Abstract
Adenylyl cyclase VI (ACVI) is one of the most abundantly expressed β adrenergic receptor (βAR)-coupled cyclases responsible for cyclic AMP (cAMP) production within the mammalian myocardium. We investigated the role of ACVI in the regulation of cardiomyocyte contractility and whether it is functionally coupled with β1 adrenergic receptor (β1AR).
Recombinant adenoviruses were generated for ACVI and for antisense to ACVI (AS). Adult rat ventricular myocytes were transfected with ACVI virus, AS or both (SAS). Adenovirus for green fluorescent protein (GFP) served as control. Myocyte contraction amplitudes (% shortening) and relaxation times (R50) were analysed. ACVI function was determined using cAMP assays.
ACVI-transfected cells demonstrated a strong 139 kDa ACVI protein band compared to controls. ACVI myocytes had higher steady-state intracellular cAMP levels than GFP myocytes when unstimulated (GFP vs ACVI=6.60±0.98 vs 14.2±2.1 fmol cAMP/viable cell, n=4, P<0.05) and in the presence of 1 μM isoprenaline or 10 μM forskolin.
ACVI myocytes had increased basal contraction (% shortening: GFP vs ACVI: 1.90±1.36 vs 3.91±2.29, P<0.0001) and decreased basal R50 (GFP vs ACVI: 62.6±24.2 ms (n=50) vs 45.0±17.2 ms (n=248), P<0.0001). ACVI myocyte responses were increased for forskolin (Emax: GFP=6.70±1.59 (n=6); ACVI=9.06±0.69 (n=14), P<0.01) but not isoprenaline.
ACVI myocyte responses were increased (Emax: GFP vs ACVI=3.16±0.77 vs 5.10±0.60, P<0.0001) to xamoterol (a partial β1AR-selective agonist) under β2AR blockade (+50 nM ICI 118, 551). AS decreased both control and ACVI-stimulated xamoterol responses (Emax: AS=2.59±1.42, SAS=1.38±0.5). ACVI response was not mimicked by IBMX. Conversely, response through β2 adrenergic receptor (β2AR) was decreased in ACVI myocytes.
In conclusion, ACVI overexpression constitutively increases myocyte contraction amplitudes by raising cAMP levels. Native ACVI did not contribute to basal cAMP production or contraction amplitude and only to a minor extent to the forskolin response. β1AR but not β2AR coupling was dependent on ACVI.
Keywords: Adenylyl cyclase VI, cardiomyocyte, contractility, lusitropy, coupling, β1AR and β2AR
Introduction
Adenylyl cyclases (ACs; EC 4.6.1.1) are large (139 kDa) transmembrane signal transduction proteins that are responsible for the production of the second messenger molecule, cAMP (Gao et al., 1998). A total of nine isoforms of AC (designated ACI–ACIX) have been identified and cloned. These AC isoforms are expressed heterogeneously in different types of mammalian tissues (Katsushika et al., 1992; Ishikawa et al., 2000; Paterson et al., 2000; Sosunov et al., 2001). The ACV and ACVI isoforms are the most predominantly expressed ACs in the mammalian heart (Raimundo et al., 1999). Although ACVI has a lower level of mRNA expression than ACV in adult rat cardiomyocytes (Espinasse et al., 1995), the reverse of this was found in the human atrium indicating that ACVI was the predominant isoform (Wang & Brown, 2001).
Located upstream of ACs are the β adrenergic receptors (βARs) which are established modulators of cardiomyocyte contractility. The βARs are transmembrane, G-protein-coupled receptors: all three subtypes (β1, β2 and β3AR) may exist in cardiac myocytes although only the β1- and β2ARs (β1- and β2 adrenergic receptors) couple to adenylate cyclase to increase contraction (Lohse et al., 2003). The β1AR exists in higher abundance, often more than two-fold, compared to the β2ARs within the ventricular membrane (Kuznetsov et al., 1995). Both βARs couple through Gs, but β2ARs can also couple through Gi in tissues including rat ventricle (Xiao et al., 2003). The βARs and ACs colocalise in microdomains within cells, known as caveolae or lipid-rafts. This form of compartmentalisation of the components of the signal transduction pathway within the membrane increases the efficiency of communication between different proteins (Schwencke et al., 1999a; Xiang et al., 2002).
Of these three components of the βAR-mediated signalling pathway, only the ACs function as rate-limiting factors in cell signalling (Gao et al., 1998). As ACs appear to be a crucial driving force in βAR signaling, they are highly likely to be involved in regulating cardiac contractility. The stimulation of both β1ARs and β2ARs generally exerts positive inotropic effects on the contractility of cardiomyocytes. This enhancement of inotropic effect by βAR stimulation is thought to be associated with the activation of protein kinase A phosphorylation processes, which occur downstream of ACs. The β2ARs are also capable of eliciting negative inotropic effects on myocyte contractility due to their association with the inhibitory Gi proteins (Gong et al., 2002; Sato et al., 2004).
The β1- and β2-ARs have been reported to display differences in coupling to ACs (Zhou et al., 2001). In both human (Kaumann et al., 1989) and rat (Xiao et al., 1994) myocardium, the β1AR is the predominant subtype mediating increases in contraction, but produces less cAMP than β2AR for a given inotropic effect. It is possible that the β1AR is coupled to an AC isoform producing a minor amount of total denosine 3′, 5′-cyclic monophosphate (cAMP) but specifically coupled to contraction. ACs produce ‘localized' cAMP which is capable of undergoing compartmentalization within a single cell (Schwencke et al., 1999b; Zaccolo & Pozzan, 2002; Cooper, 2003). One study (Xiang et al., 2002) has shown that in neonatal rat myocytes the association between the β2AR and ACVI is controlled by their colocalisation in caveolar regions of the membrane, although another group reported that overexpressed ACVI showed a greater efficiency of coupling for the β1- rather than the β2AR (Ostrom et al., 2001). However, the ultrastructure and receptor coupling in neonatal myocytes differs markedly from adult myocytes. The functionality of the heterogenous coupling between ACs and βARs in the adult heart has not been fully investigated.
The aim of this investigation was to overexpress or downregulate ACVI protein within adult rat ventricular cardiomyocytes, in order to elucidate what role ACVI plays in the regulation of contractility of myocardium. The contractility of myocytes encompasses physiological characteristics including their amplitudes of contraction (% shortening) and beat duration. The functional relationship between ACVI and both β1AR and β2AR was also characterised with the aid of specific βAR subtype agonists and blockers.
Methods
Materials
All chemicals were obtained from Sigma-Aldrich (Gillingham, Dorset, U.K.) and all restriction enzymes were purchased from New England Biolabs (Hitchin, Herts, U.K.), unless otherwise specified.
Generation of ACVI recombinant adenoviruses
The full-length 5 kb ACVI gene was obtained from a recombinant pcDNA 3.0 construct (gift from Professor Dermott Cooper, Department of Pharmacology, University of Cambridge) and cloned into pAdeno-X adenovirus expression vector (Clontech, Basingstoke, Hampshire, U.K.). First, an XbaI site was incorporated at the 5′ terminus of ACVI in pcDNA 3.0, which was originally flanked by EcoRI and XbaI restriction enzyme sites. The 5 kb ACVI cDNA was excised as an XbaI fragment and ligated into the XbaI-linearised intermediate pShuttle vector to obtain both the sense and antisense orientations of ACVI in pShuttle. The entire expression cassette in pShuttle was excised using PI-SceI and I-CeuI and cloned into PI-SceI and I-CeuI compatibly linearised pAdeno-X. The presence of ACVI in pShuttle and pAdeno-X was checked against the expected fragment sizes generated by HindIII restriction analyses and DNA sequencing. The ACVI gene was DNA sequenced at 500 bp intervals using primer pairs spanning consecutive 500 bp regions of ACVI. Each primer pair reads a sequence of at least 500 bp along the ACVI gene and the overlap of all the 500 bp sequences produced the complete DNA sequence of ACVI. This was to confirm the wild-type DNA sequence of ACVI.
The ACVI recombinant pAdeno-X was transfected into HEK 293 cells using Superfect reagent (Qiagen, Crawley, West Sussex, U.K.) for packaging of adenoviruses. Once cytopathic effect was reached, cells were lysed and the adenoviruses obtained were amplified by repeated rounds of cell infections. The adenoviruses were titered using a 96-well plating technique by serial dilutions. GFP-adenoviruses were obtained as gifts (Dr Hajjar and del Monte, CVRC, Boston, U.S.A.) and served as controls for ACVI-adenoviruses during the measurements of myocyte contractility. LacZ-adenoviruses were also obtained as gifts (Professor Peter Weissberg, CPU, Cambridge, U.K.) and were used as controls for ACVI-adenoviruses prior to the use of GFP-adenoviruses.
Isolation of adult rat cardiomyocytes
Adult rats were kept with food and water available ad libitum. They were killed in compliance with the United Kingdom Home Office Regulations Governing the Care and Use of Laboratory Animals. The ventricular cardiomyocytes were isolated from adult male Sprague–Dawley rats using a Langendorff perfusion method with the aid of enzymatic digestion. This method was previously described (Chaudhri et al., 2003). The cardiomyocytes were plated onto 12-well tissue culture plates at 104 cells well−1 and infected with recombinant green fluorescent protein (GFP) at 100 MOI (multiplicity of infection, active viral particles per adult myocyte) and 1000 MOI, sense ACVI at 50, 100 or 1000 MOI, antisense ACVI at 1000 MOI (AS), sense ACVI 100 MOI plus antisense ACVI 1000 MOI (SAS) and β2AR-adenoviruses at 50 MOI. Infected myocytes were all cultured for 48 h, with the exception of the β2AR experiments where cells were cultured for 24 h. Cell culture medium was M199 plus 5 mM creatine, 5 mM taurine, 2 mM carnitine, 0.1 μM insulin, 100 mM ascorbate and penicillin/streptomycin.
After transfection and culture, the cardiomyocytes were washed by centrifugation at 600 × g for 1 min at room temperature. The pellet was dissociated in buffer containing 120 mM NaCl, 5.4 mM KCl, 5 mM MgSO4, 5 mM pyruvate, 20 mM glucose, 20 mM taurine and 10 mM Hepes, pH 7.4 by gentle mixing. Myocytes were then transferred to the cell bath for contraction experiments.
Detection of ACVI overexpression by Western blotting
Myocytes and HEK 293 host cells were lysed in NP40 (Nonidet-P40) lysis buffer (20 mM Hepes, pH 7.0, 120 mM HCl, 1 mM dithiothreitol, 5 mM magnesium acetate, 10% v v−1 glycerol, 0.5% Nonidet-P40 containing protease inhibitors, 10 μg ml−1 each of leupeptin, aprotinin, pepstatin, 1 mg ml−1 Pefabloc). Membrane fractions of myocytes were obtained by centrifuging lysates at 14,000 × g for 15 min at 4°C. Membrane protein of myocytes and total protein lysate of HEK 293 cells was loaded onto an 8% polyacrylamide gel. The ACVI protein was detected on the transferred PVDF (polyvinyl difluoride) membrane by incubating in 1 : 100 dilution of primary rabbit anti-mouse ACVI polyclonal antibodies (Santa Cruz Biotech, CA, U.S.A.) in phosphate-buffered saline (PBS) for 1 h at room temperature. Primary antibody specificity of ACVI detection was assessed by preincubating the antibody with ACVI-blocking peptide (Santa Cruz Biotech, CA, U.S.A.) at the recommended concentration (50 μg ml−1) overnight at 4°C, prior to incubating the membrane blots with the primary anti-ACVI antibody. The specificity of antibody binding was also determined in negative controls in which either primary anti-ACVI or secondary antibodies were substituted by PBS.
After incubating the membrane with primary anti-ACVI antibody, it was rinsed for 3 × 5 min in PBS to remove nonspecific antibody binding. This was followed by a second incubation for 1 h at room temperature in 1 : 1000 dilution of goat anti-rabbit antibodies conjugated to horse radish peroxidase (Dako Cytomation, Ely, Cambridgeshire, U.K.). The ACVI protein band was detected using an enhanced chemiluminescence kit (Amersham Biosciences, Chalfont St Giles, Buckinghamshire, U.K.).
Measurement of contraction amplitude and relaxation time of single cardiomyocytes
Cardiomyocytes in buffer were allowed to settle and attach to a glass coverslip at the bottom of a Perspex bath for 5 min. The bath was located on the stage of an inverted microscope that was connected to a video camera and monitor. The cardiomyocytes were superfused with Krebs–Henseleit (KH) buffer (119 mM NaCl, 4.7 mM KCl, 0.94 mM MgSO4, 1.2 mM KH2PO4, 25 mM NaHCO3, 11.5 mM glucose, 1 mM CaCl2), equilibrated to pH 7.4 by continuous gassing in 95% O2/5% CO2. The cells were maintained at 37°C by a feedback thermostat system. The cells were electrically paced using a bipolar pulse through platinum electrodes at 0.5 Hz, 2 ms pulse width, 50 V for 15 min before performing measurements. Rod-shaped myocytes, which exhibited clear myofilament striations and demonstrated consistently stable contraction amplitude and relaxed length between beats, were used for contractility experiments.
The contraction amplitude of the cell was determined using a high-resolution, camera-screen system, incorporating an edge detection device, which monitors changes in the length of a single cell with a spatial resolution of 1 in 512 and a time resolution of 10 ms (Gong et al., 2000). Cell length was output to a chart recorder and also recorded into a computer. Data were averaged for analysis of the time to 50% relaxation (R50) using Lotus software. The measurements of basal contraction amplitudes and forskolin response were performed with the experimenter blinded to the different uninfected/GFP/ACVI/AS/SAS conditions. Concentration–response curves to forskolin, isoprenaline and xamoterol were constructed cumulatively. Blockers, where present, were superfused over cells for 15–20 min before as well as during the concentration–response curve. Carbachol, an inhibitor of cAMP production, was used to establish whether increased contraction amplitude in ACVI myocytes was cAMP-dependent.
Measurement of cAMP production in cardiomyocytes
Adult cardiomyocytes were cultured with ACVI, AS or both as above at 104 cells well−1 in a 12-well plate coated with laminin. Wells were rinsed twice to remove nonviable cells and the number of attached rod-shaped, viable cardiomyocytes was counted. The cardiomyocytes were incubated in buffer either containing 100 μM IBMX (3-isobutyl-1-methylxanthine, a nonspecific phosphodiesterase inhibitor which prevents degradation of cAMP) for 10 min, followed by addition of either 1 μM isoprenaline or 10 μM forskolin for 10 min. Cardiomyocytes were lysed for measurement of intracellular cAMP by using a specific cAMP-antibody competition assay (cAMP Biotrak Enzymeimmunoassay System, Amersham Biosciences, Little Chalfont, Buckinghamshire, U.K.). In each cAMP assay, results were analysed from triplicate wells to account for intraexperimental errors.
Statistical methods
The mean contraction amplitude and beat duration data were analysed for statistical significance using one-way ANOVA with a Bonferroni post hoc test or t-tests as appropriate in Prism 4.0 software (GraphPad, Prism, San Diego, CA, U.S.A.). Values were expressed as mean±s.e.m. except for n values exceeding 30, where s.d. is used. Comparison between curves was carried out using the F-test (Nonlinear regression analysis) between individual curve fits and a global fit in Prism 4.0.
Results
Immunodetection of ACVI overexpression by Western blotting
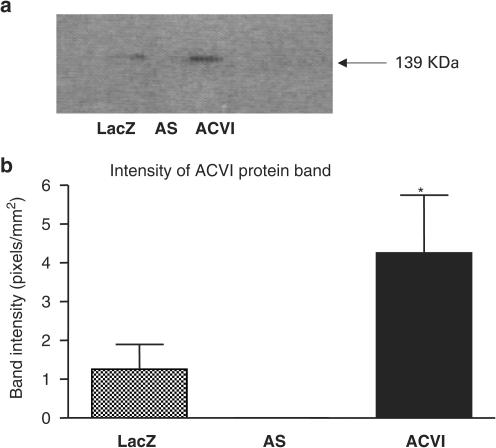
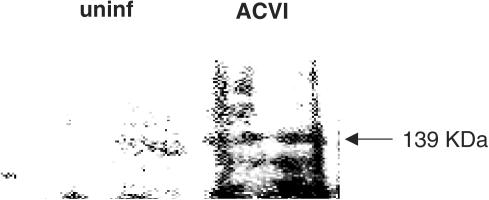
Overexpression of ACVI protein was determined in HEK 293 cells using Western blotting. Cells that were infected with the LacZ-adenoviruses as controls, antisense ACVI (AS)-adenoviruses and sense ACVI-adenoviruses were lysed. Cell lysates were probed with an anti-ACVI antibody on a Western blot. ACVI protein was detected as a strong 139 kDa protein band in ACVI HEK 293 cells (Figure 1a). HEK 293 cells, which were infected with LacZ-adenovirus, displayed a weak ACVI protein band of 139 kDa (Figure 1a, b). ACVI cells expressed a 139 kDa ACVI protein band which was three-fold higher in intensity than LacZ cells. AS did not mimic ACVI, and the weaker ACVI band in LacZ cells were abolished by expression of AS. The AS was therefore able to prevent both endogenous expression and adenovirus-mediated overexpression of ACVI (Figure 1). The intensity of the 139 kDa ACVI protein band was weak or undetectable in uninfected adult rat ventricular cardiomyocytes, but ACVI-transfected myocytes demonstrated an ACVI protein band of strong intensity (Figure 2). The ACVI-blocking peptide abolished the 139 kDa ACVI band intensity in HEK cells and myocytes but also reduced the high background detected in myocytes. Substitution of either primary or secondary antibodies with PBS in negative controls abolished the ACVI protein band.
Figure 1.
Western blot analysis of ACVI overexpression in recombinant adenovirus-infected HEK 293 cells. (a) Total cellular lysates of control LacZ (lane 1), AS (lane 2) and ACVI cells (lane 3) were subjected to Western blotting on an 8% SDS-polyacrylamide gel. (b) Intensities of the 139 kDa ACVI protein band were analysed and quantified (*one-way ANOVA, ACVI vs LacZ and AS, P<0.05). Values are means±s.e.m. from four experiments.
Figure 2.
Western blot immunodetection of ACVI overexpression in recombinant ACVI-adenovirus-infected myocytes. Membrane fractions of uninfected adult rat myocyte (lane 1) and ACVI myocytes (lane 2) were used for Western blotting on an 8% SDS-polyacrylamide gel. The overexpression of a 139 kDa ACVI protein is indicated by the arrow.
Efficiency of adenovirus infection
The GFP-adenoviruses were used as matched controls for the other groups of adenoviruses. At both 100 MOI (n=13) and 1000 MOI (n=15) infection, GFP-adenoviruses produced an infection efficiency rate of 100% by 48 h.
Cell viability
Infection of myocytes using ACVI-adenoviruses for 48 h produced a higher level of cell death compared to the other types of recombinant adenoviruses. This was concentration dependent, with 1000 MOI demonstrating higher levels of cell death than at 100 MOI. During the preparation for cAMP assays, the number of viable, rod-shaped attached myocytes was counted after dead myocytes had been removed by washing. ACVI (100 MOI) overexpression was found to reduce the final number of cells per well to 384±71, compared with 796±164 for GFP100 MOI (n=9, P<0.01). With the exception of the ACVI group, the number of cells per well in other groups was not significantly different: GFP 1000=960±196, AS=1004±217, SAS=988±181 (n=6). Both the ability of AS to prevent the cell death produced by ACVI and the lack of effect of GFP suggests that it is the increase in ACVI protein, rather than an effect of virus, which is damaging to the myocytes.
Steady-state cAMP levels in transfected myocytes
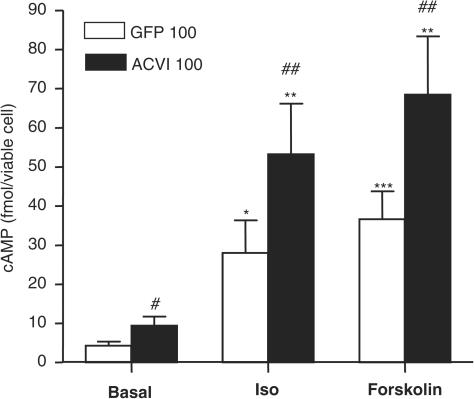
Basal cAMP was significantly increased in ACVI myocytes (Figure 3). Isoprenaline and forskolin increased cAMP production in all groups, and final cAMP in the presence of these agents was significantly higher in the ACVI cells compared to GFP (n=6, Figure 3).
Figure 3.
Increased steady-state levels of intracellular cAMP in ACVI-overexpressing myocytes. Both 100 MOI GFP and ACVI myocytes were incubated in buffer containing 100 μM IBMX at 37°C for 10 min. Myocytes were then incubated in either 1 μM isoprenaline or 10 μM forskolin in the presence of IBMX, lysed and the total intracellular cAMP levels were measured by ELISA. Statistical differences between different cells are as follows: ACVI vs GFP # P<0.05; +isoprenaline: ACVI vs GFP ## P<0.02; +forskolin: ACVI vs GFP ## P<0.02; GFP vs GFP+isoprenaline *P<0.02; ACVI vs ACVI+isoprenaline **P<0.01; GFP vs GFP+forskolin ***P<0.001; ACVI vs ACVI+forskolin **P<0.01. Values are means±s.e.m. from nine experiments.
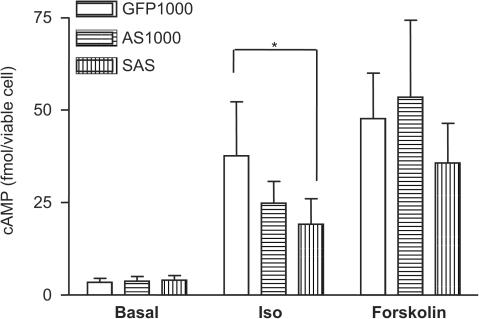
Transfection with AS was at MOI 1000 which was compared to GFP MOI 1000. There was no significant difference in levels of cAMP production between myocytes transfected with GFP MOI 100 and MOI 1000 when both were done in the same assay (data not shown). AS did not increase cAMP and prevented ACVI from increasing cAMP (SAS condition) (Figure 4). The three groups (GFP, AS and SAS) were not significantly different by ANOVA under basal or forskolin-stimulated conditions, but there was a difference for isoprenaline, with a significant linear decrease from GFP to AS and then to SAS (n=9, P<0.05).
Figure 4.
Suppression of endogenous cAMP production by AS. Myocytes transfected to express GFP (1000 MOI, open bars), AS (1000 MOI, horizontal bars) and SAS (100 MOI ACVI plus 1000 MOI AS, vertical bars) were incubated in buffer containing 100 μM IBMX at 37°C for 10 min. Myocytes were stimulated using 1 μM isoprenaline or 10 μM forskolin in the presence of IBMX for a further 10 min, lysed and their total intracellular cAMP levels were measured. Statistical differences between the three groups in the presence of isoprenaline are indicated by *(one-way ANOVA, P<0.05). Values are means±s.e.m. from six experiments.
Basal contraction amplitudes of transfected myocytes
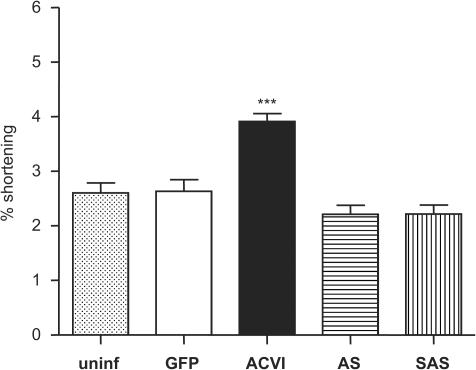
The basal contraction amplitudes of the different groups, including uninfected (uninf) cells, and cells infected with (control) GFP, sense ACVI, antisense to ACVI (AS) or sense+antisense (SAS) adenoviruses were measured (Figure 5). ACVI contractions were significantly increased, compared to the other groups as shown by one-way ANOVA analysis (all groups vs ACVI: P<0.0001). None of the other conditions differed significantly from each other (P>0.1).
Figure 5.
Increased basal amplitudes of contraction in ACVI myocytes. Myocytes were electrically paced at 0.5 Hz, 2 ms, 50 V in KH solution containing 1 mM calcium chloride. Their basal contraction amplitudes were measured. The statistical significance between ACVI and other groups is represented by ***(one-way ANOVA, P<0.0001). Values are means±s.e.m. from n myocytes (n numbers: uninfected=130, GFP=50, ACVI=248, AS=96, SAS=97).
In Figure 5, the basal contraction data was pooled together from two series of experiments. The first series measured the basal contraction amplitudes of up to six cells from each experimental day, which were used in challenged responses. In the second series of experiments, basal contraction amplitudes were obtained from 30 cells for each condition on the same experimental day: this was repeated for 6 days. The same pattern of increased contraction amplitudes in ACVI cells only was observed for both series of experiments (data not shown). For myocyte contraction in response to forskolin, isoprenaline, xamoterol and IBMX, the basal contraction amplitudes were subtracted. This compensated for the raised basal amplitudes in ACVI myocytes to reveal the overall effect on response specific to each agent.
Specific effect of carbachol on basal contraction amplitude
GFP and ACVI myocytes were treated with carbachol alone to determine whether basal contraction amplitudes were regulated by cAMP production levels. Basal ACVI contraction amplitude was significantly larger than GFP (basal: GFP; 2.37±0.49, n=10; ACVI; 6.10±0.83, n=11; P<0.01). Carbachol did not significantly decrease basal GFP contraction amplitude (GFP: basal=2.37±0.49, +1 μM carbachol=2.20±0.43, +3 μM carbachol=1.97±0.34; GFP basal vs GFP carbachol-treated at 1 μM, 3 μM: P>0.1). Basal ACVI contraction amplitude was significantly decreased by carbachol (% shortening: ACVI basal; 6.10±0.83, ACVI+1 μM carbachol; 4.82±0.84, ACVI+3 μM carbachol; 4.12±0.86; ACVI basal vs carbachol at 1 μM, 3 μM: P<0.001, n=11). This decrease in contraction amplitude of ACVI myocytes was reversed by removal of carbachol and ACVI myocytes reverted to their original basal contraction amplitudes. The GFP myocytes were next prestimulated with isoprenaline (10 and 30 nM) prior to carbachol treatment (1 and 3 μM) in the presence of 30 nM isoprenaline. Isoprenaline significantly increased GFP myocyte contraction amplitudes from 1.75±0.40 to 10.8±1.12 (n=8, P<0.0001). Carbachol significantly reduced the isoprenaline-increased GFP contraction amplitude to 4.29±0.84 (P<0.0001) and this is similar in magnitude to the 4.12% shortening shown by ACVI myocytes after carbachol treatment.
Effect of ACVI on myocyte basal relaxation time
ACVI contraction and relaxation times were significantly reduced compared to GFP. ACVI decreased myocyte basal time for relaxation to 50%, R50 (GFP vs ACVI: 62.6±24.2 vs 45.0±17.2 ms, P<0.001; n, number of experiments: GFP=50, ACVI=248). AS also produced a significant acceleration of R50, compared to GFP (AS=42.5±12.7 ms, GFP vs AS, P<0.05; n: AS=96). However, AS was able to prevent the effect of ACVI on relaxation: SAS had an R50 which was not significantly different from GFP but was significantly higher than ACVI (SAS=62.5±6.3 ms, ACVI vs SAS: P<0.05; n: SAS=97).
Myocyte contraction in response to forskolin
Both uninfected and GFP cardiomyocytes displayed similar contraction responses to increasing (cumulative) concentrations of forskolin (data not shown). The two concentration–response curves were not significantly different from each other (uninfected (n=8) vs GFP (n=6), P>0.1). GFP was used as a control for adenoviral infections in the subsequent experiments, at MOI levels matched to those for ACVI or AS.
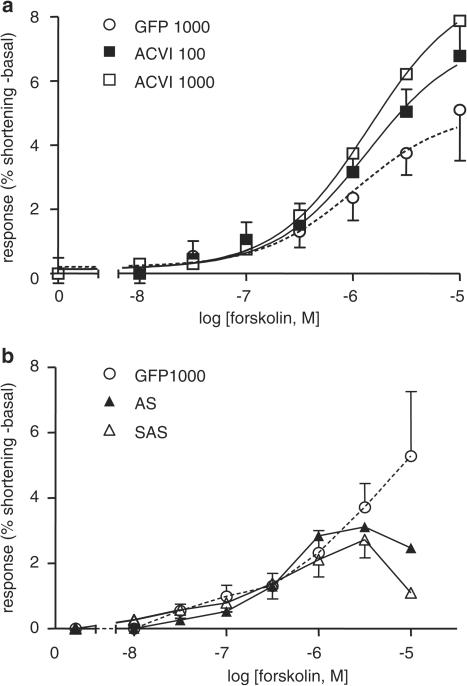
Forskolin responses were shifted upwards by ACVI 100 and 1000 MOI and were significantly increased compared to GFP, as shown by F-test analysis (Figure 6a, P<0.01). ACVI 1000 MOI responses were curtailed by development of arrhythmias, so that the number of cells in the ACVI 1000 group is small (n=2). The maximum shortening achieved was significantly increased for ACVI compared to GFP (Figure 6a). The largest % shortening, Emax, including basal contraction, reached was GFP=6.70±1.59, ACVI: 100 MOI=9.06±0.69 (P<0.01), 1000 MOI=10.9 (P>0.1). When basal contraction was subtracted, the Emax reached was GFP=5.10±1.59; ACVI: 100 MOI=6.78±0.99 (P<0.05), 1000 MOI=7.88 (P>0.1) (Figure 6a). The Log EC50 values were unchanged between ACVI and GFP myocyte response curves.
Figure 6.
Increased contraction for ACVI myocytes in response to forskolin. (a) GFP (1000 MOI) and ACVI (100 MOI and 1000 MOI) myocytes were stimulated using cumulatively increasing concentrations of forskolin. Basal contraction was subtracted from all responses. Response in ACVI myocytes was significantly larger than GFP (GFP vs ACVI 100 MOI, P<0.01; GFP vs ACVI 1000 MOI, P<0.01). (b) AS (1000 MOI) and SAS (100 MOI ACVI+1000 MOI AS). SAS contraction was significantly lower than ACVI (ACVI 100 vs SAS, P<0.001). The numbers (n) of myocytes were GFP=6, ACVI 100 MOI=19 and ACVI 1000 MOI=2, AS=5 and SAS=5. Values are means±s.e.m.
GFP myocyte contraction was similar to AS and SAS (GFP vs AS or SAS, P>0.1, Figure 6b). Although the response curves appeared to diverge between GFP, AS and SAS myocytes at 10−5 M forskolin, numbers were reduced at this point due to the occurrence of arrhythmias in some myocytes, especially SAS, so that the effects were not significant.
The maximum contraction amplitude with forskolin was lower in AS and SAS than in ACVI (AS=1.44±0.43, SAS=1.12±0.29; ACVI vs AS or SAS, P<0.0001). AS reduced the maximum % shortening of ACVI 100 MOI although not in GFP myocytes (Emax: ACVI 100 MOI=6.78±0.99, GFP=2.05±0.65, AS=1.44±0.43, SAS=1.12±0.29; ACVI vs AS and SAS, P<0.005). AS was therefore capable of suppressing the effects of ACVI overexpression on forskolin responses. However, AS exerted no inhibitory effects on native GFP contraction.
Decreased relaxation rates with increasing forskolin
The effect of forskolin to accelerate relaxation times was compared in uninfected myocytes with those in cells overexpressing GFP, ACVI or AS/SAS. Minimum values of R50 during the concentration–response curve to forskolin were compared with the basal values in the same cell before forskolin application. Forskolin significantly reduced R50 in uninfected myocytes, as expected, and this effect was not altered by expression of GFP (MOI 1000) (basal uninfected 60.0±7.1 ms, forskolin uninfected 35.0±3.8 ms (n=8), basal GFP 64.3±6.5 ms, forskolin GFP 40.0±6.6 ms, n=7). ACVI overexpression also decreased R50 (43.9±4.7 ms, n=18; basal ACVI vs GFP, P<0.01), but there was a further reduction by forskolin in ACVI myocytes (25.6±2.9 ms, ACVI+forskolin vs basal ACVI, P<0.01). AS was able to prevent the lusitropic effect of ACVI, since R50 in SAS was significantly longer than ACVI and similar to uninfected myocytes (62.5±6.3 ms, n=4). AS was not able to prevent the effect of forskolin itself on R50.
Myocyte contraction in response to isoprenaline (mixed β1- and β2AR responses)
There were no differences between the isoprenaline-stimulated contractions of uninfected and GFP cardiomyocytes. Using F-test analysis, both uninfected and GFP cardiomyocytes were shown to share a global response curve (GFP (n=6) vs uninfected (n=26): P>0.1).
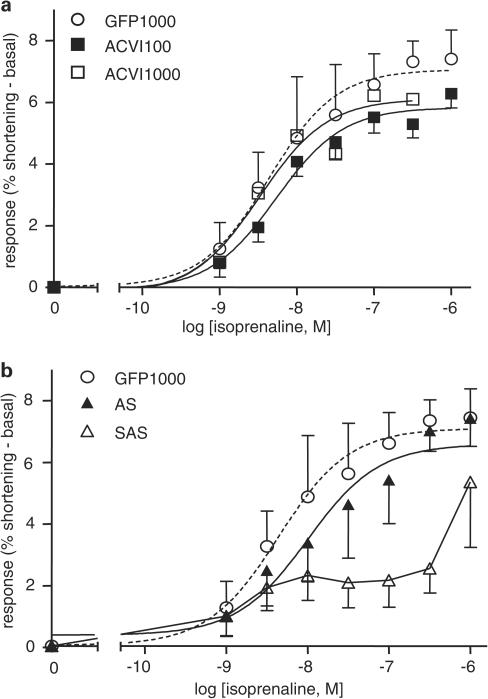
ACVI myocytes at both 100 and 1000 MOI showed no increased response to isoprenaline above GFP myocytes (Figure 7a; GFP vs ACVI, P>0.1). ACVI overexpression at 1000 MOI was proarrhythmic. Arrhythmias developed earlier during the isoprenaline challenge, so that fewer cardiomyocytes completed contraction response curves. Log EC50 values were similar for all three concentration–response curves (Figure 7a).
Figure 7.
Myocyte responses to isoprenaline were unaffected by ACVI but reduced by AS. GFP (1000 MOI, n=6), ACVI (100 MOI=35 and 1000 MOI=10), AS (1000 MOI=7) and SAS (n=6) myocytes were stimulated with cumulatively increasing concentrations of isoprenaline. Basal contraction was subtracted from all responses (a). ACVI myocytes did not differ significantly from GFP in responses to isoprenaline. (b) SAS suppressed native GFP contractions (P<0.0001). Values are means±s.e.m.
AS myocyte response was not different from GFP (Figure 7b, AS vs GFP, P>0.1). SAS contractions were significantly inhibited compared to all groups (GFP vs SAS, P<0.0001; ACVI vs SAS, P<0.01; AS vs SAS, P<0.05). The SAS contraction curve showed a biphasic pattern in contrast to the other groups.
Responses to specific β1AR stimulation
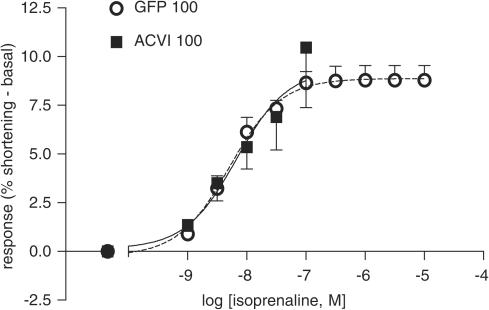
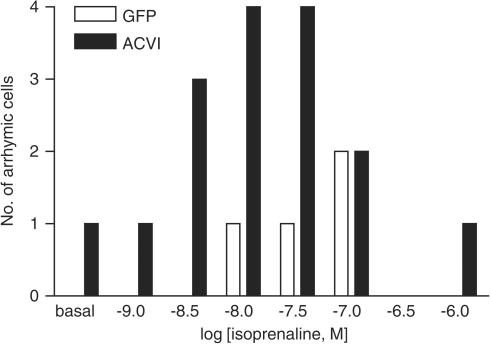
Myocytes were stimulated first through β1AR using isoprenaline, a full βAR agonist, in the presence of 50 nM ICI 118, 551 ((±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy-3-[(1-methylethyl)amino-2-butanol) hydrochloride; a specific β2AR antagonist). The ACVI myocytes (n=16) did not show an increased response compared to GFP myocytes (n=10) (ACVI vs GFP, P>0.1, Figure 8). However, the ACVI myocytes displayed increased arrhythmia which developed in the majority of these myocytes (13 out of 16 total) by 3 × 10−8 M, which is below the average maximum for the isoprenaline curve in GFP cells (Figure 9). Significantly fewer GFP myocytes (two out of 10 total) displayed arrhythmia at this concentration (Fischers exact test: ACVI vs GFP, P<0.002). The truncation of the ACVI myocyte responses to isoprenaline challenge, due to arrhythmia, suggested an increased sensitivity of the β1AR in ACVI myocytes.
Figure 8.
Myocyte responses to specific β1AR stimulation were unaffected by ACVI. GFP 100 MOI (n=10) and ACVI 100 MOI (n=16) myocytes were stimulated with cumulatively increasing isoprenaline concentrations in the presence of 50 nM ICI 118, 551 (a specific β2AR blocker). ACVI myocytes did not show an increased response above that of GFP. Values are means±s.e.m.
Figure 9.
Isoprenaline-induced arrhythmia was increased in ACVI myocytes. GFP 100 MOI (n=10) and ACVI 100 MOI (n=16) myocytes were stimulated with cumulatively increasing isoprenaline concentrations in the presence of 50 nM ICI 118, 551 (a specific β2AR blocker). Bars (open, GFP; solid ACVI) show the number of myocytes displaying arrhythmia at each isoprenaline concentration.
Responses to specific β1AR stimulation with a partial agonist
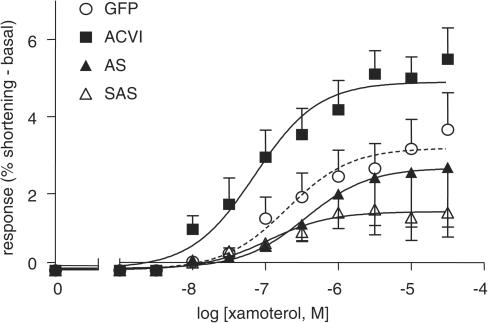
Myocytes were stimulated using increasing concentrations of xamoterol, a partial β1AR agonist, in the presence of 50 nM ICI 118, 551. ACVI responses were significantly increased compared to GFP (GFP vs ACVI, n=6, P<0.0001, Figure 10). The maximum % shortening with xamoterol for ACVI was approximately two-fold higher than GFP (Emax: GFP vs ACVI=3.16±0.77 vs 5.10±0.60, P<0.001). There were no significant differences between Log EC50 values (Log EC50: GFP=−6.75±0.10, ACVI=−7.20±0.12).
Figure 10.
Responses to xamoterol, a partial β1AR agonist, were increased in ACVI myocytes. GFP (1000 MOI), ACVI (100 MOI), AS (1000 MOI) and SAS myocytes were stimulated with cumulatively increasing concentrations of xamoterol, in the presence of 50 nM ICI 118, 551 (a specific blocker of β2ARs). ACVI contraction was significantly increased compared to GFP (GFP vs ACVI, P<0.0001). AS inhibited native GFP and ACVI contraction (SAS group) (GFP vs SAS, P<0.001). All values were mean±s.e.m. from six experiments.
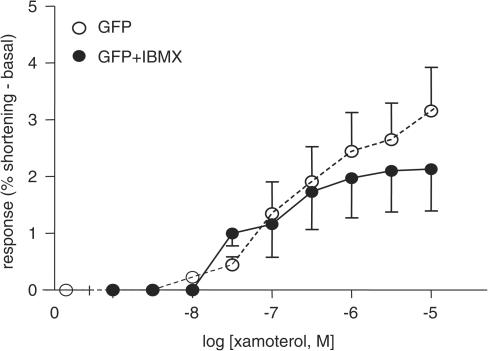
AS did not differ significantly from GFP (GFP vs AS: n=6, P>0.1), but AS contractions were significantly lower than ACVI (ACVI vs AS: P<0.0001). SAS response to xamoterol was lower than all groups (GFP or ACVI vs SAS: P<0.0001; AS vs SAS: P<0.05) (Figure 10). The increase in xamoterol response of ACVI myocytes could not be mimicked simply by raising basal cAMP (Figure 11). IBMX was titrated to increase basal GFP contraction to reach amplitudes similar to the higher basal contractions of ACVI (basal amplitude: GFP=2.06±0.25%, GFP+IBMX=4.97±0.56% shortening, ACVI=5.62±1.8%, IBMX=30–100 μM). Xamoterol was subsequently added in the presence of IBMX. However, after subtraction of basal amplitude, there was no difference in xamoterol response between GFP and GFP+IBMX-treated myocytes (P>0.1).
Figure 11.
Inability of IBMX to reproduce ACVI effects on xamoterol responses. IBMX was titrated to raise basal GFP contraction to similar extent to that achieved by ACVI transfection, after which myocytes were stimulated with cumulatively increasing xamoterol concentrations. Compared to basal GFP contraction, IBMX did not increase xamoterol-induced response (GFP: untreated (n=6) vs +IBMX (n=12), P>0.1). Values were means±s.e.m.
Responses to specific β2AR stimulation
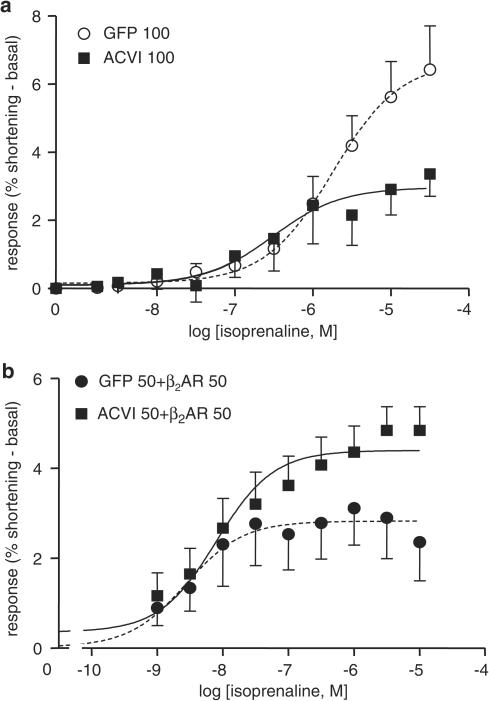
Myocytes were stimulated through β2ARs by isoprenaline, in the presence of a specific β1AR blocker, 300 nM CGP 20712A. In contrast to the previous groups, ACVI responses to β2AR stimulation were significantly lower than those in GFP cells (Figure 12; P<0.0001). The Emax values of ACVI were significantly lower than GFP (Emax, GFP vs ACVI: 6.43±1.28 vs 3.37±0.66, P<0.0001).
Figure 12.
Effect of β2AR stimulation on ACVI myocyte contraction. GFP and ACVI (100 MOI, 48 h) single infections (a) and as coinfections (b) with β2ARs (50 MOI each, 24 h) were stimulated using cumulatively increasing isoprenaline concentrations in the presence of 300 nM CGP20712A (specific blocker of β1ARs). (a) ACVI response was lower than GFP (GFP (n=11) vs ACVI (n=13), P<0.0001) (b) ACVI+β2AR contraction was significantly larger than GFP+β2AR (P<0.01, n=13). Values were mean±s.e.m.
However, when myocytes were coinfected with β2AR+GFP or β2AR+ACVI, respectively, at 50 MOI each for 24 h infection, ACVI myocyte contractions became significantly larger than those of GFP (GFP+β2AR vs ACVI+β2AR, P<0.01, Figure 12b). The Emax of ACVI myocytes was higher than those of GFP (Emax: GFP=2.36±0.86, ACVI=4.85±0.53).
Discussion
This study shows that ACVI overexpression can regulate the contractility of cardiomyocytes by increasing both contractile force (% shortening or contraction amplitude) and accelerating relaxation (R50). ACVI functionally coupled to β1AR and was capable of coupling to β2AR only when the density of β2AR was increased by adenovirus-mediated overexpression. The increased contraction amplitude achieved by β1AR-activation of ACVI could not be reproduced by IBMX, indicating specialised compartmentalisation of cAMP for ACVI. AS suppressed increases in contraction mediated by ACVI overexpression, demonstrating that the effect was mediated specifically by ACVI. However, AS had little effect on basal cAMP, contraction, relaxation or forskolin responses indicating a minor role for ACVI in these parameters in the normal myocyte. AS suppressed β1AR-mediated contraction, especially in the presence of ACVI, giving increased evidence for preferential ACVI-β1AR coupling.
Functionality of overexpressed ACVI
Effectiveness of the recombinant ACVI-adenoviruses was demonstrated by Western blot detection of a strong 139 kDa band in both HEK 293 host cells and adult rat myocytes, indicating a three-fold overexpression. The effectiveness of the recombinant AS-adenoviruses was demonstrated by its removal of the weak endogenous ACVI expression in HEK 293 cells (LacZ condition). The endogenous ACVI expression in uninfected myocytes was weak or undetectable and was not used to determine the efficiency of AS. The specificity of the primary polyclonal anti-ACVI antibody was demonstrated in HEK293 cells by the abolition of the 139 kDa ACVI band intensity following competitive binding with the ACVI blocking peptide. In adult rat myocytes, the ACVI blocking peptide reduced, but did not abolish, the 139 kDa band intensity. Although these results suggest a low background, in myocytes, of another protein with a similar epitope to the immunogenic sequence in ACVI, this is unlikely to confound our finding of increased band intensity at 139 kDa after viral infection. The increase, in both HEK293 cells and myocytes, is evidence of full-length protein translation from the infecting DNA.
Functionality of overexpressed ACVI was shown by increased steady-state cAMP levels (two-fold increase) within ACVI myocytes in the absence of agonists. The increased cAMP levels were suppressed by antisense to ACVI, confirming the efficacy of AS. AS did not decrease native basal (GFP) cAMP levels, suggesting that basal cAMP turnover in the normal rat myocyte is not dependent on the ACVI isoform. ACV is a second predominantly expressed cyclase isoform in the mammalian heart and may therefore function in maintaining basal cAMP in myocytes (Espinasse et al., 1999; Novotny et al., 2003). In addition to ACV, we have also found expression of two other AC isoforms, ACIV and ACVII in the heart (Wang & Brown, 2001).
Overexpression of ACVI and cell death
Overexpression of ACVI decreased survival of cells in culture. This was due to the increase in ACVI expression rather than the viral infection per se since there was no excess myocyte death in AS-infected cultures, and AS was able to prevent this effect of ACVI overexpression. Both necrotic and apoptotic actions of βAR stimulation have been seen in myocardium (Woolf et al., 1976; Communal et al., 1998), although apoptosis may be related more to increases in Ca2+ secondary to cAMP-dependent activation of Ca2+ entry than to cAMP itself (Saito et al., 2001; Zhu et al., 2003). Since the myocytes were largely quiescent in culture, it would be predicted that Ca2+ entry-dependent apoptosis (or necrosis) would be even higher in contracting cells. It has been suggested that AC overexpression might be a valid therapeutic strategy for heart failure (Roth et al., 2002). However, our observations indicate that cell death would be a possible complicating factor for such an approach.
Increased basal contraction and accelerated relaxation with ACVI overexpression
Overexpression of ACVI constitutively increased basal contraction and accelerated relaxation (R50), consistent with the known effects of cAMP in myocytes and resembling (although smaller than) the effects of isoprenaline or forskolin. AS was effective in reversing the effects of ACVI, with both contraction and R50 returned to uninfected levels in SAS. For both basal contraction and R50, AS reduced effects of overexpressed ACVI but did not affect control cells. However, PKA antagonists also have little effect on basal contraction and relaxation in normal rat cells (Bell & McDermott, 1994), indicating that contractility in the absence of agonists is not dependent on any AC isoform.
Cyclic AMP-dependency of basal GFP and ACVI contraction
Carbachol inhibited the increase in basal contraction produced by ACVI overexpression, indicating the cAMP-dependence of ACVI contraction. Contraction stimulated by isoprenaline was reduced by carbachol to approximately the same level as that stimulated by ACVI overexpression. Carbachol reduces ACVI contraction via M2 muscarinic receptors and Pertussis Toxin-sensitive Gi to decrease βAR/Gs-stimulated increases in cAMP production (Katano & Endoh, 1993; Sandirasegarane & Diamond, 2004). The magnitude of increases in cAMP and contraction were similar to those observed in transgenic mice overexpressing ACVI (Gao et al., 2002).
Forskolin activation of ACVI contraction and lusitropy
Forskolin is a diterpene which directly activates ACVI to increase cAMP production without prior stimulation of βARs (Seamon et al., 1981; Gao et al., 1998; Gao et al., 2002). Forskolin-stimulated cAMP was raised in ACVI myocytes and there was also significant increase in contractile response that was retained even after subtraction of the raised basal contraction levels. The sensitivity of the myocytes, in terms of EC50 concentrations of forskolin, was not shifted in ACVI myocytes but the maximum effect was increased. Higher MOI levels of ACVI exacerbated arrhythmias produced by forskolin, limiting the completion of concentration–response curves in many cases. Forskolin decreased R50 in both control (GFP or uninfected) and ACVI myocytes, indicating that neither forskolin nor ACVI alone had produced maximal effects on relaxation. This could also be interpreted as evidence for separate pools of AC activated by forskolin or ACVI overexpression, and would be consistent with a preferential activation of ACV by forskolin. Antisense prevented the effect of ACVI overexpression on forskolin-induced increases in cAMP production or contraction amplitude (SAS condition). AS had no effect on native forskolin-induced increases in cAMP and only a small and nonsignificant effect on contractile responses to forskolin in control cells, indicating that another AC isoform is mediating effects of forskolin in normal rat ventricular myocytes.
Absence of isoprenaline effects on ACVI
There was a significant increase in cAMP mediated by the mixed β1- and β2AR agonist isoprenaline levels in ACVI cells but, surprisingly, no alteration in either the sensitivity or maximum contractile response. Even a 10-fold higher level of ACVI transfection was not able to alter the contractile response to isoprenaline. AS alone was unable to suppress ACVI contraction. The SAS experiments did, however, reveal a suppression of the native response to isoprenaline in terms of both cAMP production and contraction. As for cAMP production, AS was again more potent in reducing native responses in the presence of sense ACVI (SAS). A similar tendency was noted for the forskolin series (and xamoterol, Figure 10). We speculate that the presence of both full-length sense and antisense mRNA (SAS) was able to generate regions of double-stranded RNA, some of which was cleaved to form siRNA that then acted as a gene-silencing mechanism (McManus & Sharp, 2002). We also cannot rule out the possibility that AS is suppressing other AC isoforms since there is high homology among various isoforms of AC cDNA.
Functional coupling of ACVI to β1AR
Examining the β1- and β2AR responses individually went some way towards resolving the apparent discrepancy between the cAMP and contractile responses in ACVI myocytes. If isoprenaline had stimulated both β1- and β2ARs, the decrease in β2AR response may have offset the increased response through β1ARs and resulted in the lack of change in isoprenaline concentration–response curves between control and ACVI myocytes. Stimulation with the full agonist, isoprenaline, in the presence of a β2AR blocker produced results that were difficult to analyse because of arrhythmia generation at significantly lower concentrations. This, in itself, suggests that β2AR stimulation in the previous series, where isoprenaline was used without a β2AR blocker, may have been protective. Maximum contraction of the myocyte had been reached by isoprenaline even in control cells. This would prevent the detection of increases in maximum response to the full agonist after ACVI overexpression. To reduce the generation of arrhythmias and to produce submaximal stimulation of contraction while still retaining β1AR-selectivity, we used a β1AR-selective partial agonist, xamoterol. The increase in contraction with xamoterol (in the presence of a β2AR-antagonist, ICI 118, 551) was markedly enhanced in ACVI cells, and AS was effective at reducing both the overexpressed and native response. Inhibition of contraction by AS was again most effective in SAS, as was discussed previously for cAMP production, forskolin and isoprenaline-stimulated contraction. ACVI involvement in β1AR-mediated stimulation of contraction is clearly shown, indicating that ACVI was functionally coupled to β1AR.
The effect of xamoterol on ACVI myocyte was not simply due to the increase in bulk cAMP levels, since raising contraction amplitude to the same extent with the IBMX as with ACVI overexpression did not reproduce the enhancement of xamoterol-induced response. The inability of cumulative IBMX to mimic the ACVI response for xamoterol may indicate that locally produced cAMP in a subcellular compartment, such as caveloae, could be required to mediate the effects of β1AR stimulation (Xiang et al., 2002). The increased contractility of ACVI and its coupling to β1AR indicates that ACVI may be involved in regulating β1AR-mediated inotropic responses.
The contrast between the enhanced effect of specific β1AR stimulation in ACVI myocytes and the lack of alteration under nonspecific conditions suggested that simultaneous activation of the β2AR has prevented the expression of the β1AR-ACVI coupling. Suppression of β1AR responses by the β2AR has been noted before in mouse or rat ventricular myocytes (Gong et al., 2000; Zhang et al., 2000; Sato et al., 2004) and has been associated with Gi-dependent effects of the β2AR. The final mediator of the negative inotropic effect of β2AR-Gi signalling may be the Na+/Ca2+-exchanger of the sarcolemmal membrane (Sato et al., 2004), although whether this is a direct effect or via an intermediate pathway is not yet known. While the β2AR appeared to prevent the ACVI-induced increase in isoprenaline-stimulated contraction, it did not prevent the ACVI-induced increase in isoprenaline-stimulated cAMP production. An inhibitory effect on ACVI via Gi which can reduce activity of both ACVI and ACV isoforms (Taussig & Gilman, 1995), is therefore unlikely. An enhanced negative inotropic effect through β2AR/Gi, functionally offsetting the increased β1AR/Gs/ACVI-mediated positive inotropy, may be indicated. An increase in Gi (or the downstream components of the negative inotropic pathway) may have occurred in response to the constitutively raised cAMP levels, or the β2AR may have altered their spatial localisation within the membrane to associate to a greater extent with Gi and less with Gs.
Effect of β2AR overexpresssion on ACVI contraction
Within the native cell normally populated by a low density of β2AR, our results indicate that ACVI is not coupled to β2AR. When β2ARs are overexpressed to reach a higher density, ACVI becomes coupled to this receptor subtype. There is the possibility that Gi inhibition of ACVI contraction was only overcome when the numbers of β2AR were increased to couple to the Gs-mediated contractile pathway. Overexpression of ACVI in neonatal rat myocytes similarly showed a greater efficiency of coupling for the β1- rather than the β2AR (Ostrom et al., 2001). Like us, they found that the overexpressed β2ARs, in contrast to those naturally present in the cell membrane, could couple to ACVI to the same extent as β1ARs. These authors further showed that β1- and β2ARs and ACVI were normally colocalised within caveolar domains, and that desensitisation of the β2AR (but not the β1AR) occurred as the β2AR was translocated away from the caveolae. However, contractile measurements were not made in the neonatal myocytes, so that the contribution of non-cAMP-dependent inotropic pathways could not be assessed.
Summary
The effects of ACVI overexpression and downregulation on adult ventricular myocytes has demonstrated that ACVI has an important role in the regulation of cardiac contractility. ACVI is functionally coupled to β1AR regulation of the contractile force in the heart. Although the β2AR can couple to ACVI, changes in the myocyte after ACVI overexpression lead to a predominance of the negative inotropic pathways associated with β2AR stimulation. ACVI overexpression increased myocyte maximum response but did not alter Log EC50 response for all agonists including forskolin, isoprenaline, xamoterol and IBMX. Therefore, ACVI regulates contractility by increasing the potency of contraction without changing efficacy or sensitivity of the stimulant.
There is evidence that ACVI overexpression, like β1AR stimulation, causes cell death. In chronic heart failure, the coupling between β1AR and AC becomes disrupted and β1AR-mediated AC response decreases (Ping et al., 1997). Further decreases in βAR response by the use of β-blockers have been shown to be beneficial in this disease. The discovery of ACVI as the specific AC isoform that is coupled to β1AR has identified it as a possible target for therapeutic treatment of heart failure.
Acknowledgments
We thank the BHF (British Heart Foundation) for the funding of this project. We are grateful to Professor Dermott Cooper, Department of Pharmacology, University of Cambridge for the gift of the murine ACVI/pcDNA3 clone. We also like to express our heartfelt thanks to Mr Peter O'Gara for his technical expertise in isolation of the adult rat ventricular cardiomyocytes.
Abbreviations
- cAMP
adenosine 3′, 5′-cyclic monophosphate
- AS
antisense ACVI
- βAR
β adrenergic receptor
- β1AR
β1 adrenergic receptor
- β2AR
β2 adrenergic receptor
- CGP 20712A
(±)-2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl] amino]ethoxy]-benzamide methanesulfonate salt
- Emax
maximum % shortening
- GFP
green fluorescent protein isolated from Aequorea Victoria
- IBMX
3-isobutyl-1-methylxanthine
- ICI 118,551
(±)-1-[2,3-(dihydro-7-methyl-1H-inden-4-yl)oxy-3-[(1-methylethyl)amino-2-butanol)·hydrochloride
- KH
Krebs–Henseleit solution
- MOI
multiplicity of infection
- R50
time for relaxation –to 50%
- SAS
sense ACVI+antisense ACVI
References
- BELL D., MCDERMOTT B.J. Use of the cyclic AMP antagonist, Rp-cAMPS, to distinguish between cyclic AMP-dependent and cyclic AMP-independent contractile responses in rat ventricular cardiomyocytes. J. Mol. Cell. Cardiol. 1994;26:1439–1448. doi: 10.1006/jmcc.1994.1163. [DOI] [PubMed] [Google Scholar]
- CHAUDHRI B., DEL MONTE F., HAJJAR R.J., HARDING S.E. Contractile effects of adenovirally-mediated increases in SERCA2a activity: a comparison between adult rat and rabbit ventricular myocytes. Mol. Cell. Biochem. 2003;251:103–109. [PubMed] [Google Scholar]
- COMMUNAL C., SINGH K., PIMENTEL D.R., COLUCCI W.S. Norepinephrine stimulates apoptosis in adult rat ventricular myocytes by activation of the β-adrenergic pathway. Circulation. 1998;98:1329–1334. doi: 10.1161/01.cir.98.13.1329. [DOI] [PubMed] [Google Scholar]
- COOPER D.M. Molecular and cellular requirements for the regulation of adenylate cyclases by calcium. Biochem. Soc. Trans. 2003;31:912–915. doi: 10.1042/bst0310912. [DOI] [PubMed] [Google Scholar]
- ESPINASSE I., IOURGENKO V., DEFER N., SAMSON F., HANOUNE J., MERCADIER J.J. Type V, but not type VI, adenylyl cyclase mRNA accumulates in the rat heart during ontogenic development. Correlation with increased global adenylyl cyclase activity. J. Mol. Cell. Cardiol. 1995;27:1789–1795. doi: 10.1016/0022-2828(95)90002-0. [DOI] [PubMed] [Google Scholar]
- ESPINASSE I., IOURGENKO V., RICHER C., HEIMBURGER M., DEFER N., BOURIN M.C., SAMSON F., PUSSARD E., GIUDICELLI J.F., MICHEL J.B., HANOUNE J., MERCADIER J.J. Decreased type VI adenylyl cyclase mRNA concentration and Mg(2+)-dependent adenylyl cyclase activities and unchanged type V adenylyl cyclase mRNA concentration and Mn(2+)-dependent adenylyl cyclase activities in the left ventricle of rats with myocardial infarction and longstanding heart failure. Cardiovasc. Res. 1999;42:87–98. doi: 10.1016/s0008-6363(98)00283-1. [DOI] [PubMed] [Google Scholar]
- GAO M.H., BAYAT H., ROTH D.M., YAO Z.J., DRUMM J., BURHAN J., HAMMOND H.K. Controlled expression of cardiac-directed adenylyl cyclase type VI provides increased contractile function. Cardiovasc. Res. 2002;56:197–204. doi: 10.1016/s0008-6363(02)00539-4. [DOI] [PubMed] [Google Scholar]
- GAO M., PING P., POST S., INSEL P.A., TANG R., HAMMOND H.K. Increased expression of adenylyl cyclase type VI proportionately increases β-adrenergic receptor-stimulated production of cAMP in neonatal rat cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 1998;95:1038–1043. doi: 10.1073/pnas.95.3.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONG H., ADAMSON D.L., RANU H.K., KOCH W.J., HEUBACH J.F., RAVENS U., ZOLK O., HARDING S.E. The effect of Gi-protein inactivation on basal, β1- and β2AR-stimulated contraction of myocytes from transgenic mice overexpressing the β2-adrenoceptor. Br. J. Pharmacol. 2000;131:594–600. doi: 10.1038/sj.bjp.0703591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONG H., SUN H., KOCH W.J., RAU T., ESCHENHAGEN T., RAVENS U., HEUBACH J.F., ADAMSON D.L., HARDING S.E. The specific β2AR blocker, ICI 118,551, actively decreases contraction through a Gi-coupled form of the β2AR in myocytes from failing human heart. Circulation. 2002;105:2497–2503. doi: 10.1161/01.cir.0000017187.61348.95. [DOI] [PubMed] [Google Scholar]
- ISHIKAWA Y., GRANT B.S., OKUMURA S., SCHWENCKE C., YAMAMOTO M. Immunodetection of adenylyl cyclase protein in tissues. Mol. Cell. Endocrinol. 2000;162:107–112. doi: 10.1016/s0303-7207(00)00210-0. [DOI] [PubMed] [Google Scholar]
- KATANO Y., ENDOH M. Cyclic AMP metabolism in intact rat ventricular cardiac myocytes: interaction of carbachol with isoproterenol and 3-isobutyl-1-methylxanthine. Mol. Cell. Biochem. 1993;119:195–201. doi: 10.1007/BF00926871. [DOI] [PubMed] [Google Scholar]
- KATSUSHIKA S., CHEN L., KAWABE J., NILAKANTAN R., HALNON N.J., HOMCY C.J., ISHIKAWA Y. Cloning and characterization of a sixth adenylyl cyclase isoform: types V and VI constitute a subgroup within the mammalian adenylyl cyclase family. Proc. Natl. Acad. Sci. U.S.A. 1992;89:8774–8778. doi: 10.1073/pnas.89.18.8774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUMANN A.J., HALL J.A., MURRAY K.J., WELLS F.C., BROWN M.J. A comparison of the effects of adrenaline and noradrenaline on human heart: the role of β1- and β2-adrenoceptors in the stimulation of adenylate cyclase and contractile force. Eur. Heart J. 1989;10:29–37. doi: 10.1093/eurheartj/10.suppl_b.29. [DOI] [PubMed] [Google Scholar]
- KUZNETSOV V., PAK E., ROBINSON R.B., STEINBERG S.F. β2-Adrenergic receptor actions in neonatal and adult rat ventricular myocytes. Circ. Res. 1995;76:40–52. doi: 10.1161/01.res.76.1.40. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J., ENGELHARDT S., ESCHENHAGEN T. What is the role of β-adrenergic signaling in heart failure. Circ. Res. 2003;93:896–906. doi: 10.1161/01.RES.0000102042.83024.CA. [DOI] [PubMed] [Google Scholar]
- MCMANUS M.T., SHARP P.A. Gene silencing in mammals by small interfering RNAs. Nat. Rev. Genet. 2002;3:737–747. doi: 10.1038/nrg908. [DOI] [PubMed] [Google Scholar]
- NOVOTNY J., HRBASOVA M., KOLAR F., SVOBODA P. Cardiomegaly induced by pressure overload in newborn rats is accompanied by altered expression of the long isoform of G(s)alpha protein and deranged signaling of adenylyl cyclase. Mol. Cell. Biochem. 2003;245:157–166. doi: 10.1023/a:1022828430565. [DOI] [PubMed] [Google Scholar]
- OSTROM R.S., GREGORIAN C., DRENAN R.M., XIANG Y., REGAN J.W., INSEL P.A. Receptor number and caveolar co-localization determine receptor coupling efficiency to adenylyl cyclase. J. Biol. Chem. 2001;276:42063–42069. doi: 10.1074/jbc.M105348200. [DOI] [PubMed] [Google Scholar]
- PATERSON J.M., SMITH S.M., SIMPSON J., GRACE O.C., SOSUNOV A.A., BELL J.E., ANTONI F.A. Characterisation of human adenylyl cyclase IX reveals inhibition by Ca(2+)/calcineurin and differential mRNA polyadenylation. J. Neurochem. 2000;75:1358–1367. doi: 10.1046/j.1471-4159.2000.0751358.x. [DOI] [PubMed] [Google Scholar]
- PING P., ANZAI T., GAO M., HAMMOND H.K. Adenylyl cyclase and G protein receptor kinase expression during development of heart failure. Am. J. Physiol. 1997;273:H707–H717. doi: 10.1152/ajpheart.1997.273.2.H707. [DOI] [PubMed] [Google Scholar]
- RAIMUNDO S., GIRAY J., VOLFF J.N., SCHWAB M., ALTENBUCHNER J., RATGE D., WISSER H. Cloning and sequence of partial cDNAs encoding the human type V and VI adenylyl cyclases and subsequent RNA-quantification in various tissues. Clin. Chim. Acta. 1999;285:155–161. doi: 10.1016/s0009-8981(99)00067-4. [DOI] [PubMed] [Google Scholar]
- ROTH D.M., BAYAT H., DRUMM J.D., GAO M.H., SWANEY J.S., ANDER A., HAMMOND H.K. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- SAITO S., HIROI Y., ZOU Y., AIKAWA R., TOKO H., SHIBASAKI F., YAZAKI Y., NAGAI R., KOMURO I. β-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. J. Biol. Chem. 2001;275:34528–34533. doi: 10.1074/jbc.M002844200. [DOI] [PubMed] [Google Scholar]
- SANDIRASEGARANE L., DIAMOND J. Pertussis toxin-sensitive G protein but not NO/cGMP pathway mediates the negative inotropic effect of carbachol in adult rat cardiomyocytes. Pharmacol. 2004;70:46–56. doi: 10.1159/000074242. [DOI] [PubMed] [Google Scholar]
- SATO M., GONG H., TERRACCIANO C.M., RANU H., HARDING S.E. Loss of β-adrenoceptor response in myocytes overexpressing the Na+/Ca(2+)-exchanger. J. Mol. Cell. Cardiol. 2004;36:43–48. doi: 10.1016/j.yjmcc.2003.09.010. [DOI] [PubMed] [Google Scholar]
- SCHWENCKE C., OKUMURA S., YAMAMOTO M., GENG Y.J., ISHIKAWA Y. Colocalization of β-adrenergic receptors and caveolin within the plasma membrane. J. Cell. Biochem. 1999a;75:64–72. doi: 10.1002/(sici)1097-4644(19991001)75:1<64::aid-jcb7>3.3.co;2-c. [DOI] [PubMed] [Google Scholar]
- SCHWENCKE C., YAMAMOTO M., OKUMURA S., TOYA Y., KIM S.J., ISHIKAWA Y. Compartmentation of cyclic adenosine 3′, 5′-monophosphate signaling in caveolae. Mol. Endocrinol. 1999b;13:1061–1070. doi: 10.1210/mend.13.7.0304. [DOI] [PubMed] [Google Scholar]
- SEAMON K.B., PADGETT W., DALY J.W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. U.S.A. 1981;78:3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOSUNOV S.A., KEMAIKIN S.P., KURNIKOVA I.A., ANTONI F.A., SOSUNOV A.A. Expression of adenylyl cyclase type IX and calcineurin in synapses of the central nervous system. Bull. Exp. Biol. Med. 2001;131:172–175. doi: 10.1023/a:1017556315238. [DOI] [PubMed] [Google Scholar]
- TAUSSIG R., GILMAN A.G. Mammalian membrane-bound adenylyl cyclases. J. Biol. Chem. 1995;270:1–4. doi: 10.1074/jbc.270.1.1. [DOI] [PubMed] [Google Scholar]
- WANG T., BROWN M.J. Influence of β1-adrenoceptor blockade on the gene expression of adenylate cyclase subtypes and β-adrenoceptor kinase in human atrium. Clin. Sci. 2001;101:211–217. [PubMed] [Google Scholar]
- WOOLF N., DAVIES M.J., SHAW M.J., TRICKEY R.J. Experiences with isoprenaline induced myocardial necrosis in the rat. J. Pathol. 1976;120:65–73. doi: 10.1002/path.1711200202. [DOI] [PubMed] [Google Scholar]
- XIAO R.-P., HOHL C.M., ALTSCHULD R.A., JONES L., LIVINGSTON B., ZIMAN B., TANTINI B., LAKATTA E.G. β2-Adrenergic receptor-stimulated increase in cAMP in rat heart cells is not coupled to change in Ca2+ dynamics, contractility or phospholamban phosphorylation. J. Biol. Chem. 1994;269:19151–19156. [PubMed] [Google Scholar]
- XIANG Y., RYBIN V.O., STEINBERG S.F., KOBILKA B. Caveolar localization dictates physiologic signaling of β2-adrenoceptors in neonatal cardiac myocytes. J. Biol. Chem. 2002;277:34280–34286. doi: 10.1074/jbc.M201644200. [DOI] [PubMed] [Google Scholar]
- XIAO R.P., ZHANG S.J., CHAKIR K., AVDONIN P., ZHU W., BOND R.A., BALKE C.W., LAKATTA E.G., CHENG H. Enhanced G(i) signaling selectively negates β2-adrenergic receptor (AR) – but not β1-AR-mediated positive inotropic effect in myocytes from failing rat hearts. Circulation. 2003;108:1633–1639. doi: 10.1161/01.CIR.0000087595.17277.73. [DOI] [PubMed] [Google Scholar]
- ZACCOLO M., POZZAN T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Sci. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- ZHANG S.J., CHENG H., ZHOU Y.Y., WANG D.J., ZHU W., ZIMAN B., SPURGOEN H., LEFKOWITZ R.J., LAKATTA E.G., KOCH W.J., XIAO R.P. Inhibition of spontaneous β2-adrenergic activation rescues β1-adrenergic contractile response in cardiomyocytes overexpressing β2-adrenoceptor. J. Biol. Chem. 2000;275:21773–21779. doi: 10.1074/jbc.M909484199. [DOI] [PubMed] [Google Scholar]
- ZHOU Y.Y., YANG D., ZHU W.Z., ZHANG S.-J., WANG D.J., ROHRER D., DEVIC E., KOBILKA B.K., LAKATTA E.G., CHENG H., XIAO R.-P. Spontaneous activation of β2- but not β1-adrenoceptors expressed in cardiac myocytes from β1β2 double knockout mice. Mol. Pharmacol. 2001;58:887–894. doi: 10.1124/mol.58.5.887. [DOI] [PubMed] [Google Scholar]
- ZHU W.Z., WANG S.Q., CHAKIR K., YANG D., ZHANG T., BROWN J.H., DEVIC E., KOBILKA B.K., CHENG H., XIAO R.P. Linkage of β1-adrenergic stimulation to apoptotic heart cell death through protein kinase A-independent activation of Ca2+/calmodulin kinase II. J. Clin. Invest. 2003;111:617–625. doi: 10.1172/JCI16326. [DOI] [PMC free article] [PubMed] [Google Scholar]