Abstract
The paraventricular nucleus (PVN) of the hypothalamus plays a key role in the control of appetite and energy balance. Both ghrelin and cannabinoid receptor agonists increase food intake when administered into this nucleus: this study investigated possible interactions between the two systems in relation to eating. The orexigenic effect of ghrelin (100 pmol) when infused in to the PVN was reversed by a small, systemic dose of the CB1 cannabinoid receptor antagonist SR141716 (1 mg kg−1). This is the first demonstration of a functional relationship between brain ghrelin and endocannabinoid systems, and, although it needs to be further investigated, the effect of ghrelin on food intake when injected into the PVN seems to be mediated by stimulation of cannabinoid release.
Keywords: Ghrelin, endocannabinoid, PVN, appetite, SR141716
Introduction
Ghrelin, a 28-amino-acid peptide, is synthesised in the periphery (mainly in the stomach) and in the brain (Kojima et al., 1999). It acts as an endogenous ligand of the growth hormone secretagogue receptor (Howard et al., 1996). Additionally, recent studies have shown that ghrelin will reliably stimulate food intake in animal models. Ghrelin seems to exert this hyperphagic effect through activation of specific hypothalamic areas linked to the regulation of feeding and metabolism (Nakazato et al., 2001; Wren et al., 2001; Korbonits et al., 2004). Among these areas is the paraventricular nucleus (PVN), which exerts a pivotal role in the putative network of brain nuclei involved in the control of eating and energy balance (Williams et al., 2001). Direct injection of ghrelin into this site generates a robust feeding response (Wren et al., 2001). Ghrelin-induced eating has been linked to activity in hypothalamic pathways utilising the orexigens neuropeptide Y (NPY), orexin and Agouti-related peptide (AgRP) pathways (Kamegai et al., 2000; Nakazato et al., 2001). Additionally, there is evidence that ghrelin indirectly inhibits the anorectic influence of neurotransmitters such as cocaine and amphetamine-regulated transcript (CART) and peptides derived from proopiomelanocortin (Cowley et al., 2003). It may be reasonable, therefore, to anticipate functional interactions between ghrelin and other appetite- or energy-regulating systems.
It is now well established that appetite is modulated by central endogenous cannabinoid systems (for reviews, see Kirkham & Williams, 2001; Cota et al., 2003). The administration of cannabis extracts such as Δ9-tetrahydrocannabinol (THC) stimulates appetite (Williams et al., 1998), while the CB1 cannabinoid receptor antagonist SR141716 (rimonabant) reduces food intake after systemic administration (Arnone et al., 1997). Furthermore, more recent studies have shown that appetite is increased by the endogenous cannabinoid ligands 2-arachidonoylglycerol and anandamide (Williams & Kirkham, 1999; Kirkham et al., 2002). As in the case of ghrelin, the PVN is also a sensitive site of action for the orexigenic actions of exogenous and endogenous cannabinoids (unpublished observation), and systemic cannabinoid receptor agonist and antagonist treatments will induce Fos expression in the PVN (Wenger et al., 2003).
In order to investigate the possible interactions between ghrelin and endocannabinoid systems in relation to appetite, we examined the ability of the CB1 antagonist SR141716 to reverse the orexigenic effect of intra-PVN ghrelin. Since SR141716 can suppress food intake in its own right, a sub-anorectic dose of the drug (Arnone et al., 1997) was paired with a dose of ghrelin previously reported to be hyperphagic when administered into the PVN (Wren et al., 2001; Melis et al., 2002). Our findings provide the first evidence for a functional relationship between hypothalamic ghrelin and the endocannabinoids.
Methods
Animals
Male Lister hooded rats (Harlan, U.K.) weighing between 220 and 250 g were group housed before, and singly housed after, surgery. Standard laboratory food pellets (PCD Mod. C; Special Diet Services, Witham, U.K.) and tap water were freely available, and the room in which they were housed was maintained at 21±1°C. Lights were on from 07 : 00 to 19 : 00. The experimental procedures carried out in this study were in compliance with the U.K. Animals (Scientific Procedures) Act 1986.
Drugs
N-piperidino-5(4 cholorophenyl)-1-(2,4-dicholophenyl)-4-methylpyrazolel carboxamide (SR141716; Vernalis Ltd, Wokingham, U.K.) was suspended in 10% dimethyl sulphoxide (DMSO; Sigma, Poole, U.K.) solution. Rat ghrelin (Tocris Cookson Ltd, Avonmouth, U.K.) was dissolved in isotonic saline.
Surgery
After induction of buprenorphine analgesia (0.3 mg kg−1, s.c), rats were anaesthetised using ketamine (100 mg kg−1, s.c.) and chlorpromazine (3 mg kg−1, i.m.) and positioned in the stereotaxic frame (Kopf Instruments, Tujunga, California, U.S.A.) and the flat-skull stereotaxic technique was used. Two indentations were made in the skull to accommodate screws, which together with the application of dental cement held the cannula in place. The cannula (26-gauge, Plastics One, Roanoke, VA, U.S.A.) was positioned 1.8 mm lateral to the midline, 1.8 mm caudal to bregma, and 7.4 mm below the surface of the skull, according to the atlas of Paxinos & Watson (1986). The insertion angle was offset 10° from the vertical to avoid penetration of the sagittal sinus. Cannulae were kept patent using 7.4 mm long obturators (30-gauge, Plastics One, Roanoke, VA, U.S.A.). On test days, rats were injected using 33-gauge injectors (Plastics One, Roanoke, VA, U.S.A.) that extended 1 mm below the tip of the indwelling cannulae. In order to accustom the animals to handling and to keep the indwelling cannulae patent, each day following surgery obturators were replaced. After surgery rats were allowed to recover for 7 days prior to the start of the experiments.
Experimental procedure
Animals were randomly allocated to the following four groups: vehicle–vehicle (n=6), vehicle–ghrelin (n=6) SR141716–ghrelin (n=6) and SR141716–vehicle (n=6). Rats were habituated to handling and all aspects of test procedures prior to drug testing. On the test day, each animal received an injection of SR141716 (1 mg kg−1) or vehicle, administered subcutaneously in a volume of 1 ml kg−1. After 30 min, rats received a central injection of ghrelin (100 pmol) or saline, delivered into the brain in a volume of 1 μl over 2 min using an automated microsyringe pump. Injectors were left in position for a further 30 s to allow for drug diffusion. Control animals received 1 μl infusions of vehicle at the same rate. Food was withheld during the period of drug administration. Immediately after the infusion, rats were placed in the test chambers with a pre-weighed quantity of food (chow), and food intake, corrected for spillage, was measured over 1 h. All injections were performed between 9 : 00 and 10 : 00.
Histology
After the completion of testing, rats were killed with CO2, India ink (2% in saline) was microinjected (approximately 100 nl) into the cannulae. Brains were removed, frozen and coronal sections obtained using a freezing microtome. Cannulae placement was deemed incorrect when the injection occurred outside a 0.3-mm radius from the targeted site.
Statistics
Data were analysed by two-way analyses of variance (ANOVA), with agonist treatment (ghrelin or vehicle) as one factor and antagonist treatment (SR141716 or vehicle) as the second factor. A significant ghrelin × SR141716 interaction would show the antagonism of ghrelin's effects. Comparisons between individual groups were then made with Fisher's least-square difference (LSD) test. A P-value <0.05 was considered significant.
Results
Histology
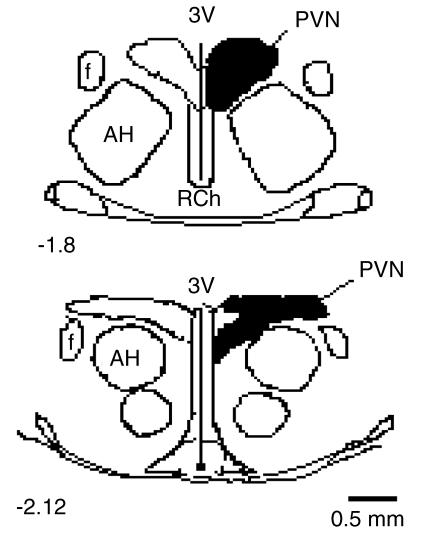
As can be seen in Figure 1, all placements were found to be within the anterior planes of 1.8–2.12 mm posterior to bregma. Cannulae placements were located in the PVN (shaded areas), with the exception of two animals which were excluded from statistical analysis (one each from the vehicle–ghrelin and SR141716–ghrelin groups).
Figure 1.
Schematic drawings of rat hypothalamus in coronal section (1.8–2.12 mm posterior to bregma), showing the area of placements accepted as falling within the PVN (shaded area). AH indicates anterior hypothalamus; f, fornix; 3V, third cerebral ventricle; RCh, retrochiasmatic area.
Reversal of ghrelin-induced feeding by SR141716
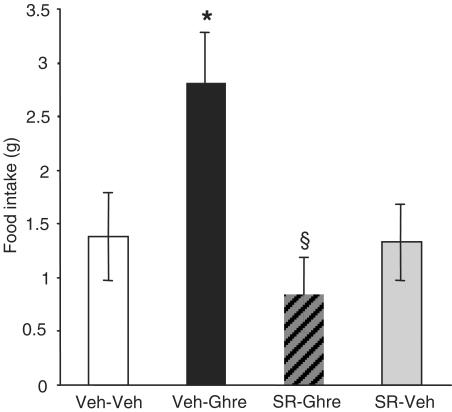
As can be seen in Figure 2, under these nondeprivation, light-phase conditions, control intakes were relatively low. Confirming previous reports, an intra-PVN injection of 100 pmol ghrelin reliably stimulated eating (F1,18=6.0; P<0.05), producing an approximate doubling of intake over control levels in the 1 h test. Importantly, the dose of SR141716, which had no effect on food intake when administered alone, abolished the hyperphagic effect of ghrelin (ghrelin × SR141716 interaction; F1,18=5.4; P<0.05).
Figure 2.
Reversal of ghrelin-induced hyperphagia in nondeprived rats by the selective CB1 antagonist SR141716. All values represent the mean (±s.e.m.) food intake (g) of rats after 1 h of free access to chow. *Significant increase in intake compared to the vehicle–vehicle group (P<0.05). §Significant reversal of ghrelin-induced feeding by SR141716 (P<0.05).
Discussion
The present findings confirm previous reports of the sensitivity of the PVN to the orexigenic actions of the peptide ghrelin (Wren et al., 2001), and support its putative role in the control of food intake and energy homeostasis. Importantly, this study also provides the first evidence for endocannabinoid involvement in ghrelin's actions on appetite. Specifically, the orexigenic effect of intra-PVN ghrelin was prevented by pretreatment with the CB1 cannabinoid receptor antagonist SR141716, at a subthreshold dose which under our experimental conditions had no discernible effect on feeding when administered alone.
These results strongly suggest that activity within endocannabinoid systems is required for the expression of ghrelin hyperphagia, and add to the list of orexigenic signals with which ghrelin is believed to interact. For example, it has been reported that the primary hypothalamic targets of ghrelin are neurons projecting to the PVN that release the potent orexigens NPY and AgRP (Kamegai et al., 2000). Acting via receptors on these neurons, ghrelin increases NPY and AgRP release (Cowley et al., 2003). The results obtained in this study indicate that the endogenous cannabinoids are another target for direct or indirect (perhaps through the release of NPY or AgRP) ghrelin actions within the PVN. The present data also extend the number of feeding-related, hypothalamic factors known to interact with the endogenous cannabinoids. For example, SR141716 has been reported to prevent the eating stimulated by PVN injections of opioids (Verty et al., 2003). In addition, the intake suppressant action of the anorexigenic neuropeptide α-melanocyte-stimulating hormone is facilitated by CB1 receptor blockade (Verty et al., 2004). There is also evidence that the orexigenic actions of glucocorticoids in the PVN may be mediated by endocannabinoids, possibly through inhibition of anorexigenic peptides such as the corticotropin-releasing hormone (CRH) (Di et al., 2003). Additionally, CB1 receptors are co-expressed with CRH, CART, prepro-orexin and melanin-concentrating hormone in mouse hypothalamic neurons (Cota et al., 2003).
In conclusion, the results obtained in this study provide the first evidence for a functional relationship between ghrelin and cannabinoid systems in the control of food intake. Further investigation is clearly needed to determine the exact nature of this interaction, but our findings consolidate existing evidence for the importance of these systems in the regulation of appetite.
Acknowledgments
This study was supported by a project grant from the U.K. Biotechnology and Biological Sciences Research Council. MK is supported by the U.K. Medical Research Council.
Abbreviations
- AgRP
agouti-related protein
- AH
anterior hypothalamus
- CART
cocaine and amphetamine-regulated transcript
- CRH
corticotropin-releasing hormone
- DMSO
dimethyl sulphoxide
- NPY
neuropeptide Y
- PVN
paraventricular nucleus of the hypothalamus
- RCh
retrochiasmatic area
- THC
Δ9-tetrahydrocannabinol
- 3V
third ventricle
References
- ARNONE M., MARUANI J., CHAPERON F., THIEBOT M., PONCELET M., SOUBRIE P., LE FUR G. Selective inhibition of sucrose and ethanol intake by SR 141716, an antagonist of central cannabinoid (CB1) receptors. Psychopharmacology. 1997;132:104–106. doi: 10.1007/s002130050326. [DOI] [PubMed] [Google Scholar]
- COTA D., MARSICANO G., LUTZ B., VICENNATI V., STALLA G., PASQUALI R., PAGOTTO U. Endogenous cannabinoid system as a modulator of food intake. Int. J. Obes. Relat. Metab. Disord. 2003;27:289–301. doi: 10.1038/sj.ijo.0802250. [DOI] [PubMed] [Google Scholar]
- COWLEY M., SMITH R., DIANO S., TSCHOP M., PRONCHUK N., GROVE K., STRASBURGER C., BIDLINGMAIER M., ESTERMAN M., HEIMAN M., GARCIA-SEGURA L., NILLNI E., MENDEZ P., LOW M., SOTONYI P., FRIEDMAN J., LIU H PINTO S., COLMERS W., CONE R., HORVATH T. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- DI S., MALCHER-LOPES R., HALMOS K., TASKER J. Nongenomic glucocorticoid inhibition via endocannabinoid release in the hypothalamus: a fast feedback mechanism. J. Neurosci. 2003;23:4850–4857. doi: 10.1523/JNEUROSCI.23-12-04850.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOWARD A., FEIGHNER S., CULLY D., ARENA J., LIBERATOR P., ROSENBLUM C., HAMELIN M., HRENIUK D., PALYHA O., ANDERSON J., PARESS P., DIAZ C., CHOU M., LIU K., MCKEE K., PONG S., CHAUNG L., ELBRECHT A., DASHKEVICZ M., HEAVENS R., RIGBY M., SIRINATHSINGHJI D., DEAN D., MELILLO D., PATCHETT A., NARGUND R., GRIFFIN P., DEMARTINO J., GUPTA S., SCHAEFFER J, SMITH R, VAN DER PLOEG L. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science. 1996;273:974–977. doi: 10.1126/science.273.5277.974. [DOI] [PubMed] [Google Scholar]
- KAMEGAI J., TAMURA H., SHIMIZU T., ISHII S., SUGIHARA H., WAKABAYASHI I. Central effect of ghrelin, an endogenous growth hormone secretagogue, on hypothalamic peptide gene expression. Endocrinology. 2000;141:4797–4800. doi: 10.1210/endo.141.12.7920. [DOI] [PubMed] [Google Scholar]
- KIRKHAM T., WILLIAMS C. Endogenous cannabinoids and appetite. Nutr. Res. Rev. 2001;14:65–86. doi: 10.1079/NRR200118. [DOI] [PubMed] [Google Scholar]
- KIRKHAM T., WILLIAMS C., FEZZA F., DI MARZO V. Endocannabinoid levels in rat limbic forebrain and hypothalamus in relation to fasting, feeding and satiation: stimulation of eating by 2-arachidonoyl glycerol. Br. J. Pharmacol. 2002;136:550–557. doi: 10.1038/sj.bjp.0704767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOJIMA M., HOSODA H., DATE Y., NAKAZATO M., MATSUO H., KANGAWA K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- KORBONITS M., GOLDSTONE A., GUEORGUIEV M., GROSSMAN A. Ghrelin – a hormone with multiple functions. Front. Neuroendocrinol. 2004;25:27–68. doi: 10.1016/j.yfrne.2004.03.002. [DOI] [PubMed] [Google Scholar]
- MELIS M., MASCIA M., SUCCU S., TORSELLO A., MULLER E., DEGHENGHI R., ARGIOLAS A. Ghrelin injected into the paraventricular nucleus of the hypothalamus of male rats induces feeding but not penile erection. Neurosci. Lett. 2002;329:339–343. doi: 10.1016/s0304-3940(02)00673-0. [DOI] [PubMed] [Google Scholar]
- NAKAZATO M., MURAKAMI M., DATE Y., KOJIMA M., MATSUO H., KANGAWA K., MATSUKURA S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press Inc; 1986. [Google Scholar]
- VERTY A., MCFARLANE J., MCGREGOR I., MALLET P.Evidence for an interaction between CB1 cannabinoid and melanocortin MCR-4 receptors in regulating food intake Endocrinology 2004. epub [DOI] [PubMed]
- VERTY A., SINGH M., MCGREGOR I., MALLET P. The cannabinoid receptor antagonist SR 141716 attenuates overfeeding induced by systemic or intracranial morphine. Psychopharmacology. 2003;168:314–323. doi: 10.1007/s00213-003-1451-9. [DOI] [PubMed] [Google Scholar]
- WENGER T., LEDENT C., TRAMU G. The endogenous cannabinoid, anandamide, activates the hypothalamo–pituitary–adrenal axis in CB1 cannabinoid receptor knockout mice. Neuroendocrinology. 2003;78:294–300. doi: 10.1159/000074882. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C., ROGERS P., KIRKHAM T. Hyperphagia in pre-fed rats following oral delta9-THC. Physiol. Behav. 1998;65:343–346. doi: 10.1016/s0031-9384(98)00170-x. [DOI] [PubMed] [Google Scholar]
- WILLIAMS C.M., KIRKHAM T.C. Anandamide induces overeating: mediation by central cannabinoid (CB1) receptors. Psychopharmacology. 1999;143:315–317. doi: 10.1007/s002130050953. [DOI] [PubMed] [Google Scholar]
- WILLIAMS G., BING C., CAI X., HARROLD J., KING P., LIU X. The hypothalamus and the control of energy homeostasis. Different circuits, different purposes. Physiol. Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- WREN A., SMALL C., ABBOTT C., DHILLO W., SEAL L., COHEN M., BATTERHAM R., TAHERI S., STANLEY SA., GHATEI M., BLOOM S. Ghrelin causes hyperphagia and obesity in rats. Diabetes. 2001;50:2540–2547. doi: 10.2337/diabetes.50.11.2540. [DOI] [PubMed] [Google Scholar]