Abstract
Melanocortin (MC) receptors are widely distributed throughout the body of chicken, like in mammals, and participate in a wide range of physiological functions.
To clarify the pharmacological impact of ligands acting in the MC system, we expressed the chicken MC1, MC2, MC3, MC4 and MC5 (cMC1–5) receptors in eukaryotic cells and performed comprehensive pharmacological characterization of the potency of endogenous and synthetic melanocortin peptides.
Remarkably, the cMC receptors displayed high affinity for ACTH-derived peptides and in general low affinity for α-MSH. It is evident that not only the cMC2 receptor but also the other cMC receptors interact with ACTH-derived peptide through an epitope beyond the sequence of α-MSH.
The synthetic ligand MTII was found to be a potent agonist whereas HS024 was a potent antagonist at the cMC4 receptor, indicating that these ligands are suitable for physiological studies in chicken.
We also show the presence of prohormone convertase 1 (PC1) and PC2 genes in chicken, and that these peptides are coexpressed with proopiomelanocortin (POMC) in various tissues.
Keywords: GPCR, MSH, ACTH, MC receptor subtypes
Introduction
The domestic chicken (Gallus gallus) is among the most investigated non-mammalian vertebrate species (Cheng, 1997). It is likely that the use of chicken as an experimental model will increase, as its genome today is completely sequenced (Burt & Pourquie, 2003). Today, the human, mouse and rat genomes as well as the genomes from the teleost fishes fugu and zebrafish have been sequenced. The chicken genome will bridge the large evolutionary gap between mammals and fish. Moreover, there is a great interest in using chicken as a model for tracking quantitative trait locus (QTLs). For example, there are large programmes in place to track genes involved in regulation of body weight homeostasis using anorectic chickens (Carlborg et al., 2003).
G-protein-coupled receptors (GPCRs) are probably the most pursued group of target proteins for drug development. The melanocortin (MC) receptors are GPCRs that are involved in a wide range of physiological functions, including skin pigmentation, stress and immune response, sexual behaviour, cardiovascular regulation and energy homeostasis. The melanocortin peptides, including α-, β-, γ-melanocyte-stimulating hormone (MSH) and adrenocorticotropic hormone (ACTH), are generated in mammals through a series of ordered proteolytic cleavages of the precursor glycoprotein proopiomelanocortin (POMC). Two kinds of endoproteases are involved in this process, the prohormone convertase 1 (PC1; also called PC3) that generates ACTH from POMC and the prohormone convertase 2 (PC2) that cleaves ACTH and other parts of POMC to produce α-, β- and γ-MSHs. The main source of circulating melanocortins is the pituitary. POMC is primarily processed into ACTH in the anterior lobe, while the intermediate lobe is the major source of α-MSH (Adan & Gispen, 1997). The intermediate lobe is well developed in lower vertebrates, but in human adults and chicken it is difficult to detect α-MSH and the quantities of circulating MSH is, in general, low in these species. Tetrapods, including chicken, have five MC receptor subtypes (Takeuchi et al., 1996; 1998; Takeuchi & Takahashi, 1998a, 1998b). The MC1 receptor is expressed in mammalian melanocytes where it regulates pigmentation. Mutations of the MC1 receptor in chicken, which renders the receptor constitutively active, result in darker feather coloration (Ling et al., 2003). The MC1 receptor also plays a role for the anti-inflammatory actions of MSH-peptides in mammals (Schiöth, 2001). The MC2 receptor, which only binds ACTH, is expressed in the cortex of the adrenal gland and at low levels in adipocytes (Mountjoy et al., 1992; Cammas et al., 1995; Boston & Cone, 1996). The receptor has an endocrine function, and regulates the biosynthesis of corticosteroids in the mammalian adrenal gland. The MC3 receptor is found in the central nervous system in mammals and MC3 receptor knockout mice exhibit a metabolic dysfunction with increased fat stores and decreased energy expenditure, indicating an important role in energy homeostasis (Chen et al., 2000). The MC4 receptor in mammals is exclusively expressed in the brain (Mountjoy et al., 1994) and the knockout of this receptor induces overeating and obesity in mice (Huszar et al., 1997). Agonists at the MC4 receptor are highly anorexic and antagonists generate orexigenic effects (Kask et al., 1998; Skuladottir et al., 1999). The MC5 receptor is expressed in the brain but is also widely distributed in the periphery (Labbe et al., 1994). Apart from involvement in exocrine gland secretion in mice, the physiological functions of this receptor remain obscure (Chen et al., 1997). The chicken MC receptors are found in a wider range of tissues. The chicken MC3 receptor seems to be expressed exclusively in the adrenal gland (Takeuchi & Takahashi, 1998b), while the chicken MC4 receptor is found in a variety of peripheral tissues as well as in the brain. Since the MC4 receptor appears to be the only MC receptor subtype present in the chick brain, it has been suggested that this receptor mediates feeding behaviour or body weight regulation in chicken. However, the pharmacological profile of the avian MC receptors has not been reported and the previous assumptions about the chicken MC receptors have been based on results from the mammalian orthologues.
In this paper, we report a comprehensive pharmacological characterization of the MC receptors in chicken. Moreover, we show the presence of convertase 1 (PC1) and PC2 genes in chicken and determine their tissue distribution.
Methods
Receptor clones and peptides
The receptor clones were received by polymerase chain reaction (PCR) using specific primers, containing HindIII and XhoI sites, and genomic phage clones as template (cMC1 from Gallus gallus Rock Cornish and cMC2, cMC3, cMC4 and cMC5 from Gallus gallus White Leghorn) (Takeuchi et al., 1996; 1998; 1999; Takeuchi & Takahashi, 1998a, 1998b). The PCR was performed, starting with 1 min 95°C denaturation, followed by 40 cycles of 50 s at 95°C, 50 s at 50°C and 65 s at 72°C. The reaction ended by 5 min final extension at 72°C. The DNA fragments were purified using QIAquick Gel extraction Kit (Qiagen, Sweden), digested with HindIII and XhoI and ligated into pCEP4 Turbo expression vector (Marklund et al., 2002). Finally, the constructs were confirmed by sequencing using ABI PRISM Dye Terminator cycle sequencing kit v2.0 (Applied Biosystems, U.S.A.). For characterization of the chicken receptors, we consistently used human melanocortin peptides. α-MSH is conserved in all positions between the two species, and also ACTH(1–24) has a high degree of conservation except that chicken has Arg15 and Ile20 that correspond to Lys15 and Val20 in the human peptide. Three positions in chicken γ-MSH, Ser4, Asn9 and Lys10 differ from the human peptide that contains the following amino acids: Gly4, Asp9 and Arg10. The β-MSH sequence differs more and is DEGPYRMEHFRWGSPPKD in the human POMC as compared with DGGSYRMRHFRWHAPLKD in the POMC sequence in chicken. The common amino-acid sequence HFRW is entirely conserved in all the human and chicken peptides.
Expression of cMC receptors
DNA for transfection was prepared using Qiagen® Plasmid Maxi Kit (Qiagen, Sweden). HEK 293 EBNA cells, 50–70% confluent on 100 mm plates, were transfected with 15 μg of the construct using FuGENE™ Transfection Reagent (Boehringer Mannheim, Biberach an der Riss, Germany), diluted in Optimem medium (Gibco BRL, Stockholm, Sweden). After transfection, cells were grown in DMEM/Nut Mix F-12 with glutamax-1 (Gibco BRL), containing 10% foetal bovine serum (Gibco BRL/Life Tech), 0.25 mg ml−1 G418, 2.5 μg ml−1 amphotericin, penicillin–streptomycin (100 U penicillin, 100 μg streptomycin ml−1) (all from Gibco BRL). Cells with semistable expression were selected for growth in the presence of 100 μg ml−1 Hygromycin B (Invitrogen), starting 48 h after transfection. Cells were harvested and frozen in −80°C in aliquots until binding assay. The MC2 receptor is known to be difficult to express, probably due to failed transport of the receptor to the membrane (Noon et al., 2002). Various types of cell lines have been evaluated and functional expression has been obtained in some melanoma and adrenocortical cell lines (Schimmer et al., 1995; Penhoat et al., 2000). The cMC2R was expressed in M3 cells that were purchased by ATCC and cultured in Kaighn's Modification of Ham's F-12 Medium (F-12K) supplemented with 15% horse serum (ATCC), 2.5% foetal bovine serum and 1% GlutaMAX (Invitrogen). The cells were kept in humidified atmosphere of 95% air and 5% CO2 at 37°C. One day before transfection, about 200,000 cells were plated in 35-mm culture dishes. Transfections were carried out with 1.5 μg DNA using Lipofectamine Reagent PLUS (Invitrogen) according to the manufacturer's instructions. Transiently transfected cells were used 72 h after transfection.
Receptor binding assays
Intact transfected cells were re-suspended in 25 mM HEPES-buffer (pH 7.4) containing 2.5 mM CaCl2, 1 mM MgCl2 and 2 g l−1 bacitracin. Saturation experiments were carried out in 96-well plates and in a final volume of 100 μl. The labelled ligand 125I-(Nle4, D-Phe7) α-MSH (NDP-MSH) was diluted in series starting with 6 nM added to the first well. The nonspecific binding was determined in the presence of 2 μM unlabelled NDP-MSH. Finally, cell suspension was added and the plates were incubated for 3 h at room temperature. In competition experiments, the cells were incubated for 3 h at room temperature with 125I-labelled NDP-MSH at a constant concentration of 0.2 nM and appropriate concentrations (starting at 1–10 μM and diluted 1 : 3) of competing unlabelled ligands. The incubations were terminated by filtration through Glass Fibre Filters, Filtermat A (Wallac Oy, Turku, Finland), which had been presoaked in 0.3% polyethylenimine, using a TOMTEC Mach III cell harvester (Orange, CT, U.S.A.). The filters were washed with 5.0 ml of 50 mM Tris (pH 7.4) at 4°C, dried at 60°C and then treated with MeltiLex A (Perkin Elmer) melt-on scintillator sheets and counted in a Wallac 1450 (Wizard automatic Microbeta counter). The results were analysed with a software package suitable for radioligand binding data analysis (Prism 3.0 – Graphpad, San Diego, CA, U.S.A.). The binding assays were performed in duplicates and repeated three times. Nontransfected HEK293-EBNA cells did not show any specific binding for (125I)NDP-MSH. NDP-MSH was radio-iodinated by the chloramine T method and purified by high-performance liquid chromatography (HPLC). NDP-MSH and α-MSH were purchased from Neosystem, France.
cAMP assay in HEK-293 EBNA cells
The transfected cells were incubated for 2 h with 2.5 μCi ml−1 (8-3H)adenine (Amersham pharmacia biotech, Uppsala, Sweden), then washed and harvested in a medium composed of 137 mM NaCl, 5 mM KCl, 0.44 mM KH2PO4, 4.2 mM NaHCO3, 1.2 mM MgCl2, 20 mM HEPES, 1 mM CaCl and 10 mM glucose, pH adjusted to 7.4. The pelleted cells were resuspended in the medium as above containing 0.5 mM 3-isobuthyl-1-methylxantine (Sigma). The ligands to be investigated were diluted in appropriate concentrations starting at 0.1 μM in a 96-well plate. The reactions were started by adding the cell suspension to the dilutions and after 10 min at 37°C stopped by centrifugation at 1300 × g for 1 min. The supernatants were decanted and 200 μl 0.33 M perchloric acid was added to each well. The cells were frozen down to −20°C, thawed and spun. The extent of conversion of [3H]ATP to [3H]cAMP was then determined by Dowex/Alumina sequential chromatography (Salomon et al., 1974). [14C]cAMP (Amersham Bioscience, Uppsala, Sweden) tracer in 0.75 ml 0.33 M perchloric acid was added to each column together with the samples. The ATP/ADP and the cAMP fractions were dissolved in scintillation cocktail (optiphase HiSafe3, Wallac, Turku, Finland) and analysed in a β-counter. The conversion to [3H]cAMP was calculated as the percentage of the total eluted [3H]ATP and was normalized to the recovery of [14C]cAMP. The cAMP assay was performed in duplicates with approximately the same amount of cells and repeated three times for each receptor.
cAMP assay in M3 cells
Intracellular cAMP production induced by MC2R expressed in M3 cells was measured by sequential chromatography on Dowex and alumina columns as described for HEK 293 EBNA cells with the following modifications (Gallo-Payet & Payet, 1989). At 72 h after transfection, MC2R transiently transfected M3 cells plated on 35 mm culture dishes were incubated for 1 h at 37°C with the complete culture medium containing 2 μCi ml−1 (3H)-adenine (NEN). The cells were then washed twice with Hank's buffer saline and equilibrated in the same buffer containing 1 mM isobutyl methylxanthine (IBMX) for 15 min at 37°C. ACTH or α-MSH (start concentration 1 or 0.1 μM, respectively) was added to the incubation medium for a further 15 min at 37°C. The reaction was ended by aspiration and addition of 1 ml ice-cold 5% (w v−1) trichloroacetic acid. Cells were harvested and 100 μM ice-cold 5 mM ATP plus 5 mM cAMP solution was added to the mixture. Cellular membranes were pelleted at 5000 × g for 15 min at 4°C and the supernatants sequentially chromatographed on Dowex and alumina columns according to the method of Salomon et al. (1974), allowing the elution of (3H)-ATP and (3H)-cAMP respectively. cAMP formation was calculated as follows: percent conversion=((3H)-cAMP/((3H)-cAMP+(3H)-ATP)) × 100 and expressed as percentage of maximum stimulation.
RT–PCR
Total RNA was prepared from various tissues of 3-day-old Rock Cornish chicks using the method of Chomczynski & Sacchi (1987). In total, 1 μg of total RNA prepared from each tissue was reverse transcribed using a SuperScript II reverse transcriptase (Gibco BRL) according to the manufacturer's directions. A one-tenth aliquot of the reactions was used in each PCR using specific primers for POMC, PC1, PC2 or GAPDH. Since both of the chicken PC1 and PC2 sequences were not available from DNA databases, we performed homology search on an EMBL DNA database with human PC1 (accession number 1813186A) and PC2 (accession number BT007928) and found chicken ESTs, BU118934 and BU119126, showing high levels of identity to the human PC1 and PC2, respectively. The primers for the chicken PC1 and PC2 designed based on the EST sequences were CTGACCAAAGAATAACAAGTGCTGA and AGACAAAAGCTTGAACCAAGG, and AGAGGCTAACTTGGATCTAACCTG and GGAACCTGAAAAGATACCACCACCAAG, respectively. The primers for the chicken POMC designed based on our reported chicken POMC gene (Takeuchi et al., 1999) were AGGCTGGTGTTTTGGCGTGT and AGTCGGCTGAGAGTTACCCCATG. The sequence of primers specific for the chicken GAPDH was described previously (Takeuchi & Takahashi, 1998a). The PCRs were carried out using Takara Taq DNA polymerase (TAKARA, Otsu, Japan) and a thermal cycler (Gene Amp PCR System 9600, Applied Biosystems). The conditions for the PCRs were as follows: 35 (for POMC), 30 (for PC1), 27 (for PC2) and 25 (for GAPDH) cycles of serial incubations including 30 s at 95°C, 30 s at 55°C (for PC1 and PC2) and 1 min at 60°C (for POMC and GAPDH) or 72°C (for PC1 and PC2) were performed, followed by additional incubation for 10 min at 60°C (for POMC and GAPDH) or 72°C (for PC1 and PC2). A one-tenth aliquot of each reaction was electrophoresed on 2.0% agarose gels, stained with ethidium bromide, and photographed under ultraviolet illumination. The gels were subsequently transferred onto Hybond-N+ (Amersham Pharmacia Biotech, Uppsala, Sweden) and subjected to Southern blot analysis using corresponding cDNA probes radio labelled using α-[32P]dCTP (3000 Ci mmol−1, Amersham Pharmacia Biotech) and a Random Primer DNA Labelling Kit Ver. 2 (Takara, Shiga, Japan). The PCR-amplified cDNA fragments were also subcloned into pGEM3Zf(+) plasmids and subjected to sequencing. Dideoxynucleotide sequencing was performed using a Big-Dye terminator cycle sequencing kit (PE Applied Biosystems, Foster City, CA, U.S.A.) and an automated DNA sequencer (PE Applied Biosystems 373A). Sequence analyses were carried out using GENETYX software. The chicken PC1 and PC2 cDNA sequences obtained in the present study are available from DDBJ, EMBL and GenBank data libraries under accession numbers AB121969 and AB121970, respectively.
Phylogenic analyses
Three different methods, Neighbour-Joining, Maximum Parsimony and Maximum likelihood, were used to gain three phylogenetic trees. The new sequences for PC1 chicken, PC2 chicken, hPC1/PC3 (1813186A), hPC2 (BT007928), hPC4 (HSU49114), hPACE4 (D87995), hPC7/hPC8/LPC (BT007578) and hFurin (1211240A) were all aligned using the UNIX version of ClustalW 1.82 (Thompson et al., 1994). The default alignment parameters were applied. The alignments were bootstrapped 1000 times using SEQBOOT from the Win32 version of the Phylip 3.6 a2.1 package (Felsenstein, 1993) to obtain a total of 1000 different alignments. Protein distances were calculated using Protdist from the Win32 version of the Phylip 3.6 a2.1 package. The Jones–Taylor–Thornton matrix was used for the calculation. The trees were calculated from the 1000 different distance matrixes, previously generated with Protdist, using NEIGHBOR from the Win32 version of the Phylip 3.6 a2.1 package, resulting in a file with 1000 trees that was analysed using Consense from the Win32 version of the Phylip 3.6a2.1 package to get a bootstrapped consensus tree. The trees were plotted using Treeview (http://taxonomy.zoology.gla.ac.uk/rod/treeview.html). Maximum parsimony trees were calculated from the same input files that were used for Protdist using Protpars from the Win32 version of the Phylip 3.6 a2.1 package. The trees were unrooted and calculated using ordinary parsimony and the topologies were obtained using the built-in tree search procedure. Consensus trees were calculated and plotted as described above. Maximum likelihood trees were calculated from the same input files as were used for PROTDIST and PROPARS. The analysis was performed using PROTML from Phylip 3.6a2.1 with 1000 bootstrap replicas and tree randomizations per alignment. The built-in tree-search algorithm was used to estimate the topology. γ-Distributed rates were used to control for rate variation among sites, with nine rate categories and a coefficient of variation of substitution of 1.24 as calculated from an α value of 0.65. The α parameter was calculated using Treepuzzle (Schmidt et al., 2002) on the same data set using the same substitution matrix.
Results
The wild-type chicken (c) MC1, cMC2, cMC3, cMC4 and cMC5 receptors were cloned into expression vectors and expressed in mammalian HEK-293 or M3 cells. Their pharmacological profiles were determined on intact cells. The natural MSH-ligands and different ACTH-fragments as well as several synthetic ligands were tested in radioligand binding for the cMC1, cMC3, cMC4 and cMC5 receptors. The Kd and Ki values obtained from saturation and competition experiments are listed in Table 1 together with previously published results for the human MC receptors for comparison, tested with the same methodological approach. It should be mentioned that these human MC receptor-binding values have been tested repeatedly for over a decade with very consistent results. Figure 1 shows the saturation curves for these four receptors. The competition curves for some of the compounds can be seen in Figure 2.
Table 1.
Kd and Ki for the chicken MC1, MC3, MC4 and MC5 receptors obtained from competition experiments, together with previously published results for the human MC receptors
| Ligand | cMC1 (nmol l−1) | hMC1a (nmol l−1) | cMC3 (nmol l−1) | hMC3b (nmol l−1) | cMC4 (nmol l−1) | hMC4b (nmol l−1) | cMC5 (nmol l−1) | hMC5c (nmol l−1) |
|---|---|---|---|---|---|---|---|---|
| [125I]NDP-MSH (Kd) | 2.76±0.41 | 0.109±0.0062 | 0.139±0.019 | 0.412±0.121 | 1.84±0.23 | 1.78±0.36 | 0.45±0.15 | 5.05±1.00 |
| NDP-MSH (Ki) | 4.25±1.42 | 0.046±0.011 | 0.31±0.11 | 0.319±0.064 | 2.47±1.12 | 1.96±0.39 | 0.84±0.18 | 2.39±0.10 |
| α-MSH (Ki) | 363±72.7 | 0.210±0.089 | 24.0±0.30 | 21.2±5.3 | 40.6±18.0 | 522±122 | 189±58.5 | 8240±1670 |
| Desacetyl α-MSH (Ki) | 70.0±2.78 | 0.279±0.084d | 1.62±0.24 | 13.9±4.8d | 41.7±11.0 | 250±150d | 47.3±4.04 | 1890±750d |
| β-MSH (Ki) | 346±362 | 2.53±0.93 | 151±35.6 | 15.1±3.4 | 50.2±15.1 | 387±208 | 166±22.1 | 14400±1670 |
| γ1-MSH (Ki) | 1039±393 | 2.68±0.35c | 3.40±0.61 | 7.45±2.55 | 586±58.8 | 51 800±12000 | 654±7.5 | 42 600±6600 |
| ACTH(1–39) (Ki) | 806±65 | 3.95±0.67d | 15.4±0.2 | 135±22d | 88.4±26.9 | 2170±120d | 107±26 | 4920±610d |
| ACTH(1–24) (Ki) | 65.7±8.34 | 0.209±0.052d | 3.69±0.86 | 32.8±6.7 | 7.48±0.54 | 755±151 | 5.36±0.69 | 2760±780d |
| ACTH(1–17) (Ki) | 17.1±4.82 | 0.230±0.061d | 0.20±0.055 | 14±4.5d | 2.01±0.38 | 419±62d | 6.56±2.57 | 4240±1200d |
| ACTH(1–16) (Ki) | 37.0±11.8 | 0.267±0.116d | 0.51±0.13 | 19±4.3d | 5.18±0.22 | 698±88d | 16.1±1.31 | 2600±595d |
| MTII (Ki) | 54.2±10.1 | 0.686±0.109e | 12.7±1.66 | 52.6±7.9 | 2.04±0.53 | 39.7±6.7 | 70.7±6.91 | 46.1±7.9e |
| HS014 (Ki) | 217±30.8 | 108±62f | 483±62.1 | 64.1±12.5 | 5.44±1.41 | 3.41±1.31 | 1569±124 | 694±237f |
| HS024 (Ki) | 28.3±6.12 | 18.6±3.3e | 6.90±0.79 | 15.1±3.0 | 0.62±0.30 | 0.341±0.089 | 72.4±15.6 | 3.29±1.15e |
Data taken from Schiöth et al. (1997b).
Data taken from Schiöth et al. (2002).
Data taken from Schiöth et al. (1995).
Data taken from Schiöth et al. (1997a).
Data taken from Kask et al. (1998).
Data taken from Schiöth et al. (1998).
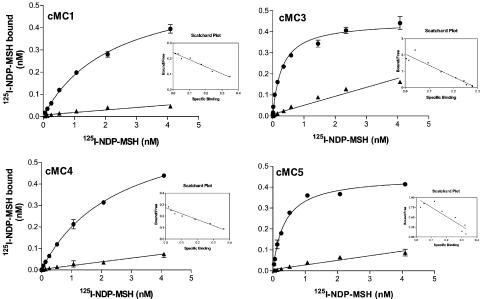
Figure 1.
Saturation curves with Scatchard plots of 125I-labelled (Nle4, D-Phe7)α-MSH obtained with transfected HEK-293 (EBNA) cells using a fixed concentration of (Nle4, D-Phe7)α-MSH at the chicken MC1, MC3, MC4 and MC5 receptors. The figure shows total binding and binding in the presence of 2 μM NDP-MSH. Data points represent means of duplicates and error bars indicate standard error of the mean (s.e.m.).
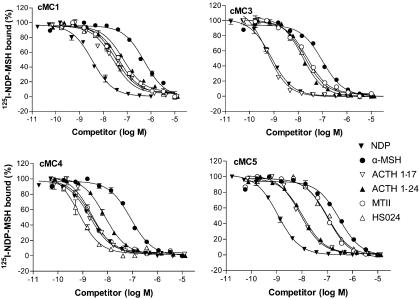
Figure 2.
Competition curves of six different ligands at the chicken MC1, MC3, MC4 and MC5 receptors. The curves were obtained using intact HEK-293 cells with semistable expression of the receptors and fixed concentration of 0.2 nM 125I-labelled NDP-MSH. Data points represent means of duplicates and error bars indicate standard error of the mean (s.e.m.).
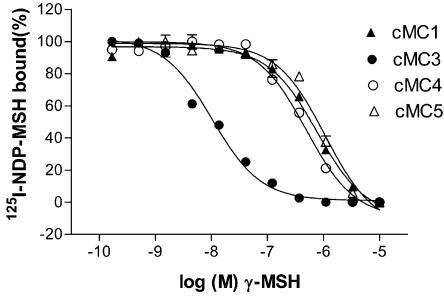
The results show that NDP-MSH binds to a single saturable site at all chicken receptors except for the cMC2 receptor that was tested separately in M3 cells (see below). The results from the saturation assay suggest that binding to the 125I-labelled NDP-MSH differs considerably from that for the human MC receptors (Figure 1). Among the chicken MC receptor subtypes, the labelled NDP-MSH binds with highest affinity to the cMC3 receptor, which is in contrast to the human receptor profile, where the MC1 receptor has clearly the highest affinity for NDP-MSH. The affinities for the cMC3 and cMC4 receptors to the labelled NDP-MSH are similar, in absolute numbers, to the affinities for the corresponding human receptors, whereas the Kd value for the cMC1 receptor is about 25-fold lower and about 11-fold higher for the cMC5 receptor as compared to the human counterparts (see Table 1). The nonlabelled NDP-MSH binds to the chicken receptors with similar affinities as the labelled, which is in agreement with previous assumptions that the iodine on Tyr2 does not affect the binding to MC receptors. The endogenous peptide α-MSH, which has very high affinity for the human MC1 receptor, showed generally lower affinities for all the chicken receptor subtypes. This is most pronounced for the cMC1 receptor as α-MSH shows 1800-fold lower affinity for this receptor in comparison to the hMC1 receptor. Moreover, the desacetylated α-MSH binds the cMC3 receptor with about 15-fold higher affinity than α-MSH. This difference in binding of α-MSH and its desacetylated counterpart is smaller for the cMC1 and cMC5 receptors, while for the cMC4 receptor no difference was found. The endogenous ligand β-MSH shows, similar to α-MSH, in general lower affinities to the chicken receptors than the human ones. β-MSH has the highest affinity for the cMC4 receptor among the chicken receptors. γ-MSH binds clearly with the highest affinity to the cMC3 receptor. γ-MSH had previously been shown to have preference for the MC3 receptor in mammals but is not selective for the MC3 subtype as the hMC1 receptor binds γ-MSH with a higher affinity than the hMC3 receptor. The selectivity of γ-MSH for the cMC3 receptor is displayed in Figure 3.
Figure 3.
Competition curve of γ-MSH at the chicken MC1, MC3, MC4 and MC5 receptors. The curves were obtained using intact HEK-293 cells with semistable expression of the receptors and fixed concentration of 0.2 nM 125I-labelled NDP-MSH. Data points represent means of duplicates and error bars indicate standard error of the mean (s.e.m.).
Our competition experiments revealed that chicken receptors bind ACTH with a higher affinity than α-MSH. When we detected the relative high affinity of ACTH(1-24), we subsequently evaluated other ACTH fragments. While ACTH(1–24) bound with equal affinity to the MC3, MC4 and MC5 receptor subtypes, it bound to the cMC1 receptor with somewhat lower affinity. This is in contrast to how α-MSH binds to the mammalian receptors, where α-MSH has the highest affinity for the MC1 receptor. In order to determine whether the positively charged amino acids beyond the α-MSH sequence in ACTH could be important to the binding, ACTH(1–16) and ACTH(1–17) were tested. Interestingly, cMC3 receptor bound the two shorter fragments with about 16- to 17-fold higher affinity on comparison to ACTH(1–24). The affinity for cMC3 to ACTH(1–17) is 0.2 nM, which is among the highest that any peptide, without artificial residues or nonendogenous sequence, has to any MC receptor. The affinities of ACTH(1–16) and ACTH(1–17) were also slightly higher to the cMC1 and cMC4 receptors, whereas they were slightly lower for the cMC5 receptor as compared with the ACTH(1–24). It is notable that the cMC4 receptor binds the ACTH-fragment with about 100-fold higher affinity and the cMC5 receptor with approximately 300- to 600-fold higher affinity in contrast to the human counterpart. Previous studies have shown that that there are no differences in the potency of ACTH(1–24) and the full-length ACTH(1–39) at the human MC receptors (Schiöth et al., 1995; 1997a). ACTH(1–39) is also suspected to be much more unstable in in vitro assays as compared with ACTH(1–24) (Schiöth et al., 1995). For the chicken MC receptors, ACTH (1–39) displays about four- to 20-fold lower affinity in comparison with ACTH (1–24).
We also tested three synthetic ligands that are widely used to delineate the role of specific MC receptors in physiological studies. The cyclic lactam MSH analogue MTII binds with high affinity to the cMC4 receptor and with about six-fold lower affinity to the cMC3 receptor. HS014, which is selective for the mammalian MC4 receptors (Schiöth et al., 1998), displays clear selectivity also for the cMC4 receptors with about 90-fold higher affinity as compared with the cMC3 receptors. HS024, which bound the human MC4 receptor with 10-fold higher affinity than HS014, shows higher affinities also for the chicken subtypes as compared with HS014. HS024 has about 10-fold higher affinity for the cMC4 receptor as for the cMC3 receptor.
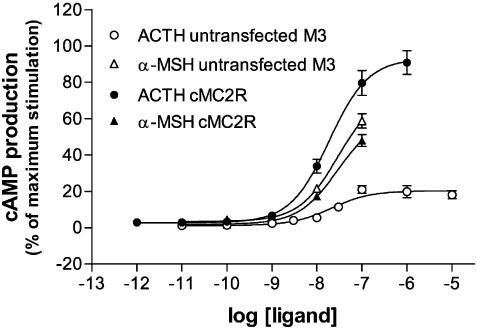
In order to investigate the ability of the chicken MC receptors to couple to intracellular messengers, we performed measurement of cAMP in the cells upon stimulation in HEK-293 cells. The results for the cMC1, cMC3, cMC4 and cMC5 receptors are shown in Figure 4. The cMC2 receptor was expressed in M3 cells and stimulated with ACTH in the same way as the receptor subtypes above. This curve can be viewed in Figure 5. Since each of the chicken receptor subtypes revealed an increase in level of cAMP after agonist stimulation, it was concluded that they were all coupled in a similar manner as the human counterparts. The EC50 values from the cAMP assay are presented in Table 2.
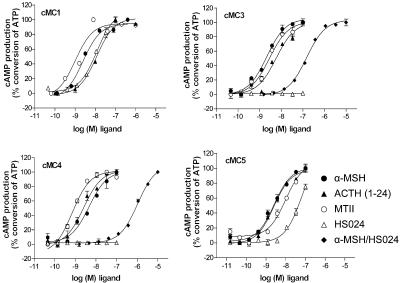
Figure 4.
Generation of cAMP in response to stimulation with α-MSH, ACTH(1–24), MTII and HS024 at the chicken MC1, MC3, MC4 and MC5 receptors. The figure also show curves obtained after stimulation with α-MSH in the presence of a fixed concentration of 0.1 μM HS024 at the cMC3 and cMC4 receptors. Each point represents the normalized mean±s.e.m. as a percentage of ATP conversion. The assay was performed in triplicates and repeated at minimum twice for each receptor and ligand.
Figure 5.
Generation of cAMP in response to ACTH and α-MSH at the cMC2 receptor expressed in M3 cells. The background response caused by the presence of endogenous MC1 receptors in the M3 cell line is also showed for ACTH and α-MSH, respectively. The values are normalized according to the maximum stimulation of the cMC2 receptor and each point represents the average±s.e.m. of values in duplicate.
Table 2.
Intracellular response in the form of EC50 values (means±s.e.m.) obtained for the chicken MC receptors on exposure to endogenous agonists; α-MSH and ACTH(1–24) as well as a synthetic agonist (MTII) and antagonist (HS024)
| Ligand | cMC1 (nM) | cMC2 (nM) | cMC3 (nM) | cMC4 (nM) | cMC5 (nM) |
|---|---|---|---|---|---|
| α-MSH | 3.80±0.64 | NS | 2.22±0.45 | 3.13±0.59 | 2.63±0.23 |
| ACTH(1–24) | 9.29±2.90 | 16.7±2.03 | 4.90±1.11 | 2.84±0.32 | 4.56±2.16 |
| MTII | 2.90±1.33 | ND | 2.72±0.59 | 1.41±0.49 | 11.9±2.81 |
| HS024 | 9.58±2.01 | ND | NS | NS | 68.0±0.86 |
NS: no stimulation; ND: not determined.
α-MSH, ACTH(1–24), and MTII functioned as full agonist at the cMC1, cMC3, cMC4 and cMC5 receptors. The EC50 values were fairly similar for the different receptors and the ligands. We also tested ACTH(1–16) at the cMC3 receptor and the results showed the similar potency as for the longer fragment (4.38 nM). It should be mentioned that the cAMP assay is by far less sensitive to detect differences between ligands or receptors as the binding assay. Upon addition of HS024, an antagonist for the mammalian MC4 and MC3 receptors, we observed increased levels of second messenger for the cMC1 and cMC5 receptor, while cMC3 and cMC4 receptor signalling were inhibited. For both the cMC1 and cMC5 receptors, HS024 appeared to function as a full agonist. When the cMC3 and cMC4 receptors were incubated with increasing concentrations of α-MSH and a fixed concentration of HS024, the α-MSH curves were shifted to a lower EC50. This indicates that HS024 is a functional antagonist for these receptors.
We expressed the cMC2 receptor in M3 cells and the intracellular response was measured after adding ACTH and α-MSH. The M3 cells possess basal activity in response to ACTH due to the presence of endogenous MC1 receptors. The cells transfected with the cMC2 receptor gave however a curve with higher maximum response than the native M3 cells (see Figure 5). The EC50 values obtained from cMC2 receptor transfected cells (EC50=15.7 nM) were about 10-fold lower on comparison with the hMC2 receptor (EC50=2.0 nM). The cells transfected with the cMC2 receptor were also tested for response to α-MSH. The intracellular cAMP level was not deviating from that received on native M3 cells, that is, the cMC2 receptor was not stimulated by α-MSH.
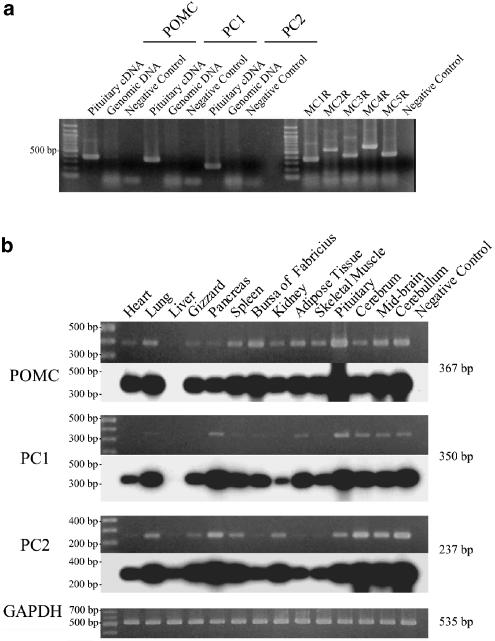
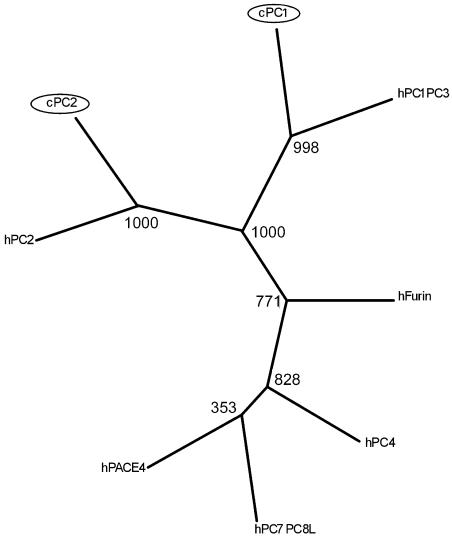
We also examined whether POMC and the processing enzymes PC1 and PC2 are co-expressed in tissues of 3-day-old chicks by RT–PCR. Except for the liver, all tissues examined were found to co-express POMC, PC1 and PC2 although their indicative expression levels vary with different tissues (see Figure 6). PC1 and PC2 belong to a family of endoproteases sharing structural characteristics, such as possessing a bacterial subtilisin-like catalytic domain and a P domain. Since the chicken PC1 and PC2 sequences were not available from DNA databases and the corresponding regions of cDNAs amplified by RT–PCR in this study were significantly conserved among the family, it might be possible that the RT–PCR amplified other members of the family, although the deduced amino-acid sequence of our PC1 and PC2 cDNAs shared 86.8 and 94.0% identities with human PC1 and PC2, respectively. The phylogenetic analyse show the chicken PC1 and PC2 group with high bootstrap values to the human orthologues. The Neighbor-Joining, Maximum Parsimony and Maximum likelihood methods resulted in trees with similar topology for PC1 and PC2. The analysis thus confirmed that our cDNAs encode chicken orthologues of the mammalian PC1 and PC2. The tree calculated using the Maximum likelihood method is displayed in Figure 7.
Figure 6.
RT–PCR analysis of the expression of POMC, PC1 and PC2 mRNAs in 3-day-old Rock Cornish chicks. (a) Assessment of the ability of each primer set to amplify its corresponding cDNA. Single band was observed in the PCR with each primer set when pituitary total cDNA was used as a template. No band was amplified in the PCR with the same primer sets when the chicken genomic DNA was used as a template. The genomic DNA used was intact because PCRs with primer sets specific for intronless genes including MC1–MC5 receptors successfully amplified DNA fragments with the predicted length. (b) Tissue distribution of the expression of POMC, PC1 and PC2 mRNAs. The upper panel shows the electrophoretic pattern of PCR products while the lower panel shows a Southern blot hybridization of the same gel to a specific chicken cDNA probe. A 100 bp ladder used as a molecular marker is indicated on the left. Negative controls in (a) and (b) indicate the PCR without template and the PCR with non-RT RNA, respectively.
Figure 7.
Molecular phylogenetic tree showing the relationship between chicken and human PC1 and PC2, together with other human prohormone convertas. The tree was calculated using the Maximum likelihood method on 1000 replicates. The numbers of the branches are bootstrap replicas. The novel chicken PCs are marked with circles.
Discussion
It is remarkable that ACTH-derived peptides seem to be the preferred ligands above α-MSH for all the MC receptors subtypes in chicken. The ACTH-derived peptides have previously not been considered to have a specific role for the MSH binding MC receptors. Earlier studies on the mammalian receptors show that MSH peptides have the highest potency at the MC1, MC3, MC4 and MC5 receptors, while ACTH-derived peptides display similar or slightly lower affinities at these receptors. Moreover, ACTH is the only peptide that is known to bind the mammalian MC2 receptors. The mammalian MC2 receptor is not only known to have very different pharmacology but also different physiology as compared with the other MC receptors.
The preference to ACTH-derived peptides is also interesting considering that the MC2 receptor in chicken seems to have a similar basic pharmacological profile (see Figure 5) as the mammalian orthologues, being specifically activated by ACTH but not responding to α-MSH. The data suggest thus that these specific characteristics of the MC2 receptors are likely to have been conserved through vertebrate evolution, while the ligand specificity of the other MC receptor subtypes may have evolved differently. This consequent conservation suggests that MC2 receptor-associated functions, such as stress response, may be of prominent importance throughout the evolution.
α-MSH has identical amino-acid sequence as the first 13 amino acids in ACTH and the peptides share a common core of four amino acids, His6-Phe7-Arg8-Trp9 (HFRW), which is believed to be crucial for activation at the MC1, MC3 MC4 and MC5 receptors. The amino acids Lys15-Lys16-Arg17-Arg18 play an important role for binding of ACTH to the MC2 receptor (Hofmann et al., 1970; Schwyzer, 1977; Buckley & Ramachandran, 1981). The core sequences, HFRW may potentiate and/or increase the effect of ACTH at the MC2 receptor as deletion of these residues from the peptide renders it inactive (Eberle, 1988). Problems related to expression, membrane insertion and radioligand binding of the MC2 receptors have however hampered detailed pharmacological characterization of this receptor. Kapas et al. (1996) analysed the mouse MC2 receptor expressed in HeLa cells for binding to ACTH fragments (ACTH1–17, 1–24, 1–39). High affinity was received for ACTH(1–17), emphasizing the importance of the four positively charged amino acids beyond the α-MSH sequence for binding to the MC2 receptor. Moreover, in a study of the pharmacological properties of ACTH-derived peptides at the other MC receptor subtypes, it was suggested that these receptors do not have a binding epitope for ACTH beyond the sequence of α-MSH (Schiöth et al., 1997a). Our new data for the chicken MC receptors suggest that the four positively charged amino acids beyond the α-MSH sequence indeed contribute to increase the affinity at all of the cMC receptor subtypes. ACTH(11–24) did not bind the chicken receptors (data not shown), which indicates that these amino acids alone cannot make strong enough interaction with the receptor to enable binding. It can be speculated that the ACTH peptide could have served as an important ligand, or even as an ‘original' ligand for the ancestral MC receptors. Subsequently, during vertebrate evolution, the other subtypes might have evolved specificity to the α-MSH (MC1), γ-MSH (MC3) and β-MSH (MC4), while the preference for ACTH was retained in the chicken lineage.
Our characterization of the cMC3 receptor is unique as it is the first non-mammalian MC3 receptor to be pharmacologically investigated. This is important as the role of the MC3 receptors is not well understood and the functional significance of γ-MSH is still unclear (Chen et al., 2000). γ-MSH has previously been suggested to play a specific role for the MC3 receptor where it acts as a relatively high-affinity ligand, while it has very low affinity for the MC4 receptor. It is indeed fascinating if a specific role of γ-MSH at the MC3 receptor is conserved through several vertebrate species. It is also intriguing that the MC3 receptor is missing from Fugu (Logan et al., 2003; Klovins et al., 2004), which together with several teleosts interestingly also lacks the γ-MSH part of the POMC (Takahashi et al., 2001).
The domesticated chicken is a widely used research animal both in physiological trials as a food intake model or to identify major QTLs that affect feeding and body weight (Cheng, 1997). Our data reveal that HS024 and MTII are suitable ligands for physiological studies in chicken as both these substances exhibit high potency at the cMC4 receptor. We confirmed that HS024 acts as effective antagonist and MTII as an agonist in the intracellular studies and it is likely that these substances act likewise in other aves. According to our data, both HS014 and HS024 show selectivity for the MC4 receptor (see Table 1 and Figure 4), indicating that these substances can be used to discriminate between the MC3 and MC4 receptors also in aves, as they have been used in rodents.
There exist three natural forms of α-MSH: desacetyl-, monoacetyl- and diacetyl-α-MSH. N-terminal acetylation of desacetylated α-MSH, the POMC cleavage product, generates α-MSH. The major fraction of desacetyl α-MSH produced by the pituitary is acetylated to α-MSH while desacetyl- α-MSH is more abundant in the brain, the foetus, human blood and amniotic fluid. The functional significance of this acetylation is unknown although it has been shown that the biological activity of many peptide hormones and neuropeptides is modified by N-terminal acetylation. In addition, it has been demonstrated that the two peptides (α-MSH and desacetyl α-MSH) give rise to different physiological effects in vivo (reviewed by Mountjoy et al., 2003). Our study is the first to address the pharmacological impact of acetylation of MSH peptides in non-mammalian species. The results suggest that there are slight differences in the binding affinities between the acetylated and non-acetylated peptide in chicken. The cMC1, cMC3 and cMC5 receptors display slightly higher preference for the desacetylated peptide, which partly correlates with the result from humans, where the MC3 receptor seemed to favour the desacetylated form of ACTH (Schiöth et al., 1997a).
There are several other features of the cMC receptor pharmacology that are worth mentioning. We found that the cMC5 receptor in general display higher affinity for the natural melanocortin peptides as compared with the human orthologue. This is particularly evident for the ACTH-fragments, which have about 200- to 500-fold higher affinities for the chicken receptor, indicating that the mammalian MC5 receptors may have lost their affinity to melanocortin peptides. Moreover, HS024, an antagonist at the mammalian MC1, MC3, MC4 and MC5 receptors, operated as a full agonist at both the cMC1 and cMC5 receptors. Interestingly, this correlates well with results received from the goldfish MC5 receptor (Cerda-Reverter et al., 2003a) that show the same agonistic profile for HS024 at the MC5 receptor. The ability of this compound to act as both agonist and antagonist at similar receptors may be useful to study the structural features that are needed for the activation of the MC receptors.
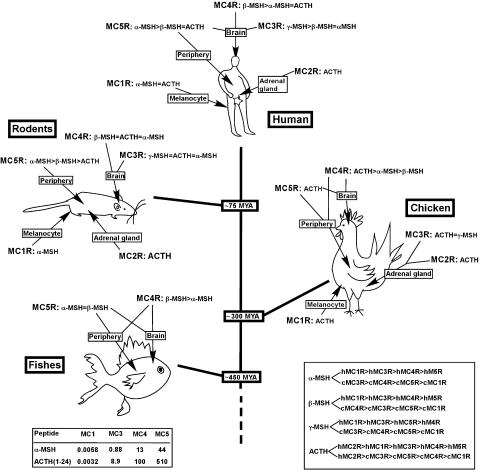
The five MC receptor genes show considerable sequence identity that seems to be highly conserved through the vertebrate evolution (Ringholm et al., 2002; Cerda-Reverter et al., 2003b, 2003c). Despite this, it is evident that the different subtypes diverged functionally early to take on their specific roles. The tissue distributions pattern is an important indicator of the functional roles, for key vertebrate species emerging, as well as the pharmacological profiles of each subtype. In order to provide an overview for how the anatomical and pharmacological evolution coincide, we highlight what is known about the tissue distribution and pharmacology of the MC receptors in Figure 8, where the main evolutionary points are represented by fish (zebrafish, goldfish), chickens, rodents (rat and mouse) and humans.
Figure 8.
Overview of the tissue distribution and pharmacology of the MC receptors in fish (zebrafish, goldfish), chickens, rodents (rat and mouse) and humans. The fishes (Carassius auratus, Danio rerio) diverged from the line leading to mammals about 450 million years ago (MYA). The lineage leading to chicken (Gallus gallus) bifurcated later about 300 MYA and the rodents (Rattus rattus, Mus musculus) line diverged about 75 MYA (Waterston et al., 2002). The endogenous ligand preferences for the human and chicken MC receptor subtypes are shown in the box to the right, and the relative binding affinities for α-MSH and ACTH(1–24) at the human and chicken MC receptors are presented in the box to the left references for humans (Chhajlani & Wikberg, 1992; Mountjoy et al., 1992; 1994; Boston & Cone 1996), chicken (Takeuchi et al., 1996; Takeuchi & Takahashi, 1998a, 1998b), rodents (Gantz et al., 1993; Labbe et al., 1994; Cammas et al., 1995) and fishes (Ringholm et al., 2002; Cerda-Reverter et al., 2003b; Logan et al., 2003).
In summary, we have performed a comprehensive study of the pharmacological properties of the entire cMC receptor repertoire. This is the first study that covers the pharmacology of all five MC receptors in a single species. The results indicate a more prominent role for the ACTH-fragments in chicken compared to mammals and hypothesis about how the specific characters of the different receptor subtypes may have developed. The study also provides applicable information setting guidelines for use of ligands in physiological trial when studying the melanocortin system in aves.
Acknowledgments
We thank Dr Robert Fredriksson, Uppsala University for assistance with phylogenetic analysis. We thank Marie Karlsson and Marie Svensson, Uppsala University for assistance with part of the pharmacological testing. Dr Nicole Gallo-Payet is a holder of a Canada Research Chair in Endocrinology of the Adrenal Gland. The studies were supported by the Swedish Medical Research council (MRC), the Swedish Society for Medical Research (SSMF), Canadian Institute for Health Research (MOP 10998), Svenska Läkaresällskapet, Åke Wibergs Stiftelse, The Novo Nordisk Foundation and Melacure Therapeutics AB, Uppsala, Sweden to H.S., and the Japanese Society for Promotion of Science (Grant-in Aid for Scientific Research) to S.T.
Abbreviations
- ACTH
adrenocorticotropic hormone
- c
chicken
- GPCR
G-protein-coupled receptor
- HEK-293 cells
human embryonic kidney cell line, strain 293
- HS024
cyclic MSH analogue, Ac-Cys-Nle-Arg-His-D-Nal(2)-Arg-Trp-Gly-Cys-NH2, disulphide bridge Cys1-Cys9
- MC receptor
melanocortin receptor
- MSH
melanocyte-stimulating hormone
- MTII
cyclic MSH analogue, Ac-Nle4-c[Asp5, D-Phe7, Lys10]α-MSH (4–10)-NH2
- MYA
million years ago
- NDP-MSH
(Nle4, D-Phe7) α-MSH
- PC
prohormone convertase
- POMC
proopiomelanocortin
- QTL
quantitative trait locus
- RT–PCR
reverse transcription PCR
References
- ADAN R.A., GISPEN W.H. Brain melanocortin receptors: from cloning to function. Peptides. 1997;18:1279–1287. doi: 10.1016/s0196-9781(97)00078-8. [DOI] [PubMed] [Google Scholar]
- BOSTON B.A., CONE R.D. Characterization of melanocortin receptor subtype expression in murine adipose tissues and in the 3T3-L1 cell line. Endocrinology. 1996;137:2043–2050. doi: 10.1210/endo.137.5.8612546. [DOI] [PubMed] [Google Scholar]
- BUCKLEY D.I., RAMACHANDRAN J. Characterization of corticotropin receptors on adrenocortical cells. Proc. Natl. Acad. Sci. U.S.A. 1981;78:7431–7435. doi: 10.1073/pnas.78.12.7431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURT D., POURQUIE O. Genetics. Chicken genome – science nuggets to come soon. Science. 2003;5626:1669. doi: 10.1126/science.1086231. [DOI] [PubMed] [Google Scholar]
- CAMMAS F.M., KAPAS S., BARKER S., CLARK A.J. Cloning, characterization and expression of a functional mouse ACTH receptor. Biochem. Biophys. Res. Commun. 1995;212:912–918. doi: 10.1006/bbrc.1995.2056. [DOI] [PubMed] [Google Scholar]
- CARLBORG Ö., KERJE S., SCHÜTZ K., JACOBSSON L., JENSEN P, ANDERSSON L. A global search reveals epistatic interaction between QTL for early growth in the chicken. Genome Res. 2003;13:413–421. doi: 10.1101/gr.528003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CERDA-REVERTER J.M., LING M.K., SCHIOTH H.B., PETER R.E. Molecular cloning, characterization and brain mapping of the melanocortin 5 receptor in the goldfish. J. Neurochem. 2003a;87:1354–1367. doi: 10.1046/j.1471-4159.2003.02107.x. [DOI] [PubMed] [Google Scholar]
- CERDA-REVERTER J.M., RINGHOLM A., SCHIÖTH H.B., PETER R.E. Molecular cloning, pharmacological characterization, and brain mapping of the melanocortin 4 receptor in the goldfish: involvement in the control of food intake. Endocrinology. 2003b;144:2336–2349. doi: 10.1210/en.2002-0213. [DOI] [PubMed] [Google Scholar]
- CERDA-REVERTER J.M., SCHIÖTH H.B., PETER R.E. The central melanocortin system regulates food intake in goldfish. Regul. Peptides. 2003c;115:101–113. doi: 10.1016/s0167-0115(03)00144-7. [DOI] [PubMed] [Google Scholar]
- CHEN A.S., MARSH D.J., TRUMBAUER M.E., FRAZIER E.G., GUAN X.M., YU H., ROSENBLUM C.I., VONGS A., FENG Y., CAO L., METZGER J.M., STRACK A.M., CAMACHO R.E., MELLIN T.N., NUNES C.N., MIN W., FISHER J., GOPAL-TRUTER S., MACINTYRE D.E., CHEN H.Y., VAN DER PLOEG L.H. Inactivation of the mouse melanocortin-3 receptor results in increased fat mass and reduced lean body mass. Nat. Genet. 2000;26:97–102. doi: 10.1038/79254. [DOI] [PubMed] [Google Scholar]
- CHEN W., KELLY M.A., OPITZ-ARAYA X., THOMAS R.E., LOW M.J., CONE R.D. Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell. 1997;91:789–798. doi: 10.1016/s0092-8674(00)80467-5. [DOI] [PubMed] [Google Scholar]
- CHENG H.H. Mapping the chicken genome. Poult. Sci. 1997;76:1101–1107. doi: 10.1093/ps/76.8.1101. [DOI] [PubMed] [Google Scholar]
- CHHAJLANI V., WIKBERG J.E. Molecular cloning and expression of the human melanocyte stimulating hormone receptor cDNA. FEBS Lett. 1992;309:417–420. doi: 10.1016/0014-5793(92)80820-7. [DOI] [PubMed] [Google Scholar]
- CHOMCZYNSKI P., SACCHI N. Single-step method of RNA isolation by acid guanidinium thiocyanate–phenol–chloroform extraction. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- EBERLE A.N. The Melanotropins; Chemistry, Physiology and Mechanisms of Action. Switzerland: Karger, Basel; 1988. [Google Scholar]
- FELSENSTEIN J. PHYLIP, phylogenetic inference package, distributed by the authors through Department of Genetics. Seattle, WA: University of Washington; 1993. [Google Scholar]
- GALLO-PAYET N., PAYET M.D. Excitation-secretion coupling: involvement of potassium channels in ACTH-stimulated rat adrenocortical cells. J. Endocrinol. 1989;120:409–421. doi: 10.1677/joe.0.1200409. [DOI] [PubMed] [Google Scholar]
- GANTZ I., MIWA H., KONDA Y., SHIMOTO Y., TASHIRO T., WATSON S.J., DELVALLE J., YAMADA T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J. Biol. Chem. 1993;268:15174–15179. [PubMed] [Google Scholar]
- HOFMANN K., WINGENDER W., FINN F.M. Correlation of adrenocorticotropic activity of ACTH analogs with degree of binding to an adrenal cortical particulate preparation. Proc. Natl. Acad. Sci. U.S.A. 1970;67:829–836. doi: 10.1073/pnas.67.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUSZAR D., LYNCH C.A., FAIRCHILD-HUNTRESS V., DUNMORE J.H., FANG Q., BERKEMEIER L.R., GU W., KESTERSON R.A., BOSTON B.A., CONE R.D., SMITH F.J., CAMPFIELD L.A., BURN P., LEE F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131–141. doi: 10.1016/s0092-8674(00)81865-6. [DOI] [PubMed] [Google Scholar]
- KAPAS S., CAMMAS F.M., HINSON J.P., CLARK A.J. Agonist and receptor binding properties of adrenocorticotropin peptides using the cloned mouse adrenocorticotropin receptor expressed in a stably transfected HeLa cell line. Endocrinology. 1996;137:3291–3294. doi: 10.1210/endo.137.8.8754753. [DOI] [PubMed] [Google Scholar]
- KASK A., MUTULIS F., MUCENIECE R., PAHKLA R., MUTULE I., WIKBERG J.E., RAGO L., SCHIÖTH H.B. Discovery of a novel superpotent and selective melanocortin-4 receptor antagonist (HS024): evaluation in vitro and in vivo. Endocrinology. 1998;139:5006–5014. doi: 10.1210/endo.139.12.6352. [DOI] [PubMed] [Google Scholar]
- KLOVINS J., HAITINA T., FRIDMANIS D., KILIANOVA Z., KAPA I., FREDRIKSSON R., GALLO-PAYET N., SCHIOTH H.B. The melanocortin system in fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol. Biol. Evol. 2004;21:563–579. doi: 10.1093/molbev/msh050. [DOI] [PubMed] [Google Scholar]
- LABBE O., DESARNAUD F., EGGERICKX D., VASSART G., PARMENTIER M. Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry. 1994;33:4543–4549. doi: 10.1021/bi00181a015. [DOI] [PubMed] [Google Scholar]
- LING M.K., LAGERSTROM M.C., FREDRIKSSON R., OKIMOTO R., MUNDY N.I., TAKEUCHI S., SCHIÖTH H.B. Association of feather colour with constitutively active melanocortin 1 receptors in chicken. Eur. J. Biochem. 2003;270:1441–1449. doi: 10.1046/j.1432-1033.2003.03506.x. [DOI] [PubMed] [Google Scholar]
- LOGAN D.W., BRYSON-RICHARDSON R.J., PAGÁN K.E., TAYLOR M.S., CURRIE P.D., JACKSON I. The structure and evolution of the melanocortin and MHC receptors in fish and mammals. Genomics. 2003;81:184–191. doi: 10.1016/s0888-7543(02)00037-x. [DOI] [PubMed] [Google Scholar]
- MARKLUND U., BYSTRÖM M., GEDDA K., LAREFALK A., JUNEBLAD K., NYSTRÖM S., EKSTRAND A.J. Intron-mediated expression of the human neuropeptide YY (1) receptor. Mol. Cell Endocrinol. 2002;188:85–97. doi: 10.1016/s0303-7207(01)00738-9. [DOI] [PubMed] [Google Scholar]
- MOUNTJOY K.G., MORTRUD M.T., LOW M.J., SIMERLY R.B., CONE R.D. Localization of the melanocortin-4 receptor (MC4-R) in neuroendocrine and autonomic control circuits in the brain. Mol. Endocrinol. 1994;8:1298–1308. doi: 10.1210/mend.8.10.7854347. [DOI] [PubMed] [Google Scholar]
- MOUNTJOY K.G., ROBBINS L.S., MORTRUD M.T., CONE R.D. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;257:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- MOUNTJOY K.G., WU C.S., CORNISH J., CALLON K.E. alpha-MSH and desacetyl-alpha-MSH signalling through melanocortin receptors. Ann. NY Acad. Sci. 2003;994:58–65. doi: 10.1111/j.1749-6632.2003.tb03162.x. [DOI] [PubMed] [Google Scholar]
- NOON L.A., FRANKLIN J.M., KING P.J., GOULDING N.J., HUNYADY L., CLARK A.J. Failed export of the adrenocorticotrophin receptor from the endoplasmic reticulum in non-adrenal cells: evidence in support of a requirement for a specific adrenal accessory factor. J. Endocrinol. 2002;174:17–25. doi: 10.1677/joe.0.1740017. [DOI] [PubMed] [Google Scholar]
- PENHOAT A., NAVILLE D., EL MOURABIT H., BURONFOSSE A., DURAND P., BEGEOT M. Functional expression of the human ACTH receptor gene. Endocr. Res. 2000;26:549–557. doi: 10.3109/07435800009048569. [DOI] [PubMed] [Google Scholar]
- RINGHOLM A., FREDRIKSSON R., POLIAKOVA N., YAN Y.L., POSTLETHWAIT J.H., LARHAMMAR D., SCHIÖTH H.B. One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J. Neurochem. 2002;82:6–18. doi: 10.1046/j.1471-4159.2002.00934.x. [DOI] [PubMed] [Google Scholar]
- SALOMON Y., LONDOS C., RODBELL M. A high sensitive adenylate cyclase assay. Anal. Biochem. 1974;58:541–548. doi: 10.1016/0003-2697(74)90222-x. [DOI] [PubMed] [Google Scholar]
- SCHIMMER B.P., KWAN W.K., TSAO J., QIU R. Adrenocorticotropin-resistant mutants of the Y1 adrenal cell line fail to express the adrenocorticotropin receptor. J. Cell Physiol. 1995;163:164–171. doi: 10.1002/jcp.1041630119. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B. The physiological role of melanocortin receptors. Vitam. Horm. 2001;63:195–232. doi: 10.1016/s0083-6729(01)63007-3. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., BOUIFROURI A.A., RUDZISH R., MUCENIECE R., WATANOBE H., WIKBERG J.E., LARHAMMAR D. Pharmacological comparison of rat and human melanocortin 3 and 4 receptors in vitro. Regul. Peptides. 2002;106:7–12. doi: 10.1016/s0167-0115(02)00025-3. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUCENIECE R., LARSSON M., WIKBERG J.E. The melanocortin 1, 3, 4 or 5 receptors do not have a binding epitope for ACTH beyond the sequence of alpha-MSH. J. Endocrinol. 1997a;155:73–78. doi: 10.1677/joe.0.1550073. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUCENIECE R., WIKBERG J.E., CHHAJLANI V. Characterisation of melanocortin receptor subtypes by radioligand binding analysis. Eur. J. Pharmacol. 1995;288:311–317. doi: 10.1016/0922-4106(95)90043-8. [DOI] [PubMed] [Google Scholar]
- SCHIÖTH H.B., MUTULIS F., MUCENIECE R., PRUSIS P., WIKBERG J. Discovery of novel melanocortin4 receptor selective MSH analogues. Br. J. Pharmacol. 1998;124:75–82. doi: 10.1038/sj.bjp.0701804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHIÖTH H.B., PETERSSON S., MUCENIECE R., SZARDENINGS M., WIKBERG J.E. Deletions of the N-terminal regions of the human melanocortin receptors. FEBS Lett. 1997b;410:223–228. doi: 10.1016/s0014-5793(97)00593-0. [DOI] [PubMed] [Google Scholar]
- SCHMIDT H.A., STRIMMER K., VINGRON M., VON HAESELER A. TREE-PUZZLE: maximum likelihood phylogenetic analysis using quartets and parallel computing. Bioinformatics. 2002;18:502–504. doi: 10.1093/bioinformatics/18.3.502. [DOI] [PubMed] [Google Scholar]
- SCHWYZER R. ACTH: a short introductory review. Ann. NY Acad. Sci. 1977;297:3–26. doi: 10.1111/j.1749-6632.1977.tb41843.x. [DOI] [PubMed] [Google Scholar]
- SKULADOTTIR G.V., JONSSON L., SKARPHEDINSSON J.O., MUTULIS F., MUCENIECE R., RAINE A., MUTULE I., HELGASON J., PRUSIS P., WIKBERG J.E., SCHIÖTH H.B. Long term orexigenic effect of a novel melanocortin 4 receptor selective antagonist. Br. J. Pharmacol. 1999;126:27–34. doi: 10.1038/sj.bjp.0702264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKAHASHI A., AMEMIYA Y., NOZAKI M., SOWER S.A., KAWAUCHI H. Evolutionary significance of proopiomelnaocortin in agnathan and chondrichthyes. Comp. Biochem. Physiol. B. 2001;129:283–289. doi: 10.1016/s1096-4959(01)00330-x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI S., KUDO T., TAKAHASHI S. Molecular cloning of the chicken melanocortin 2 (ACTH)-receptor gene. Biochim. Biophys. Acta. 1998;1403:102–108. doi: 10.1016/s0167-4889(98)00022-6. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI S., SUZUKI H., HIROSE S., YABUUCHI M., SATO C., YAMAMOTO H., TAKAHASHI S. Molecular cloning and sequence analysis of the chick melanocortin 1-receptor gene. Biochim. Biophys. Acta. 1996;1306:122–126. doi: 10.1016/0167-4781(96)00026-7. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI S., TAKAHASHI S. Melanocortin receptor genes in the chicken – tissue distributions. Gen. Comp. Endocrinol. 1998a;112:220–231. doi: 10.1006/gcen.1998.7167. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI S., TAKAHASHI S. A possible involvement of melanocortin 3 receptor in the regulation of adrenal gland function in the chicken. Biochim. Biophys. Acta. 1998b;1448:512–518. doi: 10.1016/s0167-4889(98)00165-7. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI S., TESHIGAWARA K., TAKAHASHI S. Molecular cloning and characterization of the chicken pro-opiomelanocortin (POMC) gene. Biochim. Biophys. Acta. 1999;1450:452–459. doi: 10.1016/s0167-4889(99)00046-4. [DOI] [PubMed] [Google Scholar]
- THOMPSON J.D., HIGGINS D.G., GIBSON T.J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATERSTON R.H., LINDBLAD-TOH K., BIRNEY E., ROGERS J., ABRIL J.F., AGARWAL P., AGARWALA R., AINSCOUGH R., ALEXANDERSSON M., AN P., ANTONARAKIS S.E., ATTWOOD J., BAERTSCH R., BAILEY J., BARLOW K., BECK S., BERRY E., BIRREN B., BLOOM T., BORK P., BOTCHERBY M., BRAY N., BRENT M.R., BROWN D.G., BROWN S.D., BULT C., BURTON J., BUTLER J., CAMPBELL R.D., CARNINCI P., CAWLEY S., CHIAROMONTE F., CHINWALLA A.T., CHURCH D.M., CLAMP M., CLEE C., COLLINS F.S., COOK L.L., COPLEY R.R., COULSON A., COURONNE O., CUFF J., CURWEN V., CUTTS T., DALY M., DAVID R., DAVIES J., DELEHAUNTY K.D., DERI J., DERMITZAKIS E.T., DEWEY C., DICKENS N.J., DIEKHANS M., DODGE S., DUBCHAK I., DUNN D.M., EDDY S.R., ELNITSKI L., EMES R.D., ESWARA P., EYRAS E., FELSENFELD A., FEWELL G.A., FLICEK P., FOLEY K., FRANKEL W.N., FULTON L.A., FULTON R.S., FUREY T.S., GAGE D., GIBBS R.A., GLUSMAN G., GNERRE S., GOLDMAN N., GOODSTADT L., GRAFHAM D., GRAVES T.A., GREEN E.D., GREGORY S., GUIGO R., GUYER M., HARDISON R.C., HAUSSLER D., HAYASHIZAKI Y., HILLIER L.W., HINRICHS A., HLAVINA W., HOLZER T., HSU F., HUA A., HUBBARD T., HUNT A., JACKSON I., JAFFE D.B., JOHNSON L.S., JONES M., JONES T.A., JOY A., KAMAL M., KARLSSON E.K., KAROLCHIK D., KASPRZYK A., KAWAI J., KEIBLER E., KELLS C., KENT W.J., KIRBY A., KOLBE D.L., KORF I., KUCHERLAPATI R.S., KULBOKAS E.J., KULP D., LANDERS T., LEGER J.P., LEONARD S., LETUNIC I., LEVINE R., LI J., LI M., LLOYD C., LUCAS S., MA B., MAGLOTT D.R., MARDIS E.R., MATTHEWS L., MAUCELI E., MAYER J.H., MCCARTHY M., MCCOMBIE W.R., MCLAREN S., MCLAY K., MCPHERSON J.D., MELDRIM J., MEREDITH B., MESIROV J.P., MILLER W., MINER T.L., MONGIN E., MONTGOMERY K.T., MORGAN M., MOTT R., MULLIKIN J.C., MUZNY D.M., NASH W.E., NELSON J.O., NHAN M.N., NICOL R., NING Z., NUSBAUM C., O'CONNOR M.J., OKAZAKI Y., OLIVER K., OVERTON-LARTY E., PACHTER L., PARRA G., PEPIN K.H., PETERSON J., PEVZNER P., PLUMB R., POHL C.S., POLIAKOV A., PONCE T.C., PONTING C.P., POTTER S., QUAIL M., REYMOND A., ROE B.A., ROSKIN K.M., RUBIN E.M., RUST A.G., SANTOS R., SAPOJNIKOV V., SCHULTZ B., SCHULTZ J., SCHWARTZ M.S., SCHWARTZ S., SCOTT C., SEAMAN S., SEARLE S., SHARPE T., SHERIDAN A., SHOWNKEEN R., SIMS S., SINGER J.B., SLATER G., SMIT A., SMITH D.R., SPENCER B., STABENAU A., STANGE-THOMANN N., SUGNET C., SUYAMA M., TESLER G., THOMPSON J., TORRENTS D., TREVASKIS E., TROMP J., UCLA C., URETA-VIDAL A., VINSON J.P., VON NIEDERHAUSERN A.C., WADE C.M., WALL M., WEBER R.J., WEISS R.B., WENDL M.C., WEST A.P., WETTERSTRAND K., WHEELER R., WHELAN S., WIERZBOWSKI J., WILLEY D., WILLIAMS S., WILSON R.K., WINTER E., WORLEY K.C., WYMAN D., YANG S., YANG S.P., ZDOBNOV E.M., ZODY M.C., LANDER E.S., Mouse Genome Sequencing Consortium Initial sequencing and comparative analysis of the mouse genome. Nature. 2002;420:520–556. doi: 10.1038/nature01262. [DOI] [PubMed] [Google Scholar]