Abstract
Extracts of bitter melon, soybean, dokudami and welsh onion by 40% methanol increased the accumulation of rhodamine-123 by Caco-2 cells, suggesting that these extracts inhibited P-glycoprotein (P-gp).
The extract of bitter melon was separated in a tC18 cartridge column and the eluate from 80% acetonitrile most markedly increased the [3H]-daunomycin accumulation by Caco-2 cells.
The inhibitory compounds in the bitter melon fraction were isolated by HPLC with Pegasil C4 and Pegasil ODS columns. The HPLC fraction having the highest activity was analyzed by 1H-NMR and FAB-MS, and the active compound was identified as 1-monopalmitin.
The inhibitory activities of 1-monopalmitin and its related compounds suggested that the inhibition of P-gp activity was not dependent on the degree of unsaturation of fatty acid in the monoglyceride, but on the chain length. It was also suggested that the monoglyceride structure played an important role in the inhibition of P-gp activity.
Monoglycerides could therefore alter the pharmacokinetics of drugs by inhibiting the P-gp-mediated efflux.
Keywords: P-gp, bitter melon, Caco-2, daunomycin accumulation, 1-monopalmitin
Introduction
P-glycoprotein (P-gp, P indicating permeability), a product of the MDR1 gene, is a 170-kDa membrane protein belonging to the ATP-binding cassette (ABC) transporter superfamily. P-gp has two nucleotide-binding domains (NBDs) and two highly homologous halves, each of which contains a transmembrane domain (Germann & Chambers, 1998). NBDs function as ATPase, and P-gp flushes out hydrophobic drugs from a cell by using the energy obtained by hydrolyzing ATP (Hamada & Tsuruo, 1988a, 1988b; Lelong et al., 1992).
P-gp is highly expressed in the cell membrane of tumor cells and excretes hydrophobic drugs from the cells in an ATP-dependent manner (Tanigawara et al., 1992; Yeh et al., 1992; Wang et al., 2001). The intracellular concentration of a drug in the tumor cells therefore decreases, the effect of the drug also being reduced. Furthermore, the broad substrate specificity of P-gp causes multidrug resistance, so that tumor cells gain resistance to many structurally unlike anticancer drugs. Multidrug resistance-associated proteins (MRPs), which belong to the ABC transporter superfamily but have different substrate specificity from P-gp, are also known as a factor of multidrug resistance (Cole et al., 1992; Suzuki, 1999). Since multidrug resistance is the main cause of disturbance in cancer chemotherapy, many studies have been made to investigate P-gp and MRPs from the viewpoint of multidrug resistance.
P-gp is expressed in many normal tissues, especially in the brain, liver, intestine, kidney and placenta, which have barrier functions, and also has a function as an ATP-dependent efflux pump that excretes highly hydrophobic xenobiotic compounds (Hunter et al., 1993). P-gp is expressed at the apical surface of intestinal epithelial cells (Thiebaut et al., 1987) and excretes drugs and xenobiotic compounds ingested with foods to the apical side. P-gp thus regulates the intestinal absorption of xenobiotic compounds. We examine in the present study the capability of food components, especially vegetables and fruits, to directly regulate P-gp in intestinal epithelial cells, and characterize the food factors which modulate the efflux activity of P-gp. Caco-2 cells derived from human colonic adenocarcinoma were used as a model for small intestinal epithelial cells. Caco-2 cells express P-gp and MRPs as much as normal intestinal epithelial tissues do, while they express less cytochrome P450 3A4 (CYP3A4), a drug metabolic enzyme whose substrates overlap those of P-gp, than normal intestinal epithelial tissues (Wacher et al., 1995; Kim et al., 1999; Nakamura et al., 2002). Caco-2 cells can therefore serve to assess the P-gp activity.
Methods
Materials
The Caco-2 cell line was purchased from American Type Culture Collection (Rockville, MD, U.S.A.). Dulbecco's modified Eagle's medium (DMEM) was purchased from Sigma (St Louis, MO, U.S.A.). L-glutamine and penicillin–streptomycin (10,000 units ml−1 and 10 mg ml−1 in 0.9% sodium chloride, respectively) were purchased from Gibco (Gaithersburg, MD, U.S.A.). Fetal bovine serum (FBS) was purchased from Asahi Technoglass (Chiba, Japan), and non-essential amino acids (NEAA) were purchased from Cosmobio (Tokyo, Japan). Rhodamine-123 was purchased from Kanto Kagaku (Tokyo, Japan), and [3H]-daunomycin (specific radioactivity of 5.0 Ci mmol−1) was purchased from Amersham (London, U.K.). A type-I collagen solution was purchased from Nitta Gelatin (Osaka, Japan), and Hanks' balanced salt solution (HBSS) was from Life Technologies (Grand Island, NY, U.S.A.). Pronase, 1-monopalmitin, 1-monopalmitolein, 1-monostearin, 1-monomyristin, 1-monolaurin, 1-monocaprin, 1-monocaprilin, 1-monoolein, 1-monolinolein, 1-monolinolenin, palmitic acid, glycerol, tripalmitin, 1,2-dipalmitin, 1,3-dipalmitin and 2-monopalmitin were all purchased from Sigma (St Louis, MO, U.S.A.). Lactate dehydrogenase (LDH) Test Wako was from Wako Chemicals (Osaka, Japan).
Cell culture
Caco-2 cells were cultured in 78.5 cm2 plastic dishes with a culture medium consisting of DMEM, 10% FBS, 1% NEAA, 2% glutamine, 100 U ml−1 of penicillin, 100 μg ml−1 of streptomycin and an appropriate amount of sodium bicarbonate. The cells were incubated at 37°C in a humidified atmosphere of 5% CO2 in air, the culture medium being renewed on alternate days. When they had reached confluence, the cells were passaged at a split ratio of 4 to 8 by trypsinizing with 0.1% trypsin and 0.02% EDTA in phosphate-buffered saline (PBS). All the cells used in this study were between passages 40 and 66. An accumulation experiment was performed by using Caco-2 cells that had been cultured at a density of 1.4 × 105 cells well−1 in 24-well plates precoated with collagen. The cell monolayers for the accumulation experiments were used after 14 days of culture.
Accumulation experiments
The rhodamine-123 accumulation experiments were performed in the absence or presence of an assay sample. Caco-2 monolayers were washed twice with 700 μl of PBS, and then equilibrated with 700 μl of HBSS containing 4 mM sodium bicarbonate and 10 mM HEPES, the pH being adjusted to 7.4 with KOH (the accumulation buffer). The cells were then incubated with 15 μM rhodamine-123 in 500 μl of the accumulation buffer with or without an assay sample at 37°C for 2 h. The sample solution was then removed, and each well was washed twice with 700 μl of ice-cold PBS containing 0.05% sodium azide for 5 min. The fluorescence intensity of rhodamine-123 was measured with a Fluoroskan plate reader (Labsystems, Helsinki, Finland) at an excitation wavelength of 485 nm and an emission wavelength of 544 nm. The [3H]-daunomycin accumulation experiments were performed in almost the same manner as that just described. Briefly, Caco-2 cells were incubated with 40 nM [3H]-daunomycin in 300 μl of the accumulation buffer with or without an assay sample at 37°C for 2 h. The sample solution was then removed and each well was washed twice with 700 μl of ice-cold PBS containing 0.05% sodium azide for 5 min. To each well, 300 μl of 0.1% Triton X-100 was then added, and the cell lysate was taken into 3 ml of a scintillation cocktail. The tritium content of each monolayer was finally determined with an LSC 5100 liquid scintillation analyzer (Aloka, Tokyo, Japan).
Preparation of the extracts from food materials
Vegetable and fruit samples that had been freeze-dried and powdered were provided by Kagome Co. Ltd, Japan. Inedible parts of these samples were removed. To 50 mg of each of the food materials was added 5 ml of 40% methanol, and the mixture shaken at room temperature for 15 min. After the mixture had been centrifuged at 3000 r.p.m. for 10 min at 10°C, the supernatant was recovered and evaporated at 60°C. The residue was dissolved in deionized and distilled water (DDW) and freeze-dried. The freeze-dried sample was dissolved in the accumulation buffer at a concentration of 2 mg ml−1 and served for the analysis.
Extraction of the active compounds from bitter melon
In all, 5 g of bitter melon powder was suspended in 150 ml of DDW, and the suspension shaken at room temperature for 20 min. After centrifuging the mixture at 7000 r.p.m. for 30 min at 10°C, the supernatant was filtered by suction. The resulting filtrate was applied to a Sep-Pak Vac tC18 cartridge (35-ml bead volume; Waters) that had been equilibrated with DDW. The activity of fractions recovered by step-wise elution with an increasing concentration of acetonitrile was evaluated by measuring the daunomycin accumulation in Caco-2 cells. The active fraction recovered was freeze-dried, and about 0.5 mg of dry material was obtained.
Purification of the active compounds by high-performance liquid chromatography (HPLC)
The sample solution was first injected into an HPLC equipment (Gulliver system, Jasco, Japan) fitted with a Pegasil C4 column (4.6 × 250 mm; Senshu Kagaku, Japan) that had been equilibrated with 40% acetonitrile. Elution was carried out with DDW-acetonitrile at a flow rate of 1.0 ml min−1 with a linear gradient of acetonitrile from 40 to 100% over 60 min, the eluate being monitored at 210 nm. The activity of each fraction was evaluated by measuring the daunomycin accumulation in Caco-2 cells. The fraction with the highest activity was recovered. The active material obtained was then applied to a Pegasil ODS column (4.6 × 250 mm; Senshu Kagaku, Japan) that had been equilibrated with 40% acetonitrile. Elution was carried out as described before, each fraction being recovered and freeze-dried.
Lactate dehydrogenase (LDH) assay
To evaluate the damage to the Caco-2 cells, an LDH assay was employed. The Caco-2 monolayer that had been cultured in 24-well plates for 14 days was washed twice with 500 μl of the accumulation buffer and then incubated with 500 μl of the accumulation buffer containing the sample to be tested at 37°C for 2 h. The sample solution (supernatant) was removed, and then 500 μl of 0.1% Triton X-100 was added to each well to solubilize the residual cells. The amounts of LDH in the supernatant and solubilized cell fraction were determined by the enzymatic method with an LDH-Cytotoxic Test kit (Wako, Osaka, Japan). The cytotoxicity to the Caco-2 monolayers is expressed as the relative amount being given by the sum of LDH released into the supernatant to the total amount of LDH, this latter amount being given by the sum of intracellular LDH and LDH released from the control cells.
Structural analysis
NMR analyses were conducted with a Jeol JNM-A500 spectrometer, and the FAB-MS analysis was performed with a Jeol MS700T mass spectrometer.
Statistical analysis
Each result is expressed as the mean±s.d. Differences among the experimental data were assessed by a one-way analysis of variance (ANOVA) followed by F-protected Fisher's least significant difference.
Results
Effect of extracts of vegetables and fruits on the accumulation of rhodamine-123 or daunomycin by Caco-2 cells
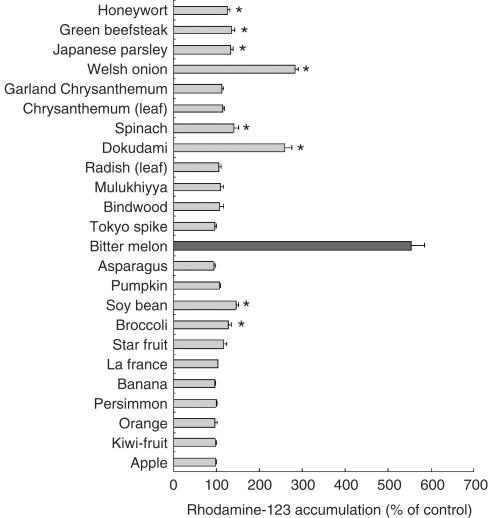
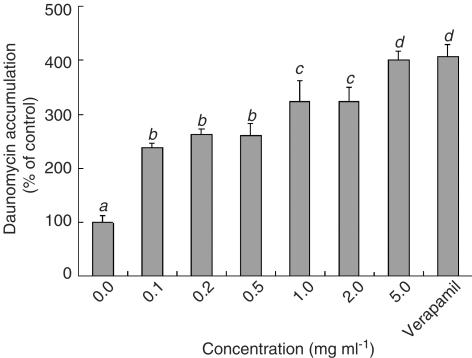
Among the 60 kinds of vegetables and fruits, the extracts of bitter melon, soybean, dokudami and welsh onion from 40% methanol significantly increased the rhodamine-123 accumulation by Caco-2 cells (Figure 1). The extract of bitter melon was the most effective of them. The [3H]-daunomycin accumulation was also increased by the extract of bitter melon in a concentration-dependent manner (Figure 2). This suggests that the extract of bitter melon inhibited the flushing out of rhodamine-123 and daunomycin by P-gp (MDR1), although the inhibitory effect was less potent than verapamil, a well-known P-gp inhibitor/substrate.
Figure 1.
Effects of vegetables and fruits on the rhodamine-123 accumulation by Caco-2 cells. The accumulation of rhodamine-123 was measured after incubating the Caco-2 cells at 37°C for 2 h with or without a 40% methanol extract of vegetables and fruits (2 mg ml−1). Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=4), *P<0.001 vs control.
Figure 2.
Effect of concentration of the bitter melon extract on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without the bitter melon extract eluted with 40% methanol. Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Effect of bitter melon fractions extracted with different concentrations of methanol on the daunomycin accumulation by Caco-2 cells
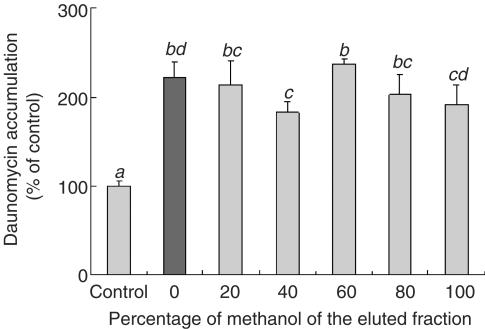
Figure 3 shows that the bitter melon fractions extracted with different concentrations of methanol increased the daunomycin accumulation. Since the bitter melon fraction extracted with DDW was also effective, the DDW extract of bitter melon was used in this study.
Figure 3.
Effect of the bitter melon extracts eluted by different concentrations of methanol on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without a bitter melon extract eluted by different concentrations of methanol (2 mg ml−1). Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Effect on the daunomycin accumulation by Caco-2 cells of the DDW extract of bitter melon boiled or treated by pronase
The effect of the DDW extract of bitter melon on the daunomycin accumulation was not affected by boiling or by the Pronase treatment (Table 1 ). It was therefore suggested that the substances inhibiting the P-gp activity in bitter melon were neither proteins nor peptides.
Table 1.
Effect on the daunomycin accumulation of the aqueous extract of bitter melon boiled or treated with pronase
| Daunomycin accumulation (% of control) | |
|---|---|
| Control | 100±6.92a |
| Extract | 366.3±32.7b |
| Extract (boiled) | 344.7±38.5b |
| Extract (treated by pronase) | 375.1±30.9b |
The aqueous extract of bitter melon was boiled at 100°C for 20 min, or treated with 0.4 U ml−1 of pronase at 37°C for 2 h and subsequently boiled at 100°C for 20 min. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without a sample (2 mg ml−1). Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abc The values not sharing common superscript letters are significantly different as P<0.01.
The values not sharing common superscript letters are significantly different as P<0.01.
Isolation of active substance from the extract by using a Sep-Pak Vac tC18 cartridge column
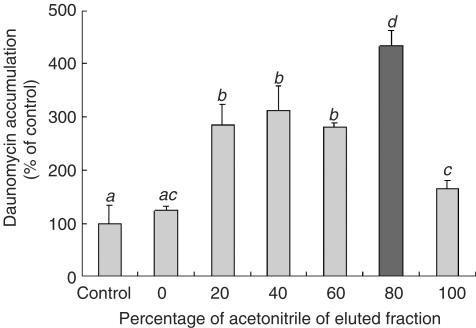
The DDW extract of bitter melon was applied to a Sep-Pak Vac tC18 cartridge column, and the absorbed materials were recovered by step-wise elution with acetonitrile. The fractions eluted with 20, 40, 60, 80 and 100% acetonitrile all increased the daunomycin accumulation, suggesting that mutiple inhibitory substances would have been present in the extract. Since the fraction eluted with 80% acetonitrile showed the greatest increase in daunomycin accumulation among them (Figure 4), this fraction seemed to contain the major factor inhibiting P-gp activity.
Figure 4.
Effect of the Sep-Pak fractions of the bitter melon extract on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without a bitter melon fraction separated in the Sep-Pak column with an increasing concentration of acetonitrile (2 mg ml−1). Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Purification and identification of the inhibitory substance by using a Pegasil C4 column
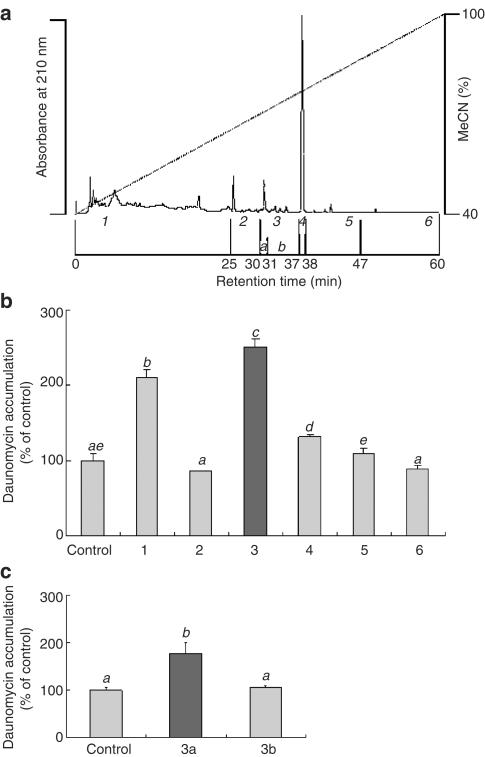
The Sep-Pak fraction eluted with 80% acetonitrile was analyzed by HPLC with a Pegasil C4 column. As shown in Figure 5a, several peaks appeared at 210 nm. The eluate was separated into six fractions (1–6) under these peaks. Figure 5b shows the effect of each fraction on the daunomycin accumulation, this being significantly increased by fractions 1 and 3, with fraction 3 having the most potent effect. Fraction 3 was further separated into two fractions (3a and 3b) under the same HPLC conditions, and the effects of these fractions were examined (Figure 5c). Only fraction 3a significantly increased the daunomycin accumulation.
Figure 5.
Purification of the active substance by HPLC with a Pegasil C4 column. (a) Typical HPLC chromatogram. Each fraction was recovered and freeze-dried. (b, c) The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without 1 mg ml−1 of each fraction. Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Analysis of the purified compound by 1H-NMR and FAB-MS
Fraction 3a was analyzed by 1H-NMR and FAB-MS. The 1H-NMR spectrum of the fraction showed signals at δ 5.37 (3H, t, J=5.5), 422 (1H, dd, J=5.0, 11.0), 4.16 (1H, dd, J=6.3, 11.0), 3.95 (1H, ‘sxt', J=4.9), 3.71 (1H, dd, J=5.0, 11.0), 3.61 (1H, dd, J=6.3, 11.0), 2.82 (2H, t, J=6.3), 2.36 (4H, t, J=7.6), 2.07 (2H, brm), 1.65 (4H, brm), 1.26 (38H, brm), 0.99 (1H, t, J=6.9) and 0.89 (3H, t, J=6.3). These results indicate that this compound was 1-monoglyceride and that it was unsaturated. On the other hand, the FAB-MS data indicated the molecular mass of this substance to be m/z 330.5, which gives a best fit to C19H38O4, although this compound is saturated. It is therefore likely that this fraction had not been completely purified.
Purification and identification of the inhibitory substance by using a Pegasil ODS column
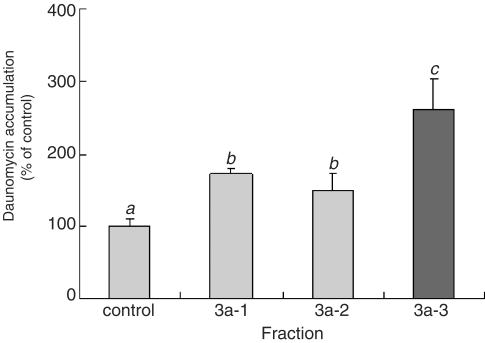
Fraction 3a was applied to a Pegasil ODS column which was eluted under the same conditions as those already described. Three fractions (3a-1, 3a-2 and 3a-3) were isolated, the major peak being observed in the fraction 3a-2. Figure 6, however, shows that 3a-3, which had no distinct peak, resulted in the greatest increase in daunomycin accumulation. When fraction 3a-3 was analyzed by 1H-NMR (data not shown), the structure of the inhibitory substance in this fraction was identified to be 1-monopalmitin. The yield of the purified compounds was about 1.0 mg from 1.3 kg of bitter melon powder.
Figure 6.
Effect of the 3a fractions on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without each fraction. Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Effect of 1-monopalmitin on the daunomycin accumulation by Caco-2 cells
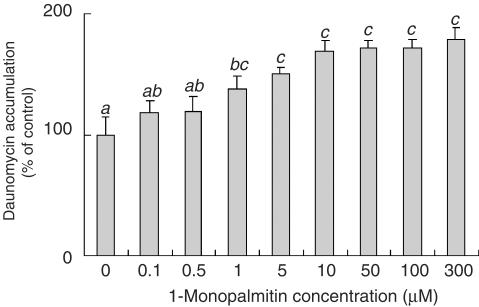
The effect of 1-monopalmitin on the daunomycin accumulation was investigated by using commercially obtained 1-monopalmitin. 1-Monopalmitin was dispersed in the accumulation buffer and solubilized by sonication before use. As shown in Figure 7, 1-monopalmitin increased the daunomycin accumulation in a dose-dependent manner. This result suggests that 1-monopalmitin was responsible for inhibiting the daunomycin efflux by P-gp.
Figure 7.
Effect of 1-monopalmitin concentration on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without different concentrations of 1-monopalmitin. Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Cytotoxic effect of 1-monopalmitin on Caco-2 cells
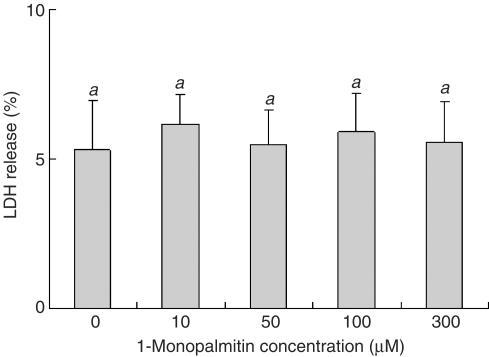
1-Monopalmitin is known to be surface active, so it was possible that the inhibition of P-gp-mediated efflux could have been due to its cytotoxic properties. The cytotoxic effect of 1-monopalmitin on Caco-2 cells was therefore examined by using a lactate dehydrogenase (LDH) assay. 1-Monopalmitin was dispersed in the accumulation buffer and solubilized by sonication before use. 1-Monopalmitin (10–300 μM) had no effect on the LDH release (Figure 8). This indicates that the inhibition of P-gp activity by Caco-2 cells caused by 1-monopalmitin was not due to cell damage.
Figure 8.
Cytotoxic effect of 1-monopalmitin on the Caco-2 cells. The amount of LDH released into the supernatant to the total amount of LDH was measured after incubating the Caco-2 cells at 37°C for 2 h with or without 1-monopalmitin. Each data value is expressed as the relative amount of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Effect of 1-monopalmitin on the amino-acid transport activities in Caco-2 cells
The effect of 1-monopalmitin on the amino-acid transport activity in the Caco-2 monolayers was investigated. 1-Monopalmitin (100 μM) did not affect amino-acid transport (data not shown), suggesting that the inhibitory effect of 1-monopalmitin was specific to the P-gp activity.
Effect of 1-monopalmitin and related compounds on the daunomycin accumulation by Caco-2 cells
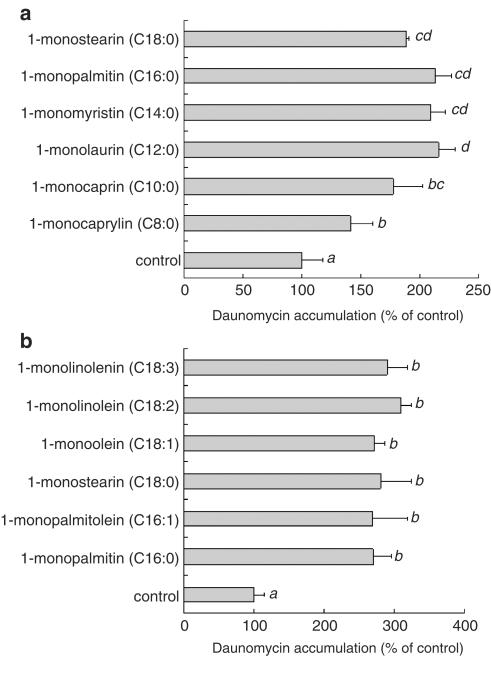
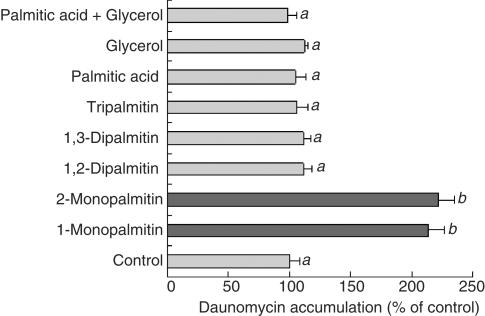
Monoglyceride samples were solubilized by sonication and served for the daunomycin accumulation experiments. As shown in Figure 9a, all of the 1-monoglycerides tested significantly increased the daunomycin accumulation. However, its accumulation in the presence of 1-monocaprin (C10:0) was lower than that of 1-monopalmitin. Furthermore, the effect of 1-monocaprylin (C8:0) on the daunomycin accumulation was significantly lower than that of 1-monocaprin (C10:0). These results suggest that the chain length of the fatty acid in a 1-monoglyceride was an important factor in the inhibition of P-gp activity. As shown in Figure 9b, all the 1-monoglycerides with unsaturated fatty acids increased the daunomycin accumulation, suggesting that being saturated or unsaturated did not affect the inhibitory activity of the monoglycerides. As shown in Figure 10, tripalmitin, dipalmitin, palmitic acid and glycerol did not have any effects on the daunomycin accumulation, while 1-monopalmitin and 2-monopalmitin each had a significant effect. This result suggests the requirement of the monoglyceride structure for the inhibition of P-gp.
Figure 9.
Effect of 1-monoglycerides with different chain length of saturated fatty acids (a) and unsaturated fatty acids (b) on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without 100 μM of a 1-monoglyceride. Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Figure 10.
Effect of 1-monoglyceride and its related compounds on the daunomycin accumulation by Caco-2 cells. The accumulation of daunomycin was measured after incubating the Caco-2 cells at 37°C for 2 h with or without 100 μM of 1-monoglyceride and its related compounds. Each data value is expressed as a percentage of the control, and is presented as the mean±s.d. (n=3). abcThe values not sharing common superscript letters are significantly different as P<0.01.
Discussion
Various studies on the regulation of P-gp by food components have been reported. Takanaga et al. (1998), for example, have reported that the ingestion of grapefruit juice (GFJ) inhibited both P-gp and the detoxication enzyme, CYP3A4, thereby changing the pharmacokinetics of the drugs. The components in GFJ that modulated the activities of P-gp and CYP3A4 were isolated by Ohnishi et al. (2000) and identified as furanocoumarin derivatives. Takanaga et al. (2000) have reported that some methoxyflavones contained in orange juice inhibited drug efflux by P-gp. An extract of the herb, rosemary (Rosemarinus officinalis Labiatae), also directly suppressed the P-gp activity by inhibiting the binding of substrates to P-gp (Plouzek et al., 1999). In the present study, a new P-gp inhibitory substance was sought in extracts of vegetables and fruits by using human intestinal epithelial Caco-2 cells.
In the first screening (Figure 1), a fluorescent probe of rhodamine-123 was used to examine the samples. However, the rhodamine fluorescence was sometimes interfered with by the pigments in food materials. We therefore used [3H]-daunomycin for further experiments, because daunomycin is also the substrate of P-gp and is not affected by food pigments. The extract of bitter melon increased the daunomycin accumulation in a concentration-dependent manner (Figure 2), suggesting that the extract contained a substance that actually inhibited the P-gp activity.
Bitter melon (Momordica charantia) is a plant that grows in tropical areas of Asia, the Caribbean and South America, and that has a long history of use as a traditional medicine. It has been reported that bitter melon has antidiabetic, antiviral, antioxidative and antineoplastic effects (Basch et al., 2003). Some functional components have already been identified in the extract, including vegetable insulin and charantin having hypoglycemic effects (Miura et al., 2001; Vikrant et al., 2001). A 30-kDa protein from the seeds and fruits of bitter melon, MAP30, having antiviral and antineoplastic effects is another example (Lee-Huang et al., 1990; 1995; Sun et al., 2001). The results of the present study demonstrate that another function of bitter melon is the inhibition of P-gp.
To isolate the P-gp inhibitory substance(s), we first investigated which concentration of methanol would be most effective for extracting the active substance. Similar activity was observed in the fractions extracted with any concentrations of methanol (Figure 3). This may indicate that the active substance was an amphipathic compound extractable with solvents of diverse polarity, or multiple inhibitory compounds with different solubility were present in bitter melon. The fractionation experiment with a Sep-Pak Vac tC18 cartridge column indicated that the active substances were eluted with a wide range of acetonitrile concentration (Figure 4). This suggests that the active substance had both hydrophilic and hydrophobic properties or that multiple inhibitory compounds were present in bitter melon extract, as had been predicted in the extraction experiment (Figure 3). The 80% acetonitrile fraction was then applied to HPLC with a Pegasil C4 column, and an active fraction with a single peak was obtained (fraction 3a), as shown in Figure 5. Further fractionation by using a Pegasil ODS column, however, indicated the peak material to be the contaminant, and the active substance was hardly detectable at 210 nm. Fraction 3a-3, having P-gp-inhibiting activity but no peak at 210 nm, was finally analyzed by 1H-NMR and identified to be 1-monopalmitin. The results of this study also demonstrate that monoglycerides with a variety of hydrocarbon-chain length were inhibitory toward P-gp (Figure 9). The results of the HPLC analysis with a Pegasil C4 column also indicate that the respective retention times for 1-monocaprylin (C8:0), 1-monocaprin (C10:0) and 1-monolaurin (C12:0) were 8.6, 14.4 and 20.3 min, and that monoglycerides with longer fatty acid chains had longer retention times (data not shown). These results suggest that the activity of Pegasil C4 fractions 1 and 4 (Figure 5) may be explained by these monoglycerides. We have not analyzed the fatty acid composition of all of the monoglycerides in the bitter melon extracts, but the diverse distribution of activity in the methanol fractions (Figure 3) and Sep-pak fractions (Figure 4) may, at least partly, be explained by the different monoglycerides present. The total effect of bitter melon would therefore be due to the monoglycerides and other inhibitory compounds containing monoglycerides.
Monopalmitin is known to be a surface-active compound. Surface-active substances often show a cytotoxic effect on cells by disrupting the cell membrane. However, 1-monopalmitin (10–300 μM) did not induce the release of lactate dehydrogenase (LDH) from Caco-2 cells, suggesting that 1-monopalmitin was not cytotoxic to Caco-2 cells. Surfactants are generally known to change the cell membrane fluidity, and this may have induced the inhibition of P-gp. Rege et al. (2002) have, however, reported that the effects of surfactants on the transporter functions in Caco-2 cells were diverse, suggesting that a change in membrane fluidity may not be the general mechanism for reducing transporter activity. Bogman et al. (2003), who studied the effects of nine different types of surfactant on the P-gp activity, have suggested that surface-active agents demonstrated transporter-specific interaction, rather than unspecific membrane permeabilization. It would therefore be possible that monoglycerides could specifically interact with P-gp in the cell membrane of Caco-2 cells.
Several possible mechanisms below may therefore be involved in the inhibition of P-gp by monoglycerides. First, monoglycerides may be substrates of P-gp. Since P-gp has broad substrate specificity for a variety of hydrophobic compounds, 1-monopalmitin could act as one of the P-gp substrates. In this case, monoglycerides would inhibit the efflux of P-gp substrates in a competitive manner, as verapamil, a well-known P-gp inhibitor/substrate, does. Accumulation experiments using [3H]-monoglyceride would be useful to reveal whether monoglycerides are substrates of P-gp. The second possibility would be direct binding of the monoglycerides to P-gp or the cell membrane, thereby inhibiting the efflux activity of P-gp. Saeki et al. (1993) and Ueda et al. (1992) have both reported that progesterone and nitrendipine directly interacted with P-gp and inhibited the efflux of P-gp substrates, although they were not transported by P-gp. Monoglycerides may also inhibit the efflux of the P-gp substrates by directly binding with P-gp without being transported by P-gp. Photoaffinity labelling using [3H]-azidopine could make out whether monoglycerides directly bind P-gp. As a final possibility, monoglycerides could modulate the ATPase activity of P-gp. P-gp has the function of hydrolyzing ATP, thereby getting the energy to transport the substrates from cells (Hamada & Tsuruo, 1988a, 1988b; Lelong et al., 1992). This mechanism would account for the reduced efflux of the substrate by monoglycerides. Orlowski et al. (1998) have reported that detergents, except for sodium deoxycholate, induced partial inhibition of P-gp ATPase activity at a nonsolubilizing concentration. They also described that specific interactions between P-gp and a detergent were more likely rather than global membrane perturbation. Photoaffinity labelling using 8-azido-α[32P]ATP could allow to evaluate effects of monoglycerides on P-gp ATPase activity.
It is surprising that 1-monopalmitin, a simple monoglyceride with an abundant fatty acid chain, was found to be one of the P-gp-inhibiting substances in bitter melon. Although a variety of functions of monoglycerides have been reported (Boyle & German, 1996; Emilio, 2003), the modulation of P-gp functions by monoglycerides has not previously been. A monoglyceride is produced from a triglyceride in dietary fat. Although hardly any of the 1-monoglyceride detected in the bitter melon extract is produced in the intestinal tract, a 2-monoglyceride with two molecules of free fatty acids is efficiently produced from dietary triglycerides by specific esterases in the small intestine. Since this 2-monoglyceride is not readily hydrolyzable by digestive enzymes and has almost identical P-gp-inhibiting activity to the 1-monoglyceride (Figure 10), the results of our study suggest that the activity of intestinal P-gp could be affected by the ingestion of dietary fat and emulsified food with monoglycerides. This may be a new function of monoglycerides, and its physiological significance to lipid absorption and metabolism in the intestines is of great interest. The results of this study allow to speculate that, whenever the drug is stable in the intestine in the presence of food, the amount of drug absorption may increase when given in the presence of lipidous food.
In conclusion, our study has demonstrated that the extracts of bitter melon, soybean, dokudami and welsh onion inhibited the P-gp activity in Caco-2 intestinal cells, bitter melon showing the most potent inhibition. 1-monopalmitin was found to be the major inhibitor of P-gp in bitter melon. Various types of monoglycerides, including 2-monoglycerides, were shown to have P-gp inhibitory activity. Monoglycerides are contained not only in natural foods such as vegetables, but also in several types of processed food as emulsified food products. Monoglycerides are also produced by the hydrolysis of dietary triglyceride in the digestive tract. The physiological role of monoglycerides should be revealed in terms of the modulation of P-gp functions.
Acknowledgments
This work was supported in part by a grant-in-aid for scientific research from the Ministry of Education, Science and Culture of Japan (S-15108002). Some of the accumulation experiments in this work were performed at the Biotechnology Research Center of the University of Tokyo (Tokyo, Japan).
Abbreviations
- ABC
ATP-binding cassette
- CYP
cytochrome P450
- DDW
deionized and distilled water
- GFJ
grapefruit juice
- HBSS
Hanks' balanced salt solution
- MRPs
multidrug resistance-associated proteins
- NBD
nucleotide-binding domain
- P-gp
P-glycoprotein
References
- BASCH E., GABARDI S., ULBLICHT C. Bitter melon (Momordica charantia): a review of efficacy and safety. Am. J. Health Syst. Pharm. 2003;60:356–359. doi: 10.1093/ajhp/60.4.356. [DOI] [PubMed] [Google Scholar]
- BOGMAN K., ERNE-BRAND F., ALSENZ J., DREWE J. The role of surfactants in the reversal of active transport mediated by multidrug resistance proteins. J. Pharm. Sci. 2003;92:1250–1261. doi: 10.1002/jps.10395. [DOI] [PubMed] [Google Scholar]
- BOYLE E., GERMAN J.B. Monoglycerides in membrane systems. Crit. Rev. Food Sci. Nutr. 1996;36:785–805. doi: 10.1080/10408399609527750. [DOI] [PubMed] [Google Scholar]
- COLE S.P.C., BHARDWAJ G., GERLACH J.H., MACKIE J.E., GRANT C.E., ALMQUIST K.C., STEWART A.J., KURZ E.U., DUNCAN A.M.V., DEELEY R.G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992;258:1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- EMILIO R. Intestinal absorption of triglyceride and cholesterol. Dietary and pharmacological inhibition to reduce cardiovascular risk. Atherosclerosis. 2003;151:357–379. doi: 10.1016/s0021-9150(00)00456-1. [DOI] [PubMed] [Google Scholar]
- GERMANN U.A., CHAMBERS T.C. Molecular analysis of the multidrug transporter, P-glycoprotein. Cytotechnology. 1998;27:31–60. doi: 10.1023/A:1008023629269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMADA H., TSURUO T. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res. 1988a;48:4926–4932. [PubMed] [Google Scholar]
- HAMADA H., TSURUO T. Purification of the 170- to 180-kilodalton membrane glycoprotein associated with multidrug resistance. J. Biol. Chem. 1988b;263:1454–1458. [PubMed] [Google Scholar]
- HUNTER J., JEPSON M.A., TSURUO T., SIMMONS N.L., HIRST B.H. Functional expression of P-glycoprotein in apical membranes of human intestinal Caco-2 cells. J. Biol. Chem. 1993;268:14991–14997. [PubMed] [Google Scholar]
- KIM R.B., WANDEL C., LEAKE B., CVETKOVIC M., FROMM M.F., DEMPSEY P.J., RODEN M.M., BELAS F., CHAUFHARY A.K., RODEN D.M., WOOD A.J.J., WILKINSON G.R. Interrelationship between substrates and inhibitors of human CYP3A and P-glycoprotein. Pharmacol. Res. 1999;16:408–414. doi: 10.1023/a:1018877803319. [DOI] [PubMed] [Google Scholar]
- LEE-HUANG S., HUANG L.P., HUANG L.P., BOURINBAIAR S.A., CHEN H., KUNG H. Inhibition of the integrase of human immunodeficiency virus (HIV) type 1 by anti-HIV plant proteins MAP30 and GAP31. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8818–8822. doi: 10.1073/pnas.92.19.8818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE-HUANG S., HUANG L.P., NARA L.P., CHEN H., KUNG H., HUANG P., HUANG I.H., HUANG L.P. MAP30: a new inhibitor of HIV-1 infection and replication. FEBS Lett. 1990;272:12–18. doi: 10.1016/0014-5793(90)80438-o. [DOI] [PubMed] [Google Scholar]
- LELONG I.H., PADMANABHAN R., LOVELACE E., PASTAN I., GOTTESMAN M.M. ATP and GTP as alternative energy sources for vinblastine transport by P-170 in KB-V1 plasma membrane vesicles. FEBS Lett. 1992;304:256–260. doi: 10.1016/0014-5793(92)80632-q. [DOI] [PubMed] [Google Scholar]
- MIURA T., ITOH C., IWAMOTO N., KATO M., KAWAI M., PRAK S.R., SUZUKI I. Hypoglycemic activity of the fruit of the Momordica charantia in type 2 diabetic mice. J. Nutr. Sci. Vitaminol. 2001;47:340–344. doi: 10.3177/jnsv.47.340. [DOI] [PubMed] [Google Scholar]
- NAKAMURA T., SAKAEDA T, OHMOTO N., TAMURA T., AOYAMA N., SHIRAKAWA T., KAMIGAKI T., NAKAMURA T., KIM K.I., KIM S.R., KURODA Y., MATSUO M., KASUGA M., OHUMURA K. Real-time quantitative polymerase chain reaction for MDR1, MRP1, MRP2, and CYP3A-mRNA levels in Caco-2 cell lines, human duodenal enterocytes, normal colorectal tissues, and colorectal adenocarcinomas. Drug Metab. Dispos. 2002;30:4–6. doi: 10.1124/dmd.30.1.4. [DOI] [PubMed] [Google Scholar]
- OHNISHI A., MATSUO H., TAMADA S., TAKANAGA H., MORIMOTO S., SHOYAMA Y., OHTANI H., SAWADA Y. Effect of furanocoumarin derivatives in grapefruit juice on the uptake of vinblastine by Caco-2 cells and on the activity of cytochrome P450 3A4. Br. J. Pharmacol. 2000;130:1369–1377. doi: 10.1038/sj.bjp.0703433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORLOWSKI S., SELOSSE M.A., BOUDON C., MICOUD C., MIR L.M., BELEHRADEK J., JR, GARRIGOS M. Effects of detergents on P-glycoprotein ATPase activity: differences in perturbation of basal and verapamil-dependent activities. Cancer Biochem. Biophys. 1998;16:85–110. [PubMed] [Google Scholar]
- PLOUZEK C.A., CIOLINO H.P., CLARKE R., YEH G.C. Inhibition of P-glycoprotein activity and reversal of multidrug resis-tance in vitro by rosemary extract. Eur. J. Cancer. 1999;35:1541–1545. doi: 10.1016/s0959-8049(99)00180-x. [DOI] [PubMed] [Google Scholar]
- REGE B.D., KAO J.P., POLLI J.E. Effects of nonionic surfactants on membrane transporters in Caco-2 cell monolayers. Eur. J. Pharm. Sci. 2002;16:237–246. doi: 10.1016/s0928-0987(02)00055-6. [DOI] [PubMed] [Google Scholar]
- SAEKI T., UEDA K., TANIGAWARA Y., HORI R., KOMANO T. P-glycoprotein-mediated transcellular transport of MDR-reversing agents. FEBS Lett. 1993;324:99–102. doi: 10.1016/0014-5793(93)81540-g. [DOI] [PubMed] [Google Scholar]
- SUN Y., HUANG L.P., Li J.J., HUANG Q.Y., ZHANG L., HUANG L.P., LEE-HAUNG S. Anti-HIV agent MAP30 modulates the expression profile of viral and cellular genes for proliferation and apoptosis in AIDS-related lymphoma cells infected with Kaposi's sarcoma-associated virus. Biochem. Biophys. Res. Commun. 2001;287:983–994. doi: 10.1006/bbrc.2001.5689. [DOI] [PubMed] [Google Scholar]
- SUZUKI H. Analaysis of xenobiotic detoxification system mediated by efflux transporters. Yakugaku Zasshi. 1999;119:822–834. doi: 10.1248/yakushi1947.119.11_822. [DOI] [PubMed] [Google Scholar]
- TAKANAGA H., OHNISHI A., MATSUO H., SAWADA Y. Inhibition of vinblastine efflux mediated by P-glycoprotein by grapefruit juice components in Caco-2 cells. Biol. Pharm. Bull. 1998;21:1062–1066. doi: 10.1248/bpb.21.1062. [DOI] [PubMed] [Google Scholar]
- TAKANAGA H., OHNISHI A., YAMADA S., MTSUO H., MORIMOTO S., SHOYAMA Y., OHTANI H., SAWADA Y. Polymethoxylated flavones in orange juice are inhibitors of P-glycoprotein but not cytochrome P450 3A4. J. Pharmacol. Exp. Ther. 2000;293:230–236. [PubMed] [Google Scholar]
- TANIGAWARA Y., OKAMURA N., HIRAI M., YASUHARA M., UEDA K. Transport of digoxin by human P-glycoprotein expressed in a porcine kidney epithelial cell line (LLC-PK1) J. Pharmacol. Exp. Ther. 1992;263:840–845. [PubMed] [Google Scholar]
- THIEBAUT F., TSURUO T., HAMADA H., GOTTESMAN M.M., PASTAN I., WILLINGHAM M.C. Proc. Natl. Acad. Sci. U.S.A. 1987. pp. 7735–7738. [DOI] [PMC free article] [PubMed]
- UEDA K., OKAMURA N., HIRAI M., TANIGAWARA Y., SAEKI T., KIOKA N., KOMANO T., HORI R. Human P-glycoprotein transports cortisol, aldosterone and dexamethasone, but not progesterone. J. Biol. Chem. 1992;267:24248–24252. [PubMed] [Google Scholar]
- VIKRANT V., GROVER J.K., TANDON N., RATHI S.S., GUPTA N. Treatment with extracts of Momordica charantia and Eugenia Jambolana prevents hyperglycemia and hyperinsulinemia in fluctose fed rats. J. Ethnopharmacol. 2001;76:139–143. doi: 10.1016/s0378-8741(01)00218-5. [DOI] [PubMed] [Google Scholar]
- WACHER V.J., WU C.Y., BENET L.Z. Overlapping substrate and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drugs delivery and activity in cancer chemotherapy. Mol. Carcinogen. 1995;13:129–134. doi: 10.1002/mc.2940130302. [DOI] [PubMed] [Google Scholar]
- WANG E.J., CASCUANO C.N., CLEMENT R.P., JOHNSON W.W. Active transport of fluorescent P-glycoprotein substrates: evaluation as markers and interaction with inhibitors. Biochem. Biophys. Res. Commun. 2001;289:580–585. doi: 10.1006/bbrc.2001.6000. [DOI] [PubMed] [Google Scholar]
- YEH G.C., LOPACZYNSKA J., POORE C.M., PHANG J.M. A new function role for P-glycoprotein: efflux pump for benzo(a)pyrene in human breast cancer MDF-7 cells. Cancer Res. 1992;52:6692–6695. [PubMed] [Google Scholar]