Abstract
Carcinine (β-alanyl histamine) is an imidazole dipeptide. The present study was designed to characterize the pharmacological effects of carcinine on histaminergic activity in the brain and on certain neurobehavior.
Carcinine was highly selective for the histamine H3 receptor over H1 or H2 receptor (Ki (μM)=0.2939±0.2188 vs 3621.2±583.9 or 365.3±232.8 μM, respectively).
Carcinine at a dose of 20 mg kg−1 slightly increased histidine decarboxylase (HDC) activity in the cortex (from 0.186±0.069 to 0.227±0.009 pmol mg protein−1 min−1). In addition, carcinine (10, 20, and 50 mg kg−1) significantly decreased histamine levels in mice brain.
Like thioperamide, a histamine H3 receptor antagonist, carcinine (20, 50 μM) significantly increased 5-HT release from mice cortex slices, but had no apparent effect on dopamine release.
Carcinine (20 mg kg−1) significantly inhibited pentylenetetrazole-induced kindling. This inhibition was completedly reversed by (R)-α-methylhistamine, a representative H3 receptor agonist, and α-fluromethylhistidine, a selective HDC inhibitor.
Carcinine (20 mg kg−1) ameliorated the learning deficit induced by scopolamine. This amelioration was reversed by (R)-α-methylhistamine as evaluated by the passive avoidance test in mice.
Like thioperamide, carcinine dose-dependently increased mice locomotor activity in the open-field test.
The results of this study provide first and direct evidence that carcinine, as a novel histamine H3 receptor antagonist, plays an important role in histaminergic neurons activation and might be useful in the treatment of certain diseases, such as epilepsy, and locomotor or cognitive deficit.
Keywords: Histamine, carcinine, histamine H3 receptor, locomotor activity, seizure, learning
Introduction
It is well known that histamine acts as a neurotransmitter and neuromodulator in the peripheral and central nervous systems (Panula et al., 1984; Watanabe et al., 1984; Schwartz et al., 1991). In the peripheral system, histamine plays an important role in various allergies, such as asthma, rhinitis, and atopic dermatitis (Hill, 1990). On the other hand, the histaminergic neuron system seems to be involved in many physiological and behavioral functions through H1, H2, and H3 receptors including the sleep–wake cycle, emotion, appetite control, locomotor activity, stress-related behavior, neuroendocrine interactions, learning, and memory (Schwartz et al., 1991; Leurs et al., 1998; Brown et al., 2001). Histamine H3 receptor antagonists, which are able to penetrate blood–brain barrier, have been reported to be effective in the treatment of neuronal diseases, such as cognitive deficit, attention deficit, hyperactivity disorder, narcolepsy, and epilepsy (Stark et al., 1996; Leurs et al., 1998; Tozer & Kalindjian, 2000).
Carcinine (β-alanyl histamine) is an imidazole dipeptide first discovered in the crustacean Carcinus maenas (Arnould & Frentz, 1975), and has subsequently been found in the hearts of several mammalian species (Brotman et al. 1989; 1990). It has been demonstrated that carcinine is metabolically related to histamine, histidine, and carnosine (β-alanyl histidine), and could be synthesized from histamine and β-alanine (Flancbaum et al., 1990). In addition, previous studies have shown that carcinine contains an imidazole group with flexible ethylene side chain known to be important for histamine H3 receptor–ligand interactions (Ali et al., 1999; Tedford et al., 1999; Yates et al., 1999; Tozer & Kalindjian, 2000). From these findings, it seems that a certain relationship exists between brain histamine and carcinine, and that carcinine might be a new histamine H3 receptor antagonist. However, only few reports have explored this relationship and little is known about the pharmacological and physiological role of carcinine. In one of those reports, carcinine was shown to act as a natural antioxidant (Babizhayev et al., 1994) and to play a role in regulating stress and shock with a 1000-fold less potent hypotensive effect than histamine (Arnould & Frentz, 1977; Brotman et al., 1990), suggesting that carcinine might have therapeutic use.
The objective of this study was to investigate the possible pharmacological effects of carcinine on brain histamine and on certain physiological and behavioral functions, such as locomotor activity, passive avoidance response, and kindling-induced seizure.
Methods
Animals
Animals were housed in individual cages in an air-conditioned room with controlled temperature (23±3°C) and humidity (50±20%) under a 12 h light–dark cycle (lights on from 06:00–18:00). Water and food were available ad libitum. Behavioral experiments were carried out each day between 10:00–18:00. All experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Chemical kindling
ICR mice were intraperitoneally administered with pentylenetetrazole (PTZ, 60 mg kg−1). After PTZ injection, seizure intensity was measured for 30 min using the following scale: Stage 0, no response; Stage 1, myoclonic body jerks; Stage 2, clonic forelimb convulsions; Stage 3, generalized clonic convulsions or generalized clonic–tonic convulsions; and Stage 4, death within 30 min. The onset and duration of each seizure were also recorded (Chen et al., 2002; 2003).
Locomotor activity
Mice locomotor activity in an open field was measured using a photo-beam system (BTA-1®, Muromachi-Kikai, Tokyo, Japan). Values of distance of ambulation were accumulated in 9 min bins and logged onto a personal computer.
Step-through passive avoidance test
Mice learning ability was evaluated using a step-through passive avoidance memory test (Meguro et al., 1995; Molinengo et al., 1999; Chen & Shen, 2002). The training apparatus was a box composed of a small lighted compartment (15 × 10 × 10 cm3) and a large dark compartment (18 × 12 × 10 cm3). The two compartments were separated by a 10 × 10 cm2 guillotine door. The light compartment was made of clear Plexiglas, and was illuminated by a lamp (60 W) from the outside. The dark compartment had a series of stainless-steel rods (3 mm in diameter, 1 cm apart) through which a constant electrical current was delivered. Mice were first habituated to the box on 2 consecutive days. On the first day, the mice were placed in the light compartment and allowed to explore the box. The latency to enter the dark compartment was recorded. As soon as mice entered the dark compartment, the door was closed, and the mice were kept inside for 15 s before being returned to their cages. On the second training day, on entry into the dark compartment, mice were given 0.5 mA current for 5 s. In the test trial, the same procedure was carried out except that the electric shock was not given. The latency for mice to move into the dark compartment was recorded up to a maximum time of 300 s. Scopolamine (1 mg kg−1) was injected 30 min before the acquisition trial, thioperamide or carcinine was given 30 min before scopolamine.
Binding assay for histamine H1, H2, and H3 receptors
Rat forebrain preparations were used for the binding assay of histamine H1 and H3 receptors, and guinea-pig brain preparations were used for binding assay of histamine H2 receptor. Rats and guinea-pigs were killed and their brains were removed and dissected on ice. Rat forebrains were stored at −80°C until use. Guinea-pig brains were homogenized in a Polytron (setting 5, 30 s) with 40 volumes of ice-cold Na+–K+–phosphate buffer (50 mM, pH 7.5) and centrifuged twice at 50,000 × g for 20 min. H1 and H3 receptors binding was measured as previously described (Chang et al., 1979; Yanai et al., 1994) using [3H]pyrilamine (4 nM) and [3H](R)-α-methylhistamine (1.5 nM), respectively. H2 receptor binding was measured as previously described (Foreman et al., 1985) using [methyl-3H]tiotidine (1.65 nM).
Measurements of endogenous 5-HT and dopamine releases from brain slices
Mice forebrain slices (450 μm thickness) including the cerebral cortex were obtained using a McIlwain tissue chopper and incubated for 5 min at 4°C with a physiological saline solution (Yanai et al., 1998; Son et al., 2001). After washing with the same physiological saline solution, tissue samples were transferred to a superfusing chamber (0.5 ml capacity) and superfused with the physiological salt solution (K+=2.2 mM, 37°C) at a rate of 80 ml min−1 (peristaltic pump, Ismatec MV-MS/CA). The physiological salt solution contained (mmol l−1): NaCl 120, KCl 1, MgSO4 1.2, NaHCO3 27.5, D-glucose 10, CaCl2 1.5, KH2PO4 1.2, and was gassed with O2/CO2 (95/5, v v−1). The slices were first perfused with the saline solution (K+=2.2 mM) for 60 min and then stimulated (S1) for 30 min with a solution containing 31.2 mM K+ (composition in mM: NaCl 91, KCl 30, MgSO4 1.2, NaHCO3 27.5, D-glucose 10, CaCl2 1.5, KH2PO4 1.2). After 45 min reperfusion with the physiological saline solution (K+=2.2 mM), brain slices were again stimulated (S2) for 30 min with the solution containing 31.2 mM K+, and finally superfused with the physiological saline solution for 60 min. Drugs were added 22.5 min before the second stimulation (S2) and were present throughout the experiment.
The perfusion solution was collected with a fraction collector at 5 min intervals, and dopamine and 5-HT in it were separated and detected using an HPLC system with an electrochemical detector (Model ECD-100, Eikom Co., Kyoto, Japan) at 30°C and a reverse-phase analytical column (DS-80TM, 4.6 mm i.d. × 15 cm) (Yanai et al., 1998). The column was eluted with a 0.1 M sodium acetate-citric acid buffer (pH 3.5) containing 15% methanol, 200 mg l−1 sodium l-octanesulfonate, and 5 mg l−1 Na2-EDTA. The first and second K+-evoked releases were denoted as S1 and S2, respectively.
Measurements of brain histamine content and histidine decarboxylase (HDC) activity
Mouse brain was homogenized with 0.2 M perchloric solution containing 100 μM EDTA-2Na and centrifuged at 20,000 × g for 15 min at 0°C. The supernatant was adjusted to pH 3.0 by adding 1 M sodium acetate. Histamine release was determined fluorometrically with o-phthalaldehyde after separation on an HPLC column as described previously (Yamatodani et al., 1985).
HDC activity was measured as describe before (Yamakami et al., 2000; Watanabe & Ohtsu, 2002). In short, mice brains were homogenized in 10-fold volume of ice-cold HDC buffer (0.1 M potassium phosphate buffer, pH=6.8, 0.01 mM pyridoxal-5′-phosphate, 0.2 mM dithiothreitol, 1% polyethyleneglycol with average molecular weight of 300, 100 μg ml−1 phenylmethane sulfonylfluoride) using a Polytron homogenizer (Kinematica, Lucerne, Switzerland). The homogenates were centrifuged at 20,000 × g for 30 min at 4°C, and the supernatant was dialyzed three times against HDC buffer at 4°C overnight. The reaction was started by adding 0.5 mM L-histidine at 37°C and the histamine produced during a 3 h reaction was measured using HPLC.
Drugs
Carcinine, thioperamide, (R)-α-methylhistamine, scopolamine, and PTZ were obtained from Sigma (St Louis, MO, U.S.A.). α-Fluromethylhistidine was supplied by Dr Kollonitsch, Merck Sharp and Dohme Research Laboratories (Rahway, NJ, U.S.A.). All other chemicals were of reagent grade and commercially available.
Statistical analysis
All data are expressed as the mean±s.d. One-way analysis of variance with Dunnett's comparison test was used for calculating statistical significance. Statistical analysis between two groups was carried out using Mann–Whitney's U-test. Statistical significance was evaluated using two-tailed test with P<0.05.
Results
In vitro histamine receptors binding study
Ki values of histamine and carcinine for radioligand binding at H1, H2, and H3 receptors in the membranes of rat forebrain (H1 and H3 receptors) or guinea-pig brain (H2 receptor) are shown in Table 1. As compared with histamine, carcinine showed modest affinity for all three types of histamine receptors. Among the three receptors, carcinine showed the highest affinity for the histamine H3 receptor (Ki=0.2939±0.2188 μM). In contrast, carcinine exhibited minimal affinity for histamine H1 and H2 receptors with Ki values of 3621.2±583.9 and 365.3±232.8 μM, respectively.
Table 1.
Ki values of histamine and carcinine for radioligand binding at H1, H2, and H3 receptors in the membranes of rat forebrain (H1and H3 receptors) or guinea-pig brain (H2 receptor)
| Receptors | Histamine | Carcinine |
|---|---|---|
| H1 | 82.5±8.9 | 3621.2±583.9 |
| H2 | 20.9±1.9 | 365.3±232.8 |
| H3 | 0.0031±0.0001 | 0.2939±0.2188 |
H1, H2, and H3 receptors binding was measured using [3H]pyrilamine, [3H](R)-(α)-methylhistamine and [3H]tiotidine, respectively, as tracers. Each value (μM) is expressed as mean±s.d.
Effects of carcinine on histamine content in the brain
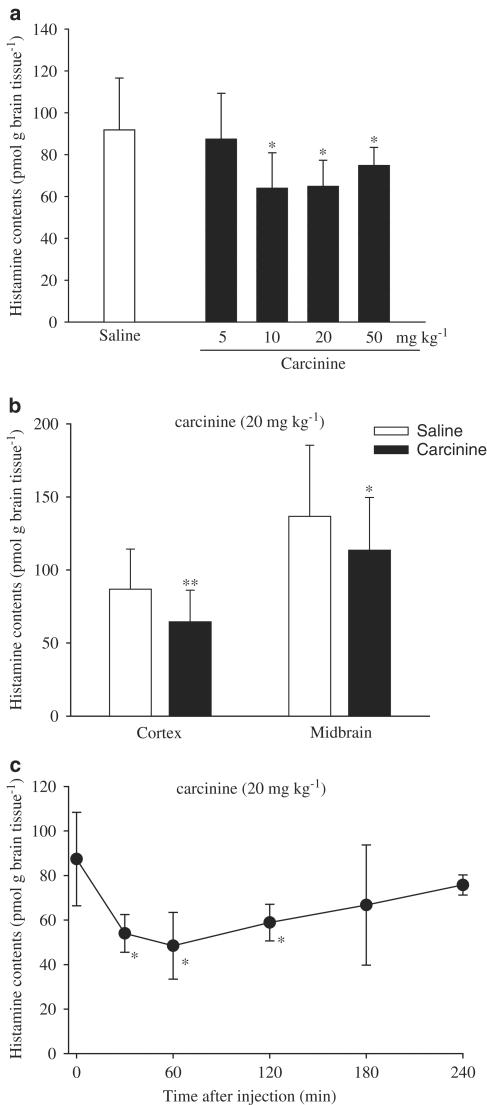
Carcinine decreased histamine levels in the cortex in a dose-dependent manner (Figure 1a). At a dose of 5 mg kg−1, carcinine slightly decrease histamine levels, although no significant effect was observed. However, at doses of 10, 20, and 50 mg kg−1, carcinine significantly decreased histamine content in the cortex (P<0.05). At a dose of 20 mg kg−1, carcinine significantly decreased histamine contents both in the cortex and midbrain (Figure 1b). As shown in Figure 1c, carcinine (20 mg kg−1) significantly decreased histamine levels in the brain 30, 60, and 120 min after injection (P<0.05) with maximum effect 60 min after administration (44.6% (n=10) as compared with control).
Figure 1.
Dose-dependent (a), region-dependent (b) and time-dependent (c) effects of carcinine on histamine levels in mice brain. Histamine contents were measured 60 min after injection of carcinine (a, b) or at different time points after injection of carcinine (c). Each value is expressed as the mean±s.d. of 12 rats. *P<0.05, **P<0.01, statistical significance of difference between the control- and carcinine-treated groups.
Effects of carcinine on histamine HDC activity
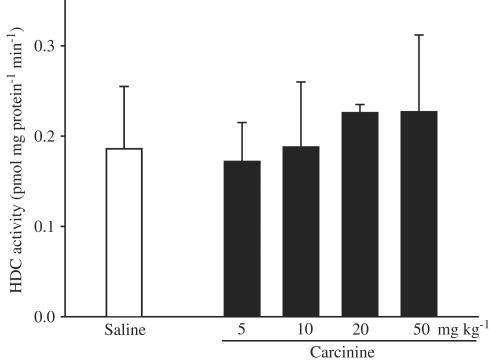
HDC activity in normal mice brain was 0.186±0.069 pmol mg protein−1 min−1. Carcinine at a dose of 5 mg kg−1 produced no appreciable effect on HDC activity. However, at doses of 10, 20, and 50 mg kg−1, carcinine slightly increased HDC activity with maximum effect at a dose of 20 mg kg−1(0.227±0.009 pmol mg protein−1 min−1, Figure 2).
Figure 2.
Effects of carcinine on HDC activity in mice. Mouse brains were removed 60 min after injection of carcinine, and HDC activity was measured as described in the methods section. Each value is expressed as the mean±s.d. of 10 to 12 mice.
Effects of carcinine on endogenous 5-HT and dopamine release from slices of mice forebrain
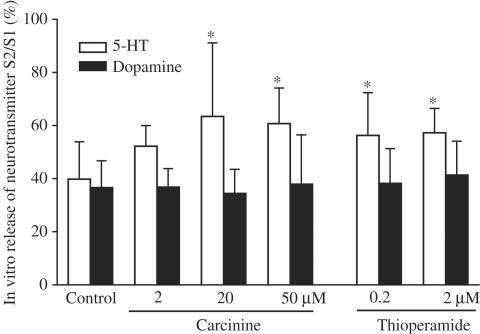
While carcinine at a concentration of 2 μM produced no apparent effect on endogenous 5-HT release from mice forebrain slices, it significantly increased 5-HT release at concentrations of 20 and 50 μM (P<0.05) (Figure 3). On the other hand, thioperamide showed a more potent effect than carcinine on endogenous 5-HT release, in which it produced a marked increase of 5-HT release at the low doses of 0.2 and 2 μM (P<0.05). Both carcinine and thioperamide at all doses showed no appreciable effect on K+-evoked release of endogenous dopamine from mice brain slices.
Figure 3.
Effects of carcinine on endogenous 5-HT and dopamine release from slices of mice forebrain. Each value is expressed as the mean±s.d. of 14 to 15 mice. *P<0.05, statistical significance of difference between saline- and carcinine- or thioperamide-treated groups.
Effects of carcinine on PTZ (60 mg kg−1)-induced kindling in mice
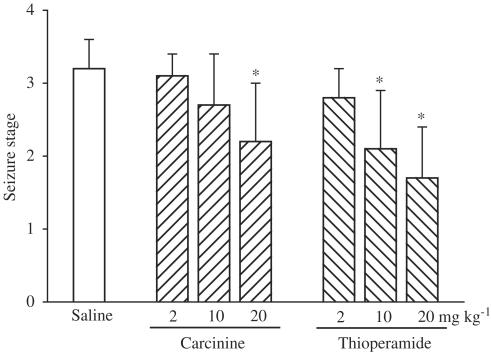
Thioperamide at doses of 10 and 20 mg kg−1 significantly decreased seizure stage (P<0.05) and prolonged the latency to both myoclonic jerks and clonic generalized seizure induced by PTZ (Figure 4). Similarly, carcinine inhibited PTZ-induced seizure in mice in a dose-dependent manner. As shown in Figure 4, at doses of 2 and 10 mg kg−1 carcinine showed no marked effect, while at 20 mg kg−1 it significantly attenuated PTZ-induced seizure, extended the latency for myoclonic jerks and clonic generalized seizure, and decreased seizure stage (P<0.05).
Figure 4.
Effects of carcinine on PTZ (60 mg kg−1)-induced kindling in mice. *P<0.05, statistical significance of difference between saline- and carcinine- or thioperamide-treated groups.
Effects of (R)-α-methylhistamine and α-fluromethylhistidine on the protective effect of carcinine against PTZ-induced kindling in mice
As shown in Table 2 , (R)-α-methylhistamine, a histamine H3 receptor agonist, significantly reversed the protective effect of carcinine against PTZ-induced seizure. At a dose of 1 μg, (R)-α-methylhistamine significantly increased seizure stage, and slightly shortened the latency for myoclonic jerks and clonic generalized seizure. In addition, at a dose of 2 μg, (R)-α-methylhistamine significantly inhibited all seizure types (P<0.05). α-Fluromethylhistidine, a selective HDC inhibitor given at doses of 10 and 20 mg kg−1, also significantly inhibited the protective effect of carcinine against PTZ-induced seizure (P<0.05).
Table 2.
Effect of carcinine on PTZ-induced kindling in mice
| Drug | Dose | Seizure stage | Latency to myoclonic jerks (s) | Latency to clonic generalized seizures (s) |
|---|---|---|---|---|
| PTZ+saline | — | 3.2±0.4 | 66.9±10.7 | 266.9±234.7 |
| PTZ+carcinine | 20 mg kg−1, i.p. | 2.2±0.8* | 155.4±92.3** | 838.4±433.9** |
| PTZ+carcinine+ | 20 mg kg−1, i.p. | |||
| (R)-α-methylhistamine | 1 μg, i.c.v. | 2.9±0.6# | 118.3±67.1 | 535.4±323.1 |
| 2 μg, i.c.v. | 3.2±0.4# | 95.4±30.7# | 326.6±102.6# | |
| PTZ+carcinine+ | 20 mg kg−1, i.p. | |||
| α-fluoromethylhistidine | 10 mg kg−1, i.p. | 2.7±0.8# | 85.2±57.8# | 672.1±460.5 |
| 20 mg kg−1, i.p. | 3.0±0.5# | 77.0±18.0# | 368.0±211.0# |
Each value is expressed as the mean±s.d. of 16 to 30 rats.
P<0.05 represent statistically significant difference as compared to the PTZ+saline control group.
P<0.01 represent statistically significant difference as compared to the PTZ+saline control group.
P<0.05 represent statistically significant difference as compared to the PTZ+carcinine group.
Effects of carcinine on the learning deficit induced by scopolamine (1 mg kg−1) as evaluated by passive avoidance test in mice
Behavioral testing in the passive avoidance test demonstrated that initial training latencies in the carcinine- and control-treated mice were equivalent (Table 3). In the 24 h retention test, serious memory deficit was caused by pretraining injection of scopolamine (1 mg kg−1) (P<0.01). Thioperamide (10 mg kg−1) significantly ameliorated memory deficit induced by scopolamine in the passive avoidance test (P<0.05). Similarly, carcinine at a dose of 20 mg kg−1 significantly prolonged transfer latency of passive avoidance test (P<0.05), although only a slight effect was obtained at a dose of 10 mg kg−1. On the other hand, (R)-α-methylhistamine dose-dependently inhibited the ameliorating effect of carcinine with significant effect at doses of 2 and 5 μg. α-Fluoromethylhistidine at a dose of 10 μg also markedly reversed the ameliorating effect of carcinine (Table 3).
Table 3.
Effects of carcinine on the learning deficit induced by scopolamine (1 mg kg−1) as evaluated by passive avoidance test in mice
| Drug | Dose | Training trial | Test trial |
|---|---|---|---|
| Saline+saline | — | 8.0±4.3 | 288.0±36.0 |
| Scopolamine+saline | 1 mg kg−1, i.p. | 8.2±3.5 | 56.6±29.5** |
| Scopolamine+thioperamine | 10 mg kg−1, i.p. | 9.2±4.2 | 216.7±62.8# |
| Scopolamine+carcinine | 5 mg kg−1, i.p. | 7.8±3.3 | 75.6±27.6 |
| 10 mg kg−1, i.p. | 6.9±3.0 | 105.8±38.8 | |
| 20 mg kg−1, i.p. | 7.2±4.4 | 156.6±70.2# | |
| Scopolamine+carcinine | 20 mg kg−1, i.p. | ||
| +(R)-α-methylhistamine | 1 μg, i.c.v. | 8.0±2.6 | 108.3±47.1 |
| 2 μg, i.c.v. | 7.7±2.9 | 58.9±24.7& | |
| 5 μg, i.c.v. | 8.2±3.5 | 32.1±14.2&& | |
| Scopolamine+carcinine | 20 mg kg−1, i.p. | ||
| +α-fluoromethylhistidine | 10 mg kg−1, i.p. | 10.6±4.5 | 80.6±43.7 |
| 20 mg kg−1, i.p. | 9.3±3.3 | 50.0±29.8& |
Each value is expressed as the mean±s.d. of 15 to 18 rats.
P<0.01 represents statistically significant difference as compared to the saline+saline control group.
P<0.05 represents statistically significant difference as compared to the scopolamine+saline group.
P<0.05
P<0.01 represent statistically significant difference as compared to the scopolamine+carcinine (20 mg kg−1)-treated group.
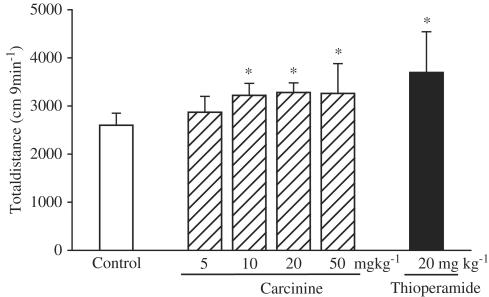
Effects of carcinine on the locomotor activity as evaluated by open-field test in mice
Mice locomotor activity was measured using an open-field test 60 min after carcinine or thioperamide injection (Figure 5). Carcinine dose-dependently increased the total distance with significant effects at doses of 10, 20, and 50 mg kg−1 (P<0.05). Thioperamide at a dose of 20 mg kg−1 also significantly increased mice locomotor activity (P<0.05).
Figure 5.
Effects of carcinine on locomotor activity in mice as evaluated by the open-field test. Carcinine or thioperamide was intraperitoneally administrated 60 min before the test. Distances of ambulation were accumulated every 9 min. Each value was expressed as the mean±s.d. of 13 to 15 mice. *P<0.05, statistical significance of difference between saline- and carcinine- or thioperamide-treated groups.
Discussion
Histamine receptors are highly expressed in the central nervous system, while low levels are observed in peripheral tissues (Panula et al., 1984; Watanabe et al., 1984; Schwartz et al., 1991; Haas & Panula, 2003). The synthesis and release of histamine in the CNS are considered to be under tonic inhibitory control of presynaptic histamine H3 autoreceptors (Arrang et al., 1983; Schwartz et al., 1991). Accordingly, histamine H3 receptor antagonists are generally known to increase histamine synthesis and release in the histamine presynaptic terminals (Schwartz et al., 1991; Leurs et al., 1998; Brown et al., 2001). In the present studies, carcinine was evaluated for its pharmacological effects on histaminergic neurons and its selectivity for the histamine H3 receptor. Carcinine slightly increased HDC activity in mice cortex, and significantly decreased histamine contents both in mice cortex and midbrain. These results indicate that carcinine increases histamine synthesis and release, especially histamine release from histamine presynaptic terminals, suggesting that carcinine could activate histaminergic neurons in the brain. These findings are similar to those obtained with thioperamide, a histamine H3 antagonist, known to significantly increase HDC activity and decrease histamine levels in mouse brain (Arrang et al., 1983; Schwartz et al., 1991). In contrast, (R)-α-methylhistamine, a histamine H3 receptor agonist, has been shown to cause no significant change in HDC activity or histamine levels (Sakai et al., 1992; Meguro et al., 1995). From the above, it is assumed that carcinine can act on histaminergic neurons in the mammalian brain, and that some of its biochemical characteristics are similar to those of H3 antagonists.
Like most histamine H3 receptor antagonists, such as clobenpropit, iodoproxyfan, and vernongamine, carcinine has a flexible ethylene side chain known to be important for H3 receptor binding (Tedford et al., 1999; Yates et al., 1999). Indeed, in our in vitro binding study, we found that carcinine has a higher affinity for histamine H3 receptor than for histamine H1 or H2 receptor (Ki (μM)=0.2939 vs 3621.2 or 365.3, respectively). In addition, our behavioral study provides evidence supporting the hypothesis that carcinine acts as a histamine H3 receptor antagonist. Like thioperamide, carcinine significantly inhibited PTZ-induced kindling and ameliorated memory deficit induced by scopolamine in mice. Interestingly, these effects were completely reversed by (R)-α-methylhistamine, a selective histamine H3 receptor agonist, which given alone had no apparent effects in mice. From these findings, it is assumed that carcinine exerts its protective effects by facilitating endogenous histamine release and blocking autoinhibitory presynaptic H3 receptors. Taken together, our in vitro and in vivo results indicate that carcinine is a novel histamine H3 receptor antagonist, although further biochemical studies are needed to elucidate the mechanism of its action.
It is interesting that in the present study α-fluromethylhistidine, a selective HDC inhibitor, reversed carcinine inhibition of PTZ-induced kindling in mice. We have previously reported that α-fluromethylhistidine inhibits the protective effect of clobenprobit, or thioperamide on PTZ-induced development kindling seizure and the kindled seizure, respectively (Chen et al., 2002; Zhang et al., 2003a, 2003b). In addition, in the present study carcinine produced no marked effect on HDC activity in mouse brain. It is therefore suggested that the decrease in histamine synthesis caused by α-fluromethylhistidine results in a decrease in carcinine-induced histamine release.
The existence of H3 receptor, its functional role, and pharmacological importance have been demonstrated by several laboratories. Accordingly, H3 receptors antagonists have been indicated to be involved into various neuronal diseases such as cognitive deficits, attention deficit hyperactivity disorder, narcolepsy, and epilepsy (Stark et al., 1996; Leurs et al., 1998; Tozer & Kalindjian, 2000). For instance, H3 receptor-deficient mice have been shown to have an overall decreased locomotion in wheel-running test and to be resistant to the amnesic effect of scopolamine in the passive avoidance test (Toyota et al., 2002). In addition, Rizk et al. (2004) has recently reported that H3 receptor-deficient mice show enhanced spatial learning and memory in the Barnes maze test and reduced anxiety in the elevated plus maze. Moreover, H3 antagonists, such as thioperamide and clobenpropit, which are conventionally used to study the precise function of brain histamine, have been reported to ameliorate memory deficits induced by scopolamine (Miyazaki et al., 1995; Chen & Kamei, 2000), protect from developing kindling seizure (Scherkl et al., 1991; Chen et al., 2002), and improve locomotor activity (Sakai et al., 1991). Carcinine also produced similar behavioral effects, such as ameliorating the learning deficit induced by scopolamine in the passive avoidance test and inhibiting PTZ-induced kindling. Furthermore, carcinine significantly increased locomotor activity as evaluated by the open-field test and markedly decreased histamine levels in the cortex and midbrain. Taken altogether, these results suggest that the mechanism by which carcinine inhibits PTZ-induced kindling, protects against scopolamine-induced learning deficit, and ameliorates locomotor activity in mice is based on an increase in histamine release from histamine presynapses caused by a high occupancy of H3 receptors. Thus, the present study provides initial and direct evidence of the pharmacological role of carcinine in histaminergic neurons function in the mammalian brain.
It has been demonstrated that histamine H3 receptors exist as heteroreceptors: they are present not only in the histaminergic nerve terminals in the brain but also in the terminals of other neurotransmitter systems (Schlicker et al., 1988; 1993). In addition, several reports have demonstrated the functional interactions between histaminergic, serotoninergic, and dopaminergic neurotransmission (Schlicker et al., 1988; 1993; Ryu et al., 1994; Son et al., 2001). In the present study, we found that like thioperamide, carcinine significantly increased endogenous 5-HT release from mice forebrain slices, although both carcinine and thioperamide had no appreciable effect on K+-evoked release of endogenous dopamine from mice brain slices.
In conclusion, the present study provides direct evidence that carcinine is a new histamine H3 receptor antagonist that might be useful not only as an antiepileptic drug or as an adjunct to existing antiepileptic drugs but also as therapeutic treatment for memory deficits including Alzheimer disease.
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 30371638) and by the Grants-in-Aid for Scientific Research B (No.12557007 and 14370027) from the Ministry of Education, Culture, Sports, Science and Technology, Japan. A part of the project was supported by a grant from the Zhejiang Provincial Natural Science Foundation of China (R303779, 2004C34002).
Abbreviations
- Carcinine
β-alanyl histamine
- HDC
histidine decarboxylase
- PTZ
pentylenetetrazole
References
- ALI S.M., TEDFORD C.E., GREGORY R., HANDLEY M.K., YATES S.L., HIRTH W.W., PHILLIPS J.G. Design, synthesis, and structure–activity relationships of acetylene-based histamine H3 receptor antagonists. J. Med. Chem. 1999;42:903–909. doi: 10.1021/jm980310g. [DOI] [PubMed] [Google Scholar]
- ARNOULD J.M., FRENTZ R. Mice an evidence, isolement et structure chimique d'une substance carateristique du Coeur de Carcinus maenas (L): La β-alanyl-histamine. Comp. Biochem. Physiol. 1975;50:59–66. [PubMed] [Google Scholar]
- ARNOULD J.M., FRENTZ R. Carcinine (β-alanyl-histamine): rapid synthesis and action on vertebrate blood pressure. Arch. Int. Physiol. Biochim. 1977;85:339–350. doi: 10.3109/13813457709058766. [DOI] [PubMed] [Google Scholar]
- ARRANG J.M., GARBARG M., SCHWARTZ J.C. Autoinhibition of histamine release mediated by a novel class (H3) of histamine receptor. Nature. 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- BABIZHAYEV M.A., SEGUIN M.C., GUEYNE J., EVSTIGNEEVA R.P., AGEYEVA E.A., ZHELTUKHINA G.A. L-Carnocine (β-alanyl-L-histidine) and carcinine (β-alanylhistamine act as natural antioxidants with hydroxyl-radical-scavenging and lipid-peroxidase activities. J. Biochem. 1994;304:509–516. doi: 10.1042/bj3040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROTMAN D.N., FLANCBAUM L., FITZPATRICK J.C., FISHER H. Presence of carcinine (β-alanylhistamine) in mammalian tissues. FASEB J. 1989;3:1028. [Google Scholar]
- BROTMAN D.N., FLANCBAUM L., KANG Y.H., MERRILL G.F., FISHER H. Positive inotropic effects of carcinine in the isolated, perfused guinea pig heart. Crit. Care. Med. 1990;18:317–321. doi: 10.1097/00003246-199003000-00015. [DOI] [PubMed] [Google Scholar]
- BROWN R.E., STEVENS D.R., HASS H.L. The physiology of brain histamine. Progr. Neurobiol. 2001;63:637–672. doi: 10.1016/s0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- CHANG R.S., TRAN V.T., SNYDER S.H. Heterogeneity of histamine H1-receptors: species variations in [3H]mepyramine binding of brain membranes. J. Neurochem. 1979;32:1653–1663. doi: 10.1111/j.1471-4159.1979.tb02276.x. [DOI] [PubMed] [Google Scholar]
- CHEN Z., KAMEI C. Facilitating effects of histamine on spatial memory deficit induced by scopolamine on radial maze performance in rats. Acta Pharmacol. Sin. 2000;21:814–818. [PubMed] [Google Scholar]
- CHEN Z., LI W.D., ZHU L.J., SHEN Y.J., WEI E.Q. Effects of histidine, a precursor of histamine, on pentylenetetrazole-induced seizures in rats. Acta Pharmacol. Sin. 2002;23:361–366. [PubMed] [Google Scholar]
- CHEN Z., LI Z.Y., SAKURAI E., MOBARAKEH J.I., OHTSU H., WATANABE T., WATANABE T., IINUMA K., YANAI K. Chemical kindling induced by pentylenetetrazol in histamine H1 receptor gene knockout mice (H1KO), histidine decarboxylase-deficient mice (HDC−/−) and mast cell-deficient W/Wv mice. Brain Res. 2003;968:162–166. doi: 10.1016/s0006-8993(03)02229-7. [DOI] [PubMed] [Google Scholar]
- CHEN Z., SHEN Y.J. Effects of histamine on memory deficit induced by nucleus basalis-lesion on passive avoidance test and radial maze performance in rats. Acta Pharmacol. Sin. 2002;23:66–70. [PubMed] [Google Scholar]
- FLANCBAUM L., BROTMAN D.N., FITZPATRICK JC VAN ES T., KASZIBA E., FISHER H. Existence of carcinine, a histamine-related compound, in mammalian tissues. Life Sci. 1990;47:1587–1593. doi: 10.1016/0024-3205(90)90188-w. [DOI] [PubMed] [Google Scholar]
- FOREMAN J.C., RISING T.J., WEBBER S.E. A study of the histamine H2-receptor mediating relaxation of the parenchymal lung strip preparation of the guinea-pig. Br. J. Pharmacol. 1985;86:465–473. doi: 10.1111/j.1476-5381.1985.tb08916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAAS H., PANULA P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nat. Rev. Neurosci. 2003;4:121–130. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- HILL S.J. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol. Rev. 1990;42:45–83. [PubMed] [Google Scholar]
- LEURS R., BLANDINA P., TEDFORD C., TIMMERMAN H. Therapeutic potential of histamine H3 receptor agonists and antagonists. Trends Pharmacol. Sci. 1998;19:177–183. doi: 10.1016/s0165-6147(98)01201-2. [DOI] [PubMed] [Google Scholar]
- MEGURO K., YANAI K., SAKAI N., SAKURAI E., MAEYAMA K., SASAKI H., WATANABE T. Effects of thioperamide, a histamine H3 antagonist, on the step-through passive avoidance response and histidine decarboxylase activity in senescence-accelerated mice. Pharmacol. Biochem. Behav. 1995;50:321–325. doi: 10.1016/0091-3057(95)00248-u. [DOI] [PubMed] [Google Scholar]
- MIYAZAKI S., IMAIZUMI M., ONODERA K. Effects of thioperamide, a histamine H3-receptor antagonist, on a scopolamine-induced learning deficit using an elevated plus-maze test in mice. Life Sci. 1995;57:2137–2144. doi: 10.1016/0024-3205(95)02206-x. [DOI] [PubMed] [Google Scholar]
- MOLINENGO L., DI CARLO G., GHI P. Combined action of thioperamide plus scopolamine, diphenhydramine, or methysergide on memory in mice. Pharmacol. Biochem. Behav. 1999;63:221–227. doi: 10.1016/s0091-3057(98)00229-9. [DOI] [PubMed] [Google Scholar]
- PANULA P., YANG H.Y., COSTA E. Histamine-containing neurons in the rat hypothalamus. Proc. Natl. Acad. Sci. U.S.A. 1984;81:2572–2576. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZK A., CURLEY J., ROBERTSON J., RABER J. Anxiety and cognition in histamine H3 receptor−/− mice. Eur. J. Neurosci. 2004;19:1992–1996. doi: 10.1111/j.1460-9568.2004.03251.x. [DOI] [PubMed] [Google Scholar]
- RYU J.-H., YANAI K., IWATA R., IDO T., WATANABE T. Heterogeneous distributions of histamine H3, dopamine D1 and D2 receptors in rat brain. Neuroreport. 1994;5:621–624. doi: 10.1097/00001756-199401000-00022. [DOI] [PubMed] [Google Scholar]
- SAKAI N., ONODERA K., MAEYAMA K., YANAI K., WATANABE T. Effects of thioperamide, a histamine H3 receptor antagonist, on locomotor activity and brain histamine content in mast cell-deficient W/Wv mice. Life Sci. 1991;48:2397–2404. doi: 10.1016/0024-3205(91)90373-j. [DOI] [PubMed] [Google Scholar]
- SAKAI N., SAKURAI A., SAKURAI E., YANAI K., MAEYAMA K., WATANABE T. Effects of the histamine H3 receptor ligands thioperamide and (R)-alpha-methylhistamine on histidine decarboxylase activity of mouse brain. Biochem. Biophys. Res. Commun. 1992;185:121–126. doi: 10.1016/s0006-291x(05)80964-7. [DOI] [PubMed] [Google Scholar]
- SCHERKL R., HASHEM A., FREY H.H. Histamine formation in rat brain – its role in regulation of seizure susceptibility. Epilepsy Res. 1991;10:111–118. doi: 10.1016/0920-1211(91)90003-x. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., BETZ R., GOTHERT M. Histamine H3 receptor-mediated inhibition of serotonin release in the rat brain cortex. Naunyn-Schimied. Arch. Pharmacol. 1988;337:558–590. doi: 10.1007/BF00182737. [DOI] [PubMed] [Google Scholar]
- SCHLICKER E., FINK K., DETZNER M., GOTHERT M. Histamine inhibits dopamine release in the mouse striatum via presynaptic H3 receptors. J. Neural. Transm. 1993;93:1–10. doi: 10.1007/BF01244933. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ J.C., ARRANG J.M., GARBARG M., PALLARD H., RUST M. Histaminergic transmission in the mammalian brain. Physiol. Rev. 1991;71:1–51. doi: 10.1152/physrev.1991.71.1.1. [DOI] [PubMed] [Google Scholar]
- SON L.Z., YANAI K., MOBARAKEH J.I., KURAMASU A., LI Z.Y., SAKURAI E., HASHIMOTO Y., WATANABE T., WATANABE T. Histamine H1 receptor-mediated inhibition of potassium-evoked release of 5-hydroxytryptamine from mouse forebrains. Behav. Brain Res. 2001;124:113–120. doi: 10.1016/s0166-4328(01)00220-0. [DOI] [PubMed] [Google Scholar]
- STARK H., SCHLICKER E., SCHUNACK W. Developments of histamine H3-receptor antagonists. Drugs Future. 1996;21:507–520. [Google Scholar]
- TEDFORD C.E., PHILLIPS J.G., GREGORY R., PAWLOWSKI G.P., FADNIS L., KHAN M.A., ALI S.M., HANDLEY M.K., YATES S.L. Development of trans-2-[1H-imidazol-4-yl] cyclopropane derivatives as new high-affinity histamine H3 receptor ligands. J. Pharmacol. Exp. Ther. 1999;289:1160–1168. [PubMed] [Google Scholar]
- TOYOTA H., DUGOVIC C., KOEHL M., LAPOSKY A.D., WEBER C., NGO K., WU Y., LEE D.H., YANAI K., SAKURAI E., WATANABE T., LIU C., CHEN J., BARBIER A.J., TUREK F.W., FUNG-LEUNG W.P., LOVENBERG T.W. Behavioral characterization of mice lacking histamine H(3) receptors. Mol. Pharmacol. 2002;62:389–397. doi: 10.1124/mol.62.2.389. [DOI] [PubMed] [Google Scholar]
- TOZER M.J., KALINDJIAN S.B. Histamine H3 receptor antagonists. Expert Opin. Ther. Patents. 2000;10:1045–1055. [Google Scholar]
- WATANABE T., OHTSU H. L-histidine decarboxylase as a probe in studies on histamine. Chem. Rec. 2002;2:369–376. doi: 10.1002/tcr.10036. [DOI] [PubMed] [Google Scholar]
- WATANABE T., TAGUCHI Y., YAYASHI Y., SHIOSAKA J., KUBOTA H., TERANO Y., TOHYAMA M., KUBOTA H., TERANO Y., WADA H. Distribution of the histaminergic neuron system in the central nervous system of rats: α-fluorescent immunohistochemical analysis with histidine decarboxylase as a marker. Brain Res. 1984;295:13–25. doi: 10.1016/0006-8993(84)90811-4. [DOI] [PubMed] [Google Scholar]
- YAMAKAMI J., SAKURAI E., KURAMASU A., SAKURAI E., YANAI K., WATANABE T., TANAKA Y. L-Histidine decarboxylase protein and activity in rat brain microvascular endothelial cells. Inflamm. Res. 2000;49:231–235. doi: 10.1007/s000110050584. [DOI] [PubMed] [Google Scholar]
- YAMATODANI A., FUKUDA H., WADA H., IWAEDA T., WATANABE T. High-performance liquid chromatographic determination of plasma and brain histamine without previous purification of biological samples: cation-exchange chromatography coupled with post-column derivatization fluorometry. J. Chromatogr. 1985;344:115–123. doi: 10.1016/s0378-4347(00)82012-5. [DOI] [PubMed] [Google Scholar]
- YANAI K., RYU J.-H., SAKAI N., TAKAHASHI T., IWATA R., IDO T., MURAKAMI K., WATANABE T. Binding characteristics of a histamine H3-receptor antagonist, [3H]S-methylthioperamide: comparison with [3H](R)alpha-methylhistamine binding to rat tissues. Jpn. J. Pharmacol. 1994;65:107–112. doi: 10.1254/jjp.65.107. [DOI] [PubMed] [Google Scholar]
- YANAI K., SON L.Z., NAKAGAWASAI O., TADANO T., KISARA K., WATANABE T., ENDOU M., SAKURAI E., WATANABE T. Behavioral characterization and amounts of brain monoamines and their metabolites in mice lacking histamine H1 receptors. Neuroscience. 1998;87:479–487. doi: 10.1016/s0306-4522(98)00167-5. [DOI] [PubMed] [Google Scholar]
- YATES S.L., PHILLIPS J.G., GREGORY R., PAWLOWSKI G.P., FADNIS L., KHAN M.A., ALI S.M., TEDFORD C.E. Identification and pharmacological characterization of a series of new 1H-4-substituted-imidazoyl histamine H3 receptor ligands. J. Pharmacol. Exp. Ther. 1999;289:1151–1159. [PubMed] [Google Scholar]
- ZHANG L.S., CHEN Z., HUANG Y.W., HU W.W., WEI E.Q., YANAI K. Effects of endogenous histamine on seizure development of pentylenetetrazole-induced kindling in rats. Pharmacology. 2003a;69:27–32. doi: 10.1159/000071263. [DOI] [PubMed] [Google Scholar]
- ZHANG L.S., CHEN Z., REN K.M., LEURS R., CHEN J.C., ZHANG W.B., YE B., WEI E.Q., TIMMERMAN H. Effects of clobenpropit on pentylenetetrazole-kindled seizures in rats. Eur. J. Pharmacol. 2003b;482:169–175. doi: 10.1016/j.ejphar.2003.09.066. [DOI] [PubMed] [Google Scholar]