Abstract
ATP is an important vasoactive mediator, which acts via two receptor classes: P2X and P2Y. Activation of P2X receptors has traditionally been associated with the well-characterised vasoconstrictor properties of ATP.
In the current study, we have shown that the P2X1 & 3 receptor ligand, α, β methylene ATP, induces vasodilation of rat isolated mesenteric arteries and that P2X1 receptors are abundantly expressed in the endothelium of these vessels.
Second-order rat mesenteric arteries were mounted in myographs and vasomotor responses recorded. Both ATP and α, β methylene ATP induced a constriction followed by a vasodilation. The dilator effects of either ATP or α, β methylene ATP were slower in onset than those induced by acetylcholine. By contrast, the traditional vasodilator P2Y ligand, ADP, induced vasodilation without contraction.
Vasodilation induced by α, β methylene ATP was endothelial dependent, but was not affected by treatment of the vessels with l-NAME plus indomethacin alone. Dilation was, however, partially inhibited by the combination of apamin plus charybdotoxin and blocked by treating vessels with all four drugs.
Using confocal microscopy, P2X1 receptor immunoreactivity was localised to the endothelial, smooth muscle and adventitial layers of mesenteric vessels. P2X1 protein migrated as a primary band at around 50–60 kDa in vascular tissue.
These results show for the first time that P2X1 receptors are expressed on the endothelium and that a selective ligand of this receptor results in vasoconstriction followed by vasodilation. These observations have important implications for our understanding of the role of purines in biological responses.
Keywords: ATP; α, β methylene ATP; P2X1 receptor; purinergic; nitric oxide; endothelial derived hyperpolarising factor (EDHF)
Introduction
ATP is an important mediator in the cardiovascular system. It is released by platelets, vascular and cardiac cells in response to common receptor-mediated ligands (Yang et al., 1994), physical forces and after ischemia–reperfusion (Burnstock, 1999). Once released, ATP then acts on specific receptors or is rapidly metabolised by a group of ecto-enzymes (Zimmermann, 2000) to products including ADP, AMP and adenosine. ATP and its metabolites are potent mediators of vasomotor tone in most vascular beds (Kunapuli & Daniel, 1998); however, the mechanism by which it acts is yet to be clarified.
ATP and other purines activate purinergic (P) receptors to cause their effects in blood vessels. The vasodilator actions of ATP are mediated by the activation of P2Y receptors located on the endothelium and are caused by the release of endothelial derived relaxing factors such as nitric oxide (NO) and prostacyclin (Ralevic & Burnstock, 1991; Boeynaems et al., 2000) or endothelium derived hyperpolarising factor (EDHF) (Stanford et al., 2001; Malmsjo et al., 2002).
P2X receptors are ligand-gated ion channels (Ralevic & Burnstock, 1988). Arterial P2X receptors are most commonly described as smooth muscle receptors (Vulchanova et al., 1996). However, some studies show that P2X receptors are expressed on the endothelium (Hansen et al., 1999; Yamamoto et al., 2000; Glass et al., 2002b; Ray et al., 2002), but this observation is controversial. The vasoconstrictor role of P2X receptors on vascular smooth muscle is well studied, although, in contrast, the potential role of P2X receptors on endothelial cells is not known. Activation of any receptor on endothelial cells, which leads to elevation of intracellular calcium, would almost certainly release endothelial derived vasoactive mediators, including NO, prostacyclin or EDHF. It has been suggested that P2X receptor activation induces vasodilation of the intact mesenteric arterial bed of the rat (Ralevic, 2002); however, this phenomenon was described as endothelium independent. Definitive studies of endothelial dependence of any ligand are not possible in the intact vascular bed because removal of the endothelial layer is technically difficult.
Thus, in the current report, we have studied isolated segments of mesenteric arteries mounted in wire myographs, where the endothelium can be removed easily, to definitively elucidate the role of the endothelium in the vasodilator effects of the putative P2X ligand α, β methylene ATP (Burnstock & Kennedy, 1985; North, 2002). Secondly, we have used immunofluorescence labelling and laser-scanning confocal microscopy to demonstrate the presence of P2X1 receptors on the luminal surface of the endothelium lining these vessels. Some of this work has been published in abstract form (Harrington & Mitchell, 2003).
Methods
Male Wistar rats (200±15.4 g) were killed by lethal exposure to CO2, followed by cervical dislocation. The rats were maintained and killed in accordance with the European Community guidelines for the use of experimental animals.
The entire mesenteric bed was removed using ligatures, and placed into physiological salt solution (PSS; composition in mM) containing NaCl 119, KCl 4.7, CaCl2 2.5, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.027 and glucose 5.5. The mesentery was pinned flat on a dissecting dish containing PSS, to allow second-order arteries to be cleaned of fat and connective tissue; these arteries were stored in fresh PSS solution at room temperature (RT) until use. In this study, the second-order arteries had a mean internal diameter of 247 μm.
Isometric myograph recordings
Using tungsten wire, segments of 2 mm length of artery were mounted in a four-channel Mulvany-Halpern myograph (Model 610M, Danish Myo Technology, Aarhus, Denmark). The vessels were equilibrated to 37°C and the solution bubbled with 95% O2 and 5% CO2 for 30 min. The tension of the vessel was normalised to a tension equivalent to that generated at 90% of the diameter of the vessel at 100 mmHg, using standard procedures as described previously (Mulvany & Halpern, 1977). Changes in arterial tone were recorded via a PowerLab/800 recording unit (ADI instruments Pty Ltd, Australia), and analysed using Chart 4.0 acquisition system (ADI instruments) on a Macintosh personal computer.
In order to assess the viability of the vessels, they were challenged three times with high potassium solution (KPSS; composition in mM: KCl 123.7, CaCl2 2.5, MgSO4 1.17, NaHCO3 25, KH2PO4 1.18, EDTA 0.027 and glucose 5.5). Tissues were then pre-contracted with 10−5 M methoxamine, and once a plateau was reached 10−5 M acetylcholine was added to the organ bath. Vessels that relaxed >80% in response to acetylcholine were considered to have an intact endothelium. Occasionally vessels failed this test and were disregarded.
Drug treatments
Vessels were contracted to approximately 80% of the response obtained with high potassium solution using methoxamine (10−5 M), and the effects of single additions of either ATP (10−4 M), or α, β methylene ATP (10−6–10−4 M) were determined. At the end of each experiment, the ability of the tissue to dilate maximally was determined by the addition of sodium nitroprusside (SNP 10−5 M). Since α, β methylene ATP induced a constrictor response prior to vasodilation, concentration–response curves were constructed using single concentrations on individual tissues, and were therefore not cumulative. A concentration–response curve to ADP was also determined cumulatively.
In some experiments, the NO synthase inhibitor, L-NG-nitro-L-arginine (L-NAME; 10−3 M), the cyclooxygenase inhibitor indomethacin (10−5 M), or apamin (5 × 10−7 M) plus charybdotoxin (10−7 M), which together inhibit EDHF responses, were added. All inhibitors were added 30 min prior to the addition of methoxamine. Finally, some of the protocols were repeated in the present of depolarising concentrations of K+ (123 mM).
In some experiments, the endothelial layer lining the lumen of the vessels was removed physically using a human hair. Complete endothelial removal was documented by an absence in the ability of acetylcholine to induce relaxation of vessels contracted with methoxamine.
Confocal imaging
Mesenteric arteries were mounted and stretched to 100 mmHg in the isometric myograph, and fixed with the addition of 4% formaldehyde solution in PSS for 30 min at RT. Fixed vessels were dissected longitudinally with fine spring scissors and stored for up to a week in PBS containing 0.1% v v−1 Triton X-100 (TPBS) at 4°C.
In order to stain for P2X1 receptors, fixed vessels were incubated overnight with P2X1 goat polyclonal primary antibody (1 : 50) in TPBS 5% BSA at RT. Peptide neutralisation of the primary antibody was performed using commercial peptide used in its generation (antigen-blocking peptide). For these experiments, a ratio for antibody : peptide of 1 : 20 (μg) was used, as suggested by the supplier. In the respective protocols, antibody and peptide or antibody alone was incubated at 37°C for 2 h and then added to the fixed arteries overnight at 4°C. Vessels were washed in TPBS 3 × 20 min prior to incubation with rabbit anti-goat secondary antibody conjugated to Texas Red (1 : 100 in TPBS 5% BSA) for 4 h. In order to define the nuclei, the fixed arteries were incubated in 2 × 10−5 M DAPI for 10 min, and then washed 3 × 20 min at RT. Vessels were mounted onto slides with aqueous fluorescent mounting medium (DAKO) and set with nail varnish, prior to images being obtained by oil emersion confocal microscopy (magnification × 400). Images of the arteries were taken through the Z-axis, using both the DAPI staining to identify the different morphologies of the cell nuclei through the vessel, their direction in relation to each cell type layer and their proximity to the basal membrane.
Western blotting
Antibody specificity was determined by Western blotting analysis using standard procedures. Briefly, tissues were taken from a 250 g male Sprague–Dawley rat, dissected from connective tissue and snap frozen in liquid nitrogen, and stored at −80°C. Tissues were homogenised glass on glass in × 10(v) of lysis buffer (10 nM HEPES pH 7.5; 1 mM MgCl2; 40 mM KCl; 5% glycerol; 3% NP-40; 1 mM PMSF). The samples were centrifuged at 21,000 r.p.m. for 10 min at 4°C, and the supernatants removed and stored in fresh ice-cold eppendorfs, and kept at −20°C. The protein content was determined by Bradford assay, and the samples diluted 1 : 1 (v/v−1) with loading buffer, and boiled for 10 min, followed by cooling on ice for 2 min. In all, 83–90 μg of each sample was loaded onto 10% SDS–PAGE gels. Perfect markers were run in parallel for accurate size determination of the proteins. Proteins were transferred to a Hybond nitrocellulose membrane, and were blocked (blocking medium: PBS containing 5% non-fat milk and 0.1% Tween 20) at RT for 1 h. Peptide neutralisation was performed by incubating P2X1 primary antibody with or without blocking peptide (1 : 20) for 2 h at 37°C. The Western blots were incubated with primary antibodies for 16 h at 4°C, and washed 3 × 20 min in PBS Tween. The blots were incubated with secondary antibody (rabbit anti-goat horseradish peroxidase (HRP) conjugated) and 0.5 μl S-protein conjugate (for detection of the perfect marker) for 1 h at RT. Following 3 × 20 min wash in PBS Tween, antibody-positive bands were visualised using ECL chemiluminescence on Hyperfilm ECL.
Data and statistical analysis
Contractile or relaxant responses were calculated as a percentage of methoxamine-induced tone. Data are given as the mean±s.e.m. for experiments (one animal per experiment). Data were analysed statistically using Student's t-test or one-way analysis of variance, followed by a Dunnett's multiple comparison test using Prism 4.0 as appropriate. A P-value of less than 0.05 was considered statistically significant.
Drugs and antibodies
All drugs were purchased from Sigma Chemical Co., Gillingham, Dorset, U.K. Drugs were prepared each day, except for α, β, methylene ATP, charybdotoxin and apamin, which were prepared in high-concentration ‘stock' solutions and were stored at −20°C until used. All drugs were dissolved in aqueous solutions except for indomethacin, which was dissolved in dimethyl sulphoxide (DMSO). The final concentration of DMSO in the bath was 0.1%, which had no effect on the tissue.
Both antibodies, P2X1 and anti-goat secondary and blocking peptide, were purchased from Santa Cruz (U.S.A.). The P2X1 antibody was raised against a peptide mapping within an internal region of P2X1 of human origin. All Western blot reagents were from Amersham (U.K.), except for Perfect markers which were from Novogen (U.K.).
Results
P2X receptor ligands induce vasodilation of methoxamine-contracted mesenteric vessels
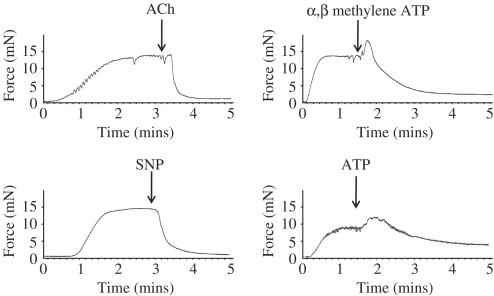
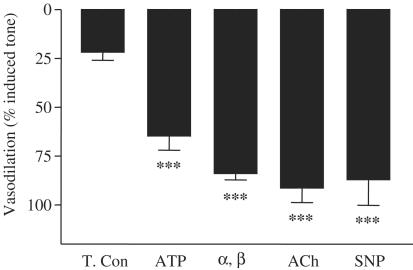
Profound relaxation of methoxamine-contracted vessels was induced by 10−5 M acetylcholine, 10−4 M ATP, 10−4 M α, β methylene ATP or 10−5 M SNP (Figures 1 and 2). In contrast to acetylcholine or SNP, both ATP or α, β methylene ATP induced contractile responses prior to vasodilation (Figure 1). Interestingly, the vasodilator response induced by either ATP or α, β methylene ATP was slower in onset than that induced by acetylcholine.
Figure 1.
Representative traces of vasodilator responses of rat mesenteric arteries induced by 10−4 M ATP, 10−4 M α, β methylene ATP, 10−5 M acetylcholine or 10−5 M SNP. Vessels were first contracted with 10−5 M methoxamine.
Figure 2.
Histogram depicting vasodilation of rat mesenteric arteries induced by 10−4 M ATP, 10−4 M α, β methylene ATP (α, β), 10−5 M acetylcholine (ACh) or 10−5 M SNP. Vessels were first contracted using methoxamine (10−5 M). The decline in baseline tone, which occurs over time without addition of additional drugs, is depicted in the column for ‘time control' (T.Con). Results are the mean±the s.e.m. (n=4). Statistical differences were calculated using one-way ANOVA followed by Dunnett's multiple comparison. Differences were considered statistical significant where P was found to be <0.05 and where ***P<0.001 is shown.
Characterisation of P2X-mediated response
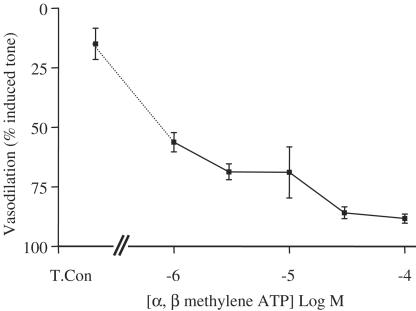
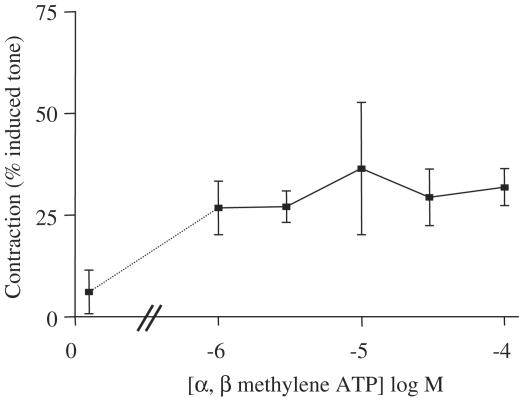
The vasodilator actions of α, β methylene ATP were concentration dependent. α, β methylene ATP had an apparent EC50 of approximately 10−6 M (Figure 3). By contrast, the contractile actions of α, β methylene ATP were not concentration related in the range used in this study (Figure 4).
Figure 3.
Concentration–response curve illustrating the vasodilator effects of α, β methylene ATP. Isolated mesenteric vessels were contracted with methoxamine. The data shown are the mean±the s.e.m. for n=3–4 experiments. In these experiments, a single concentration was administered to each tissue, cumulative response curves were not possible because of desensitisation of the drug.
Figure 4.
Effects of α, β methylene ATP on contractile responses of mesenteric vessels precontracted with methoxamine. The concentrations shown are selected from those depicted in Figure 3 where concentration-dependent dilation is seen. The data shown are the mean±the s.e.m. for n=3–4 experiments. Again, a single concentration of α, β methylene ATP was administered to each tissue, since cumulative response curves were not possible due to desensitisation effect of the drug.
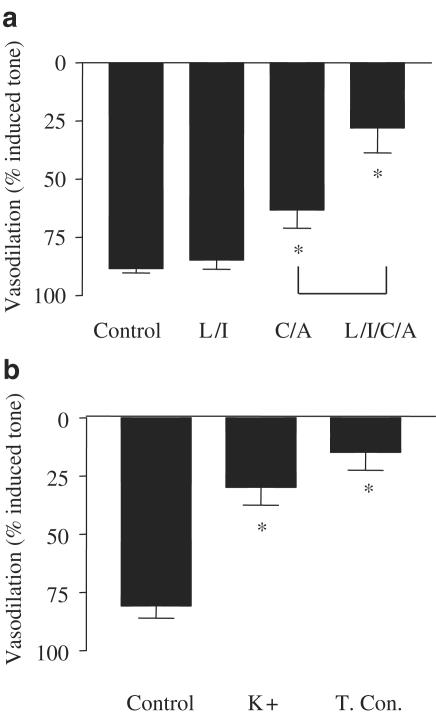
The vasodilator actions of α, β methylene ATP (10−4 M) were not inhibited by the combination of L-NAME (10−4 M) plus indomethacin (10−5 M). However, dilator responses were significantly inhibited by either ‘high' K+ (124 mM) or the combination of charybdotoxin plus apamin, and abolished by the combination of L-NAME, indomethacin, charybdotoxin and apamin (Figure 5).
Figure 5.
Characterisation of the dilator function of α, β methylene ATP. Panel a shows the effects of the combination of L-NAME (10−4 M) plus indomethacin (10−5 M; L+I), apamin (10−6 M) plus charybdotoxin (10−7 M; A+C), L+I plus A+C (L/I/A/C). Panel b shows the effects of KCl (1.2410−2 M) on the vasodilator actions of α, β methylene ATP (10−4 M) on methoxamine precontracted arteries. Data are shown as the mean±s.e.m. for n=4 experiments, in each case the control and drug-treated columns are ‘paired' data. Statistical significance was established using one-way ANOVA, followed by Bonferroni's multiple comparison test. Statistically significant differences between drug treatments and control responses are represented as follows: *P<0.05. Difference between responses in the presence of A+C versus A+C+L+I are illustrated by ‘f<0.05.'
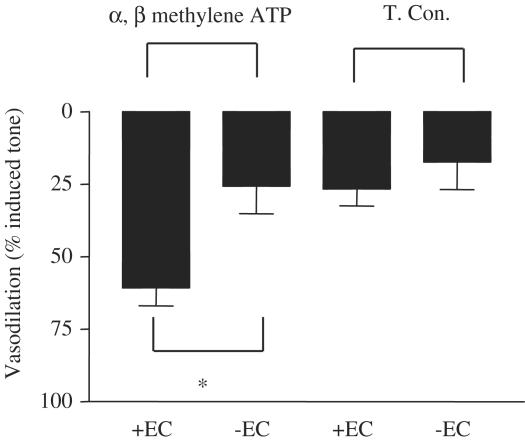
The dilator actions of α, β methylene ATP were abolished when mesenteric vessels were denuded of endothelium (Figures 6, 7 and 8). The control arteries, which were not stimulated after pre-contraction with methoxamine, lost tone over the period of these experiments, by approximately 25%. Such basal drift is an unfortunate, but normal, phenomenon in arteries contracted in this manner for a long period of time.
Figure 6.
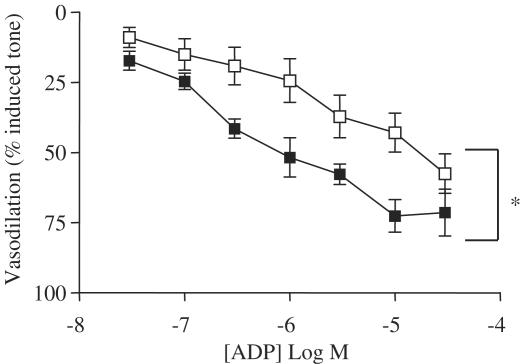
Effects of ADP on mesenteric vessels. The figure shows the dilator effects of ADP in methoxamine-contracted mesenteric vessels (filled squares). Responses obtained in the presence of L-NAME (10−4 M) plus indomethacin (10−5 M) are depicted by the open square symbols. Data are shown as the mean±s.e.m. for n=4–6 experiments. Statistical differences were calculated using two-way ANOVA and a P-value of <0.05 taken significant and is illustrated by *.
Figure 7.
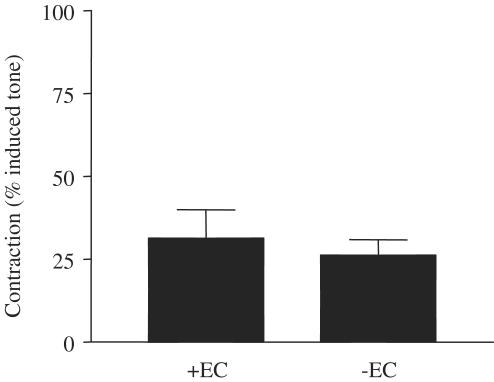
Role of the endothelium in the vasodilator actions of α, β methylene ATP. The figure shows responses of vessels prepared either with (+EC) or without (−EC) an intact endothelium to α, β methylene ATP (10−4 M). Data are mean±s.e.m. for n=3 experiments. Statistical significance was established by Student's t-test, where a P-value of <0.05 was taken as significant and is illustrated by *.
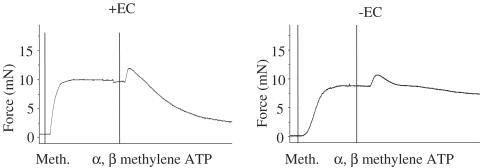
Figure 8.
Representative recording of the effect of removal of endothelial cells (±EC) on α, β methylene ATP-induced vasodilation. Meth, methoxamine (10−5 M).
In contrast, the constrictor actions of α, β methylene ATP were unaffected by endothelial removal (Figures 8 and 9).
Figure 9.
Effect of removal of endothelial cells (±EC) on α, β methylene ATP-induced contraction of methoxamine precontracted mesenteric arteries. Data are shown as the mean±s.e.m. for n=3 experiments.
Characterisation of P2Y-mediated response
The classical P2Y vasodilator ligand, ADP, also induced concentration-dependent dilator responses. Consistent with the literature, in the case of ADP, drug could be added in a cumulative fashion (Figure 6). The vasodilator actions of ADP were inhibited by the combination of L-NAME plus indomethacin.
Immunohistochemical detection of P2X1 receptor
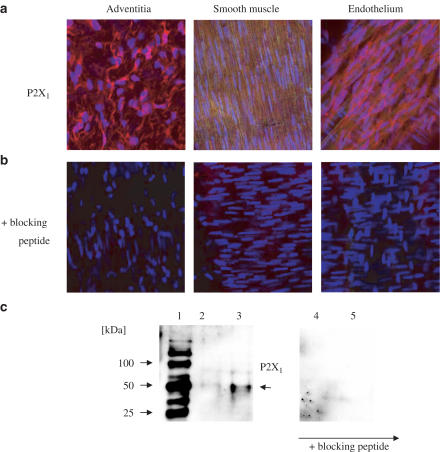
P2X1 receptor like-immunoreactivity was localised in all three layers of the artery, namely the endothelial, smooth muscle and adventitial regions of the vessel, where nerve-like structures could clearly be identified as positive (shown by red staining in Figure 10). No evidence for P2X1 immunoreactivity was found in the basement membrane (not shown). The primary antibody used recognised the two reported forms of P2X1 (migrating at approximately 50–60 and 80–90 kDa). No other bands were detected (Figure 10). When primary anti-P2X1 antibody was pre-incubated with excess antigen-blocking peptide, immunoreactive staining in the whole tissue or in the Western blot was lost (Figure 10).
Figure 10.
Expression of P2X1-like immunoreactivity in vascular tissue. Immunohistochemical visualisation of P2X1 receptors in rat mesenteric arteries. The image was captured using confocal microscopy, using the Z-axis to focus on the three layers of cells of mesenteric artery: pictures show the adventitial, smooth muscle and endothelial cell layers. Experiments were performed with primary anti-P2X1 antibody alone (a) or following pre-incubation with excess amounts of specific antigen-blocking peptide (b) (see Methods). Red colour (Texas Red) indicates positive staining for P2X1, while blue (DAPI) defines the nucleus and green represents autofluorescence. Magnification was × 400. Western blot analysis for P2X1 receptors of crude tissue extract from vascular tissue (c). Two separate blots are shown. The blot on the left-hand side of the figure was primed with anit-P2X1 antibody, while the primary antibody used to prime the blot on the right-hand side of the figure was pre-blocked with excess antigen-blocking peptide (see Methods). Lane 1 contains molecular weight markers, lanes 2 and 4 contain tissue extract from mesenteric arteries (83 μg) and lanes 3 and 5 contain tissue extract from aorta (90 μg).
Discussion
P2X receptors have been firmly linked to vasoconstrictor function. Where a dilator action of P2X receptors have been investigated, this phenomenon was attributed to a functional rebound effect of contraction and suggested to be endothelium independent. However, we show here that the P2X receptor ligand, α, β methylene ATP, induces vasodilation, which is endothelium dependent. Furthermore, we show that the vasodilator actions of α, β methylene ATP are not directly linked to its constrictor function as suggested by others (Ralevic, 2002). Finally, we demonstrate the clear presence of P2X1-like immunoreactivity on the surface of the endothelium in mesenteric arteries.
We have previously shown that ATP induces three phases of vascular response in the perfused intact mesenteric bed of the rat (Stanford & Mitchell, 1998). ATP initially induces a transient dilation mediated by the co-release of NO and prostacyclin, which is followed by a constrictor response and finally a sustained dilation, which is slow in onset and mediated by EDHF (Stanford et al., 2001). Interestingly, there is a clear demarcation of vascular response associated with concentration range of ATP used. Thus, at lower concentrations, there is no constrictor response. At doses of 10−7 mol or above, ATP induced vasoconstriction, followed by the two phases on dilation (Stanford & Mitchell, 1998). Thus, the constrictor and second-phase dilation induced by ATP coincide in the same dose range. This observation clearly demonstrates that a common receptor may be involved in both the constrictor and the second-phase dilator response induced by ATP.
From our previous work with ATP in the perfused mesenteric bed of the rat, it was suggested that P2X receptor activation could result in vasodilation. In the current study, we have indeed established this to be the case. In fact, α, β methylene ATP induces vasoconstriction, followed by a profound and sustained dilation, similar in nature to the second-phase dilator response induced by ATP in the intact arterial mesenteric bed (Stanford & Mitchell, 1998). We found this response to be inhibited by high potassium and by the combination of apamin plus charybdotoxin, and was absent in tissues where the endothelium had been removed. This pharmacological profile of results is consistent with EDHF being a key mediator responsible for the P2X induced vasodilation. Interestingly we found that whilst the combination of L-NAME plus indomethacin had no effect on the dilator actions of α, β methylene ATP when give alone, this combination of drugs ‘synergised' with apamin plus charybdotoxin to inhibit vasodilation. We cannot comment at this time why this observation occurred; however, the entire dilator actions of α, β methylene ATP were abolished in the presence of depolarising concentrations of K+. These observations are consistent with those we have made previously using ATP, and with the notion that α, β methylene ATP induces vasodilation by the release of EDHF.
α, β methylene ATP is a selective ligand for both P2X1 and P2X3 subunits (Virginio et al., 1998; Surprenant et al., 2000; North, 2002). However, we provide evidence to suggest that P2X1 seems the most likely receptor responsible for the responses we observed. P2X1 immunoreactivity is highly localised to the endothelium, as shown in Figure 10 and by Hansen et al. (1999). The biochemical properties of the P2X1-like immunoreactivity we report here is consistent with published data using rat tissue. Specifically, we found that P2X1 migrated as a principle protein of approximately 50–60 kDa, which is consistent with observations in rat bladder (65 kDa; Yunaev et al., 2000) or rat thyroid (70 kDa; Glass et al., 2002a, 2002b; Glass & Burnstock, 2001). In addition, in blood vessels (our study) or thyroid P2X1 antibodies also recognise a heavier form of the receptor migrating at 80–90 kDa (this study) or 140 kDa (thyroid; Glass & Burnstock, 2001). The heavier form of P2X1 has been explained by glycosylation of the protein, which would, of course, result in a variable molecular weight depending upon tissue and cellular conditions. Interestingly, Lewis & Evans (2000) failed to detect P2X1 on the endothelium of vessels used in their study. The explanation for the apparent conflict in data between their paper and ours may be related to the use of conventional sectioning techniques where transverse sections are cut and labelled with FITC-conjugated secondary antibodies. This may have made the differentiating between the strong autofluorescence caused by the elastic lamina, taking into account the proximity between the endothelial cells and the basal membrane rather difficult.
It should be noted that, while the overwhelming numbers of publications in the literature described α, β methylene ATP as a specific P2X ligand (Simon et al., 1995), there are some isolated reports suggesting that α, β methylene ATP can act weakly on P2Y11 receptors, and unlike other more traditional P2Y receptors the potential role of P2Y11 in vasomotor responses has not been fully explored. It remains technically possible therefore, that some of the constrictor or dilator actions of α, β methylene ATP are mediated via activation of P2Y11 receptors. ADP, which activates classical vasodilator P2Y receptors, like α, β methylene ATP, induced vasodilator responses in vessels used in this study. However, two key differences were noted between the dilator actions of α, β methylene ATP and ADP. Firstly, ADP induced dilation without constriction. Secondly, the dilator actions of ADP, unlike those of α, β methylene ATP, were partially mediated by the co-release of NO and/or prostacyclin.
It has been widely noted that the desensitisation of P2X1 & 3 receptors by α, β methylene ATP occurs rapidly. In our experiments, we found that the constrictor effects of α, β methylene ATP were relatively transient, and could reflect the time course of receptor desensitisation. However, the dilator actions of α, β methylene ATP were more long lasting. This phenomenon may well be due to amplification by the release of longer-acting dilator mediators associated with EDHF.
To evaluate a possible nonspecific effect of the α, β methylene ATP-induced vasodilation, high K+ solution was added to the organ bath without washing out the ligand (unpublished observations). The arteries were still able to contract, suggesting that the effect of P2X receptor desensitisation and consequent vasodilation was not due to an unspecific effect of α, β methylene ATP.
Thus, in summary, we have shown, for the first time, that the P2X1 receptor ligand α, β methylene ATP is an endothelium-dependent vasodilator of rat isolated mesenteric vessels and that EDHF is involved in this response and that P2X1 receptors are expressed on the endothelium of the vessels used in our study. This work illustrates a novel bifunctional role for P2X receptors, causing constriction when activated on smooth muscle and dilation when activated on the endothelium. This would be comparable to the variable functions of P2Y receptors, which mediate relaxation when located on the endothelium and contraction when situated on the smooth muscle cells (Ralevic & Burnstock, 1998).
Abbreviations
- DMSO
dimethyl sulphoxide
- EDHF
endothelial derived hyperpolarising factor
- KPSS
high potassium physiological salt solution
- L-NAME
L-NG-nitro-L-arginine
- PSS
physiological salt solution
- SNP
sodium nitroprusside
References
- BOEYNAEMS J.M., COMMUNI D., SAVI P., HERBERT J.M. P2Y receptors: in the middle of the road. Trends Pharmacol. Sci. 2000;21:1–3. doi: 10.1016/s0165-6147(99)01415-7. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J. Anat. 1999;194 Part 3:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., KENNEDY C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen. Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- GLASS R., BURNSTOCK G. Immunohistochemical identification of cells expressing ATP-gated cation channels (P2X receptors) in the adult rat thyroid. J. Anat. 2001;198:569–579. doi: 10.1046/j.1469-7580.2001.19850569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASS R., LOESCH A., BODIN P., BURNSTOCK G. P2X4 and P2X6 receptors associate with VE-cadherin in human endothelial cells. Cell Mol. Life Sci. 2002a;59:870–881. doi: 10.1007/s00018-002-8474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GLASS R., TOWNSEND-NICHOLSON A., BURNSTOCK G. P2 receptors in the thymus: expression of P2X and P2Y receptors in adult rats, an immunohistochemical and in situ hybridisation study. Cell Tissue Res. 2002b;300:295–306. doi: 10.1007/s004410000206. [DOI] [PubMed] [Google Scholar]
- HANSEN M.A., DUTTON J.L., BALCAR V.J., BARDEN J.A., BENNETT M.R. P2X (purinergic) receptor distributions in rat blood vessels. J. Auton. Nerv. Syst. 1999;75:147–155. doi: 10.1016/s0165-1838(98)00189-1. [DOI] [PubMed] [Google Scholar]
- HARRINGTON L.S., MITCHELL J.A.Endothelium dependant relaxation induced by P2X receptor activation. pA2 online 2003. pA2 online, 1 (abst. 026P)
- KUNAPULI S.P., DANIEL J.L. P2 receptor subtypes in the cardiovascular system. Biochem. J. 1998;336 Part 3:513–523. doi: 10.1042/bj3360513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS C.J., EVANS R.J. Lack of run-down of smooth muscle P2X receptor currents recorded with the amphotericin permeabilized patch technique, physiological and pharmacological characterization of the properties of mesenteric artery P2X receptor ion channels. Br. J. Pharmacol. 2000;131:1659–1666. doi: 10.1038/sj.bjp.0703744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MALMSJO M., CHU Z.M., CROFT K., ERLINGE D., EDVINSSON L., BEILIN L.J. P2Y receptor-induced EDHF vasodilatation is of primary importance for the regulation of perfusion pressure in the peripheral circulation of the rat. Acta Physiol. Scand. 2002;174:301–309. doi: 10.1046/j.1365-201x.2002.00956.x. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., HALPERN W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ. Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- NORTH R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- RALEVIC V. The involvement of smooth muscle P2X receptors in the prolonged vasorelaxation response to purine nucleotides in the rat mesenteric arterial bed. Br. J. Pharmacol. 2002;135:1988–1994. doi: 10.1038/sj.bjp.0704663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Actions mediated by P2-purinoceptorsubtypes in the isolated perfused mesenteric bed of the rat. Br. J. Pharmacol. 1988;95:637–645. doi: 10.1111/j.1476-5381.1988.tb11686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Effects of purines and pyrimidines on the rat mesenteric arterial bed. Circ. Res. 1991;69:1583–1590. doi: 10.1161/01.res.69.6.1583. [DOI] [PubMed] [Google Scholar]
- RALEVIC V., BURNSTOCK G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- RAY F.R., HUANG W., SLATER M., BARDEN J.A. Purinergic receptor distribution in endothelial cells in blood vessels: a basis for selection of coronary artery grafts. Atherosclerosis. 2002;162:55–61. doi: 10.1016/s0021-9150(01)00681-5. [DOI] [PubMed] [Google Scholar]
- SIMON J., WEBB T.E., KING B.F., BURNSTOCK G., BARNARD E.A. Characterisation of a recombinant P2Y purinoceptor. Eur. J. Pharmacol. 1995;291:281–289. doi: 10.1016/0922-4106(95)90068-3. [DOI] [PubMed] [Google Scholar]
- STANFORD S., MITCHELL J.A. ATP-induced vasodilatation in the rat isolated mesenteric bed exhibits two apparent phases. Br. J. Pharmacol. 1998;125:94. [Google Scholar]
- STANFORD S.J., GITLIN J.M., MITCHELL J.A. Identification of two distinct vasodilator pathways activated by ATP in the mesenteric bed of the rat. Br. J. Pharmacol. 2001;133:825–832. doi: 10.1038/sj.bjp.0704139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SURPRENANT A., SCHNEIDER D.A., WILSON H.L., GALLIGAN J.J., NORTH R.A. Functional properties of heteromeric P2X(1/5) receptors expressed in HEK cells and excitatory junction potentials in guinea-pig submucosal arterioles. J. Auton. Nerv. Syst. 2000;81:249–263. doi: 10.1016/s0165-1838(00)00123-5. [DOI] [PubMed] [Google Scholar]
- VIRGINIO C., ROBERTSON G., SURPRENANT A., NORTH R.A. Trinitrophenyl-substituted nucleotides are potent antagonists selective for P2X1, P2X3, and heteromeric P2X2/3 receptors. Mol. Pharmacol. 1998;53:969–973. [PubMed] [Google Scholar]
- VULCHANOVA L., ARVIDSSON U., RIEDL M., WANG J., BUELL G., SURPRENANT A., NORTH R.A., ELDE R. Differential distribution of two ATP-gated channels (P2X receptors) determined by immunocytochemistry. Proc. Natl. Acad. Sci. U.S.A. 1996;93:8063–8067. doi: 10.1073/pnas.93.15.8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAMAMOTO K., KORENAGA R., KAMIYA A., QI Z., SOKABE M., ANDO J. P2X(4) receptors mediate ATP-induced calcium influx in human vascular endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H285–H292. doi: 10.1152/ajpheart.2000.279.1.H285. [DOI] [PubMed] [Google Scholar]
- YANG S., CHEEK D.J., WESTFALL D.P., BUXTON I.L. Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ. Res. 1994;74:401–407. doi: 10.1161/01.res.74.3.401. [DOI] [PubMed] [Google Scholar]
- YUNAEV M.A., BARDEN J.A., BENNETT M.R. Changes in the distribution of different subtypes of P2X receptor clusters on smooth muscle cells in relation to nerve varicosities in cthe pregnant rat urinary bladder. J. Neurocytol. 2000;29:99–108. doi: 10.1023/a:1007152428481. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN H. Extracellular metabolism of ATP and other nucleotides. Naunyn-Schmiedeberg's Arch.Pharmacol. 2000;362:299–309. doi: 10.1007/s002100000309. [DOI] [PubMed] [Google Scholar]