Abstract
Chronic alcohol exposure modifies endocannabinoid levels in different brain regions, while pharmacological targeting of the endocannabinoid system has been reported to influence ethanol intake in laboratory animals.
The present study was aimed at evaluating the pattern of changes of endocannabinoids and their receptors, with emphasis on reward-related brain areas, in Wistar rats subjected to consecutive phases of alcoholization, alcohol deprivation (abstinence), and voluntary consumption of alcohol (relapse).
We observed that, in the limbic forebrain, anandamide (AEA) and 2-arachidonoylglycerol (2-AG) contents increased after 7 days of alcoholization, then to dramatically decrease after 48 h of alcohol deprivation and, in the case of 2-AG, to further decrease when rats were allowed to relapse to alcohol consumption. By contrast, in the midbrain, there was a marked reduction in AEA, but not 2-AG, content, after alcoholization. This decrease was not affected during alcohol abstinence, but both AEA and 2-AG contents were then significantly reduced when rats were allowed to relapse to alcohol consumption.
Based on these data, we examined whether pharmacological activation/blockade of endocannabinoid transmission might influence ethanol intake in rats allowed to relapse to alcohol consumption after subsequent periods of alcoholization and alcohol deprivation.
Treatment with either Δ9-tetrahydrocannabinol or CP55,940, two cannabinoid agonists, reduced both total liquid and ethanol intake but did not affect ethanol preference. Treatment with SR141716, a selective cannabinoid CB1 receptor antagonist, also produced a significant reduction in both total liquid and ethanol intake without affecting ethanol preference. Accordingly, none of these effects on ethanol intake were accompanied by changes in dopamine and GABA in limbic structures.
In summary, the levels of endocannabinoids underwent significant changes in reward-related areas during alcoholization, alcohol deprivation, and relapse, showing the lowest values in this latter phase. Treatment with cannabinoid agonists or a selective CB1 receptor antagonist resulted in a reduction of ethanol intake by rats allowed to relapse to alcohol consumption after periods of alcoholization and alcohol deprivation, but these effects did not appear to be due to changes in neurobiological substrates currently involved in alcohol reinforcement/relapse.
Keywords: Endocannabinoids, CB1 receptors, reward-related regions, alcohol addiction, alcohol abstinence, alcohol intake
Introduction
Alcohol is possibly the habit-forming drug that has recently been more studied for its relationships with the endocannabinoid signaling system (for a review, see Hungund & Basavarajappa, 2000a). This can be concluded from genetic studies that have proved a greater frequency for the appearance of a genetic polymorphism for the cannabinoid CB1 receptor in several subpopulations of alcoholic patients, in particular in alcoholics with severe withdrawal signs, such as delirium or seizures (Schmidt et al., 2002), or with antecedents of childhood attention deficit/hyperactivity (Ponce et al., 2003); and also from biochemical studies that examined the effects of alcohol exposure on endocannabinoid signaling in laboratory animals or cultured nerve cells (Basavarajappa et al., 1998, 2000; Basavarajappa & Hungund, 1999a, 1999b, 2001; Hungund & Basavarajappa, 2000b; González et al., 2002a, 2002b). These latter studies allowed to postulate that hyperactivity of endocannabinoid transmission might be involved in the addictive properties of alcohol (for a review, see Basavarajappa & Hungund, 2002). This hypothesis was confirmed by studying voluntary alcohol consumption in CB1 receptor knockout mice (Hungund et al., 2003; Wang et al., 2003; Racz et al., 2003) or in wild rodents administered with SR141716, a selective CB1 receptor antagonist (Arnone et al., 1997; Colombo et al., 1998; Freedland et al., 2001; Serra et al., 2001; Wang et al., 2003; see Basavarajappa & Hungund, 2002, for a recent review). However, most of these last pharmacological data were obtained in rodent strains genetically selected for their high ethanol preference and, moreover, they mainly addressed the reinforcing properties of alcohol during the first contact with this substance. By contrast, only very few studies have been carried out in rat strains with no special preference for ethanol (Gallate & McGregor, 1999; Lallemand et al., 2001), while there is no data on the effects during alcohol relapse. Interestingly, Lallemand et al. (2001) have recently reported that, in nonpreferring rats, the time of SR141716 administration might be crucial to determine its beneficial effects in alcohol dependence. On the other hand, we recently described that chronic alcohol exposure in rats resulted in marked changes in the levels of anandamide (AEA) and 2-arachidonoyl-glycerol (2-AG) in the limbic forebrain and the midbrain (ventral-tegmental area), two key regions for drug reinforcement processes (González et al., 2002b). However, no data have been reported yet on the status of the endocannabinoid transmission in these limbic structures or in other brain regions in conditions of alcohol abstinence or relapse.
Based on these previous data, in the present study, we first evaluated the possible changes in the levels of endocannabinoids and their receptors in reward-related brain regions, using a classic model of alcohol addiction developed in rats with variable preference for alcohol (reviewed recently by Lê & Shaham, 2002). This model includes the analysis of three consecutive phases: (i) forced chronic exposure (alcoholization), (ii) alcohol deprivation (abstinence), and (iii) voluntary consumption of alcohol (relapse). This objective was pursued to provide further support to the hypothesis that the status of the endocannabinoid signaling system might be a determinant of ethanol intake/preference (reviewed recently by Basavarajappa & Hungund, 2002). As a second objective, and based on the results obtained in the first part of the study, we explored in the same animal model the therapeutic potential of the endocannabinoid system for the treatment of alcohol relapse, by examining whether pharmacological activation or blockade of the endocannabinoid signaling would influence ethanol intake in rats allowed to relapse to alcohol consumption after subsequent periods of alcoholization and deprivation. We also wanted to elucidate whether these potential effects might be related to changes in classic neurochemical substrates involved in drug reinforcement/reinstatement, such as dopaminergic and/or GABAergic transmission in limbic structures (McBride & Li, 1998; Lê & Shaham, 2002). For all these experiments, in order to better mimic the situation occurring in the human population, we used rats that had not been genetically selected for their ethanol preference and that were proportionally assigned to the different experimental groups.
Methods
Animals, treatments, and sampling
Male Wistar rats were housed in a room with controlled photoperiod (08:00–20:00 darkness; experiments were always conducted in the dark phase and under red light) and temperature (23±1°C). They had free access to standard food and water and were used at adult age (3 month-old; 250–300 g weight) for two different experiments conducted according to European rules (directive 86/609/EEC). The first experiment addressed the analysis of endocannabinoid ligands and their receptors in rats subjected to consecutive phases of alcoholization, alcohol deprivation (abstinence), and voluntary consumption of alcohol (relapse), following a classic method to induce alcoholism in rats that do not have a special preference for alcohol (for a review, see McBride & Li, 1998; Koob, 2000; Lê & Shaham, 2002). To this end, rats were housed individually and allowed to drink ethanol (7.2% v v−1) in the liquid diet for a period of 7 days (forced chronic alcohol group). The alcohol solution was prepared daily and presented at the same time of the day (10:00 h), and the animals had access to this solution all over the day. On day 7 after the onset of this treatment, the animals were passed to drink water alone for a period of 2 weeks (alcohol abstinence group), and, afterwards, they were allowed to reinstate alcohol consumption by placing the animals in a paradigm of free choice of either water or ethanol (alcohol relapse group). The length of alcoholization and alcohol deprivation periods, which can determine the efficacy of reinstatement of alcohol-drinking behavior in rats that do not have a special preference for alcohol, was chosen according to the data recently reviewed by Lê & Shaham (2002). Animals were rapidly and carefully decapitated at selected days of these three phases: (i) after 7 days of forced alcoholization, (ii) after 48 h of being passed to drink water alone (abstinent rats), (iii) after 24 h of being passed to a paradigm of voluntary consumption of ethanol or water (alcohol relapsing rats), and (iv) controls (animals allowed to drink water alone). The times for decapitation in each phase were selected according to previous studies demonstrating that important molecular and behavioral changes associated with alcoholization and alcohol deprivation occurred at these times, and, in the case of alcohol relapse, because this response is progressively extinguished as a function of time (see details in Lê & Shaham, 2002). After decapitation, brains were quickly and carefully removed. Those brains to be used for analysis of endocannabinoids were used, before freezing, to dissect the limbic forebrain and the midbrain, which were then rapidly frozen in dry ice. Those brains to be used for analysis of CB1 receptors were rapidly frozen by immersion in 2-methyl-butane in dry ice. Samples were stored at −80°C until processed. In the second experiment, we used rats allowed to relapse to alcohol consumption after subsequent periods of alcoholization and abstinence, following the same model of alcohol exposure and deprivation than for the first objective. Therefore, 24 h after passing the rats to this paradigm, we analyzed ethanol and water intake and animals were assigned proportionally to the different treatment groups: (i) treated with vehicle (Tween 80-saline solution), (ii) treated with an acute dose of Δ9-tetrahydrocannabinol (Δ9-THC) (3 mg kg−1), (iii) treated with an acute dose of CP55,940 (0.1 mg kg−1, a dose that does not produce motor impairment (et al., 2003)), (iv) treated with an acute dose of SR141716 (3 mg kg−1), and (v) treated with an acute dose of SR141716 (3 mg kg−1) combined with each of the two cannabinoid agonists, Δ9-THC (3 mg kg−1) or CP55,940 (0.1 mg kg−1). All injections were carried out i.p. and the doses were similar to those used in the previous pharmacological experiments to test the effects on ethanol intake (see references in the Introduction). Liquid intake (either ethanol or water) or ethanol preference (volume of ethanol intake/volume of total liquid intake) were measured after 6 h of the corresponding treatment, and used to calculate, for each animal, the percentage of variation versus the same parameter measured the day before treatment. To control that the potential changes in liquid intake induced by cannabinoid agonists are not indirectly caused by their well-known capabilities to depress motor behavior, a separate group of animals treated with Δ9-THC was also analyzed in the open-field test (et al., 2003). Afterwards, they were rapidly and carefully decapitated, their brains quickly and carefully removed and rapidly frozen by immersion in 2-methyl-butane in dry ice. All samples were stored at −80°C until processed for analysis of GABA, dopamine (DA), and related enzymes.
Measurement of endocannabinoid contents
Procedure of extraction
Pools for each brain region, in amount ranging from 0.5 to 3 g wet weight, were extracted immediately after killing to avoid the post-mortem rise in the concentrations of long-chain N-acylethanolamines, occurring approximately 30 min after the killing when tissue is left at room temperature (Schmid et al., 1995). The tissue was homogenized in 5 volumes of chloroform/methanol/Tris HCl 50 mM (2 : 1 : 1) containing 1 nmol of d8-AEA and d8-2-AG, synthesized from d8 arachidonic acid and ethanolamine or glycerol as described, respectively, in Devane et al. (1992) and Bisogno et al. (1997). Homogenates were centrifuged at 13000 × g for 16 min (4°C), the acqueous phase plus debris were collected and extracted again twice with 1 volume of chloroform. The organic phases from the three extractions were pooled and the organic solvents evaporated in a rotating evaporator. Lyophilized samples were then stored frozen at −80°C under nitrogen atmosphere until analysis.
Analysis of endocannabinoid contents by gas chromatography/mass spectrometry (GC-MS)
Lyophilized extracts were resuspended in chloroform/methanol 99 : 1 by vol. The solutions were then purified by open-bed chromatography on silica as described in Fontana et al. (1995). Fractions eluted with chloroform/methanol 9 : 1 by vol. (containing AEA and 2-AG) were further fractionated by normal phase-high-pressure liquid chromatography (NP-HPLC) carried out using a semipreparative silica column (Spherisorb S5W, Phase Sep, Queensferry, CLWYD, U.K.) eluted under conditions allowing the separation of 1(3)- and 2-acyl-glycerols (retention time of 18 and 20 min, respectively) from N-acylethanolamines (retention time=26–27 min) (Bisogno et al., 1997). NP-HPLC fractions containing AEA and 2-AG were derivatized with 20 μl N-methyl-N-trimethylsilyl-trifluoroacetamide+1% trimethylchlorosylane for 2 h at room temperature and analyzed by GC-MS carried out under conditions described previously (Bisogno et al., 1997) and allowing the separations of monoacylglycerols or N-acylethanolamines with different fatty acid chains. MS detection was carried out in the selected ion monitoring mode using m/z values of 427 and 419 (molecular ions for deuterated and undeuterated AEA), 412 and 404 (loss of 15 mass units from deuterated and undeuterated AEA), 530 and 522 (molecular ions for deuterated and undeuterated 2-AG), and 515 and 507 (loss of 15 mass units from deuterated and undeuterated 2-AG). The amounts of endocannabinoids were quantified by isotope dilution (González et al., 2002b) and expressed as pmols or nmols per gram of wet tissue extracted. Further details on the GC/MS technique, including the detection and quantification limits, have been published (De Petrocellis et al., 1999).
Autoradiographic techniques
Coronal sections 20 μm-thick were cut in a cryostat, according to Paxinos & Watson (1986) atlas. The sections were thaw-mounted onto RNAse-free gelatin/chrome alum-coated slides and dried briefly at 30°C and stored at −80°C until used. They were used to analyze CB1 receptor binding according to the autoradiographic method described by Herkenham et al. (1991), using 10 nM [3H]-CP-55,940 (Du Pont NEN) as radioactive ligand. The analysis of CB1 receptor mRNA levels was carried out according to the in situ hybridization method described by Rubino et al. (1994), using a mixture (1 : 1 : 1) of the three 48-mer oligonucleotide probes complementary to bases 4–51, 349–396, and 952–999 of the rat CB1 receptor cDNA (Du Pont) labelled with [35S]dATP. In all cases, autoradiograms were generated by apposing the labelled tissues to appropriate sensitive films for specific periods of time, and then developed (D-19, Kodak). Developed films were analyzed by measuring the grey levels in the films with a computer-assisted videodensitometer (see details in González et al., 2002a).
Determinations of GABA and DA indices by HPLC with electrochemical detection
Brains were used to manually obtain coronal slices (around 500 μm thick) at levels containing the substantia nigra/ventral tegmental area, or the caudate-putamen/nucleus accumbens (Palkovits & Brownstein, 1988). Subsequently, the four structures were dissected and homogenized in 20–40 vol of cold 150 mM potassium phosphate buffer, pH 6.8. Each homogenate was distributed for the analysis, using well-described HPLC methods coupled to electrochemical determination, of: (i) GABA contents (Smith & Sharp, 1994), (ii) glutamate decarboxylase (GAD) activity (Nicoletti et al., 1985), (iii) DA and DOPAC contents (Romero et al., 1995; González et al., 1999), and (iv) tyrosine hydroxylase (TH) activity (Nagatsu et al., 1979). Details of these methods have been previously published (et al., 2003). An aliquot of each homogenate was used to analyze protein concentration (Lowry et al., 1951).
Statistics
Data were assessed by one-way analysis of variance followed by the Bonferroni multiple comparison test.
Results
Experiment. I: Fluctuations of endocannabinoid transmission in the rat brain during alcoholization, alcohol abstinence, or alcohol relapse
The objective of the first group of experiments was to evaluate the pattern of changes in endocannabinoids and their receptors, in reward-related brain regions, in rats subjected to consecutive phases of alcoholization, alcohol deprivation (abstinence), and voluntary consumption of alcohol (relapse). The results showed that CB1 receptor binding (Table 1) and mRNA levels (Table 2) did not vary in most of the brain regions analyzed during these three phases, except in the case of the superficial layer of the cerebral cortex for binding (F(3,23)=3.982, P<0.05), and the medial caudate-putamen (F(3,23)=7.086, P<0.005), the superficial (F(3,23)=5.025, P<0.01) and deep (F(3,23)=6.946, P<0.05) layers of the cerebral cortex, the ventromedial hypothalamic nucleus (F(3,23)=4.933, P<0.01), and the hippocampus (CA1: F(3,23)=10.94, P<0.0005; dentate gyrus: F(3,23)=19.46, P<0.0001), for mRNA levels. In most of these regions, values of CB1 receptors were always higher in the phase of alcohol relapse compared with the controls and the other two phases, or with some of them (Tables 1 and 2), except in the case of the ventromedial hypothalamic nucleus where the maximal value for CB1 receptor-mRNA levels was found during the abstinence phase (Table 2). Another aspect deserving comments is that CB1 receptor binding (Table 1) and mRNA levels (Table 2) did not show parallel changes along these phases, as it has been previously reported in other physiological or pathological conditions (Berrendero et al., 1999; Romero et al., 2000; González et al., 2002a).
Table 1.
CB1 receptor binding (fmol mg−1 of tissue) in different brain regions of rats during chronic exposure (alcoholization), abstinence, or voluntary consumption (relapse) of ethanol
| Brain region | Controls | Alcohol | ||
|---|---|---|---|---|
| Chronic exposure | Abstinence | Voluntary consumption | ||
| Limbic structures | ||||
| Nucleus accumbens | 85.9±8.1 | 75.0±11.0 | 80.3±8.5 | 65.9±8.7 |
| Basal ganglia | ||||
| Lateral caudate-putamen | 159.4±9.2 | 148.0±8.8 | 137.2±11.0 | 164.5±11.2 |
| Medial caudate-putamen | 119.5±7.9 | 108.8±10.3 | 107.0±8.2 | 111.8±12.9 |
| Globus pallidus | 136.7±11.6 | 165.2±24.2 | 146.9±8.8 | 145.7±9.7 |
| Entopeduncular nucleus | 179.2±20.4 | 191.6±16.5 | 173.3±10.6 | 136.9±28.7 |
| Substantia nigra | 214.9±26.2 | 203.4±17.6 | 195.1±22.8 | 217.7±14.9 |
| Hippocampus | ||||
| Ammon's horn (CA1) | 91.5±4.9 | 95.9±8.0 | 84.0±9.1 | 87.4±2.6 |
| Ammon's horn (CA2) | 90.3±4.1 | 90.6±6.9 | 79.2±10.1 | 87.9±2.1 |
| Ammon's horn (CA3) | 76.8±1.7 | 79.4±6.1 | 72.8±7.3 | 73.1±3.1 |
| Dentate gyrus | 111.9±8.3 | 117.2±5.6 | 101.9±9.7 | 114.5±2.1 |
| Cerebral cortex | ||||
| Superficial layer (I–II) | 75.3±6.0 | 49.1±10.9 | 76.2±9.5 | 83.9±5.5* |
| Deep layer (V–VI) | 85.7±8.4 | 75.8±7.5 | 75.5±7.2 | 86.4±2.8 |
| Other regions | ||||
| Cerebellum | 269.0±9.3 | 267.1±17.2 | 251.0±19.5 | 286.3±11.9 |
Data are expressed as means±s.e.m. of 4–6 determinations per group. Data were assessed by one-way analysis of variance followed by the Bonferroni multiple comparison test (*P<0.05 versus chronic exposure).
Table 2.
CB1 receptor mRNA levels (optical density) in different brain regions of rats during chronic exposure (alcoholization), abstinence, or voluntary consumption (relapse) of ethanol
| Brain region | Controls | Alcohol | ||
|---|---|---|---|---|
| Chronic exposure | Abstinence | Voluntary consumption | ||
| Limbic structures | ||||
| Septum nuclei | 0.51±0.04 | 0.41±0.03 | 0.51±0.11 | 0.56±0.01 |
| Basolateral amygdala | 0.58±0.11 | 0.52±0.03 | 0.61±0.03 | 0.65±0.05 |
| Basal ganglia | ||||
| Lateral caudate-putamen | 0.53±0.04 | 0.48±0.02 | 0.60±0.10 | 0.57±0.02 |
| Medial caudate-putamen | 0.31±0.02a | 0.31±0.03ab | 0.42±0.02b | 0.40±0.03b |
| Hippocampus | ||||
| Ammon's horn (CA1) | 0.58±0.02ab | 0.55±0.02a | 0.51±0.02a | 0.64±0.02b |
| Ammon's horn (CA2) | 0.70±0.04 | 0.59±0.04 | 0.58±0.05 | 0.71±0.05 |
| Ammon's horn (CA3) | 0.60±0.03 | 0.60±0.04 | 0.59±0.02 | 0.63±0.05 |
| Dentate gyrus | 0.55±0.01a | 0.55±0.02a | 0.59±0.02a | 0.69±0.02b |
| Cerebral cortex | ||||
| Superficial layer (II–III) | 0.25±0.01a | 0.29±0.05ab | 0.38±0.05ab | 0.41±0.03b |
| Deep layer (V–VI) | 0.27±0.02a | 0.27±0.03a | 0.38±0.05b | 0.39±0.02b |
| Other regions | ||||
| Ventromedial hypothalamic nucleus | 0.57±0.06a | 0.62±0.05ab | 0.72±0.02b | 0.68±0.02ab |
| Cerebellum | 1.15±0.05 | 1.05±0.04 | 1.17±0.04 | 1.02±0.05 |
| Habenula | 0.54±0.03 | 0.55±0.05 | 0.60±0.05 | 0.59±0.05 |
Data are expressed as means±s.e.m. of 4–6 determinations per group. Data were assessed by one-way analysis of variance followed by the Bonferroni multiple comparison test (different letters between two groups mean that their differences reached statistical significance: P<0.05).
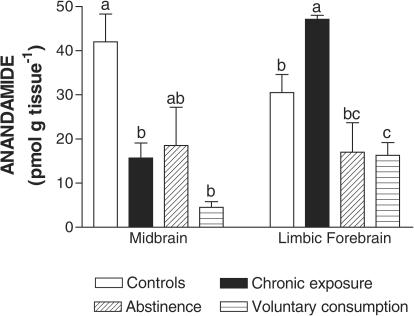
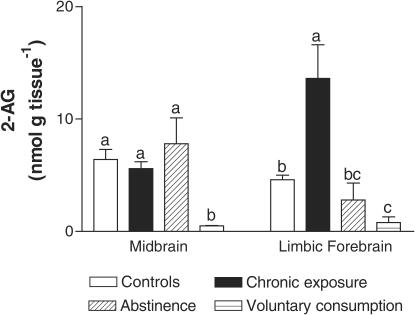
In contrast with the small magnitude of changes observed for CB1 receptors, the concentrations of endocannabinoids were markedly altered in the two reward-related brain regions under investigation, the limbic forebrain and the midbrain, during the phases of alcoholization, alcohol deprivation, and alcohol relapse in laboratory animals. Thus, in the limbic forebrain, AEA (F(3,15)=11.82, P<0.001) and, in particular, 2-AG (F(3,15)=10.95, P<0.001) contents increased after 7 days of alcoholization, but the contents of both endocannabinoids dramatically decreased after 48 h of alcohol abstinence and these reductions were stronger, in the case of 2-AG, when animals were allowed to reinstate ethanol consumption (Figures 1 and 2). By contrast, in the midbrain, there was a marked reduction in AEA (F(3,15)=7.721, P<0.005), but not 2-AG, contents, after alcoholization (Figures 1 and 2). This decrease was not affected during alcohol deprivation, but both AEA and, in particular, 2-AG (F(3,15)=6.27, P<0.01) contents were significantly reduced when animals were passed to a schedule of voluntary consumption allowing ethanol relapse (Figures 1 and 2).
Figure 1.
Anandamide contents in the limbic forebrain and midbrain of rats during chronic exposure (alcoholization), abstinence, or voluntary consumption (relapse) of ethanol. See details in the text. Data are expressed as means±s.e.m. of four determinations per group and were assessed by one-way analysis of variance followed by the Bonferroni multiple comparison test (different letters between two groups mean that their differences reached statistical significance: P<0.05).
Figure 2.
2-Arachidonoylglycerol contents in the limbic forebrain and midbrain of rats during chronic exposure (alcoholization), abstinence, or voluntary consumption (relapse) of ethanol. See details in the text. Data are expressed as means±s.e.m. of four determinations per group and were assessed by one-way analysis of variance followed by the Bonferroni multiple comparison test (different letters between two groups mean that their differences reached statistical significance: P<0.05).
Experiment II: Effects of Δ9-THC, CP55,940, or SR141716 on alcohol relapse in abstinent rats
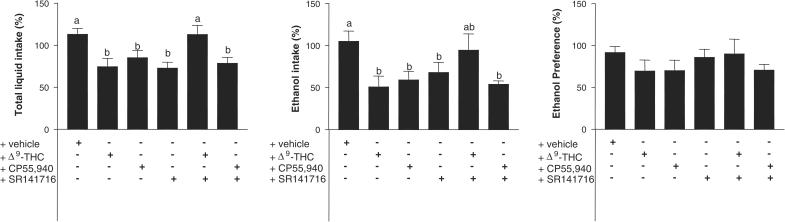
Since we observed profound reductions in endocannabinoid levels in the ‘relapse' group, we examined whether pharmacological activation or blockade of cannabinoid receptors might influence ethanol intake and/or preference in rats allowed to relapse to ethanol consumption after previous consecutive phases of alcoholization and alcohol deprivation. The treatment with either Δ9-THC or CP55,940 reduced both total liquid (F(5,67)=5.224, P<0.0005) and ethanol (F(5,66)= 2.53, P<0.05) intake but it did not affect ethanol preference (F(5,67)=0.606, ns; see Figure 3). These effects were not the consequence of the well-known capability of both cannabinoid agonists to produce motor impairment, since, in the case of CP55,940, we used a dose that does not alter open-field response in rats (et al., 2003), and in the case of Δ9-THC, we controlled this response and observed that this cannabinoid did not alter ambulation (+vehicle: 102.0±10.3 (n=6) versus + Δ9-THC: 76.8±20.5 (n=6), t=1.098, ns) and inactivity (+vehicle: 10.6±7.1 (n=6) versus + Δ9-THC: 24.3±13.8 (n=6), t=0.931, ns). The treatment with SR141716, a selective cannabinoid CB1 receptor antagonist, also produced a significant reduction in both total liquid and ethanol intake and did not affect ethanol preference (Figure 3). We observed no potentiation of the lowering effects on ethanol intake when agonists and antagonists were coadministrated, thus suggesting that they might be acting through different subpopulations of cannabinoid receptors. Thus, the coadministration of both SR141716 and Δ9-THC abolished the effects observed with both compounds alone, whereas the coadministration of SR141716 and CP55,940 produced an effect similar to that produced by each compound when administered alone (Figure 3).
Figure 3.
Voluntary liquid and ethanol intake, and ethanol preference, in abstinent rats allowed to reinstate alcohol-drinking behavior and administered with Δ9-THC, CP55,940, or SR141716, alone or in combination. See details on treatments in the text. Data correspond to the percentage of intake or preference after treatment versus the data collected the day before treatment, and are expressed as means±s.e.m. of more than six determinations per group. Data were assessed by one-way analysis of variance followed by the Bonferroni multiple comparison test (different letters between two groups mean that their differences reached statistical significance: P<0.05).
Based on the fact that reinstatement of alcohol-drinking behavior in animals with a previous story of alcoholism has been related to substrates typically involved in drug reinforcement, such as DA or GABA transmission in reward-related areas (Lê & Shaham, 2002), we explored whether the above effects described for cannabinoid-based compounds in ethanol intake and/or preference might be related to changes in the activity of these two neurotransmitters. In our hands, neither of the two agonists, nor the antagonist were able to modify the activity of GABA or DA observed in vehicle-treated animals in the nucleus accumbens (GABA contents: 2.9±0.3 μg mg−1; GAD activity: 119.2±19.9 μg mg−1 h−1; DA contents: 54.3±6.4 ng mg−1; TH activity: 292.9±45.3 ng mg−1 h−1) and the ventral-tegmental area (GABA contents: 2.6±0.3 μg mg−1; GAD activity: 42.9±7.3 μg mg−1 h−1; DA contents: 15.1±1.1 ng mg−1; TH activity: 353.5±38.1 ng mg−1 h−1). We also analyzed these neurochemical parameters in motor-related areas in order to discard any potential effect of cannabinoid-based compounds, which might have influenced their effects on the ethanol intake and/or preference. In concordance with the absence of effects on ambulation and inactivity, we did not observe any significant changes in dopaminergic and GABAergic activities in the basal ganglia (caudate-putamen and substantia nigra) (data not shown).
Discussion
The evidence relating alcohol and cannabinoids is relatively recent, despite the fact that these are two drugs that are frequently consumed together by humans (for a review, see Smart & Ogborne, 2000), and is based on recent genetic, pharmacological, and biochemical data that have been largely detailed in the Introduction. Despite this evidence, however, many questions remain unanswered, some of them being explored in the present study.
Status of the endocannabinoid transmission during alcohol addiction
As a first objective, we wanted to examine the potential changes in endocannabinoid transmission in conditions of alcohol deprivation or of reinstatement of alcohol-drinking behavior, conditions that are neurochemically and behaviorally different from the situation of chronic alcohol exposure, the only one examined in previous studies (see the Introduction for key references). The analysis of endocannabinoid contents and their receptors in the phase of alcoholization yielded results mostly similar to those of our previous studies (González et al., 2002a, 2002b). Thus, chronic exposure to alcohol resulted in an increase of both endocannabinoids in the limbic forebrain, whereas AEA, but not 2-AG, decreased in the midbrain, as observed and largely discussed in our previous study (González et al., 2002b). Also, as in that previous study (González et al., 2002b), we did not find here any differences in CB1 receptors between rats chronically exposed to alcohol and controls. As will be discussed later, this increase in endocannabinoid levels might play a role in reinforcing potential of alcohol. The interruption of alcoholization resulted, in the limbic forebrain, in the reversal of the increase of endocannabinoids measured during the previous phase. Endocannabinoid levels were reduced to values similar to, or even lower than, controls, while this did not occur in the midbrain, where AEA levels remained similar to those seen during alcoholization. These observations might be indicative that endocannabinoids in the limbic forebrain might also be associated with the occurrence of somatic and/or neurovegetative signs of alcohol abstinence. We did not record the occurrence of withdrawal signs in our study, but other authors have reported that rats subjected to alcohol deprivation after a prolonged period of alcoholization exhibit, at times comparable to those used here, responses such as anxiety, hyperlocomotion, hyperexcitability, and other signs that are at the same time characteristic of alcohol abstinence (for a review, see Becker, 2000) and of a decreased endocannabinoid tone. However, Racz et al. (2003) recently reported that withdrawal symptoms after the cessation of chronic ethanol administration, rather than being enhanced, are absent in CB1 receptor knockout mice. Therefore, the demonstration of a relationship between endocannabinoid levels and alcohol withdrawal signs remains an issue to be investigated in future experiments.
When abstinent animals were allowed to reinstate alcohol-drinking behavior, the levels of both endocannabinoids were dramatically reduced in the midbrain, and only those of 2-AG in the limbic forebrain, supporting the existence of region-dependent differences in the regulation and, possibly, action of the two major endocannabinoids (see below). This decrease in endocannabinoid levels was rather unexpected, in particular, for the limbic forebrain, since it suggests that alcohol exposure is associated with opposing endocannabinoid activities in this area depending on whether alcohol consumption is forced, as in alcohol-naive rats (enhancement), or it follows the free choice between water and alcohol, as in abstinent rats (reduction). This finding supports the notion, proposed by several authors (reviewed by Basavarajappa & Hungund, 2002), that the interactions between the output of the endocannabinoid signaling system and the alcohol-drinking behavior are quite complex.
An interesting fact is that, in the three phases examined, we always recorded differences in the response of AEA as compared to 2-AG, particularly in the midbrain. This presumably indicates a slightly different role in reward processes for either endocannabinoid, as reported for other neurobiological functions (see Di Marzo et al., 1998, for a review), as well as in other studies carried out with chronic treatment with other substances of abuse (Di Marzo et al., 2000; González et al., 2002b; Viganò et al., 2003). Since alcohol appears to affect the concentrations of endocannabinoids at the level of both their biosynthesis and inactivation in isolated neurons (Basavarajappa & Hungund, 1999b; Basavarajappa et al., 2003), these differential effects might be produced through a region-selective modulation of the proteins involved in endocannabinoid anabolic and catabolic processes, which are different for AEA and 2-AG. Importantly, the levels of AEA (and, to some extent, 2-AG) appeared to change in a complementary way in the limbic forebrain and midbrain during the first two phases, suggesting a possible regulatory feedback mechanism between endocannabinoid neurons in these two areas. Indeed, the activity of dopaminergic neurons running from the ventral-tegmental area (included in the midbrain) to the nucleus accumbens (included in the limbic forebrain) might be under the direct control of the endocannabinoid system, since a recent study by Wenger et al. (2003) has demonstrated for the first time colocalization of tyrosine hydroxylase and CB1 receptors in these structures. These dopaminergic neurons are known to be implicated in reward/craving mechanisms. Therefore, the specular changes in AEA levels in these two regions might represent a feedback regulatory loop on endocannabinoid tone, and possibly result in the fine regulation of the mesolimbic dopaminergic pathways of reward from, and craving for, ethanol (see van der Stelt & Di Marzo (2003) for a review). However, reinstatement of ethanol consumption lowered the levels of the two endocannabinoids, and of 2-AG in particular, in both the midbrain and, to a smaller extent, the limbic forebrain. If this treatment can be taken as a model of relapse to alcohol abuse, it appears that this phenomenon resets the output of the endocannabinoid system to a much lower signal than in alcohol-naive rats. This might also suggest that mesolimbic endocannabinoid levels play a role in relapse to alcohol different from that proposed for the relapse to heroin and cocaine, which, in rat models, were blocked by CB1 receptor antagonists, thus suggesting an enhanced endocannabinoid tone prior to reinstatement of the use of these two drugs (De Vries et al., 2001; Fattore et al., 2003). Given the possible role of endocannabinoids against anxiety (Berrendero & Maldonado, 2002), the decrease of mesolimbic endocannabinoid activity during voluntary consumption of ethanol after an abstinence period might represent an adaptive response to the cessation of an anxiogenic situation such as that produced from abstinence.
Effects of cannabinoid agonists/antagonists on alcohol relapse in abstinent Wistar rats
Based on the present finding that alcoholization, followed by alcohol deprivation and reinstament of alcohol-drinking behavior in rats, is associated with fluctuations in the endocannabinoid transmission in brain regions involved in addictive states, it could be hypothesized that the pharmacological targeting of this system might serve to reduce the incentive properties of alcohol, the signs of alcohol withdrawal, and/or the vulnerability to relapse. However, based on the observation that endocannabinoid levels change in opposite directions, at least in the limbic forebrain, it was expected a priori that cannabinoid agonists might be useful in some conditions, while antagonists might be appropriate in others. Indeed, the increase observed for endocannabinoid levels in the limbic forebrain would explain why compounds that block endocannabinoid transmission, such as the selective antagonist of CB1 receptors SR141716, are effective in attenuating the primary reinforcing properties of alcohol thus reducing the intake of this drug in rodents (Arnone et al., 1997; Colombo et al., 1998; Freedland et al., 2001; Lallemand et al., 2001; Wang et al., 2003), whereas the agonists increase the motivation to drink (Gallate et al., 1999; Colombo et al., 2002). As mentioned in the Introduction, however, most of these effects were observed in alcohol-naive rats genetically selected for their preference for alcohol. In our study, we focused on alcohol relapse using a model of alcoholism designed to increase alcohol-drinking behavior in rat strains, such as Wistar rats, which do not have a congenital preference for alcohol (see Lê & Shaham (2002) for details). We found that SR141716 was able to reduce alcohol intake also in these abstinent rats. This finding is consistent with the previous reports that this antagonist blocks reinstatement of heroin and cocaine (see above), but it is in contrast with our present finding of reduced endocannabinoid levels in the mesolimbic system of these rats. However, a reduction of ethanol intake was evident also when using cannabinoid agonists, an observation that was also reported by McMillan & Snodgrass (1991) using acute or chronic treatment with Δ9-THC. In our opinion, this similarity of the effects produced by the agonists and the antagonist might be explained by assuming that, possibly due to pharmacokinetic reasons, the agonists activate preferentially cannabinoid receptors located onto neuronal populations different from those affected by the CB1 receptor blockade, and possibly located onto different, although related, neuronal circuits. This assumption is supported by three experimental observations. First, the reductions of ethanol intake by both the agonists and the antagonist were not, in any case, additive. Second, these pharmacological data were obtained in the phase of reinstatement of alcohol-drinking behavior when the levels of endocannabinoids in regions related to addictive states were found to be significantly low compared with those observed in the alcoholization and/or abstinence phases. Therefore, while the effect of cannabinoid agonists might represent a pharmacological compensation for the reduced cannabinoid activity in this phase (as shown by Fattore et al. (1999) to explain the effects of cannabinoid agonists on cocaine self-administration), the effect of SR141716 cannot be explained with a counteraction of enhanced endocannabinoid tone in the mesolimbic, and might have been due to antagonism of upregulated CB1 receptors in other brain regions. Lastly, neither the agonists nor the antagonist inhibited ethanol intake selectively, all compounds being active also on water intake; this might suggest that, in our model, SR141716 might target preferentially CB1 receptors involved, for example, in the control of liquid intake and/or appetite in hypothalamic regions, and possibly overactivated during a period of abstinence (Kirkham et al., 2002). In support of this, we did not find any statistically significant effects in limbic areas of both types of cannabinoid compounds on DA and GABA transmission, which are involved in reinforcing properties of alcohol during both alcoholization and relapse (McBride & Li, 1998; Lê & Shaham, 2002).
Conclusion
In conclusion, despite the general lack of alterations in the status of CB1 receptors during alcohol addiction, the levels of their endogenous ligands underwent significant changes in reward-related regions, such as the limbic forebrain and the midbrain, during the phases of alcoholization, alcohol deprivation, and reinstatement of alcohol-drinking behavior, the lowest levels being found in this latter phase. In the limbic forebrain, a crucial region for alcohol addiction, our data suggest that endocannabinoid levels during alcoholization, abstinence, and relapse might be first enhanced to reinforce the hedonic properties of this substance of abuse and then reduced, possibly in an attempt to avoid its further consumption. During voluntary alcohol consumption, the pharmacological manipulation of the endocannabinoid system with either cannabinoid agonists or a selective CB1 receptor antagonist resulted in a reduction of ethanol intake, although these effects were not additive and affected mostly mechanisms other than the classic substrates mediating the reinforcing properties of alcohol.
Acknowledgments
This work has been supported by grants from CICYT (PM99-0056), CAM-PRI (08.8/0013/2003.1), Plan Nacional sobre Drogas (Madrid, Spain) and MURST (grants 3933). Sara González is a predoctoral fellow supported by the Plan Nacional sobre Drogas. We thank Ottavio De Luca and Ana Jurado for technical assistance.
Abbreviations
- AEA
arachidonoyl-ethanolamide
- 2-AG
2-arachidonoyl-glycerol
- DA
dopamine
- GAD
glutamate decarboxylase
- GC-MS
gas chromatography-mass spectrometry
- NP-HPLC
normal phase-high pressure liquid chromatography
- TH
tyrosine hydroxylase
- Δ9-THC
Δ9-tetrahydrocannabinol
References
- ARNONE M., MARUANI J., CHAPERON F., THIÉBOT M.H., PONCELOR M., SOUBRIÉ P., LE FUR G. Selective inhibition of sucrose and ethanol intake by SR141716, an antagonist of central cannabinoid (CB1) receptors. Pyschopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., COOPER T.B., HUNGUND B.L. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;79:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., HUNGUND B.L. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTPγS binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999a;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., HUNGUND B.L. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J. Neurochem. 1999b;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., HUNGUND B.L. Cannabinoid receptor agonist-stimulated [35S]guanosine triphosphate-γ-S binding in the brain of C57BL/6 and DBA/2 mice. J. Neurosci. Res. 2001;64:429–436. doi: 10.1002/jnr.1094. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., HUNGUND B.L. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostag. Leukot. Essent. Fatty Acids. 2002;66:287–299. doi: 10.1054/plef.2001.0352. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., SAITO M., COOPER T.B., HUNGUND B.L. Stimulation of cannabinoid receptor agonist 2-arachidonoylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim. Biophys. Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- BASAVARAJAPPA B.S., SAITO M., COOPER T.B., HUNGUND B.L. Chronic ethanol inhibits the anandamide transport and increases extracellular anandamide levels in cerebellar granule neurons. Eur. J. Pharmacol. 2003;466:73–83. doi: 10.1016/s0014-2999(03)01557-7. [DOI] [PubMed] [Google Scholar]
- BECKER H.C. Animal models of alcohol withdrawal. Alcoh. Res. Health. 2000;24:105–113. [PMC free article] [PubMed] [Google Scholar]
- BERRENDERO F., MALDONADO R. Involvement of the opioid system in the anxiolytic-side effects induced by Δ9-tetrahydrocannabinol. Psychopharmacology. 2002;163:111–117. doi: 10.1007/s00213-002-1144-9. [DOI] [PubMed] [Google Scholar]
- BERRENDERO F., SEPE N., RAMOS J.A., DI MARZO V., FERNÁNDEZ-RUIZ J.J. Analysis of cannabinoid receptor binding and mRNA expresion and endogenous cannabinoid contents in the developing rat brain during late gestation and early postnatal period. Synapse. 1999;33:181–191. doi: 10.1002/(SICI)1098-2396(19990901)33:3<181::AID-SYN3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- BISOGNO T., SEPE N., MELCK D., MAURELLI S., DE PETROCELLIS L., DI MARZO V. Biosynthesis, release and degradation of the novel endogenous cannabimimetic metabolite 2-arachidonoylglycerol in mouse neuroblastoma cells. Biochem. J. 1997;322:671–677. doi: 10.1042/bj3220671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLOMBO G., AGABIO R., FA M., GUANO L., LOBINA C., LOCHE A., REALI R., GESSA G.L. Reduction of voluntary ethanol intake in ethanol preferring SP by the cannabinoid antagonist SR141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- COLOMBO G., SERRA S., BRUNETTI G., GÓMEZ R., MELIS S., VACCA G., CARAI M.M., GESSA G.L. Stimulation of voluntary ethanol intake by cannabinoid receptor agonists in ethanol preferring SP rats. Psychopharmacology. 2002;159:181–187. doi: 10.1007/s002130100887. [DOI] [PubMed] [Google Scholar]
- DE PETROCELLIS L., MELCK D., BISOGNO T., MILONE A., DI MARZO V. Finding of endocannabinoid signalling system in Hydra, a very primitive organism: possible role in the feeding response. Neuroscience. 1999;92:377–387. doi: 10.1016/s0306-4522(98)00749-0. [DOI] [PubMed] [Google Scholar]
- DEVANE W.A., HANUS L., BREUER A., PERTWEE R.G., STEVENSON L.A., GRIFFIN G., GIBSON D., MANDELBAUM A., ETINGER A., MECHOULAM R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- DE VRIES T.J., SHAHAM Y., HOMBERG J.R., CROMBAG H., SCHUURMAN K., DIEBEN J., VANDERSCHUREN L.J.M.J., SCHOFFELMEER A.N.M. A cannabinoid mechanism in relapse to cocaine seeking. Nat. Med. 2001;7:1151–2254. doi: 10.1038/nm1001-1151. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., BERRENDERO F., BISOGNO T., GONZÁLEZ S., CAVALIERE P., ROMERO J., CEBEIRA M., RAMOS J.A., FERNÁNDEZ-RUIZ J.J. Enhancement of anandamide formation in the limbic forebrain an reduction of endocannabinoid contents in the striatum of Δ9-tetrahydrocannabinol-tolerant rats. J. Neurochem. 2000;74:1627–1635. doi: 10.1046/j.1471-4159.2000.0741627.x. [DOI] [PubMed] [Google Scholar]
- DI MARZO V., MELCK D., BISOGNO T., DE PETROCELLIS L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- FATTORE L., MARTELLOTA M.C., COSSU G., MASCIA M.S., FRATTA W. CB1 cannabinoid receptor agonist WIN55,212-2 decreases intravenous cocaine self-administration in rats. Behav. Brain Res. 1999;104:141–148. doi: 10.1016/s0166-4328(99)00059-5. [DOI] [PubMed] [Google Scholar]
- FATTORE L., SPANO M.S., COSSU G., DEIANA S., FRATTA W. Cannabinoid mechanism in reinstatement of heroin-seeking after a long period of abstinence in rats. Eur. J. Neurosci. 2003;17:1723–1726. doi: 10.1046/j.1460-9568.2003.02607.x. [DOI] [PubMed] [Google Scholar]
- FONTANA A., DI MARZO V., CADAS H., PIOMELLI D. Analysis of anandamide, an endogenous cannabinoid substance, and other natural N-acylethanolamines. Prostaglandins, Leukot. Essent. Fatty Acids. 1995;53:301–308. doi: 10.1016/0952-3278(95)90130-2. [DOI] [PubMed] [Google Scholar]
- FREEDLAND C.S., SHARPE A.L., SAMSON H.H., PORRINO L.J. Effects of SR141716A on ethanol and sucrose self-administration. Alcohol Clin. Exp. Res. 2001;25:277–282. [PubMed] [Google Scholar]
- GALLATE J.E., MCGREGOR I.S. The motivation for beer in rats: effects of ritanserin, naloxone and SR141716. Psychopharmacology. 1999;142:302–308. doi: 10.1007/s002130050893. [DOI] [PubMed] [Google Scholar]
- GALLATE J.E., SAHAROV T., MALLET P.E., MCGREGOR I.S. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur. J. Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- GONZÁLEZ S., CASCIO M.G., FERNÁNDEZ-RUIZ J., FEZZA F., DI MARZO V., RAMOS J.A. Changes in endocannabinoid contents in the brain of rats chronically exposed to nicotine, ethanol or cocaine. Brain Res. 2002b;954:73–81. doi: 10.1016/s0006-8993(02)03344-9. [DOI] [PubMed] [Google Scholar]
- GONZÁLEZ S., FERNÁNDEZ-RUIZ J.J., SPARPAGLIONE V., PAROLARO D., RAMOS J.A. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB1 receptor binding and mRNA levels. Drug Alcoh. Depend. 2002a;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- GONZÁLEZ S., ROMERO J., DE MIGUEL R., LASTRES-BECKER I., VILLANÚA M.A., MAKRIYANNIS A., RAMOS J.A., FERNÁNDEZ-RUIZ J.J. Extrapyramidal and neuroendocrine effects of AM404, an inhibitor of the carrier-mediated transport of anandamide. Life Sci. 1999;65:327–336. doi: 10.1016/s0024-3205(99)00251-9. [DOI] [PubMed] [Google Scholar]
- HERKENHAM M., LYNN A.B., JOHNSON M.R., MELVIN L.S., DE COSTA B.R., RICE K.C. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in situ autoradiographic study. J. Neurosci. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNGUND B.L., BASAVARAJAPPA B.S. Are anandamide and cannabinoid receptors involved in ethanol tolerance? A review of the evidence. Alcohol Alcohol. 2000a;35:126–133. doi: 10.1093/alcalc/35.2.126. [DOI] [PubMed] [Google Scholar]
- HUNGUND B.L., BASAVARAJAPPA B.S. Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. J. Neurosci. Res. 2000b;60:122–128. doi: 10.1002/(SICI)1097-4547(20000401)60:1<122::AID-JNR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- HUNGUND B.L., SZAKALL I., ADAM A., BASAVARAJAPPA B.S., VADASZ C. Cannabinoid CB1 receptor knockout mice exhibit markedly reduced voluntary alcohol consumption and lack alcohol-induced dopamine release in the nucleus accumbens. J. Neurochem. 2003;84:698–704. doi: 10.1046/j.1471-4159.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- KIRKHAM T., WILLIAMS C., FEZZA F., DI MARZO V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoylglycerol. Br. J. Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOOB G.F. Animal models of craving for ethanol. Addiction. 2000;95:73–81. doi: 10.1080/09652140050111663. [DOI] [PubMed] [Google Scholar]
- LALLEMAND F., SOUBRIE P.H., DE WITTE P.H. Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic ethanol administration. Alcohol Clin. Exp. Res. 2001;25:1317–1323. [PubMed] [Google Scholar]
- LASTRES-BECKER I., DE MIGUEL R., DE PETROCELLIS L., MAKRIYANNIS A., DI MARZO V., FERNÁNDEZ-RUIZ J.J. Compounds acting at the endocannabinoid and/or endovanilloid systems reduce hyperkinesia in a rat model of Huntington's disease. J. Neurochem. 2003;84:1097–1109. doi: 10.1046/j.1471-4159.2003.01595.x. [DOI] [PubMed] [Google Scholar]
- LÊ A.D., SHAHAM Y. Neurobiology of relapse to alcohol in rats. Pharmacol. Ther. 2002;94:137–156. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSEBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MCBRIDE W.J., LI T.K. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit. Rev. Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- MCMILLAN D.E., SNODGRASS S.H. Effects of acute and chronic administration of Δ9-tetrahydrocannabinol or cocaine on ethanol intake in a rat model. Drug Alcoh. Depend. 1991;27:263–274. doi: 10.1016/0376-8716(91)90009-n. [DOI] [PubMed] [Google Scholar]
- NAGATSU T., OKA K., KATO T. Highly sensitive assay for tyrosine-hydroxylase activity by high-performance liquid chromatography. J. Chromat. 1979;163:247–252. doi: 10.1016/s0378-4347(00)81411-5. [DOI] [PubMed] [Google Scholar]
- NICOLETTI F., GRANDISON L., MEEK J.L. Effects of repeated administration of estradiol benzoate on tubero-infundibular GABAergic activity in male rats. J. Neurochem. 1985;44:1217–1220. doi: 10.1111/j.1471-4159.1985.tb08746.x. [DOI] [PubMed] [Google Scholar]
- PALKOVITS M., BROWNSTEIN J. Amsterdam: Elsevier; 1988. Maps and Guide to Microdissection of the Rat Brain. [Google Scholar]
- PAXINOS G., WATSON C. London: Academic Press; 1986. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- PONCE G., HOENICKA J., RUBIO G., AMPUERO I., JIMÉNEZ-ARRIERO M.A., RODRÍGUEZ-JIMÉNEZ R., PALOMO T., RAMOS J.A. Association between cannabinoid receptor gene (CNR1) and childhood attention deficit/hyperactivity disorder in Spanish male alcoholic patients. Mol. Psychiatry. 2003;8:466–467. doi: 10.1038/sj.mp.4001278. [DOI] [PubMed] [Google Scholar]
- RACZ I., BILKEI-GORZO A., TOTH Z.E., MICHEL K., PALKOVITS M., ZIMMER A. A critical role for the cannabinoid-CB1 receptor in alcohol dependence and stress-stimulated alcohol drinking. J. Neurosci. 2003;23:2453–2458. doi: 10.1523/JNEUROSCI.23-06-02453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMERO J., BERRENDERO F., PÉREZ-ROSADO A., MANZANARES J., ROJO A., FERNÁNDEZ-RUIZ J.J., DE YÉBENES J.G., RAMOS J.A. Unilateral 6-hydroxydopamine lesions of nigrostriatal dopaminergic neurons increased CB1 receptor mRNA levels in the caudate-putamen. Life Sci. 2000;66:485–494. doi: 10.1016/s0024-3205(99)00618-9. [DOI] [PubMed] [Google Scholar]
- ROMERO J., DE MIGUEL R., GARCÍA-PALOMERO E., FERNÁNDEZ-RUIZ J.J., RAMOS J.A. Time-course of the effects of anandamide, the putative endogenous cannabinoid receptor ligand, on extrapyramidal function. Brain Res. 1995;694:223–232. doi: 10.1016/0006-8993(95)00835-e. [DOI] [PubMed] [Google Scholar]
- RUBINO T., MASSI P., PATRINI G., VENIER I., GIAGNONI G., PAROLARO D. Chronic CP-55,940 alters cannabinoid receptor-mRNA in the rat brain: an in situ hybridization study. NeuroReport. 1994;5:2493–2496. doi: 10.1097/00001756-199412000-00022. [DOI] [PubMed] [Google Scholar]
- SCHMID P.C., KREBSBACH R.J., PERRY S.R., DETTMER T.M., MAASSON J.L., SCHMID H.H.O. Occurrence and postmortem generation of anandamide and other long-chain N-acylethanolamines in mammalian brain. FEBS Lett. 1995;375:117–120. doi: 10.1016/0014-5793(95)01194-j. [DOI] [PubMed] [Google Scholar]
- SCHMIDT L.G., SAMOCHOWIEC J., FINCKH U., FISZER-PIOSIK E., HORODNICKI J., WENDEL B., ROMMELSPACHER H., HOEHE M.R. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- SERRA S., CARAI M.A., BRUNETTI G., GÓMEZ R., MELIS S., VACCA G., COLOMBO G., GESSA G.L. The cannabinoid receptor antagonist SR141716 prevents acquisition of drinking behavior in alcohol-preferring rats. Eur. J. Pharmacol. 2001;430:369–371. doi: 10.1016/s0014-2999(01)01379-6. [DOI] [PubMed] [Google Scholar]
- SMART R.G., OGBORNE A.C. Drug use and drinking among students in 36 countries. Addict. Behav. 2000;25:455–460. doi: 10.1016/s0306-4603(99)00013-1. [DOI] [PubMed] [Google Scholar]
- SMITH S., SHARP T. Measurement of GABA in rat brain microdialysates using o-phtaldialdehyde-sulphite derivatization and high-performance liquid chromatography with electrochemical detection. J. Chromat. 1994;652:228–233. doi: 10.1016/0378-4347(93)e0391-3. [DOI] [PubMed] [Google Scholar]
- VAN DER STELT M., DI MARZO V. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: implications for neurological and psychiatric disorders. Eur. J. Pharmacol. 2003;480:133–150. doi: 10.1016/j.ejphar.2003.08.101. [DOI] [PubMed] [Google Scholar]
- VIGANÒ D., CASCIO M.G., RUBINO T., FEZZA F., VACCANI A., DI MARZO V., PAROLARO D. Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoylglycerol, in rat brain. Neuropsychopharmacology. 2003;28:1160–1167. doi: 10.1038/sj.npp.1300117. [DOI] [PubMed] [Google Scholar]
- WANG L., LIU J., HARVEY-WHITE J., ZIMMER A., KUNOS G. Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:1393–1398. doi: 10.1073/pnas.0336351100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WENGER T., MOLDRICH G., FURST S. Neuromorphological background of cannabis addiction. Brain Res. Bull. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]