Abstract
Vasorelaxation to β2-adrenoceptor stimulation occurs through both endothelium-dependent and endothelium-independent mechanisms, and the former is mediated through Ca2+-independent activation of endothelial-type nitric oxide synthase (NOS-3). Since Ca2+-independent NOS-3 activation may occur through its serine phosphorylation via protein kinase A (PKA) or Akt, we determined the PKA and Akt dependency of β2-adrenergic relaxation of rat aorta.
Rat aortic rings were pre-incubated with the PKA inhibitor H-89 (10−7 M), the phosphatidylinositol 3-kinase (PI3K) inhibitor wortmannin (5 × 10−7 M), Akt inhibitor (10−5 M), or vehicle, in the absence or presence of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 10−4 M). Rings were then contracted with phenylephrine (10−7 M), and concentration–relaxation responses determined to the β2-adrenoceptor agonist albuterol.
Rings exhibited a concentration-dependent relaxation to albuterol: pEC50 6.9±0.2, Emax 88.2±4.0%. L-NAME attenuated Emax to 60.2±3.5% (P<0.001).
In the presence of L-NAME, wortmannin or Akt inhibitor did not influence albuterol responses, whereas H-89 reduced Emax further, to 27.5±2.2% (P<0.001).
In the absence of L-NAME, Emax to albuterol was reduced by H-89, wortmannin or Akt inhibitor, to 56.2±2.2, 56.0±1.6 and 55.4±1.8%, respectively (P<0.001 for each); the combinations H-89 plus wortmannin or H-89 plus Akt inhibitor reduced Emax further still.
Western blotting of NOS-3 immunoprecipitates from rat aortas confirmed that albuterol increased serine phosphorylation of NOS-3, and this increase was attenuated by H-89 or Akt inhibitor.
Our results indicate that β2-adrenoceptor stimulation relaxes rat aorta through both NO-dependent and independent mechanisms. The latter is predominantly PKA-mediated, whereas the former occurs through both PKA and PI3K/Akt activation.
Keywords: Akt, β2-adrenoceptors, endothelium, nitric oxide, phosphatidylinositol 3-kinase, protein kinase A, rat aorta
Introduction
Nitric oxide (NO) is an important endothelium-derived mediator which contributes to vasorelaxation, and is released in response to a number of mechanical and neurohormonal stimuli (Furchgott & Zawadzki, 1980; Katusic et al., 1984; Rubanyi et al., 1986; Vanhoutte & Miller, 1989; Yang et al., 1989). Following release from endothelial cells, NO diffuses to the subjacent vascular smooth muscle cells, where it gives rise to vasorelaxation through activation of the soluble isoform of the enzyme guanylyl cyclase, which catalyses the formation of cGMP and hence activation of cGMP-dependent protein kinase (Ignarro et al., 1987; Palmer et al., 1987). Classically, it is released from endothelial cells following activation of the endothelial or type 3 isoform of NO synthase (NOS-3), which is a Ca2+- and calmodulin-dependent enzyme, so that many endothelium-dependent vasodilators cause NO generation through inducing a rise in intracellular Ca2+.
It has been recognised for many years that vascular endothelial cells express β-adrenoceptors (βAR), but for a long time their physiological function was unclear (Steinberg et al., 1984; Buxton et al., 1987; Summers et al., 1987; Howell et al., 1988; Molenaar et al., 1988). Over the last two decades, however, evidence has accumulated that they contribute to vasorelaxation through stimulation of endothelial NO biosynthesis (Rubanyi & Vanhoutte, 1985; Kamata et al., 1989; Gardiner et al., 1991; Gray & Marshall, 1992; Blankesteijn & Thien, 1993; Graves & Poston, 1993; Parent et al., 1993; Rebich et al., 1995; Dawes et al., 1997; Priest et al., 1997; Ferro et al., 1999; Xu et al., 2000), and in at least some vessels βAR-mediated NO production may greatly outweigh any direct vasorelaxant effect of βAR located on vascular smooth muscle (Ferro et al., 1999; Xu et al., 2000). However, the mechanisms by which endothelial βAR may couple to NO biosynthesis remain unclear.
We have previously demonstrated, in cultured human umbilical vein endothelial cells, that β2AR, but not β1AR, stimulate NOS activity, and that they do so in a Ca2+-independent manner (Ferro et al., 1999). The mechanism by which this occurs is not known, but may involve protein kinase modifications of NOS-3, since serine phosphorylation of NOS-3 by both protein kinase A (PKA) and Akt (otherwise known as protein kinase B) is known to increase the activity of NOS-3 in a Ca2+-independent manner through increasing its sensitivity to Ca2+-calmodulin (Dimmeler et al., 1999; Butt et al., 2000; Fisslthaler et al., 2000; Boo et al., 2002); however, this has never been shown to occur with βAR stimulation. β2AR on vascular smooth muscle, on the other hand, also mediate vasorelaxation, and although this appears to occur principally through the cyclic adenosine-3′,5′-monophosphate (cAMP) – PKA pathway (Murray, 1990), the possible role of other kinase pathways remains unclear.
We have recently shown that β2AR stimulation increases NOS-3 serine phosphorylation, in endothelial cells in culture (Yao et al., 2003). We hypothesised that β2AR-mediated activation of endothelial NOS may be mediated through this serine phosphorylation of NOS-3, and that this may occur via PKA, Akt or both. In the present study, therefore, we determined the contribution of NO to β2AR-mediated vasorelaxation, and examined the involvement of the PKA and Akt systems in both NO-dependent and NO-independent β2-adrenergic relaxation, in rat aortic rings in vitro.
Methods
Animals and preparation of vascular rings
Male Wistar rats (175–200 g) were from Harlan, U.K. They were provided with food and water ad libitum and, on the day of experimentation, were humanely killed by cervical dislocation. The thoracic aorta was removed and placed into Krebs solution, of the following composition (mM): NaCl 125, KCl 4.8, NaHCO3 25, KH2PO4 1.2, MgSO4 1.2, glucose 11, EDTA 0.3, CaCl2 2.5, pH 7.4, continuously oxygenated with 95% O2/5% CO2. The vessels were cleaned of adipose and connective tissue, and cut into 2 mm-long rings, which were then mounted in 3 ml organ baths containing Krebs (gassed with 95% O2/5% CO2, at 37°C). Preliminary length–tension curves suggested that a resting tension of 20 mN gave optimal contractile responses to KCl 45 mM; therefore, this level of resting tension was used in all experiments.
Organ bath pharmacology
Following tensioning as above with 1 h equilibration, aortic rings were repeatedly contracted with KCl 45 mM, with washouts in between, until stable and reproducible contractions were obtained. Vessels were then washed extensively, and subsequently contracted with the α-adrenergic agonist phenylephrine (10−7 M). In preliminary experiments (n=6), we confirmed that phenylephrine at this concentration does not exert a significant βAR agonist effect, since co-incubation with propranolol (10−6 M) did not affect phenylephrine-induced constriction (22.0±4.2 mN in the absence of propranolol vs 24.2±3.8 mN in its presence, P>0.05).
Following attainment of plateau constriction in response to phenylephrine, acetylcholine (10−6 M) was added; only rings which demonstrated ⩾60% relaxation to acetylcholine were considered to have functional endothelium, and were used for further experiments (the range of acetylcholine relaxations was 60–70%).
Following further extensive washing, rings were exposed to NG-nitro-L-arginine methyl ester (L-NAME, a NOS inhibitor, 10−4 M) or corresponding vehicle (0.9% saline, final dilution 1 : 1000). In addition, H-89 (10−7 M, a selective PKA inhibitor), wortmannin (5 × 10−7 M, a selective inhibitor of phosphatidylinositol 3- kinase (PI3K)), Akt inhibitor (1L-6-hydroxymethyl-chiro-inositol 2-(R)-2-O-methyl-3-O-octadecylcarbonate, 10−5 M, a selective inhibitor of Akt) or corresponding vehicle (dimethyl sulphoxide, final dilution 1 : 1000) were added to the bathing medium. After 15 min incubation with these, phenylephrine (10−7 M) was re-applied and, following attainment of plateau of contraction, cumulative concentration–relaxation curves were performed to the selective β2AR agonist albuterol (concentration range 10−9–10−4 M). In some preliminary experiments, to determine the functional βAR subtypes present in rat aortic rings, concentration–relaxation responses were performed to the nonselective βAR agonist isoproterenol (concentration range 10−9–10−4 M), following 15 min pre-incubation with the selective β1AR antagonist CGP 20712A (1-[2-((3-carbamoyl-4-hydroxy)phenoxy)ethylamino]-3-[4-(1-methyl-4-trifluoromethyl-2-imidazolyl)phenoxy]-2-propanol dihydrochloride, concentration 3 × 10−7 M), the selective β2AR antagonist ICI 118551 (1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy]-3-[(1-methylethyl)amino]-2-butanol hydrochloride, concentration 10−7 M), or vehicle (dimethyl sulphoxide, final dilution 1 : 1000), as described previously (Ferro et al., 1999).
Determination of serine phosphorylation of NOS-3
Rat aortic rings, prepared and tensioned in organ baths as above, were exposed to H-89 (10−7 M), Akt inhibitor (10−5 M), the combination of H-89 and Akt inhibitor, or corresponding vehicle (dimethyl sulphoxide, final dilution 1 : 1000); after 15 min, albuterol (10−6 M) or vehicle (dimethyl sulphoxide, final dilution 1 : 1000) was added for a further 5 min. Rings were then snap frozen in liquid nitrogen, and homogenised in ice-cold lysis buffer (composition: Tris–HCl 25 mM, NaCl 150 mM, phenylmethanesulphonyl fluoride 1 mM, aprotinin 1 μg ml−1, leupeptin 10 μg ml−1, EDTA 1 mM, NaF 50 mM, sodium orthovanadate 1 mM, Triton-X 1%, pH 7.6) using a glass–glass tissue homogeniser. The resulting homogenates were clarified by centrifugation at 11,000 × g for 10 min, and the resulting supernatants stored at −70°C.
These supernatants subsequently underwent immunoprecipitation using a mouse monoclonal anti-NOS-3 antibody, and NOS-3 expression as well as serine phosphorylation of NOS-3 were analysed by Western blotting, as described previously (Xu et al., 2003).
Materials and drugs
CGP 20712A was graciously provided by Novartis International AG (Basel, Switzerland), and ICI 118551 by Zeneca Pharmaceuticals (Macclesfield, Cheshire, U.K.). Akt inhibitor (Hu et al., 2000) was from Calbiochem-Novabiochem Ltd (Nottingham, U.K.). Mouse monoclonal anti-NOS-3 antibody was from BD Biosciences Pharmingen (San Diego, U.S.A.). All other chemicals were from Sigma-Aldrich Company Ltd (Poole, Dorset, U.K.).
Statistical analysis
All results are presented as mean±s.e.m. of six experiments. Relaxations are expressed as the percentage of initial phenylephrine contraction. Data were plotted using GraphPad Prism version 4, with sigmoidal curve fitting performed by nonlinear regression using the Prism software; these curves were used to derive Emax (maximal relaxant response) and pEC50 (the negative logarithm of the agonist concentration giving a response 50% of Emax). Data were analysed by repeated-measures two-way ANOVA with Bonferroni post-testing, using StatView version 5.0.1. Statistical significance was taken as P<0.05 (two-tailed).
Results
Lack of involvement of NOS, PKA and PI3K/Akt in phenylephrine-mediated responses in rat aorta
In initial experiments, concentration–constriction curves were determined to phenylephrine in rat thoracic aortic rings; we also examined the effect of L-NAME (10−4 M), H-89 (10−7 M), wortmannin (5 × 10−7 M) and Akt inhibitor (10−5 M) on phenylephrine responses. Phenylephrine evoked a concentration-dependent vasoconstriction, which was not affected by L-NAME or by any of the kinase inhibitors (Table 1). Since phenylephrine at a concentration of 10−7 M elicited a submaximal response (approximately 70% of maximum), this concentration was used in all the following experiments.
Table 1.
Contractile responses (in mN) of rat thoracic aorta to phenylephrine at different concentrations, and the effects of L-NAME (10−4 M), H-89 (10−7 M), wortmannin (5 × 10−7 M) and Akt inhibitor (10−5 M) on these responses
| Control | L-NAME | H-89 | Wortmannin | Akt inhibitor | |
|---|---|---|---|---|---|
| Phenylephrine 10−9 M | 3.6±1.2 | 4.2±1.6 | 4.0±0.8 | 3.8±1.0 | 3.9±0.9 |
| Phenylephrine 10−8 M | 10.6±3.2 | 11.1±3.8 | 12.2±2.8 | 10.4±2.6 | 11.5±3.0 |
| Phenylephrine 10−7 M | 25.8±5.1 | 27.9±4.7 | 24.8±4.4 | 26.0±4.8 | 26.2±4.9 |
| Phenylephrine 10−6 M | 37.8±5.8 | 36.2±6.4 | 38.4±5.4 | 38.3±5.2 | 37.7±5.8 |
| Phenylephrine 10−5 M | 32.4±4.2 | 35.2±5.0 | 36.1±4.8 | 36.5±4.8 | 35.8±5.2 |
Functional βAR subtypes in rat aorta
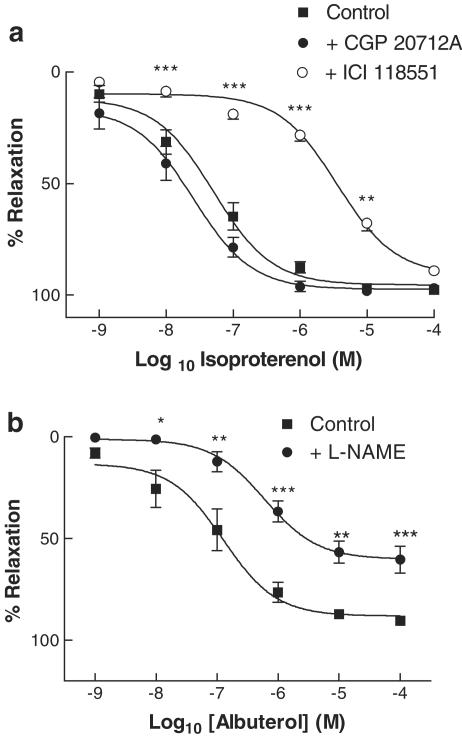
In aortic rings pre-constricted with phenylephrine (10−7 M), isoproterenol induced a concentration-dependent vasorelaxation, with pEC50 7.3±0. 1 and Emax 93.9±3.3% (Figure 1a). CGP 20712A had no effect on isoproterenol responses; by contrast, in the presence of ICI 118551, a significant rightward shift was seen in the isoproterenol concentration–response curve, with pEC50 5.4±0.1, with no change in Emax (Figure 1a). This degree of rightward shift is consistent with displacement of isoproterenol from a pure population of β2AR by ICI 118551 (Ferro et al., 1999). In view of this finding that β2AR were the sole βAR subtype mediating relaxation of rat aorta, the selective β2AR agonist albuterol was used in all further experiments.
Figure 1.
(a) Concentration–relaxation curves to isoproterenol in rat aortic rings, in the absence or presence of the β1AR-selective antagonist CGP 20712A (3 × 10−7 M) or the β2AR-selective antagonist ICI 118551 (10−7 M). (b) Concentration–relaxation curves to albuterol in rat aortic rings, in the absence or presence of the NOS inhibitor L-NAME (10−4 M). All data are shown as mean±s.e.m. of experiments on aortic rings derived from six different rats. *P<0.05, **P<0.01 and ***P<0.001 as compared with control.
NO dependence of β2AR-mediated relaxation of rat aorta
Aortic rings preincubated with either L-NAME (10−4 M) or vehicle were preconstricted with phenylephrine (10−7 M), following which concentration–relaxation responses were determined to albuterol. Albuterol induced a concentration-dependent vasorelaxation, with pEC50 6.9±0.2 and Emax 88.2±4.0% (Figure 1b). L-NAME did not significantly affect pEC50, but reduced Emax to 60.2±3.5% (P<0.001), suggesting that β2AR-mediated vasorelaxation occurs through both NO-dependent and -independent pathways.
Roles of PKA and of the PI3K/Akt system in NO-dependent and -independent β2AR-mediated relaxation of rat aorta
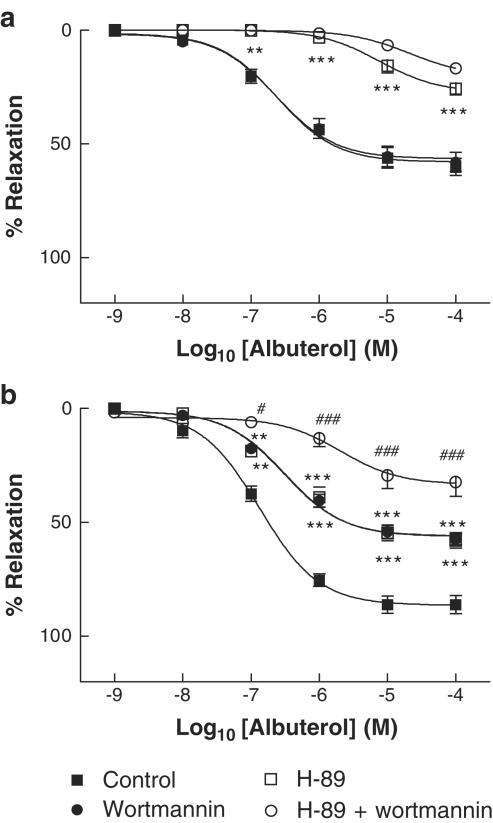
To dissect out the roles of PKA, PI3K (the upstream activator of Akt) and Akt in the NO-dependent and -independent components of β2AR-mediated vasorelaxation, experiments were performed using specific inhibitors of each of these kinases. We used H-89 (10−7 M), wortmannin (5 × 10−7 M) and Akt inhibitor (10−5 M); at these concentrations, they specifically inhibit PKA, PI3K and Akt maximally or near maximally, with little or no crossreactivity with other pathways (Chijiwa et al., 1990; Davies et al., 2000; Hu et al., 2000). In the presence of L-NAME, wortmannin did not affect albuterol responses, whereas H-89 caused a substantial attenuation of albuterol-induced relaxation, reducing the Emax for albuterol from 58.1±2.1 to 27.5±2.2% (P<0.001); the combination of H-89 and wortmannin was as effective in this regard as H-89 alone (Figure 2a). These results suggest that PKA is the principal mediator involved in β2-adrenergic NO-independent relaxation, and that the PI3K/Akt pathway is not involved. By contrast, in the absence of L-NAME, H-89 and wortmannin each significantly reduced Emax for albuterol, from 86.5±2.0 to 56.2±2.2 and 56.0±1.6%, respectively (P<0.001 for each), and the combination of H-89 and wortmannin decreased the albuterol Emax further, to 33.5±3.5% (P<0.001 as compared to H-89 alone), suggesting the involvement both of PKA and PI3K where the NO system is intact (Figure 2b).
Figure 2.
Concentration–relaxation curves to albuterol in rat aortic rings, in the presence (a) or absence (b) of the NOS inhibitor L-NAME (10−4 M). Responses are shown in the concomitant absence or presence of the PKA inhibitor H-89 (10−7 M), the PI3K inhibitor wortmannin (5 × 10−7 M), or both. All data are shown as mean±s.e.m. of experiments on aortic rings derived from six different rats. **P<0.01 and ***P<0.001 as compared with control. #P<0.05 and ###P<0.001 as compared with H-89 alone.
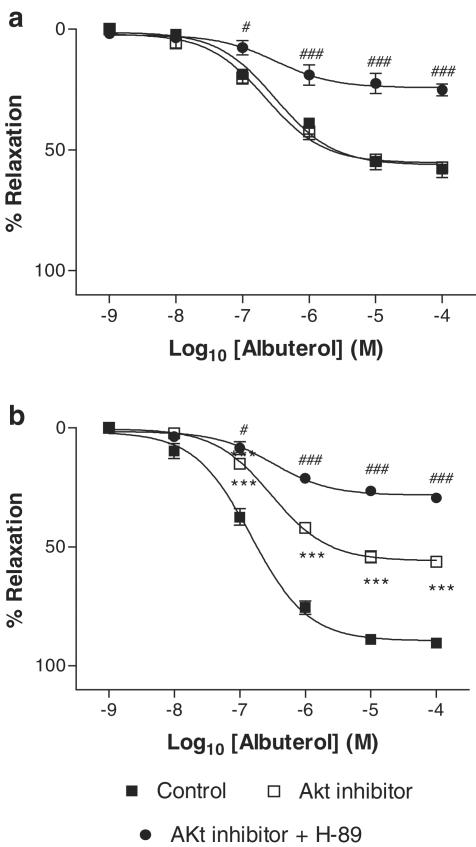
Similarly, we found that, in the presence of L-NAME, Akt inhibitor caused no further diminution of albuterol-mediated vasorelaxation (Figure 3a), suggesting a lack of involvement of Akt in NO-independent β2AR-mediated responses. In the absence of L-NAME, Akt inhibitor decreased Emax to albuterol, from 89.5±1.6 to 55.4±1.8% (P<0.001), and this was decreased further by the combination of Akt inhibitor and H-89, to 28.3±1.1% (P<0.001 as compared with Akt inhibitor alone), suggesting the involvement of both PKA and Akt where the NO system is intact (Figure 3b).
Figure 3.
Concentration–relaxation curves to albuterol in rat aortic rings, in the presence (a) or absence (b) of the NOS inhibitor L-NAME (10−4 M). Responses are shown in the concomitant absence or presence of Akt inhibitor (10−5 M) or the combination of Akt inhibitor (10−5 M) and the PKA inhibitor H-89 (10−7 M). All data are shown as mean±s.e.m. of experiments on aortic rings derived from six different rats. ***P<0.001 as compared with control. #P<0.05 and ###P<0.001 as compared with Akt inhibitor alone.
Roles of PKA and Akt in β2AR-mediated serine phosphorylation of NOS-3 in rat aorta
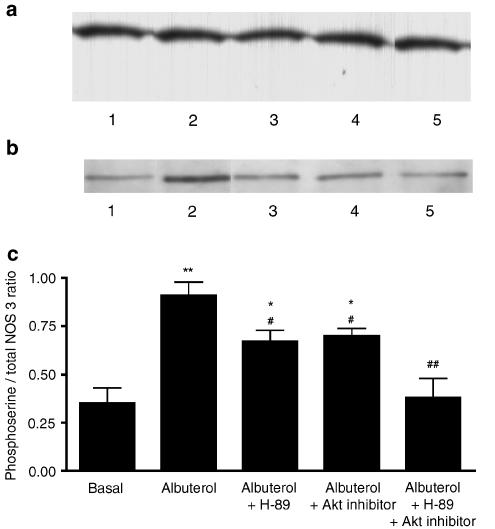
To confirm that both PKA and Akt are involved in serine phosphorylation of NOS-3, rat aortic rings were exposed to albuterol (10−6 M) or vehicle, in the absence or presence of H-89 (10−7 M), Akt inhibitor (10−5 M), or the combination. Following these incubations, aortic homogenates underwent immunoprecipitation of NOS-3, and the resulting immunoprecipitates were subjected to Western blotting, the blots being probed with anti-NOS-3 antibody or anti-phosphoserine IgG (Figure 4a and b). Both of these antibodies detected a band at 135 kDa, which is the known molecular mass of NOS-3. The densitometric ratio of the phosphoserine to the NOS-3 band was taken to indicate the degree of serine phosphorylation of NOS-3. Albuterol increased NOS-3 serine phosphorylation, and this increase was attenuated by either H-89 or by Akt inhibitor, although not to basal levels; the combination of H-89 and Akt inhibitor completely inhibited the albuterol-induced serine phosphorylation of NOS-3, so that this was not different from basal serine phosphorylation (Figure 4c).
Figure 4.
Western blots depicting the presence of a 135 kDa band (the known molecular mass of NOS-3) in NOS-3 immunoprecipitates prepared from rat aortic homogenates, the blots being probed with (a) anti-NOS-3 antibody or (b) anti-phosphoserine IgG. Lanes: 1=basal (vehicle treatment); 2=albuterol (10−6 M); 3=albuterol (10−6 M)+H-89 (10−7 M); 4=albuterol (10−6 M)+Akt inhibitor (10−5 M); 5=albuterol (10−6 M)+H-89 (10−7 M)+Akt inhibitor (10−5 M). (c) Densitometric ratio of 135 kDa phosphoserine/NOS-3 bands, in rat aortas treated with each of these combinations, expressed as mean±s.e.m. of experiments on aortic rings derived from six different rats. *P<0.05 and **P<0.01 as compared with basal; #P<0.05 and ##P<0.01 as compared with albuterol alone.
Discussion
The principal aims of this study were to determine firstly the relative contributions of NO-dependent and -independent mechanisms to βAR-mediated vasorelaxation, and secondly the involvement of PKA and Akt in these NO-dependent and -independent components, in rat aorta. We found that β-adrenergic relaxation of rat aorta occurred entirely through β2AR. Some other studies have suggested a component of β-adrenergic relaxation in rat aorta that is mediated by β1AR and possibly also by atypical βAR, but these components are minor compared with the β2AR relaxant effect (O'Donnell & Wanstall, 1984; Brawley et al., 2000); in our experiments, we found no evidence of β-adrenergic relaxation attributable to receptors other than β2AR, and this may be related to differences in experimental conditions used. We also found that NOS inhibition decreased the Emax of albuterol by approximately 30%, suggesting that, although the major component of β2-adrenergic relaxation in this vessel was NO-independent, nevertheless a substantial component was dependent on the integrity of the NO system. This accords with previously published reports, which suggest similarly that β2AR in a variety of different blood vessels may cause vasorelaxation through both endothelial NO generation and through direct relaxation of vascular smooth muscle (Rubanyi & Vanhoutte, 1985; Kamata et al., 1989; Gardiner et al., 1991; Gray & Marshall, 1992; Blankesteijn & Thien, 1993; Graves & Poston, 1993; Parent et al., 1993; Rebich et al., 1995; Dawes et al., 1997; Priest et al., 1997; Ferro et al., 1999; Xu et al., 2000).
In the present series of experiments, we found no evidence that phenylephrine-induced vasoconstriction was augmented by NOS inhibition with L-NAME, suggesting that basal NO production was negligible from rat aortic rings under the conditions used here. Indeed, different reports in the literature suggest that α-adrenergic contractions of rat aorta may or may not be increased by NOS inhibition, depending on the experimental conditions used (Martin et al., 1986; Topouzis et al., 1991; Kaneko & Sunano, 1993; Kamata & Makino, 1997). Furthermore, whereas NO production is actively increased by endothelial α2-adrenergic receptor stimulation, the same does not appear to occur with endothelial α1-adrenergic receptor stimulation (Vanhoutte, 2001).
In previous work, we have demonstrated that β2AR-mediated NOS activation in cultured endothelial cells occurs with no change in intracellular Ca2+ levels (Ferro et al., 1999); this contrasts with ‘traditional' endothelial cell NOS agonists, such as histamine, which cause a marked increase in intracellular Ca2+, thereby activating NOS-3 through increased binding of Ca2+-calmodulin. Other workers have shown that NOS-3 activation in endothelial cells can indeed occur in a Ca2+-independent manner, for example, in response to shear stress, and this can occur through increased serine phosphorylation of NOS-3 by kinases such as PKA and Akt (by contrast, protein kinase C phosphorylation of NOS-3 causes a decrease in its activity), thereby increasing its binding to intracellular Ca2+-calmodulin (Dimmeler et al., 1999; Butt et al., 2000; Fisslthaler et al., 2000; Boo et al., 2002). Indeed, we have recently shown, in cultured human umbilical vein endothelial cells, that β2AR, but not β1AR, stimulation gives rise to an increase in the degree of serine phosphorylation of NOS-3 (Yao et al., 2003), which might explain the Ca2+-independent activation of NOS-3 in response to β2AR agonists. In the present work, therefore, we wished to determine whether PKA and/or Akt were important in mediating β2AR-mediated NO-dependent relaxation, in rat aorta.
In aortic rings incubated with the NOS inhibitor L-NAME, β2-adrenergic vasorelaxation was not affected by PI3K inhibition with wortmannin or by Akt inhibitor, but was substantially reduced by PKA inhibition with H-89; the combination of H-89 with wortmannin was not significantly more effective in inhibiting β2AR responses than H-89 alone. This implies that the NO-independent component of β2-adrenergic vasorelaxation is mediated principally through activation of PKA, in line with long-established views of β-adrenergic signalling; the presence of some residual dilatation in the presence of H-89 may be consistent either with the involvement of a different pathway (not PI3K/Akt) or submaximal blockade of PKA. In the absence of L-NAME, on the other hand, H-89, wortmannin and Akt inhibitor each caused a partial attenuation of β2-adrenergic vasorelaxation, whereas the combination either of H-89 with wortmannin or of H-89 with Akt inhibitor attenuated β2AR-mediated relaxation to a much larger degree. This suggests that the NO-dependent component of β2-adrenergic relaxation is mediated through activation both of PKA and of the PI3K/Akt system.
To confirm the involvement of both PKA and Akt in modulation of NOS-3 in rat aorta, we determined the degree of serine phosphorylation of NOS-3 in this tissue in response to albuterol, and the degree of inhibition by H-89 and by Akt inhibitor. We found that albuterol increases NOS-3 serine phosphorylation, and that this increase is attenuated by either H-89 or Akt inhibitor, and completely abolished by the combination of both inhibitors. Since NOS-3 in rat aorta is expressed predominantly in the endothelium (Stumm et al., 2002), these experiments demonstrate that both PKA and Akt are involved in mediating β2-adrenergic serine phosphorylation, and hence activation, of endothelial NOS-3 in rat aorta.
The signal-transduction pathway by which βAR can activate PKA has long been established. βAR couple, via the stimulatory G-protein Gs, to adenylyl cyclase, which catalyses the conversion of adenosine triphosphate to cAMP. Subsequently, cAMP activates PKA through binding to its regulatory subunit, causing this to dissociate from the catalytic subunit, thereby rendering it active. In recent years, it has become apparent that β2AR, but not β1AR, can also activate the inhibitory G-protein Gi (Xiao et al., 1995; 1999), and this may provide a mechanism whereby β2AR can stimulate Akt, since β/γ subunits derived from Gi following its activation can stimulate PI3K, which in turn activates Akt (Brock et al., 2003). Whether this is the pathway involved in PI3K/Akt activation in our system remains to be determined.
The question arises as to the specificities of the PKA, PI3K and Akt inhibitors used in the present experiments. Based on previously published activity and selectivity data for each of these inhibitors (Chijiwa et al., 1990; Davies et al., 2000; Hu et al., 2000), we were careful to use concentrations which would cause maximal or near-maximal inhibition of the chosen kinase, with little or no crossreactivity with other pathways. We are confident, therefore, that our data truly reflect selective kinase inhibition as stated.
The data presented here shed important insight into the mechanisms by which β2AR couple to NO generation physiologically. It has previously been shown that different polymorphisms of the β2AR may give rise to differential coupling to endothelial NO generation (Garovic et al., 2003), but investigation of the mechanisms of such differences was beyond the scope of the present work. Furthermore, cardiovascular disease states may give rise to impairment in vascular β2AR-mediated NO generation, as has been shown in patients with type II diabetes (Chowienczyk et al., 1999). The mechanisms underlying such impairment merit further study.
In conclusion, there is now abundant evidence that endothelial β2AR play an important role in mediating β-adrenergic vasorelaxation in a variety of blood vessels through stimulation of NO production. The data presented here provide a mechanism by which this occurs. Our results suggest that, in rat aorta, β2AR stimulate both the PKA and PI3K/Akt pathways, both of which are known to have the ability to cause serine phosphorylation – and hence Ca2+-independent activation – of NOS-3. Our study provides important novel information about the physiological mechanisms underlying β-adrenergic regulation of vascular tone.
Acknowledgments
Yong Ji is funded by a Wellcome Trust Travelling Research Fellowship, and Lindsay Queen by a Project Grant from the Guy's and St Thomas' Charitable Foundation. Albert Ferro receives funding also from the British Heart Foundation, Diabetes UK, the Coronary Research Fund, the Friends of St Thomas' Hospital and Pfizer Ltd.
Abbreviations
- βAR
β-adrenoceptors
- cAMP
cyclic adenosine-3′,5′-monophosphate
- L-NAME
NG-nitro-L-arginine methyl ester
- NO
nitric oxide
- NOS
nitric oxide synthase
- PI3K
phosphatidylinositol 3-kinase
- PKA
protein kinase A
References
- BLANKESTEIJN W.M., THIEN T. Effect of NG-monomethyl-L-arginine on the β-adrenoceptor-mediated relaxation of rat mesenteric resistance arteries. Life Sci. 1993;52:PL135–PL139. doi: 10.1016/0024-3205(93)90178-6. [DOI] [PubMed] [Google Scholar]
- BOO Y.C., SORESCU G., BOYD N., SHIOJIMA I., WALSH K., DU J., JO H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at Ser1179 by Akt-independent mechanisms: role of protein kinase A. J. Biol. Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- BRAWLEY L., SHAW A.M., MacDONALD A. Beta 1-, beta 2- and atypical beta-adrenoceptor-mediated relaxation in rat isolated aorta. Br. J. Pharmacol. 2000;129:637–644. doi: 10.1038/sj.bjp.0703091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROCK C., SCHAEFER M., REUSCH H.P., CZUPALLA C., MICHALKE M., SPICHER K., SCHULTZ G., NURNBERG B. Roles of G beta gamma in membrane recruitment and activation of p110 gamma/p101 phosphoinositide 3-kinase gamma. J. Cell. Biol. 2003;160:89–99. doi: 10.1083/jcb.200210115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTT E., BERNHARDT M., SMOLENSKI A., KOTSONIS P., FROHLICH L.G., SICKMANN A., MEYER H.E., LOHMANN S.M., SCHMIDT H.H.H.W. Endothelial nitric-oxide synthase (type III) is activated and becomes calcium independent upon phosphorylation by cyclic nucleotide-dependent protein kinases. J. Biol. Chem. 2000;275:5179–5187. doi: 10.1074/jbc.275.7.5179. [DOI] [PubMed] [Google Scholar]
- BUXTON B.F., JONES C.R., MOLENAAR P., SUMMERS R.J. Characterization and autoradiographic localization of β-adrenoceptor subtypes in human cardiac tissues. Br. J. Pharmacol. 1987;92:299–310. doi: 10.1111/j.1476-5381.1987.tb11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHIJIWA T., MISHIMA A., HAGIWARA M., SANO M., HAYASHI K., INOUE T., NAITO K., TOSHIOKA T., HIDAKA H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5- isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J. Biol. Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- CHOWIENCZYK P.J., KELLY R.P., MacCALLUM H., MILLASSEAU S.C., ANDERSSON T.L., GOSLING R.G., RITTER J.M., ANGGARD E.E. Photoplethysmographic assessment of pulse wave reflection: blunted response to endothelium-dependent β2-adrenergic vasodilation in type II diabetes mellitus. J. Am. Coll. Cardiol. 1999;34:2007–2014. doi: 10.1016/s0735-1097(99)00441-6. [DOI] [PubMed] [Google Scholar]
- DAVIES S.P., REDDY H., CAIVANO M., COHEN P. Specificity and mechanism of action of some commonly used protein kinase inhibitors. Biochem. J. 2000;351:95–105. doi: 10.1042/0264-6021:3510095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAWES M., CHOWIENCZYK P.J., RITTER J.M. Effects of inhibition of the L-arginine/nitric oxide pathway on vasodilation caused by β-adrenergic agonists in human forearm. Circulation. 1997;95:2293–2297. doi: 10.1161/01.cir.95.9.2293. [DOI] [PubMed] [Google Scholar]
- DIMMELER S., FLEMING I., FISSLTHALER B., HERMANN C., BUSSE R., ZEIHER A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- FERRO A., QUEEN L.R., PRIEST R.M., XU B., RITTER J.M., POSTON L., WARD J.P.T. Activation of nitric oxide synthase by β2-adrenoceptors in human umbilical vein endothelium in vitro. Br. J. Pharmacol. 1999;126:1872–1880. doi: 10.1038/sj.bjp.0702512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., DIMMELER S., HERMANN C., BUSSE R., FLEMING I. Phosphorylation and activation of the endothelial nitric oxide synthase by fluid shear stress. Acta Physiol. Scand. 2000;168:81–88. doi: 10.1046/j.1365-201x.2000.00627.x. [DOI] [PubMed] [Google Scholar]
- FURCHGOTT R.F., ZAWADZKI J.V. The obligatory role of the endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methyl ester on vasodilator responses to acetylcholine, 5′-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br. J. Pharmacol. 1991;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAROVIC V.D., JOYNER M.J., DIETZ N.M., BOERWINKLE E., TURNER S.T. β2-Adrenergic receptor polymorphism and nitric oxide-dependent forearm blood flow responses to isoproterenol in humans. J. Physiol. 2003;546:583–589. doi: 10.1113/jphysiol.2002.031138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAVES J., POSTON L. β-Adrenoceptor agonist mediated relaxation of rat isolated resistance arteries: a role for the endothelium and nitric oxide. Br. J. Pharmacol. 1993;108:631–637. doi: 10.1111/j.1476-5381.1993.tb12853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRAY D.W., MARSHALL I. Novel signal transduction pathway mediating endothelium-dependent β-adrenoceptor vasorelaxation in rat thoracic aorta. Br. J. Pharmacol. 1992;107:684–690. doi: 10.1111/j.1476-5381.1992.tb14507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWELL R.E., ALBEDA S.M., DAISE M.L., LEVINE E.M. Characterization of β-adrenergic receptors in cultured human and bovine endothelial cells. J. Appl. Physiol. 1988;65:1251–1257. doi: 10.1152/jappl.1988.65.3.1251. [DOI] [PubMed] [Google Scholar]
- HU Y., QIAO L., WANG S., RONG S.B., MEUILLET E.J., BERGGREN M., GALLEGOS A., POWIS G., KOZIKOWSKI A.P. 3-(Hydroxymethyl)-bearing phosphatidylinositol ether lipid analogues and carbonate surrogates block PI3-K, Akt, and cancer cell growth. J. Med. Chem. 2000;43:3045–3051. doi: 10.1021/jm000117y. [DOI] [PubMed] [Google Scholar]
- IGNARRO L.J., BUGA G.M., WOOD K.S., BYRNS R.E. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U.S.A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., MAKINO A. A comparative study on the rat aorta and mesenteric arterial bed of the possible role of nitric oxide in the desensitization of the vasoconstrictor response to an α1-adrenoceptor agonist. Br. J. Pharmacol. 1997;120:1221–1228. doi: 10.1038/sj.bjp.0701031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., MIYATA N., KASUYA Y. Involvement of endothelial cells in relaxation and contraction responses of the aorta to isoproterenol in naive and streptozotocin-induced diabetic rats. J. Pharmacol. Exp. Ther. 1989;249:890–894. [PubMed] [Google Scholar]
- KANEKO K., SUNANO S. Involvement of α-adrenoceptors in the endothelium-dependent depression of noradrenaline-induced contraction in rat aorta. Eur. J. Pharmacol. 1993;240:195–200. doi: 10.1016/0014-2999(93)90898-r. [DOI] [PubMed] [Google Scholar]
- KATUSIC Z.S., SHEPHERD J.T., VANHOUTTE P.M. Vasopressin causes endothelium-dependent relaxations of the canine basilar artery. Circ. Res. 1984;55:575–579. doi: 10.1161/01.res.55.5.575. [DOI] [PubMed] [Google Scholar]
- MARTIN W., FURCHGOTT R.F., VILLANI G.M., JOTHIANANDAN D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J. Pharmacol. Exp. Ther. 1986;237:529–538. [PubMed] [Google Scholar]
- MOLENAAR P., MALTA E., JONES C.R., BUXTON B.F., SUMMERS R.J. Autoradiographic localization and function of β-adrenoceptors on the human internal mammary artery and saphenous vein. Br. J. Pharmacol. 1988;95:225–233. doi: 10.1111/j.1476-5381.1988.tb16568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY K.J. Cyclic AMP and mechanisms of vasodilation. Pharmacol. Ther. 1990;47:329–345. doi: 10.1016/0163-7258(90)90060-f. [DOI] [PubMed] [Google Scholar]
- O'DONNELL S.R., WANSTALL J.C. Beta-1 and beta-2 adrenoceptor-mediated responses in preparations of pulmonary artery and aorta from young and aged rats. J. Pharmacol. Exp. Ther. 1984;228:733–738. [PubMed] [Google Scholar]
- PALMER R.M., FERRIGE A.G., MONCADA S. Nitric oxide accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- PARENT R., AL-OBAIDI M., LAVALLÉE M. Nitric oxide formation contributes to β-adrenergic dilation of resistance coronary vessels in conscious dogs. Circ. Res. 1993;73:241–251. doi: 10.1161/01.res.73.2.241. [DOI] [PubMed] [Google Scholar]
- PRIEST R.M., HUCKS D., WARD J.P.T. Noradrenaline, β-adrenoceptor mediated vasorelaxation and nitric oxide in large and small pulmonary arteries of the rat. Br. J. Pharmacol. 1997;122:1375–1384. doi: 10.1038/sj.bjp.0701528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REBICH S., DEVINE J.O., ARMSTEAD W.M. Role of nitric oxide and cAMP in β-adrenoceptor-induced pial artery vasodilation. Am. J. Physiol. 1995;268:H1071–H1076. doi: 10.1152/ajpheart.1995.268.3.H1071. [DOI] [PubMed] [Google Scholar]
- RUBANYI G., VANHOUTTE P.M. Endothelium-removal decreases relaxations of canine coronary arteries caused by β-adrenergic agonists and adenosine. J. Cardiovasc. Pharmacol. 1985;7:139–144. doi: 10.1097/00005344-198501000-00023. [DOI] [PubMed] [Google Scholar]
- RUBANYI G.M., ROMERO J.C., VANHOUTTE P.M. Flow-induced increase of endothelium-derived relaxing factor. Am. J. Physiol. 1986;250:H1145–H1149. doi: 10.1152/ajpheart.1986.250.6.H1145. [DOI] [PubMed] [Google Scholar]
- STEINBERG S.F., JAFFE E.A., BILEZIKIAN J.P. Endothelial cells contain beta adrenoceptors. Naunyn-Schmiedeberg's Arch Pharmacol. 1984;325:310–313. doi: 10.1007/BF00504374. [DOI] [PubMed] [Google Scholar]
- STUMM M.M., D'ORAZIO D., SUMANOVSKI L.T., MARTIN P.Y., REICHEN J., SIEBER C.C. Endothelial, but not the inducible, nitric oxide synthase is detectable in normal and portal hypertensive rats. Liver. 2002;22:441–450. doi: 10.1034/j.1600-0676.2002.01653.x. [DOI] [PubMed] [Google Scholar]
- SUMMERS R.J., MOLENAAR P., STEPHENSON J.A., JONES C.R. Autoradiographic localization of receptors in the mammalian cardiovascular system. Clin. Exp. Pharmacol. Physiol. 1987;14:437–447. doi: 10.1111/j.1440-1681.1987.tb00995.x. [DOI] [PubMed] [Google Scholar]
- TOPOUZIS S., SCHOTT C., STOCLET J.C. Participation of endothelium-derived relaxing factor and role of cyclic GMP in inhibitory effects of endothelium on contractile responses elicited by α-adrenoceptor agonists in rat aorta. J. Cardiovasc. Pharmacol. 1991;18:670–678. doi: 10.1097/00005344-199111000-00004. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M. Endothelial adrenoceptors. J. Cardiovasc. Pharmacol. 2001;38:796–808. doi: 10.1097/00005344-200111000-00016. [DOI] [PubMed] [Google Scholar]
- VANHOUTTE P.M., MILLER V.M. α2-Adrenoceptors and endothelium-derived relaxing factor. Am. J. Med. 1989;87 Suppl 3C:S1–S5. doi: 10.1016/0002-9343(89)90496-8. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., AVDONIN P., ZHOU Y.Y., CHENG H., AKHTER S.A., ESCHENHAGEN T., LEFKOWITZ R.J., KOCH W.J., LAKATTA E.G. Coupling of β2-adrenoceptor to Gi proteins and its physiological relevance in murine cardiac myocytes. Circ. Res. 1999;84:43–52. doi: 10.1161/01.res.84.1.43. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., JI X., LAKATTA E.G. Functional coupling of the β2-adrenoceptor to a pertussis toxin-sensitive G protein in cardiac myocytes. Mol. Pharmacol. 1995;47:322–329. [PubMed] [Google Scholar]
- XU B., CHIBBER R., RUGGIERO D., KOHNER E., RITTER J., FERRO A. Impairment of vascular endothelial nitric oxide synthase activity by advanced glycation end products. FASEB J. 2003;17:1289–1291. doi: 10.1096/fj.02-0490fje. [DOI] [PubMed] [Google Scholar]
- XU B., LI J., GAO L., FERRO A. Nitric oxide-dependent vasodilatation of rabbit femoral artery by β2-adrenergic stimulation or cyclic AMP elevation in vivo. Br. J. Pharmacol. 2000;129:969–974. doi: 10.1038/sj.bjp.0703155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG Z., DIEDERICH D., SCHNEIDER K., SIEBENMANN R., STULZ P., VON SEGESSER L., TURINA M., BUHLER F.R., LÜSCHER T.F. Endothelium-derived relaxing factor and protection against contractions induced by histamine and serotonin in the internal mammary artery and saphenous vein. Circulation. 1989;80:1041–1048. doi: 10.1161/01.cir.80.4.1041. [DOI] [PubMed] [Google Scholar]
- YAO K., XU B., LIU Y.P., FERRO A. Effects of β-adrenoceptor stimulation on endothelial nitric-oxide synthase phosphorylation of human umbilical vein endothelial cells. Acta Pharmacol. Sin. 2003;24:219–224. [PubMed] [Google Scholar]