Abstract
The reward circuitry of the brain consists of neurons that synaptically connect a wide variety of nuclei. Of these brain regions, the ventral tegmental area (VTA) and the nucleus accumbens (NAc) play central roles in the processing of rewarding environmental stimuli and in drug addiction. The psychoactive properties of marijuana are mediated by the active constituent, Δ9-THC, interacting primarily with CB1 cannabinoid receptors in a large number of brain areas. However, it is the activation of these receptors located within the central brain reward circuits that is thought to play an important role in sustaining the self-administration of marijuana in humans, and in mediating the anxiolytic and pleasurable effects of the drug. Here we describe the cellular circuitry of the VTA and the NAc, define the sites within these areas at which cannabinoids alter synaptic processes, and discuss the relevance of these actions to the regulation of reinforcement and reward. In addition, we compare the effects of Δ9-THC with those of other commonly abused drugs on these reward circuits, and we discuss the roles that endogenous cannabinoids may play within these brain pathways, and their possible involvement in regulating ongoing brain function, independently of marijuana consumption. We conclude that, whereas Δ9-THC alters the activity of these central reward pathways in a manner that is consistent with other abused drugs, the cellular mechanism through which this occurs is likely different, relying upon the combined regulation of several afferent pathways to the VTA.
Keywords: Drug abuse, nucleus accumbens, ventral tegmental area, dopamine, plasticity, long-term depression, tolerance, self-administration, conditioned place preference, intracranial self-stimulation
Introduction
Marijuana (Cannabis sativa) intoxication is a complex phenomenon involving many physiological systems. Whereas tachycardia, hypothermia, analgesia, and the appetite-enhancing effects of the drug are mediated by central and peripheral mechanisms regulating autonomic and phylogenetically primitive physiological states, clear effects are also observed upon close examination of higher brain function. Among the best known of these actions is the ability of marijuana, and congeners of its active ingredient, Δ9-tetrahydrocannabinol (Δ9-THC), to disrupt sensory processing and learning and memory in animals and humans (Deadwyler et al., 1990; Hampson & Deadwyler, 1999; Sullivan, 2000). However, the pharmacological properties of marijuana that undoubtedly sustain its use in humans are the sense of euphoria and well being that are produced. In fact, this ‘high' along with the sense of relaxation produced by marijuana are among the most often cited properties of the drug in subjective human reports (Green et al., 2003). It is likely that the euphorigenic properties of marijuana, and virtually all other abused drugs, result from interactions with the brain's intrinsic ‘reward circuitry' that has evolved so that pleasure may be found in environmental stimuli possessing survival value (i.e. food, water, social interaction, sex) (Wise, 1996; Gardner, 2002).
The actions of marijuana, its primary psychoactive active ingredient Δ9-THC, and more potent synthetic analogs (collectively known as ‘cannabinoids') on the brain reward circuitry is the subject of this review. In addition, because the largest impediment to the development of therapeutic cannabinoid medications in the U.S.A. is the occurrence of untoward cognitive and euphoric side effects of these drugs, understanding the mechanisms through which these actions occur is essential to developing rational approaches to medication development.
The psychoactive Δ9-THC was isolated and identified from the concentrated resins of the marijuana plant (hashish) in the early 1960s (Gaoni & Mechoulam, 1964). However, it was nearly 30 years after this discovery that a binding site that interacted with Δ9-THC was cloned from brain and identified as the cannabinoid CB1 receptor (Matsuda et al., 1990). Although CB1 and the subsequently identified CB2 receptors are both members of a seven-transmembrane spanning class that couple to inhibitory Gi/Go proteins, only the CB1 receptor is normally found in the CNS (Pertwee, 1997). Furthermore, although CB1 and CB2 represent the only cannabinoid receptors cloned to date, there is pharmacological evidence to suggest the existence of at least one novel, yet uncloned, cannabinoid receptor in the brain (Breivogel et al., 2001; Hajos et al., 2001; Hajos & Freund, 2002). The CB1 receptor is located in brain areas known to mediate the effects of Δ9-THC (Herkenham et al., 1990; 1991; Hohmann & Herkenham, 2000), where it is coupled to several signal transduction mechanisms, including the activation of potassium channels, the inhibition of voltage-dependent calcium channels, the inhibition of adenylyl cyclase, and the activation of MAP kinase (Bidaut-Russell et al., 1990; Henry & Chavkin, 1995; Twitchell et al., 1997; Hoffman & Lupica, 2000). Following the identification of the CB1 receptor, several naturally occurring lipid agonists were discovered in brain tissue, which are now known as endocannabinoids. Whereas the list of lipid molecules that bind to cannabinoid receptors continues to grow, the best understood are the arachidonic acid-containing lipids known as anandamide and 2-arachidonylglycerol (Mechoulam et al., 1998). Distinct physiological roles in which endocannabinoids act as ‘retrograde messengers' have been described in several brain regions, including the NAc and VTA (Robbe et al., 2002; Melis et al., 2004). In this capacity, endocannabinoids that are released from postsynaptic neurons upon depolarization activate presynaptic CB1 receptors and inhibit neurotransmitter release. This suggests that the endocannabinoid system may play additional important roles in the regulation of ongoing synaptic brain function (Alger, 2002; Wilson & Nicoll, 2002).
Cannabis and brain reward circuits
Smoked marijuana produces subjective feelings of well being and euphoria in humans, which are blocked by the CB1 receptor antagonist SR141716A (rimonabant) (Rinaldi-Carmona et al., 1994), suggesting that many, if not all, of the psychological properties of the drug are mediated by these receptors (Huestis et al., 2001). Furthermore, because the pleasurable subjective effects of this drug are thought to contribute to its use in humans, similarities between the actions of Δ9-THC and other commonly abused drugs on brain circuitry underlying reward and reinforcement processes have been investigated. The central neuronal circuits known to be involved in mediating the rewarding aspects of most abused drugs originate with a subgroup of dopamine (DA) neurons located in an area of the mesencephalon known as the ventral tegmental area (VTA, Figure 1). These DA neurons possess axons that target GABAergic medium spiny neurons located rostrally in an area of the limbic forebrain known as the ventral striatum or nucleus accumbens (NAc), as well as neurons in the frontal cortex (Wise & Bozarth, 1984; 1985; Gardner, 2002). The VTA also contains at least two additional neuronal phenotypes that are not DAergic (Cameron et al., 1997). A substantial number of these non-DA neurons are GABAergic and their output forms substantial and discrete projections to the NAc (Van Bockstaele & Pickel, 1995), and the prefrontal cortex (Carr & Sesack, 2000). In addition, since it is well established that VTA DA neurons are also inhibited by local circuit GABAergic axon terminals, it is possible that these same GABA projection neurons also locally synapse on DA neurons via axon collaterals (Johnson & North, 1992; Steffensen et al., 1998). In addition to these diverse outputs, the VTA also receives input from a large array of brain nuclei that are involved in integrating sensory information and motor output (e.g. glutamatergic inputs from the medial prefrontal cortex, amygdala, pedunculopontine nucleus, and the subthalamic nucleus), whereas the NAc also sends reward-relevant information to the ventral globus pallidus (VP). In addition, the VTA, NAc, and VP are interconnected via reciprocal axon collaterals that are critical for the performance of reward-relevant behaviors. In recent years, it has become clear that these brain reward nuclei also receive glutamatergic and GABAergic inputs, whose functional integrity is necessary to observe drug-related reward behavior (Carlezon & Wise, 1996a, 1996b).
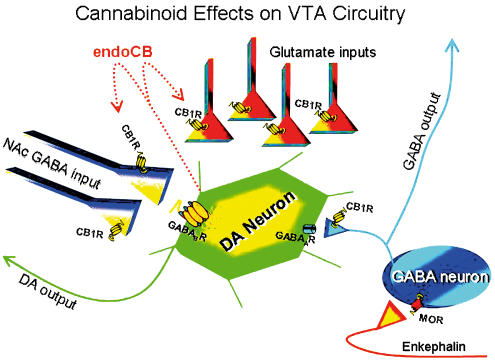
Figure 1.
Simplified schematic demonstrating the known cellular components involved in mediating cannabinoid actions in the VTA. Presynaptic cannabinoid CB1 receptor locations are based upon electrophysiological studies cited in the text. MOR, μ opioid receptor; DA, dopamine; endoCB, endocannabinoid; NAc, nucleus accumbens. Note that the extrinsic GABAergic input originating in the NAc provides input to GABAB receptors located on DA neurons, whereas input to GABAA receptors is derived from GABA neurons thought to be intrinsic to the VTA. Also note the hypothetical location of an enkephalin containing input to the intrinsic GABA neuron, present to account for data demonstrating that the MOR antagonist, naloxone, can block the effects of Δ9-THC in vivo. It is speculated that Δ9-THC may somehow act to enhance enkephalinergic input to the intrinsic GABA neurons, thereby decreasing GABA release, and enhancing DA neuron activity.
Reward-relevant behavioral effects of cannabinoids
A variety of behavioral assays are used to determine the rewarding or reinforcing properties of commonly abused drugs (for reviews, see Bardo, 1998; Gardner & Vorel, 1998; Gardner, 2002). In general, behavioral assays in animals that have high validity and predictability for the rewarding potential of drugs in humans have demonstrated that Δ9-THC and other cannabinoids act upon reward substrates in a manner that is consistent with other abused drugs. For example, it is now well established that cannabinoids support conditioned place preference (CPP) in animals, seemingly through the activation of CB1 receptors, since the antagonist SR141716A can reverse this effect (Gardner, 2002; Tanda & Goldberg, 2003). Another assay of the rewarding effects of pharmacological agents is the self-administration (SA) paradigm. As SA requires the animal to freely exhibit an operant response in order to receive intravenous, or intracranial, injections of a drug, it is thought to have a great deal of validity in its ability to emulate human drug SA (Gardner, 2002; Tanda & Goldberg, 2003). More simply, the paradigm can provide information as to drug preferences exhibited by animals that can be compared to those exhibited by humans. For many years, it appeared that Δ9-THC did not support SA in laboratory animals (for a review, see Tanda & Goldberg, 2003). However, when carefully controlled studies were performed in which close attention was paid to the dose, the vehicle in which it was dissolved, and the speed at which Δ9-THC injections were delivered, it was found that robust SA could be maintained in non-human primates at doses comparable to those obtained from smoked marijuana (Tanda et al., 2000; Tanda & Goldberg, 2003). In addition, this same study demonstrated that the operant responses required to receive Δ9-THC injections could be extinguished when the CB1 antagonist SR141617A was co-administered with the agonist.
Consistent with the actions of many other abused drugs, Δ9-THC can also lower the threshold for intracranial electrical brain self-stimulation (ICSS) of the medial forebrain bundle in animals (Gardner & Lowinson, 1991). In general, there is a strong positive correlation between a drug's ability to increase NAc DA accumulation, its ability to support SA, and its threshold-lowering effect on ICSS. Furthermore, because animals will also readily generate operant responses for the opportunity for ICSS, and because this stimulation activates DA neuron axons projecting to the NAc, the threshold-lowering effect of Δ9-THC on this phenomenon is interpreted to indicate clear rewarding effects of the drug on the central VTA-NAc circuitry (Gardner, 2002).
Physiology of cannabinoids in reward circuits: modulation of DA function in the VTA
As described above, ample behavioral, biochemical, and pharmacological evidence indicates that marijuana and other cannabinoids act upon the central drug reward circuitry in the mammalian brain. One of the hallmarks of this action for other abused drugs is the ability to increase DA function in the axon terminal regions of the VTA DA neurons (Di Chiara & Imperato, 1988). Some drugs, such as cocaine, increase DA levels in the NAc by inhibiting the DA transporter, whereas amphetamine shares this mechanism with cocaine, but also initiates the release of DA in the NAc. In contrast, heroin, morphine and other opioids appear to increase the activity of DA neurons in the VTA via the activation of μ opioid receptors located on GABAergic axon terminals, inhibiting GABA release onto these cells (Johnson & North, 1992). The inhibition of inhibitory neurotransmitter release, referred to as ‘disinhibition', is a common theme throughout the CNS, and it represents a mechanism to globally increase the spontaneous activity of principal neurons via local circuit interactions.
With the intent of examining the actions of cannabinoids on reward circuitry in the context of other abused drugs, researchers have studied the ability of Δ9-THC to increase DA function in the NAc, and its ability to alter DA neuron activity in the VTA (Figure 1). Early studies demonstrated that i.v. administration of Δ9-THC, at concentrations that enhanced ICSS, could increase the concentration of DA in the NAc, as measured by microdialysis in freely moving rats, and by electrochemistry (Ng Cheong Ton et al., 1988; Chen et al., 1990). This augmentation of DA release in the NAc was blocked by the sodium channel blocker tetrodotoxin, by removing calcium from the dialysis perfusate, and by systemic injection of the opioid receptor antagonist naloxone (Chen et al., 1990). A subsequent study replicated these effects of Δ9-THC, and further demonstrated that the synthetic cannabinoid agonist WIN55,212-2 also increased NAc DA release that was blocked by systemic injection of either the CB1 antagonist SR141716A, or the opioid receptor antagonist naloxone, or by the selective μ1 opioid receptor antagonist, naloxonazine, infused into the VTA (Tanda et al., 1997). It should be noted here that systemic naloxone also attenuates the enhancement of ICSS caused by Δ9-THC (Gardner & Lowinson, 1991). Collectively, these data provided evidence that, like other abused drugs, Δ9-THC enhances DA function in the NAc through action potential- and calcium-dependent mechanisms, but also demonstrates that endogenous opioids in the VTA are somehow involved. Whereas these studies provided a wealth of information and placed the effects of Δ9-THC on a similar footing as other psychoactive drugs, the specific mechanism of the DA-enhancing effect of the cannabinoids was not identified.
As indicated above, drugs such as heroin and morphine appear to act within the VTA itself to increase DA neuron activity, leading to the enhancement of extracellular DA concentrations in the NAc. To evaluate this possibility, the effects of Δ9-THC and other cannabinoid receptor agonists were examined in several laboratories using single-unit electrophysiological recordings from neurons in the rodent VTA. These studies demonstrated that Δ9-THC and the more potent and efficacious cannabinoid receptor agonists WIN55,212-2, HU210, and CP55940 increased neuronal firing rates in anesthetized and unanesthetized rats (French et al., 1997; Gessa et al., 1998; Wu & French, 2000), as well as in brain slices containing the VTA (Cheer et al., 2000). Also noteworthy in these studies were the observations that the cannabinoid effects could be blocked with the CB1 receptor antagonist SR141716A, and that the increase in average DA neuron-firing rate was accompanied by an increase in DA neuron burst activity (French et al., 1997; Diana et al., 1998), which is associated with a more efficacious terminal release of DA than a simple increase in firing rate alone (Gonon, 1988).
Together, these studies suggest that the cannabinoid-induced increase in DA accumulation in the NAc may result from an increase in DA neuron firing and burst rates as a result of CB1 receptor activation. Furthermore, the increased activity of VTA DA neurons caused by the agonist HU210 in brain slices (Cheer et al., 2000) implies that the cannabinoids must act either directly upon the DA neurons themselves (which is unlikely, given the absence of cannabinoid receptors on these neurons; Herkenham et al., 1991), or upon the local circuitry of the VTA to increase DA neuron activity. In fact, this study also reported that prior application of the GABAA receptor antagonist, bicuculline, blocked the excitatory effect of HU210 (Cheer et al., 2000), suggesting that, like opioid receptors (Johnson & North, 1992), CB1 receptors may increase DA neuron activity via a local disinhibitory mechanism. This hypothesis has recently gained more direct support with the finding that WIN55,212-2 application in brain slices containing the VTA could reduce electrically evoked inhibitory postsynaptic currents (IPSCs) mediated by the activation of GABAA receptors (Szabo et al., 2002). Furthermore, this effect appeared to be mediated by CB1 receptors located on the inhibitory terminals of GABAergic neurons intrinsic to the VTA, since it was blocked by SR141716A and was not observed with ion currents evoked by dendritic application of the GABAA receptor agonist muscimol (Szabo et al., 2002). In addition, miniature spontaneously occurring IPSCs that were resistant to tetrodotoxin were also unaffected by WIN55,212-2, further suggesting that this drug did not act postsynaptically to diminish GABAergic IPSCs. Since the presynaptic inhibition of neurotransmitter release is one of the most frequently observed and best characterized effects of CB1 receptor activation in the CNS (Hoffman & Lupica, 2000; 2001; Schlicker & Kathmann, 2001; Hoffman et al., 2003b), and subpopulations of GABAergic terminals throughout the CNS are densely populated by CB1 receptors (Freund et al., 2003), it is perhaps not surprising that this effect was observed. However, it is also worth noting in this context that the VTA contains a heterogeneous population of at least three distinct neuron subtypes, only one of which is clearly DAergic (Cameron et al., 1997). The study by Szabo et al. (2002) did not sufficiently characterize the neurons to permit conclusions as to their DAergic identity, thereby precluding an unambiguous conclusion as to the disinhibitory mechanism of cannabinoids on DA neurons in the VTA. In addition to this potential caveat, the following observations argue for more complex interactions between cannabinoids and DA neurons, possibly involving the modulation of additional neuronal circuits either within the VTA, or extrinsic projections to this structure. First, the ability of systemic cannabinoids to increase extracellular DA concentrations in the NAc is reversed by systemic and intra-VTA opioid antagonist administration (Chen et al., 1990; Tanda et al., 1997), but the increase in DA neuron-firing rates caused by Δ9-THC are not (French, 1997). Second, the direct infusion of Δ9-THC into the VTA does not increase DA accumulation in the NAc (Chen et al., 1993). Third, it has recently been demonstrated that synthetic cannabinoid agonists and endocannabinoids, acting in a retrograde manner, can also inhibit glutamate release onto neurons in the VTA in vitro (Melis et al., 2004), which would tend to diminish the excitatory input to DA neurons in the VTA and reduce the probability of bursting (Johnson et al., 1992; Kitai et al., 1999). Finally, preliminary data from our laboratory indicate that CB1 receptors are also located on GABAergic terminals believed to originate from NAc medium spiny output neurons (Walaas & Fonnum, 1980; Heimer et al., 1991) that target GABAB receptors on DA neurons in the VTA (Sugita et al., 1992), suggesting a second possible disinhibitory mechanism (Riegel et al., 2003). This latter study, taken together with that of Szabo et al. (2002), implies that cannabinoids acting at CB1 receptors can inhibit the release of GABA in the VTA that is derived from both intrinsic and extrinsic sources, and further that the inputs from the NAc to the VTA may represent a critical pathway for the expression of cannabinoid reward. Collectively, the studies examining the effects of cannabinoids on VTA DA neurons suggest that, whereas the activation of CB1 receptors within the VTA may account for some of the reward-relevant aspects of cannabinoid exposure, additional sites both within and external to this critical brain structure must also be considered. In particular, it is intriguing to speculate that a functional opioid pathway that inhibits intrinsic GABA neurons that normally regulate DA neuron firing may be present, and cannabinoids somehow act to increase this opioidergic inhibition of GABA release onto the DA neurons (e.g. Figure 1). However, in order to account for the absence of an effect of naloxone on the spontaneous firing and bursting rates of DA neurons in vivo (French, 1997), it is necessary to propose that redundant mechanisms that do not involve an opioid component may exist to regulate DA neuron excitability. It may be that the effects of cannabinoids on the net VTA DA neuron output depend upon the relative contribution of several neurotransmitter systems (e.g. intrinsic and extrinsic GABAergic, glutamatergic and opioidergic pathways) at a given time and under specific behavioral conditions that involve the activation of these pathways. For example, the activation of CB1 receptors located on inputs to the VTA DA neurons may only be relevant to an increase in DA neuron firing when the appropriate GABAergic and glutamatergic afferents to the VTA are activated under specific behavioral conditions (e.g. during increased NAc and cortical output to the VTA). Similarly, presumed activation of endogenous opioidergic inputs to the inhibitory neurons that impinge upon the VTA DA neurons may require a specific environmental or behavioral stimulus. Clearly, more data are needed before firm conclusions regarding the effects of cannabinoids and their activation of DA neurons in the VTA can be reached.
Physiology of cannabinoids in reward circuits: modulation of DA-independent function in the NAc
As mentioned above, the NAc represents a brain area that is critical to the expression of the rewarding and addictive properties of several classes of abused drugs (Wise & Bozarth, 1987; Zahm, 2000). In recent years, it has become apparent that many of these abused drugs have direct effects on synaptic processes in reward-relevant brain areas, including the NAc that may not rely upon DAergic neurotransmission. In fact, several of these drugs, including opioids, psychomotor stimulants, and phencyclidine (PCP), are self-administered by animals directly into the NAc (Carlezon & Wise, 1996b; McBride et al., 1999). In addition, many of these drugs have been shown to inhibit GABA and glutamate synaptic transmission in the NAc, either by pre- or postsynaptic mechanisms (Harvey & Lacey, 1997; Martin et al., 1997; Chieng & Williams, 1998; Nicola & Malenka, 1998). Based upon these observations, we have hypothesized that at least part of the rewarding actions of these drugs may be mediated via direct interactions with the NAc neuronal circuitry (Figure 2), and we have studied the acute effects of cannabinoid agonists on neurotransmitter release in brain slices containing the NAc (Hoffman & Lupica, 2001). This study demonstrated that WIN55,212-2 could reduce GABA release onto medium spiny projection neurons in the rat NAc via the activation of CB1 receptors located on inhibitory axon terminals (Hoffman & Lupica, 2001). A subsequent study verified these findings in the mouse NAc, and demonstrated that the presynaptic inhibition of GABA release could also be observed with the cannabinoid agonist CP55940 (Manzoni & Bockaert, 2001). As the GABAergic medium spiny projection neurons in the dorsal and ventral striatum (NAc) are thought to receive GABAergic inputs from intrinsic interneurons (Koós & Tepper, 1999), as well as via interconnections from recurrent axon collaterals (Plenz, 2003), it is unclear whether CB1 receptors inhibit GABA release from one or both sources (Figure 2). However, the net effect of the inhibition of GABA release by Δ9-THC and other cannabinoid drugs onto medium spiny projection neurons in the NAc might be the disinhibition of this GABAergic output to the VTA and other target structures (Figure 2). However, this situation is made more complicated by the finding that glutamate release onto NAc medium spiny neurons is also inhibited by cannabinoid agonists, apparently as a result of the activation of CB1 receptors coupled to voltage-dependent K+ channels in the glutamatergic nerve terminals (Robbe et al., 2001). Since the glutamatergic afferents to the NAc are thought to arise from neurons in the prefrontal cortex, it was hypothesized by Robbe et al. (2001) that the reduction of glutamatergic input to the NAc by CB1 receptor activation would reduce the excitation of GABAergic NAc medium spiny neurons projecting to DA neurons in the VTA, and thereby decrease the inhibition of the DA neurons. However, these data, considered with those above demonstrating that robust inhibition of GABA release onto these same neurons is a consequence of CB1 receptor activation in the NAc (Hoffman & Lupica, 2001; Manzoni & Bockaert, 2001), suggest that a more complex interaction must explain the rewarding properties of Δ9-THC. Again, the relative contribution of the CB1 receptor modulation of GABAergic and glutamatergic synaptic transmission to the output of the NAc might rely upon the circumstances under which each system predominates.
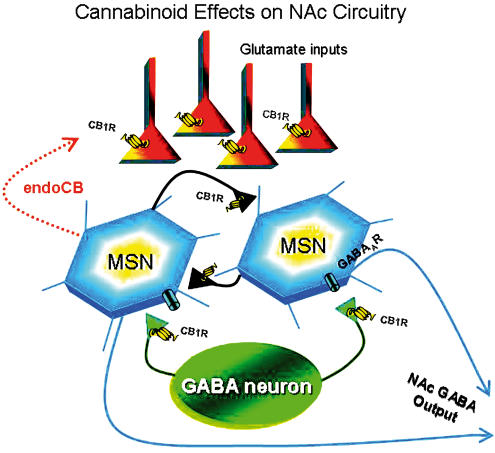
Figure 2.
Simplified schematic demonstrating sites of presynaptic cannabinoid action in the NAc. Abbreviations: MSN, medium spiny neuron. Note that MSNs are shown receiving GABAergic inputs from both axon collaterals (black connections), and from a GABA neuron intrinsic to the NAc. CB1Rs are shown located on both MSN collaterals, and on intrinsic GABA neuron terminals, although the actual location of these receptors on these precise inhibitory elements in the NAc has not been resolved. The strong cortical glutamatergic input to the intrinsic GABA neurons that is thought to drive feed-forward inhibition of the MSNs is not shown.
Physiology of cannabinoids in reward circuits: endocannabinoid-dependent synaptic plasticity in the NAc
A long-term change in synaptic transmission resulting from prior drug experience represents an attractive mechanism to explain some of the changes in neural circuits that may occur during recreational and compulsive drug use (Gerdeman et al., 2003). In this view, abused drugs are thought to usurp brain mechanisms that have evolved to support beneficial forms of synaptic plasticity, such as those that occur during learning and memory (Gerdeman et al., 2003). In keeping with this hypothesis, several recent studies have demonstrated that synaptic plasticity in brain reward circuits can be by initiated or modified by commonly abused drugs (Thomas et al., 2001; Robbe et al., 2002; Saal et al., 2003).
Shortly after the discovery of a role for endocannabinoids in the regulation of synaptic transmission (Wilson & Nicoll, 2001), it was reported that endocannabinoids and functional CB1 receptors were required to observe the long-term depression (LTD) of glutamatergic cortical synaptic inputs to dorsal striatal medium spiny neurons following high-frequency (100 Hz, 1 s) stimulation (Gerdeman et al., 2002). Later, this phenomenon was also described in the NAc following low-frequency stimulation (13 Hz, 10 min), where it was additionally shown that endocannabinoid release was secondary to the activation of type 5 metabotropic glutamate receptors, and an increase in postsynaptic Ca2+ in medium spiny neurons (Robbe et al., 2002). Together, these studies demonstrated that CB1 receptors and retrograde endocannabinoid signaling were required to observe LTD in the dorsal and ventral striatum. Although the precise role that LTD plays in regulating the function of the dorsal and ventral striatum is not presently known, it has been speculated that the long-term changes in synaptic efficacy mediated by this system may be involved in communicating enduring information as to reward salience, and in the establishment of motor habits associated with compulsive drug use (Gerdeman et al., 2003). In a further effort to evaluate the possible relevance of LTD to enduring changes in brain reward circuitry, we examined the effects of chronic exposure to Δ9-THC on synaptic function and plasticity in the NAc (Hoffman et al., 2003a). As mentioned above, CB1 receptors are located on both GABAergic and glutamatergic axon terminals in the NAc, where they inhibit the release of each of these neurotransmitters. However, following in vivo treatment with Δ9-THC for 1 week, the inhibition of glutamatergic and GABAergic synaptic transmission by WIN55,212-2 was greatly diminished, confirming CB1 receptor tolerance (Hoffman et al., 2003a). In addition, the normally robust inhibition of glutamate release by μ opioid receptor activation was also greatly diminished following chronic Δ9-THC treatment, indicating that cross-tolerance had developed at μ opioid receptors in the NAc. When endocannabinoid-dependent LTD was examined in the NAc, we found that this form of synaptic plasticity was blocked following chronic Δ9-THC exposure, possibly as a result of CB1 receptor tolerance (Hoffman et al., 2003a). A more recent brief communication has shown that, 24 h following a single in vivo injection of Δ9-THC, endocannabinoid-dependent LTD in the NAc and LTD at inhibitory synapses in the hippocampus were blocked (Mato et al., 2004), suggesting that alterations in synaptic plasticity resulting from Δ9-THC can occur very rapidly, and in brain regions other than those directly involved in mediating drug reward. Together, these data suggested that tolerance at CB1 receptors resulting from prior exposure to Δ9-THC is sufficient to explain both the diminished inhibitory presynaptic actions of WIN55,212-2 on neurotransmitter release, and the elimination of the endocannabinoid-mediated LTD in the NAc. In addition, cross-tolerance between CB1 and μ opioid receptors was observed, further demonstrating that these two systems interact within this reward pathway, and suggesting that the NAc may be another critical site to observe opioid–cannabinoid interactions in reward pathways. Future studies will focus on the significance of synaptic plasticity in reward-relevant circuits to determine whether the cellular adaptations resulting from prior Δ9-THC exposure play a significant role in drug use and addiction.
Conclusion
The euphorigenic and anxiolytic properties of marijuana and hashish have been appreciated by man for centuries. However, it has not been until recent times that we have acquired the experimental tools to evaluate the substrates upon which the pharmacologically active constituents of the drug act upon the brain. With the discovery of endogenous ligands possessing biological activity at cannabinoid receptors, and the recognition of their abilities to initiate relatively enduring changes in synaptic processes, an entirely new avenue of exploration has been revealed in which these endocannabinoids may play important fundamental roles in regulating ongoing brain function. Superimposed on the expansion of our knowledge of the basic neurobiology of endocannabinoids is the increasing understanding of the consequences of acute and prolonged recreational use of marijuana. It is now clear that Δ9-THC has effects on core brain reward circuits that are fundamentally similar to those other abused drugs, although the exact mechanisms may differ. In addition, it is now clear that, like other abused drugs, animals will ‘work' to be given the opportunity to self-administer Δ9-THC.
Thus far, a unifying theme in the mechanisms of acute cannabinoid action in the brain has been that of presynaptic inhibition. Since the NAc, and to a larger extent the VTA, receives afferents from a large variety of brain regions, it will be important to determine the effects of cannabinoids on each of these pathways, and to take into account the relative activation of these pathways during cannabinoid consumption. Unfortunately, at present, the most sensitive techniques for resolving pre- versus postsynaptic effects of pharmacological agents rely upon in vitro brain slice studies in which afferent connections cannot be accurately traced. Therefore, it will be important to extend these studies with those performed in vivo, combined with techniques for tracing afferents. Another issue that remains to be resolved is the apparent disparity between the electrophysiological studies described herein, demonstrating clear effects of cannabinoids on presumed CB1 receptors in the VTA, despite receptor binding and immunohistochemical studies that have failed to identify these receptors in this brain region (Herkenham et al., 1991; Matsuda et al., 1993). One possibility is that CB1 receptors are located on a small number of afferent axon terminals impinging upon the VTA, which nevertheless provide strong synaptic input to the DA neurons. If this is indeed the case, then high-resolution immunohistochemical studies might detect CB1 receptors at low levels in the VTA. Clearly, more information is needed in this regard.
Although it is too early to determine whether the direct effects of Δ9-THC on fast amino-acid-mediated synaptic transmission in the NAc and VTA are related to the process of addiction, and to the acute rewarding effects of this ubiquitously consumed drug, the similarities between its actions and those of other drugs on these brain areas suggest that it is not premature to draw parallels. Furthermore, the observations that exogenous and endogenous cannabinoids can alter synaptic process in both the NAc and VTA suggest that the regulation of local and distal afferent input may be essential for the expression of reward-relevant behaviors, and that the continued regulation of these processes by endocannabinoids may contribute to addiction to several classes of drugs. Since our neurobiological understanding of the mechanisms of cannabinoid actions in the brain has increased dramatically in recent years, it is likely that more precise descriptions of the actions of Δ9-THC and of the endocannabinoids in these critical reward circuits will further our understanding of the addictive process in the future.
Abbreviations
- CPP
conditioned place preference
- DA
dopamine
- ICSS
intracranial self-stimulation
- LTD
long-term depression
- NAc
nucleus accumbens
- SA
self-administration
- Δ9-THC
Δ9-tetrahydrocannabinol
- VTA
ventral tegmental area
References
- ALGER B.E. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog. Neurobiol. 2002;68:247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- BARDO M.T. Neuropharmacological mechanisms of drug reward: beyond dopamine in the nucleus accumbens. Crit. Rev. Neurobiol. 1998;12:37–67. doi: 10.1615/critrevneurobiol.v12.i1-2.30. [DOI] [PubMed] [Google Scholar]
- BIDAUT-RUSSELL M., DEVANE W.A., HOWLETT A.C. Cannabinoid receptors and modulation of cyclic AMP accumulation in the rat brain. J. Neurochem. 1990;55:21–26. doi: 10.1111/j.1471-4159.1990.tb08815.x. [DOI] [PubMed] [Google Scholar]
- BREIVOGEL C.S., GRIFFIN G., DI M.V., MARTIN B.R. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol. Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- CAMERON D.L., WESSENDORF M.W., WILLIAMS J.T. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- CARLEZON W.A.J., WISE R.A. Microinjections of phencyclidine (PCP) and related drugs into nucleus accumbens shell potentiate medial forebrain bundle brain stimulation reward. Psychopharmacology (Berl.) 1996a;128:413–420. doi: 10.1007/s002130050151. [DOI] [PubMed] [Google Scholar]
- CARLEZON W.A.J., WISE R.A. Rewarding actions of phencyclidine and related drugs in nucleus accumbens shell and frontal cortex. J. Neurosci. 1996b;16:3112–3122. doi: 10.1523/JNEUROSCI.16-09-03112.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR D.B., SESACK S.R. GABA-containing neurons in the rat ventral tegmental area project to the prefrontal cortex. Synapse. 2000;38:114–123. doi: 10.1002/1098-2396(200011)38:2<114::AID-SYN2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- CHEER J.F., MARSDEN C.A., KENDALL D.A., MASON R. Lack of response suppression follows repeated ventral tegmental cannabinoid administration: an in vitro electrophysiological study. Neuroscience. 2000;99:661–667. doi: 10.1016/s0306-4522(00)00241-4. [DOI] [PubMed] [Google Scholar]
- CHEN J., MARMUR R., PULLES A., PAREDES W., GARDNER E.L. Ventral tegmental microinjection of delta 9-tetrahydrocannabinol enhances ventral tegmental somatodendritic dopamine levels but not forebrain dopamine levels: evidence for local neural action by marijuana's psychoactive ingredient. Brain Res. 1993;621:65–70. doi: 10.1016/0006-8993(93)90298-2. [DOI] [PubMed] [Google Scholar]
- CHEN J.P., PAREDES W., LI J., SMITH D., LOWINSON J., GARDNER E.L. Delta 9-tetrahydrocannabinol produces naloxone-blockable enhancement of presynaptic basal dopamine efflux in nucleus accumbens of conscious, freely-moving rats as measured by intracerebral microdialysis. Psychopharmacology (Berl.) 1990;102:156–162. doi: 10.1007/BF02245916. [DOI] [PubMed] [Google Scholar]
- CHIENG B., WILLIAMS J.T. Increased opioid inhibition of GABA release in nucleus accumbens during morphine withdrawal. J. Neurosci. 1998;18:7033–7039. doi: 10.1523/JNEUROSCI.18-17-07033.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEADWYLER S.A., HEYSER C.J., MICHAELIS R.C., HAMPSON R.E. The effects of delta-9-THC on mechanisms of learning and memory. NIDA Res. Monogr. 1990;97:79–93. [PubMed] [Google Scholar]
- DIANA M., MELIS M., GESSA G.L. Increase in meso-prefrontal dopaminergic activity after stimulation of CB1 receptors by cannabinoids. Eur. J. Neurosci. 1998;10:2825–2830. doi: 10.1111/j.1460-9568.1998.00292.x. [DOI] [PubMed] [Google Scholar]
- DI CHIARA G., IMPERATO A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc. Natl. Acad. Sci. U.S.A. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH E.D. Delta9-tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci. Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- FRENCH E.D., DILLON K., WU X.F. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- FREUND T.F., KATONA I., PIOMELLI D. Role of endogenous cannabinoids in synaptic signaling. Physiol. Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- GAONI Y., MECHOULAM R. Isolation, structure and partial synthesis of an active constituent of hashish. J. Am. Chem. Soc. 1964;86:1646. [Google Scholar]
- GARDNER E.L. Addictive potential of cannabinoids: the underlying neurobiology. Chem. Phys. Lipids. 2002;121:267–290. doi: 10.1016/s0009-3084(02)00162-7. [DOI] [PubMed] [Google Scholar]
- GARDNER E.L., LOWINSON J.H. Marijuana's interaction with brain reward systems: update 1991. Pharmacol. Biochem. Behav. 1991;40:571–580. doi: 10.1016/0091-3057(91)90365-9. [DOI] [PubMed] [Google Scholar]
- GARDNER E.L., VOREL S.R. Cannabinoid transmission and reward-related events. Neurobiol. Dis. 1998;5:502–533. doi: 10.1006/nbdi.1998.0219. [DOI] [PubMed] [Google Scholar]
- GERDEMAN G.L., PARTRIDGE J.G., LUPICA C.R., LOVINGER D.M. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- GERDEMAN G.L., RONESI J., LOVINGER D.M. Postsynaptic endocannabinoid release is critical to long-term depression in the striatum. Nat. Neurosci. 2002;5:446–451. doi: 10.1038/nn832. [DOI] [PubMed] [Google Scholar]
- GESSA G., MELIS M., MUNTONI A., DIANA M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur. J. Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- GONON F.G. Nonlinear relationship between impulse flow and dopamine released by rat midbrain dopaminergic neurons as studied by in vivo electrochemistry. Neuroscience. 1988;24:19–28. doi: 10.1016/0306-4522(88)90307-7. [DOI] [PubMed] [Google Scholar]
- GREEN B., KAVANAGH D., YOUNG R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- HAJOS N., FREUND T.F. Pharmacological separation of cannabinoid sensitive receptors on hippocampal excitatory and inhibitory fibers. Neuropharmacology. 2002;43:503–510. doi: 10.1016/s0028-3908(02)00157-0. [DOI] [PubMed] [Google Scholar]
- HAJOS N., LEDENT C., FREUND T.F. Novel cannabinoid-sensitive receptor mediates inhibition of glutamatergic synaptic transmission in the hippocampus. Neuroscience. 2001;106:1–4. doi: 10.1016/s0306-4522(01)00287-1. [DOI] [PubMed] [Google Scholar]
- HAMPSON R.E., DEADWYLER S.A. Cannabinoids, hippocampal function and memory. Life Sci. 1999;65:715–723. doi: 10.1016/s0024-3205(99)00294-5. [DOI] [PubMed] [Google Scholar]
- HARVEY J., LACEY M.G. A postsynaptic interaction between dopamine D1 and NMDA receptors promotes presynaptic inhibition in the rat nucleus accumbens via adenosine release. J. Neurosci. 1997;17:5271–5280. doi: 10.1523/JNEUROSCI.17-14-05271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIMER L., ZAHM D.S., CHURCHILL L., KALIVAS P.W., WOHLTMANN C. Specificity in the projection patterns of accumbal core and shell in the rat. Neuroscience. 1991;41:89–125. doi: 10.1016/0306-4522(91)90202-y. [DOI] [PubMed] [Google Scholar]
- HENRY D.J., CHAVKIN C. Activation of inwardly rectifying potassium channels (GIRK1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neurosci. Lett. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., LITTLE M.D., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Cannabinoid receptor localization in brain. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN A.F., LUPICA C.R. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J. Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN A.F., LUPICA C.R. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J. Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- HOFFMAN A.F., OZ M., CAULDER T., LUPICA C.R. Functional tolerance and blockade of long-term depression at synapses in the nucleus accumbens after chronic cannabinoid exposure. J. Neurosci. 2003a;23:4815–4820. doi: 10.1523/JNEUROSCI.23-12-04815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN A.F., RIEGEL A.C., LUPICA C.R. Functional localization of cannabinoid receptors and endogenous cannabinoid production in distinct neuron populations of the hippocampus. Eur. J. Neurosci. 2003b;18:524–534. doi: 10.1046/j.1460-9568.2003.02773.x. [DOI] [PubMed] [Google Scholar]
- HOHMANN A.G., HERKENHAM M. Localization of cannabinoid CB1 receptor mRNA in neuronal subpopulations of rat striatum: a double-label in situ hybridization study. Synapse. 2000;37:71–80. doi: 10.1002/(SICI)1098-2396(200007)37:1<71::AID-SYN8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- HUESTIS M.A., GORELICK D.A., HEISHMAN S.J., PRESTON K.L., NELSON R.A., MOOLCHAN E.T., FRANK R.A. Blockade of effects of smoked marijuana by the CB1-selective cannabinoid receptor antagonist SR141716. Arch. Gen. Psychiatry. 2001;58:322–328. doi: 10.1001/archpsyc.58.4.322. [DOI] [PubMed] [Google Scholar]
- JOHNSON S.W., NORTH R.A. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J. Neurosci. 1992;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON S.W., SEUTIN V., NORTH R.A. Burst firing in dopamine neurons induced by N-methyl-D-aspartate: role of electrogenic sodium pump. Science. 1992;258:665–667. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- KITAI S.T., SHEPARD P.D., CALLAWAY J.C., SCROGGS R. Afferent modulation of dopamine neuron firing patterns. Curr. Opin. Neurobiol. 1999;9:690–697. doi: 10.1016/s0959-4388(99)00040-9. [DOI] [PubMed] [Google Scholar]
- KOÓS T., TEPPER J.M. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat. Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- MANZONI O.J., BOCKAERT J. Cannabinoids inhibit GABAergic synaptic transmission in mice nucleus accumbens. Eur. J. Pharmacol. 2001;412:R3–R5. doi: 10.1016/s0014-2999(01)00723-3. [DOI] [PubMed] [Google Scholar]
- MARTIN G., NIE Z., SIGGINS G.R. μ-opioid receptors modulate NMDA receptor-mediated responses in nucleus accumbens neurons. J. Neurosci. 1997;17:11–22. doi: 10.1523/JNEUROSCI.17-01-00011.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATO S., CHEVALEYRE V., ROBBE D., PAZOS A., CASTILLO P.E., MANZONI O.J. A single in-vivo exposure to Delta9THC blocks endocannabinoid-mediated synaptic plasticity. Nat. Neurosci. 2004;7:585–586. doi: 10.1038/nn1251. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., BONNER T.I., LOLAIT S.J. Localization of cannabinoid receptor mRNA in rat brain. J. Comp. Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- MATSUDA L.A., LOLAIT S.J., BROWNSTEIN M.J., YOUNG A.C., BONNER T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA (see comments) Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- MCBRIDE W.J., MURPHY J.M., IKEMOTO S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav. Brain Res. 1999;101:129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., FRIDE E., DI M.V. Endocannabinoids. Eur. J. Pharmacol. 1998;359:1–18. doi: 10.1016/s0014-2999(98)00649-9. [DOI] [PubMed] [Google Scholar]
- MELIS M., PISTIS M., PERRA S., MUNTONI A.L., PILLOLLA G., GESSA G.L. Endocannabinoids mediate presynaptic inhibition of glutamatergic transmission in rat ventral tegmental area dopamine neurons through activation of CB1 receptors. J. Neurosci. 2004;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NG CHEONG TON J.M., GERHARDT G.A., FRIEDEMANN M., ETGEN A.M., ROSE G.M., SHARPLESS N.S., GARDNER E.L. The effects of delta 9-tetrahydrocannabinol on potassium-evoked release of dopamine in the rat caudate nucleus: an in vivo electrochemical and in vivo microdialysis study. Brain Res. 1988;451:59–68. doi: 10.1016/0006-8993(88)90749-4. [DOI] [PubMed] [Google Scholar]
- NICOLA S.M., MALENKA R.C. Modulation of synaptic transmission by dopamine and norepinephrine in ventral but not dorsal striatum. J. Neurophysiol. 1998;79:1768–1776. doi: 10.1152/jn.1998.79.4.1768. [DOI] [PubMed] [Google Scholar]
- PERTWEE R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- PLENZ D. When inhibition goes incognito: feedback interaction between spiny projection neurons in striatal function. Trends Neurosci. 2003;26:436–443. doi: 10.1016/S0166-2236(03)00196-6. [DOI] [PubMed] [Google Scholar]
- RIEGEL A.C., WILLIAMS J.T., LUPICA C.R.Cannabinoid CB1 receptors inhibit GABAB-mediated synaptic currents in midbrain dopamine neurons Soc. Neurosci. Abstr. 200333(abstract no. 462.6) [Google Scholar]
- RINALDI-CARMONA M., BARTH F., HEAULME M., SHIRE D., CALANDRA B., CONGY C., MARTINEZ S., MARUANI J., NELIAT G., CAPUT D. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- ROBBE D., ALONSO G., DUCHAMP F., BOCKAERT J., MANZONI O.J. Localization and mechanisms of action of cannabinoid receptors at the glutamatergic synapses of the mouse nucleus accumbens. J. Neurosci. 2001;21:109–116. doi: 10.1523/JNEUROSCI.21-01-00109.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBBE D., KOPF M., REMAURY A., BOCKAERT J., MANZONI O.J. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAAL D., DONG Y., BONCI A., MALENKA R.C. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., KATHMANN M. Modulation of transmitter release via presynaptic cannabinoid receptors. Trends Pharmacol. Sci. 2001;22:565–572. doi: 10.1016/s0165-6147(00)01805-8. [DOI] [PubMed] [Google Scholar]
- STEFFENSEN S.C., SVINGOS A.L., PICKEL V.M., HENRIKSEN S.J. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J. Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGITA S., JOHNSON S.W., NORTH R.A. Synaptic inputs to GABAA and GABAB receptors originate from discrete afferent neurons. Neurosci. Lett. 1992;134:207–211. doi: 10.1016/0304-3940(92)90518-c. [DOI] [PubMed] [Google Scholar]
- SULLIVAN J.M. Cellular and molecular mechanisms underlying learning and memory impairments produced by cannabinoids. Learn. Mem. 2000;7:132–139. doi: 10.1101/lm.7.3.132. [DOI] [PubMed] [Google Scholar]
- SZABO B., SIEMES S., WALLMICHRATH I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur. J. Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- TANDA G., GOLDBERG S.R. Cannabinoids: reward, dependence, and underlying neurochemical mechanisms – a review of recent preclinical data. Psychopharmacology (Berl.) 2003;169:115–134. doi: 10.1007/s00213-003-1485-z. [DOI] [PubMed] [Google Scholar]
- TANDA G., MUNZAR P., GOLDBERG S.R. Self-administration behavior is maintained by the psychoactive ingredient of marijuana in squirrel monkeys. Nat. Neurosci. 2000;3:1073–1074. doi: 10.1038/80577. [DOI] [PubMed] [Google Scholar]
- TANDA G., PONTIERI F.E., DI CHIARA G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common μ1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- THOMAS M.J., BEURRIER C., BONCI A., MALENKA R.C. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat. Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- TWITCHELL W., BROWN S., MACKIE K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J. Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- VAN BOCKSTAELE E.J., PICKEL V.M. GABA-containing neurons in the ventral tegmental area project to the nucleus accumbens in rat brain. Brain Res. 1995;682:215–221. doi: 10.1016/0006-8993(95)00334-m. [DOI] [PubMed] [Google Scholar]
- WALAAS I., FONNUM F. Biochemical evidence for gamma-aminobutyrate containing fibres from the nucleus accumbens to the substantia nigra and ventral tegmental area in the rat. Neuroscience. 1980;5:63–72. doi: 10.1016/0306-4522(80)90071-8. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- WILSON R.I., NICOLL R.A. Endocannabinoid signaling in the brain. Science. 2002;296:678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- WISE R.A. Addictive drugs and brain stimulation reward. Annu. Rev. Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- WISE R.A., BOZARTH M.A. Brain reward circuitry: four circuit elements ‘wired' in apparent series. Brain Res. Bull. 1984;12:203–208. doi: 10.1016/0361-9230(84)90190-4. [DOI] [PubMed] [Google Scholar]
- WISE R.A., BOZARTH M.A. Brain mechanisms of drug reward and euphoria. Psychiatr. Med. 1985;3:445–460. [PubMed] [Google Scholar]
- WISE R.A., BOZARTH M.A. A psychomotor stimulant theory of addiction. Psychol. Rev. 1987;94:469–492. [PubMed] [Google Scholar]
- WU X., FRENCH E.D. Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment (in process citation) Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- ZAHM D.S. An integrative neuroanatomical perspective on some subcortical substrates of adaptive responding with emphasis on the nucleus accumbens. Neurosci. Biobehav. Rev. 2000;24:85–105. doi: 10.1016/s0149-7634(99)00065-2. [DOI] [PubMed] [Google Scholar]