruitment regulates beta-adrenoceptor switching from Gs to Gi. Proc. Natl. Acad. Sci. U.S.A. 2003;100:940–945. doi: 10.1073/pnas.262787199. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- BERGDAHL A., GOMEZ M.F., DREJA K., XU S.Z., ADNER M., BEECH D.J., BROMAN J., HELLSTRAND P., SWARD K. Cholesterol depletion impairs vascular reactivity to endothelin-1 by reducing store-operated Ca2+ entry dependent on TRPC1. Circ. Res. 2003;93:839–847. doi: 10.1161/01.RES.0000100367.45446.A3. [DOI] [PubMed] [Google Scholar]
- BOCKAERT J., MARIN P., DUMUIS A., FAGNI L. The ‘magic tail' of G protein-coupled receptors: an anchorage for functional protein networks. FEBS Lett. 2003;546:65–72. doi: 10.1016/s0014-5793(03)00453-8. [DOI] [PubMed] [Google Scholar]
- BRADY J.D., RICH T.C., LE X., STAFFORD K., FOWLER C.J., LYNCH L., KARPEN J.W., BROWN R.L., MARTENS J.R. Functional role of lipid raft microdomains in cyclic nucleotide-gated channel activation. Mol. Pharmacol. 2004;65:503–511. doi: 10.1124/mol.65.3.503. [DOI] [PubMed] [Google Scholar]
- CARMAN C.V., LISANTI M.P., BENOVIC J.L. Regulation of G protein-coupled receptor kinases by caveolin. J. Biol. Chem. 1999;274:8858–8864. doi: 10.1074/jbc.274.13.8858. [DOI] [PubMed] [Google Scholar]
- CHUN M., LIYANAGE U.K., LISANTI M.P., LODISH H.F. Signal transduction of a G protein-coupled receptor in caveolae: colocalization of endothelin and its receptor with caveolin. Proc. Natl. Acad. Sci. U.S.A. 1994;91:11728–11732. doi: 10.1073/pnas.91.24.11728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPHAM D.E., NEER E.J. G protein beta gamma subunits. Annu. Rev. Pharmacol. Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E., RUNNELS L.W., STRUBING C. The TRP ion channel family. Nat. Rev. Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- COOPER D.M. Molecular and cellular requirements for the regulation of adenylate cyclases by calcium. Biochem. Soc. Trans. 2003;31:912–915. doi: 10.1042/bst0310912. [DOI] [PubMed] [Google Scholar]
- COUET J., SARGIACOMO M., LISANTI M.P. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins. Caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J. Biol. Chem. 1997;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- DARBY P.J., KWAN C.Y., DANIEL E.E. Caveolae from canine airway smooth muscle contain the necessary components for a role in Ca(2+) handling. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279:L1226–L1235. doi: 10.1152/ajplung.2000.279.6.L1226. [DOI] [PubMed] [Google Scholar]
- DE WEERD W.F., LEEB-LUNDBERG L.M. Bradykinin sequesters B2 bradykinin receptors and the receptor-coupled Galpha subunits Galphaq and Galphai in caveolae in DDT1 MF-2 smooth muscle cells. J. Biol. Chem. 1997;272:17858–17866. doi: 10.1074/jbc.272.28.17858. [DOI] [PubMed] [Google Scholar]
- DOHLMAN H.G., THORNER J. RGS proteins and signaling by heterotrimeric G proteins. J. Biol. Chem. 1997;272:3871–3874. doi: 10.1074/jbc.272.7.3871. [DOI] [PubMed] [Google Scholar]
- ENGELMAN J.A., CHU C., LIN A., JO H., IKEZU T., OKAMOTO T., KOHTZ D.S., LISANTI M.P. Caveolin-mediated regulation of signaling along the p42/44 MAP kinase cascade in vivo. A role for the caveolin-scaffolding domain. FEBS Lett. 1998;428:205–211. doi: 10.1016/s0014-5793(98)00470-0. [DOI] [PubMed] [Google Scholar]
- FAGAN K.A., SMITH K.E., COOPER D.M. Regulation of the Ca2+-inhibitable adenylyl cyclase type VI by capacitative Ca2+ entry requires localization in cholesterol-rich domains. J. Biol. Chem. 2000;275:26530–26537. doi: 10.1074/jbc.M001369200. [DOI] [PubMed] [Google Scholar]
- FERGUSON S.S. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol. Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- FERON O., SALDANA F., MICHEL J.B., MICHEL T. The endothelial nitric-oxide synthase-caveolin regulatory cycle. J. Biol. Chem. 1998;273:3125–3128. doi: 10.1074/jbc.273.6.3125. [DOI] [PubMed] [Google Scholar]
- FERON O., SMITH T.W., MICHEL T., KELLY R.A. Dynamic targeting of the agonist-stimulated m2 muscarinic acetylcholine receptor to caveolae in cardiac myocytes. J. Biol. Chem. 1997;272:17744–17748. doi: 10.1074/jbc.272.28.17744. [DOI] [PubMed] [Google Scholar]
- FREDRIKSSON R., LAGERSTROM M.C., LUNDIN L.G., SCHIOTH H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- GAGNON A.W., KALLAL L., BENOVIC J.L. Role of clathrin-mediated endocytosis in agonist-induced down-regulation of the beta2-adrenergic receptor. J. Biol. Chem. 1998;273:6976–6981. doi: 10.1074/jbc.273.12.6976. [DOI] [PubMed] [Google Scholar]
- GALBIATI F., RAZANI B., LISANTI M.P. Emerging themes in lipid rafts and caveolae. Cell. 2001;106:403–411. doi: 10.1016/s0092-8674(01)00472-x. [DOI] [PubMed] [Google Scholar]
- GARCÍA-CARDEÑA G., MARTASEK P., MASTERS B.S., SKIDD P.M., COUET J., LI S., LISANTI M.P., SESSA W.C. Dissecting the interaction between nitric oxide synthase (NOS) and caveolin. Functional significance of the nos caveolin binding domain in vivo. J. Biol. Chem. 1997;272:25437–25440. doi: 10.1074/jbc.272.41.25437. [DOI] [PubMed] [Google Scholar]
- GOODMAN O.B., JR, KRUPNICK J.G., SANTINI F., GUREVICH V.V., PENN R.B., GAGNON A.W., KEEN J.H., BENOVIC J.L. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- GUZZI F., ZANCHETTA D., CASSONI P., GUZZI V., FRANCOLINI M., PARENTI M., CHINI B. Localization of the human oxytocin receptor in caveolin-1 enriched domains turns the receptor-mediated inhibition of cell growth into a proliferative response. Oncogene. 2002;21:1658–1667. doi: 10.1038/sj.onc.1205219. [DOI] [PubMed] [Google Scholar]
- HAKAK Y., SHRESTHA D., GOEGEL M.C., BEHAN D.P., CHALMERS D.T. Global analysis of G-protein-coupled receptor signaling in human tissues. FEBS Lett. 2003;550:11–17. doi: 10.1016/s0014-5793(03)00762-2. [DOI] [PubMed] [Google Scholar]
- HALL R.A., PREMONT R.T., LEFKOWITZ R.J. Heptahelical receptor signaling: beyond the G protein paradigm. J. Cell Biol. 1999;145:927–932. doi: 10.1083/jcb.145.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANOUNE J., DEFER N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- HERMANS E. Biochemical and pharmacological control of the multiplicity of coupling at G-protein-coupled receptors. Pharmacol. Ther. 2003;99:25–44. doi: 10.1016/s0163-7258(03)00051-2. [DOI] [PubMed] [Google Scholar]
- HILL J., HOWLETT A., KLEIN C. Nitric oxide selectively inhibits adenylyl cyclase isoforms 5 and 6. Cell Signal. 2000;12:233–237. doi: 10.1016/s0898-6568(99)00082-0. [DOI] [PubMed] [Google Scholar]
- HNASKO R., LISANTI M.P. The biology of caveolae: lessons from caveolin knockout mice and implications for human disease. Mol. Interventions. 2003;3:445–464. doi: 10.1124/mi.3.8.445. [DOI] [PubMed] [Google Scholar]
- HOOPER N.M. Detergent-insoluble glycosphingolipid/cholesterol-rich membrane domains, lipid rafts and caveolae (review) Mol. Membr. Biol. 1999;16:145–156. doi: 10.1080/096876899294607. [DOI] [PubMed] [Google Scholar]
- INSEL P.A. Location, location, location. Trends Endocrinol. Metab. 2003;14:100–102. doi: 10.1016/s1043-2760(03)00029-8. [DOI] [PubMed] [Google Scholar]
- KAWASAKI H., SPRINGETT G.M., MOCHIZUKI N., TOKI S., NAKAYA M., MATSUDA M., HOUSMAN D.E., GRAYBIEL A.M. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- KENAKIN T. Ligand-selective receptor conformations revisited: the promise and the problem. Trends Pharmacol. Sci. 2003;24:346–354. doi: 10.1016/S0165-6147(03)00167-6. [DOI] [PubMed] [Google Scholar]
- KOHOUT T.A., LEFKOWITZ R.J. Regulation of G protein-coupled receptor kinases and arrestins during receptor desensitization. Mol. Pharmacol. 2003;63:9–18. doi: 10.1124/mol.63.1.9. [DOI] [PubMed] [Google Scholar]
- KOPPERUD R., KRAKSTAD C., SELHEIM F., DOSKELAND S.O. cAMP effector mechanisms. Novel twists for an ‘old' signaling system. FEBS Lett. 2003;546:121–126. doi: 10.1016/s0014-5793(03)00563-5. [DOI] [PubMed] [Google Scholar]
- KRUPNICK J.G., BENOVIC J.L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- LAHTINEN U., HONSHO M., PARTON R.G., SIMONS K., VERKADE P. Involvement of caveolin-2 in caveolar biogenesis in MDCK cells. FEBS Lett. 2003;538:85–88. doi: 10.1016/s0014-5793(03)00135-2. [DOI] [PubMed] [Google Scholar]
- LEFKOWITZ R.J. G protein-coupled receptors. III. New roles for receptor kinases and beta-arrestins in receptor signaling and desensitization. J. Biol. Chem. 1998;273:18677–18680. doi: 10.1074/jbc.273.30.18677. [DOI] [PubMed] [Google Scholar]
- LEVITZKI A., BAR-SINAI A. The regulation of adenylyl cyclase by receptor-operated G proteins. Pharmacol. Ther. 1991;50:271–283. doi: 10.1016/0163-7258(91)90045-n. [DOI] [PubMed] [Google Scholar]
- LLOYD P.G., HARDIN C.D. Caveolae and the organization of carbohydrate metabolism in vascular smooth muscle. J. Cell. Biochem. 2001;82:399–408. doi: 10.1002/jcb.1170. [DOI] [PubMed] [Google Scholar]
- LOCKWICH T.P., LIU X., SINGH B.B., JADLOWIEC J., WEILAND S., AMBUDKAR I.S. Assembly of Trp1 in a signaling complex associated with caveolin-scaffolding lipid raft domains. J. Biol. Chem. 2000;275:11934–11942. doi: 10.1074/jbc.275.16.11934. [DOI] [PubMed] [Google Scholar]
- LOHN M., FURSTENAU M., SAGACH V., ELGER M., SCHULZE W., LUFT F.C., HALLER H., GOLLASCH M. Ignition of calcium sparks in arterial and cardiac muscle through caveolae. Circ. Res. 2000;87:1034–1039. doi: 10.1161/01.res.87.11.1034. [DOI] [PubMed] [Google Scholar]
- LUDWIG M.G., SEUWEN K. Characterization of the human adenylyl cyclase gene family: cDNA, gene structure, and tissue distribution of the nine isoforms. J. Recept. Signal Transduct. Res. 2002;22:79–110. doi: 10.1081/rrs-120014589. [DOI] [PubMed] [Google Scholar]
- LUTTRELL L.M., LEFKOWITZ R.J. The role of beta-arrestins in the termination and transduction of G-protein-coupled receptor signals. J. Cell. Sci. 2002;115:455–465. doi: 10.1242/jcs.115.3.455. [DOI] [PubMed] [Google Scholar]
- MARTENS J.R., O'CONNELL K., TAMKUN M. Targeting of ion channels to membrane microdomains: localization of KV channels to lipid rafts. Trends Pharmacol. Sci. 2004;25:16–21. doi: 10.1016/j.tips.2003.11.007. [DOI] [PubMed] [Google Scholar]
- MARTENS J.R., SAKAMOTO N., SULLIVAN S.A., GROBASKI T.D., TAMKUN M.M. Isoform-specific localization of voltage-gated K+ channels to distinct lipid raft populations: targeting of Kv1.5 to caveolae. J. Biol. Chem. 2000;13:13. doi: 10.1074/jbc.M009948200. [DOI] [PubMed] [Google Scholar]
- MCVEY M., HILL J., HOWLETT A., KLEIN C. Adenylyl cyclase, a coincidence detector for nitric oxide. J. Biol. Chem. 1999;274:18887–18892. doi: 10.1074/jbc.274.27.18887. [DOI] [PubMed] [Google Scholar]
- MICHEL J.J., SCOTT J.D. AKAP mediated signal transduction. Annu. Rev. Pharmacol. Toxicol. 2002;42:235–257. doi: 10.1146/annurev.pharmtox.42.083101.135801. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G. The stoichiometry of expression of protein components of the stimulatory adenylyl cyclase cascade and the regulation of information transfer. Cell Signal. 1996;8:87–95. doi: 10.1016/0898-6568(95)02034-9. [DOI] [PubMed] [Google Scholar]
- MILLIGAN G., GRASSIE M.A., WISE A., MACEWAN D.J., MAGEE A.I., PARENTI M. G-protein palmitoylation: regulation and functional significance. Biochem. Soc. Trans. 1995;23:583–587. doi: 10.1042/bst0230583. [DOI] [PubMed] [Google Scholar]
- MUMBY S.M. Reversible palmitoylation of signaling proteins. Curr. Opin. Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- NAIR K.S., BALASUBRAMANIAN N., SLEPAK V.Z. Signal-dependent translocation of transducin, RGS9-1-Gbeta5L complex, and arrestin to detergent-resistant membrane rafts in photoreceptors. Curr. Biol. 2002;12:421–425. doi: 10.1016/s0960-9822(02)00691-7. [DOI] [PubMed] [Google Scholar]
- OH P., SCHNITZER J.E. Immunoisolation of caveolae with high affinity antibody binding to the oligomeric caveolin cage. Toward understanding the basis of purification. J. Biol. Chem. 1999;274:23144–23154. doi: 10.1074/jbc.274.33.23144. [DOI] [PubMed] [Google Scholar]
- OH P., SCHNITZER J.E. Segregation of heterotrimeric G proteins in cell surface microdomains. G(q) binds caveolin to concentrate in caveolae, whereas g(i) and g(s) target lipid rafts by default. Mol. Biol. Cell. 2001;12:685–698. doi: 10.1091/mbc.12.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKA N., YAMAMOTO M., SCHWENCKE C., KAWABE J., EBINA T., OHNO S., COUET J., LISANTI M.P., ISHIKAWA Y. Caveolin interaction with protein kinase C. Isoenzyme-dependent regulation of kinase activity by the caveolin scaffolding domain peptide. J. Biol. Chem. 1997;272:33416–33421. doi: 10.1074/jbc.272.52.33416. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., SCHLEGEL A., SCHERER P.E., LISANTI M.P. Caveolins, a family of scaffolding proteins for organizing ‘preassembled signaling complexes' at the plasma membrane. J. Biol. Chem. 1998;273:5419–5422. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- ORLANDI P.A., FISHMAN P.H. Filipin-dependent inhibition of cholera toxin: evidence for toxin internalization and activation through caveolae-like domains. J. Cell Biol. 1998;141:905–915. doi: 10.1083/jcb.141.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSTROM R.S., BUNDEY R.A., INSEL P.A. Nitric oxide inhibition of adenylyl cyclase type 6 activity is dependent upon lipid rafts and caveolin signaling complexes. J. Biol. Chem. 2004;279:19846–19853. doi: 10.1074/jbc.M313440200. [DOI] [PubMed] [Google Scholar]
- OSTROM R.S., GREGORIAN C., DRENAN R.M., XIANG Y., REGAN J.W., INSEL P.A. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 2001;276:42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- OSTROM R.S., LIU X., HEAD B.P., GREGORIAN C., SEASHOLTZ T.M., INSEL P.A. Localization of adenylyl cyclase isoforms and G protein-coupled receptors in vascular smooth muscle cells: expression in caveolin-rich and noncaveolin domains. Mol. Pharmacol. 2002;62:983–992. doi: 10.1124/mol.62.5.983. [DOI] [PubMed] [Google Scholar]
- OSTROM R.S., NAUGLE J.E., HASE M., GREGORIAN C., SWANEY J.S., INSEL P.A., BRUNTON L.L., MESZAROS J.G. Angiotensin II enhances adenylyl cyclase signaling via Ca2+/calmodulin. Gq-Gs cross-talk regulates collagen production in cardiac fibroblasts. J. Biol. Chem. 2003;278:24461–24468. doi: 10.1074/jbc.M212659200. [DOI] [PubMed] [Google Scholar]
- OSTROM R.S., POST S.R., INSEL P.A. Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving Gs. J. Pharmacol. Exp. Ther. 2000a;294:407–412. [PubMed] [Google Scholar]
- OSTROM R.S., VIOLIN J.D., COLEMAN S., INSEL P.A. Selective enhancement of beta-adrenergic receptor signaling by overexpression of adenylyl cyclase type 6: colocalization of receptor and adenylyl cyclase in caveolae of cardiac myocytes. Mol. Pharmacol. 2000b;57:1075–1079. [PubMed] [Google Scholar]
- PAGE E., WINTERFIELD J., GOINGS G., BASTAWROUS A., UPSHAW-EARLEY J. Water channel proteins in rat cardiac myocyte caveolae: osmolarity-dependent reversible internalization. Am. J. Physiol. 1998;274:H1988–H2000. doi: 10.1152/ajpheart.1998.274.6.H1988. [DOI] [PubMed] [Google Scholar]
- PAPOUCHEVA E., DUMUIS A., SEBBEN M., RICHTER D.W., PONIMASKIN E.G. The 5-hydroxytryptamine(1A) receptor is stably palmitoylated, and acylation is critical for communication of receptor with Gi-protein. J. Biol. Chem. 2004;279:3280–3291. doi: 10.1074/jbc.M308177200. [DOI] [PubMed] [Google Scholar]
- PERRY S.J., BAILLIE G.S., KOHOUT T.A., MCPHEE I., MAGIERA M.M., ANG K.L., MILLER W.E., MCLEAN A.J., CONTI M., HOUSLAY M.D., LEFKOWITZ R.J. Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science. 2002;298:834–836. doi: 10.1126/science.1074683. [DOI] [PubMed] [Google Scholar]
- PIERCE K.L., LUTTRELL L.M., LEFKOWITZ R.J. New mechanisms in heptahelical receptor signaling to mitogen activated protein kinase cascades. Oncogene. 2001;20:1532–1539. doi: 10.1038/sj.onc.1204184. [DOI] [PubMed] [Google Scholar]
- PIKE L.J. Lipid rafts: bringing order to chaos. J. Lipid Res. 2003;44:655–667. doi: 10.1194/jlr.R200021-JLR200. [DOI] [PubMed] [Google Scholar]
- POST S.R., HILAL-DANDAN R., URASAWA K., BRUNTON L.L., INSEL P.A. Quantification of signalling components and amplification in the beta-adrenergic-receptor-adenylate cyclase pathway in isolated adult rat ventricular myocytes. Biochem. J. 1995;311:75–80. doi: 10.1042/bj3110075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAPACCIUOLO A., SUVARNA S., BARKI-HARRINGTON L., LUTTRELL L.M., CONG M., LEFKOWITZ R.J., ROCKMAN H.A. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J. Biol. Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- RAZANI B., COMBS T.P., WANG X.B., FRANK P.G., PARK D.S., RUSSELL R.G., LI M., TANG B., JELICKS L.A., SCHERER P.E., LISANTI M.P. Caveolin-1-deficient mice are lean, resistant to diet-induced obesity, and show hypertriglyceridemia with adipocyte abnormalities. J. Biol. Chem. 2002a;277:8635–8647. doi: 10.1074/jbc.M110970200. [DOI] [PubMed] [Google Scholar]
- RAZANI B., LISANTI M.P. Caveolin-deficient mice: insights into caveolar function human disease. J. Clin. Invest. 2001;108:1553–1561. doi: 10.1172/JCI14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZANI B., RUBIN C.S., LISANTI M.P. Regulation of cAMP-mediated signal transduction via interaction of caveolins with the catalytic subunit of protein kinase A. J. Biol. Chem. 1999;274:26353–26360. doi: 10.1074/jbc.274.37.26353. [DOI] [PubMed] [Google Scholar]
- RAZANI B., WANG X.B., ENGELMAN J.A., BATTISTA M., LAGAUD G., ZHANG X.L., KNEITZ B., HOU H., JR, CHRIST G.J., EDELMANN W., LISANTI M.P. Caveolin-2-deficient mice show evidence of severe pulmonary dysfunction without disruption of caveolae. Mol. Cell. Biol. 2002b;22:2329–2344. doi: 10.1128/MCB.22.7.2329-2344.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZANI B., WOODMAN S.E., LISANTI M.P. Caveolae: from cell biology to animal physiology. Pharmacol. Rev. 2002c;54:431–467. doi: 10.1124/pr.54.3.431. [DOI] [PubMed] [Google Scholar]
- ROSS E.M., WILKIE T.M. GTPase-activating proteins for heterotrimeric G proteins: regulators of G protein signaling (RGS) and RGS-like proteins. Annu. Rev. Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- RYBIN V.O., GRABHAM P.W., ELOUARDIGHI H., STEINBERG S.F. Caveolae-associated proteins in cardiomyocytes: caveolin-2 expression and interactions with caveolin-3. Am. J. Physiol. Heart Circ. Physiol. 2003;285:H325–H332. doi: 10.1152/ajpheart.00946.2002. [DOI] [PubMed] [Google Scholar]
- RYBIN V.O., XU X., LISANTI M.P., STEINBERG S.F. Differential targeting of beta-adrenergic receptor subtypes and adenylyl cyclase to cardiomyocyte caveolae. A mechanism to functionally regulate the cAMP signaling pathway. J. Biol. Chem. 2000;275:41447–41457. doi: 10.1074/jbc.M006951200. [DOI] [PubMed] [Google Scholar]
- RYBIN V.O., XU X., STEINBERG S.F. Activated protein kinase C isoforms target to cardiomyocyte caveolae: stimulation of local protein phosphorylation. Circ. Res. 1999;84:980–988. doi: 10.1161/01.res.84.9.980. [DOI] [PubMed] [Google Scholar]
- SCHERER P.E., LEWIS R.Y., VOLONTÉ D., ENGELMAN J.A., GALBIATI F., COUET J., KOHTZ D.S., VAN DONSELAAR E., PETERS P., LISANTI M.P. Cell-type and tissue-specific expression of caveolin-2. Caveolins 1 and 2 co-localize and form a stable hetero-oligomeric complex in vivo. J. Biol. Chem. 1997;272:29337–29346. doi: 10.1074/jbc.272.46.29337. [DOI] [PubMed] [Google Scholar]
- SCHERER P.E., OKAMOTO T., CHUN M., NISHIMOTO I., LODISH H.F., LISANTI M.P. Identification, sequence, and expression of caveolin-2 defines a caveolin gene family. Proc. Natl. Acad. Sci. U.S.A. 1996;93:131–135. doi: 10.1073/pnas.93.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNITZER J.E., OH P. Aquaporin-1 in plasma membrane and caveolae provides mercury-sensitive water channels across lung endothelium. Am. J. Physiol. 1996;270:H416–H422. doi: 10.1152/ajpheart.1996.270.1.H416. [DOI] [PubMed] [Google Scholar]
- SCHNITZER J.E., OH P., PINNEY E., ALLARD J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J. Cell. Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHUBERT W., FRANK P.G., WOODMAN S.E., HYOGO H., COHEN D.E., CHOW C.W., LISANTI M.P. Microvascular hyper-permeability in caveolin-1 (−/−) knock-out mice: treatment with a specific NOS inhibitor, L-NAME, restores normal microvascular permeability in Cav-1 null mice. J. Biol. Chem. 2002;277:40091–40098. doi: 10.1074/jbc.M205948200. [DOI] [PubMed] [Google Scholar]
- SCHWENCKE C., OKUMURA S., YAMAMOTO M., GENG Y.J., ISHIKAWA Y. Colocalization of beta-adrenergic receptors and caveolin within the plasma membrane. J. Cell. Biochem. 1999a;75:64–72. doi: 10.1002/(sici)1097-4644(19991001)75:1<64::aid-jcb7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- SCHWENCKE C., YAMAMOTO M., OKUMURA S., TOYA Y., KIM S.J., ISHIKAWA Y. Compartmentation of cyclic adenosine 3′,5′-monophosphate signaling in caveolae. Mol. Endocrinol. 1999b;13:1061–1070. doi: 10.1210/mend.13.7.0304. [DOI] [PubMed] [Google Scholar]
- SHAUL P.W., SMART E.J., ROBINSON L.J., GERMAN Z., YUHANNA I.S., YING Y., ANDERSON R.G., MICHEL T. Acylation targets endothelial nitric-oxide synthase to plasmalemmal caveolae. J. Biol. Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- SMART E.J., YING Y.S., MINEO C., ANDERSON R.G. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc. Natl. Acad. Sci. U.S.A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH K.E., GU C., FAGAN K.A., HU B., COOPER D.M. Residence of adenylyl cyclase type 8 in caveolae is necessary but not sufficient for regulation by capacitative Ca2+ entry. J. Biol. Chem. 2002;277:6025–6031. doi: 10.1074/jbc.M109615200. [DOI] [PubMed] [Google Scholar]
- SONG K.S., SARGIACOMO M., GALBIATI F., PARENTI M., LISANTI M.P. Targeting of a G alpha subunit (Gi1 alpha) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol. Biol. (Noisy-le-grand) 1997;43:293–303. [PubMed] [Google Scholar]
- SONG K.S., SCHERER P.E., TANG Z., OKAMOTO T., LI S., CHAFEL M., CHU C., KOHTZ D.S., LISANTI M.P. Expression of caveolin-3 in skeletal, cardiac, and smooth muscle cells. Caveolin-3 is a component of the sarcolemma and co-fractionates with dystrophin and dystrophin-associated glycoproteins. J. Biol. Chem. 1996a;271:15160–15165. doi: 10.1074/jbc.271.25.15160. [DOI] [PubMed] [Google Scholar]
- SONG S.K., LI S., OKAMOTO T., QUILLIAM L.A., SARGIACOMO M., LISANTI M.P. Co-purification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains. Detergent-free purification of caveolae microdomains. J. Biol. Chem. 1996b;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- SOWA G., PYPAERT M., SESSA W.C. Distinction between signaling mechanisms in lipid rafts vs caveolae. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14072–14077. doi: 10.1073/pnas.241409998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEINBERG S.F., BRUNTON L.L. Compartmentation of g protein-coupled signaling pathways in cardiac myocytes. Annu. Rev. Pharmacol. Toxicol. 2001;41:751–773. doi: 10.1146/annurev.pharmtox.41.1.751. [DOI] [PubMed] [Google Scholar]
- TANG C.M., INSEL P.A. GPCR expression in the heart; ‘new' receptors in myocytes and fibroblasts. Trends Cardiovasc. Med. 2004;14:94–99. doi: 10.1016/j.tcm.2003.12.007. [DOI] [PubMed] [Google Scholar]
- TANG Z., SCHERER P.E., OKAMOTO T., SONG K., CHU C., KOHTZ D.S., NISHIMOTO I., LODISH H.F., LISANTI M.P. Molecular cloning of caveolin-3, a novel member of the caveolin gene family expressed predominantly in muscle. J. Biol. Chem. 1996;271:2255–2261. doi: 10.1074/jbc.271.4.2255. [DOI] [PubMed] [Google Scholar]
- TASKEN K., AANDAHL E.M. Localized effects of cAMP mediated by distinct routes of protein kinase A. Physiol. Rev. 2004;84:137–167. doi: 10.1152/physrev.00021.2003. [DOI] [PubMed] [Google Scholar]
- TORIHASHI S., FUJIMOTO T., TROST C., NAKAYAMA S. Calcium oscillation linked to pacemaking of interstitial cells of Cajal: requirement of calcium influx and localization of TRP4 in caveolae. J. Biol. Chem. 2002;277:19191–19197. doi: 10.1074/jbc.M201728200. [DOI] [PubMed] [Google Scholar]
- TOYA Y., SCHWENCKE C., COUET J., LISANTI M.P., ISHIKAWA Y. Inhibition of adenylyl cyclase by caveolin peptides. Endocrine. 1998;139:2025–2031. doi: 10.1210/endo.139.4.5957. [DOI] [PubMed] [Google Scholar]
- VALLEJO J., HARDIN C.D. Metabolic organization in vascular smooth muscle: distribution and localization of caveolin-1 and phosphofructokinase. Am. J. Physiol. Cell. Physiol. 2004;286:C43–C54. doi: 10.1152/ajpcell.00483.2002. [DOI] [PubMed] [Google Scholar]
- VASSILATIS D.K., HOHMANN J.G., ZENG H., LI F., RANCHALIS J.E., MORTRUD M.T., BROWN A., RODRIGUEZ S.S., WELLER J.R., WRIGHT A.C., BERGMANN J.E., GAITANARIS G.A. The G protein-coupled receptor repertoires of human and mouse. Proc. Natl. Acad. Sci. U.S.A. 2003;100:4903–4908. doi: 10.1073/pnas.0230374100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON ZASTROW M. Mechanisms regulating membrane trafficking of G protein-coupled receptors in the endocytic pathway. Life Sci. 2003;74:217–224. doi: 10.1016/j.lfs.2003.09.008. [DOI] [PubMed] [Google Scholar]
- WILLIAMS T.M., LISANTI M.P. The caveolin proteins. Genome Biol. 2004;5:214. doi: 10.1186/gb-2004-5-3-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WONG W., SCHLICHTER L.C. Differential recruitment of Kv1.4 and Kv4.2 to lipid rafts by PSD-95. J. Biol. Chem. 2004;279:444–452. doi: 10.1074/jbc.M304675200. [DOI] [PubMed] [Google Scholar]
- WOODMAN S.E., PARK D.S., COHEN A.W., CHEUNG M., CHANDRA M., SHIRANI J., TANG B., JELICKS L.A., KITSIS R.N., CHRIST G.J., FACTOR S.M., TANOWITZ H.B., LISANTI M.P. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAP kinase cascade. J. Biol. Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- XIANG Y., DEVIC E., KOBILKA B. The PDZ binding motif of the beta1 adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J. Biol. Chem. 2002a;277:33783–33790. doi: 10.1074/jbc.M204136200. [DOI] [PubMed] [Google Scholar]
- XIANG Y., KOBILKA B. The PDZ-binding motif of the beta2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10776–10781. doi: 10.1073/pnas.1831718100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIANG Y., RYBIN V.O., STEINBERG S.S., KOBILKA B. Caveolar localization dictates physiologic signaling of beta2 adrenoceptors in neonatal cardiac myocytes. J. Biol. Chem. 2002b;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., AVDONIN P., ZHOU Y.Y., CHENG H., AKHTER S.A., ESCHENHAGEN T., LEFKOWITZ R.J., KOCH W.J., LAKATTA E.G. Coupling of beta2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., HOHL C., ALTSCHULD R., JONES L., LIVINGSTON B., ZIMAN B., TANTINI B., LAKATTA E.G. Beta 2-adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to changes in Ca2+ dynamics, contractility, or phospholamban phosphorylation. J. Biol. Chem. 1994;269:19151–19156. [PubMed] [Google Scholar]
- YAMABHAI M., ANDERSON R.G. Second cysteine-rich region of epidermal growth factor receptor contains targeting information for caveolae/rafts. J. Biol. Chem. 2002;277:24843–24846. doi: 10.1074/jbc.C200277200. [DOI] [PubMed] [Google Scholar]
- YARBROUGH T.L., LU T., LEE H.C., SHIBATA E.F. Localization of cardiac sodium channels in caveolin-rich membrane domains: regulation of sodium current amplitude. Circ. Res. 2002;90:443–449. doi: 10.1161/hh0402.105177. [DOI] [PubMed] [Google Scholar]
- YATANI A., TAJIMA Y., GREEN S.A. Coupling of beta-adrenergic receptors to cardiac L-type Ca2+ channels: preferential coupling of the beta1 versus beta2 receptor subtype and evidence for PKA-independent activation of the channel. Cell Signal. 1999;11:337–342. doi: 10.1016/s0898-6568(98)00050-3. [DOI] [PubMed] [Google Scholar]
- ZACCOLO M., POZZAN T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- ZACHARIAS D.A., VIOLIN J.D., NEWTON A.C., TSIEN R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- ZHENG B., DE VRIES L., GIST FARQUHAR M. Divergence of RGS proteins: evidence for the existence of six mammalian RGS subfamilies. Trends Biochem. Sci. 1999;24:411–414. doi: 10.1016/s0968-0004(99)01474-7. [DOI] [PubMed] [Google Scholar]
- ZHENG B., MA Y.C., OSTROM R.S., LAVOIE C., GILL G.N., INSEL P.A., HUANG X.Y., FARQUHAR M.G. RGS-PX1, a GAP for Galpha s and sorting Nexin in vesicular trafficking. Science. 2001;294:1939–1942. doi: 10.1126/science.1064757. [DOI] [PubMed] [Google Scholar]
- ZHONG H., WADE S.M., WOOLF P.J., LINDERMAN J.J., TRAYNOR J.R., NEUBIG R.R. A spatial focusing model for G protein signals. Regulator of G protein signaling (RGS) protein-mediated kinetic scaffolding. J. Biol. Chem. 2003;278:7278–7284. doi: 10.1074/jbc.M208819200. [DOI] [PubMed] [Google Scholar]