Abstract
A biphasic cardiovascular response to bolus i.v. injection of human urotensin II (hUII, 3 nmol kg−1) in conscious, male, Sprague–Dawley (SD) rats was identified and underlying mechanisms were explored. Initially (0–5 min) there was tachycardia, hypotension and mesenteric and hindquarters vasodilatation; later (30–120 min), tachycardia, hindquarters vasodilatation and a modest rise in blood pressure occurred.
Pretreatment with indomethacin or NG nitro-L-arginine methylester (L-NAME) reduced the mesenteric vasodilator response to hUII, and abolished the late tachycardia and hindquarters vasodilatation. Indomethacin also abolished the hypotension and early hindquarters vasodilatation, and substantially reduced the initial tachycardia. Indomethacin and L-NAME together prevented all haemodynamic responses to hUII.
Inhibition of inducible NOS had no effect on responses to hUII, whereas inhibition of neuronal NOS reduced the delayed tachycardic response to hUII but did not significantly affect the vasodilatation. Only the initial tachycardic response to hUII was antagonised by propranolol.
In spontaneously hypertensive rats (SHR), the initial haemodynamic responses to hUII were qualitatively similar to those in SD rats, although there was also a modest renal vasodilatation. The secondary response comprised a smaller tachycardia and a small rise in blood pressure, with no significant hindquarters vasodilatation.
Haemodynamic responses to hUII were not enhanced by endothelin and angiotensin receptor antagonism in either SD rats or in SHRs.
One interpretation of these results is that the primary response to bolus injection of hUII is prostanoid- or prostanoid- and NO-mediated (mesenteric vasodilatation) and that this triggers secondary events, which are dependent on eNOS (hindquarters vasodilatation) and neuronal NOS (tachycardia).
Keywords: Urotensin II, haemodynamics, spontaneously hypertensive rats, nitric oxide, cyclooxygenase
Introduction
There is an extensive, and growing, literature on the in vitro vascular actions of human urotensin II (hUII), the recently identified endogenous ligand for the G protein-coupled receptor 14 (Ames et al., 1999), and there is unquestionably marked species and regional variability in the extent and nature of the responses obtained (see Maguire & Davenport, 2002; Douglas (2003) for reviews), although surprisingly few studies have dealt with the in vivo vascular actions of the peptide to date.
The landmark study of Ames et al. (1999) showed decreases in total peripheral conductance with bolus doses of hUII given i.v. to anaesthetised cynomolgus monkeys. However, these effects were not associated with rises in systemic arterial blood pressure, due to an accompanying marked fall in cardiac output. Since then, several groups have reported cardiovascular actions of bolus i.v. doses of hUII in conscious (Gardiner et al., 2001; Lin et al., 2003a) and anaesthetised (Abdelrahman & Pang, 2002; Hassan et al., 2003) rats, and all described depressor responses, consistent with earlier reports, using the structurally related fish UII (Gibson et al., 1986; Hasegawa et al., 1992). Our study also showed that the fall in blood pressure was accompanied by marked, regionally selective, peripheral vasodilatation (Gardiner et al., 2001), which contrasted strikingly with the data in primates (see above). Recently, Watson et al. (2003) reported small, transient pressor effects of a bolus i.v. dose of hUII in conscious sheep, but, again, in marked contrast to the primate data, there was no accompanying reduction in total peripheral conductance. Very few studies have explored the in vivo cardiovascular effects of hUII in man, and the currently available data are conflicting showing ‘potent' vasoconstriction (Böhm & Pernow, 2002) or no effect (Wilkinson et al., 2002) in the forearm, and vasodilatation in the cutaneous vasculature (Lim et al., 2004) in normal subjects. Affolter et al. (2002) also failed to detect any blood pressure effect of a systemic i.v. infusion of the peptide in man.
In a recent preliminary study, we found an unexpected biphasic response to bolus injection of hUII (Gardiner et al., 2004). The initial response consisted of mesenteric and hindquarters vasodilatation, tachycardia and a small fall in blood pressure, which waned after 30–60 min as described previously (Gardiner et al., 2001). However, thereafter, a second phase developed, which peaked around 90–120 min postinjection and comprised tachycardia and hindquarters vasodilatation with a modest rise in blood pressure. The temporal relation between the first and second phases of the response to hUII indicated that the former might have triggered the latter, and/or the first phase was a direct action and the second phase was an indirect action. Therefore, the present experiments were designed to explore the possible mechanisms involved in the biphasic response to bolus injection of hUII.
Some of the results have been presented to the British Pharmacological Society (Bennett et al., 2004).
Methods
Animals and surgical preparation
Male, Sprague–Dawley (SD) rats (400–450 g) and male spontaneously hypertensive rats (SHR) (25–30 weeks old) were obtained from Charles River, U.K. Inbred male Wistar–Kyoto (WKY/NHsd), Lewis (LEW/SsNHsd) and Fischer 344 (F344/NHsd) rats (15–20 weeks old) were obtained from Harlan, U.K. Rats were housed in the Biomedical Services Unit for at least 10 days after delivery, before any surgical interventions took place. The procedures were approved by the University of Nottingham Ethical Review Committee and were performed under Home Office Project Licence authority. Surgery was performed in two stages under general anaesthesia (fentanyl and medetomidine, 300 μg kg−1 of each i.p.). Anaesthetic reversal and the provision of analgesia was achieved using atipamezole and nalbuphine, respectively (1 mg kg−1 of each s.c.). Initially, miniaturised pulsed Doppler flow probes were sutured around the left renal and superior mesenteric arteries, and around the distal abdominal aorta (to monitor hindquarters flow). At least 10 days later, under anaesthesia (as above), catheters were implanted in the distal abdominal aorta (via the ventral caudal artery), for monitoring arterial blood pressure and heart rate, and in the right jugular vein for the administration of substances. The fitness of the animals between surgical stages was certified by the named Veterinary Surgeon.
Cardiovascular recordings began on the day following catheterisation, when the animals were fully conscious and freely moving, with access to food and water ad libitum.
Cardiovascular recordings
Continuous recordings of cardiovascular variables (heart rate, arterial blood pressure, renal, mesenteric and hindquarters Doppler shifts (flow)) were made using a customized, computer-based system (Haemodynamics Data Acquisition System (HDAS), University of Limburg, Maastricht, The Netherlands) connected to the transducer amplifier (Gould model 13-4615-50) and the Doppler flowmeter (Crystal Biotech (Holliston MA, U.S.A.) VF-1 mainframe (pulse repetition frequency 125 kHz) fitted with high velocity (HVPD-20) modules). Raw data were sampled by HDAS every 2 ms, averaged every cardiac cycle, and stored to disc at 5 s intervals. Data were analysed offline using software (Datview, University of Limburg, Maastricht, The Netherlands) that interfaced with HDAS.
Experimental protocols
In all the main experiments, animals were given a bolus injection of hUII (3 nmol kg−1) on two occasions separated by 48 h, with no treatments being given in the intervening period. Pilot experiments confirmed the reproducibility of responses to hUII under these conditions. Furthermore, in other studies, we have established the dose-dependent actions of hUII (Gardiner et al., 2001), and demonstrated that the chosen dose reliably evokes responses with all the components described. Lower doses of the peptide (up to 300 pmol kg−1) did not evoke a secondary response (unpublished observations).
Experiment 1. Effects of indomethacin and/or NG-nitro-L-arginine methyl ester (L-NAME) on responses to hUII in SD rats
It has been reported that in anesthetised rats, indomethacin abolishes the hypotensive response to fish UII (Hasegawa et al., 1992), and that the hypotensive response to hUII involves nitric oxide (NO; Abdelrahman & Pang, 2002). Therefore, in this part of the study, one group of SD rats (n=8) was given hUII (3 nmol kg−1) in the presence of indomethacin vehicle (10 mM Na2CO3) on day 1, and 90 min after the onset of administration of i.v. indomethacin (5 mg kg−1 h−1 infusion, Gardiner et al., 1990a) on day 3. A second group of SD rats (n=17) was given i.v. saline (0.1 ml bolus, 0.4 ml h−1 infusion) on day 1 and L-NAME (3 mg kg−1 bolus, 3 mg kg−1 h−1 infusion, Wakefield et al., 2003) on day 3, starting 90 min before administration of hUII (3 nmol kg−1). To control for the effects of L-NAME on baseline haemodynamics, a third group of SD rats (n=8) was given hUII (3 nmol kg−1) 90 min after the onset of infusion of saline on day 1 and angiotensin II (AII, 200 ng kg−1 h−1) and arginine vasopressin (AVP, 20 ng kg−1 h−1) on day 3. The combination of AII plus AVP was used to achieve a haemodynamic status which most closely matched that of L-NAME (Gardiner et al., 1998). Since indomethacin and L-NAME given separately both influenced the haemodynamic responses to hUII (see Results), a fourth group of SD rats (n=4) was given hUII (3 nmol kg−1) 90 min after the onset of infusion of AII plus AVP (doses as above) on day 1 and indomethacin plus L-NAME (doses as above) on day 3.
Experiment 2. Effects of N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide dihydrochloride (1400W hydrochloride) or Nω-propyl-L-arginine (NPA) on responses to hUII in SD rats
Since L-NAME abolished the delayed hindquarters vasodilatation and tachycardia evoked by bolus injection of hUII (see Results), we speculated that the delayed events might have been due to activation of inducible NOS (iNOS) and/or involvement of neuronal NOS (nNOS). Therefore, SD rats (n=17) were given saline (0.1 ml bolus, 0.4 ml h−1 infusion) on day 1, and either 1400W (iNOS inhibitor (Garvey et al., 1997); 3 mg kg−1 bolus, 3 mg kg−1 h−1 infusion, n=5) or NPA (nNOS inhibitor (Zhang et al., 1997); 3 mg kg−1 bolus, 3 mg kg−1 h−1 infusion, n=12) on day 3, starting 90 min before administration of hUII (3 nmol kg−1). The dose of 1400W was chosen on the basis of the study of Cheng et al. (2003) showing cardiovascular effects under conditions where iNOS is activated. The dose of NPA was chosen on the basis of its selectivity profile (Zhang et al., 1997) and evidence for in vivo effectiveness of an i.v. dose of 1 mg kg−1 h−1 (Kakoki et al., 2001).
Experiment 3. Effects of propranolol on responses to hUII in SD rats
There is evidence that hUII may release adrenaline (Watson et al., 2003), and it is known that the latter causes tachycardia and marked hindquarters vasodilatation in conscious rats (Gardiner et al., 1991a). Therefore, we assessed the effects of propranolol on responses to hUII. SD rats (n=9) were given either saline (0.1 ml bolus, 0.4 ml h−1 infusion) or propranolol (1 mg kg−1 bolus, 0.5 mg kg−1 h−1 infusion, Janssen et al., 1991) on day 1 and the other treatment on day 3, starting 60 min before administration of hUII (3 nmol kg−1).
Experiment 4. Responses to hUII in the absence and presence of [(+)-(1S, 2R, 3S)-3-(2-carboxymethoxy-4-methoxyphenyl)-1-13,4-methylenedioxyphenyl-5-(prop-1-yloxy)indane-2-carboxylic acid] (SB 209670) and losartan in SD rats and SHR
Lin et al. (2003b) have recently reported exaggerated pressor responses to central administration of hUII in SHR, but we know of no studies in which responses to peripherally administered hUII have been measured in SHR. Furthermore, although there is evidence that activation of the sympathetic nervous system may limit the hypotensive effect of hUII in anaesthetised rats (Abdelrahman & Pang, 2002), it is not known to what extent vasoconstrictor effects of endogenous angiotensin and endothelin serve to offset the vasodilator response to hUII. Therefore, rats (n=8 SHR and n=8 SD) were given saline (0.4 ml h−1) on day 1 and the ETA-, ETB-receptor antagonist, SB 209670 (600 μg kg−1 bolus, 600 μg kg−1 h−1 infusion) together with the AT1-receptor antagonist, losartan (10 mg kg−1 bolus) on day 3, starting 90 min before administration of hUII.
Although not included in this series of experiments, we have previously shown that following combined administration of SB 209670 and losartan, cardiovascular variables are quite stable for a period of several hours, once the new steady state has been reached (Gardiner et al., 1996).
Experiment 5. Responses to hUII in inbred WKY, LEW and F344 rats
Since there were differences between the effects of hUII in SHR compared with SD rats (see Results from Experiment 4), small groups of inbred WKY (n=4), LEW (n=3) and F344 (n=3) rats were given hUII (3 nmol kg−1) to determine whether or not the effects seen in SD rats were also apparent in other normotensive strains.
Data analysis
Within-group analyses were carried out using a nonparametric ANOVA (Friedman's test). Between-group analyses were by Wilcoxon's test or the Mann–Whitney U-test, as appropriate; P⩽0.05 was taken as significant.
Drugs
Fentanyl citrate was obtained from Janssen-Cilag (High-Wycombe, U.K.); medetomidine hydrochloride (Domitor) and atipamezole hydrochloride (Antisedan) were obtained from Pfizer (Kent, U.K.); nalbuphine hydrochloride (Nubain) was obtained from Bristol-Myers-Squibb (Houslow, U.K.). hUII was purchased from Peptide Institute Inc. (Scientific Marketing Associates, Barnet, U.K.). L-NAME was purchased from Sigma (Dorset, U.K.). NPA and 1400W hydrochloride were purchased from Tocris (Avonmouth, U.K.). Indomethacin was purchased from Merck Biosciences Ltd (Nottingham, U.K.). AII and AVP were purchased from Bachem (St Helens, U.K.) Ltd. SB 209670 was a gift from Dr E. Ohlstein (GSK, U.S.A.). Losartan potassium was a gift from Dr R.D. Smith (DuPont, U.S.A.).
All drugs and peptides were dissolved in sterile saline with the exception of indomethacin which was dissolved in 10 mM Na2CO3. Bolus injections were in a volume of 0.1 ml and infusions were at a rate of 0.4 ml h−1.
Results
Experiment 1. Effects of indomethacin and/or L-NAME on responses to hUII in SD rats
Group 1: Indomethacin
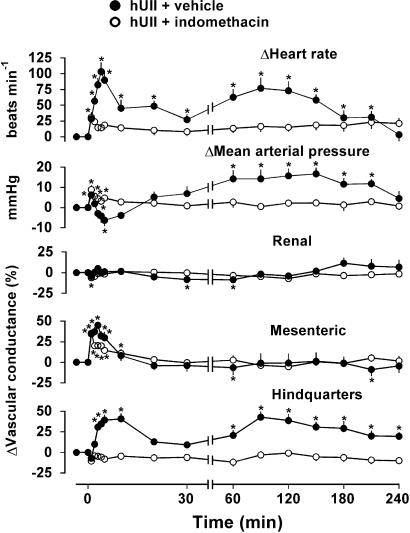
In the presence of vehicle, there was an initial response to administration of hUII which comprised tachycardia, mesenteric vasodilatation, a rapid, transient (1 min) rise followed by a fall in mean arterial blood pressure, accompanied by a slowly developing hindquarters vasodilatation (Figure 1). Thereafter, a second phase of response developed (between 60 and 120 min after hUII administration) comprising tachycardia, a rise in mean arterial blood pressure and hindquarters vasodilatation (Figure 1).
Figure 1.
Haemodynamic responses to human urotensin II (hUII; 3 nmol kg−1) in the presence of indomethacin (5 mg kg−1 h−1; open circles) or its vehicle (10 mM Na2CO3 at 0.4 ml h−1; closed circles) in conscious SD rats (n=8). Values are mean and vertical bars show s.e.m. *P⩽0.05 vs baseline (Friedman's test). Between-group differences are given in the text. Note the change in time scale between 30 and 60 min.
Indomethacin had no significant effect on resting cardiovascular variables (Table 1 ). However, in the presence of indomethacin, the initial hypotensive and hindquarters vasodilator effects of hUII were abolished, and there was little or no tachycardia. The integrated (0–5 min) increase in mesenteric vascular conductance in response to hUII was reduced (P<0.05) from 164±9 to 104±15 % min (Figure 1). In addition, in the presence of indomethacin, the delayed hUII-induced increase in heart rate, mean arterial pressure and hindquarters vascular conductance were absent (Figure 1).
Table 1.
Resting cardiovascular variables in Experiment 1
| Group 1 (n=8) vehicle | Group 1 (n=8) Indo | Group 2 (n=17) saline | Group 2 (n=17) L-NAME | Group 3 (n=8) saline | Group 3 (n=8) AII+AVP | |
|---|---|---|---|---|---|---|
| Heart rate (beats min−1) | 330±10 | 297±12 | 343±12 | 293±13* | 344±15 | 294±14* |
| Mean BP (mmHg) | 108±2 | 105±3 | 109±4 | 134±5* | 100±3 | 139±4* |
| Renal VC ((kHz mmHg−1)103) | 68±8 | 69±5 | 77±10 | 46±7* | 89±10 | 67±9* |
| Mesenteric VC ((kHz mmHg −1)103) | 65±6 | 62±5 | 89±9 | 41±5* | 77±7 | 36±3* |
| Hindquarters VC ((kHz mmHg−1)103) | 38±4 | 37±4 | 35±5 | 18±2* | 31±2 | 18±1* |
All values are mean±s.e.m. for measurements 90 min after the onset of treatment, immediately prior to administration of hUII. VC=vascular conductance. Rats were given vehicle on day 1 and indomethacin (Indo, Group 1), L-NAME (Group 2) or AII plus AVP (Group 3) on day 3.
P⩽0.05 vs day 1 (Wilcoxon's test).
In a separate series of experiments (n=4, preliminary data), the changes in cardiovascular variables during infusion of indomethacin alone for the period of time shown in Figure 1 were heart rate, +16±9 beats min−1; mean arterial blood pressure, +1±3 mmHg; renal vascular conductance, +6±7%; mesenteric vascular conductance, +12±13%; hindquarters vascular conductance, −10±10%. Hence, there was little consistent change during this time period.
Groups 2 and 3: L-NAME
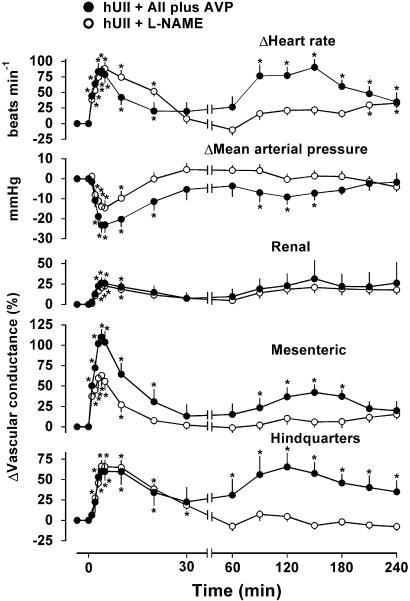
In the presence of vehicle for L-NAME or vehicle for AII plus AVP (i.e., saline), responses to bolus administration of hUII were as described above (data not shown). Administration of L-NAME or AII plus AVP caused similar degrees of hypertension, bradycardia and widespread vasoconstriction, such that, with the exception of renal vascular conductance, haemodynamic variables were not different in the presence of L-NAME compared with AII plus AVP (Table 1). In the presence of AII plus AVP, responses to hUII were generally enhanced relative to the control condition (compare Figures 1 and 2). When the initial (0-5 min) effects of hUII in the presence of L-NAME were compared with those in the presence of AII plus AVP, the increase in mesenteric vascular conductance was significantly (P<0.05) reduced (233±42 vs 386±37 % min, respectively), there was a trend towards a diminution in the hypotension which did not reach significance (P=0.065), and the hindquarters vasodilatation was unaffected (Figure 2). Furthermore, in the presence of L-NAME, there were no delayed responses to hUII, whereas, in the presence of AII plus AVP, the late-onset hindquarters vasodilatation and tachycardia were marked, and there was a slight mesenteric vasodilatation (Figure 2).
Figure 2.
Haemodynamic responses to human urotensin II (hUII; 3 nmol kg−1) in the presence of AII and AVP (200 and 20 ng kg−1 h−1, respectively; closed circles; n=8) or L-NAME (3 mg kg−1 h−1; open circles; n=17) in conscious SD rats. Values are mean and vertical bars show s.e.m. *P⩽0.05 vs baseline (Friedman's test). Between-group differences are given in the text. Note the change in time scale between 30 and 60 min.
In a separate series of experiments (n=6, preliminary data), the changes in cardiovascular variables during infusion of L-NAME alone for the period of time shown in Figure 2 were heart rate, +21±2 beats min−1; mean arterial blood pressure, +4±3 mmHg; renal vascular conductance, +12±9%; mesenteric vascular conductance, −3±5%; hindquarters vascular conductance, −9±3%. Hence, there was little consistent change during this time period.
Group 4: L-NAME plus indomethacin
In the presence of L-NAME plus indomethacin, all haemodynamic responses to hUII were abolished (data not shown).
Experiment 2. Effects of 1400W or NPA on responses to hUII in SD rats
Neither 1400W nor NPA had any significant cardiovascular effects.
In the presence of 1400W, cardiovascular responses to hUII were not different from those seen in the presence of saline (data not shown). In the presence of NPA, the initial cardiovascular responses to hUII were not different from those in the presence of saline, but the delayed (at 90 min) increase in heart rate and pressor responses (+19±10 beats min−1, +2±2 mmHg, respectively) were smaller (P⩽0.05) than the corresponding changes seen in the presence of saline (+68±12 beats min−1, +10±3 mmHg), but the increase in hindquarters vascular conductance was not significantly affected (+20±6 and +33±5% in presence and absence of NPA, respectively).
Experiment 3. Effects of propranolol on responses to hUII in SD rats
Administration of propranolol had no significant cardiovascular effects.
In the presence of propranolol, the initial integrated (0–5 min) tachycardic response to hUII (+115±16 beats) was smaller (P<0.05) than the corresponding change in the presence of saline (+258±31 beats), but, otherwise, responses to hUII were not different from those seen in the presence of saline.
In other experiments (n=8), we have found no significant changes in cardiovascular variables over a 240 min period starting 90 min after administration of propranolol (heart rate, −5±9 beats min−1; mean arterial blood pressure, +8±3 mmHg; renal vascular conductance, +4±5%; mesenteric vascular conductance, −8±4%; hindquarters vascular conductance, −3±2%. Hence, there was little consistent change during this time period.
Experiment 4. Responses to hUII in the absence and presence of SB 209670 and losartan in SD rats and in SHR
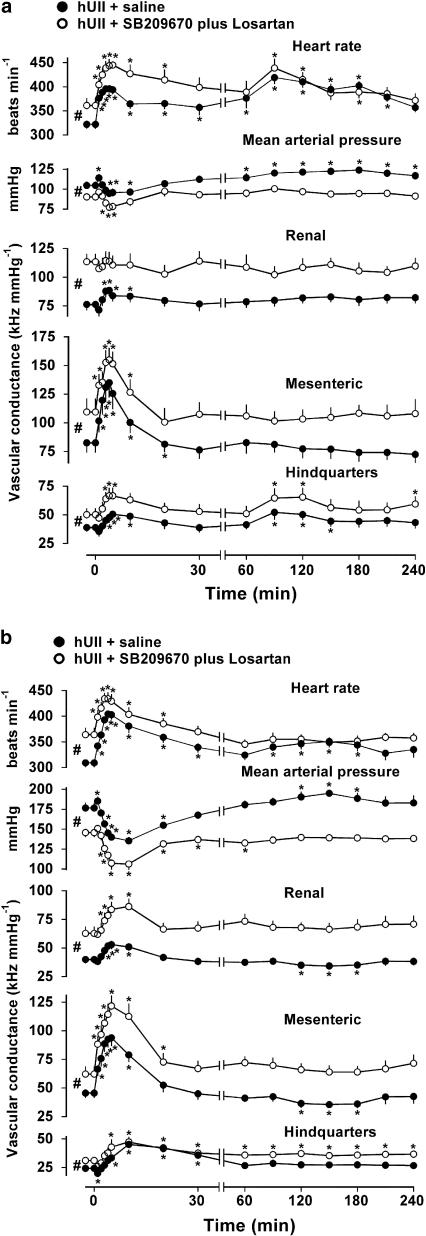
In SD rats, combined administration of SB 209670 plus losartan caused hypotension, tachycardia and vasodilatation such that all baseline cardiovascular variables just prior to administration of hUII in the presence of SB 209670 plus losartan or saline were significantly different (Figure 3a). In the presence of saline (Figure 3a), cardiovascular responses to hUII were as described above, although in this group of rats, there was a small renal vasodilatation. In the presence of SB 209670 plus losartan, the initial cardiovascular responses to hUII were similar to those seen in the presence of saline, and the delayed tachycardic and hindquarters vasodilator responses were unaffected, although there was no secondary rise in blood pressure (Figure 3a).
Figure 3.
Cardiovascular variables before and after administration (at time 0) of human urotensin II (hUII; 3 nmol kg−1) in conscious SD rats (a; n=8) and spontaneously hypertensive rats (b; n=8) in the presence of saline (closed circles) or SB 209670 plus losartan (600 μg kg−1, 600 mg kg−1 h−1 and 10 mg kg−1, respectively, open circles). Values are mean and vertical bars show s.e.m. *P⩽0.05 vs baseline (Friedman's test). #P⩽0.05 between baseline values (Wilcoxon's test). Note the change in time scale between 30 and 60 min.
In SHR, in the presence of saline, the initial responses to hUII were generally similar to those seen in SD rats, although the hindquarters vasodilatation and hypotension were more marked. However, there was no significant secondary hindquarters vasodilator response to hUII in SHR, and smaller delayed increases in heart rate and blood pressure (Figure 3b). Combined administration of SB 209670 plus losartan caused a marked fall in blood pressure, tachycardia and widespread vasodilatation in SHR (Figure 3b). In the presence of SB 209670 plus losartan, the cardiovascular responses to hUII were largely unchanged (Figure 3b).
Experiment 5. Responses to hUII in inbred WKY, LEW and F344 rats
At 5 min following administration of hUII, there was tachycardia (+142±12, +71±22, +41±2 beats min−1), increases in mesenteric vascular conductance (+21±4, +59±13, +34±6%) and increases in hindquarters vascular conductance (+23±5, +32±10, +27±5%) in WKY, LEW and F344, respectively. Thereafter, a secondary response developed such that, at 90 min following hUII administration, there was tachycardia (+62±8, +52±22, +26±6 beats min−1), a rise in blood pressure (+16±4, +11±2, +10±2 mmHg) and increases in hindquarters vascular conductance (+20±3, +24±9, +36±6%) in WKY, LEW and F344, respectively.
Discussion
The finding which prompted these studies was that, with prolonged continuous monitoring, the regional haemodynamic effects of a bolus dose of hUII in conscious rats were seen to be more complex than hitherto appreciated. Thus, in addition to the initial response, which we have described previously (Gardiner et al., 2001), we found an unexpected delayed phase, which appeared about 60 min after administration of the bolus, and which resembled the initial response, in that it consisted of a tachycardia and hindquarters vasodilatation, but differed inasmuch as there was no mesenteric vasodilatation, and blood pressure tended to rise rather than fall (Gardiner et al., 2004).
The initial effects of bolus doses of hUII were qualitatively similar to those reported in our earlier study (Gardiner et al., 2001), but quantitatively less. Since our previous protocol involved giving incremental hUII injections about 60 min apart, and we now know there is a clear secondary response to hUII, beginning about 60 min after the injection, it is feasible that, in our earlier experiments, responses to doses other than the first were augmented by the then unknown secondary response to the first dose, and so on. Others (Abdelrahman & Pang, 2002) have used 5 min dosing schedules for hUII, and so their results also could have been affected by the phenomenon described here. However, it may be that the biphasic response to hUII is only seen in conscious animals, because Hasegawa et al. (1992) observed marked tachyphylaxis to repeated doses of UII given at 45 min intervals in anaesthetised Wistar rats. Interestingly, in the recent study of Watson et al. (2003) in conscious sheep, there was no evidence for a second phase of response to an i.v. bolus dose of hUII.
Regarding mechanisms, it was striking that indomethacin abolished the initial hypotensive, tachycardic and hindquarters vasodilator effect of hUII, and reduced the mesenteric vasodilatation by about 50%. These findings with respect to the initial hypotension are consistent with the earlier observations of Hasegawa et al. (1992) using fish UII in anaesthetised rats, and extend them by showing regional differentiation, inasmuch as the mesenteric vasodilatation appeared to be only partly dependent on prostanoids, whereas the initial hindquarters response seemed to be entirely prostanoid-mediated. Furthermore, we found some indication of an involvement of NO in the initial hypotensive effect of hUII, which is consistent with an earlier report using hUII in anaesthetised rats (Abdelrahman & Pang, 2002). Again, we extended those observations showing that NO was involved in the mesenteric, but not in the initial hindquarters, vasodilator response to hUII. At first sight, the increase in hUII-induced mesenteric vasodilatation seen in the presence of L-NAME, compared with the saline condition, seems inconsistent with a vasodilator role for NO. However, when the baseline changes were accounted for by the use of AII plus AVP, we observed a relative inhibition of the effect of hUII by L-NAME. Elsewhere, we have shown apparent enhancement of vasodilator responses to bolus injections of classical endothelium-dependent, NO-mediated, vasodilators in the presence of L-NAME (Gardiner et al., 1990b). We cannot dismiss the possibility that the augmentation of the response to hUII in the presence of AII plus AVP was due to an interaction between the peptides, rather than to the change in baseline. However, the results of the experiment where we gave indomethacin and L-NAME together and showed total abolition of the mesenteric vasodilator effect of hUII are consistent with the suggestion that the residual response seen in the presence of indomethacin was NO-mediated. It is notable that the residual, NO-mediated mesenteric vasodilatation in the presence of indomethacin was not sufficient to evoke an hypotensive response, possibly indicating a predominant role for hindquarters vasodilatation in this event.
Surprisingly, all the delayed responses to the peptide were abolished by either indomethacin, or L-NAME. One possible interpretation of these findings is that the delayed responses to hUII were β-adrenoceptor-mediated consequences of sympathoadrenal activation (Watson et al., 2003), which may be modulated by arachidonic acid metabolites (Yokotani et al., 2001) and which may be sensitive to L-NAME (Gardiner et al., 1991a, 1991b). However, the present results with propranolol showed no involvement of β-adrenoceptors in the second phase of response to hUII. The suppression of the initial tachycardic effect of hUII by propranolol, as reported previously in anaesthetised rats using fish UII (Gibson et al., 1986) and hUII (Abdelrahman & Pang, 2002), is consistent with this being, at least in part, attributable to a reflex sympathetic response to the fall in blood pressure. However, it should be noted that the tachycardia was far more prolonged than the hypotension, possibly indicating an additional effect of hUII on sympathetic drive, and/or on the heart itself.
Another possible interpretation of our finding that the delayed responses to the peptide were abolished by either indomethacin or L-NAME is that the primary event was hUII-induced activation of prostanoid production which, in turn, stimulated NOS (Borda et al., 2002; Madrigal et al., 2003). Alternatively, it is feasible that hUII directly stimulated NOS expression which was inhibited by indomethacin (DiGirolamo et al., 2003). A further possibility is that hUII stimulated NO production and that this activated cyclooxygenase (Salvemini et al., 1993). It seems that such effects did not involve iNOS since inhibition of iNOS with 1400W (Garvey et al., 1997) did not replicate any of the effects of L-NAME. However, inhibition of nNOS with NPA (Zhang et al., 1997) inhibited the delayed tachycardia and the associated rise in blood pressure, indicating a role for nNOS-derived NO in those responses. There are a number of ways in which NO can influence heart rate, either directly (e.g., Chowdhary et al., 2002) or by influencing autonomic control (e.g., Jumrussirikul et al., 1998; Choate et al., 2001). However, whether or not the hUII-induced tachycardia, which was sensitive to NPA, was a direct or an indirect effect of NO cannot be determined from our experiments. Since the small, delayed, pressor response to hUII was not associated with vasoconstriction, and was also inhibited by NPA, it is feasible that it was due to a rise in cardiac output, possibly associated with the tachycardia. An interesting observation was that the modest delayed rise in blood pressure was accompanied by an increase in pulse pressure (data not shown), further supporting the proposition that it was due to a rise in cardiac output possibly due to an hUII-induced positive inotropic effect (see Douglas, 2003).
The difference between the effects of L-NAME and NPA on responses to hUII, and the lack of effect of 1400W, indicate a major role for endothelial NOS (eNOS) in the delayed hindquarters vasodilator response to the peptide. This is intriguing against the background of a lack of involvement of NO in the early hindquarters vasodilator effect of hUII and a clear involvement of NO in the mesenteric vasodilatation which was monophasic and short-lived. Since NPA abolished the tachycardia and modest rise in blood pressure yet, under those conditions, hindquarters vasodilatation still occurred, it does not seem likely that the L-NAME-sensitive delayed hindquarters vasodilatation was secondary to an increase in pulsatility brought about by a positive inotropic action of hUII (see above).
It is notable that the renal vascular bed showed no consistent response to hUII in SD rats (see also Gardiner et al., 2001) because Zhang et al. (2003) have reported that hUII caused significant, NO-mediated renal vasodilatation in anaesthetised rats. However, in their experiments the peptide was administered intrarenally, so it is feasible that a local renal vasodilator action of hUII is opposed by other mechanisms when the peptide is given systemically (but see below).
Lin et al. (2003b) have recently reported exaggerated pressor responses to central administration of hUII in SHR, but as far as we are aware, ours is the first study in which responses to peripherally administered hUII have been measured in SHR. We found that the initial responses were qualitatively similar to those seen in SD rats, but the magnitude of effects tended to be greater, and there was a small, but significant, renal vasodilator response to hUII. Interestingly, however, there was a smaller delayed response to hUII in SHR, suggesting that, whatever the mechanism(s) (see above), they were less in that strain of rat. In small groups of inbred strains of normotensive rat, we were able to demonstrate secondary responses to hUII, albeit to varying degrees. Thus, on the basis of the experiments performed to date, the absence of a secondary hindquarters vasodilator response to hUII in SHR appears to be unique to that strain.
In the presence of the endothelin and angiotensin receptor antagonists, SB 209670 and losartan, there was no significant enhancement of the hypotensive or vasodilator effects of hUII, in either SHR or SD rats. Hence, it appears that the absence of renal vasodilator responses to hUII in SD rats was not due to compensatory activation of these vasoconstrictor mechanisms, but rather, the resting vasoconstriction in SHR might have unmasked the renal vasodilator action of this peptide, consistent with the finding that in SD rats given AII plus AVP, a small renal vasodilator effect of hUII was seen. The lack of influence of SB 209670 plus losartan on the overt mesenteric and hindquarters vasodilator responses to hUII indicates that vasoconstrictor influences of endogenous endothelin and angiotensin II do not simply oppose these effects of the peptide.
From the present results it appears that the variability in responses to hUII as reported in the literature could be due to variations in degrees of involvement of NO and prostanoids in different experimental conditions (see Introduction; Katano et al., 2000; Gray et al., 2001). Indeed, the very recent study of Lim et al. (2004) shows cutaneous vasodilator responses to hUII in normal subjects, but vasoconstrictor responses in patients with congestive heart failure. Although the latter did not show significantly reduced endothelium-dependent vasodilator responses, the group size was small, and responses to acetylcholine were in fact reduced by 31%. However, under no conditions have we been able to demonstrate vasoconstrictor responses to hUII, and the failure of Wilkinson et al. (2002) to demonstrate vasoconstrictor responses to hUII in the human forearm is not likely due to opposing action of NO and prostanoids, since they performed experiments in the presence of L-NMMA and aspirin.
In conclusion, the regional vasodilator responses to bolus injection of hUII in conscious rats include an initial, short-lived mesenteric vasodilatation which is largely prostanoid-mediated with a small contribution from NO, and a biphasic hindquarters vasodilatation, the first phase of which is entirely prostanoid-mediated. Since the second phase was inhibited by either indomethacin or L-NAME, one interpretation is that a prostanoid-mediated event triggers NO-dependent vasodilatation. Interestingly, an analogous sequence of events has been shown to be involved in hypoxia-induced vasodilatation in rats (Ray et al., 2002), whereby the primary mediator (adenosine) increases prostaglandin synthesis which, in turn, increases synthesis and release of NO via the production of cAMP.
Acknowledgments
This work was supported by the British Heart Foundation. We are grateful to Drs Anthony Davenport and Katherine Wiley for useful discussions of the work.
Abbreviations
- AII
angiotensin II
- AVP
arginine vasopressin
- HDAS
haemodynamic data acquisition system
- hUII
human urotensin II
- L-NAME
NG nitro-L-arginine methylester
- NO
nitric oxide
- NPA
Nω-propyl-L-arginine
- SD
Sprague–Dawley
- SHR
spontaneously hypertensive rat
- 1400W hydrochloride
(N-[[3-(aminomethyl)phenyl]methyl]-ethanimidamide dihydrochloride)
References
- ABDELRAHMAN A.M., PANG C.C.Y. Involvement of the nitric oxide/L-arginine and sympathetic nervous systems on the vasodepressor action of human urotensin II in anesthetized rats. Life Sci. 2002;71:819–825. doi: 10.1016/s0024-3205(02)01743-5. [DOI] [PubMed] [Google Scholar]
- AFFOLTER J.T., NEWBY D.E., WILKINSON I.B., WINTER M.J., BALMENT R.J., WEBB D.J. No effect on central or peripheral blood pressure of systemic urotensin II infusion in humans. Br. J. Clin. Pharmacol. 2002;54:617–621. doi: 10.1046/j.1365-2125.2002.t01-1-01704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMES R.S., SARAU H.M., CHAMBERS J.K., WILLETTE R.N., AIYAR N.V., ROMANIC A.M., LOUDEN C.S., FOLEY J.J., SAUERMEICH C.F., COATNEY R.W., AO Z., DISA J., HOLMES S.D., STADEL J.M., MARTIN J.D., LIU W.-S., GLOVER G.I., WILSON S., MCNULTY D.E., ELLIS C.E., ELSHOURBAGY N.A., SHABON U., TRILL J.J., HAY D.W.P., OHLSTEIN E.H., BERGSMA D.J., DOUGLAS S.A. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- BENNETT T., MARCH J.E., KEMP P.A., GARDINER S.M.Effects of NG nitro-L-arginine methyl ester (L-NAME) on the haemodynamic responses to human urotensin II (hUII) in conscious rats 2004. Proceedings of the British Journal of Pharmacology at [DOI] [PMC free article] [PubMed]
- BÖHM F., PERNOW J. Urotensin II evokes potent vasoconstriction in humans in vivo. Br. J. Pharmacol. 2002;135:25–27. doi: 10.1038/sj.bjp.0704448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORDA E., HEIZIG G., BUSCH L., STERIN-BORDA L. Nitric oxide synthase/PGE2 cross-talk in rat submandibular gland. Prost. Leuk. Essent. Fatty Acids. 2002;67:39–44. doi: 10.1054/plef.2002.0379. [DOI] [PubMed] [Google Scholar]
- CHENG X., LEUNG S.W.S., LO L.S., PANG C.C.Y. Selective versus non-selective suppression of nitric oxide synthase on regional hemodynamics in rats with or without LPS-induced endotoxemia. Naunyn-Schmied. Arch. Pharmacol. 2003;367:372–379. doi: 10.1007/s00210-002-0684-1. [DOI] [PubMed] [Google Scholar]
- CHOATE J.K., DANSON E.J.F., MORRIS J.F., PATERSON D.J. Peripheral vagal control of heart rate is impaired in neuronal NOS knockout mice. Am. J. Physiol. 2001;281:H2310–H2317. doi: 10.1152/ajpheart.2001.281.6.H2310. [DOI] [PubMed] [Google Scholar]
- CHOWDHARY S., HARRINGTON D., BONSER R.S., COOTE J.H., TOWNEND J.N. Chronotropic effects of nitric oxide in the denervated human heart. J. Physiol. 2002;541:645–651. doi: 10.1113/jphysiol.2001.015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIGIROLAMO G., FARINA M., RIBERIO M.L., OGANDO D., AISEMBERG J., DE LOS SANTOS A.R., MARTI M.L., FRANCHI A.M. Effects of cyclooxygenase inhibitor pre-treatment on nitric oxide production, nNOS and iNOS expression in rat cerebellum. Br. J. Pharmacol. 2003;139:1164–1170. doi: 10.1038/sj.bjp.0705315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOUGLAS S.A. Human urotensin-II as a novel cardiovascular target: ‘heart' of the matter or simply a fishy ‘tail'. Curr. Opin. Pharmacol. 2003;3:159–167. doi: 10.1016/s1471-4892(03)00012-2. [DOI] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., BENNETT T. Effect of indomethacin on the regional haemodynamic responses to low doses of endothelins and sarafotoxin. Br. J. Pharmacol. 1990a;100:158–162. doi: 10.1111/j.1476-5381.1990.tb12069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., COMPTON A.M., KEMP P.A., BENNETT T. Regional and cardiac haemodynamic responses to glyceryl trinitrate, acetylcholine, bradykinin and endothelin-1 in conscious rats: effects of Ng-nitro-L-arginine methyl ester. Br. J. Pharmacol. 1990b;101:632–639. doi: 10.1111/j.1476-5381.1990.tb14132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methylester on the vasodilator responses to adrenaline or BRL 38227 in conscious rats. Br. J. Pharmacol. 1991a;104:731–737. doi: 10.1111/j.1476-5381.1991.tb12496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., BENNETT T. Effects of NG-nitro-L-arginine methylester on the vasodilator responses to acetylcholine, 5′-N-ethylcarboxamidoadenosine or salbutamol in conscious rats. Br. J. Pharmacol. 1991b;103:1725–1732. doi: 10.1111/j.1476-5381.1991.tb09854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., KEMP P.A., MARCH J.E., BENNETT T. Temporal differences between the involvement of angiotensin II and endothelin in the cardiovascular responses to endotoxaemia in conscious rats. Br. J. Pharmacol. 1996;119:1619–1627. doi: 10.1111/j.1476-5381.1996.tb16081.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. The contribution of nitric oxide to cardiovascular status and responses to vasodilators in conscious, hypertensive, transgenic ((mRen-2)27) rats. Br. J. Pharmacol. 1998;124:299–306. doi: 10.1038/sj.bjp.0701838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARDINER S.M., MARCH J.E., KEMP P.A., BENNETT T. Haemodynamic effect of human urotensin II (hUII) in the absence and presence of the corticotropin releasing factor (CRF) receptor antagonist, astressin, in conscious rats. Br. J. Pharmacol. 2004.
- GARDINER S.M., MARCH J.E., KEMP P.A., DAVENPORT A.P., BENNETT T. Depressor and regionally-selective vasodilator effects of human and rat urotensin II in conscious rats. Br. J. Pharmacol. 2001;132:1625–1629. doi: 10.1038/sj.bjp.0704051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GARVEY E.P., OPLINGER J.A., FURFINE E.S., KIFF R.J., LASZLO F., WHITTLE B.J.R., KNOWLES R.G. 1400W is a slow, tight binding and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J. Biol. Chem. 1997;272:4959–4963. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- GIBSON A., WALLACE P., BERN H.A. Cardiovascular effects of urotensin II in anesthetized and pithed rats. Gen. Comp. Endocrinol. 1986;64:435–439. doi: 10.1016/0016-6480(86)90080-8. [DOI] [PubMed] [Google Scholar]
- GRAY G.A., JONES M.R., SHARIF I. Human urotensin II increases coronary perfusion pressure in the isolated rat heart. Potentiation by nitric oxide synthase and cyclooxygenase inhibition. Life Sci. 2001;69:175–180. doi: 10.1016/s0024-3205(01)01101-8. [DOI] [PubMed] [Google Scholar]
- HASEGAWA K., KOBAYASHI Y., KOBAYASHI H. Vasodepressor effects of urotensin II in rats. Neuroendocrinol. Lett. 1992;14:357–363. [Google Scholar]
- HASSAN G.S., CHOUIALI F., SAITO T., HU F., DOUGLAS S.A., AO Z., WILLETTE R.N., OHLSTEIN E.H., GIAID A. Effect of human urotensin-II infusion on hemodynamics and cardiac function. Can. J. Physiol. Pharmacol. 2003;81:125–128. doi: 10.1139/y03-004. [DOI] [PubMed] [Google Scholar]
- JANSSEN P.J.J.M., GARDINER S.M., COMPTON A.M., BENNETT T. Mechanisms contributing to the differential haemodynamic effects of bombesin and cholecystokinin in conscious, Long Evans rats. Br. J. Pharmacol. 1991;102:123–134. doi: 10.1111/j.1476-5381.1991.tb12143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUMRUSSIRIKUL P., DINERMAN J., DAWSON T.M., DAWSON V.L., EKELUND U., GEORGAKOPOULOS D., SCHRAMM L.P., CALKINS H., SNYDER S.H., HARE J.M., BERGER R.D. Interaction between neuronal nitric oxide synthase and inhibitory G protein activity in heart rate regulation in conscious mice. J. Clin. Invest. 1998;102:1279–1285. doi: 10.1172/JCI2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAKOKI M., ZOU A.-P., MATTSON D.L. The influence of nitric oxide synthase 1 on blood flow and interstitial nitric oxide in the kidney. Am. J. Physiol. 2001;281:R91–R97. doi: 10.1152/ajpregu.2001.281.1.R91. [DOI] [PubMed] [Google Scholar]
- KATANO Y., ISHIHATA A., AITA T., OGAKI T., HORIE T. Vasodilator effect of urotensin II, one of the most potent vasoconstricting factors, on rat coronary arteries. Eur. J. Pharmacol. 2000;402:209–211. doi: 10.1016/s0014-2999(00)00506-9. [DOI] [PubMed] [Google Scholar]
- LIM M., HONISETT S., SPARKES C.D., KOMESAROFF P., KOMPA A., KRUM H. Differential effect of urotensin II on vascular tone in normal subjects and patients with chronic heart failure. Circulation. 2004;109:1212–1214. doi: 10.1161/01.CIR.0000121326.69153.98. [DOI] [PubMed] [Google Scholar]
- LIN Y., TSUCHIHASHI T., MATSUMURA K., ABE I., IIDA M. Central cardiovascular action of urotensin II in conscious rats. J. Hypertens. 2003a;21:159–165. doi: 10.1097/00004872-200301000-00026. [DOI] [PubMed] [Google Scholar]
- LIN Y.Z., TSUCHIHASHI T., MATSUMURA K., FUKUHARA M., OHYA Y., FUJII K., IIDA M. Central cardiovascular action of urotensin II in spontaneously hypertensive rats. Hypertens. Res. 2003b;26:839–845. doi: 10.1291/hypres.26.839. [DOI] [PubMed] [Google Scholar]
- MADRIGAL J.L.M., GARCIA-BUENO B., MORO M.A., LIZASOAIN I., LORENZO P., LEZA J.C. Relationship between cyclooxygenase-2 and nitric oxide synthase-2 in rat cortex after stress. Eur. J. Neurosci. 2003;18:1701–1705. doi: 10.1046/j.1460-9568.2003.02888.x. [DOI] [PubMed] [Google Scholar]
- MAGUIRE J.J., DAVENPORT A.P. Is urotensin-II the new endothelin. Br. J. Pharmacol. 2002;137:579–588. doi: 10.1038/sj.bjp.0704924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY C.J., ABBAS M.R., CONEY A.M., MARSHALL J.M. Interactions of adenosine, prostaglandins and nitric oxide in hypoxia-induced vasodilatation: in vivo and in vitro studies. J. Physiol. 2002;544:195–209. doi: 10.1113/jphysiol.2002.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALVEMINI D., MISKO T.P., MASFERRER J.L., SEIBERT K., CURRIE M.G., NEEDLEMAN P. Nitric oxide activates cyclooxygenase enzymes. Proc. Natl. Acad. Sci. U.S.A. 1993;90:7240–7244. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKEFIELD I.D., MARCH J.E., KEMP P.A., VALENTIN J.-P., BENNETT T., GARDINER S.M. Comparative regional haemodynamic effects of the nitric oxide synthase inhibitors, S-methyl-L-thiocitrulline and L-NAME, in conscious rats. Br. J. Pharmacol. 2003;139:1235–1243. doi: 10.1038/sj.bjp.0705351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON A.M.D., LAMBERT G.W., SMITH K.J., MAY C.N. Urotensin II acts centrally to increase epinephrine and ACTH release and cause potent inotropic and chronotropic actions. Hypertension. 2003;42:373–379. doi: 10.1161/01.HYP.0000084633.85427.E6. [DOI] [PubMed] [Google Scholar]
- WILKINSON I.B., AFFOLTER J.T., DE HAAS S.L., PELLEGRINI M.P., BOYD J., WINTER M.J., BALMENT R.J., WEBB D.J. High plasma concentrations of human urotensin II do not alter local or systemic hemodynamics in man. Cardiovasc. Res. 2002;53:341–347. doi: 10.1016/s0008-6363(01)00485-0. [DOI] [PubMed] [Google Scholar]
- YOKOTANI K., MURAKAMI Y., OKADA S., HIRATA M. Role of brain arachidonic acid cascade on central CRF1 receptor-mediated activation of sympatho-adrenomedullary outflow in rats. Eur. J. Pharmacol. 2001;419:183–189. doi: 10.1016/s0014-2999(01)00987-6. [DOI] [PubMed] [Google Scholar]
- ZHANG A.Y., CHEN Y.-F., ZHANG D.X., YI F.-X., QI J., ANDRADE-GORDON P., DE GARAVILLA L., LI P.-L., ZOU A.-P. Urotensin II is a nitric oxide-dependent vasodilator and natriuretic peptide in the rat kidney. Am. J. Physiol. 2003;285:F792–F798. doi: 10.1152/ajprenal.00342.2002. [DOI] [PubMed] [Google Scholar]
- ZHANG H.Q., FAST W., MARLETTA M.A., MARTASEK P., SILVERMAN R.B. Potent and selective inhibition of neuronal nitric oxide synthase by Nω-propyl-L-arginine. J. Med. Chem. 1997;40:3869–3870. doi: 10.1021/jm970550g. [DOI] [PubMed] [Google Scholar]