Abstract
Serotonin 5-HT7 receptors are present in astrocytes. Understanding their role in this type of cell would greatly benefit from the identification of astroglial cell lines expressing this receptor type.
The aim of the present study was to assess the expression of native 5-HT7 receptors and 5-HT7 receptor mRNA in a number of human glioblastoma cell lines, by means of cAMP measurements, Western blot analysis and reverse transcriptase–polymerase chain reaction (RT–PCR) analysis.
5-Hydroxytryptamine (5-HT), 5-carboxamidotryptamine (5-CT), 5-methoxytryptamine (5-MeOT) and 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT) induced concentration-dependent stimulations of cAMP accumulation in the human glioblastoma cell lines, U-373 MG, U-138 MG, U-87 MG, DBTRG-05MG, T98G, H4, CCF-STTG1 and Hs 683. The rank order of potency was 5-CT>5-HT=5-MeOT≫8-OH-DPAT.
The effect of 5-CT was inhibited in a concentration-dependent manner by the selective 5-HT7 receptor antagonist SB-269970 in all human glioblastoma cells. Schild analyses yielded slope factors close to unity (0.89–1.13) and pA2 values of 8.69–9.05.
Western blot analysis revealed the presence of immunoreactive bands corresponding to the human 5-HT7 receptor in extracts of all human glioblastoma cell lines.
The presence of the three splice variants of the 5-HT7 receptor (5-HT7(a/b/d)) was visualized by RT–PCR analysis with specific primers in all human glioblastoma cell lines.
In conclusion, human glioblastoma cell lines express functional 5-HT7 receptors and the three splice variants of the corresponding mRNA. These cell lines could serve as model systems of native 5-HT7 receptors in glial cells to investigate their putative role in processes like release of neurotrophic factors or inflammatory cytokines.
Keywords: 5-HT7 receptor, human glioblastoma, astrocytes, glial cells, SB-269970
Introduction
Receptors for serotonin (5-hydroxytryptamine (5-HT)) are classified into seven major classes (5-HT1–7), based on structural, functional and pharmacological criteria (Hoyer et al., 1994). The 5-HT7 receptor, cloned from mouse (Plassat et al., 1993), rat (Lovenberg et al., 1993; Meyerhof et al., 1993; Ruat et al., 1993; Shen et al., 1993), guinea-pig (Tsou et al., 1994) and human (Bard et al., 1993), is positively coupled to adenylyl cyclase and cAMP accumulation through the stimulatory G protein Gs and displays a unique pharmacological profile which is consistent across species. Sequence alignment shows a high degree of interspecies homology (95%) but a low overall homology (<40%) with other 5-HT receptors. A number of splice variants of both the human (5-HT7(a/b/d)) and rat (5-HT7(a/b/c)) receptors have been identified. They display similar pharmacological and functional characteristics when expressed in cell lines, and a similar tissue distribution (Heidmann et al., 1997; Jasper et al., 1997; Krobert et al., 2001). The most abundant isoform (5-HT7(a)) consists of a 445-amino-acid polypeptide with a relatively short third intracellular loop and a long carboxy-terminus.
The 5-HT7 receptor mRNA has been found in the brain, where it is located in the thalamus, hypothalamus and various limbic and cortical regions in rat (Lovenberg et al., 1993; Ruat et al., 1993; Shen et al., 1993), guinea-pig (To et al., 1995) and man (Bard et al., 1993; Hagan et al., 2000), as well as in smooth muscles from cardiovascular and gastrointestinal tissues (Bard et al., 1993; Schoeffter et al., 1996; Hagan et al., 2000). Receptor distribution and pharmacological studies have suggested that 5-HT7 receptors may play a role in the control of circadian rhythms (Lovenberg et al., 1993; Ying & Rusak, 1997) and smooth muscle tone (Eglen et al., 1997). As far as the nervous system is concerned, selective 5-HT7 receptor ligands may have potential therapeutic applications in sleep disorders, depression, migraine and pain (for a review, see Thomas & Hagan, 2004).
Electrophysiological studies have shown that 5-HT7 receptors modulate neuronal function in slices of rat hippocampus (Bacon & Beck, 2000; Gill et al., 2002) and thalamus (Chapin & Andrade, 2001; Goaillard & Vincent, 2002). Besides this neuronal localization, there is also evidence that 5-HT7 receptors are present in glial cells. Primary cultures of rat (Shimizu et al., 1996; Hirst et al., 1997) and human (Cohen et al., 1999) brain astrocytes express these receptors. Glial 5-HT7 receptors have also been detected in situ in the mouse suprachiasmatic nucleus by electron microscopic immunocytochemistry (Belenky & Pickard, 2001). Although astrocytes have long been regarded as neuron-supporting, ancillary cells in the brain, their active role in reciprocal neuron–glia interactions is being increasingly recognized. They now appear to be regulators of synaptic activity, synaptogenesis and neurogenesis. However, the molecular pathways involved in this regulatory activity are poorly understood (for a review, see Ransom et al., 2003).
In this context, the knowledge of the role of astroglial 5-HT7 receptors would greatly benefit from the identification of cell lines expressing this receptor type, as has been the case with rat C6 glioma cells for the 5-HT2A receptor (Elliott et al., 1995). It was the aim of the present study to assess the expression of native 5-HT7 receptors and 5-HT7 receptor mRNA in a number of human glioblastoma cell lines, by means of cAMP measurements, Western blot analysis and reverse transcriptase–polymerase chain reaction (RT–PCR) analysis.
Methods
Cell lines and culture
The human glioblastoma cell lines, U-373 MG, U-138 MG, U-87 MG, DBTRG-05MG, T98G, H4, CCF-STTG1 and Hs 683 were purchased from the American Type Culture Collection (Rockville, MD, U.S.A.). Dulbecco's modified Eagle medium (DMEM), minimum essential medium (MEM), RPMI 1640 medium and non-essential amino acids were purchased from Gibco BRL Life Technologies (Rockville, MD, U.S.A.). The glioblastoma cell lines U-373 MG, U-138 MG, U-87 MG, T98G and DBTRG-05MG were maintained in MEM, supplemented with non-essential amino acids, the H4 and Hs 683 cell lines in DMEM and the CCF-STTG1 cell line in RPMI 1640 medium. All media were supplemented with 10% foetal calf serum, penicillin G (100 U ml−1) and streptomycin (100 μg ml−1). Cells were grown at 37°C in a humidified atmosphere containing 5% CO2. For cAMP measurements, the cells were subcultured in 24-well plates. For RT–PCR and Western blot experiments, cells were subcultured in six-well culture plates.
cAMP measurements and analysis of data
cAMP accumulation was measured in intact cells seeded in 24-well plates, using the standard [3H]adenine prelabelling technique, as described previously (Schoeffter et al., 1997; 1999). Cells were deprived of serum 24 h before the assay. They were incubated with agonists/antagonists for 15 min in the presence of 1 mM isobutylmethylxanthine. The [3H]cAMP/([3H]cAMP+[3H]ATP) d.p.m. ratio (cAMP conversion rate) was calculated for each sample. Concentration–response curves were fitted to the nonlinear logistic function of the Origin 6.1 software package (OriginLab Corporation, Northampton, MA, U.S.A.). Emax and EC50 values were derived from these analyses. Results are given as mean values±s.e.m. of the indicated n number of experiments. The pA2 values of the antagonist compound (R)-3-(2-(2-(4-methylpiperin-1-yl)-ethyl)pyrrolidine-1-sulphonyl)phenol (SB-269970) were estimated from Schild analyses (Arunlakshana & Schild, 1959). Incomplete curves in the presence of the highest concentration of the antagonist were constrained to the Emax of the control curve to obtain EC50 estimates.
Western blot analysis
Cells were first washed twice with cold phosphate-buffered saline and then scraped in 100 μl of a buffer containing 20 mM Tris at pH 8, 137 mM NaCl, 1% Nonidet P40, 10% glycerol, 0.5 mM orthovanadate, 1 mM phenylmethane sulphonyl fluoride and a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany), sonicated and centrifuged at 13,000 r.p.m. for 5 min at 4°C. The proteins (25 μg per lane) were separated by sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) on a 4–20% Tris-glycine polyacrylamide gel (Cambrex, Rockland, ME, U.S.A.) and transferred onto a PVDF membrane. The membranes were blocked with blocking buffer, consisting of 5% milk, 1% BSA in TBST buffer (0.2 mM Tris–HCl, pH 7.5, 150 mM NaCl and 0.05% Tween 20) and then incubated for 2 h with a rabbit polyclonal antibody to the serotonin 5-HT7 receptor (Imgenex, San Diego, CA, U.S.A.) diluted in TBST to a final concentration of 0.5 μg ml−1. This antibody was raised against a sequence identical for all human receptor splice variants. Membranes were then washed and incubated for 1 h with an anti-rabbit peroxidase-conjugated antibody (Sigma-Aldrich, Buchs, Switzerland) diluted 1 : 100,000 in blocking buffer, followed by application of an enhanced chemiluminescent system (Socochim SA Supersignal® West Femto, Pierce, Perbio Science, Lausanne, Switzerland).
Primers for polymerase chain reaction (PCR)
To identify the three splice variants of the human 5-HT7 receptor (which differ from each other only by their carboxy-terminus tails), primers were designed in such a way that the length of the amplification products was specific to each splice variant:
 |
The sequences of the primers were as follows:
 |
RNA extraction and RT–PCR analysis
Total RNA was isolated from glioblastoma cell lines by using the SNAP™ Total RNA Isolation Kit (Invitrogen, Carlsbad, CA, U.S.A.). The quantity of total RNA was determined by Ribogreen® staining (Ribogreen™ RNA Quantitation Kit; Molecular Probes, Inc., Eugene, OR, U.S.A.). The quality of the RNA was checked by electrophoretic separation of the RNA on a 1.2% SeaKem LE agarose gel (Karlan, Santa Rosa, CA, U.S.A.). For each RNA sample, 400 ng of total RNA was first digested with DNAse I (Qiagen, Hilden, Germany) in order to remove traces of genomic DNA contamination. The DNAse I enzyme was inactivated by addition of EDTA and heating up to 65°C for 2 min. DNAse I-treated total RNA was reverse transcribed into cDNA for 60 min at 42°C with 300 ng of random hexamer primers and 50 U of StrataScript™ reverse transcriptase (Stratagene, La Jolla, CA, U.S.A.). The reaction was stopped by heating up to 95°C for 5 min. In all, 20 ng of RNA/cDNA was used as template for the PCR. PCR amplification was performed in a final volume of 20 μl containing 130 μM of dNTPs, 0.5 μM of each primers, 1 U of Taq DNA polymerase (Amersham Biosciences Europe, Otelfingen, Switzerland) and its corresponding buffer. The PCR amplification took place in a Biometra thermocycler with the following conditions: initial denaturation at 95°C for 5 min, then 40 cycles with the following profile: 95°C for 40 s, 60°C for 40 s and 72°C for 40 s, final extension at 72°C for 7 min. After amplification, the PCR products were separated electrophoretically on a 3% NuSieve 3 : 1 agarose gel and visualized by ethidium bromide staining. Each RT–PCR product was excised from agarose gels, extracted and purified using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany). The eluted DNA fragments were sequenced by A Wanner (Novartis Sequencing Facility).
Drugs
5-Hydroxytryptamine creatinine sulphate (5-HT), 5-carboxamidotryptamine maleate (5-CT) and 8-hydroxy-2-(di-n-propylamino)tetralin hydrobromide (8-OH-DPAT) were obtained from Sigma-Aldrich (Buchs, Switzerland). 5-Methoxytryptamine (5-MeOT) and (SB-269970) hydrochloride were synthesized at Novartis Pharma AG, Basel. Millimolar stock solutions of test compounds were made on the day of the experiment in dimethylsulphoxide or distilled water. Further dilutions were made in distilled water.
Results
Identification of functional 5-HT7-like receptors in human glioblastoma cell lines by cAMP measurements
A series of human glioblastoma cell lines (U-373 MG, U-138 MG, U-87 MG, DBTRG-05MG, T98G, H4, CCF-STTG1 and Hs 683) were examined for their cAMP response to 5-HT. The basal cAMP conversion rate (see Methods) ranged from 0.23 × 10−3 (U-373 MG cells) to 0.72 × 10−3 (U-138 MG cells). Stimulation of cAMP accumulation by 5-HT was observed in all cells, maximal effects varying from 1.7- (CCF-STTG1 cells) to 33-fold (H4 cells) increases above basal levels (Table 1 ).
Table 1.
Maximal effects (Emax) of 5-HT for stimulation of cAMP accumulation, pEC50 values of 5-HT, 5-CT, 5-MeOT and 8-OH-DPAT and pA2 values of SB-269970 versus 5-CT in human glioblastoma cell lines
| Emax (5-HT) | pEC50 | pA2 | ||||
|---|---|---|---|---|---|---|
| Cell line | Fold basal | 5-HT | 5-CT | 5-MeOT | 8-OH-DPAT | SB-269970 |
| U-373 MG | 9.5±0.5 | 6.09±0.12 | 6.98±0.03 | 5.89±0.09 | <4 | 8.82±0.05 |
| DBTRG-05MG | 8.3±0.3 | 6.18±0.07 | 7.22±0.09 | 6.13±0.03 | <4 | 8.95±0.05 |
| T98G | 12.9±1.5 | 5.86±0.06 | 6.84±0.03 | 5.59±0.06 | <4 | 8.81±0.13 |
| H4 | 32.7±0.9 | 6.03±0.03 | 7.03±0.06 | 5.65±0.02 | <4 | 8.92±0.06 |
| U-138 MG | 7.6±1.5 | 5.69±0.04 | 6.58±0.08 | ND | ND | 9.05±0.07 |
| U-87 MG | 2.0±0.1 | 6.09±0.04 | 7.09±0.03 | ND | ND | 8.69±0.05 |
| CCF-STTG1 | 1.7±0.1 | 6.26±0.05 | 7.33±0.08 | ND | ND | 8.98±0.04 |
| Hs 683 | 1.9±0.2 | 6.11±0.17 | 7.32±0.08 | ND | ND | 8.75±0.10 |
Data are mean values±s.e.m. from three or four individual experiments. Maximal effects are expressed as fold increases above basal cAMP accumulation. Since no maximum was reached for 8-OH-DPAT (see Figure 1), pEC50 values for this agonist are rough estimates. ND, not determined.
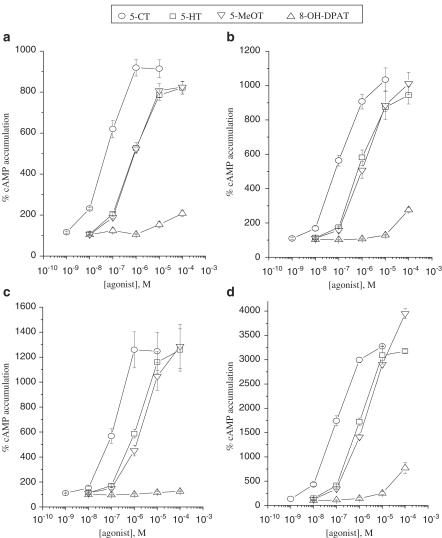
Concentration–response relationships for cAMP stimulation were obtained using the four agonists 5-HT, 5-CT, 5-MeOT and 8-OH-DPAT. Results with U-373 MG, DBTRG-05MG, T98G and H4 cells are illustrated in Figure 1. The rank order of potency was 5-CT>5-HT=5-MeOT≫8-OH-DPAT in these cell lines. Similar potencies were found for 5-CT and 5-HT in U-138 MG, U-87 MG, CCF-STTG1 and Hs 683 (Table 1).
Figure 1.
Concentration–response curves of 5-CT, 5-HT, 5-MeOT and 8-OH-DPAT for stimulation of cAMP accumulation in (a) U-373 MG, (b) DBTRG-05MG, (c) T98G and (d) H4 cells. Data are mean values±s.e.m. from three individual experiments. Results are expressed as percentage of basal cAMP accumulation.
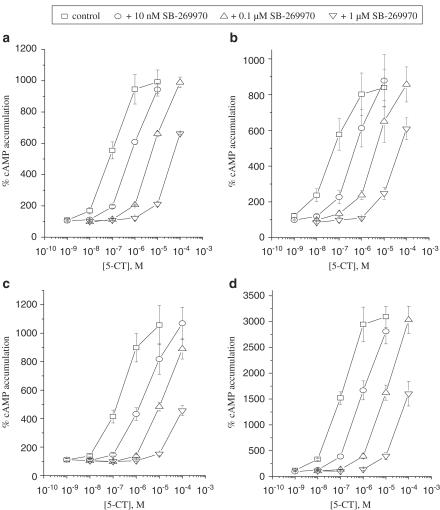
The potent and selective 5-HT7 receptor antagonist SB-269970 (10 nM–1 μM) induced incremental shifts in the concentration–response curve of 5-CT to the right in a parallel manner in all cell lines. Data with U-373 MG, DBTRG-05MG, T98G and H4 cells are illustrated in Figure 2. Schild analyses yielded slope factors close to unity (0.89–1.13) and pA2 values in the range 8.69–9.05 (Table 1).
Figure 2.
Concentration–response curves of 5-CT in the absence (control) and in the presence of 10 nM, 0.1 and 1 μM SB-269970 in (a) U-373 MG, (b) DBTRG-05MG, (c) T98G and (d) H4 cells. Data are mean values±s.e.m. from three or four individual experiments. Results are expressed as percentage of basal cAMP accumulation.
Western blot analysis
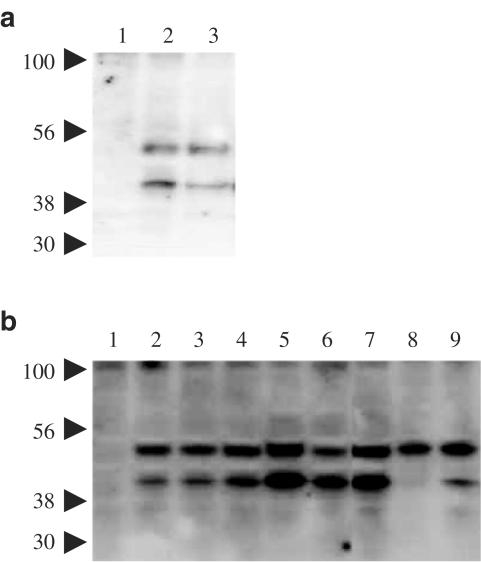
Western blot analysis was performed to investigate the occurrence of the 5-HT7 receptor protein in human glioblastoma cells. The antiserum revealed two bands with apparent molecular masses of approximately 45 and 50 kDa in extracts of Chinese hamster ovary (CHO) cells stably transfected with the human 5-HT7(a) receptor cDNA, but not in untransfected CHO cells (Figure 3a). These two bands were also present in extracts of all the human glioblastoma cell lines tested, with the exception of Hs 683 cells for which only the 50 kDa band was apparent (Figure 3b). This pattern of data was observed in at least two independent experiments for each cell line.
Figure 3.
Western blot analysis of human glioblastoma cell and control cell extracts. (a) Lane 1, untransfected CHO cells; lanes 2 and 3, CHO cells transfected with the human 5-HT7(a) receptor. (b) Lane 1, untransfected CHO cells; lane 2, T98G cells; lane 3, H4 cells; lane 4, U-373 MG cells; lane 5, U-138 MG cells; lane 6, DBTRG-05MG cells; lane 7, U-87 MG cells; lane 8, Hs 683 cells; lane 9, CCF-STTG1 cells. Molecular mass markers (in kDa) are indicated on the left.
Reverse transcriptase–polymerase chain reaction (RT–PCR)
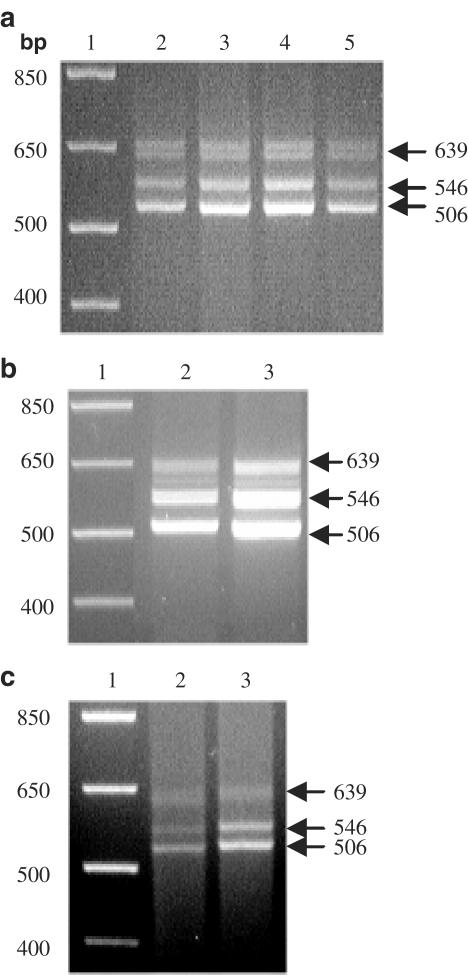
The presence of the different splice variants of the 5-HT7 receptor was investigated by RT–PCR analysis with specific primers that give specific amplification products for each of them (5-HT7(a), 5-HT7(b) and 5-HT7(d)). As a control, the cDNA of the constitutively expressed β-actin gene was also amplified. Three cDNA bands of the expected sizes (506 bp for 5-HT7(a), 546 bp for 5-HT7(b) and 639 bp for 5-HT7(d)) were amplified in the reverse transcribed products from all human glioblastoma cell lines tested (Figure 4). The band corresponding to the 5-HT7(d) splice variant consistently appeared to be less intense than the others. The different bands obtained for each cell line were excised, re-amplified with the same primers and sequenced. Sequencing confirmed that the PCR products corresponded to the amplification of the three cDNA splice variants in all cell lines. This pattern of data was observed in at least two independent experiments for each cell line.
Figure 4.
RT–PCR analysis of the 5-HT7 receptor splice variants in human glioblastoma cells. Lane 1, DNA length standards of the indicated size (in base pairs, bp). (a) Lane 2, U-138 MG cells; lane 3, U-373 MG cells; lane 4, DBTRG-05MG cells; lane 5, T98G cells. (b) Lane 2, H4 cells; lane 3, U-87 MG cells. (c) Lane 2, CCF-STTG1 cells; lane 3, Hs 683 cells. Fragment sizes corresponding to amplified fragments of each of the three variants 5-HT7(a) (506 bp), 5-HT7(b) (546 bp) and 5-HT7(d) (639 bp) are indicated on the right.
Discussion
The objective of the present study was to assess the validity of human glioblastoma cell lines as model systems for the study of 5-HT7 receptors in astroglial cells. Functional 5-HT7 receptors and 5-HT7 receptor mRNA are known to be present and expressed in human and rat brain astrocytes (Shimizu et al., 1996; Hirst et al., 1997; Cohen et al., 1999). However, such primary cells cannot be propagated in culture and are therefore of limited help for extensive and detailed investigations. The present results show that several human glioblastoma cell lines (U-373 MG, U-138 MG, U-87 MG, DBTRG-05MG, T98G, H4, CCF-STTG1 and Hs 683) express native, functional 5-HT7 receptors coupled to cAMP accumulation. These receptors could also be visualized by immunodetection (Western blotting) and the corresponding mRNA by RT–PCR.
In functional studies with human glioblastoma cells, the rank order of agonist potency for stimulation of cAMP accumulation was 5-CT>5-HT=5-MeOT≫8-OH-DPAT. This is the ‘fingerprint' of 5-HT7 receptors. Of the three 5-HT receptor classes positively coupled to adenylyl cyclase (5-HT4, 5-HT6 and 5-HT7), 5-HT7 is the only one showing higher affinity for 5-CT than for 5-HT (see Hoyer et al., 1994). The relatively low pEC50 values of agonists in glioblastoma cells, as compared to their affinities for the cloned 5-HT7 receptor (Bard et al., 1993), are probably to be ascribed to a limited number of receptors in the former situation. In line with this view, it is pertinent to say that no specific radioligand binding could be detected in human glioblastoma cells, using [3H]-5-HT, [3H]-5-CT, [3H]-lysergic acid diethylamide or [3H]-mesulergine (C. Mahé, personal observations). Also, similar agonist pEC50 values to those reported in the present study have been observed at native 5-HT7 receptors in rat primary astrocytes (Hirst et al., 1997).
Definitive evidence that the effect of 5-CT is mediated by 5-HT7 receptors in human glioblastoma cells was shown in antagonist studies with SB-269970. This compound has been introduced as a potent and selective 5-HT7 receptor antagonist, with a pKi value of 8.9 and a functional pA2 value of 8.5 (Hagan et al., 2000; Lovell et al., 2000). Values of pA2 found in human glioblastoma cells (8.69–9.05) were quite in line with these reported data.
To confirm the expression of 5-HT7 receptors in the human glioblastoma cells, the presence of this protein was then investigated by Western blotting, using a specific antibody raised against a sequence identical for all human receptor splice variants. Two bands, with apparent molecular masses of approximately 45 and 50 kDa, were detected for all glioblastoma cells except for Hs 683 cells, in which only the upper band was observed. These two bands were also present in CHO cells stably transfected with the human 5-HT7 receptor cDNA. The 45–50 kDa range corresponds to the anticipated molecular mass of the 5-HT7 receptor, which in addition possesses two putative sites for N-linked glycosylation in its amino-terminal region and several putative sites for phosphorylation (Boess & Martin, 1994). The results suggest that two protein forms of the 5-HT7 receptor are expressed in human glioblastoma cells (and control CHO cells), perhaps with different degrees of glycosylation and/or phosphorylation. For some reason, Hs 683 cells do not express one of these forms.
Pharmacological and immunological detection of 5-HT7 receptors in human glioblastoma cells was corroborated by results of molecular biology studies, showing the presence of the three splice variants of the human 5-HT7 receptor. RT–PCR amplifications were conducted using specific primers designed in such a way that the length of the amplification products was specific for each splice variant of the human 5-HT7 receptor. Three bands of the expected sizes were generated, suggesting the presence of the three splice variants in all glioblastoma cell lines. Sequencing of the different bands confirmed that the amplified fragments correspond to the cDNA sequences of the human 5-HT7 receptor splice variants. The 5-HT7(a) and 5-HT7(b) splice variants seem to be predominantly expressed in all glioblastoma cells compared to the 5-HT7(d) variant, in agreement with findings in some other tissues, including the brain (Heidmann et al., 1997; Krobert et al., 2001).
Taken together, the present results indicate that human glioblastoma cells may be used as model systems of native astroglial 5-HT7 receptors. The physiological role of these astroglial receptors, as pointed out in earlier studies, remains speculative (Hirst et al., 1997). The various ‘classical' functions of glial cells include nutritive support for neurons and uptake of excess neurotransmitters and K+. However, glial cells now appear to be involved in a broader spectrum of neuron-associated processes, for example, synaptogenesis, neurogenesis and neuroinflammation (Ransom et al., 2003). Since 5-HT7 receptors are positively linked to cAMP accumulation, cellular events associated with the cAMP/protein kinase A pathway in glial cells may be considered as candidate functions of these receptors. Interestingly enough, among these are the release of neurotrophic factors and of inflammatory cytokines (Huneycutt & Benveniste, 1995).
In conclusion, human glioblastoma cell lines express functional 5-HT7 receptors and the three splice variants of the corresponding mRNA. These cell lines could serve as model systems of native 5-HT7 receptors in glial cells to investigate their putative role in processes like release of neurotrophic factors or inflammatory cytokines.
Acknowledgments
We are indebted to Dr Bernard Bucher (CNRS UMR 7034, France) for helpful discussions and to Dr Daniel Hoyer (Novartis Pharma, NIBR Basel) for carefully reading the manuscript.
Abbreviations
- CHO
Chinese hamster ovary
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine, serotonin
- 5-MeOT
5-methoxytryptamine
- 8-OH-DPAT
8-hydroxy-2-(di-n-propylamino)tetralin
- RT–PCR
reverse transcriptase-polymerase chain reaction
- SB-269970
(R)-3-(2-(2-(4-methylpiperin-1-yl)-ethyl)pyrrolidine-1-sulphonyl)phenol
References
- ARUNLAKSHANA O., SCHILD H.O. Some quantitative uses of drug antagonists. Br. J. Pharmacol. Chemother. 1959;14:48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACON W.L., BECK S.G. 5-Hydroxytryptamine7 receptor activation decreases slow afterhyperpolarization amplitude in CA3 hippocampal pyramidal cells. J. Pharmacol. Exp. Ther. 2000;294:672–679. [PubMed] [Google Scholar]
- BARD J.A., ZGOMBICK J., ADHAM N., VAYSSE P., BRANCHEK T.A., WEINSHANK R.L. Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- BELENKY M.A., PICKARD G.E. Subcellular distribution of 5-HT1B and 5-HT7 receptors in the mouse suprachiasmatic nucleus. J. Compar. Neurol. 2001;432:371–388. doi: 10.1002/cne.1109. [DOI] [PubMed] [Google Scholar]
- BOESS F.G., MARTIN I.L. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- CHAPIN E.M., ANDRADE R. A 5-HT7 receptor-mediated depolarization in the anterodorsal thalamus. I. Pharmacological characterization. J. Pharmacol. Exp. Ther. 2001;297:395–402. [PubMed] [Google Scholar]
- COHEN Z., BOUCHELET I., OLIVIER A., VILLEMURE J.G., BALL R., STANIMIROVIC D.B., HAMEL E. Multiple microvascular and astroglial 5-hydroxytryptamine receptor subtypes in human brain: molecular and pharmacologic characterization. J. Cereb. Blood Flow Metabol. 1999;19:908–917. doi: 10.1097/00004647-199908000-00010. [DOI] [PubMed] [Google Scholar]
- EGLEN R.M., JASPER J.R., CHANG D.J., MARTIN G.R. The 5-HT7 receptor: orphan found. Trends Pharmacol. Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- ELLIOTT J.M., NEWBERRY N.R., CHOLEWINSKI A.J., BARTRUP J.T., BRIDDON S.J., CAREY J.E., FLANIGAN T.P., NEWTON R.A., PHIPPS S.L., REAVLEY A.C., SMITH C., WIGMORE M., GRAHAME-SMITH D.G., LESLIE R.A. Characterization of the 5-hydroxytryptamine2A receptor-activated cascade in rat C6 glioma cells. Neuroscience. 1995;69:1119–1131. doi: 10.1016/0306-4522(95)00323-b. [DOI] [PubMed] [Google Scholar]
- GILL C.H., SOFFIN E.M., HAGAN J.J., DAVIES C.H. 5-HT7 receptors modulate synchronized network activity in rat hippocampus. Neuropharmacology. 2002;42:82–92. doi: 10.1016/s0028-3908(01)00149-6. [DOI] [PubMed] [Google Scholar]
- GOAILLARD J.M., VINCENT P. Serotonin suppresses the slow afterhyperpolarization in rat intralaminar and midline neurones by activating 5-HT7 receptors. J. Physiol. 2002;541:453–465. doi: 10.1113/jphysiol.2001.013896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGAN J.J., PRICE G.W., JEFFREY P., DEEKS N.J., STEAN T., PIPER D., SMITH M.I., UPTON N., MEDHURST A.D., MIDDLEMISS D.N., RILEY G.J., LOVELL P.J., BROMIDGE S.M., THOMAS D.R. Characterization of SB-269970-A, a selective 5-HT7 receptor antagonist. Br. J. Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEIDMANN D.E.A., METCALF M.A., KOHEN R., HAMBLIN M.W. Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron–exon organization. J. Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- HIRST W.D., PRICE G.W., RATTRAY M., WILKIN G.P. Identification of 5-hydroxytryptamine receptors positively coupled to adenylyl cyclase in rat cultured astrocytes. Br. J. Pharmacol. 1997;120:509–515. doi: 10.1038/sj.bjp.0700921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYER D., CLARKE D.E., FOZARD J.R., HARTIG P.R., MARTIN G.R., MYLECHARANE E.J., SAXENA F.R., HUMPHREY P.P.A. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol. Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- HUNEYCUTT B.S., BENVENISTE E.N. Regulation of astrocyte cell biology by the cAMP/protein kinase A signaling pathway. Adv. Neuroimmunol. 1995;5:261–269. doi: 10.1016/0960-5428(95)00022-t. [DOI] [PubMed] [Google Scholar]
- JASPER J.R., KOSAKA A., TO Z.P., CHANG D.J., EGLEN R.M. Cloning, expression and pharmacology of a truncated splice variant of the human 5-HT7 receptor (h5-HT7(b)) Br. J. Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROBERT K.A., BACH T., SYVERSVEEN T., KVINGEDAL A.M., Levy F.O. The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363:620–632. doi: 10.1007/s002100000369. [DOI] [PubMed] [Google Scholar]
- LOVELL P.J., BROMIDGE S.M., DABBS S., DUCKWORTH D.M., FORBES I.T., JENNINGS A.J., KING F.D., MIDDLEMISS D.N., RAHMAN S.K., SAUNDERS D.V., COLLIN L.L., HAGAN J.J., RILEY G.J., THOMAS D.R. A novel, potent, and selective 5-HT7 antagonist: (R)-3-(2-(2-(4-methylpiperin-1-yl)-ethyl)pyrrolidine-1-sulfonyl)phenol (SB-269970) J. Med. Chem. 2000;43:342–345. doi: 10.1021/jm991151j. [DOI] [PubMed] [Google Scholar]
- LOVENBERG T.W., BARON B.M., DE LECEA L., MILLER J.D., PROSSER R.A., REA M.A., FOYE P.E., RACKE M., SLONE A.L., SIEGEL B.W., DANIELSON P.E., SUTCLIFFE J.G., ERLANDER M.G. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- MEYERHOF W., OBERMÜLLER F., FEHR S., RICHTER D. A novel rat serotonin receptor: primary structure, pharmacology, and expression pattern in distinct brain regions. DNA Cell Biol. 1993;12:401–409. doi: 10.1089/dna.1993.12.401. [DOI] [PubMed] [Google Scholar]
- PLASSAT J.L., AMLAIKI N., HEN R. Molecular cloning of a mammalian serotonin receptor that activates adenylate cyclase. Mol. Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- RANSOM B., BEHAR T., NEDERGAARD M. New roles for astrocytes (stars at last) Trends Neurosci. 2003;26:520–522. doi: 10.1016/j.tins.2003.08.006. [DOI] [PubMed] [Google Scholar]
- RUAT M., TRAIFFORT E., LEURS R., TARDIVEL-LACOMBE J., DIAZ J., ARRANG J.M., SCHWARTZ J.-C. Molecular cloning, characterization, and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U.S.A. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOEFFTER P., BOBIRNAC I., BODDEKE E., HOYER D. Inhibition of cAMP accumulation via recombinant human serotonin 5-HT1A receptors: considerations on receptor effector coupling across systems. Neuropharmacology. 1997;36:429–437. doi: 10.1016/s0028-3908(97)00043-9. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., FEUERBACH D., BOBIRNAC I., GAZI L., LONGATO R. Functional, endogenously expressed corticotropin-releasing factor receptor type 1 (CRF1) and CRF1 receptor mRNA expression in human neuroblastoma SH-SY5Y cells. Fund. Clin. Pharmacol. 1999;13:484–489. doi: 10.1111/j.1472-8206.1999.tb00007.x. [DOI] [PubMed] [Google Scholar]
- SCHOEFFTER P., ULLMER C., BOBIRNAC I., GABBIANI G., LÜBBERT H. Functional, endogenously expressed 5-hydroxytryptamine 5-ht7 receptors in human vascular smooth muscle cells. Br. J. Pharmacol. 1996;117:993–994. doi: 10.1111/j.1476-5381.1996.tb16687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN Y., MONSMA F.J., JR, METCALF M.A., JOSE P.A., HAMBLIN M.W., SIBLEY D.R. Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- SHIMIZU M., NISHIDA A., ZENSHO H., YAMAWAKI S. Chronic antidepressant exposure enhances 5-hydroxytryptamine7 receptor-mediated cyclic adenosine monophosphate accumulation in rat frontocortical astrocytes. J. Pharmacol. Exp. Ther. 1996;279:1551–1558. [PubMed] [Google Scholar]
- THOMAS D.R., HAGAN J.J. 5-HT7 receptors. Curr. Drug Targets CNS Neurol. Disord. 2004;3:81–90. doi: 10.2174/1568007043482633. [DOI] [PubMed] [Google Scholar]
- TO Z.P., BONHAUS D.W., EGLEN R.M., JAKEMAN L.B. Characterization and distribution of putative 5-ht7 receptors in guinea-pig brain. Br. J. Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSOU A.P., KOSAKA A., BACH C., ZUPPAN P., YEE C., TOM L., ALVAREZ R., RAMSEY S., BONHAUS D.W., STEFANICH E., JAKEMAN L., EGLEN R.M., CHAN H.W. Cloning and expression of a 5-hydroxytryptamine7 receptor positively coupled to adenylate cyclase. J. Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- YING S.W., RUSAK B. 5-HT7 receptors mediate serotonergic effects on light-sensitive suprachiasmic nucleus neurons. Brain Res. 1997;755:246–254. doi: 10.1016/s0006-8993(97)00102-9. [DOI] [PubMed] [Google Scholar]