Abstract
Expression of two isoforms of Rho-kinase (ROCK) and its functional role in the physiological control of smooth muscle contraction in the sheep ureter were investigated. Helical strips of the ureteric smooth muscle were stimulated by electrical field stimulation (EFS, 60 V, 1 mS, 2, 4, 8, 16 and 32 Hz, for 20 S), KCl (80 mM), carbachol (CCh, 10−8–10−4 M) or phenylephrine (Phe, 10−8–10−4 M).
EFS produced a reproducible contractile activity, which was abolished by tetrodotoxin (3 × 10−6 M), a Na+ channel blocker. A muscarinic receptor antagonist, atropine (2 × 10−6 M), and an adrenergic neuron blocker, guanethidine (10−5 M), significantly suppressed the contraction induced by EFS. However, this contraction was augmented in the presence of NG-nitro-L-arginine (L-NA, 10−4 M), a nitric oxide synthase inhibitor.
Two Rho-kinase inhibitors, Y-27632 (5 × 10−5 M) and fasudil (5 × 10−5 M), markedly attenuated the EFS-elicited contraction. CCh and Phe produced concentration-dependent contraction in the sheep ureter. pD2 values for Phe and CCh were 5.04±0.11 and 5.00±0.22, respectively. Y-27632 (5 × 10−5 M) and fasudil (5 × 10−5 M) also significantly inhibited CCh- and Phe-induced contractions. Moreover, these ROCK inhibitors produced relaxations in the KCl-elicited contraction in a concentration-dependent manner. pD2 values for Y-27632 and fasudil were, respectively, 5.17±0.07 and 4.58±0.08 (P<0.001).
Furthermore, the influences of these agents were also tested on spontaneous phasic contractions of the tissue. Among Y-27632, fasudil, TTX, L-NA, guanethidine and atropine, only the ROCK inhibitors (10−6–10−5 M) were able to suppress the spontaneous contractile activity.
Western blot analysis has revealed that both isoforms of Rho-kinase (ROCK-1 and ROCK-2) are expressed in the sheep ureter. Densitometric analysis has indicated that these enzymes are less expressed in the sheep ureter than are in the sheep aorta in a significant manner.
These results show that a contractile enzyme, Rho-kinase, is expressed, and it mediates agonist- and EFS-induced contractions as well as spontaneous contractile activity of the isolated sheep ureter. Since Y-27632 and fasudil depressed the contractions, it seems plausible to postulate that Rho-kinase inhibitors may be beneficial in the treatment of renal colic.
Keywords: Electrical field stimulation, fasudil, Rho kinase, ureter, Y-27632
Introduction
One of the most important functions of the pyloureteral complex is to ensure the unidirectional transport of urine from kidney to the urinary bladder. It has been proposed that there might be some putative pacemakers responsible for initiation of peristaltic waves in the renal pelvis as well as ureteral smooth muscle (Santicioli & Maggi, 1998). On the other hand, the ureter is innervated by a large population of afferents that produce an intense pain following distention of the ureter by ureteral calculi. Electrical field stimulation (EFS) of the ureter also induces pain and muscular hyperalgesia in rats (Cervero, 1994). Furthermore, the implantation of an experimental calculus in rats have caused hyperalgesia and abdominal stretching, a hallmark of visceral pain (Giamberardino et al., 1995a). Stretching of the ureter may cause the release of prostanoids and tachykinins which amplify ureteral motility (Laird et al., 1997; Lang et al., 2002). The drugs with spasmolytic and/or anti-inflammatory effects can be beneficial in the treatment of renal colic (Giamberardino et al., 1995b). Moreover, installation of capsaicin resulted in a pain-relieving effect in patients (Bultitude, 1995). Several mechanisms are proposed in the physiological control of ureteral peristalsis and smooth muscle tone (Santicioli & Maggi, 1998). Nevertheless, the cellular mechanisms underlying neurogenic and myogenic contractions are not exactly elucidated.
The increase in cytoplasmic free Ca2+ concentration ([Ca2+]i) was regarded to be the principle mechanism in smooth muscle contraction. Upon activation of calmodulin, Ca2+ activates myosin light chain kinase (MLCK) to phosphorylate the myosin light chain (MLC) to induce smooth muscle contraction (Kamm & Stull, 1985). However, the studies in which [Ca2+]i and concomitant contractile force were detected have shown that Ca2+ concentration does not always parallel the degree of MLC phosphorylation and subsequent contraction of the muscle. In other words, regulation of MLC phosphorylation and contractile force is independent of changes in [Ca2+]i. This is due to a phenomenon, the so-called Ca2+ sensitization (Somlyo & Somlyo, 1994), raising the possibility that there must be some additional pathways, which can modulate the contractile process at a constant [Ca2+]i (Fukata et al., 2001). Indeed, it has been reported that a monomeric small GTP-binding protein, Rho, and its downstream effector, Rho-kinase (ROCK, ROK), play a substantial role in the Ca2+ sensitization (Somlyo & Somlyo, 2003). The activation of heterotrimeric G proteins such as Gq (G11) and G12/13 following the stimulation of G protein-coupled receptors leads to some cellular events. On activation, Gq or G11 proteins cause phosphatidylinositol 4,5-bis-phosphate hydrolysis to diacylglycerol (DAG) and inositol 1,4,5-triphosphate (IP3). However, following the activation of another heterotrimeric G protein, G12/13, the signal is transmitted to the Rho/Rho-kinase pathway (Fukata et al., 2001). This novel pathway provides an alternative vasoconstrictor mechanism, which is independent of [Ca2+]i elevation (Somlyo & Somlyo, 1994). Indeed, it has been well established that the smooth muscle contraction can be induced also in the absence of an increase in the [Ca2+]i (Bradley & Morgan, 1987).
It has been reported that Rho/Rho-kinase pathway could be involved in the contractile activity of various tissues such as aorta (Uehata et al., 1997), mesenteric artery (Büyükafşar et al., 2004), ileum (Sward et al., 2000), gastric fundus (Büyükafşar & Levent, 2003), corpus cavernosum (Büyükafşar & Ün, 2003), urinary bladder (Wibberley et al., 2003), vas deferens (Büyükafşar et al., 2003) and uterus (Tahara et al., 2002). However, this signalling pathway has not been studied in ureteric smooth muscle. For that reason, first we detected the expression of ROCK proteins of both isoforms by Western blotting in the tissue, and then investigated the functional importance of this enzyme by using its selective inhibitor, (+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride monohydrate (Y-27632) (Ishizaki et al., 2000), in the contractions, which are induced by EFS, KCl, phenylephrine and carbachol. We also tested another Rho kinase inhibitor, fasudil in these contractions. Furthermore, possible contribution of the enzyme to the physiological control of spontaneous contractile activity of the tissue was also examined, which would provide a further insight into mechanisms underlying pyeloureteral motility, and so display any physiological and pharmacological relevence of Rho GTPase signalling in the ureteric smooth muscle contraction. Moreover, since decreasing of calculus-induced ureteral motility and stretch may result in a remedy for the visceral pain, we aimed to point out Y-27632 as a possible antispasmodic agent in the ureteral colic.
Methods
Tissue preparation
Sheep ureters were obtained from a local slaughterhouse in Mersin, Turkey. The ureter was cut in the middle section of the whole tissue and then cleared of fat and other connective tissues. It was helically cut by placing onto a steel rod. Strips (about 2 cm long and 2–3 mm width) were prepared and suspended between two ring electrodes connected to the Biopac stimulator (Biopac system Inc., CA, U.S.A.) in an organ bath filled with Krebs' solution (composition in mM: NaCl 118, KCl 4.7, CaCl2 2.5, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, glucose 11, Na2EDTA 0.3) gassed with 95% O2 and 5% CO2 under an initial tension of 0.5 g, which was chosen as the optimum resting tension after preliminary experiments. The bath temperature was maintained at 37°C. Tension was recorded isometrically with a force transducer (COMMAT, Ankara, Turkey) and displayed on a Biopac acquisition system (Biopac system Inc., CA, U.S.A.).
Experimental protocol
Following an equilibration period of 1 h, the helical strips of the sheep ureter were contracted by EFS (60 V, 1 mS, 2, 4, 8, 16 and 32 Hz, for 20 S), carbachol (10−8–10−4 M, cumulatively) or phenylephrine (10−8–10−4 M, cumulatively). These contraction series were regarded as the first series of contraction. After washing with fresh Krebs solution, the strips were incubated for 1 h. Thereafter, they were contracted with the same manner (the second series). In another series of experiments between the first and second series, the agents, which would be tested, namely, atropine (2 × 10−6 M for 20 min), guanethidine (10−5 M for 60 min), NG-nitro-L-arginine (L-NA, 10−4 M for 20 min), fasudil (5 × 10−5 M for 30 min), Y-27632 (5 × 10−5 M for 30 min) and tetrodotoxin (TTX, 3 × 10−6 M for 15 min), were incubated. In the other series of experiments, effects of Y-27632 (10−8–10−4 M, cumulatively) and fasudil (10−8–10−4 M, cumulatively) were also tested on KCl (80 mM)-induced contraction. Finally, the compounds were also tested on spontaneous contractile activity of the tissue. The compounds were incubated for the above-mentioned durations. The corresponding control series without the agents were also performed. The concentrations and incubation duration of the agents used in this study were determined after preliminary experiments.
Western blot analysis for Rho-kinase
The ureteral as well as aortic smooth muscles of sheep were homogenized with the lyses buffer solution (composition in mM; Tris–HCl (pH=7.4) 50 mM, NaCl 400 mM, EGTA 2 mM, EDTA 1 mM, dithiothreitol 1 mM, phenylmethylsulfonyl fluoride 10 μM, leupeptin 10 μg ml−1, pepstatin 1 μg ml−1, benzamidine 1 mM). The homogenate was centrifuged at 1500 × g for 10 min at 4°C to remove nuclei and unlysed cells, and the supernatant was removed. It was then used for protein analysis (with Lowry method) and Western blot analysis. Equal amounts of proteins (400 μg) were loaded in wells, electrophoresed on 8% polyacrylamide–sodium dodecyl sulfate (SDS) gels and then transferred to a nitrocellulose membrane overnight. The membrane was blocked with the blocking agent of enhanced chemiluminescence (ECL advance) kit (Amersham Biosciences, Freiburg, Germany) in Tris-buffered solution containing 0.05% Tween-20 (TBS-T) for 1 h. It was then probed with primary antibodies raised against ROCK-1 (ROKβ) or ROCK-2 (ROKα, Polyclonal IgG, Santa Cruz Biotechnology Inc., CA, U.S.A.) at 1 : 200 dilution, followed by horseradish peroxidase-conjugated secondary antibody (donkey antigoat, 1 : 1000, Santa Cruz Biotechnology Inc., CA, U.S.A.). The blots were then detected with the advanced chemiluminescence detection kit (Amersham Biosciences, Freiburg, Germany).
Chemicals used
Ethylene glycol-bis(β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), ethylenediamine tetraacetic acid (EDTA) disodium, dithiothreitol, phenylmethylsulfonyl fluoride (PMSF), leupeptin, benzamidine, Tris–HCl, atropine sulfate, guanethidine sulfate, carbachol hydrochloride, L-NA and phenylephrine hydrochloride were obtained from Sigma Chemical Co (St Louis, U.S.A.). Y-27632 and fasudil were obtained from Tocris Cookson Ltd (Bristol, U.K.), and TTX from Alomone (Jerusalem, Israel). Potassium chloride (KCl), glycine and dodecyl sulfate sodium salt were purchased from Merck Co (Darmstadt, Germany). Primary antibodies for ROCK-1 and ROCK-2, and HRP-conjugated secondary antibody was obtained from Santa Cruz Biotechnology Inc. (CA, U.S.A.). ECL advance kit was purchased from Amersham Biosciences (Freiburg, Germany). The kit was used according to the manufacturer's guide. Potassium chloride, phenylephrine hydrochloride, endothelin-1, angiotensin-2, fasudil and Y-27632 were dissolved in distilled water.
Statistical evaluations
All data represent means±standard error of the mean (s.e.m.) of n observations. Contractions to phenylephrine and carbachol were expressed as milliNewton (mN) of tension per g of wet tissue. The relaxations to Y-27632 and fasudil were evaluated as percent reductions of active tone induced by KCl. For spontaneous contractions, the amplitude of the contractions at a given time was expressed as percentage of the contraction amplitude obtained at the time zero. After the application of test drugs, the spontaneous contractions were evaluated at the end of 15, 20 or 60 min in the same manner. For statistical comparison, one-way analysis of variance (ANOVA) followed by the Bonferroni post hoc test or Student's t-test, if appropriate, was used. A P-value less than 0.05 was considered significant. Graphs were drawn using the GraphPad Prism 3.0 program (GraphPad software, San Diego, CA, U.S.A.).
Results
Effects of TTX, atropine, guanethidine, L-NA, fasudil and Y-27632 on EFS-elicited contractions and spontaneous contractile activity
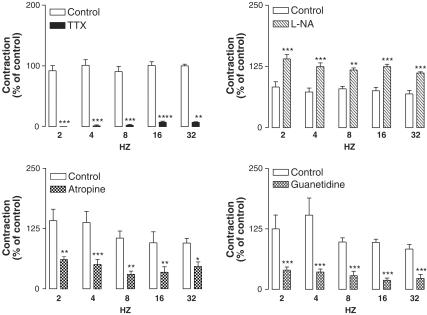
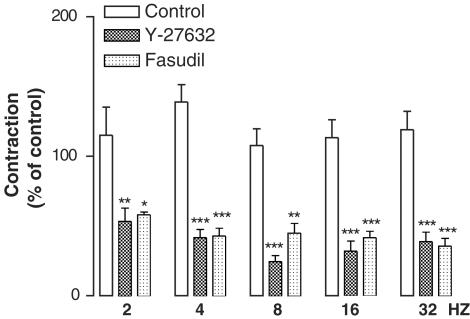
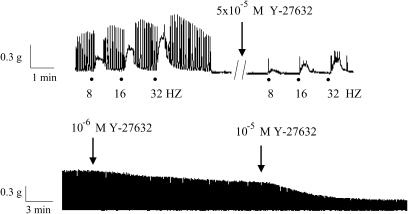
TTX (3 × 10−6 M) almost abolished EFS-induced contractions in that at 2, 4, 8, 16 and 32 Hz EFS-evoked contractions were, respectively, 0±0, 1. 4±1.4, 2.4±1.2, 7.5±1.7 and 7.5±1.6% of the corresponding control series (Figure 1). Atropine (2 × 10−6 M) and guanethidine (10−5 M) markedly inhibited these contractions (Figure 1). Electrically induced contractions were significantly augmented in the presence of L-NA (10−4 M, Figure 1). The Rho-kinase inhibitors, Y-27632 (5 × 10−5 M) and fasudil (5 × 10−5 M), produced a significant inhibition on the EFS-elicited contractions (Figure 2 and 3). The sheep ureteral helical strips exhibited spontaneous contractile activity with the frequency of 0.2±0.007 Hz (n=51). The mean amplitude of the spontaneous contractions was 11.0±0.8 mN (n=51). None of these agents but Y-27632 (10−6 and 10−5 M) and fasudil (10−6 and 10−5 M) had suppressing effects on the spontaneous contractions (Figure 3 and Table 1).
Figure 1.
Effects of TTX (left upper panel, 3 × 10−6 M, n=8), L-NA (right upper panel, 10−4 M, n=11), atropine (left lower panel, 2 × 10−6 M, n=8) and guanethidine (right lower panel, 10−5 M, n=8) on the EFS (60 V, 1 mS, 2, 4, 8, 16, 32 Hz, 20 S)-elicited contractions. The contractions were expressed as percent of control (the first series). Data represent means±s.e.m. *P<0.05, **P<0.01, ***P<0.001. Comparison was made by ANOVA, followed by Bonferroni post hoc test.
Figure 2.
Effects of Y-27632 (5 × 10−5 M, n=7) and fasudil (5 × 10−5 M, n=5) on the EFS (60 V, 1 mS, 2, 4, 8, 16, 32 Hz, 20 S)-elicited contractions. The contractions were expressed as percent of control (the first series). Data represent means±s.e.m. *P<0.05, **P<0.01, ***P<0.001. Comparison was made by one-way ANOVA, followed by Bonferroni post hoc test.
Figure 3.
Original tracings showing the effects of the Rho-kinase inhibitor Y-27632 (10−6–5 × 10−5 M) on EFS (60 V, 1 mS, 8, 16, 32 Hz, 20 S, upper panel)-elicited and spontaneous (lower panel) contractions in the helical strips of the sheep ureter. Note that Y-27632 suppresses not only EFS-induced contractions but also spontaneous contractile activity.
Table 1.
Influence of NG-nitro-L-arginine (L-NA), tetrodotoxin, atropine, guanethidine, fasudil and Y-27632 on the spontaneous contractile activity of the isolated sheep ureteral helical strips
| Control | 90.9±2.8%, n=12 |
| L-NAME (10−4 M) | 96.2±2.9%, n=7, NS |
| Control | 94.3±2.1%, n=15 |
| TTX (3 × 10−6 M) | 93.1±5.2%, n=4, NS |
| Control | 90.9±2.8%, n=12 |
| Atropin (10−6 M) | 97.1±2.1%, n=7, NS |
| Control | 76.1±7.5%, n=7 |
| Guanethidine (10−5 M) | 89.8±3.8%, n=7, NS |
| Control | 94.3±2.1%, n=15 |
| Y-27632 (10−6 M) | 55.7±3.0%***, n=7 |
| Y-27632 (10−5 M) | 15.2±3.1%***, n=6 |
| Fasudil (10−6 M) | 67.1±3.4%***, n=4 |
| Fasudil (10−5 M) | 43.0±2.4%***, n=4 |
The amplitude of contractions at a given time was expressed as the percentage of the contraction amplitude obtained at time zero. After the application of L-NA and atropine, the spontaneous contractions were evaluated at the end of 20 min; for Y-27632, fasudil and TTX at the end of 15 min and for guanethidine at the end of 60 min. For comparison, one-way ANOVA followed by Bonferroni post hoc test was used.
P<0.001, NS: not significant.
Effect of Rho-kinase inhibitors, Y-27632 and fasudil, on KCl-, phenylephrine- and carbachol-induced contractions
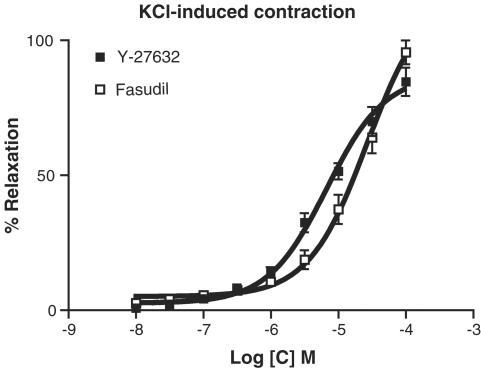
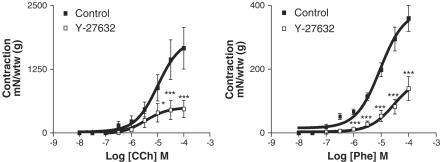
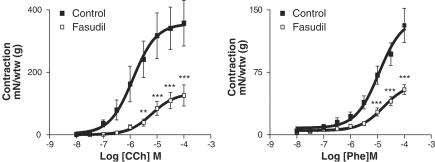
Y-27632 (10−8–10−4 M) and fasudil (10−8–10−4 M) produced relaxation in a concentration-dependent manner on KCl (80 mM)-elicited contraction (Figure 4). pD2 values for Y-27632 and fasudil were 5.17±0.07 and 4.58±0.08, respectively (P<0.001). Y-27632 (5 × 10−5 M) and fasudil (5 × 10−5 M) conspicuously inhibited the contractions induced by phenylephrine and carbachol (Figures 5 and 6).
Figure 4.
Effect of Y-27632 (10−8–10−4 M, cumulatively, n=7) and fasudil (10−8–10−4 M, cumulatively, n=6) on KCl (80 mM)-induced tonic contraction. Relaxations were expressed as percent reductions in the KCl-induced contraction. Data represent means±s.e.m.
Figure 5.
Effects of carbachol, a cholinergic agonist (CCh, 10−8–10−4 M, left panel, n=7), and phenylephrine, an α-adrenoceptor agonist (Phe, 10−8–10−4 M, right panel, n=7) in the absence and presence of the Rho-kinase inhibitor, Y-27632 (5 × 10−5 M). Contractions were expressed as mN of the wet tissue weight (g). Data represent means±s.e.m. *P<0.05, ***P<0.001. Comparison was made by one-way ANOVA, followed by Bonferroni post hoc test.
Figure 6.
Effects of carbachol, a cholinergic agonist (CCh, 10−8–10−4 M, left panel, n=7), and phenylephrine, an α-adrenoceptor agonist (Phe, 10−8–10−4 M, right panel, n=4), in the absence and presence of the Rho-kinase inhibitor, fasudil (5 × 10−5 M). Contractions were expressed as mN of the wet tissue weight (g). Data represent means±s.e.m. **P<0.01, ***P<0.001. Comparison was made by one-way ANOVA, followed by Bonferroni post hoc test.
Expression of Rho-kinase in the sheep ureter and aorta
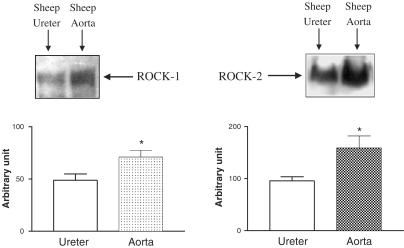
Western blot analysis revealed that both isoforms of ROCK (ROCK-1 and ROCK-2) were expressed in the sheep ureteric smooth muscle at the molecular weight about 160 kDa. When compared with expression levels in the sheep aorta, the ureteral smooth muscle expressed these proteins significantly less (Figure 7).
Figure 7.
Demonstration of the expressions of ROCK-1 and ROCK-2 in the sheep ureteric (n=4) and aortic (n=4) smooth muscle homogenates by Western blotting. The homogenates were submitted to SDS–PAGE with 8% polyacrylamide and then transferred to a nitrocellulose membrane (PVDF). The membrane was blocked with an ECL Advance blocking agent in Tris-buffered solution containing 0.05% Tween-20 (TBS-T) for 1 h. It was then probed with a primary antibody raised against ROCK-1 and ROCK-2 (Polyclonal IgG) at 1 : 200 dilution, followed by horseradish peroxidase-conjugated secondary antibody (donkey antigoat, 1 : 1000). Proteins bound with the antibodies were then visualized by the ECL Advance kit. Densitometric analysis of the protein bands was evaluated by a computer program (Scion image, U.S.A.).
Discussion
In this study, we examined the possible involvement of Rho/Rho-kinase pathway in the spontaneous as well as EFS, KCl, phenylephrine and carbachol-induced contractions of the sheep ureter. Furthermore, we detected the expression of two isoforms of Rho-kinase in this tissue, and compared its expression level to that in the sheep aortic smooth muscle.
EFS of the sheep ureteral smooth muscle induced contraction, which was abolished by the sodium channel blocker TTX, indicating that the mediators responsible for the contractile activity could be released from nerves. The mammalian ureter is innervated by afferent sensory, noradrenergic, cholinergic, nitrergic and some other nerves. Acetylcholine, noradrenaline and tachykinins such as substance P and neurokinin A exert as excitatory neurotransmitters, whereas calcitonine gene-related peptide and nitric oxide (NO) act as inhibitory neurotransmitters (Santicioli & Maggi, 1998). Accordingly, in the present study, atropine and guanethidine inhibited EFS-induced contraction. Moreover, L-NA augmented these contractions, indicating that concomitant release of NO upon EFS may modulate the contractile effects of excitatory neurotransmitters. However, myogenic spontaneous contractile activity of the ureter is somewhat different. This contraction was not inhibited in the presence of TTX, excluding any neurogenic components. Neither was it affected by atropine or guanethidine, indicating that neither cholinergic nor adrenergic components are involved in this contractile activity. In support, it has been demonstrated that spontaneous waves of the renal pelvic smooth muscle are resistant to cholinergic, noradrenergic and neuronal blockers, suggesting that this activity is purely myogenic in origin (Seki & Suzuki, 1990). Finally, the NOS inhibitor, L-NA had no influence on spontaneous contraction, excluding any modulation of NO in the contractions. In addition, NO synthase blockers did not affect the motility of the guinea-pig (Maggi et al., 1995) and sheep ureter (Garcia-Pascual et al., 1996). It is know that functional capsaicin-sensitive sensory afferents and endogenous release of both tachykinins and prostanoids are essential in the maintenance of normal peristalsis (Lang et al., 2002). In confirmation, Patacchini et al. (1998) have reported that a tachykinin, neurokinin A, produced concentration-dependent contraction of human and guinea-pig ureter, which was inhibited by a selective NK2 receptor antagonist. In this study, however, we did not have any decisive findings over the main mechanism(s) of the myogenic spontaneous contractions. Nevertheless, since Y-27632 and fasudil had suppressor effects on the contractile activity, the Rho/Rho-kinase pathway seems to be involved in the spontaneous contractions, regardless of the nature of excitatory mediator(s) or upstream mechanisms. It has been proposed that there might be some putative pacemakers responsible for the initiation of peristaltic waves in the renal pelvis as well as ureteral smooth muscle (Maggi et al., 1988; Lammers et al., 1996). It has been reported that normal ureteral peristalsis is physiologically due to the myogenic properties of pyeloureteral smooth muscle; however, in pathological conditions such as those occurring during a passage of a stone or a bacterial infection, local efferent and function of capsaicin-sensitive sensory nerves may possibly affect ureteral peristalsis (Santicioli & Maggi, 1998). In the present study, however, we did not try any exogenously applied tachykininergic compounds like neurokinin A or substance P. Therefore, we did not examine the effect of Y-27632 and fasudil on these agents-induced contractions. However, it has been demonstrated that Rho/Rho-kinase signalling is also involved in the tachykinin receptor-induced signal transduction in the rat urinary bladder smooth muscle (Wibberley et al., 2003). Therefore, it is possible that Y-27632 and fasudil would be able to inhibit the effects of excitatory tachykinin agonists in the sheep ureteric smooth muscle.
With respect to the agonists, phenylephrine-induced contraction was suppressed by Y-27632 and fasudil in the sheep ureter, as shown in the mouse vas deferens (Büyükafşar et al., 2003), indicating that α-adrenoceptors could be involved in the Rho/Rho-kinase signalling. Indeed, it has been reported by many researchers that phenyephrine- and noradrenaline-induced contractions were dramatically inhibited by Rho-kinase inhibitors (see for reviews, Fukata et al., 2001; Wettschureck & Offermanns, 2002). Adrenaline, noradrenaline and phenylephrine produced concentration-dependent increases in both the phasic activity and basal tone of ureteral preparations, which were mediated by α-adrenoceptors (Hernandez et al., 1992). On the other hand, carbachol-induced contraction was also attenuated in the presence of Y-27632 and fasudil. This shows that cholinergic receptors may be coupled with this novel signalling pathway. In the isolated canine ureteric spiral preparation, it has been demonstrated that carbachol induced contraction, and this contraction could be mediated by M3 cholinergic receptor (Tomiyama et al., 2003). These are consistent with the data obtained in the EFS series, where atropine and guanethidine inhibited EFS-evoked contraction.
It has been known that Rho-signalling can be activated by the stimulation of heterotrimeric G protein-coupled receptors (Somlyo & Somlyo, 2000). However, in the present study, Y-27632 and fasudil relaxed the ureteral smooth muscle, which was contracted by KCl. It has been known that K+ stimulation induces an increase in intracellular free Ca2+ concentration of smooth muscles. Although we did not check whether KCl might cause the release of any excitatory neurotransmitters from the nerves supplying the tissue, it may act on the smooth muscle as in gastric fundus (Büyükafşar & Levent, 2003) and vas deferens (Büyükafşar et al., 2003). Consequently, Ca2+ entry may activate Rho-signalling pathway, like Ca2+-induced Ca2+ sensitization in smooth muscle cells. In order to ascertain this hypothesis, further studies are needed.
In conclusion, the results of this descriptive study indicate that Rho-kinase of both isoforms are expressed in the sheep ureteric smooth muscle, and it plays a substantial role in the spontaneous activity as well as the contractions induced by EFS, KCl, phenylephrine and carbachol. Since Y-27632 and fasudil have the marked inhibitions on these contractions, the ROCK inhibitors seem to be potential therapeutic agents in the renal colic treatment.
Acknowledgments
This work has been supported by the Turkish Academy of Sciences, in the framework of the Young Scientist Award Program (K.B./TÜBA-GEBİP/2002-1-5). We are indebted to N. Tiftik, H. Kubat and M. Ark for their some help in Western blotting.
Abbreviations
- ANOVA
one-way analysis of variance
- [Ca2+]i
intracellular Ca2+
- CCh
carbachol
- EFS
electrical field stimulation
- L-NA
NG-nitro-L-arginine
- NO
nitric oxide
- Phe
phenylephrine
- ROCK
Rho-kinase
- TTX
tetrodotoxin
- Y-27632
(+)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl) cyclohexanecarboxamide dihydrochloride monohydrate
References
- BRADLEY A.B., MORGAN K.G. Alterations in cytoplasmic calcium sensitivity during porcine coronary artery contractions as detected by aequorin. J. Physiol. (Lond.) 1987;385:437–448. doi: 10.1113/jphysiol.1987.sp016500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULTITUDE M.I. Capsaicin in treatment of loin pain/haematuria syndrome. Lancet. 1995;345:921–922. [PubMed] [Google Scholar]
- BÜYÜKAFŞAR K., ARIKAN O., ARK M., SEÇILMIŞ A., ÜN İ., ŞINGIRIK E. Rho-kinase expression and its contribution to the control of perfusion pressure in the isolated rat mesenteric vascular bed. Eur. J. Pharmacol. 2004;485:263–268. doi: 10.1016/j.ejphar.2003.11.076. [DOI] [PubMed] [Google Scholar]
- BÜYÜKAFŞAR K., LEVENT A. Involvement of Rho/Rho-kinase signaling in the contractile activity and acetylcholine release in the mouse gastric fundus. Biochem. Biophys. Res. Commun. 2003;303:777–781. doi: 10.1016/s0006-291x(03)00422-4. [DOI] [PubMed] [Google Scholar]
- BÜYÜKAFŞAR K., LEVENT A., ARK M. Expression of Rho-kinase and its functional role in the contractile activity of the mouse vas deferens. Br. J. Pharmacol. 2003;140:743–749. doi: 10.1038/sj.bjp.0705479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜYÜKAFŞAR K., ÜN İ. Effects of Rho-kinase inhibitors, Y-27632 and fasudil on the corpus cavernosum from diabetic mice. Eur. J. Pharmacol. 2003;472:235–238. doi: 10.1016/s0014-2999(03)01905-8. [DOI] [PubMed] [Google Scholar]
- CERVERO F. Sensory innervation of the viscera: peripheral bases of visceral pain. Physiol. Rev. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- FUKATA Y., MUTSUKI A., KAIBUCHI K. Rho–Rho-kinase pathway in smooth muscle contraction and cytoskeletal reorganisation of non-muscle cells. Trends Pharmaco.l Sci. 2001;22:32–39. doi: 10.1016/s0165-6147(00)01596-0. [DOI] [PubMed] [Google Scholar]
- GARCIA-PASCUAL A., COSTA G., LABADIA A., PERSSON K., TRIGUERO D. Characterization of nitric oxide synthase activity in sheep urinary tract: functional implications. Br. J. Pharmacol. 1996;118:905–914. doi: 10.1111/j.1476-5381.1996.tb15485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIAMBERARDINO M.A., VALENTE R., DE BIGONTINA P., IEZZI S., VECCHIET L. Effects of spasmolytic and/or non-steroidal antiinflammatory drugs on muscle hyperalgesia of ureteral origin in rats. Eur. J. Pharmacol. 1995a;278:97–101. doi: 10.1016/0014-2999(95)00104-s. [DOI] [PubMed] [Google Scholar]
- GIAMBERARDINO M.A., VALENTE R., DE BIGONTINA P., VECCHIET L. Artificial ureteral calculosis in rats: behavioural characterization of visceral pain episodes and their relationship with referred lumbar muscle hyperalgesia. Pain. 1995b;61:459–469. doi: 10.1016/0304-3959(94)00208-V. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ M., PRIETO D., SIMONSEN U., RIVERA L., BARAHONA M.V., GARCIA-SACRISTAN A. Noradrenaline modulates smooth muscle activity of the isolated intravesical ureter of the pig through different types of adrenoceptors. Br. J. Pharmacol. 1992;107:924–931. doi: 10.1111/j.1476-5381.1992.tb13387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZAKI T., UEHATA M., TAMECHIKA I., KEEL J., NONOMURA K., MAEKAWA M., NARUMIYA S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]
- KAMM K.E., STULL J.T. The function of myosin and myosin light chain kinase phosphorylation in smooth muscle. Annu. Rev. Pharmacol. Toxicol. 1985;25:593–603. doi: 10.1146/annurev.pa.25.040185.003113. [DOI] [PubMed] [Google Scholar]
- LAIRD J.M.A., ROSA C., CERVERO F. Effects of artificial calculosis on rat ureter motility: peripheral contribution to the pain of ureteral colic. Am. J. Physiol. 1997;41:R1409–R1416. doi: 10.1152/ajpregu.1997.272.5.R1409. [DOI] [PubMed] [Google Scholar]
- LAMMERS W.J.E.P., AHMAD H.R., ARAFAT K. Spatial and temporal variations in pacemaking and conduction in the isolated renal pelvis. Am. J. Physiol. 1996;270:F567–F574. doi: 10.1152/ajprenal.1996.270.4.F567. [DOI] [PubMed] [Google Scholar]
- LANG R.J., DAVIDSON M.E., EXINTARIS B. Pyeloureteral motility and ureteral peristalsis: essential role of sensory nerves and endogenous prostaglandins. Exp. Physiol. 2002;87:129–146. doi: 10.1113/eph8702290. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., PARLANI M., ASTOLFI M., SANTICIOLI P., ROVERO P., ABELLI V., SOMMA V., GIULIANI S., REGOLI D., PATACCHINI R., MELI A. Neurokinin receptors in the rat lower urinary tract. J. Pharmacol. Exp. Ther. 1988;246:308–315. [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., GIULIANI S. Role of cyclic AMP and protein kinase A in K channel activation by CGRP in the guinea-pig ureter. J. Autonom. Pharmacol. 1995;15:403–419. doi: 10.1111/j.1474-8673.1995.tb00406.x. [DOI] [PubMed] [Google Scholar]
- PATACCHINI R., SANTICIOLI P., ZAGORODNYUK V., LAZZERI M., TURINI D., MAGGI C.A. Excitatory motor and electrical effects produced by tachykinins in the human and guinea-pig isolated ureter and guinea-pig renal pelvis. Br. J. Pharmacol. 1998;125:987–996. doi: 10.1038/sj.bjp.0702147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANTICIOLI P., MAGGI C.A. Myogenic and neurogenic factors in the control of pyeloureteral motility and ureteral peristalsis. Pharmacol. Rev. 1998;50:683–721. [PubMed] [Google Scholar]
- SEKI N., SUZUKI H. Electrical properties of smooth muscle cell membrane in renal pelvis of rabbits. Am. J. Physiol. 1990;259:F888–F894. doi: 10.1152/ajprenal.1990.259.6.F888. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. doi: 10.1038/372231a0. [DOI] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Signal transduction by G-proteins, Rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J. Physiol. (Lond.) 2000;522:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMLYO A.P., SOMLYO A.V. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol. Rev. 2003;83:1325–1358. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- SWARD K., DREJA K., SUSNJAR M., HELLSTRAND P., HARTSHORNE D.J., WALSH M.P. Inhibition of Rho-associated kinase blocks agonist-induced Ca2+ sensitization of myosin phosphorylation and force in guinea-pig ileum. J. Physiol. 2000;522:33–49. doi: 10.1111/j.1469-7793.2000.0033m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAHARA M., MORISHIGE K., SAWADA K., IKEBUCHI Y., KAWAGISHI R., TASAKA K., MURATA Y. RhoA/Rho-kinase cascade is involved in oxytocin-induced rat uterine contraction. Endocrinology. 2002;143:920–929. doi: 10.1210/endo.143.3.8696. [DOI] [PubMed] [Google Scholar]
- TOMIYAMA Y., WANAJO I., YAMAZAKI Y., MURAKAMI M., KOJIMA M., SHIBATA N. Functional muscarinic cholinoceptors in the isolated canine ureter. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003;367:348–352. doi: 10.1007/s00210-003-0697-4. [DOI] [PubMed] [Google Scholar]
- UEHATA M., ISHIZAKI T., SATOH H., ONO T., KAWAHARA T., MORISHITA T., TAMAKAWA H., YAMAGAMI K., INUI J., MAEKAWA M., NARUMIYA S. Calcium sensitization of smooth muscle mediated by a Rho-associated protein kinase in hypertension. Nature. 1997;389:990–994. doi: 10.1038/40187. [DOI] [PubMed] [Google Scholar]
- WETTSCHURECK N., OFFERMANNS S. Rho/Rho-kinase signalling in physiology and pathophysiology. J. Mol. Med. 2002;80:629–638. doi: 10.1007/s00109-002-0370-2. [DOI] [PubMed] [Google Scholar]
- WIBBERLEY A., CHEN Z., HU E., HIEBLE J.P., WESTFALL T.D. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. Br. J. Pharmacol. 2003;138:757–766. doi: 10.1038/sj.bjp.0705109. [DOI] [PMC free article] [PubMed] [Google Scholar]