Abstract
We examined bone-marrow in mice receiving subcutaneous implants of heat-coagulated egg white, which are known to present chronic eosinophilic inflammation at the implant site. Egg white implants (EWIs) induced marked bone-marrow eosinophilia, and increased bone-marrow cell responses to granulocyte-macrophage colony-stimulating factor and interleukin-5 in culture. These effects were observed as early as 24 h and lasted for, at least, 30 days in implant recipients.
We found, however, that increased eosinophil production was also observed in control mice which underwent surgery but received no EWI (sham-implanted mice), up to 15 days post-surgery. As this suggests an important contribution of nonspecific stress mechanisms to eosinopoiesis, we further evaluated the role of stress hormones produced by the adrenal glands in the bone-marrow eosinophilia of sham-implanted mice.
Bone-marrow eosinophilia in mice undergoing surgery was dissociated from increases in other haemopoietic lineages. Surgery by itself increased circulating corticosterone levels by 24 h, and the increase was prevented by inhibition of adrenal glucocorticoid production by metyrapone. The effect of surgery on bone-marrow eosinophilia was prevented by pretreatment with both the glucocorticoid receptor antagonist, mifepristone, and metyrapone, and by surgical adrenalectomy.
By contrast, cathecolamine receptor antagonists (propranolol, prazosin and yohimbine) were ineffective, indicating that cathecolamine release from the adrenal glands was not responsible for the effects on bone-marrow.
These results highlight a critical role for stress-induced glucocorticoid hormones in selectively upregulating bone-marrow eosinopoiesis in mice submitted to surgery.
Keywords: Surgery, haematopoiesis, eosinophils, adrenal glands, stress, IL-5, glucocorticoids
Introduction
Eosinophils are important effector cells in allergic and parasitic diseases (Adamko et al., 2003; Lemanske & Busse, 2003). Glucocorticoids (GCs), widely used in the treatment of allergic diseases and asthma due to their well-established anti-inflammatory actions (Lemanske & Busse, 2003), have been shown by many studies to inhibit cytokine production and action (Leung & Bloom, 2003; Padgett & Glasser, 2003). As several studies have documented the ability of GCs to suppress the synthesis and action of interleukin (IL)-5, IL-3, and granulocyte-macrophage colony-stimulating factor (GM-CSF), which support eosinopoiesis and promote eosinophil survival, it is often assumed that GCs will always counteract the effects of these cytokines, thereby contributing to blood and tissue eosinopenia (Adcock & Caramori, 2001). However, our recent studies (Gaspar Elsas et al., 2000), along with those from others (reviewed by Xavier Elsas et al. (2003) and Sehmi et al., 2003) raise the possibility that GCs can actually potentiate the effects of IL-5 and GM-CSF, on bone-marrow eosinopoiesis, pointing to a more complex interaction between cytokines and GCs that might critically depend on the maturation stage of the target cells.
Recently, a murine model of insoluble antigen-induced eosinophilic inflammation has been described (Siqueira et al., 1997). In this model, recipients of a subcutaneous heat-coagulated egg white implant (EWI) present intense eosinophilic infiltration at the implant site, and, if further exposed to an intratracheal ovalbumin challenge, develop profuse eosinophilic infiltration of the lungs, accompanied by asthma-like functional abnormalities (Siqueira et al., 1997). The EWI model is unique in that it presents sustained eosinophilia despite surgical trauma, which is expected to trigger systemic release of stress-induced glucocorticoids (DeKeyser et al., 2000; Ogawa et al., 2000). As eosinophils are produced in the bone-marrow, we conducted an evaluation of eosinophil production in the bone-marrow of EWI recipients as a function of time after surgery. We also included in this evaluation mice submitted to surgery which received no implant (sham-implanted mice), in which the effects of surgery itself on eosinophil production could be assessed. In the course of these studies, we obtained direct evidence of a stimulatory effect of surgery on eosinophil production in the bone-marrow. We have further evaluated whether this effect depended on stress-stimulated production of glucocorticoids by the adrenal glands.
Methods
Animal procedures
BALB/c mice, bred at FIOCRUZ, Rio de Janeiro (Brazil), were used at 6–8 weeks of age. EWI followed the procedures described by Siqueira et al. (1997). Briefly, coagulated egg white pellets were rehydrated in sterile saline for 2 h. Mice were anaesthesiated with tribromoethanol (250 mg kg−1, i.p.) and a dorsal incision was made after local trichotomy. Rehydrated egg white pellets were implanted subcutaneously through the dorsal incision, and the skin was sutured. Control (sham-implanted) mice were submitted to the surgery, but received no implant. Animals were killed at the indicated times post-surgery. In selected experiments, mice were treated, 2 h before surgery, with the glucocorticoid receptor antagonist, mifepristone (RU486; 20 mg kg−1 dissolved in 0.5% methylcellulose, orally), or the glucocorticoid synthesis inhibitor, metyrapone, using a chronic administration protocol (30 mg kg−1, i.p., daily through 8 days, surgery being performed 2 h after the last dose). Control mice received vehicle only. Animals were bilaterally adrenalectomized (ADX) under tribromoethanol anaesthesia (250 mg kg−1, i.p.), using the dorsal approach. All ADX animals received 0.9% NaCl solution ad libitum after surgery, and experimental procedures were carried out 2 weeks after adrenalectomy. Where indicated, metyrapone was administered as above to mice which were ADX at day 8, 2 h after the last dose. This was done because the removal of one adrenal gland already involves a surgical procedure, during which rapid glucocorticoid release from the contralateral gland can occur. For the adrenalectomy experiments, experimental procedures were carried out 2 weeks after adrenalectomy, hence 22 days after the initial metyrapone dose. A separate control group received metyrapone as above, but underwent no adrenalectomy, undergoing the experimental procedures 22 days after initiation of metyrapone treatment.
Haematologic studies
Bone-marrow cell harvest and culture followed procedures described previously (Gaspar Elsas et al., 1997). For bone-marrow culture, we used the conditions that were previously defined for BALB/c mice, on the basis of dose–response curves for IL-5-driven eosinophil differentiation in liquid culture (precursor assay; Gaspar Elsas et al., 2000). Optimal concentration was defined as the lowest concentration that yielded plateau responses. Liquid bone-marrow cultures (106 cells in a 1 ml volume, in a 24-well cluster) were seeded in RPMI 1640 medium, with 10% foetal calf serum (FCS), 2 mM L-glutamine, and penicillin–streptomycin, at 37°C, 5% CO2 and 95% air, for 7 days, at least in duplicate, in the presence of IL-5 (1 ng ml−1). Control cultures established in the absence of IL-5 present no eosinophils after 7 days (Gaspar Elsas et al., 1997). The frequency of cells stained for eosinophil peroxidase (EPO) was determined in cytocentrifuge smears of freshly harvested bone-marrow cells (day 0) or after 7-day liquid cultures as previously described (Gaspar Elsas et al., 1997; 2000). The cytochemical and staining pattern of cells in bone-marrow preparations was identical to that described elsewhere (Horton et al., 1996). We have previously confirmed that these conditions are adequate for evaluating eosinophil differentiation in murine bone-marrow, and that the increase in % EPO+cells, as directly determined on cytocentrifuge smears, was paralleled by increases in the total numbers of EPO+ cells in these cultures, as indirectly determined from cell counts (Gaspar Elsas et al., 2000). For colony formation assays, 1 ml semi-solid cultures were established in 35 mm culture dishes, from 2 × 105 cells, in a mixture of Iscove's modified Dulbecco's medium with 20% FCS and agar to 0.3% final concentration (Kurland et al., 1977; Bagby, 1994), in the presence of GM-CSF (2 ng ml−1), at least in triplicate. Colonies (progenitor-derived ensembles >50 cells; Bagby, 1994) were scored at day 7. We have previously confirmed that these conditions were adequate for counting total myeloid colonies and for accurate differential counts of myeloid colony types on dried agar layers (Gaspar Elsas et al., 2000).
Determination of corticosterone levels
Circulating corticosterone levels were evaluated using a radioimmunoassay (RIA) assay kit, following the manufacturer's guidelines. Plasma was collected between 08:00 and 09:00 h, when circadian glucocorticoid levels are decreased. For operated mice, this corresponds to 18 h after surgery. Heparinized blood was collected, plasma was obtained by centrifugation, and stored at −20°C, until use.
Reagents
Dehydrated egg white was from Ito Avicultura (São Paulo, SP, Brazil). Egg white pellets used for implantation were prepared as described (Siqueira et al., 1997) with slight modifications. Briefly, dehydrated egg white was solubilized in distilled water to achieve a 10% (w v−1) concentration, and dispensed on a 96-well plate (45 μl per well, 4.5 mg) and coagulated in a microwave oven (90 s at 900 Hz). Pellets were recovered and dehydrated in absolute ethanol for 48 h. Dehydrated pellets were allowed to dry at room temperature for 24 h and stored at 4°C until use. Recombinant murine IL-5 (rmIL-5) and recombinant murine GM-CSF (rmGM-CSF) were from R&D Systems (Minneapolis, MN, U.S.A.). Agar noble, methylcellulose, propranolol, prazosin, and mifepristone (RU486) were from Sigma Chemical Co. (St Louis, MO, U.S.A.). Metyrapone (2-methyl-1,2-di-3-pyridyl-1-propanone) and 2,2,2-tribromoethanol were from Aldrich (Milwaukee, WI, U.S.A.). Yohimbine was a kind gift from Professor Paulo Melo (UFRJ, Brazil). Propranolol, yohimbine, and prazosin were solubilized in sterile saline and administered intraperitoneally. Propranolol and prazosin were administered 30 min before surgery at 10 mg kg−1. Yohimbine was administered 15 min before surgery at 5 mg kg−1. These treatments should result in complete blockade, as established from the studies: (a) by Karalis et al. (1999) and Carmo et al. (2003), for propranolol; (b) by Pinardi et al. (2002) and Zarrindast et al. (2003), for prazosin; and (c) by Zarrindast et al. (2003) and Choy et al. (2003) for yohimbine. [125I]corticosterone RIA kit was purchased from ICN Pharmaceuticals (Costa Mesa, CA, U.S.A.). Culture medium and FCS were from Hyclone (Logan, UT, U.S.A.). Harris' haematoxylin acidified was from Shandon Inc. (Pittsburgh, PA, U.S.A.).
Statistical analysis
Pairwise comparisons were carried out using the two-tailed t-test. For multiple comparisons between different treatments within each experimental group, we used factorial analysis of variance with the Tukey (HSD) correction, using Systat for Windows 4.0.
Results
Effect of subcutaneous EWI on bone-marrow eosinopoiesis
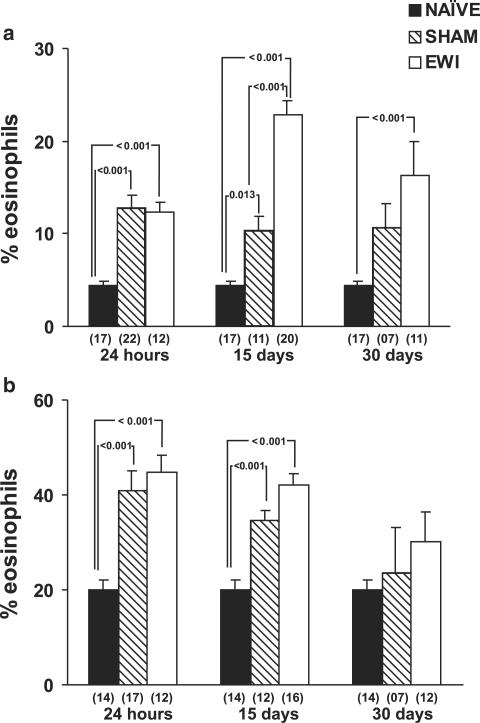
We initially evaluated changes in bone-marrow eosinopoiesis in EWI recipients, as well as sham-implanted mice, and compared both to naïve controls. Both bone-marrow eosinophilia at the time of harvest and the ex vivo responses to IL-5 in culture were analysed at various time points. As shown in Figure 1, panel a, a rapid and sustained increase in bone-marrow eosinophilia was detected in EWI mice relative to naïve controls. It was detectable as early as 24 h after surgery and lasted for, at least, a 30-day period. Interestingly, sham-implanted control mice also showed a very significant increase in bone-marrow eosinophilia at 24 h after surgery, which persisted for at least 2 weeks, but was no longer significant after 1 month (P=0.808 at day 30, relative to naïve controls). For the earliest time point, the surgery-associated component, as evidenced in sham-implanted mice, accounted for all of the increase observed in EWI recipients. Even at 15 days, it remained a significant fraction of the overall effect of EWI. By contrast, no effect of the anaesthesia by itself, in the absence of surgical wound, was observed (data not shown).
Figure 1.
Bone-marrow eosinopoiesis in egg white-implanted and sham-implanted mice. Data are means±s.e.m. of the percent eosinophils in cytocentrifuge smears of (a) bone-marrow cells, or (b) cells present in 7-day bone-marrow liquid cultures. NAÏVE, naïve controls, SHAM, sham-implanted, and EWI, egg white-implanted mice. Bone-marrow counts and cultures were made at the indicated times after surgery. Multiple comparisons between groups were carried out within each time point. P-values are indicated for all significant differences. In (a), numbers of experiments for the three groups were, respectively, 17, 22, and 12 at 24 h; 17, 11, and 20 at 15 days; and 17, 7, and 11 at 30 days. In (b), numbers of experiments for the three groups were, respectively, 14, 17, and 12 at 24 h; 14, 12, and 16 at 15 days; and 14, ,7 and 12 for 30 days.
As shown in Figure 1, panel b, the proliferation and differentiation of eosinophil precursors in liquid bone-marrow culture, induced by the eosinophil-specific haematopoietic growth factor IL-5, showed a similar increase in EWI mice, relative to naïve controls. EWI induced a rapid increase in bone-marrow responses to IL-5 at 24 h and 15 days after implantation. However, this enhancement was no longer significant at day 30 (P=0.363, relative to naïve controls). On the other hand, sham-implanted mice also showed significantly increased bone-marrow responses to IL-5 at 24 h and 15 days, but not at 30 days after surgery (P=0.906).
These results show that, as expected, EWI upregulates bone-marrow eosinopoiesis, as well as bone-marrow responses to IL-5. They also show a major contribution of the surgical procedure itself, independently of the heat-coagulated EWI, suggesting that surgical stress-dependent mechanisms contribute to eosinophilia. For this reason, in the experiments described below, we have restricted our observations to sham-implanted mice, as a model to analyse the role of stress and surgical trauma in these phenomena.
Role of endogenous glucocorticoids in upregulation of bone-marrow eosinopoiesis in sham-implanted mice
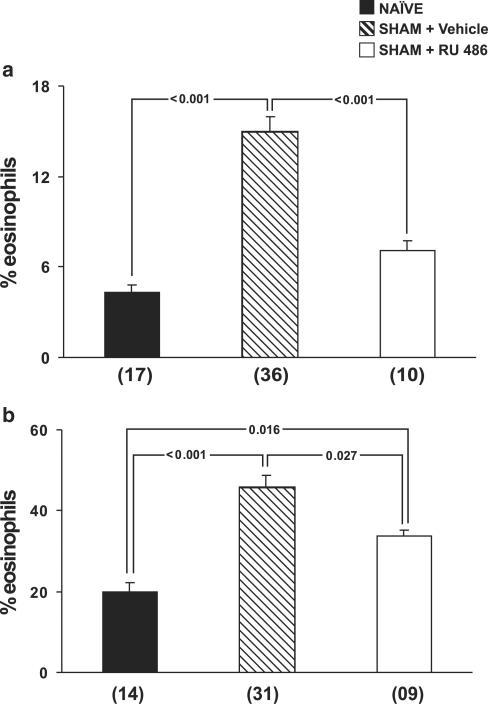
To evaluate the contribution of endogenous GCs to the upregulation of eosinopoiesis by surgical trauma, we used the GC receptor antagonist mifepristone (RU486). As shown in Figure 2, panel a, RU486 pretreatment abolished the effect of surgery on bone-marrow eosinophilia, as shown by the comparison of drug-treated and vehicle-treated sham-implanted mice. As a consequence, eosinophilia in RU486-treated sham-implanted mice was not significantly different from that in naïve controls (P=0.148). However, as shown in panel b, RU486 treatment was only partly effective in preventing the effects of surgery on bone-marrow responses to IL-5. These results suggest that the contribution of surgery to increased eosinopoiesis is, at least in part, mediated by the effects of endogenous GCs released in response to surgical stress.
Figure 2.
Effect of RU486 on the upregulation of bone-marrow eosinopoiesis by surgery. Data are means±s.e.m. of the percent eosinophils present in (a) bone-marrow and (b) 7-day bone-marrow liquid cultures. NAÏVE, naïve controls, SHAM+Vehicle, vehicle-treated, sham-implanted mice, SHAM+RU486, RU486-treated, sham-implanted mice. Bone-marrow counts and culture were established 24 h after surgery. Multiple comparisons were carried out and P-values are indicated for all significant differences. Numbers of experiments for the three groups were, respectively, 17, 36, and 10 in panel a; and 14, 31, and 9 in panel b.
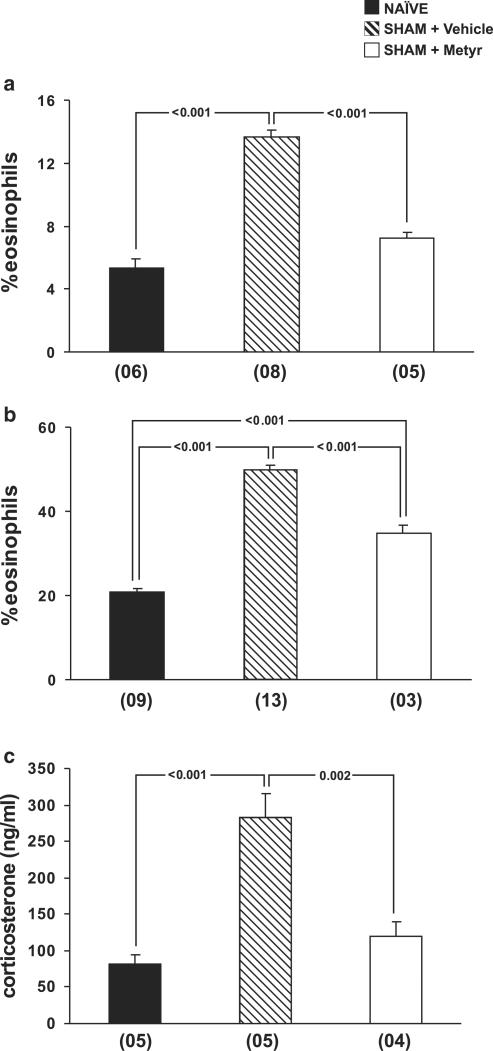
We have also evaluated the role of endogenous GCs in upregulating bone-marrow eosinopoiesis in sham-implanted mice by pretreating the animals with the inhibitor of GC synthesis, metyrapone. As shown in Figure 3, panel a, metyrapone pretreatment blocked the effects of surgical stress on bone-marrow eosinophilia, as evidenced in the comparison of drug-treated and vehicle-treated sham-implanted mice. As a result, eosinophilia in metyrapone-pretreated, sham-implanted mice was not significantly different from that in naïve controls (P=0.069). However, as shown in panel b, metyrapone treatment was only partly effective in preventing the effects of surgery on bone-marrow responses to IL-5. Metyrapone by itself had no effect on naïve bone-marrow responses to IL-5 (data not shown). The ability of metyrapone to counteract the effects of surgical trauma was correlated to its ability to inhibit corticosterone production in response to trauma, as shown in panel c. GC levels were significantly increased in sham-implanted mice relative to naïve controls, and this increase was blocked by metyrapone pretreatment, as evidence in the comparison between drug-treated and vehicle-treated, sham-implanted mice. As a consequence, corticosterone levels in metyrapone-pretreated, sham-implanted mice were not significantly different from those of naïve controls (P=0.518).
Figure 3.
Effect of metyrapone treatment on the upregulation of bone-marrow eosinopoiesis by surgery. Data are means±s.e.m. of the percent eosinophils present in (a) bone-marrow or in (b) 7-day bone-marrow liquid cultures, and (c) of the plasma corticosterone concentration. NAÏVE, naïve controls, SHAM+Vehicle, vehicle-treated, sham-implanted mice, SHAM+Metyr, metyrapone-treated, sham-implanted mice. Bone-marrow counts and cultures were established 24 h after surgery. Multiple comparisons were carried out and P-values are indicated for all significant differences. Numbers of experiments for the three groups were, respectively, 6, 8, and 5 in panel a; 9, 13, and 3 in panel b; and 5, 5, and 4 in panel c.
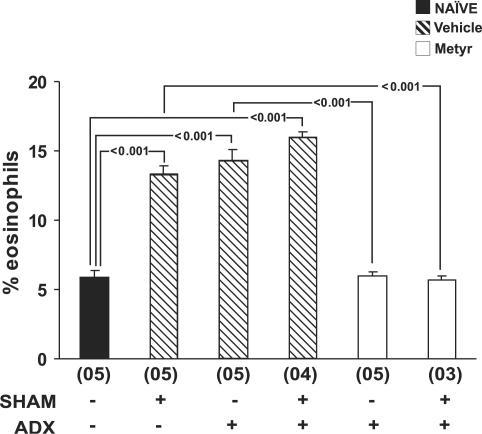
Finally, to directly confirm the involvement of the adrenal gland in the effects of surgical stress, we evaluated bone-marrow responses to surgery in ADX mice. As shown in Figure 4, adrenalectomy by itself significantly upregulated bone-marrow eosinophilia in mice analysed 2 weeks after removal of the adrenal glands. However, adrenalectomy also prevented further bone-marrow responses to surgical stress (P=0.305, for the differences between ADX sham-implanted mice and ADX controls). Both effects were paralleled by an increased bone-marrow response to IL-5 in ADX mice, and by the refractoriness of the bone-marrow to subsequent surgery (data not shown). By contrast, in ADX mice that were pretreated with metyrapone before adrenalectomy, and subsequently sham-implanted, neither the first nor the second surgical procedure increased bone-marrow eosinophilia (P=0.999 for the difference between this group and naïve controls). On the other hand, animals which received metyrapone but underwent no adrenalectomy responded fully to sham-implantation surgery 2 weeks after the last dose of metyrapone (11.89±0.40% eosinophils in bone-marrow 24 h after surgery). This indicates that metyrapone was effective only by preventing release of GCs during adrenalectomy and had no residual effect 2 weeks later. Taken together, data from these three independent lines of evidence show that stress-induced GC secretion by the adrenal gland is required for upregulation of bone-marrow eosinophil production in the sham-operated implant model.
Figure 4.
Effect of adrenal gland blockade and removal on the response of bone-marrow to subsequent surgery. Data are means±s.e.m. of the percent eosinophils present at day 0 in bone-marrow cytocentrifuge smears. NAÏVE, naïve controls, Vehicle, vehicle-treated mice, Metyr, metyrapone-treated mice. Mice were submitted or not to surgical removal of the adrenal glands (ADX) and further submitted or not to sham implantation surgery (SHAM), 15 days after ADX. Bone-marrow counts were made 24 h after the sham implantation surgical procedure. Multiple comparisons were carried out and P-values are indicated for all significant differences. Numbers of experiments for the six groups were, respectively, 5, 5, 5, 4, 5, and 3.
Lineage selectivity of the effect of surgical stress on bone-marrow
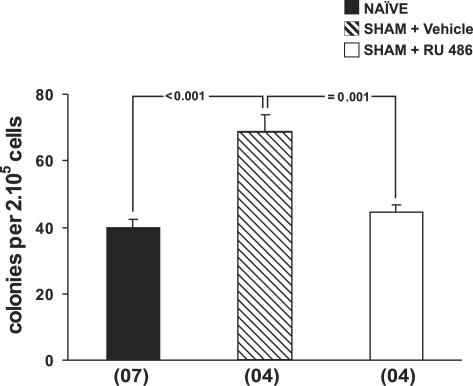
To evaluate whether increased eosinopoiesis reflects a nonspecific stimulation of myelopoiesis by surgical stress, we analysed the effects of surgery on colony formation by bone-marrow cells in culture. As shown in Figure 5, surgical trauma induced a very significant increase in GM-CSF-stimulated bone-marrow colony formation 24 h after surgery. Moreover, this increase was GC-dependent, because the effect of surgery on bone-marrow colony formation was abolished by RU486 pretreatment, as evidenced by the comparison between vehicle-treated and drug-treated, sham-implanted mice. As a result, colony formation did not differ significantly between RU486-treated sham-implanted and naïve control mice (P=0.604). This indicates that surgical trauma primes bone-marrow cells for increased colony formation in response to GM-CSF, through GC-dependent mechanisms. We further evaluated whether operated mice had significantly increased bone-marrow cellularity relative to naïve controls. To do so, we compared bone-marrow nucleated cell counts of EWI mice and the control groups at all time points. As shown in Table 1, bone-marrow cellularity was unaltered at all time points for all the different treatments (P>0.214). In contrast, eosinophil numbers were significantly increased in the sham-implanted group relative to naïve controls at 24 h (P=0.006). However, the change in this minority myeloid lineage was not sufficient to detectably increase total bone-marrow cellularity. This result demonstrates that the effect of surgical trauma on bone-marrow cell populations in vivo is eosinophil-lineage specific, even though priming for increased responses to GM-CSF when bone-marrow cells were subsequently cultured could also be demonstrated.
Figure 5.
Colony formation by bone-marrow cells from sham-implanted mice. Data are means±s.e.m. of the number of myeloid colonies formed by 2 × 105 bone-marrow cells in response to GM-CSF (2 ng ml−1) in semi-solid cultures. NAÏVE, naïve controls, SHAM+Vehicle, vehicle-treated, sham-implanted mice, SHAM+ RU486, RU486-treated, sham-implanted mice. Bone-marrow cultures were established 24 h after surgery. Multiple comparisons were carried out and P-values are indicated for all significant differences. Numbers of experiments for the three groups were, respectively, 7, 4, and 4.
Table 1.
Effect of surgical procedure on bone-marrow cellularity
| 24 h | 15 days | 30 days | |
|---|---|---|---|
| NAÏVE | Cells: 20.51 (±1.98) | ||
| eos: 1.06 (±0.19) | |||
| SHAM | Cells: 20.25 (±2.28) | Cells: 23.09 (±1.55) | Cells: 22.32 (±1.30) |
| eos: 2.8 (±0.49)* | eos: 2.56 (±0.52)* | eos: 2.39 (±0.63) | |
| EWI | Cells: 17.95 (±1.68) | Cells: 21.56 (±1.26) | Cells: 25.23 (±1.61) |
| eos: 2.1 (±0.18)* | eos: 5.06 (±0.54)* | eos: 4.39 (±1.09)* |
Data are means (±s.e.m.) of the number of total nucleated cells (cells) or eosinophils (eos) (× 10−6) per femur of the indicated groups of mice at the indicated times after surgery. Data are derived from at least seven experiments.
Indicates significant differences from naïve controls (P<0.01 in all cases).
Role of endogenous catecholamines in upregulation of bone-marrow eosinopoiesis
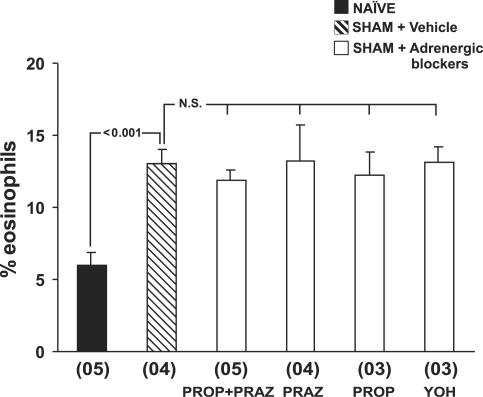
As stress responses also involve enhanced production and secretion of catecholamines, we decided to evaluate the contribution of adrenergic mechanisms to the observed bone-marrow eosinophilia in our model. To do so, we treated mice with the β-adrenergic antagonist, propranolol, the α1-specific blocker, prazosin, or both, or with the α2-adrenergic blocker, yohimbine, before surgery, and evaluated bone-marrow eosinophilia 24 h after surgery. As shown in Figure 6, adrenergic antagonists, administered either alone or in association, did not significantly affect bone-marrow responses to surgery. These results show that catecholamines are not involved in bone-marrow modulation in our model.
Figure 6.
Ineffectiveness of adrenergic blockade in preventing the upregulation of bone-marrow eosinophilia by surgery. Data are means±s.e.m. of the percent eosinophils present in bone-marrow. NAÏVE, naïve mice, SHAM+Vehicle, vehicle-treated, sham-implanted mice, SHAM+Adrenergic blockers, sham-implanted mice pretreated with prazosin (PRAZ), propranolol (PROP), prazosin and propranolol (PROP+PRAZ) or yohimbine (YOH). Bone-marrow counts were made 24 h after surgery. Multiple comparisons were carried out. P-value is shown for the difference between vehicle-treated, sham-implanted mice and naïve controls. All differences among sham-implanted (vehicle-treated and drug-treated) mice were nonsignificant (NS). Numbers of experiments for the six groups were, respectively, 5, 4, 5, 4, 3, and 3.
Discussion
This study was originally intended to evaluate the modifications of bone-marrow eosinophil production in a murine model of chronic eosinophilic inflammation, which is initiated by surgical implantation of heat-coagulated egg white under the skin, presumably in response to allergens contained in the implant (Siqueira et al., 1997). In the course of this study, however, we also obtained evidence for a strong stimulation of eosinophil production in a control group of mice which underwent surgery but received no EWI. This suggested that surgery itself was a major determinant of bone-marrow eosinophilia. As surgical stress induces release of glucocorticoids and catecholamines from the adrenal glands (DeKeyser et al., 2000; Ogawa et al., 2000), and because glucocorticoids enhance eosinophil production from bone-marrow cells of murine (Gaspar Elsas et al., 2000) and human (Sehmi et al., 2003) origin, we further evaluated whether adrenal secretion of stress hormones played a role in the upregulation of bone-marrow eosinopoiesis by surgery.
We could demonstrate by three different experimental approaches (pharmacological blockade of the glucocorticoid receptor, pharmacological inhibition of adrenal glucocorticoid synthesis, and surgical removal of the adrenals) that endogenous GCs, released in response to the surgical stress inherent to this model, were the critical mediators of the increased in vitro and in vivo eosinopoiesis observed. It is important to realize that, because surgical stress is itself the critical variable, this issue could not be settled by the classical approach of removing the adrenal glands in the absence of pharmacological blockade, since stress hormones can be released from the contralateral gland while one adrenal is being removed. This limitation of the classical approach, however, could be overcome by a combination of pharmacological inhibition (metyrapone) and surgical removal of the adrenals, for mice submitted to this sequential treatment no longer respond to surgical stress, even when all the effects of metyrapone have disappeared.
Our observations support the concept that stress-mediated GC release work in parallel with specific immune mechanisms to induce the sustained eosinophilia observed in EWI carriers.
One important issue is whether eosinophilia induced by surgery is restricted to our murine model, or whether similar phenomena have been described in other species, including humans. Several early studies in surgically operated patients have reported that profound eosinopenia is found immediately after surgery, but that after day 3 blood eosinophil counts rise above levels noted preoperatively (Gabrilove, 1950; Roche et al., 1950). It is important to realize that these studies described eosinophilia in peripheral blood, not bone-marrow (which can only be studied through invasive procedures, with their associated practical and ethical limitations). However, eosinophils in the blood come from the bone-marrow, and bone-marrow eosinophilia is required for blood eosinophilia to develop. These human studies interpreted their findings in terms of glucocorticoids being primarily able to induce eosinopenia. According to this paradigm, eosinophilia, which exceeds normal (i.e., preoperative) eosinophil counts, is due to glucocorticoid levels returning to normal sometime after surgery. In our study, however, we have directly examined bone-marrow, and have shown glucocorticoids to be necessary for eosinophilia in the bone-marrow. In our study, sham-implanted mice also presented the profound peripheral blood eosinopenia immediately after surgery, which was originally reported in surgically operated humans (unpublished observations). This indicates that profound blood eosinopenia can coexist with bone-marrow eosinophilia. This apparent paradox may in part reflect the ability of glucocorticoids to suppress exit of eosinophils from bone-marrow to peripheral blood (Das et al., 1997).
Previous studies from our group demonstrated the enhancement of ex vivo eosinopoiesis by exogenously added GCs, as well as in vivo priming for subsequent increased responses to IL-5 and GM-CSF (Gaspar Elsas et al., 2000). However, in those studies, administration of exogenous GCs did not induce bone-marrow eosinophilia in vivo, in contrast with our observations in sham-implanted mice. This suggests that, in this model, even though GCs are essential, additional mediator(s) contribute to the observed effects. The nature of such additional mediator(s) is at present unclear. Catecholamines are also released in response to stress and were demonstrated by others to have a modulatory activity on bone-marrow haematopoiesis. For example, norepinephrine was able to rescue bone-marrow haematopoiesis from lethal doses of carboplatin, and this effect was counteracted by the α1-adrenergic antagonist, prazosin (Maestroni, 2000). In our model, the administration of adrenergic antagonists was unable to interfere with the effects of surgery on bone-marrow eosinopoiesis, thus ruling out the involvement of catecholamines. Together, these observations suggest that GCs may act in concert with a yet unknown mediator, which is unlikely to be catecholamines.
These considerations – namely the site in which eosinophilia is observed, as well as the participation of hitherto unidentified additional mediators – explain why our results apparently differ from those of numerous other studies (reviewed in Sehmi et al., 2003; Xavier Elsas et al., 2003), which reported that glucocorticoids, including synthetic compounds such as dexamethasone, reduce eosinophilia in blood and in tissues other than bone-marrow. It is indeed true that glucocorticoids can reduce circulating eosinophils. However, this does not mean that they will disappear from bone-marrow after dexamethasone treatment, as clearly shown by Das et al. (1997). Our interpretation is that bone-marrow is protected from the eosinopenic actions of glucocorticoids (Xavier Elsas et al., 2003), which will therefore critically depend on the site one examines. Furthermore, all enhancing effects of exogenous dexamethasone (Gaspar Elsas et al., 2000) or of stress-induced glucocorticoids (this study) required additional factors: IL-5 or GM-CSF in vitro, or unknown mediators released by surgical trauma in vivo. Our interpretation is that glucocorticoid interactions with these factors, especially in the privileged environment of bone-marrow, leads to effects distinct from those observed with glucocorticoids in the absence of these factors, and outside the bone-marrow.
Studies from other groups demonstrate that surgical procedures can activate the HPA axis and lead to increased GC production (DeKeyser et al., 2000; Ogawa et al., 2000). However, the mechanisms underlying HPA axis activation by surgery have not been thoroughly explored. A recent study demonstrates the involvement of centrally released norepinephrine and IL-1 in stimulating the release of corticotrophin-releasing hormone (CRH) by median eminence neurons (DeKeyser et al., 2000). While this describes a central pathway of HPA axis activation by surgical stress, it remains to be established how surgical trauma leads to increased central norepinephrine and IL-1. One possibility is the direct signalling to the central nervous system by skin nociceptive neurons, which may be activated by surgical trauma (Ohtori et al., 2000). On the other hand, the central nervous system may be activated indirectly, by mast cell-derived pro-inflammatory cytokines, such as tumour necrosis factor (TNF)-α and IL-1β, released in response to skin trauma (Edston & van Hage-Hamsten, 2003). In any case, further experimentation is required to dissect this process.
The biological consequences of bone-marrow eosinophilia induced by surgery are at present unclear. However, one setting in which this might play a role is wound healing, which is expected to follow surgery, since a number of studies have pointed to eosinophils as an important component of healing wounds (Wong et al., 1993; Yang et al., 1997). Eosinophils were originally thought to contribute to wound healing through their ability to secrete transforming growth factor-α (TGF-α) and transforming growth factor-β1 (TGF-β1), which act in different phases of the healing process (reviewed in Werner & Grose, 2003). A recent study, however, showed that eosinophil depletion following in vivo treatment of hamsters with neutralizing monoclonal antibody to IL-5 was associated with accelerated wound healing, thereby suggesting that eosinophils contribute to retard, rather than accelerate, wound healing (Yang et al., 1997), even though the mechanism of this retarding effect was not established. On the other hand, glucocorticoids have important effects on collagen deposition and wound healing, and some of their effects may involve complex changes in TGF-β production and signalling (Frank et al., 1996). As eosinophils are an important source of TGF-β in healing wounds even though the secreted protein is subsequently found throughout the tissue (Wong et al., 1993), eosinophils may mediate some of the glucocorticoid effects on wound healing, especially those associated with changes in TGF-β secretion. While it is potentially of great interest to evaluate whether bone-marrow eosinophils eventually contribute to modulate wound healing in sham-implanted mice, and whether this can be affected by topically or systemically applied glucocorticoids, this should be left for future studies, given the need to quantitate eosinophils in blood and wound tissue at different times after surgery, and to distinguish between the effects of glucocorticoids on bone-marrow and those on circulating and tissue eosinophils.
Acknowledgments
We acknowledge the equal contributions of P.X. Elsas and H.A. Paula Neto to the completion of this work. This work was supported by grants from PAPES-FIOCRUZ, FINEP, CNPq-RHAE, INSERM-FIOCRUZ, CAPES-COFECUB, Institut Pasteur-INSERM and fellowships from CAPES and CNPq.
Abbreviations
- ADX
adrenalectomized
- CRH
corticotrophin-releasing hormone
- EPO
eosinophil peroxidase
- EWI
egg white implant
- FCS
foetal calf serum
- GC
glucocorticoid
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- HPA
hypothalamic–pituitary–adrenal
- IL
interleukin
- TGF
transforming growth factor
References
- ADAMKO D., ODEMUYIWA S.O., MOQBEL R. The eosinophil as a therapeutic target in asthma: beginning of the end or end of the beginning. Curr. Opin. Pharmacol. 2003;3:227–232. doi: 10.1016/s1471-4892(03)00040-7. [DOI] [PubMed] [Google Scholar]
- ADCOCK I.M., CARAMORI G. Cross-talk between pro-inflammatory transcription factors and glucocorticoids. Immunol. Cell Biol. 2001;79:376–384. doi: 10.1046/j.1440-1711.2001.01025.x. [DOI] [PubMed] [Google Scholar]
- BAGBY C.G.Haemopoiesis Molecular Basis of Blood Diseases 1994Philadelphia: W.B. Saunders; 71–106.ed. Nienhuis, G.A.W., Majerus, P.W. & Varmus, H. pp [Google Scholar]
- CARMO H., REMIÃO F., CARVALHO F., FERNANDES E., DE BOER D., DOS REYS L.A., BASTOS M.L. 4-Methylthioamphetamine-induced hyperthermia in mice: influence of serotonergic and catecholaminergic pathways. Toxicol. Appl. Pharmacol. 2003;190:262–271. doi: 10.1016/s0041-008x(03)00190-x. [DOI] [PubMed] [Google Scholar]
- CHOY S.S., HANA K.-J., LEEA J.-K., LEEA H.-K., HANA E.-J., KIMB D.H., SUHA H.-W. Antinociceptive mechanisms of orally administered decursinol in the mouse. Life Sci. 2003;73:471–485. doi: 10.1016/s0024-3205(03)00311-4. [DOI] [PubMed] [Google Scholar]
- DAS A.M., FLOWER R.J., HELLEWELL P.G., TEIXEIRA M.M., PERRETTI M. A novel murine model of allergic inflammation to study the effect of dexamethasone on eosinophil recruitment. Br. J. Pharmacol. 1997;121:97–104. doi: 10.1038/sj.bjp.0701122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEKEYSER F.G., LEKER R.R., WEIDENFELD J. Activation of the adrenocortical axis by surgical stress: involvement of central norepinephrine and interleukin-1. Neuroimmunomodulation. 2000;7:182–188. doi: 10.1159/000026437. [DOI] [PubMed] [Google Scholar]
- EDSTON E., VAN HAGE-HAMSTEN M. Mast cell tryptase and hemolysis after trauma. Forensic Sci. Int. 2003;131:8–13. doi: 10.1016/s0379-0738(02)00383-3. [DOI] [PubMed] [Google Scholar]
- FRANK S., MADLENER M., WERNER S. Transforming growth factors β1, β2 and β3 and their receptors are differentially regulated during normal and impaired wound healing. J. Biol. Chem. 1996;271:10188–10193. doi: 10.1074/jbc.271.17.10188. [DOI] [PubMed] [Google Scholar]
- GABRILOVE J.L. The level of the circulating eosinophils following trauma. J. Clin. Endocrinol. 1950;10:637–640. doi: 10.1210/jcem-10-6-637. [DOI] [PubMed] [Google Scholar]
- GASPAR ELSAS M.I., JOSEPH D., ELSAS P.X., VARGAFTIG B.B. Rapid increase in bone marrow eosinophil production and responses to eosinopoietic interleukins triggered by intranasal allergen challenge. Am. J. Respir. Cell Mol. Biol. 1997;17:404–413. doi: 10.1165/ajrcmb.17.4.2691. [DOI] [PubMed] [Google Scholar]
- GASPAR ELSAS M.I., MAXIMIANO E.S., JOSEPH D., ALVES L., TOPILKO A., VARGAFTIG B.B., ELSAS P.X. Upregulation by glucocorticoids of responses to eosinopoietic cytokines in bone-marrow from normal and allergic mice. Br. J. Pharmacol. 2000;129:1543–1552. doi: 10.1038/sj.bjp.0703145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORTON M.A., LARSON K.A., LEE J.J., LEE N.A. Cloning of the murine eosinophil peroxidase gene (mEPO): characterization of a conserved subgroup of mammalian hematopoietic peroxidases. J. Leukoc. Biol. 1996;60:285–294. doi: 10.1002/jlb.60.2.285. [DOI] [PubMed] [Google Scholar]
- KARALIS K., KONTOPOULOS E., MUGLIA L.J., MAJZOUB J.A. Corticotropin-releasing hormone deficiency unmasks the proinflammatory effect of epinephrine. Proc. Natl. Acad. Sci. U.S.A. 1999;96:7093–7097. doi: 10.1073/pnas.96.12.7093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURLAND J.I., HADDEN J.W., MOORE M.A.S. Role of cyclic nucleotides in the proliferation of committed granulocyte-macrophage progenitor cells. Cancer Res. 1977;37:4534–4538. [PubMed] [Google Scholar]
- LEMANSKE R.F., JR, BUSSE W.W. Asthma. J. Allergy Clin. Immunol. 2003;111:S502–519. doi: 10.1067/mai.2003.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEUNG D.Y.M., BLOOM J.W. Update on glucocorticoid action and resistance. J. Allergy Clin. Immunol. 2003;111:3–22. doi: 10.1067/mai.2003.97. [DOI] [PubMed] [Google Scholar]
- MAESTRONI G.J.M. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann. N. Y. Acad. Sci. 2000;917:29–37. doi: 10.1111/j.1749-6632.2000.tb05370.x. [DOI] [PubMed] [Google Scholar]
- OGAWA K., HIRAI M., KATSUBE T., MURAYAMA M., HAMAGUCHI K., SHIMAKAWA T., NARITAKE Y., HOSOKAWA T., KAJIWARA T. Suppression of cellular immunity by surgical stress. Surgery. 2000;127:329–336. doi: 10.1067/msy.2000.103498. [DOI] [PubMed] [Google Scholar]
- OHTORI S., TAKAHASHI K., CHIBA T., TAKAHASHI Y., YAMAGATA M., SAMEDA H., MORIYA H. Fos expression in the rat brain and spinal cord evoked by noxious stimulation to low back muscle and skin. Spine. 2000;25:2425–2430. doi: 10.1097/00007632-200010010-00002. [DOI] [PubMed] [Google Scholar]
- PADGETT D.A., GLASSER R. How stress influences the immune response. Trends Immunol. 2003;24:444–448. doi: 10.1016/s1471-4906(03)00173-x. [DOI] [PubMed] [Google Scholar]
- PINARDI G., SIERRALTA F., MIRANDA H.F. Adrenergic mechanisms in antinociceptive effects of non steroidal anti-inflammatory drugs in acute thermal nociception in mice. Inflamm. Res. 2002;51:219–222. doi: 10.1007/pl00000296. [DOI] [PubMed] [Google Scholar]
- ROCHE M., THORN G.W., HILLS A.G. The levels of circulating eosinophils and their response to ACTH in surgery. Their use as an index of adrenocortical function. New Engl. J. Med. 1950;242:307–314. doi: 10.1056/NEJM195003022420901. [DOI] [PubMed] [Google Scholar]
- SEHMI R., BAATJES A.J., DENBURG J.A. Hemopoietic progenitor cells and hemopoietic factors: potential targets for treatment of allergic inflammatory diseases. Curr. Drug Targets Inflamm. Allergy. 2003;2:271–278. doi: 10.2174/1568010033484007. [DOI] [PubMed] [Google Scholar]
- SIQUEIRA A.L.P., RUSSO M., STEIL A.A., FACINCONE S., MARIANO M., JANCAR S. A new murine model of pulmonary eosinophilic hyper-sensitivity: contribution to experimental asthma. J. Allergy Clin. Immunol. 1997;100:383–388. doi: 10.1016/s0091-6749(97)70253-7. [DOI] [PubMed] [Google Scholar]
- WERNER S., GROSE R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003;83:835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- WONG D.T.W., DONOFF R.B., YANG J., SONG B.-Z., MATOSSIAN K., NAGURA N., ELOVIC A., McBRIDE J., GALLAGHER G., TODD R., CHIANG T., CHOU L.S.S., YUNG C.M., GALLI S.J., WELLER P.F. Sequential expression of transforming growth factors α and β1 by eosinophils during cutaneous wound healing in the hamster. Am. J. Pathol. 1993;143:130–142. [PMC free article] [PubMed] [Google Scholar]
- XAVIER ELSAS P., MAXIMIANO E.S., VARGAFTIG B.B., GASPAR ELSAS M.I. The effects of allergen and anti-allergic drugs on murine hemopoietic cells: moving targets, unusual mechanisms, and changing paradigms. Curr. Drug Targets Inflamm. Allergy. 2003;2:329–337. doi: 10.2174/1568010033484089. [DOI] [PubMed] [Google Scholar]
- YANG J., TORIO A., DONOFF R.B., GALLAGHER G.T., EGAN R., WELLER P.F., WONG D.T.W. Depletion of eosinophil infiltration by anti-IL-5 monoclonal antibody (TRFK-5) accelerates open skin wound epithelial closure. Am. J. Pathol. 1997;151:813–819. [PMC free article] [PubMed] [Google Scholar]
- ZARRINDAST M.-R., SADEGHI S., SAHEBGHARANI M. Influence of α-adrenoceptor agonists and antagonists on imipramine-induced hypothermia in mice. Pharmacol. Toxicol. 2003;93:48–53. doi: 10.1034/j.1600-0773.2003.930107.x. [DOI] [PubMed] [Google Scholar]