Abstract
In hypertension, a decrease of the vascular β-adrenergic relaxation has been described. However, the specific involvement of each β-adrenoceptor (β-AR) subtype, in particular the low-affinity state of β1-AR, has not yet been evaluated. We investigated whether the low-affinity state of β1-AR-induced relaxation was impaired in Spontaneously Hypertensive Rats (SHR).
The relaxant responses to CGP 12177 and cyanopindolol, low-affinity state β1-AR agonists (with β1-/β2-AR antagonistic and partial β3-AR agonistic properties) were evaluated on thoracic aortic rings isolated from 12-weeks-old Wistar Kyoto rats (WKY) and SHR.
In WKY, CGP 12177 and cyanopindolol produced an endothelium and nitric oxide (NO)-independent relaxation. CGP 12177-induced endothelium-independent relaxation was not modified either by β1-, β2-AR (nadolol) or β3-AR (L-748337 or SR 59230A) antagonists but was significantly reduced by high concentrations of CGP 20712A (P<0.05). This relaxation was also reduced by adenylyl cyclase inhibitors, SQ 22536 or MDL 12330A.
In SHR, CGP 12177 produced mainly an endothelium and NO-dependent relaxation. This effect was not modified by nadolol, but was strongly reduced by β3-AR blockade. Endothelium-independent relaxation to CGP 12177 was not altered by adenylyl cyclase inhibition, but was amplified in preparations from pertussis toxin-pretreated SHR.
The immunohistochemical analysis revealed an upregulation of β3-AR in the endothelial layer of SHR aorta, whereas the β3-AR-induced relaxation was not modified.
In conclusion, we demonstrated an impaired low-affinity state of the β1-AR-induced relaxation and an upregulation of the β3-AR in hypertension. Some clinical implications of those findings are discussed.
Keywords: Hypertension, vasodilation, receptors, adrenergic, beta, CGP 12177
Introduction
Vascular tone is generated via a net balance between vasoconstriction and vasorelaxation established through the effects of numerous neurotransmitters and hormones (epinephrine, norepinephrine, acetylcholine, angiotensin II, nitric oxide (NO), etc.). Thus, hypertension results mainly from an imbalance between vasoconstrictive and vasodilatory mechanisms, partly due to pre- and postsynaptic sympathetic dysfunctions (De Champlain, 2001). Several studies reported a decreased β-adrenoceptors (β-ARs)-mediated relaxation of isolated vascular preparations from Spontaneously Hypertensive Rats (SHR) (Fujimoto et al., 1987; Arribas et al., 1994; Gros et al., 2000). The main identified defects included β-AR downregulation, alteration of G protein levels and impairment of β-AR/G protein/effectors coupling (Werstiuk & Lee, 2000). However, some studies described that β-AR-mediated relaxation of thoracic aorta and mesenteric arteries was unchanged (Borkowski et al., 1992; Boonen et al., 1993) or enhanced (Carvalho et al., 1987) comparatively to what was observed in Wistar Kyoto (WKY) rats. Several explanations may exist for those controversial findings regarding β-AR-induced relaxation in arterial hypertension. Thus, the β-AR-mediated relaxation was dependent on the following: (1) the stage of hypertension (Borkowski et al., 1992), (2) the agent used for preconstriction (Konishi & Su, 1983; Carvalho et al., 1987) and (3) the precontraction level (Konishi & Su, 1983; Carvalho et al., 1987). It is important to note that in all those studies, the β-AR relaxant responses have been investigated by using isoprenaline, a nonselective β-AR agonist, and the contribution of each β-AR subtype in the β-AR-mediated relaxation in SHR was not determined.
At the present time, at least three populations of β-AR subtypes have been shown to be involved in the β-adrenergic response in blood vessels (Guimaraes & Moura, 2001). Stimulation of β1- and β2-ARs produces a vasorelaxation. In some vascular beds, in addition to β1- and β2-ARs, the functional expression of a third β-AR subtype, β3, has been described in several in vivo and in vitro studies using β1-/β2-AR antagonists and β3-AR agonists (Tavernier et al., 1992; Oriowo, 1994; Hom et al., 2001). In rat thoracic aorta, β3-AR stimulation produced an endothelium and NO-dependent relaxation (Trochu et al., 1999; Rautureau et al., 2002). Furthermore, vascular relaxation induced by BRL 37344 (a β3-AR agonist), cyanopindolol or CGP 12177 suggested the presence of an atypical β-AR in vessels (Shafiei & Mahmoudian, 1999; Brawley et al., 2000a, 2000b). Such an atypical β-AR has been previously described in heart (Kaumann et al., 2001) and adipose tissue (Granneman, 2001) and firstly qualified as ‘putative' β4-AR. Recently, β4-AR has been demonstrated to actually correspond to a low-affinity state of the β1-AR (Granneman, 2001). It was activated by cyanopindolol and CGP 12177, which also possesses β1-/β2-AR antagonistic and partial β3-AR agonistic properties.
To date, no available data has been reported on the role of the vascular low-affinity state of the β1-AR in experimental hypertension. So, the present investigation was carried out in order to evaluate the effect of the low-affinity state of the β1-AR in the model of SHR by analysing the relaxing responses to CGP 12177.
Methods
Animals
Male SHR at 12 weeks of age and age-matched WKY rats were purchased from CER Janvier (Laval, France). They were housed with standard rat chow and water was provided ad libitum.
Systolic blood pressure (SBP) of conscious rats was determined by the tail-cuff method (Bioseb, Chaville, France). The measurements, performed from 16:00 to 18:00 hours by the same investigator, were repeated at least 10 times and the mean values were determined for each animal. SBP was 117.3±2.8 mmHg in WKY (n=73) and 182.8±3.7 mmHg in SHR (n=50; P<0.05 vs WKY).
Preparation of aortic rings
Rats were anaesthetized with pentobarbital (30 mg kg−1 i.p.) and exsanguinated. Intact and endothelium-denuded aortic rings were prepared as previously described (Trochu et al., 1999). Each aortic ring was suspended on stainless steel wires in a 5 ml organ bath containing Krebs solution maintained at 37°C (Trochu et al., 1999). Then, the preparations were progressively stretched in order to get a resting tension of 1 g and allowed to equilibrate for 1 h. Isometric tension was recorded by a force displacement transducer (Electromike, Electrocorporation, Sarasota, U.S.A.) on a potentiometric recorder (Bryans-BS 272, U.S.A.).
Vascular relaxation measurements
Functional endothelium was checked by application of 1 μM acetylcholine resulting in the presence of at least 60% relaxation of precontracted rings with 0.3 μM phenylephrine (PE), an α1-AR agonist. After washout, aortic rings were contracted with PE at concentrations ranging from 0.1 to 1 μM in order to obtain similar values of the sustained tension for each ring studied (0.9±0.05 g). Then, cumulative concentration–response curves (CCRC) to relaxant agents were conducted after reaching the steady-state amplitude of PE contraction. Some rings were incubated during 30 min in Krebs containing nadolol (β1-/β2-AR antagonist), L-748337 (Candelore et al., 1999) or SR 59230A (two β3-AR antagonists), NG-monomethyl-L-arginine monoacetate (L-NMMA, an NO synthase inhibitor), SQ 22536 or MDL 12330A (two adenylyl cyclase inhibitors) before CCRC establishment. CCRC to CGP 12177 were also constructed in aortic rings isolated from rats pretreated with pertussis toxin (PTX, an inhibitor of Gi/0 proteins) (10 μg kg−1 once daily during 3 days).
Immunohistochemistry analysis
The method used was already described by Rautureau et al. (2002). After freezing in 2-methyl butane solution (−50°C), 10 μm sections of thoracic aorta were cut on cryostat microtome and stored at −20°C until use. The presence of the endothelial layer of the preparations was tested by incubation with a von Willebrand factor antibody (vWf Ab; DAKO, Trappes). The rat β3-AR antibody (rβ3-AR Ab) (Santa Cruz Biotechnology Inc., U.S.A.) is an affinity-purified goat polyclonal antibody raised against a peptide mapping at the carboxy terminus of the β3-AR of mouse origin. This antibody reacts with β3-ARs of mouse and rat origin, without crossreactive signal with β1- and β2-ARs. After the secondary hybridization with an anti-goat IgG peroxidase conjugated (Sigma, France), the antibody complexes were revealed for sensitive detection of the enzymatic activity with peroxidase substrate kit AEC (SK4200 – Vector, France). Controls for specificity of the rβ3-AR antiserum involved the incubation of the primary antiserum overnight at 4°C with a five excess by weight of blocking peptide, the sequence used for the immunization procedure without the immunogenic carrier.
Statistics
Results were expressed as the means±s.e.m. of n experiments, which refers to the number of rats. Relaxation was expressed as percentage relaxation of contraction induced by PE. Comparison of the different CCRC was performed by a two-way ANOVA with repeated measures. Maximal effect (Emax) obtained for the highest concentration of agonist was compared using Student's t-test. A level of P<0.05 was considered statistically significant.
Drugs
Phenylephrine hydrochloride (HCl), acetylcholine chloride, nadolol, 5′-(N-ethylcarbamidoadenosine (NECA), CGP 20712A ((±)-2-hydroxy-5-[2-[[2-hydroxy-3-[4-[1-methyl-4-(trifluoromethyl)-1H-imidazol-2-yl]phenoxy]propyl]amino]ethoxy]-benzamide methanesulphonate), (−) cyanopindolol and SR 59230A (3-(2-ethylphenoxy)-1-[(1S)1,2,34-tetrahydronapht-1-ylaminol]-(2S)-2-propanol oxalate) were obtained from Sigma-Aldrich (France). NG-monomethyl-L-arginine monoacetate (L-NMMA), SQ 22536, MDL 12330A and PTX were purchased from Calbiochem (France). CGP 12177 (4-[3-t-butylamino-2-hydroxypropoxy] benzimidazol-2-one) was purchased from RBI (France). SR 58611A [(RS)-N-[(25)-7-ethoxycarbonylmethoxy-1,2,3,4-tetrahydronapht-2-yl]-(2R)-2-(3-chlorophenyl)-2 hydroethanamine hydrochloride] was a generous gift from Sanofi Synthelabo Recherche (France) and L-748337 from Merck (U.S.A.). All drugs were prepared as stock solutions in distilled water, with the exception of nadolol, which was dissolved in HCl and neutralized with NaOH to pH 7.4, and L-748337, MDL 12330A, NECA and cyanopindolol that were dissolved in dimethylsulphoxide (DMSO, Sigma-Aldrich). The final concentration of the solvents in the organ bath was less than 0.1% v v−1 and has been tested to induce per se no effect on the tissue responses.
Results
Effects of CGP 12177 in WKY aorta
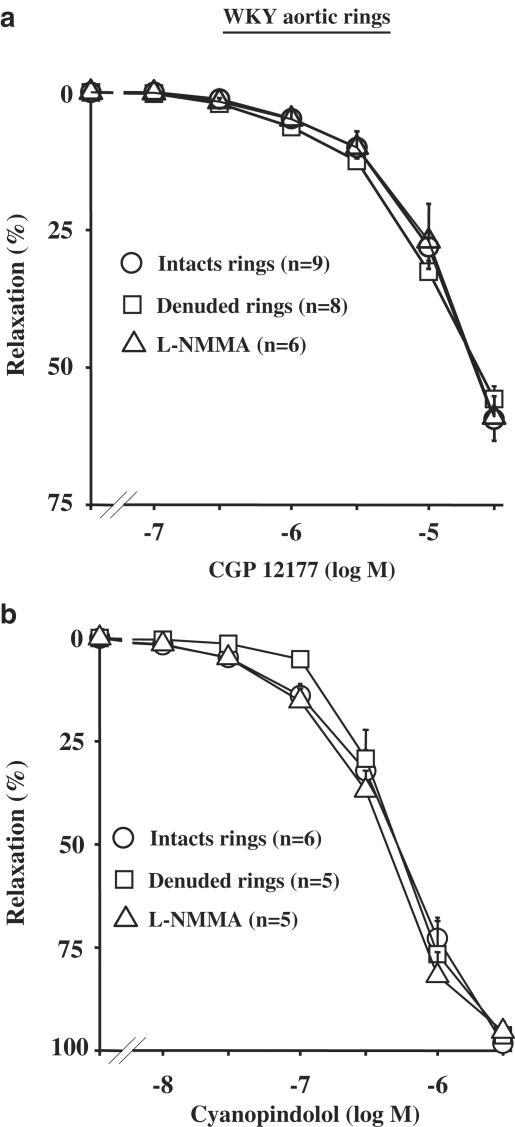
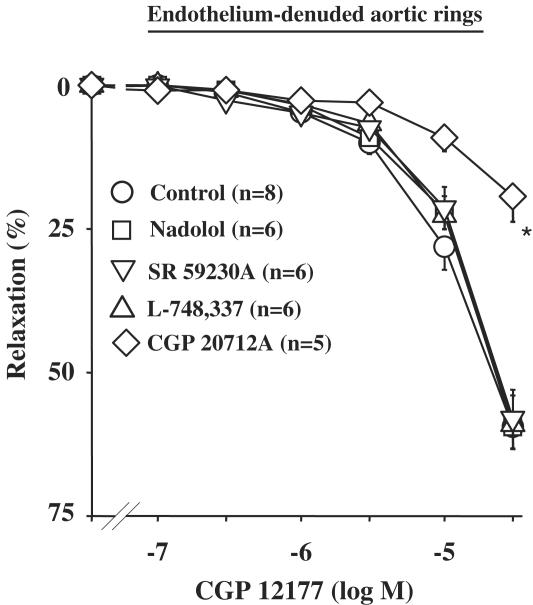
In intact aortic rings from WKY, CGP 12177 (0.1–30 μM) produced a concentration-dependent relaxation (Emax=55.8±2.3%; n=9). This relaxation was unaffected by endothelium removal (Emax=59.4±3.3%; n=8), or after pretreatment with 100 μM L-NMMA (Figure 1a). Similar results were obtained with cyanopindolol (10 nM to 3 μM; Figure 1b). In order to determine the β-AR subtype involved in the CGP 12177-induced relaxation, CCRC in endothelium-denuded rings were constructed in the presence of 10 μM nadolol, or 3 μM L-748337 or 1 μM SR 59230A. In all these conditions, the CGP 12177-induced effects were not significantly modified (Figure 2). Conversely, the CGP 12177-mediated relaxation was antagonized by a high concentration (10 μM) of CGP 20712A, a β1-AR antagonist (Figure 2).
Figure 1.
Concentration–response curves to CGP 12177 (a) and cyanopindolol (b) in WKY rats. Curves were performed in intact, denuded rings or in intact rings pretreated with 100 μM L-NMMA for 30 min.
Figure 2.
Concentration–response curves to CGP 12177 in denuded aortic rings from WKY rats. Curves were performed in the absence or presence of 10 μM nadolol, 3 μM L-748337, 1 μM SR 59230A or 10 μM GCP 20712A. *P<0.05 vs CGP 12177 alone (the curve in the presence of nadolol is mostly obscured under the control one).
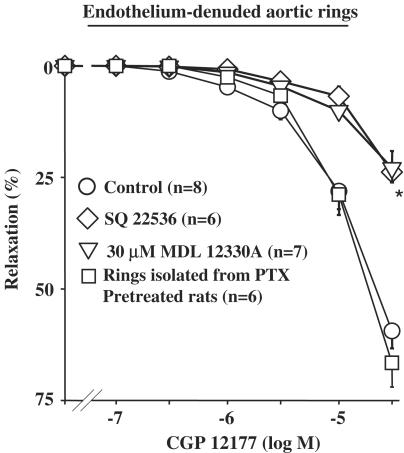
In order to determine a putative involvement of the adenylyl cyclase/adenosine-3′,5′-monophosphate (cAMP) pathway, CCRC to CGP 12177 were constructed in endothelium-denuded rings pretreated by adenylyl cyclase inhibitors, SQ 22536 or MDL 12330A (Satake et al., 1996; Hourani et al., 2001). These compounds were first tested by performing CCRC to NECA, a nonselective adenosine A2 receptor agonist, which produces a vasorelaxation through the activation of A2A,B receptors linked to adenylyl cyclases via Gs proteins (Fahim et al., 2001). In denuded aortic rings, CCRC to NECA (Emax=91.7±0.6%; n=6) was significantly inhibited in the presence of 200 μM SQ 22536 (Emax=58.2±6.3; n=6) or 30 μM MDL 12330A (Emax=38.4±2.78; n=7). Then, the effect of CGP 12177 was tested and was significantly inhibited in the presence of either 200 μM SQ 22536 (Emax=23.8±5.9, n=6; P<0.05 vs CGP 12177 alone) or 30 μM MDL 12330A (Emax=25.5±3.8, n=7; P<0.05 vs CGP 12177 alone) (Figure 3). Finally, in another set of experiments, we evaluated the CGP 12177-mediated response in aortic rings isolated from rats pretreated with PTX. Gi proteins inhibitory effect of PTX was previously confirmed by establishing CCRC to UK 14304, a selective α2-AR agonist (Rautureau et al., 2002). The endothelium-independent effect of CGP 12177 was not modified by PTX pretreatment (Emax=66.9±7.3%; n=6) compared to control rats.
Figure 3.
Concentration–response curves to CGP 12177 in denuded aortic rings from WKY rats. Curves were performed in the absence or presence of 200 μM SQ 22536, 30 μM MDL 12330A or after pretreatment of rats with 10 μg kg−1 PTX during 3 days. *P<0.05 vs CGP 12177 alone.
Alteration of the CGP 12177-induced vasorelaxation in SHR aorta
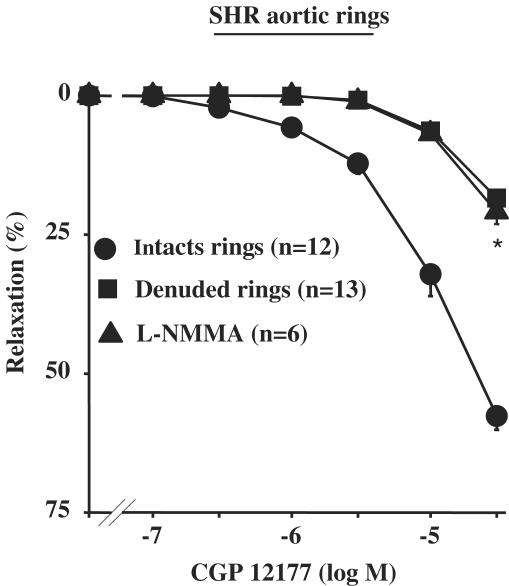
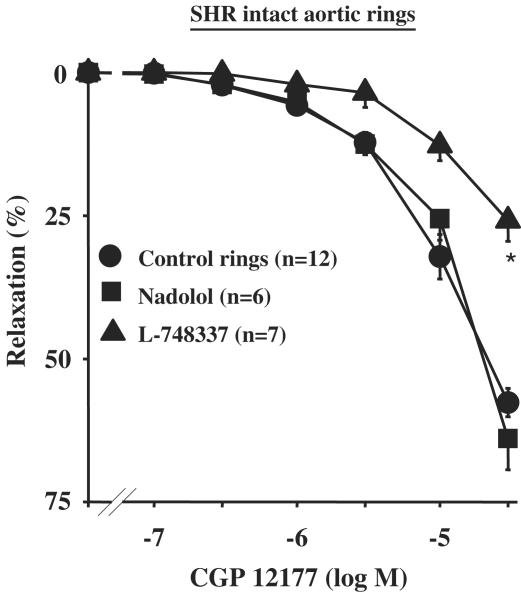
In intact aortic rings from SHR, CGP 12177 produced a concentration-dependent relaxation (Emax=57.6±2.5%, n=12) (Figure 4). This relaxation was greatly inhibited by endothelium removal or after pretreatment with 100 μM L-NMMA (Figure 4). Interestingly, endothelium-dependent relaxation of CGP 12177 was not modified in the presence of 10 μM nadolol, but was significantly inhibited in the presence of 3 μM L-748337 (Emax=25.9±3.3%; n=7; P<0.05 vs CGP 12,177 alone) (Figure 5).
Figure 4.
Concentration–response curves to CGP 12177 in SHR rats. Curves were performed in intact, denuded rings or in intact rings pretreated with 100 μM L-NMMA. *P<0.05 vs CGP 12177 alone.
Figure 5.
Concentration–response curves to CGP 12177 in intact aortic rings from SHR. Curves were performed in the absence or in the presence of 3 μM L-748337 or 10 μM nadolol. *P<0.05 vs CGP 12177 alone.
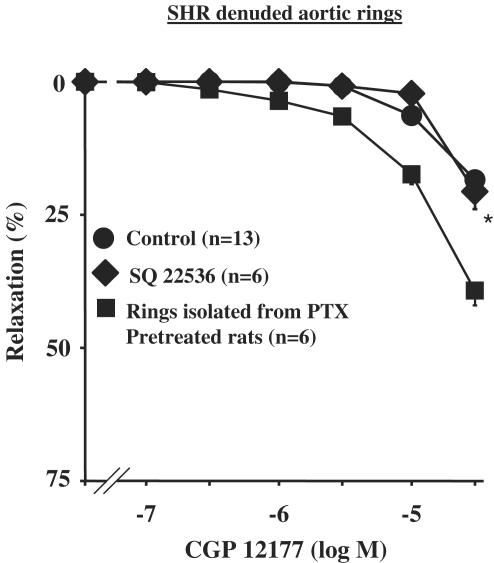
Endothelium-independent relaxation to CGP 12177 was not altered in the presence of SQ 22536 (Figure 6) but was amplified by PTX pretreatment (Emax=36.9±3.4%; n=5; P<0.05 vs rings without PTX by ANOVA) (Figure 6).
Figure 6.
Concentration–response curves to CGP 12177 in denuded aortic rings from SHR. Curves were performed in the absence or in the presence of 200 μM SQ 22536 or after pretreatment of rats with 10 μg kg−1 PTX during 3 days. *P<0.05 vs CGP 12177 alone.
Modification of β3-AR expression without alteration of the functional response
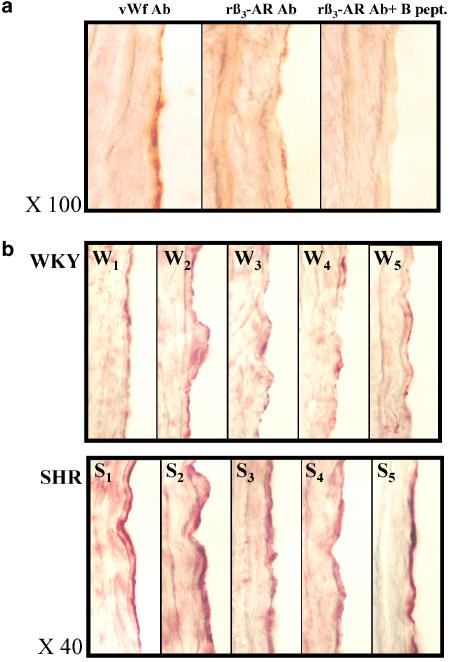
As the endothelium-dependent relaxation to CGP 12177 was blunted by a β3-AR antagonist in SHR, we evaluated the expression level of β3-AR in both strains of WKY rats and SHR by immunohistochemical analysis. The pattern of rat β3-AR immunoreactivity was compared with the vWf expression profile (Figure 7a). vWf was used as a marker of the endothelial layer. The rat β3-AR antibody (rβ3-AR Ab) highly stained cells from the endothelial layer in a similar distribution and form, to those revealed with the vWf antiserum. Furthermore, the preabsorption of rβ3-AR antiserum with the synthetic peptide, used for the procedure of immunization, totally abolished the staining observed in the endothelial layer. The same rβ3-AR Ab staining experiment was performed in WKY rats and SHR aorta (n=5, Figure 7b). In both rat strains, the rβ3-AR Ab revealed a staining for the endothelial layer. A light and discontinuous signal was observed in the endothelial layer of WKY aorta. Conversely, a strong, large and continuous labelling was stained in the endothelial layer of SHR aorta. Furthermore, a light and diffuse signal was observed with the same distribution and intensity in smooth muscle layers of both strains.
Figure 7.
β3-AR expression in WKY and SHR aorta. Panel a: adjacent 10 μm thick sections were incubated with either von Willebrand factor antibody (vWf Ab) or rat β3-AR antibody (rβ3-AR Ab) or after preabsorption of rβ3-AR Ab with the blocking peptide (rβ3-AR Ab+B pept.). Panel b: the same rβ3-AR Ab staining experiment was performed in WKY (W1–W5) and SHR (S1–S5) aorta. vWf and rβ3-AR antibodies were revealed with peroxidase-conjugated second serum.
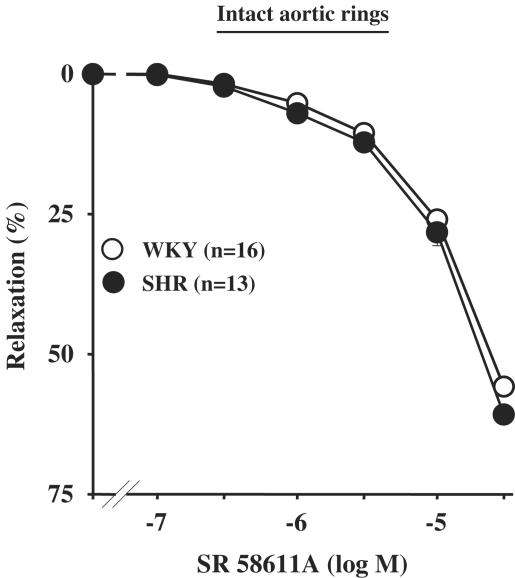
On the basis of the results showing an upregulation of β3-AR, we determined whether β3-AR-induced effect was potentiated in SHR rats. Then, CCRC to SR 58611A, a preferential β3-AR agonist, was performed. In SHR intact aortic rings, SR 58611A (0.1–30 μM) induced a concentration-dependent relaxation (Emax=60.8±1.6%; n=13), which was similar to that obtained in WKY rats (Emax=55.8±1.6%; n=16) (Figure 8).
Figure 8.
Concentration–response curves to SR 58611A in intact aortic rings from WKY rats or SHR.
Discussion
In the present study, CGP 12177, a low-affinity state β1-AR agonist (with β1-/β2-AR antagonistic and partial β3-AR agonistic properties) produced an endothelium-independent relaxation of aortic rings isolated from normotensive WKY rats. This finding corroborates results obtained in Wistar rat aorta, where the relaxation to CGP 12177 was found to be mainly endothelium-independent (Brawley et al., 2000b). Furthermore, we have shown that the relaxant effect of CGP 12177 was not modified by pretreatment with L-NMMA, an NO synthase inhibitor, suggesting that an endothelial NO pathway was not involved. Thus, present results showed that CGP 12177 activated a receptor located in vascular smooth muscle cells.
CGP 12177 has been demonstrated to activate an atypical β-AR in adipose tissue (Granneman, 2001), in heart (Kaumann et al., 2001) and in blood vessels such as aorta (Brawley et al., 2000b) and carotid artery (Oriowo, 1994; MacDonald et al., 1999). In the present work, the endothelium-independent effect of CGP 12177 was not modified after β1-/β2-AR or β3-AR blockade, but was inhibited by CGP 20712A, a low-affinity state β1-AR antagonist at high concentration. Altogether, those results suggested that CGP 12177 acted through the interaction with a β-AR pharmacologically distinct from the well-characterized β1-, β2- and β3-AR subtypes. Using hamster cells stably expressing either human β1-AR, human β2-AR or rat β1-AR, Pak & Fishman (1996) have already found that CGP 12177 behaved as an agonist of β1-AR in a lower affinity, guanine nucleotide-sensitive state precoupled to Gs proteins. Only the high-affinity uncoupled receptors recognized CGP 12177 as an antagonist. Recent evaluation of recombinant β-AR subtypes, as well as the characterization of the β-adrenergic response of several β-AR-deficient mice, have established the identity of the atypical β-AR as a novel state of the β1-AR (Kaumann et al., 2001) and CGP 12177 produced its agonistic effects by interacting with the lower affinity state of the β1-AR.
It should be noted that in rat aorta, Brahmadevara et al. (2003) proposed that the CGP 12177-mediated relaxation resulted from an α1-AR antagonism, but not from the activation of the low-affinity state of β1-AR. However, in the present study, in endothelium-denuded aortic rings of WKY, CGP 12177-induced relaxation was significantly decreased in the presence of adenylyl cyclase inhibitors, suggesting that a cAMP pathway was partly involved in the CGP 12177-mediated relaxation rather than an effect on α1-AR. The role of cAMP in the response to CGP 12177 has also been reported in rat aorta (Brawley et al., 2000b), in rat isolated atria (Kaumann & Lynham, 1997), in guinea-pig taenia caecum (Koike et al., 1995) and in mouse adipose tissue (Konkar et al., 2000).
Conversely to normotensive rats, the removal of endothelium in aortic rings from SHR greatly reduced the relaxant effect of CGP 12177. Furthermore, adenylyl cyclase inhibitors did not modify the remaining endothelium-independent relaxation to CGP 12177 in SHR aorta. An increase of expression and/or activity of Gi proteins has been shown to occur in hypertension and could contribute to the alteration of the cAMP pathway (Anand-Srivastava, 1996). Interestingly, in our study, the inhibition of Gi/0 proteins by PTX partly restored the CGP 12177-induced endothelium-independent relaxation. Two hypotheses might explain this effect: (i) the inhibition of Gi proteins restored the adenylyl cyclase activity and/or (ii) the low-affinity state of β1-AR could be linked to Gi proteins in SHR. Concerning the latter, it has already been shown in a rat model of heart failure, where there was an increase of Gi proteins, that the cardiostimulatory effect of CGP 12177 was lost. However, this cardiostimulatory effect was restored by blockade of Gi proteins with PTX treatment, suggesting that the low-affinity state of β1-AR was linked to Gi proteins in the rat's failing heart (Kompa & Summers, 1999). Thus, the increased expression and/or activity of Gi proteins could be a mechanism to protect the cardiovascular system against a sustained activation of the sympathetic nervous system, during the hypertensive state. Nevertheless, this compensation could be deleterious by impairing the relaxation induced by activation of receptors linked positively to adenylyl cyclases. It is noticeable that only partial restoration of the low-affinity state of β1-AR-induced relaxation was achieved by PTX pretreatment. This observation indicates that Gi proteins are not exclusively involved in the impairment of relaxation and other mechanisms could participate in this alteration such as a decrease of the low-affinity state β1-AR density or an enhanced β-AR kinase activity.
In the present work, the relaxant effect to CGP 12177 was significantly inhibited by a β3-AR antagonist in SHR, suggesting that CGP 12177 could activate β3-AR in hypertensive rats. By contrast with a full agonist, a partial agonist, such as CGP 12177, can induce an effect only in a tissue where the receptor density and/or the coupling efficiency are high. Therefore, the unveiled β3-AR effect in SHR aorta should be related to a high density and/or a high coupling efficiency of β3-AR. In this respect, the immunohistochemical analysis has revealed the presence of an upregulation of β3-AR in the endothelial layer of SHR aorta. However, experiments performed with a full β3-AR agonist, SR 58611A, demonstrated similar relaxant responses in WKY and SHR, indicating that β3-AR-mediated responses were not enhanced in hypertensive rats. In the same way, in rat brown adipose tissue, the increase in the blood flow in response to a β3-AR agonist, BRL 26830A, was found to be unaltered in SHR compared to WKY (Iwase et al., 2000). As β3-AR-mediated relaxation is mainly NO-dependent (Trochu et al., 1999), the decrease of the NO bioavailability observed in SHR aorta (Kerr et al., 1999) might explain why the functional response to SR 58611A was not enhanced.
In conclusion, our results showed an impaired relaxation of aortic rings, induced by the stimulation of the low-affinity state of the β1-AR by CGP 12177 in 12-week-old SHR. We also demonstrated, for the first time, an upregulation of the β3-AR expression in an animal model of hypertension. However, this upregulation was not associated with an increase in the β3-adrenergic-induced vasorelaxation. As the aorta is a conductance vessel, the results obtained in the present study could not be extended to the resistance vessels. Then, additional experiments will be needed to investigate the role of the low-affinity state of β1-AR in the resistance vessels in hypertension.
In clinics, decreased vascular resistance by vasodilating β-blockers, known as third-generation drugs, are associated with β3-AR agonism (nebivolol, bucindolol), β2-AR activation (celiprolol), α-AR blockade (bucindolol), antioxidant activity (carvedilol), potassium channel opening properties (tilisolol) or NO synthesis (Strosberg, 1997; Gosgnach et al., 2001; de Groot et al., 2003; Toda, 2003). The relaxant effect demonstrated in our work through the activation of the low-affinity state of β1-AR could represent another possible mechanism underlying vasorelaxant actions of vasodilating β-blockers. In this respect, carvedilol or alprenolol have already been described to activate the human low-affinity state β1-AR in CHO cells (Baker et al., 2003). This activation might include intracellular changes at the short term of secondary messenger and/or at long term of gene transcription. However, it remains to be established in clinical studies whether the beneficial effects of these β-AR antagonists can be partly attributed to the activation of low-affinity state of β1-AR in blood vessels.
Acknowledgments
This work was supported by grants from the ‘Institut National de la Santé et de la Recherche Médicale'. SS was supported by a grant from ‘Le Conseil Général de Loire Atlantique'. We thank GlaxoSmithKline, Harlow, U.K. for the gift of human β3-AR antibody, Dr Jacques Noireaud for reading and criticizing the manuscript, and Patrick Guyot and Francis Prual for animal care.
Abbreviations
- β-AR
β-adrenoceptor
- cAMP
adenosine-3′,5′-monophosphate
- CCRC
cumulative concentration–response curve
- L-NMMA
NG-monomethyl-L-arginine monoacetate
- NECA
5′-(N-ethylcarbamidoadenosine)
- NO
nitric oxide
- PE
phenylephrine
- PTX
pertussis toxin
- rβ3-AR Ab
rat β3-AR antibody
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rat
- vWf
von Willebrand factor
- WKY
Wistar Kyoto
References
- ANAND-SRIVASTAVA M.B. G-proteins and adenylyl cyclase signalling in hypertention. Mol. Cell. Biochem. 1996;157:163–170. doi: 10.1007/BF00227895. [DOI] [PubMed] [Google Scholar]
- ARRIBAS S., MARIN J., PONTE A., BALFAGON G., SALAICES M. Norepinephrine-induced relaxations in rat aorta mediated by endothelial beta adrenoceptors. Impairment by ageing and hypertension. J. Pharmacol. Exp. Ther. 1994;270:520–527. [PubMed] [Google Scholar]
- BAKER J.G., HALL I.P., HILL S.J. Agonist actions of ‘β-blockers' provide evidence for two agonist activation sites or conformations of the human β1-adrenoceptor. Mol. Pharmacol. 2003;63:1312–1321. doi: 10.1124/mol.63.6.1312. [DOI] [PubMed] [Google Scholar]
- BOONEN H.C., DAEMEN M.J., EERDMANS P.H., FAZZI G.E., VAN KLEEF E.M., SCHIFFERS P.M., DE MEY J.G. Mesenteric small artery changes after vasoconstrictor infusion in young rats. J. Cardiovasc. Pharmacol. 1993;22:388–395. doi: 10.1097/00005344-199309000-00007. [DOI] [PubMed] [Google Scholar]
- BORKOWSKI K.R., GROS R., SCHNEIDER H. Vascular beta-adrenoceptor-mediated responses in hypertension and ageing in rats. J. Auton. Pharmacol. 1992;12:389–401. doi: 10.1111/j.1474-8673.1992.tb00387.x. [DOI] [PubMed] [Google Scholar]
- BRAHMADEVARA N., SHAW A.M., MACDONALD A. Evidence against beta 3-adrenoceptors or low affinity state of beta 1-adrenoceptors mediating relaxation in rat isolated aorta. Br. J. Pharmacol. 2003;138:99–106. doi: 10.1038/sj.bjp.0705017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAWLEY L., SHAW A.M., MACDONALD A. β1–β2 and atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Br. J. Pharmacol. 2000a;129:637–644. doi: 10.1038/sj.bjp.0703091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAWLEY L., SHAW A.M., MACDONALD A. Role of endothelium/nitric oxide in atypical β-adrenoceptor-mediated relaxation in rat isolated aorta. Eur. J. Pharmacol. 2000b;398:285–296. doi: 10.1016/s0014-2999(00)00319-8. [DOI] [PubMed] [Google Scholar]
- CANDELORE M.R., DENG L., TOTA L., GUAN X.M., AMEND A., LIU Y., NEWBOLD R., CASCIERI M.A., WEBER A.E. Potent and selective human beta(3)-adrenergic receptor antagonists. J. Pharmacol. Exp. Ther. 1999;290:649–655. [PubMed] [Google Scholar]
- CARVALHO M.H., SCIVOLETTO R., FORTES Z.B., NIGRO D., CORDELLINI S. Reactivity of aorta and mesenteric microvessels to drugs in spontaneously hypertensive rats: role of the endothelium. J. Hypertens. 1987;5:377–382. doi: 10.1097/00004872-198706000-00019. [DOI] [PubMed] [Google Scholar]
- De CHAMPLAIN J. Do most antihypertensive agents have a sympatholytic action. Curr. Hypertens. Rep. 2001;3:305–313. doi: 10.1007/s11906-001-0093-8. [DOI] [PubMed] [Google Scholar]
- DE GROOT A.A., MATHY M.J., VAN ZWIETEN P.A., PETERS S.L. Involvement of the beta3 adrenoceptor in nebivolol-induced vasorelaxation in the rat aorta. J. Cardiovasc. Pharmacol. 2003;42:232–236. doi: 10.1097/00005344-200308000-00012. [DOI] [PubMed] [Google Scholar]
- FAHIM M., HUSSAIN T., MUSTAFA S.J. Relaxation of rat aorta by adenosine in diabetes with and without hypertension: role of endothelium. Eur. J. Pharmacol. 2001;412:51–59. doi: 10.1016/s0014-2999(00)00869-4. [DOI] [PubMed] [Google Scholar]
- FUJIMOTO S., DOHI Y., AOKI K., ASANO M., MATSUDA T. Diminished beta-adrenoceptor-mediated relaxation of arteries from spontaneously hypertensive rats before and during development of hypertension. Eur. J. Pharmacol. 1987;136:179–187. doi: 10.1016/0014-2999(87)90710-2. [DOI] [PubMed] [Google Scholar]
- GOSGNACH W., BOIXEL C., NEVO N., POIRAUD T., MICHEL J.B. Nebivolol induces calcium-independent signaling in endothelial cells by a possible β-adrenergic pathway. J. Cardiovasc. Pharmacol. 2001;38:191–199. doi: 10.1097/00005344-200108000-00004. [DOI] [PubMed] [Google Scholar]
- GRANNEMAN J.G. The putative β4-adrenergic receptor is a novel state of the β1-adrenergic receptor. Am. J. Physiol. 2001;43:E199–E202. doi: 10.1152/ajpendo.2001.280.2.E199. [DOI] [PubMed] [Google Scholar]
- GROS R., CHORASYCZEWSKI J., MEEK M.D., BENOVIC J.L., FERGUSON S.S., FELDMAN R.D. G-protein-coupled receptor kinase activity in hypertension: increased vascular and lymphocyte G-protein receptor kinase-2 protein expression. Hypertension. 2000;35:38–42. doi: 10.1161/01.hyp.35.1.38. [DOI] [PubMed] [Google Scholar]
- GUIMARAES S., MOURA D. Vascular adrenoceptors: an update. Pharmacol. Rev. 2001;53:319–356. [PubMed] [Google Scholar]
- HOM G.J., FORREST M.J., BACH T.J., BRADY E., CANDELORE M.R., CASCIERI M.A., FLETCHER D.J., FISHER M.H., ILIFF S.A., MATHVINK R., METZGER J., PECORE V., SAPERSTEIN R., SHIH T., WEBER A.E., WYVRATT M., ZAFIAN P., MacINTYRE D.E. Beta(3)-adrenoceptor agonist-induced increases in lipolysis, metabolic rate, facial flushing, and reflex tachycardia in anesthetized rhesus monkeys. J. Pharmacol. Exp. Ther. 2001;297:299–307. [PubMed] [Google Scholar]
- HOURANI S.M., BOON K., FOOKS H.M., PRENTICE D.J. Role of cyclic nucleotides in vasodilations of the rat thoracic aorta induced by adenosine analogues. Br. J. Pharmacol. 2001;133:833–840. doi: 10.1038/sj.bjp.0704140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWASE M., ICHIKAWA K., TASHIRO K., IINO K., SHINOHARA N., IBAYASHI S., YOSHINARI M., FUJISHIMA M. Effects of monosodium glutamate-induced obesity in spontaneously hypertensive rats vs Wistar Kyoto rats: serum leptin and blood flow to brown adipose tissue. Hypertens. Res. 2000;23:503–510. doi: 10.1291/hypres.23.503. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., ENGELHARDT S., HEIN L., MOLENAAR P., LOHSE M. Abolition of (−)-CGP 121777-evoked cardiostimulation in double β1/β2-adrenoceptor knockout mice. Obligatory role of β1-adrenoceptors for putative β4-adrenoceptor pharmacology. Naunyn-Schmiedeberg's. Arch. Pharmacol. 2001;363:87–93. doi: 10.1007/s002100000336. [DOI] [PubMed] [Google Scholar]
- KAUMANN A.J., LYNHAM J.A. Stimulation of cyclic AMP-dependent protein kinase in rat atria by (–)-CGP 12177 through an atypical beta adrenoceptor. Br. J. Pharmacol. 1997;120:1187–1189. doi: 10.1038/sj.bjp.0701053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KERR S., BROSNAN M.J., McINTYRE M., REID J.L., DOMINICZAK A.F., HAMILTON C.A. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- KOIKE K., HORINOUCHI T., TAKAYANAGI I. Signal transduction pathway involved in beta 3-adrenoceptor-mediated relaxation in guinea pig taenia caecum. Jpn. J. Pharmacol. 1995;68:41–46. doi: 10.1254/jjp.68.41. [DOI] [PubMed] [Google Scholar]
- KOMPA A.R., SUMMERS R.J. Desensitization and resensitization of β1- and putative β4-adrenoceptor-mediated response occur in parallel in a rat model of cardiac failure. Br. J. Pharmacol. 1999;128:1399–1406. doi: 10.1038/sj.bjp.0702920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONISHI M., SU C. Role of endothelium in dilator response of spontaneously hypertensive rat arteries. Hypertension. 1983;5:881–886. doi: 10.1161/01.hyp.5.6.881. [DOI] [PubMed] [Google Scholar]
- KONKAR A.A., ZHU Z., GRANNEMAN J.G. Aryloxypropranolamine and catecholamine ligand interactions with the β1-adrenergic receptors: evidence for interaction with distinct conformations of β1-adrenergic receptors. J. Pharmacol. Exp. Ther. 2000;294:923–932. [PubMed] [Google Scholar]
- MACDONALD A., MCLEAN M., MACAULAY L., SHAW A.M. Effects of propranolol and L- NAME on beta-adrenoceptor-mediated relaxation in rat carotid artery. J. Auton. Pharmacol. 1999;19:145–149. doi: 10.1046/j.1365-2680.1999.00128.x. [DOI] [PubMed] [Google Scholar]
- ORIOWO M.A. Atypical beta-adrenoceptors in the rat isolated common carotid artery. Br. J. Pharmacol. 1994;113:699–702. doi: 10.1111/j.1476-5381.1994.tb17049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAK M.D., FISHMAN P.H. Anomalous behavior of CGP 12177A on beta1-adrenergic receptors. J. Recept. Signal. Transduct. Res. 1996;16:1–23. doi: 10.3109/10799899609039938. [DOI] [PubMed] [Google Scholar]
- RAUTUREAU Y., TOUMANIANTZ G., SERPILLON S., JOURDON P., TROCHU J.N., GAUTHIER C. Beta 3-adrenoceptor in rat aorta: molecular and biochemical characterization and signalling pathway. Br. J. Pharmacol. 2002;137:153–161. doi: 10.1038/sj.bjp.0704867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SATAKE N., SHIBATA M., SHIBATA S. The inhibitory effects of iberiotoxin and 4-aminopyridine on the relaxation induced by beta 1- and beta 2-adrenoceptor activation in rat aortic rings. Br. J. Pharmacol. 1996;119:505–510. doi: 10.1111/j.1476-5381.1996.tb15700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAFIEI M., MAHMOUDIAN M. Atypical β-adrenoceptors of rat thoracic aorta. Gen. Pharmacol. 1999;32:557–562. doi: 10.1016/s0306-3623(98)00283-3. [DOI] [PubMed] [Google Scholar]
- STROSBERG A.D. Structure and function of the β3-adrenergic receptor. Ann. Rev. Pharmacol. Toxicol. 1997;37:421–450. doi: 10.1146/annurev.pharmtox.37.1.421. [DOI] [PubMed] [Google Scholar]
- TAVERNIER G., GALITZKY J., BOUSQUET-MELOU A., MONTRASTRUC J.L., BERLAN M. The positive chronotropic effect induced by BRL 37344 and CGP 12177, two beta-3 adrenergic agonists, does not involve cardiac beta adrenoceptors but baroreflex mechanisms. J. Pharmacol. Exp. Ther. 1992;263:1083–1090. [PubMed] [Google Scholar]
- TODA N. Vasodilating β-adrenoceptor blockers as cardiovascular therapeutics. Pharmacol. Ther. 2003;100:215–234. doi: 10.1016/j.pharmthera.2003.09.001. [DOI] [PubMed] [Google Scholar]
- TROCHU J.N., LEBLAIS V., RAUTUREAU Y., BEVERELLI F., LE MAREC H., BERDEAUX A., GAUTHIER C. Beta 3-adrenoceptor stimulation induces vasorelaxation mediated essentially by endothelium-derived nitric oxide in rat thoracic aorta. Br. J. Pharmacol. 1999;128:69–76. doi: 10.1038/sj.bjp.0702797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WERSTIUK E.S., LEE R.M. Vascular beta-adrenoceptor function in hypertension and in ageing. Can. J. Physiol. Pharmacol. 2000;78:433–452. [PubMed] [Google Scholar]