Abstract
The concept that nitric oxide (NO) release can be beneficial in inflammatory conditions has raised more attention in the recent years, particularly with the development of nitric oxide-releasing anti-inflammatory drugs. There is considerable evidence that NO is capable of enhancing the anti-inflammatory benefits of conventional anti-inflammatory drugs.
Since hydrocortisone is the most widely used anti-inflammatory drug for the treatment of skin inflammation, we compared the anti-inflammatory effects of hydrocortisone to an NO-releasing derivative of hydrocortisone, NCX 1022, in a murine model of irritant contact dermatitis, induced by epidermal application of benzalkonium chloride.
Topical pre- and post-treatment with NCX 1022 (3 nmol) in C57BL6 mice not only reduced ear oedema formation in a dose-dependent manner, but also was significantly more effective than the parent compound during the initial stages of inflammation (from 1 to 5 h). NCX 1022, but not hydrocortisone, significantly inhibited granulocyte recruitment (tissue myeloperoxidase activity). Histological samples of mouse ears treated with NCX 1022 showed significant reduction in both the number of infiltrated cells and disruption of the tissue architecture compared to hydrocortisone-treated tissues.
With intravital microscopy, we observed that both pre- and post-treatments with NCX 1022 were more effective than hydrocortisone in terms of inhibiting benzalkonium chloride-induced leukocyte adhesion to the endothelium, without affecting the flux of rolling leukocytes or venule diameter.
These results suggest that by releasing NO, NCX 1022 modulates one of the early events of skin inflammation: the recruitment of leukocytes to the site of inflammation. Overall, we have shown that NO-hydrocortisone provided faster and greater protective effects, reducing major inflammatory parameters (leukocyte adhesion and recruitment, oedema formation, tissue disruption) compared to its parental compound.
Keywords: Inflammation, nitric oxide, skin, neutrophils, dermatitis
Introduction
Topical treatment with glucocorticoids (GCs) in general and hydrocortisone in particular constitutes the most common form of treatment for inflammatory dermatological disorders (Ahluwalia, 1998). The anti-inflammatory effects of GCs start by interaction with the glucocorticoid receptor, resulting in the formation of glucocorticoid–glucocorticoid receptor complex, which translocates to the nucleus. This complex can then modulate inflammatory responses through several different mechanisms: (i) by interacting with specific DNA sequences, known as glucocorticoid responsive elements (GRE), for activation or repression of genes; (ii) via protein–protein interaction, inhibiting the activities of various pro-inflammatory transcriptional factors such as activator protein-1 (AP-1) and the nuclear factor (NF)-κB; or (iii) by enhancing the degradation of specific mRNAs (DiDonato et al., 1996; Saklatvala, 2002). Through these mechanisms, GCs are able to inhibit the synthesis of most of the pro-inflammatory mediators (examples include pro-inflammatory cytokines, adhesion molecules, cyclooxygenases, phospholipases A2, chemokines), and to promote the synthesis of anti-inflammatory molecules such as annexin 1, secretory leukocyte inhibitory protein, interleukin-1 receptor antagonist, and IκBα (Goulding et al., 1998; Perretti & Ahluwalia, 2000). However, steroid action is not limited to molecules involved in inflammation, and long-term use of GCs is associated with multiple side effects on metabolism, hypothalamopituitary axis (HPA) function, and cell growth. Although suppression of HPA function is negligible with topical administration of low doses of GCs on the skin, this effect may occur with higher doses (Ellison et al., 2000). Moreover, a strong allergic potential of topical GCs has been described in the skin of patients who need chronic GC treatments (Leung & Bieber, 2003).
Other side effects of skin exposure to GCs include visual loss with increased risk for glaucoma (Aggarwal et al., 1993), or development of Cushing syndrome (Teelucksingh et al., 2002), and skin atrophia. Thus, the generation of modified GC's with reduced side effects and higher potency will have important clinical benefits. The recent development of nitric oxide (NO)-releasing anti-inflammatory drugs has highlighted the important therapeutic benefits of modulating NO pathways. Recently, a derivative of prednisolone, NCX 1015, has been shown to be a more potent anti-inflammatory agent than its parent compound in different models (Paul-Clark et al., 2000). In a rodent model of collagen-induced arthritis, and in acute peritonitis, NCX 1015 was shown to reduce inflammation with minimal side effects as compared to prednisolone (Paul-Clark et al., 2000; 2002). The studies performed with NCX 1015 suggested that a new class of glucocorticoids, the nitro-steroids, were endowed with enhanced anti-inflammatory properties and reduced side effects (Perretti et al., 2003). Considering that corticosteroids in general, and hydrocortisone in particular, represent the treatment of choice for most of the inflammatory diseases of the skin, there is a strong need to access the potential properties of nitro-steroids in skin inflammation.
The aim of this study was to compare the efficacy of NO-hydrocortisone, NCX 1022, to its parent compound at inhibiting inflammatory processes in a model of acute toxic contact dermatitis. In order to monitor cutaneous inflammation, we measured tissue myeloperoxidase (MPO) activity as an index of granulocyte infiltration, we also measured oedema formation and performed histological analysis of the tissue to evaluate morphological changes. Finally, we used intravital microscopy to examine the effects of NCX 1022 and its parent compound on leukocyte/endothelial cell interactions. We investigated both preventive and curative properties of hydrocortisone and NCX 1022.
Methods
Animals
Male C57BL6 mice (6–8-weeks old) were obtained from Charles River Laboratories (Montreal, Quebec, Canada). The mice were kept at room temperature and had free access to food and water. The Animal Care and Ethic Committees of the University of Calgary approved all experimental protocols, which followed the guidelines of the Canadian Council on Animal Care.
Chemicals
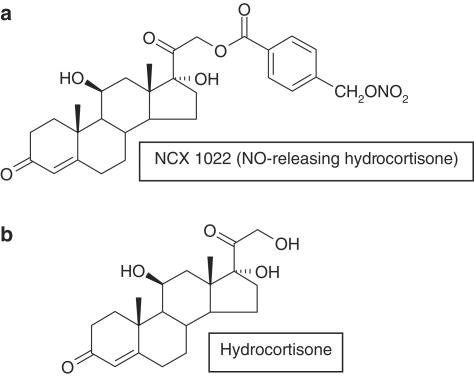
NCX 1022 (NO-hydrocortisone) and hydrocortisone were obtained from NicOx (Sophia-Antipolis, France). Figure 1 refers to the chemical structures of NCX 1022 and hydrocortisone. Benzalkonium chloride was obtained from Sigma (St Louis, U.S.A.). Diethylenetriamine-NONOate (DETA-NONOate) was purchased from Caiman (Ann Arbor, MI, U.S.A.).
Figure 1.
Chemical structure of NCX 1022 (NO-hydrocortisone): hydrocortisone 21-[4′-(nitrooxymethyl) benzoate] (a) and hydrocortisone (b).
Dermatitis induction and treatments
Irritant contact dermatitis was induced by applying 5% benzalkonium chloride (Sigma, St Louis, MO, U.S.A.), dissolved in olive oil : acetone (1 : 5 v v−1) on the surface of either the dorsal aspect of both ears (20 μl per site), or on the observed unshaved abdominal skin (intravital microscopy experiments) as previously described (Seeliger et al., 2003). Groups of mice (6–8-weeks old) have received topically (100 μl per site applied directly on the skin) treatment with hydrocortisone (0.3, 3 or 30 nmol per site), NO-hydrocortisone (0.3, 3 or 30 nmol per site) or their vehicle (ethanol : sterile water 1 : 1), 15 min before (pre-treatment), or 5 min after (post-treatment) the induction of irritant contact dermatitis (Yamaguchi et al., 2000). Animals were treated with DETA-NONOate (3 nmol) or its vehicle (ethanol : sterile water 1 : 1), topically 5 min after benzalkonium chloride application. The dose of DETA-NONOate was selected such that it could have an equal number of NO-releasing moieties, on a molar basis, to that of NCX 1022. DETA-NONOate spontaneously releases NO in aqueous solution.
Ear oedema and MPO activity measurements
Using an electronic calliper (Mitutoyo, Canada, resolution 0.01 mm), ear thickness was measured as a parameter for oedema formation, before and hourly for 8 h, after contact dermatitis induction. After the 8-h time-point measurements, the mice were euthanized, the left ear was harvested for histological examination, and the right ear was harvested for MPO activity measurement as an index of granulocyte infiltration. Tissues collected to measure MPO activity were processed as previously described (Steinhoff et al., 2000; Cenac et al., 2002). Briefly, tissue samples were cut into smaller pieces and homogenized in a solution of 0.5% hexadecyltrimethylammonium bromide dissolved in phosphate buffer solution (pH 6.0) for 1 min. The homogenized tissues were centrifuged at 13,000 × g for 2 min in a refrigerated centrifuge. Supernatants were added to a buffer supplemented with 1% hydrogen peroxide, and O-dianisidine dihydrochloride solution into well plate. Optical density readings were taken for 1 min at 30 s interval at 450 nm.
Histology
The left ears of mice were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections of 6 μm were cut and mounted on colourfrost microslide (VWR scientific, Edmonton, AB, Canada). The sections were dried overnight and stained with haematoxylin and eosin (H&E) in accordance with classical methods of histology. Photographs of sections representing each treatment group were taken using a SONY DSC-S75 digital camera attached to a microscope (Zeiss).
Intravital microscopy experiments
C57BL6 male mice were anaesthetized by intraperitoneal (i.p.) injection of a mixture of 10 mg kg−1 xylazine (MTC Pharmaceuticals, Cambridge, Ontario, Canada) and 200 mg kg−1 ketamine hydrochloride (Rogar/STB, London, Ontario, Canada). Intravital microscopy was performed on skin flaps, the thickness of which does not permit visualizing leukocyte/endothelial cells interaction by simple trans-illumination. Therefore, after anaesthesia, mice received an intravenous injection of a fluorescent dye, rhodamine 6G (Sigma, St Louis, MO, U.S.A., 0.3 mg kg−1). At this dose, rhodamine 6G labels leukocytes and platelets, and has been shown to have no effect on leukocyte kinetics (Nolte et al., 1994; Baatz et al., 1995). Then, a midline abdominal incision was performed, from the diaphragm, extending to the pelvic region. The skin was carefully separated from the underlying tissue, but remained attached laterally, so the blood supply to the skin flap remained intact (Nolte et al., 1994; Hickey et al., 1999). The skin flap was extended over a viewing pedestal to expose the dermal microvasculature and secured along the edges using 4.0 sutures. The exposed dermal tissues were superfused with a bicarbonate-buffered saline pH 7.4, to avoid tissue dehydration. The microcirculation was observed using an inverted microscope (Nikon) with a × 20 objective lens, and rhodamine 6G allowed visualization and quantification of the number of rolling and adherent leukocytes, by epi-illumination at 510–560 nm, using a 590-nm emission filter. Single unbranched venules (20–40 μm in diameter) were selected for the study. Images of the selected venule were recorded for 5 min, after a 15-min equilibration period, and the end of this 5-min interval was considered as time 0 (Vergnolle, 1999; Vergnolle et al., 2002; Seeliger et al., 2003). Leukocyte adherence was determined upon video playback, on 100 μm vessel length. A leukocyte was considered adherent to the endothelium if it remained stationary for 30 s or more. Leukocyte flux was defined as the number of leukocytes per minute moving at a velocity less than that of erythrocytes, which passed a reference point in the venule. The changes in flux of rolling leukocytes were evaluated as differences between the number of rolling leukocytes at each interval and the basal number of rolling leukocytes.
Images of the selected venule were then recorded for 5 min before the induction of dermatitis, and then at different intervals beginning 15, 30, 45, 60, 75, 90, and 120 min after the induction of contact dermatitis.
Statistical analysis
The results are expressed as mean±s.e.m. Statistical comparisons among groups were performed using a one-way analysis of variance followed by the Student–Newman–Keuls test, where a probability (P-value) of less than 0.05 was considered to be significant.
Results
Effects of preventive treatment with hydrocortisone and NCX 1022 on the development of dermatitis
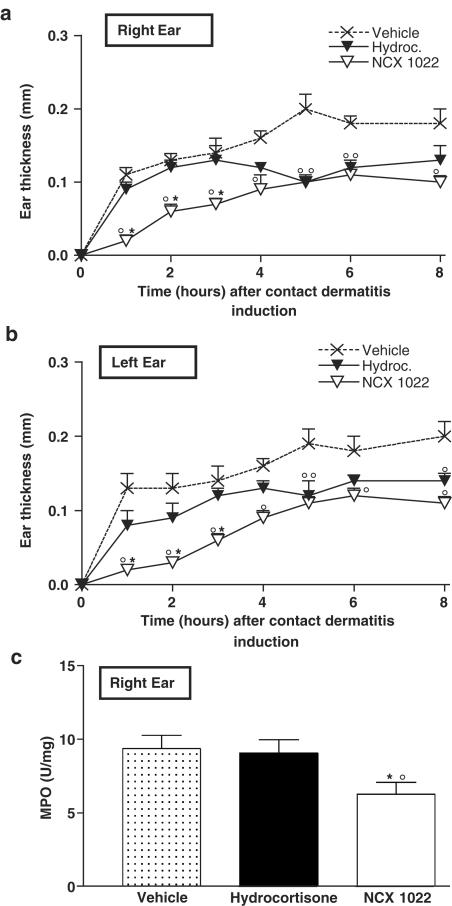
Epidermal application of benzalkonium chloride to mouse ear provoked skin inflammation characterized by two of the main features of inflammation: swelling (oedema) and increased granulocyte infiltration, as measured by increased MPO activity (Figure 2a–c). Hydrocortisone pre-treatment (3 nmol per ear) significantly reduced ear oedema formation from 5 h after the induction of dermatitis until 8 h. In mice that had received a pre-treatment with NCX 1022 (3 nmol per ear), oedema formation was significantly reduced as early as the first hour after the induction of dermatitis, and this reduction was still significant 8 h later (Figure 2a, b). The inhibition of irritant dermatitis-induced oedema by NCX 1022 was significantly greater as compared to the effects of hydrocortisone in the initial phase of inflammation (for the first 3 h).
Figure 2.
Changes in ear thickness (a, b) and MPO activity (c), after the induction of irritant contact dermatitis in male C57BL6 mice pre-treated with NCX 1022 (3 nmol), hydrocortisone (3 nmol), or their vehicle (ethanol : sterile water, 1 : 1). MPO was measured 8 h after the induction of contact dermatitis. N=8 per group, *significantly different from hydrocortisone-treated group, °significantly different from vehicle-treated group (P<0.05).
While benzalkonium chloride-induced contact dermatitis caused a significant increase in granulocyte infiltration in mouse ear, hydrocortisone pre-treatment failed to reduce this infiltration of inflammatory cells. However, NO-hydrocortisone pre-treatment significantly reduced MPO activity in mouse ear after the induction of dermatitis (Figure 2c).
Effects of curative treatment with hydrocortisone and NCX 1022 on the development of dermatitis
Similar to pre-treatment, post-treatment (5 min after dermatitis induction) with hydrocortisone started to significantly reduce ear oedema formation only 5 h after the induction of dermatitis, while NCX 1022 was effective as soon as the first hour (Figure 3a, b). Comparing Figure 2a, b and Figure 3a, b, it is interesting to note that curative treatment with NCX 1022 seems to be even more effective than preventive treatment to reduce the formation of oedema. The benzalkonium-induced increase in ear thickness was inferior to 0.04 mm in mice post-treated with NCX 1022, while it could reach 0.1 mm in mice that have received a pre-treatment with NCX 1022. The inhibitory effects of NCX 1022 on oedema formation were dose-dependent (0.3–30 nmol per ear), as shown in Figure 3c, and lasted for at least 8 h (Figure 3a, b).
Figure 3.
Changes in ear thickness (a, b, c, and e) and MPO activity (d and f), after the induction of irritant contact dermatitis in male C57BL6 mice post-treated with 3 nmol (a, b) of NCX 1022, hydrocortisone, or their vehicle (ethanol : sterile water, 1 : 1), or 3 nmol of DETA-NONOate or its vehicle (ethanol : sterile water, 1 : 1) (e). MPO was measured 8 and 6 h after the induction of contact dermatitis respectilively in (d) and (f). Dosage response was performed using reported doses of NO-hydrocortisone and hydrocortisone (0.3, 3, and 30 nmol); measuring change in ear thickness (c), 1 h after the induction of dermatitis. N=8 per group. *significantly different from hydrocortisone-treated group, °significantly different from vehicle-treated group (P<0.05).
Post-treatment with hydrocortisone did not modify the increased granulocyte infiltration induced by benzalkonium application, but NCX 1022 reduced by 63% the MPO activity (Figure 3d), producing a maximum effect at the dose of 3 nmol per ear (dose-response not shown).
Post-treatments with both hydrocortisone and NCX 1022 were more effective at reducing dermatitis-induced oedema than pre-treatments, the increased ear thickness being back to normal values for both drugs given 5 h after dermatitis induction. Hence, NCX 1022 was more effective and acted earlier than its parent compound.
Post-treatment with the NO donor DETA-NONOate had no effect on the dermatitis-induced generation of ear oedema (Figure 3e) or granulocyte infiltration, as observed by similar MPO levels in vehicle or NONOate-treated mice (Figure 3f).
Effects of hydrocortisone and NO-releasing hydrocortisone on tissue architecture
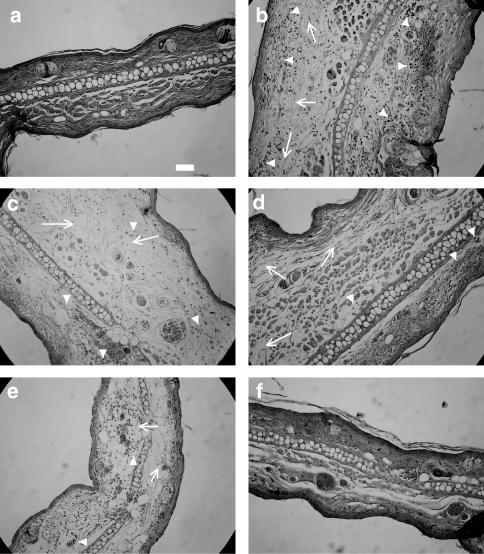
As illustrated in Figure 4, the induction of dermatitis (8-h time point) caused large tissue disruption and inflammatory cell infiltration (arrow heads in panel b) compared to normal tissues (panel a). Tissues from mice that had received pre- or post-treatment with hydrocortisone (Figure 4, panels c and d, respectively) showed very slight reduction of tissue architecture disruption (arrows) compared to vehicle-treated mouse ears (Figure 4, panel b), and still a large infiltration of inflammatory cells. In contrast, 8 h after the induction of dermatitis, the architecture of conjunctive tissues from mice treated with NCX 1022 (Figure 4, panels e and f), was less disrupted than what was observed in vehicle- or hydrocortisone-treated mouse ears. In the case of post-treatment with NCX 1022 (Figure 4, panel f), the histological appearance of tissues was very similar to that of naïve mice (Figure 4, panel a), and no clear inflammatory cell infiltration was observed.
Figure 4.
Histological appearance of mouse ear (left ear), 8 h after the induction of dermatitis (panels b–f) or of naïve animals (panel a). Mice received a topical treatment (5 min before or after the induction of dermatitis) with vehicle (panel b), pre- and post-hydrocortisone (panels c and d, respectively) or pre- and post-NCX 1022 (panels e and f, respectively). Hydrocortisone and NCX 1022 were used at the dose of 3 nmol. Scale bar: 50 μm.
Effects of NCX 1022 and hydrocortisone on vessel diameter, leukocyte rolling, and adhesion
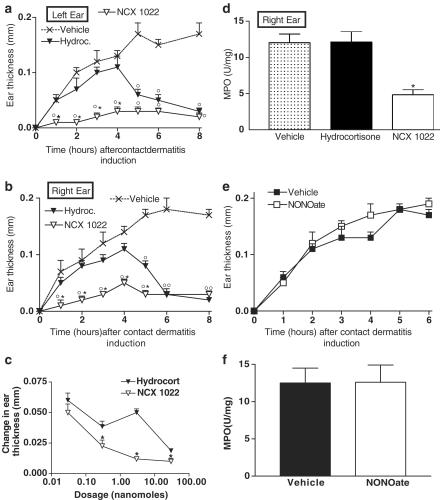
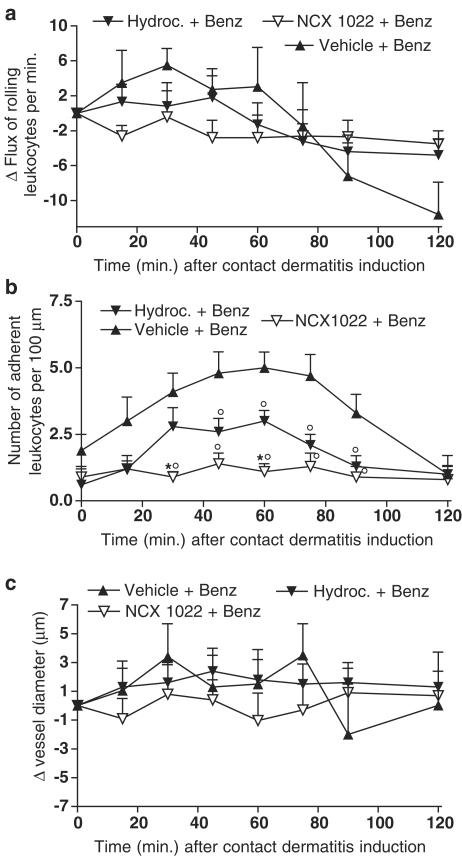
In an attempt to elucidate the mechanism behind the anti-inflammatory effects of NCX 1022, we used intravital microscopy to test whether the movement of leukocytes was modulated by NCX 1022 treatment. The induction of irritant contact dermatitis on the abdominal skin of mice did not provoke significant variations in flux of rolling leukocytes for the first 120 min (Figure 5a), but did provoke a significant increase in the number of leukocytes adherent to the vessel wall as compared to basal values (Figure 5b). Hydrocortisone pre-treatment reduced contact dermatitis-induced increased leukocyte adhesion from 50 to 90 min after dermatitis induction (Figure 5b), but had no significant effect on flux of rolling leukocytes (Figure 5a). NCX 1022 was significantly more potent than hydrocortisone in reducing contact dermatitis-induced leukocyte adhesion, particularly at the early time points (e.g., 30–60 min after dermatitis induction; Figure 5b). At each time point, NCX 1022 was able to keep the number of adherent leukocytes similar to basal levels, completely abolishing the effects of dermatitis on leukocyte/endothelium adherence (Figure 5b). Like hydrocortisone, NCX 1022 had no effect on flux of rolling leukocytes (Figure 5a). Vessel diameter was not affected by benzalkonium treatment compared to basal values (Figure 5c). Hydrocortisone and NCX 1022 treatments did not affect either venule diameter (Figure 5c).
Figure 5.
Changes in flux of rolling leukocytes (a), number of adherent leukocytes (b) or vessel diameter (c), in naïve mice and mice that had irritant contact dermatitis (+ Benz.) and that were pre-treated with NCX 1022, hydrocortisone or their vehicle. Hydrocortisone and NCX 1022 were used at the dose of 3 nmol. Naïve group n=7; Vehicle + Benz. group n=8; Hydroc. + Benz. n=8; NCX 1022. + Benz. n=9; *significantly different from hydrocortisone-treated group (P<0.05), °significantly different from vehicle-treated group (P<0.05).
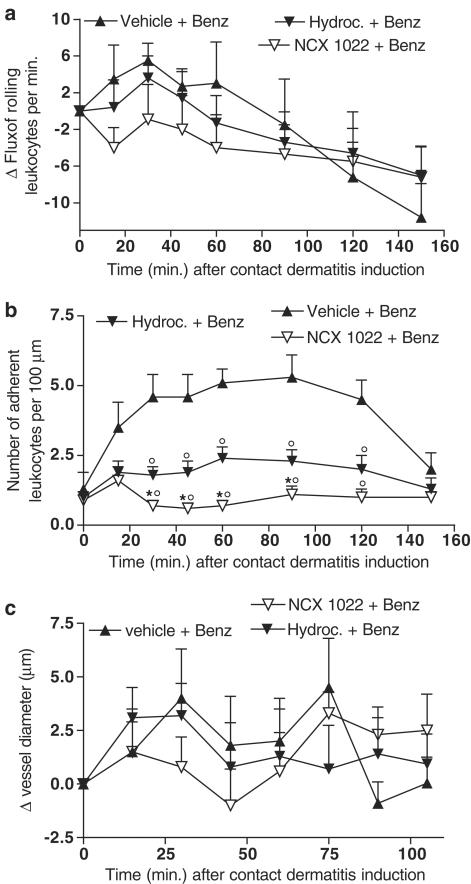
Post-treatment with hydrocortisone reduced contact dermatitis-induced increases in leukocyte adhesion to the vessel wall (Figure 6b). However, NCX 1022 was significantly more potent than hydrocortisone at reducing the number of leukocytes adhering to the endothelial wall, keeping that level similar to basal level (Figure 6b). As seen with pre-treatment, vehicle, hydrocortisone, and NCX 1022 post-treatment had no significant effect on flux of rolling leukocytes or vessel diameter (Figure 6a, c). Comparing curative versus preventive effects of hydrocortisone and NO-hydrocortisone, it appears that for both drugs, curative treatment is more effective, keeping the number of adherent leukocytes inferior to a mean of 2.5 leukocytes per 100 μm vessel length (Figure 6b compared to Figure 5b).
Figure 6.
Changes in flux of rolling leukocytes (a), number of adherent leukocytes (b) or vessel diameter (c), in naïve mice and mice that had irritant contact dermatitis (+ Benz.) and that were treated 5 min after the induction of dermatitis, with NCX 1022 (30 nmol), hydrocortisone (30 nmol) or their vehicle. Vehicle + Benz. group n=8; Hydroc. + Benz. n=9; NCX 1022. + Benz. n=9; *significantly different from hydrocortisone-treated group (P<0.05), °significantly different from the vehicle-treated group (P<0.05).
Discussion
NO is one of the most dynamic compounds affecting various physiological and cellular processes in the body, and in particular inflammatory processes (Knowles & Moncada, 1994). Depending on the concentration of NO released into the tissue microenvironment, and the type and stage of inflammation, NO seems to exhibit either anti- or pro-inflammatory effects (Moncada et al., 1991; Moilanen & Vapaatalo, 1995; Muijsers et al., 1997). It has been well documented that excessive production of NO via iNOS is involved in the pathogenesis of several inflammatory disorders, including dermatitis (Ross et al., 1998; Sahin et al., 2001). On the other hand, studies with iNOS-deficient mice indicated that the production of NO might be needed as a protective factor in various models of acute or chronic inflammation (McCafferty et al., 1999; Kenyon et al., 2002). In a model of contact hypersensitivity, inhibition of NO synthesis caused the release of inflammatory mediators such as histamine and platelet-activating factor (PAF) from mast cells (Kubes et al., 1993). Further studies have shown that exogenous NO can modulate leukocyte recruitment and mast cell-induced microvascular permeability alterations, raising the possibility that the use of NO donors may be a reasonable therapeutic approach to reducing mast cell-dependent inflammation (Gaboury et al., 1996). Anti-inflammatory drugs that have been modified to include a NO-releasing moiety have been recently developed and have shown enhanced anti-inflammatory activities together with reduced side-effects (Burgaud et al., 2002; Wallace & Del Soldato, 2003).
Nitro-steroids such as NCX 1015 displayed enhanced efficacy as an anti-inflammatory agent, without at least some of the unwanted side effects associated with its parental compound (Paul-Clark et al., 2000; 2002). Here, we have examined NCX 1022, an NO-releasing derivative of hydrocortisone, for its anti-inflammatory effects in a model of acute contact dermatitis. Our study shows that both pre- and post-treatments with NCX 1022 significantly reduced the inflammatory response compared to hydrocortisone. These effects of NCX 1022 were particularly prominent during the early phase of inflammation (during the first 5 h) and affected both oedema formation and granulocyte recruitment. The effects of steroids in general, and the anti-inflammatory effects of glucocorticoids in particular, are mediated through the modulation of protein transcription (DiDonato et al., 1996; Saklatvala, 2002). Thus, the effects of hydrocortisone on irritant contact dermatitis are somehow delayed, depending on the fixation of hydrocortisone to the glucocorticoid receptor and further modulation of transcriptional events. However, NO has been shown to have a very rapid effect on several inflammatory parameters including leukocyte recruitment (Kubes et al., 1991; 1994). NO release from NO-hydrocortisone might then be responsible for the immediate anti-inflammatory effects observed in this dermatitis model.
Although hydrocortisone was effective at reducing dermatitis-induced oedema, it only partially inhibited leukocyte adhesion to the vessel wall (Figure 5b, 6b), and failed to inhibit granulocyte recruitment to the site of inflammation, as observed by the lack of effect of hydrocortisone on MPO activity (see Figures 2c, 3d). Interestingly, the effects of hydrocortisone on leukocyte adhesion to the vessel wall were observed within minutes, suggesting that nongenomic mechanisms might be involved in this inhibitory process. Indeed, nongenomic responses have been described for all classes of steroids, they occur within minutes, are reversible immediately following steroid removal, and are insensitive to blockers of RNA transcription and translation (Wehling, 1997; Borski, 2000). However, such mechanism did not seem to be sufficient for hydrocortisone to fully abolish leukocyte adherence (in contrast to NCX 1022 which completely inhibited adherence), and to further inhibit granulocyte recruitment to the site of inflammation, as MPO was not reduced in hydrocortisone-treated mice compared to vehicle-treated mice. Both pre- and post-treatments with NO-hydrocortisone (NCX 1022) were able to significantly reduce the MPO activity in ear tissues, 8 h after the induction of dermatitis. This result suggests an additional effect of NCX 1022 compared to hydrocortisone on the recruitment of inflammatory cells. Indeed, the results generated with intravital microscopy demonstrated a direct, rapid (within seconds), and potent effect of NCX 1022, which completely abolished the increased number of leukocyte adherent to the vessel wall in this model of dermatitis (see Figures 5, 6). Here again, this effect of NCX 1022 might be attributed, at least in part, to the release of NO, since previous studies have demonstrated inhibitory effects for NO donors and endogenous NO on leukocyte/endothelium interactions (Kubes et al., 1991; 1993; 1994; Gaboury et al., 1996) and adhesion molecule expression (Khan et al., 1996). NO has also been shown to modulate neutrophil functions such as chemotaxis and phagocytosis by stimulating ADP ribosylation of actin (Clancy et al., 1995). No significant change in leukocyte rolling between mice treated with NCX 1022 or hydrocortisone was observed, suggesting that the expression of E-, L- and P-selectins was unaffected by those treatments. Histological examination of tissue samples taken 8 h after induction of dermatitis revealed a strong correlation between the reduction of tissue architecture destruction and a low number of infiltrated inflammatory cells. This further suggests that the mechanisms through which NCX 1022 reduces more efficiently irritant contact dermatitis than hydrocortisone, involves the inhibition of neutrophil adhesion and recruitment.
Recently, a molecular mechanism involving the modulation of glucocorticoid receptor binding and function by NO-releasing glucocorticoid drugs has been proposed to explain the enhanced therapeutic effects of NO-prednisolone (Paul-Clark et al., 2003). In that study, the authors showed that specific nitration of the glucocorticoid receptor by NO-prednisolone (NCX 1015) resulted in the enhancement of glucocorticoid receptor-mediated (i) binding to dexamethasone, (ii) dissociation from heat shock protein 90, and (iii) nuclear translocation. It is possible that NCX 1022, via release of NO, is also capable of post-translational modification of the glucocorticoid receptor, resulting in the enhancement of anti-inflammatory activity in the present model of dermatitis. As some of the effects of hydrocortisone, particularly on leukocyte adhesion, might be mediated by nongenomic mechanisms, it can also be hypothesized that NO released from NCX 1022 can also enhance glucocorticoid nongenomic effects. This potential synergestic effect of NO and hydrocortisone is further suggested in the present model by the fact that an NO-donor alone had no effect on the development of dermatitis in terms of oedema formation and granulocyte recruitment (see Figure 3e, f). Similar findings in carrageenan-induced inflammation of an airpouch in rats have been shown for NO prednisolone: administration of DETA-NONOate alone did not inhibit inflammatory cell recruitment, while co-administration of DETA-NONOate and prednisolone resulted in markedly reduced leukocyte infiltration (Turesin et al., 2003).
In summary, we showed that an NO-releasing derivative of hydrocortisone, NCX 1022, was more effective than hydrocortisone at reducing major inflammatory parameters (leukocyte recruitment, oedema formation, tissue disruption), and that the inhibitory effects of NCX 1022 on the development of inflammation were observed several hours before that of hydrocortisone. We have further shown, via intravital microscopy, that the reduction in inflammatory parameters was in part due to inhibition by NCX 1022 of neutrophil adhesion to the endothelium. These results suggest that NCX 1022 could be an attractive alternative to hydrocortisone for acute dermatitis, being more potent and faster acting than its parent compound.
Acknowledgments
This study was supported by operating grants from the Canadian Institutes for Health Research (CIHR) (to N.V.) through a University-Industry Program partnered with Nicox S.A. France, by the Rosacea Foundation (U.S.A.), and by JZKF D-16, SFB 293, SFB 492 grants to M.S. N.V. is a CIHR scholar, and an Alberta Heritage Foundation for Medical Research scholar.
Abbreviations
- AP-1
activator protein-1
- DETA-NONOate
diethylenetriamine-NONOate
- GC
glucocorticoide
- GRE
glucocorticoid-responsive element
- HPA
hypothalamopituitary axis
- MPO
myeloperoxidase
- NF
nuclear factor
- NO
nitric oxide
References
- AGGARWAL R.K., POTAMITIS T., CHONG N.H., GUARRO M., SHAH P., KHETERPAL S.Extensive visual loss with topical facial steroids Eye 19937664–666.(Part 5) [DOI] [PubMed] [Google Scholar]
- AHLUWALIA A. Topical glucocorticoids and the skin – mechanisms of action: an update. Mediators Inflamm. 1998;7:183–193. doi: 10.1080/09629359891126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAATZ H., STEINBAUER M., HARRIS A.G., KROMBACH F. Kinetics of white blood cell staining by intravascular administration of rhodamine 6G. Int. J. Microcirc. Clin. Exp. 1995;15:85–91. doi: 10.1159/000178955. [DOI] [PubMed] [Google Scholar]
- BORSKI R.J. Nongenomic membrane actions of glucocorticoids in vertebrates. Trends Endocrinol. Metab. 2000;11:427–436. doi: 10.1016/s1043-2760(00)00325-8. [DOI] [PubMed] [Google Scholar]
- BURGAUD J.L., ONGINI E., DEL SOLDATO P. Nitric oxide-releasing drugs: a novel class of effective and safe therapeutic agents. Ann. N.Y. Acad. Sci. 2002;962:360–371. doi: 10.1111/j.1749-6632.2002.tb04080.x. [DOI] [PubMed] [Google Scholar]
- CENAC N., COELHO A., NGUYEN C., COMPTON S., ANDRADE-GORDON P., MACNAUGHTON W.K., WALLACE J.L., HOLLENBERG M.D., BUNNETT N.W., GARCIA-VILLAR R., BUENO L., VERGNOLLE N. Induction of intestinal inflammation in mouse by activation of proteinase-activated receptor-2. Am. J. Pathol. 2002;161:1903–1915. doi: 10.1016/S0002-9440(10)64466-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLANCY R., LESZCZYNSKA J., AMIN A., LEVARTOVSKY D., ABRAMSON S.B. Nitric oxide stimulates ADP ribosylation of actin in association with the inhibition of actin polymerization in human neutrophils. J. Leukoc. Biol. 1995;58:196–202. doi: 10.1002/jlb.58.2.196. [DOI] [PubMed] [Google Scholar]
- DIDONATO J.A., SAATCIOGLU F., KARIN M. Molecular mechanisms of immunosuppression and anti-inflammatory activities by glucocorticoids. Am. J. Respir. Crit. Care Med. 1996;154:S11–S15. doi: 10.1164/ajrccm/154.2_Pt_2.S11. [DOI] [PubMed] [Google Scholar]
- ELLISON J.A., PATEL L., RAY D.W., DAVID T.J., CLAYTON P.E. Hypothalamic–pituitary–adrenal function and glucocorticoid sensitivity in atopic dermatitis. Pediatrics. 2000;105:794–799. doi: 10.1542/peds.105.4.794. [DOI] [PubMed] [Google Scholar]
- GABOURY J.P., NIU X.F., KUBES P. Nitric oxide inhibits numerous features of mast cell-induced inflammation. Circulation. 1996;93:318–326. doi: 10.1161/01.cir.93.2.318. [DOI] [PubMed] [Google Scholar]
- GOULDING N.J., EUZGER H.S., BUTT S.K., PERRETTI M. Novel pathways for glucocorticoid effects on neutrophils in chronic inflammation. Inflamm. Res. 1998;47 Suppl 3:S158–S165. doi: 10.1007/s000110050310. [DOI] [PubMed] [Google Scholar]
- HICKEY M.J., KANWAR S., MCCAFFERTY D.M., GRANGER D.N., EPPIHIMER M.J., KUBES P. Varying roles of E-selectin and P-selectin in different microvascular beds in response to antigen. J. Immunol. 1999;162:1137–1143. [PubMed] [Google Scholar]
- KENYON N.J., VAN DER VLIET A., SCHOCK B.C., OKAMOTO T., MCGREW G.M., LAST J.A. Susceptibility to ozone-induced acute lung injury in iNOS-deficient mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2002;282:L540–L545. doi: 10.1152/ajplung.00297.2001. [DOI] [PubMed] [Google Scholar]
- KHAN B.V., HARRISON D.G., OLBRYCH M.T., ALEXANDER R.W., MEDFORD R.M. Nitric oxide regulates vascular cell adhesion molecule 1 gene expression and redox-sensitive transcriptional events in human vascular endothelial cells. Proc. Natl. Acad. Sci. U.S.A. 1996;93:9114–9119. doi: 10.1073/pnas.93.17.9114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOWLES R.G., MONCADA S.Nitric oxide synthases in mammals Biochem. J. 1994298249–258.(Part 2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUBES P., KANWAR S., NIU X.F., GABOURY J.P. Nitric oxide synthesis inhibition induces leukocyte adhesion via superoxide and mast cells. FASEB J. 1993;7:1293–1299. doi: 10.1096/fasebj.7.13.8405815. [DOI] [PubMed] [Google Scholar]
- KUBES P., KUROSE I., GRANGER D.N. NO donors prevent integrin-induced leukocyte adhesion but not P-selectin-dependent rolling in postischemic venules. Am. J. Physiol. 1994;267:H931–H937. doi: 10.1152/ajpheart.1994.267.3.H931. [DOI] [PubMed] [Google Scholar]
- KUBES P., SUZUKI M., GRANGER D.N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc. Natl. Acad. Sci. U.S.A. 1991;88:4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG D.Y., BIEBER T. Atopic dermatitis. Lancet. 2003;361:151–160. doi: 10.1016/S0140-6736(03)12193-9. [DOI] [PubMed] [Google Scholar]
- MCCAFFERTY D.M., MIAMPAMBA M., SIHOTA E., SHARKEY K.A., KUBES P. Role of inducible nitric oxide synthase in trinitrobenzene sulphonic acid induced colitis in mice. Gut. 1999;45:864–873. doi: 10.1136/gut.45.6.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOILANEN E., VAPAATALO H. Nitric oxide in inflammation and immune response. Ann. Med. 1995;27:359–367. doi: 10.3109/07853899509002589. [DOI] [PubMed] [Google Scholar]
- MONCADA S., PALMER R.M., HIGGS E.A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- MUIJSERS R.B., FOLKERTS G., HENRICKS P.A., SADEGHI-HASHJIN G., NIJKAMP F.P. Peroxynitrite: a two-faced metabolite of nitric oxide. Life Sci. 1997;60:1833–1845. doi: 10.1016/s0024-3205(96)00651-0. [DOI] [PubMed] [Google Scholar]
- NOLTE D., SCHMID P., JAGER U., BOTZLAR A., ROESKEN F., HECHT R., UHL E., MESSMER K., VESTWEBER D. Leukocyte rolling in venules of striated muscle and skin is mediated by P-selectin, not by L-selectin. Am. J. Physiol. 1994;267:H1637–H1642. doi: 10.1152/ajpheart.1994.267.4.H1637. [DOI] [PubMed] [Google Scholar]
- PAUL-CLARK M., DEL SOLDATO P., FIORUCCI S., FLOWER R.J., PERRETTI M. 21-NO-prednisolone is a novel nitric oxide-releasing derivative of prednisolone with enhanced anti-inflammatory properties. Br. J. Pharmacol. 2000;131:1345–1354. doi: 10.1038/sj.bjp.0703704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL-CLARK M.J., MANCINI L., DEL SOLDATO P., FLOWER R.J., PERRETTI M. Potent antiarthritic properties of a glucocorticoid derivative, NCX-1015, in an experimental model of arthritis. Proc. Natl. Acad. Sci. U.S.A. 2002;99:1677–1682. doi: 10.1073/pnas.022641099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUL-CLARK M.J., ROVIEZZO F., FLOWER R.J., CIRINO G., SOLDATO P.D., ADCOCK I.M., PERRETTI M. Glucocorticoid receptor nitration leads to enhanced anti-inflammatory effects of novel steroid ligands. J. Immunol. 2003;171:3245–3252. doi: 10.4049/jimmunol.171.6.3245. [DOI] [PubMed] [Google Scholar]
- PERRETTI M., AHLUWALIA A. The microcirculation and inflammation: site of action for glucocorticoids. Microcirculation. 2000;7:147–161. [PubMed] [Google Scholar]
- PERRETTI M., PAUL-CLARK M.J., MANCINI L., FLOWER R.J. Generation of innovative anti-inflammatory and anti-arthritic glucocorticoid derivatives that release NO: the nitro-steroids. Dig. Liver Dis. 2003;35 Suppl 2:S41–S48. doi: 10.1016/s1590-8658(03)00051-3. [DOI] [PubMed] [Google Scholar]
- ROSS R., GILLITZER C., KLEINZ R., SCHWING J., KLEINERT H., FORSTERMANN U., RESKE-KUNZ A.B. Involvement of NO in contact hypersensitivity. Int. Immunol. 1998;10:61–69. doi: 10.1093/intimm/10.1.61. [DOI] [PubMed] [Google Scholar]
- SAHIN S., ONDER M., SANCAK B., BUKAN N., GURER M.A. The role of nitric oxide in allergic contact dermatitis. Arch. Dermatol. Res. 2001;293:214–217. doi: 10.1007/s004030100207. [DOI] [PubMed] [Google Scholar]
- SAKLATVALA J. Glucocorticoids: do we know how they work. Arthritis Res. 2002;4:146–150. doi: 10.1186/ar398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEELIGER S., DERIAN C.K., VERGNOLLE N., BUNNETT N.W., NAWROTH R., SCHMELZ M., DER WEID P.Y., BUDDENKOTTE J., SUNDERKOTTER C., METZE D., ANDRADE-GORDON P., HARMS E., VESTWEBER D., LUGER T.A., STEINHOFF M. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003;17:1871–1885. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- STEINHOFF M., VERGNOLLE N., YOUNG S., TOGNETTO M., AMADESI S., ENNES H., TREVISANI M., HOLLENBERG M.D., WALLACE J.L., CAUGHEY G., MITCHELL S., WILLIAMS L., GEPPETTI P., MAYER E., BUNNETT N. Agonists of proteinase-activated receptor 2 induce inflammation by a neurogenic mechanism. Nat. Med. 2000;6:151–158. doi: 10.1038/72247. [DOI] [PubMed] [Google Scholar]
- TEELUCKSINGH S., BALKARAN B., GANESHMOORTHI A., ARTHUR P. Prolonged childhood Cushing's syndrome secondary to intralesional triamcinolone acetonide. Ann. Trop. Paediatr. 2002;22:89–91. doi: 10.1179/027249302125000229. [DOI] [PubMed] [Google Scholar]
- TURESIN F., DEL SOLDATO P., WALLACE J.L. Enhanced anti-inflammatory potency of a nitric oxide-releasing prednisolone derivative in the rat. Br. J. Pharmacol. 2003;139:966–972. doi: 10.1038/sj.bjp.0705324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERGNOLLE N. Proteinase-activated receptor-2-activating peptides induce leukocyte rolling, adhesion, and extravasation in vivo. J. Immunol. 1999;163:5064–5069. [PubMed] [Google Scholar]
- VERGNOLLE N., DERIAN C.K., D'ANDREA M.R., STEINHOFF M., ANDRADE-GORDON P. Characterization of thrombin-induced leukocyte rolling and adherence: a potential pro-inflammatory role for proteinase-activated receptor-4 (PAR-4) J. Immunol. 2002;169:1467–1473. doi: 10.4049/jimmunol.169.3.1467. [DOI] [PubMed] [Google Scholar]
- WALLACE J.L., DEL SOLDATO P. The therapeutic potential of NO-NSAIDs. Fundam. Clin. Pharmacol. 2003;17:11–20. doi: 10.1046/j.1472-8206.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- WEHLING M. Specific, nongenomic actions of steroid hormones. Annu. Rev. Physiol. 1997;59:365–393. doi: 10.1146/annurev.physiol.59.1.365. [DOI] [PubMed] [Google Scholar]
- YAMAGUCHI S., TOMOMATSU N., KOMATSU H. Effect of Y-24180, a receptor antagonist to platelet-activating factor (PAF), on allergic cutaneous reactions in actively sensitized mice. Inflamm. Res. 2000;49:584–590. doi: 10.1007/s000110050635. [DOI] [PubMed] [Google Scholar]