Abstract
Upon various stimuli, cells metabolize sphingomyelin from the cellular plasma membrane to form sphingosylphosphorylcholine (SPC) or ceramide. The latter can be further metabolized to sphingosine and then sphingosine-1-phosphate (S1P). Apart from local formation, S1P and SPC are major constituents of blood plasma.
All four sphingomyelin metabolites (SMM) can act upon intracellular targets, and at least S1P and probably also SPC can additionally act upon G-protein-coupled receptors. While the molecular identity of the SPC receptors remains unclear, several subtypes of S1P receptors have been cloned and their distribution in cardiovascular tissues is described.
In the heart SMM can alter intracellular Ca2+ release, particularly via the ryanodine receptor, and conductance of various ion channels in the plasma membrane, particularly IK(Ach). While the various SMM differ somewhat in their effects, the above alterations of ion homeostasis result in reduced cardiac function in most cases, and ceramide and/or sphingosine may be the mediators of the negative inotropic effects of tumour necrosis factor.
In the vasculature, SMM mainly act as acute vasoconstrictors in most vessels, but ceramide can be a vasodilator. SMM-induced vasoconstriction involves mobilization of Ca2+ from intracellular stores, influx of extracellular Ca2+ via L-type channels and activation of a rho-kinase.
Extended exposure to SMM, particularly S1P, promotes several stages of the angiogenic process like endothelial cell activation, migration, proliferation, tube formation and vascular maturation.
We propose that SMM are an important class of endogenous modulators of cardiovascular function.
Keywords: Sphingosine-1-phosphate, sphingosylphosphorylcholine, sphingosine, ceramide, heart, vasoconstriction, angiogenesis
Introduction
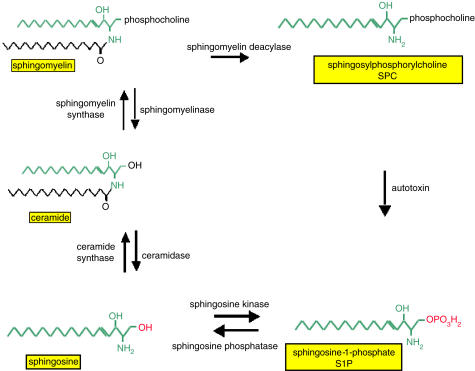
The biologically active sphingomyelin metabolites (SMM) sphingosine-1-phosphate (S1P), sphingosylphosphorylcholine (SPC), sphingosine and ceramide are important lipid mediators which are generated upon cell activation from membrane phospholipids as part of the sphingomyelin cycle (Hannun et al., 2001; Kolesnick, 2002). Various stressful stimuli activate sphingomyelinase, which catalyses the hydrolysis of sphingomyelin to ceramide, which can then be metabolized by ceramidase to sphingosine (Figure 1). Phosphorylation of sphingosine by sphingosine kinase, a ubiquitous enzyme in the cytosol and endoplasmic reticulum, finally yields S1P (Figure 1). Momentarily, less is known about the formation and degradation of SPC under physiological conditions (Meyer zu Heringdorf et al., 2002). Under pathological conditions, SPC was shown to be formed from sphingomyelin by the enzyme sphingomyelin deacylase (Higuchi et al., 2000). Interestingly, it has been shown recently that SPC can be hydrolysed into S1P by autotoxin, an ectoenzyme that belongs to the nucleotide and pyrophosphatase and phosphodiesterase family (Clair et al., 2003).
Figure 1.
Sphingomyelin metabolism.
S1P is present in plasma and serum in high nanomolar concentrations. Recently, SPC was also detected in plasma and serum at concentrations similar to that of S1P (Liliom et al., 2001). Both, S1P and SPC in plasma and serum are associated with lipoproteins, especially the high-density lipoprotein (HDL) (Okajima, 2002). The major source for S1P in plasma and serum are platelets. High concentrations of S1P are stored in platelets as these lack the enzyme S1P lyase, an enzyme that catalyses the degradation of S1P to hexadecanal and ethanolamine-phosphate (Yatomi et al., 1997). The stored S1P is released upon activation of the platelets.
Many of the actions of S1P are shared by the structurally related SPC, and these two lipids may thus share common mechanisms and sites of action (Meyer zu Heringdorf et al., 2002). With regard to cellular signalling, S1P is most thoroughly investigated, whereas the exact targets of SPC remain largely unknown. S1P has unique properties as it can act both as an intracellular messenger and also as an intercellular messenger (Pyne & Pyne, 2000; Payne et al., 2002; Spiegel & Milstien, 2003). To date, the intracellular effects of S1P are not that well understood, as the putative intracellular target(s) that mediates these effects has not been identified yet. However, evidence is increasing that some of the S1P effects cannot be explained by intracellular generated S1P acting extracellularly (Spiegel & Milstien, 2003).
The extracellular effects of S1P are much easier to investigate as these are mediated via its interaction with G-protein-coupled receptors. To date, a family of five structurally related receptors with high affinity for S1P has been identified. Recently, Uhlenbrock et al. (2002) reported the identification of three additional members of the S1P receptor family, gpr3, gpr6 and gpr12. Very recently, S1P was also found to be a low-affinity agonist for the GPR63 receptor (Niedernberg et al., 2003), and the existence of even more S1P receptors cannot be excluded, particularly since not all S1P effects which apparently are receptor-mediated can be explained based on the presently identified receptor subtypes. It has been speculated that specific G-protein-coupled receptors also exist for SPC (Xu et al., 2000), but recent evidence could not confirm the cloned protein to be an SPC receptor (Bektas et al., 2003). However, SPC can act as a low-affinity agonist at S1P receptors (Pyne & Pyne, 2000; Spiegel & Milstien, 2000). Ceramide, one of the precursors of S1P, is also an important signalling molecule in pathways that are mediated by diverse stress stimuli. The mechanisms how ceramide functions in the stress signal cascade still need to be elucidated.
Studying the functional effects and signal transduction mechanisms of SMM is rather complex as the various SMM can be converted into each other. The functional effect found for a particular SMM may, in some cases, not be the effect of the actual compound itself, but of one of its metabolites. For example, the functional effects found for sphingosine are most likely explained by its conversion either to ceramide or to S1P. Thus, it has been reported that the administered sphingosine is rapidly converted into S1P by sphingosine kinase upon intracoronary administration in rat hearts perfused in Langendorff mode (Cavalli et al., 2002).
Signalling characteristics of SMM and its receptors
The first S1P receptor identified was the S1P1 receptor. The receptor was initially named endothelium differentiation gene (Edg) 1 receptor, because the corresponding gene is abundantly expressed in differentiating endothelial cells (Hla & Maciag, 1990b). According to the official IUPHAR nomenclature, Edg receptors were renamed as S1P receptors (Chun et al., 2002). Rapidly after cloning of the S1P1 receptor the S1P2 (formerly Edg-5), S1P3 (formerly Edg-3), S1P4 (formerly Edg-6) and S1P5 (formerly Edg-8) receptors have been cloned (Pyne & Pyne, 2000).
The S1P receptors, being part of the G-protein-coupled receptor family, transduce their signals via heterotrimeric G-proteins. As S1P receptor subtypes show subtype-specific G-protein coupling, the set of intracellular signalling pathways activated by a particular S1P receptor is unique (for a review, see Takuwa et al., 2001). The signal transduction mechanism of S1P receptors will only be discussed here shortly, as this has been the subject of several recent comprehensive reviews (Takuwa et al., 2001; English et al., 2002; Spiegel & Milstien, 2002).
The S1P1 receptor is coupled exclusively via Gi/o, leading to an inhibition of adenylyl cyclase and activation of Ras and extracellular signal-regulated kinases (ERKs) of the mitogen-activated protein kinase (MAPK) family. Stimulation of S1P1 receptors also results in activation of phospholipase C, probably through the βγ subunit of Gi/o. Like the S1P1 receptor, the S1P2 and the S1P3 receptors are also coupled to Ras/ERK via Gi/o. These receptors are, like the S1P1 receptor, also found to activate phospholipase C and Ca2+ mobilization. In contrast to the S1P1 receptors, phospholipase C activation and Ca2+ mobilization by these receptors is via both, pertussis toxin (PTX)-insensitive and PTX-sensitive G-proteins, most likely Gq/11 and Gi/o, respectively. In addition, the S1P2 and S1P3 receptors are coupled to stimulation of Rho via G12/13. For the S1P2 receptor, the most efficient coupling is via Gq/11 to phospholipase C and via G12/13 to Rho. The S1P2 receptor is also coupled to p38 MAPK and c-Jun N-terminal protein kinase via a PTX-insensitive G-protein (Gonda et al., 1999).
Signal transduction pathways of the S1P4 and S1P5 receptor are not that well defined yet. S1P4 is reported to couple to Gi/o, leading to stimulation of phospholipase C and ERK in a PTX-sensitive manner. The S1P5 receptor was shown to couple to Gi and G12 and to inhibit cAMP production in a PTX-sensitive manner. This receptor, however, did not activate ERK, but rather inhibited the serum-induced ERK activation. The mechanism by which S1P5 mediates this effect is not understood yet (Takuwa, 2002).
The signal transduction pathways mediated by the recently identified gpr3, gpr6, gpr12 and GPR63 have not been extensively studied to date. In transiently transfected HEK-293 cells, the gpr3, gpr6 and gpr12 receptors were found to constitutively activate adenylyl cyclase (Uhlenbrock et al., 2002). As PTX affected the basal and S1P-induced cAMP levels in HEK-293 cells transiently transfected with the gpr3, gpr6 or gpr12 receptor, the authors concluded that these receptors also constitutively couple to Gi. The authors, however, also showed that untransfected HEK-293 cells already express a wide variety of other S1P receptor subtypes. Thus, the effect of PTX found could also originate from uncoupling of background S1P receptors as all S1P receptor subtypes are known to couple to Gi-proteins. This explanation could be excluded in case PTX would not affect the basal cAMP production in mock-transfected cells, but unfortunately this has not been tested within the above study. Strong evidence for constitutive Gi coupling of the gpr3, gpr6 and gpr12 receptors, for example, by reconstitution experiments in Sf9 cells, is thus still lacking.
S1P is a low-affinity agonist for the GPR63 receptor. The receptor can thus not really be classified as a S1P receptor, as the existence of another endogenous ligand with higher affinity cannot be excluded. The GPR63 receptor is shown to couple to Gq and Gi proteins (Niedernberg et al., 2003).
As described above, besides its low affinity for S1P receptors, the target for SPC is not known. It is speculated that SPC also acts via a G-protein-coupled receptor based upon findings, such as stereo-selectivity and PTX-sensitivity, of some of its effects, but the receptor mediating this has not been identified yet (Meyer zu Heringdorf et al., 2002). Although the signal transduction mechanisms for ceramide are also not that clear, it is known that this lipid does not act via G-protein-coupled receptors. A number of enzymes can be activated by ceramide, including both protein kinases, such as protein kinase C ζ and c-Jun N-terminal protein kinases, and protein phosphatases such as protein phosphatase 1 (Ruvolo, 2003). The mechanisms by which ceramide directly activates these enzymes still need to be elucidated.
Various studies in the heart or in cardiac myocytes have indicated that SMM can also regulate the activity of various ion channels, including L-type Ca2+ channels, K+ channels and Na+ channels. The effects of SMM on ion channels are rather complex and will be discussed in more detail in the section describing the cardiac effects of SMM.
Localization of S1P receptor subtypes in the cardiovascular system
To date the S1P receptor subtypes that are found to play a functional role in the cardiovascular system are the S1P1, S1P2 and S1P3 receptor. A summary of the expression of S1P receptor subtypes at the mRNA and protein level in various cell types of the cardiovascular system is presented in Table 1. Tissue expression analysis of S1P receptor subtypes in mice indicates that the heart and lung have the highest overall expression of S1P1, S1P2 and S1P3 mRNA transcripts (Zhang et al., 1999; Ishii et al., 2001). Expression of the S1P4 receptor is mainly limited to lymphoid and haematopoietic tissues as well as the lung (Graler et al., 1998), and transcripts for this receptor were not detected in the heart. Overall expression of the S1P5 receptor is also limited and is highest in the brain, particularly in the midbrain, hindbrain and spinal cord (Im et al., 2000).
Table 1.
Detection of S1P receptor subtypes in cardiovascular tissue at the mRNA and protein level
| Receptor | S1P1 | S1P2 | S1P3 | S1P4 | S1P5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | mRNA | Protein | |
| Ventricular cardiomyocytes | +++a, ++b | +++a | +a, ++b | +a | ++ab | ++a | −b | ++b | ||
| Septal cardiomyocytes | +++a | +++a | +a | +a | ++a | ++a | −b | ++b | ||
| Atrial cardiomyocytes | +++a, ++b,c | +++a | +a, ++b,c | +a | ++a,b,c | ++a | −b | ++b | ||
| Coronary artery endothelial cells | +++a | +++a | −a | −a | ||||||
| Coronary artery smooth muscle cells | −a | −a, +d | −a | −a | ++a | ++a | ||||
| Aortic endothelial cells | −a, +++e,f | −a,e, +f | −a, +++e | |||||||
| Aortic smooth muscle cells | −a,g, +h,i,k,l | −a, +d | +a, +++g,h,i | +a, ++d | +++a,h,i, ++g | +++a, ++d | −g,j | −g,j | ||
| Umbilical vein endothelial cells | +++f,g | −g, +f | ++f | −g | −g | |||||
+++: high expression; ++: intermediate expression; +: low expression; −: not detected.
Cardiac expression of S1P receptors has been studied in mice, rats and humans. The murine heart expresses transcripts for the S1P1, S1P2 and S1P3 but not the S1P4 and S1P5 receptor (Ishii et al., 2001). While S1P1, S1P2 and S1P3 receptor mRNA expression also dominates in rat heart (Nakajima et al., 2000; Liliom et al., 2001), RT–PCR analysis additionally detected the presence of transcripts for the S1P5 (but not S1P4) receptor in rat atrial and ventricular myocytes (Liliom et al., 2001). When assessing these potential species differences it should be noted that the experiments in the mouse heart were performed on total RNA isolated from cardiac tissue whereas those in rats were done with RNA specifically isolated from cardiomyocytes. If overall expression of the S1P5 receptor in the heart is low and restricted to certain areas, its signal might evade detection in whole tissue lysates. The localization of S1P1, S1P2 and S1P3 receptors within the human heart was analyzed using in situ hybridization and immunohistochemical techniques (Mazurais et al., 2002). While expression of S1P2 and S1P3 receptors was lower than that of S1P1 receptors, all three subtypes exhibited a similar distribution pattern. Thus, they were present in left and right ventricle, septum and atrium. Himmel et al. (2000) had also detected transcripts for these three subtypes of S1P receptors in human atrial cardiomyocytes.
The S1P1, S1P2 and S1P3 receptor are also expressed in human aorta as indicated by Northern and Western blot analysis (Mazurais et al., 2002). The S1P1 transcript is strongly and equally expressed in the aorta as in different compartments of the adult human heart. The S1P2 receptor is also equally expressed in the aorta and in the different regions of the human heart although the abundance of this transcript is generally lower than that of the S1P1 transcript. In line with these findings the S1P2 receptor protein was also equally present in the aorta and the human heart (Mazurais et al., 2002). The mRNA for the S1P3 receptor, in contrast, was more abundant in the aorta than in the human heart regions. In agreement with these results the protein expression of this receptor was also higher in the aorta then in the heart (Mazurais et al., 2002). RT–PCR detection studies in rat showed, besides the transcripts for the S1P1, S1P2 and S1P3 receptor, also transcripts for the S1P5 receptor (Salomone et al., 2003).
More precise studies on the localization of S1P receptors in aorta were carried out using immunohistochemical and other techniques in aortic smooth muscle and endothelial cells. The data on the expression profile of S1P receptors in aortic smooth muscle cells are not conclusive as some groups report the expression of S1P1 (Coussin et al., 2000; Liu et al., 2000; Kluk & Hla, 2001; Tamama et al., 2001), whereas others did not detect its expression in these cells (Mazurais et al., 2002; Ryu et al., 2002). The expression profile in a particular cell type might also reflect particular ages of the animals at the time of the smooth muscle cell isolation, as indicated by a study of Kluk & Hla (2001), who showed a higher S1P1 expression level in vascular smooth muscle cells isolated from rat pups than in adult medial smooth muscle cells.
In general, it can be concluded from all reports that the S1P2 receptor and the S1P3 receptor are the receptors that are expressed most abundantly in aortic vascular smooth muscle cells (Table 1). The S1P1 receptor is probably also expressed in these cells, but at much lower levels than the other two subtypes. In aortic endothelial cells the expression pattern of S1P receptors is completely different. In these cells, the S1P1 receptor is the most prominently expressed receptor (Wang et al., 1999; Kimura et al., 2000). Expression of the S1P2 receptor in these cells is generally reported to be much lower than that of the S1P1 receptor (Wang et al., 1999), and in some studies the receptor is even undetectable (Kimura et al., 2000; Mazurais et al., 2002). Aortic endothelial cells in addition also express low levels of S1P3 receptors (Wang et al., 1999; Kimura et al., 2000).
Interestingly, the expression pattern of S1P receptors in the aorta does not necessarily reflect the expression pattern found in other vessels since, in contrast to many other blood vessels, aorta does not contract in response to S1P (see below). The expression profile of the different S1P receptor subtypes may thus vary among different arteries. RT–PCR studies in rat, using RNA isolated from different arteries, however, did not indicate any difference in expression profile between different arteries (Salomone et al., 2003). Basilar, carotid, femoral, coronary arteries and aorta all contained mRNA for the S1P1, S1P2, S1P3 and S1P5 receptors (Salomone et al., 2003). In contrast to these results, no S1P2 receptor expression was detected in human cardiac vessels, although not as extensively studied as the aorta, whereas this receptor was highly expressed in aortic smooth muscle cells of different species (Coussin et al., 2000; Liu et al., 2000; Hobson et al., 2001; Kluk & Hla, 2001; Tamama et al., 2001). Human coronary smooth muscle cells were only shown to express S1P3 receptors (Mazurais et al., 2002). In line with the findings in aortic endothelial cells, cardiac endothelial cells also express high levels of the S1P1 receptor (Mazurais et al., 2002). Transcripts for the S1P2 and S1P3 receptor were not detected in these cells (Mazurais et al., 2002).
High expression levels of S1P1 receptors seem to be a general characteristic of endothelial cells. Besides aortic and cardiac endothelial cells, human umbilical vein endothelial cells also predominantly express the S1P1 receptor (Wang et al., 1999; Ryu et al., 2002). Expression of the S1P2 receptor in endothelial cells is generally low, if present at all. Interestingly, in smooth muscle cells the S1P expression profile seems to be just the opposite (see above).
As discussed earlier, a few years ago, three potential additional members of the S1P receptor family, gpr3, gpr6 and gpr12, have been identified (Uhlenbrock et al., 2002). Information about the precise distribution of these receptors in the cardiovascular system is lacking. However, it was shown by Uhlenbrock et al. (2003) using RT–PCR analysis that most primary human endothelial cells and most vascular smooth muscle cells are positive for gpr3, gpr6 and gpr12 transcripts.
Acute effects of sphingolipids in the cardiovascular system
Cardiac effects
SMM effects on cardiac function have been determined at the levels of cell signalling, isolated tissue function and in vivo. Not unexpectedly, ceramide, sphingosine, S1P and SPC have at least partly yielded different effects at each of these levels. Therefore, we will discuss the levels of cardiac SMM effects in this order with separate descriptions for each of the SMM.
Several studies have addressed SMM effects on cardiac function at the signal transduction level. Although inhibition of adenylyl cyclase is a prototypical signal transduction mechanism of SMM receptors (see above), such inhibition has not consistently been found in the heart. Sphingosine reduced basal and isoprenaline-stimulated cAMP levels in neonatal rat cardiomyocytes, but failed to inhibit forskolin-stimulated values (Benediktsdottir et al., 2002). Studies in membrane preparations from adult rat right atrium or left ventricle (Sugiyama et al., 2000a) or dog right atrium or right ventricle (Sugiyama et al., 2000b) did not detect inhibition of adenylyl cyclase by S1P. Although inhibition of adenylyl cyclase can be difficult to measure and hence false-negative results cannot be excluded in the absence of a positive control, the above data certainly do not support the idea that SMM primarily affect cardiac function by modulating intracellular cAMP levels.
Numerous studies have indicated that SMM can alter intracellular ion concentrations in the heart by regulating the activity of various ion channels in the membranes of the sarcoplasmic reticulum and/or in the cell membrane. However, the net effect may differ among the SMM. Thus, sphingosine was consistently found to reduce free intracellular Ca2+ concentrations in neonatal rat (McDonough et al., 1994), adult rat (McDonough et al., 1994; Amadou et al., 2002), guinea-pig (Sugishita et al., 1999) and feline cardiomyocytes (Oral et al., 1997). Moreover, sphingosine appears to be the mediator of tumour necrosis factor (TNF)-α-induced lowering of cardiac Ca2+ concentrations (Oral et al., 1997; Sugishita et al., 1999; Amadou et al., 2002). Other SMM have not been systematically studied in this regard, but it has been reported that the sphingosine precursor ceramide and the sphingosine metabolite S1P can increase free intracellular Ca2+ concentrations in cardiomyocytes from adult (Liu & Kennedy, 2003; Relling et al., 2003) and neonatal rats (Nakajima et al., 2000).
The observed SMM-induced alterations in free intracellular Ca2+ concentration could result from alterations in intracellular Ca2+ handling and/or from alterations in the influx of extracellular Ca2+. With regard to the former, the ryanodine receptor is an important mediator of Ca2+-induced Ca2+ release from the sarcoplasmic reticulum. Caffeine and doxorubicin enhance high-affinity [3H]ryanodine binding to isolated sarcoplasmic reticulum membranes from the canine heart and promote Ca2+ release from this organelle; presumably this involves ryanodine receptors. Both effects were concentration-dependently inhibited by sphingosine (Dettbarn et al., 1994). Similar observations were also made in sarcoplasmic reticulum from rabbit heart (Webster et al., 1994). These observations are well in line with the consistently reported lowering of cardiac Ca2+ concentration upon sphingosine administration. With regard to SPC effects on the ryanodine receptor, conflicting data have been reported. Thus, it was reported that SPC can strongly induce Ca2+ release from the sarcoplasmic reticulum of the canine heart via the ryanodine receptor, possibly due to an SPC-induced increase in the Ca2+-sensitivity of the ryanodine receptor (Betto et al., 1997). Interestingly, sphingosine was found to inhibit this SPC effect (Betto et al., 1997). On the other hand, studies with incorporation of rabbit sarcoplasmic reticulum membranes into lipid bilayers have found that SPC reduces the open probability of ryanodine receptor following cytoplasmic but not luminal application (Uehara et al., 1999; Yasukochi et al., 2003). Whether these are true species differences, remains to be determined. Moreover, it remains unclear how these alterations in ryanodine receptor activity relate to overall SPC-induced changes in free intracellular Ca2+ concentrations. Whether ceramide or S1P affects the cardiac ryanodine receptor has not yet been reported.
Other studies have looked at SMM effects upon ion channel activity in the plasma membrane. Sphingosine-induced inhibition of L-type Ca2+ channels have been reported in cardiomyocytes from neonatal rats (McDonough et al., 1994), adult rats (McDonough et al., 1994; Yasui & Palade, 1996) and cats (Friedrichs et al., 2002). The latter was accompanied by decreases of action potential duration and a lower action potential height (Friedrichs et al., 2002). A reduced L-type Ca2+ channel activity was also reported in rat cardiomyocytes for SPC (Yasui & Palade, 1996) and ceramide (Liu & Kennedy, 2003). In rabbit isolated sinoatrial node cells, S1P did not affect the basal activity of L-type Ca2+ channels but reversed the effects of the β-adrenergic agonist isoprenaline (Guo et al., 1999). Thus, it appears that all SMM exert some inhibitory effect upon cardiac L-type Ca2+ channels. Moreover, sphingosine, SPC and S1P were reported to inhibit rat cardiac Na+ channels (Yasui & Palade, 1996; MacDonell et al., 1998). Interestingly, the S1P effects on the latter channel were not PTX-sensitive (MacDonell et al., 1998).
Most studies have investigated SMM effects on cardiac K+ channels. They have consistently found that S1P activates IK(ACh) in guinea-pig atrial myocytes (Bünemann et al., 1995; 1996; van Koppen et al., 1996; Himmel et al., 2000; Liliom et al., 2001). A similar S1P-induced activation of IK(ACh) was seen in freshly isolated myocytes from mouse and human atrium (Himmel et al., 2000) and in rabbit isolated sinoatrial node cells (Guo et al., 1999). The S1P effects in the guinea-pig atrium were inhibited by pre-treatment with PTX (Bünemann et al., 1995) or with suramin (Himmel et al., 2000). SPC was found to mimic the S1P effects in guinea-pig atrial myocytes, and the two SMM caused cross-desensitization of each other (Bünemann et al., 1996; Liliom et al., 2001). Since these studies observed no cross-desensitization with acetylcholine acting on muscarinic receptors, it was proposed that S1P and SPC may act on the same receptor. It was further proposed that S1P and SPC account for the plasma-induced activation of IK(ACh) in guinea-pig atrial myocytes (Liliom et al., 2001). On the other hand, in contrast to S1P, SPC did not activate IK(ACh) in freshly isolated myocytes from mouse and human atrium (Himmel et al., 2000). In rabbit isolated sinoatrial node cells, S1P did not alter basal activity of the If channel but reversed the isoprenaline effects (Guo et al., 1999). In rat ventricular myocardium, S1P did not significantly affect the inward rectifier K+ channel (MacDonell et al., 1998).
Taken together, sphingosine inhibits both Ca2+ release from the sarcoplasmic reticulum via ryanodine receptor channels and Ca2+ influx via L-type channels, and hence lowers free intracellular Ca2+ concentrations; its effects upon Na+ and K+ channels have not been established (Table 2). S1P inhibits IK(ACh) and, perhaps, If K+ channels, Na+ channels and L-type Ca2+ channels, but its effect upon ryanodine receptors is unknown. SPC mimics S1P effects upon IK(ACh) in guinea-pigs (possibly even acting via the same receptor) but not in mice or humans; SPC possibly shares inhibitory S1P effects upon Na+ channels and L-type Ca2+ channels, but whether it stimulates or inhibits ryanodine receptor channels remains controversial. Too few data are available to determine ceramide effects on cardiac ion homeostasis. Based upon these actions, it would be expected that all SMM primarily inhibit frequency and force of myocardial contraction, albeit perhaps using somewhat different mechanisms.
Table 2.
Sphingolipid effect on cardiac ion homeostasis
| Ceramide | Sphingosine | S1P | SPC | |
|---|---|---|---|---|
| Ca2+ concentration | ↑ | ↓ | ↑ | ND |
| Ryanodine receptor | ND | ↓ | ↓ | ↓ |
| L-type Ca2+ channel | ↓ | ↓ | ↓ | ↓ |
| Na+ channel | ND | ND | ↓ | ↓ |
| IK(ACh) channel | ND | ND | ↑ | → a |
| If channel | ND | ND | ↓ | ND |
| Inward rectifier K+ channel | ND | ND | → | ND |
Arrows indicate increased, decreased or unchanged concentration or activity. Note that only sphingosine effect in general and S1P effects on IK(ACh) channels have been confirmed by multiple investigators and/or in multiple studies. ND: not determined.
No effect in mouse and human; but increase in guinea-pig. For details and references, see text.
SMM effects on cardiac rhythm have been determined using cultured cardiomyocytes, isolated sinoatrial node cells, isolated hearts and in vivo studies. These studies support the notion that SMM lower heart rate under most but not all conditions. Thus, experiments at the level of the isolated pacemaker have found S1P-induced increases of sinoatrial rate in dogs (Sugiyama et al., 2000b) but reductions of basal rate and reversal of the effects of the β-adrenergic agonist isoprenaline in rabbits (Guo et al., 1999). Sphingosine reduced the beating rate of neonatal rat isolated cardiomyocytes (Benediktsdottir et al., 2002) and of skinned myocytes from rabbit heart (Webster et al., 1994). SPC reduced the rate of isolated guinea-pig hearts (Liliom et al., 2001). The systemic administration of S1P reduced heart rate in rats (Sugiyama et al., 2000a; Benediktsdottir et al., 2002), but in some studies this effect was seen only at high doses (Friedrichs et al., 2002) or not at all (Bischoff et al., 2000b; 2001a). In one study in which S1P-induced reductions of heart rate had been observed, this was not affected by changes of atrio-ventricular or intra-ventricular conduction (Sugiyama et al., 2000a). Two recent studies in mice also described S1P-induced lowering of heart rate (Forrest et al., 2004; Sanna et al., 2004). Most interestingly, this effect was abolished in S1P3 receptor knockout mice (Sanna et al., 2004), and the ability of several S1P receptor agonists to lower heart rate in wild-type mice correlated better with their potency at S1P3 than at S1P1 receptors (Forrest et al., 2004). Taken together, it appears that SMM, particularly S1P, lower heart rate under most conditions, but these effects may not be of sufficient magnitude to be detectable in all cases, particularly in vivo.
The above signalling studies would also predict SMM to cause negative inotropic effects. This has been tested at the level of isolated cardiomyocytes, isolated myocardial strips, isolated hearts and in vivo. Sphingosine was shown to inhibit contractions of isolated cardiomyocytes from both cats (Oral et al., 1997) and guinea-pigs (Sugishita et al., 1999). In either case, sphingosine not only mimicked the negative inotropic effects of TNF-α but mediated them. Sphingosine also mimicked the negative inotropic effects of TNF-α in rat right ventricular trabeculae, and this occurred by impairing the economy of chemo-mechanical energy transduction (Hofmann et al., 2003). In line with these in vitro observations, sphingosine caused negative inotropy upon systemic administration in rats in vivo (Sugiyama et al., 2000b). Moderate negative inotropic effects have also been reported for S1P in isolated dog heart (Sugiyama et al., 2000b), but these were largely attributed to its prominent contractile effect on the coronary vessels. Studies on inotropic effects of ceramide are less consistent. While it was found that ceramide increased contraction of cardiomyocytes and hearts isolated from adult rats (Liu & Kennedy, 2003; Relling et al., 2003), another study detected negative inotropic effects of ceramide upon i.v. administration in rats (Friedrichs et al., 2002). Thus, the majority of studies support the notion that SMM, particularly sphingosine, will reduce cardiac contractility.
The negative chronotropic and inotropic effects of SMM make them a logical candidate to mediate impaired cardiac function under pathophysiological conditions, particularly in the context of hypoxia and ischaemia. It is well established that cardiac hypoxia and ischaemia are associated with release of TNF-α. Hypoxia and ischaemia also lead to the formation and release of ceramide, for example, in neonatal rat cardiomyocytes (Bielawska et al., 1997), and of sphingosine, for example, in adult rat isolated cardiomyocytes (Cavalli et al., 2002), in Langendorff-perfused rabbit heart (Cavalli et al., 2002) and in dog heart in vivo (Thielmann et al., 2002). This is further supported by the detection of hypoxia-induced activation of neutral sphingomyelinase in cardiac myocytes (Hernandez et al., 2000). Since a TNF receptor-blocking antibody prevented hypoxia-induced sphingosine release in adult rat cardiomyocytes, sphingosine formation occurs secondary to TNF receptor activation (Cavalli et al., 2002). Accordingly, the ceramidase inhibitor N-oleoylethanolamine prevented microembolization-induced sphingosine formation but not TNF release in dog heart (Thielmann et al., 2002). Hypoxia/ischaemia-induced ceramide and sphingosine formation appear to primarily have negative effects on cardiac function. Thus, ceramide and sphingosine can induce apoptosis in neonatal cardiomyocytes (Krown et al., 1996; Bielawska et al., 1997). Moreover, the increase in ceramide content upon ischaemia in myocytes in vitro and in adult heart in vivo are accompanied by apoptosis (Bielawska et al., 1997). Additional evidence for a deleterious role, particularly of sphingosine, comes from findings that the ceramidase inhibitor N-oleoylethanolamine prevented microembolization-induced contractile dysfunction in anaesthetized dogs (Thielmann et al., 2002) and also improved survival after acute myocardial infarction in rats (Friedrichs et al., 2002). Since cardiac tissue can rapidly and effectively convert sphingosine to S1P (Cavalli et al., 2002), it was important to determine whether these adverse effects are indeed mediated by ceramide and sphingosine or by its metabolite S1P. In studies with neonatal rat cardiomyocytes, S1P protected against hypoxia-induced cell death, whereas dimethylsphingosine, an inhibitor of sphingosine kinase, enhanced cell death; moreover, ganglioside GM-1, an activator of sphingosine kinase, prevented the adverse effects of dimethylsphingosine (Karliner et al., 2001). A similar protection against ischaemia-induced cardiac damage was found for S1P and the sphingosine kinase activator ganglioside GM-1 in mice (Jin et al., 2002). Interestingly, this protection was absent in the hearts of protein kinase C-ɛ knockout mice, indicating a mediator role of this kinase (Jin et al., 2002). Taken together, these data demonstrate that ceramide and sphingosine are formed during cardiac hypoxia/ischaemia, contribute to the myocardial damage under these conditions and mediate at least some of the adverse effects of TNF-α in this regard. In contrast, conversion of ceramide and sphingosine to S1P will counteract these effects and is cardioprotective. Therefore, we propose that manipulation of the relative balance between ceramide and sphingosine on the one hand and S1P on the other hand may be a possible target for the treatment of myocardial ischaemia.
Vascular effects
While cardiac SMM effects had originally been studied at the level of cell signalling and extended only later to that of isolated tissue function, studies on vascular SMM effects were pioneered by experiments on the contraction of isolated blood vessels. While the effects of ceramide on vascular tone remain controversial, S1P and the other SMM cause vasoconstriction in most blood vessels (for exceptions see below, for summary see Table 3). Rat mesenteric microvessels and intra-renal microvessels (inter-lobar arteries) were the first isolated vascular preparations in which S1P-induced vasoconstriction was reported (Bischoff et al., 2000a) (Figure 2); these effects were confirmed in subsequent studies by the same group (Bischoff et al., 2001a; Altmann et al., 2003d; Hedemann et al., 2004). S1P can also constrict many other rat blood vessels including basilar artery (Salomone et al., 2003), cerebral artery (Coussin et al., 2002; Salomone et al., 2003), and coronary arteries (Salomone et al., 2003), but apparently causes little if any contraction of aorta (Coussin et al., 2002), carotid and femoral arteries (Salomone et al., 2003). This pattern would suggest that S1P is more important for the contractile function of resistance than of conduit vessels. In line with this proposal, we have recently observed that agonist-induced force of contraction strongly decreases with vessel diameter for the α-adrenergic agonist noradrenaline, but only little if any for S1P (unpublished observations). In this regard, it appears to be interesting that maximum S1P-induced contraction was consistently only approximately half of maximum noradrenaline-induced, α1A-adrenoceptor-mediated effects in rat mesenteric and renal vessels. Nevertheless, S1P can elicit at least some additional vasoconstriction when added to maximally effective concentrations of an α-adrenergic agonist (Czyborra et al., 2002).
Table 3.
Sphingolipid effects on the tone of isolated blood vessels
| Ceramide | Sphingosine | S1P | SPC | |
|---|---|---|---|---|
| Aorta | ↓ | ND | → | ND |
| Carotid artery | ND | ND | → | ND |
| Femoral artery | ND | ND | → | ND |
| Mesenteric arteries | ↓ | ↑ | ↑a↓ | ↑ |
| Intra-renal arteries | ND | ↑ | ↑a | ↑ |
| Basilar artery | ND | ND | ↑a | ↑ |
| Cerebral artery | ND | ND | ↑a | ↑ |
| Coronary artery | ↑ | ↑ | ↑a | ↑↓ |
Arrows indicate increased, decreased or unchanged vascular tone. While several findings were shown for multiple species (rat, mouse, dog, cow, pig), not all effects have been tested in more than one species. ND: not determined.
Confirmed in vivo studies. For details and references, see text.
Figure 2.
Effects of diastereomers of SPC (upper panel) and effect of PTX in SPC-induced contraction (lower panel) of rat isolated mesenteric microvessels vasculature in vitro. Adapted from Bischoff et al. (2000a).
S1P-induced vasoconstriction was also found in rats in vivo following local or systemic injection, for example, for mesenteric and renal blood flow which were dose-dependently and transiently reduced in anaesthetized rats (Bischoff et al., 2000b; 2001a, 2001b; Wecking et al., 2001). Upon direct injection into the renal artery, the effects of S1P on renal blood flow were stronger than upon systemic administration (Bischoff et al., 2000b). Continuous infusions of S1P also lowered mesenteric and renal blood flow in anaesthetized rats (Bischoff et al., 2001b). Comparison of the time courses of S1P-induced blood flow reductions upon bolus injection vs continuous infusion indicates that both pharmacokinetic effects and tachyphylaxis may contribute to the transient nature of in vivo vasoconstriction by this agonist. S1P has also been shown to reduce rat cerebral blood flow upon intra-arterial infusion (Salomone et al., 2003), and S1P administration into cerebrospinal fluid was reported to cause a long-lasting reduction of the diameter of the basilar artery in dogs (Tosaka et al., 2001).
Additional studies have demonstrated that S1P is not the only SMM which can elicit vascular smooth muscle cell contraction and that SMM-induced vasoconstriction is not limited to rats. The initial study reporting S1P-induced contraction of rat mesenteric and intra-renal vessels found that SPC, sphingosine and glucopsychosine also induced a concentration-dependent contraction in both vessels (Bischoff et al., 2000a). SPC-induced contraction was also found in murine isolated mesenteric vessels (Hedemann et al., 2004), bovine isolated middle cerebral artery (Shirao et al., 2002), canine basilar artery (Tosaka et al., 2001) and porcine coronary artery smooth muscle cells (Todoroki-Ikeda et al., 2000). Sphingosine and sphingomyelinase were found to contract porcine coronary arteries (Murohara et al., 1996). One group of investigators described ceramide-induced contraction and inhibition of endothelium-dependent dilation of bovine isolated small coronary arteries (Li et al., 1999; Zhang et al., 2001), but ceramide was found to cause vasodilatation in all other preparations (see below). S1P-induced vasoconstriction was also found in dogs in situ following intra-coronary administration (Sugiyama et al., 2000b), in murine isolated mesenteric vessels (Hedemann et al., 2004) and in human coronary smooth muscle cells (Ohmori et al., 2003). Nevertheless, as compared to S1P, SPC, sphingosine and glucopsychosine caused much less if any alterations of mesenteric and renal blood flow upon in vivo injection (Bischoff et al., 2000b; 2001a) or infusion (Bischoff et al., 2001b; Wecking et al., 2001). Thus, several SMM, most importantly S1P and SPC, can contract vascular smooth muscle cells in various vascular beds of several species both in vitro and in vivo. In line with this addition of the enzyme, sphingomyelinase can also cause vasoconstriction (Murohara et al., 1996). Moreover, studies with forced expression of sphingosine kinase or a dominant-negative mutant thereof in hamster resistance arteries suggest that SMM may also play a crucial role in the development of basal tone and myogenic responses of blood vessels (Bolz et al., 2003a).
Nevertheless, most studies failed to detect any major alterations of blood pressure upon injection or infusion of S1P or other SMM (Bischoff et al., 2000b; 2001a, 2001b; Wecking et al., 2001; Forrest et al., 2004). This could be explained if many vascular beds were largely insensitive to SMM-induced vasoconstriction, but this is unlikely in light of the sensitivity of many vascular beds in vitro. A more likely explanation for the lack of blood pressure alteration would be concomitant cardio-depressant effects, that is, a reduced cardiac output. While these have not been determined by direct in vivo studies, many of the above in vitro studies make them likely to occur. Finally, the lack of blood pressure effect in anaesthetized animals could relate to experimental circumstances because SMM-induced hypertension was prominent in conscious rats and mice (Forrest et al., 2004).
Based upon the original debate whether SMM effects occur via intracellular sites and/or specific SMM receptors in the cell membrane, several investigators have studied the involvement of receptors and, more recently, specific SMM receptor subtypes in vasoconstriction. One characteristic of receptor- vs non-receptor-mediated SMM effects is stereo-selectivity of SPC responses (Meyer zu Heringdorf et al., 1997). In this regard, SPC-induced contraction of rat isolated mesenteric microvessels was stereo-selective with D-erythro-SPC being more potent than L-threo-SPC (Bischoff et al., 2000a) (Figure 2). Another characteristic of receptor-mediated SMM effects is sensitivity towards inhibition by PTX (Meyer zu Heringdorf et al., 1997). Pre-treatment with PTX markedly inhibited SMM-induced contraction of rat mesenteric microvessels in vitro (Bischoff et al., 2000a) (Figure 2) and of S1P-induced reductions of rat mesenteric and renal blood flow in vivo (Bischoff et al., 2000b; 2001b) (Figure 3). While S1P-induced contraction of rat cerebral arteries was largely resistant to PTX treatment (Salomone et al., 2003), this does not exclude receptor involvement.
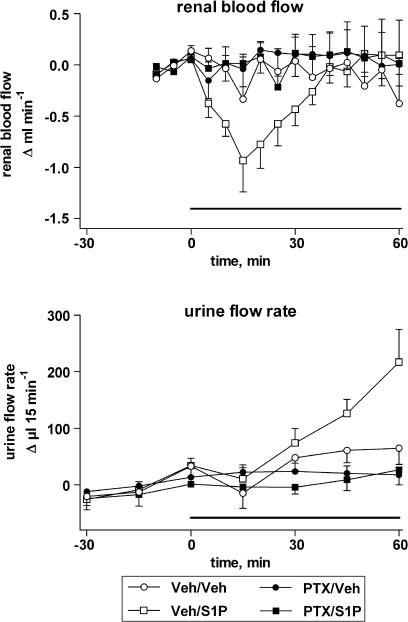
Figure 3.
S1P infusion reduces renal blood flow (upper panel) and increases urine flow rate (lower panel), effects of PTX. The solid line indicates the period of S1P infusion. Adapted from Bischoff et al. (2001b).
Due to the lack of widely available, specific SMM-receptor antagonists with good selectivity, the following approaches have been used to identify the subtypes involved in SMM-induced vasoconstriction: agonist order of potencies, the nonspecific but moderately subtype-selective antagonist suramin, antisense approaches and receptor knockout mice. The initial study reporting S1P-induced contraction of rat mesenteric and intra-renal vessels found that several SMM induced a concentration-dependent contraction with a rank order of potency of SPC>S1P>sphingosine⩾psychosine⩾glucopsychosine; in this regard, all five SMM acted in the micromolar range and the latter three were considerably less efficacious than SPC and S1P (Bischoff et al., 2000a). Two observations are noteworthy in this context: Firstly, in this as in most other studies, micromolar SMM concentrations were required to elicit vasoconstriction. This contrasts the nanomolar affinity of the SMM for their cloned receptors but fits reported plasma concentrations of S1P and SPC (see above). Secondly, in both rat preparations (Bischoff et al., 2000a) and in mouse vessels (Hedemann et al., 2004), S1P and SPC were active in a similar concentration range. While a similar situation had also been found for the activation of IK(ACh) in guinea-pig atria (see above), none of the cloned SMM receptors has similar affinity for both SMM (see above), indicating that they either act on a common site distinct from all known SMM receptors or via multiple concomitantly expressed receptors.
It had been reported that rat cerebral artery is much more responsive to S1P than aorta, and, based upon the finding that cerebral artery expresses considerably more S1P2 and S1P3 receptors but similar amounts of S1P1 receptors as aorta, it had been proposed that one of the former mediates contraction of this vessel (Coussin et al., 2002). A more detailed study regarding the receptor subtype(s) mediating S1P-induced contraction was presented for a closely related vessel, that is, rat basilar artery, using the antagonist suramin and antisense constructs (Salomone et al., 2003). Suramin has been proposed to be an antagonist at SMM receptors with some selectivity for the S1P3 relative to the S1P1 and S1P2 subtypes (Ancellin & Hla, 1999). In rat basilar artery, suramin produced some inhibition of S1P-induced contraction, and a similar degree of inhibition was observed with adenoviral transfer of S1P3 receptor antisense (Salomone et al., 2003). In contrast, S1P2 receptor antisense did not cause inhibition, and suramin did not inhibit responses to serotonin in that study. Moreover, the authors also reported antagonism of S1P-induced reductions of rat cerebral blood flow in vivo by suramin (Salomone et al., 2003). Based upon these data, it appears that S1P3 receptors contribute to contraction of rat basilar artery, but additional contributions of other subtypes cannot be excluded. In another study, suramin also caused inhibition of S1P-induced contraction of rat mesenteric microvessels; this inhibition was stronger than that of SPC-induced contraction in the same preparation and that caused by S1P in murine mesenteric vessels of similar size (Hedemann et al., 2004). In vivo studies have detected blood pressure elevations by SMM receptor agonists in wild-type but not in S1P3 receptor knockout mice (Forrest et al., 2004). While all of these findings point towards an involvement of S1P3 receptors, S1P-induced contraction of human coronary smooth muscle cells was inhibited by the S1P2-selective antagonist JTE-013 (Ohmori et al., 2003). Thus, considerable additional efforts will be required to establish which subtype(s) of SMM receptors is/are important for vasoconstriction.
Key mechanisms to elicit vascular smooth muscle contractions are an elevation of free intracellular Ca2+ concentrations, through mobilization from intracellular stores and/or through influx of extracellular Ca2+, and a sensitization of the contractile filaments towards ambient Ca2+ concentrations, frequently involving a rho-kinase. Both mechanisms have been implicated in SMM-induced vasoconstriction. S1P- and/or SPC-induced, PTX-sensitive Ca2+ elevations have directly been demonstrated in rat (Bischoff et al., 2000a; Coussin et al., 2002; 2003) and porcine vascular smooth muscle cells (Chin & Chueh, 1998; 2000; Nakao et al., 2002), but apparently were absent in bovine cerebral artery smooth muscle (Shirao et al., 2002). Moreover, forced overexpression of a sphingosine kinase in hamster resistance vessels elevated basal intracellular Ca2+ concentrations (Bolz et al., 2003a).
A role for a mobilization of Ca2+ from intracellular stores was indicated by several findings: Firstly, SPC can activate a phospholipase C in porcine vascular smooth muscle cells (Chin & Chueh, 1998). Secondly, 2-aminoethoxydiphenylborate, an inhibitor of inositol-trisphosphate receptors, reduced S1P-induced Ca2+mobilization in rat cerebral arteries (Coussin et al., 2003). Thirdly, pre-treatment with thapsigargin, an inhibitor of sarcoplasmic calcium-ATPase, ryanodine or the phospholipase C inhibitor, U 73,122 (but not its negative control, U 73,343) almost completely prevented S1P- and SPC-induced Ca2+ elevation in rat basilar artery (Coussin et al., 2002) and porcine aorta smooth muscle cells (Chin & Chueh, 1998), respectively. Finally, U 73,122 but not U 73,343 markedly inhibited SPC-induced contraction of rat mesenteric microvessels (Altmann et al., 2003d). In contrast, SK&F 96,365, an inhibitor of store-operated Ca2+-channels, or the phospholipase D inhibitor butan-1-ol relative to its negative control butan-2-ol did not affect SPC-induced contraction of mesenteric microvessels (Altmann et al., 2003d). These data demonstrate that mobilization from an intracellular store contributes to SMM-induced Ca2+ elevations in vascular smooth muscle cells; this appears to occur largely via inositol-trisphosphate- and perhaps also ryanodine-sensitive stores, whereas store-operated Ca2+-channels or phospholipase D do not appear to play a major role.
A contribution of mobilization from intracellular stores does not necessarily exclude additional contributions from the influx of extracellular Ca2+, but the available data are contradictory. Removal of extracellular Ca2+ and/or blockade of L-type Ca2+-channels was reported to have little if any effects on S1P- or SPC-induced Ca2+ elevation in vascular smooth muscle cells from rat cerebral artery (Coussin et al., 2002) or porcine aorta (Chin & Chueh, 1998), respectively. In contrast to the above, other investigators reported nifedipine to block at least part of the S1P-induced Ca2+ increase in rat cerebral artery (Coussin et al., 2003). Similarly, removal of extracellular Ca2+ markedly attenuated S1P- and SPC-induced contraction of rat mesenteric microvessels (Bischoff et al., 2000a; 2001a). L-type Ca2+-channel inhibitors significantly inhibited S1P-induced contraction of rat intra-renal microvessels (Bischoff et al., 2001a) and, to a lesser extent, SPC-induced contraction of mesenteric microvessels (Bischoff et al., 2000a). Nifedipine also dose-dependently attenuated S1P-induced reductions of renal blood flow in anaesthetized rats in vivo (Bischoff et al., 2001a). Direct electrophysiological proof of SMM-induced activation of L-type Ca2+-channels was obtained in smooth muscle cells from rat cerebral arteries, where it was found to occur secondary to inhibition of voltage-gated K+-channels (Coussin et al., 2003). Thus, the role of extracellular Ca2+ and specifically of L-type, dihydropyridine-sensitive Ca2+-channels may depend upon the blood vessel under investigation. In the only vessel where ceramide was reported to cause vasoconstriction, that is, bovine small coronary arteries, this was accompanied by an inhibition of the activity of Ca2+-activated K+-channels (Li et al., 1999).
Several protein kinases may be involved in coupling the above primary signals to smooth muscle contraction. Controversial results have been obtained with regard to protein kinase C. Thus, protein kinase C was reportedly not involved in SPC-induced Ca2+ sensitization in bovine cerebral artery (Shirao et al., 2002), but on the other hand found to mediate S1P-induced inhibition of voltage-gated K+-channels and hence activation of L-type Ca2+-channels in rat cerebral artery smooth muscle (Coussin et al., 2003). Sphingosine-induced contraction of porcine isolated coronary arteries was insensitive to the protein kinase C inhibitor staurosporine (Murohara et al., 1996). One study in rat mesenteric microvessels looked at the role of phosphatidyl-inositol-3′-kinase (PI3K) in SPC-induced vasoconstriction; it reported ambiguous results with the kinase inhibitor LY 294,002 and, to a smaller extent, its negative control LY 303,511 causing inhibition of the SPC effect, but wortmannin being ineffective as an inhibitor (Altmann et al., 2003d). Within the same study, the tyrosine kinase inhibitors genistein (relative to its negative control daidzein) and the src-type tyrosine kinase inhibitor PP2 (relative to its negative control PP3) also produced little inhibition (Altmann et al., 2003d). In contrast, the src-kinase inhibitors PP1 (but not PP3) and EPA inhibited S1P-induced Ca2+ elevation, fyn (but not c-src) translocation, rho-kinase translocation and contraction in porcine coronary artery (Nakao et al., 2002). Another group of studies has determined a possible role of ERKs in SMM-induced vasoconstriction. SPC- and S1P-induced activation of such kinases was described in cultured porcine aortic smooth muscle (Chin & Chueh, 1998) and rat aorta and cerebral artery (Coussin et al., 2002), respectively. Although the activation was of similar magnitude in the latter two, S1P induced contraction only in cerebral artery but not in aorta, calling into doubt the role of this kinase in smooth muscle contraction. Studies with pharmacological inhibitors have yielded equivocal results, since an inhibitor of ERK activation, PD 98,059, attenuated SPC-induced contraction of rat mesenteric microvessels, whereas a chemically distinct inhibitor, U 126, or its inactive analogue, U 124, did not share the effect (Altmann et al., 2003d). Thus, the overall data indicate an SMM-induced activation of such kinases, but make it doubtful that they are important for the control of vasoconstriction.
As stated above, vasoconstriction can not only be brought about by elevations of intracellular Ca2+ but also by a Ca2+ sensitization of the contractile filaments, which frequently involves a rho-kinase. Indeed, S1P and SPC were found to cause Ca2+ sensitization in hamster skeletal muscle microvessels (Bolz et al., 2003b) and in bovine cerebral artery (Shirao et al., 2002). Moreover, S1P was found to activate rho A in rat cerebral artery (Coussin et al., 2002). Accordingly, inhibition of the rho A/rho kinase pathway inhibited SMM-induced contraction of bovine cerebral artery (Shirao et al., 2002), dog basilar artery (Tosaka et al., 2001), rat cerebral artery (Coussin et al., 2002), rat mesenteric microvessels (Altmann et al., 2003d), hamster skeletal muscle microvessels (Bolz et al., 2003b), porcine coronary artery (Nakao et al., 2002) and human coronary smooth muscle cells (Ohmori et al., 2003). Such inhibition was achieved using the rho-kinase inhibitors Y 27,632 (Tosaka et al., 2001; Coussin et al., 2002; Nakao et al., 2002; Shirao et al., 2002; Altmann et al., 2003d; Ohmori et al., 2003; Salomone et al., 2003) and fasudil (Altmann et al., 2003d), C3 exotoxin (Bolz et al., 2003b; Ohmori et al., 2003; Salomone et al., 2003), or dominant-negative mutants of rho kinase (Shirao et al., 2002).
Taken together, it appears that SMM-induced vasoconstriction is mediated by a combination of Ca2+ mobilization from inositol-trisphosphate-sensitive stores, Ca2+ influx through L-type channels and Ca2+ sensitization of contractile filaments via a rho-kinase pathway. In this regard, it is interesting to note that SPC-induced and α1-adrenoceptor-mediated vasoconstriction in rat mesenteric microvessels, despite occurring via Gi and Gq, respectively, were not only well correlated in their magnitude but also exhibited an almost identical profile of sensitivity towards signal transduction inhibitors (Altmann et al., 2003d).
Although endothelial cells can express SMM receptors (see above), the role of the endothelium in SMM-induced vasoconstriction remains unclear. Thus, neither endothelial denudation nor the NO synthase (NOS) inhibitor NG-nitro-L-arginine attenuated SPC-induced contraction of rat isolated mesenteric microvessels (Bischoff et al., 2000a). While one study reported a minor attenuation of SPC-induced vasoconstriction in the presence of the cyclooxygenase inhibitor indomethacin, whether or not the NOS inhibitor NG-nitro-L-arginine was present (Bischoff et al., 2000a), another study by the same group did not confirm this effect of indomethacin (Altmann et al., 2003d). Accordingly, these authors also reported that indomethacin failed to inhibit S1P-induced reductions of mesenteric or renal blood flow in anaesthetized rats in vivo (Wecking et al., 2001). On the other hand, it was found that sphingosine-induced contraction of porcine coronary arteries was endothelium-dependent and partly indomethacin-sensitive, but insensitive to NOS inhibition (Murohara et al., 1996). Thus, the question of an endothelial involvement in SMM-induced vasoconstriction needs to be studied in additional vascular beds to get a more comprehensive view.
S1P can activate endothelial NOS in bovine endothelial cells (Mogami et al., 1999; Tanimoto et al., 2002). Accordingly, it has also been found that SPC can cause relaxation of bovine coronary arteries in an NO-dependent manner (Mogami et al., 1999). Moreover, S1P can transiently relax pressurized mesenteric vessels from rats and wild-type mice, but not those from knockout mice lacking the endothelial NOS (Dantas et al., 2003). Within the latter study, S1P-induced vasodilatation was abrogated by removal of the endothelium or an NOS inhibitor, but not by the cyclooxygenase inhibitor indomethacin or the K+-channel inhibitor 4-aminopyridine. Moreover, it was inhibited by PTX, the PI3K inhibitor wortmannin and the intracellular Ca2+-chelator BAPTA. Nevertheless, vasoconstriction appears to be the most frequent vascular response to SMM administration (see above).
The role of ceramides on vascular tone has remained controversial. In bovine small coronary arteries, ceramide was found to cause vasoconstriction (Li et al., 1999) and to inhibit endothelium-dependent vasodilatation (Zhang et al., 2001). The former may involve activation of Ca2+-activated K+-channels, while the latter may involve attenuated NO function due to increased O2− formation. In contrast, ceramide-induced vasodilatation was demonstrated in rat isolated thoracic aorta (Johns et al., 1997), a finding later confirmed by the same (Johns et al., 1998) and other authors (Zheng et al., 1999). Ceramide-induced vasodilatation was also seen in rat mesenteric microvessels (Czyborra et al., 2002). Particularly in the latter preparation, ceramide-induced relaxation was concentration-dependent and inhibited specific structure–activity relationships. However, relaxation of mesenteric microvessels was only transient, which may involve desensitization of the ceramide response as well as metabolism of the ceramides to inactive metabolites (Czyborra et al., 2002). Two findings suggest that ceramide-induced vasodilatation may be an endogenously active process. Firstly, activation of sphingomyelinase, which generates endogenous ceramides, imitates the vasodilating effect of ceramide in rat aorta (Johns et al., 1997). Secondly, the ceramidase inhibitor (1S,2R)-D-erythro-2-(N-myristoylamino)-1-phenyl-1-propanol imitated the vasodilating effect of ceramide in rat mesenteric microvessels in a concentration where it hastened the vasodilatation by exogenous ceramide (Czyborra et al., 2002). Whether the above discrepancies reflect heterogeneity in vascular ceramide responses between vascular beds and/or between species remains to be determined. Moreover, it appears possible that this reflects a methodological difference since studies reporting ceramide-induced vasodilatation have used vessel strips whereas those reporting vasoconstriction or inhibition of vasodilatation have used isolated perfused vessels.
Whether ceramide-induced vasodilatation primarily occurs at the level of the vascular smooth muscle cells or, at least partly, is endothelium-dependent has remained controversial. Thus, ceramide-induced relaxation of rat aorta (Johns et al., 1998) and bovine coronary arteries (Zhang et al., 2001) was reported to be at least partly endothelium-dependent, whereas that of rat mesenteric microvessels was not (Czyborra et al., 2002). In line with the latter study, it was shown that ceramide can attenuate elevations of intracellular Ca2+ concentrations induced by vasoconstricting agents such as phenylephrine in isolated smooth muscle cells (Zheng et al., 1999). Czyborra et al. (2002) have reported extensive studies into the molecular mechanism of ceramide-induced vasodilatation in rat mesenteric microvessels, that is, in a preparation where vasodilatation appears to occur endothelium-independently. Inhibitors of cyclic nucleotide-dependent signalling pathways such as the protein kinase A inhibitors H7 and H89 or the guanylyl cyclase inhibitor ODQ had little effect on vasodilatation, the latter observation being in line with the lack of effect of endothelium removal in this preparation. Similarly, inhibitors of various types of K+-channels, including tetraethylammonium, apamine, glibenclamide, iberiotoxin and charybdotoxin, also caused small, if any, attenuation of ceramide-induced vasodilatation. However, a combination of ODQ and charybdotoxin almost completely abolished it. Thus, both guanylyl cyclase and charybdotoxin-sensitive K+-channels may be involved in ceramide-induced vasodilatation, but only combined inhibition of both reveals this effect, possibly because they can largely compensate for each other when only one of them is inhibited. In the absence of proposals for receptor-mediated ceramide effects, it appears to be determined what the molecular ceramide targets are that activate these signalling mechanisms.
While several studies have investigated cardiac SMM responses under pathophysiological conditions, only few have addressed such possible alterations in blood vessels. One study has looked at alterations of mesenteric microvessel vasoconstriction responses to S1P and SPC and vasodilatation responses to ceramide in spontaneously hypertensive relative to normotensive Wistar-Kyoto rats (Altmann et al., 2003b). Numerous previous studies have established that vascular responsiveness to various contractile stimuli is enhanced in this model of genetic hypertension, whereas that to relaxing stimuli is attenuated in most cases. In confirmation of such findings, that study reported an enhanced vasoconstriction to the α1-adrenoceptor agonists noradrenaline and methoxamine, whereas vasodilatation in response to the β-adrenoceptor agonist isoprenaline and to nitroprusside-sodium was attenuated. In contrast, vasoconstriction responses to both S1P and SPC were not only not enhanced but rather significantly attenuated in hypertension. Similarly, the attenuation of vasodilating ceramide responses in hypertension was less pronounced than that of isoprenaline or nitroprusside-sodium. Thus, it was proposed that the regulation of vascular SMM responses in hypertension may differ from that of most established vasoactive agents, possibly related to specific effects upon expression of SMM receptors. In a second study, the same group has determined the effects of ageing on rat vascular responses to S1P and SPC (Altmann et al., 2003a) or ceramide (Altmann et al., 2003c). The authors have used mesenteric microvessels from 3 vs 23 months old male Wistar rats. Both age groups had similar blood pressure, but older rats had slightly lower heart rate and markedly larger internal vessel diameters. Nevertheless, vasoconstriction responses to noradrenaline and methoxamine or to S1P exhibited little, if any, difference between age groups. In contrast, vessels from aged rats exhibited an approximately 50% greater wall tension in response to SPC. Vasodilation in response to isoprenaline, nitroprusside-sodium and ceramide was not altered in vessels from aged rats.
Renal effects
The kidney is the organ with the greatest impact on long-term blood pressure control (Cowley, 1992). Moreover, enhancements and reductions of renal blood flow generally tend to enhance and reduce diuresis, respectively, and SMM, particularly, S1P, have been shown to cause renal vasoconstriction in vitro and in vivo (see above). Therefore, some studies have investigated the possible effects of SMM on renal function. Based on the pronounced S1P effects on renovascular tone in vitro (Bischoff et al., 2000a; 2001a) and in vivo (Bischoff et al., 2000b; 2001a), it had been expected that SMM might inhibit diuresis and natriuresis. Surprisingly, however, intravenous infusion of S1P did not reduce but rather enhanced diuresis (Figure 3), natriuresis, calciuresis and, to a lesser extent, kaliuresis (Bischoff et al., 2001b). This occurred under conditions where the S1P infusion dose-dependently but transiently reduced renal blood flow and did not cause major alterations of mean arterial pressure or glomerular filtration rate, as assessed by endogenous creatinine clearance. Moreover, the S1P effects upon tubular function occurred and abated slower than those on renovascular function. This overall pattern is typical for agents which act in distal nephron segments. Diuretic effects of S1P were confirmed by the authors in a second study (Wecking et al., 2001).
Interestingly, other SMM such as SPC, sphingosine and glucopsychosine lacked the renovascular effects of S1P, but shared its diuretic effects (Bischoff et al., 2001b). Actually, diuretic effects by the former three were larger and exhibited a clearer dose-dependency than those elicited by S1P, indicating that the renovascular effects of S1P might at least partly counteract its effect on the renal tubules. The finding that the effects of both S1P and SPC were abolished by PTX pre-treatment of the animals suggests that at least these two SMM exerted their effects in a receptor-mediated manner (Bischoff et al., 2001b). Apart from the fact that none of the cloned SMM receptors is activated by S1P and SPC in a similar concentration range (see above), another finding suggests that both SMM exerted their tubular effects via distinct receptors. Thus, pre-treatment with the cyclooxygenase inhibitor indomethacin enhanced the diuretic and natriuretic effects of S1P, but abolished those of SPC (Wecking et al., 2001). Thus, it appears that SMM can affect renal function indirectly via receptors located on the renal vasculature, and directly via one or more receptors located on the renal tubules. However, it should also be noted that the diuretic and natriuretic effects of S1P and SPC are of limited magnitude and hence, for example, the diuretic effects of SPC did not reach statistical significance under all experimental conditions (Bai et al., 2002).
Thus, it appears that SMM can affect renal function via at least two different mechanisms. One of them is a receptor located on the renal vasculature, which is preferentially activated by S1P and mediates reductions of renal blood flow and possibly indirectly inhibits diuresis. The other is more likely to be located on tubular cells, activated by several SMM, and directly enhances diuresis. The latter may be a potential target for treatment of renal dysfunction, but considerable additional studies are required.
Long-term effects of SMM in the cardiovascular system
SMM in vascular development
The formation of new blood vessels is essential for a variety of physiological processes like embryogenesis and the female reproduction system, as well as pathophysiological processes such as wound healing and neovascularization of ischaemic tissue. In the developing embryo, vasculogenesis and angiogenesis are the mechanisms responsible for the development of the blood vessels. Vasculogenesis resembles the process in which haemangioblasts differentiate to blood cells, and ultimately form a primitive vascular plexus de novo. Although also derived from the blood-forming bone marrow, vasculogenic stem cells contribute only little to the regular vascular repair mechanisms in adult life. Angiogenesis refers to the formation of capillaries from pre-existing vessels in the embryo and adult organism. While pathologic angiogenesis includes the role of post-natal neovascularization in the pathogenesis of arthritis, diabetic retinopathy, tumour growth and metastasis, therapeutic angiogenesis, either endogenously or in response to administered growth factors, involves the development of collateral blood vessels in tissue ischaemia. Major progress in understanding the underlying mechanisms regulating blood vessel growth has offered novel therapeutic options in the treatment of a variety of disease states. Since the identification of S1P1 as a G-protein-coupled receptor homologue that is induced during the in vitro differentiation of human endothelial cells (Hla & Maciag, 1990a) and the discovery that S1P is its endogenous ligand (Lee et al., 1998), it has become clear that SMM play a role in vascular development. Numerous investigations to date have shown that SMM may be involved in several steps of vascular development. Here we will discuss the involvement of S1P in several aspects of angiogenesis: endothelial cell activation, extracellular matrix degradation, proliferation and migration, tube formation and vessel maturation.
S1P, VEGF crosstalk and endothelial cell activation
Vascular endothelial growth factor (VEGF) is most likely one of the most important growth-promoting factors involved in angiogenesis. VEGF transcription is stimulated by hypoxia, which induces the generation and binding of hypoxia-inducible factor-1α to the hypoxia responsive element in the VEGF promotor region. VEGF binds to its specific receptor tyrosine kinase (VEGFR) and thereby promotes and modulates several aspects in the angiogenic process. The VEGFR family is composed of VEGFR-1 (Flt-1), VEGFR-2 (Flk-1 or KDR) and VEGFR-3 (Flt-4). The VEGFR-2 is the main receptor via which VEGF exerts its effects, such as vascular permeability, endothelial cell proliferation, migration and tube formation (for a review, see Carmeliet, 2003).
VEGF is known to stimulate PI3K- and Akt-dependent phosphorylation of eNOS, resulting in activation of eNOS and increased NO production (Fulton et al., 1999). Activation of eNOS is an essential step in the action of VEGF, since it has been shown that eNOS inhibition blocks VEGF-induced endothelial cell migration, proliferation and tube formation in vitro, as well as VEGF-induced angiogenesis in vivo (Murohara et al., 1998; Lee et al., 1999c; Rikitake et al., 2002).
Recent studies revealed that S1P binding to its S1P1 receptor also leads to eNOS activation and NO synthesis (Igarashi et al., 2001; Kwon et al., 2001; Morales-Ruiz et al., 2001; Rikitake et al., 2002). S1P treatment of bovine aortic endothelial cells leads to an Akt-mediated eNOS phosphorylation at Ser1179 and concomitant NO production (Igarashi et al., 2001). This S1P-mediated eNOS activation was PTX sensitive, indicating that these events are mediated by surface receptors rather than via intracellular mechanisms (Igarashi et al., 2001). Inhibition of Gi signaling with PTX leads to a decrease in S1P-mediated endothelial cell chemotaxis, whereas NOS inhibition by means of NG-nitro-L-arginine methyl ester was ineffective in this respect. In contrast, NOS inhibition effectively blocked the chemotactic actions of VEGF (Morales-Ruiz et al., 2001). In a later study, Rikitake et al. (2002) have shown that inhibition of PI3K, Akt or eNOS significantly decreases endothelial cell migration and tube formation, whereas NG-nitro-L-arginine methyl ester, but not the PI3K inhibitor LY294002 failed to inhibit endothelial cell viability and proliferation induced by S1P. However, another study suggests that NO is involved in S1P-mediated prevention of serum-deprived apoptosis of human umbilical vein endothelial cells (Kwon et al., 2001). These results show that, in analogy with VEGF, S1P leads to activation of Akt and eNOS. The resulting production of NO may be involved in different aspects of the angiogenic action of these two agonists.
The relation between S1P and VEGF is probably even more complex, since several groups have shown a rather complicated receptor crosstalk (receptor transactivation and upregulation) between these two compounds.
In a recent report by Shu et al. (2002), evidence was presented that sphingosine kinase mediates VEGF-induced activation of Ras and MAPK. Thus, VEGF activated sphingosine kinase, and the VEGF-induced activation of ERK1/2 type MAPK was prevented by the sphingosine kinase inhibitor dimethylsphingosine. It was proposed by the authors that sphingosine may increase the activity of Ras GTPase-activating proteins and thereby decrease Ras activity, and that S1P can block the ability of sphingosine to activate Ras GTPase-activating proteins. Thus VEGF-induced sphingosine kinase activity will result in the conversion of sphingosine to S1P and a subsequent displacement of sphingosine from Ras GTPase-activating proteins will increase the levels of activated Ras-GTP and hence activated Raf (Shu et al., 2002). In another recent report by Endo et al. (2002), it was shown that S1P induces membrane ruffling (a renowned characteristic of migratory cells) of human umbilical vein endothelial cells via the vascular endothelial growth factor receptor (VEGFR-2), Src family tyrosine kinase(s) and the CrkII adaptor protein (which is known to be involved in cellular migration and the induction of membrane ruffling). S1P-induced CrkII phosphorylation was blocked by PTX and overexpression of the carboxyl terminus of β-adrenergic receptor kinase, indicating that the βγ-subunit of Gi was required for the phosphorylation. In addition, the authors showed that S1P-induced CrkII phosphorylation was also abolished by inhibitors of VEGF receptor or Src family tyrosine kinase. Interestingly, it has been shown that the previously discussed S1P-induced eNOS activation involving Gi protein, PI3K and Akt is dependent on VEGFR-2 transactivation (Tanimoto et al., 2002).
In addition to these complex signalling interactions, it has recently been shown that VEGF can induce S1P1 expression and concomitant enhanced intracellular signalling responses to subsequent S1P treatment in endothelial cells (Igarashi et al., 2003).
Regulation of matrix degradation
The breakdown of the extracellular matrix (ECM) by proteinases is an essential step in the angiogenic process, since it creates an environment to support endothelial cell migration and proliferation. Furthermore, breakdown of the matrix will liberate additional growth factors that are normally bound to heparan sulphates in the extracellular matrix. Matrix degradation is mainly achieved by proteolitic activity of membrane-type metalloproteinases. By making use of three-dimensional collagen and fibrin matrices, it was shown that S1P potently stimulated human endothelial cell invasion (Bayless & Davis, 2003). The S1P-induced invasion response was PTX sensitive and completely dependent on integrins and matrix metalloproteinases. In another study, treatment of bovine aortic endothelial cells overexpressing membrane type 1 matrix metalloproteinase with antisense oligonucleotides directed against S1P1 and S1P3 significantly inhibited membrane type 1 matrix metalloproteinase-dependent cell migration and morphogenic differentiation (Langlois et al., 2004).
Endothelial cell proliferation and migration
Subsequent to matrix degradation, endothelial cell proliferation and migration are critical steps in the angiogenic process and in both of these processes SMM do play a regulatory role. Since VEGF is a potent mitogen for endothelial cells and S1P and VEGF share many signalling pathways as discussed previously, it is not surprising that also S1P induces endothelial cell proliferation (Lee et al., 1999b; Wang et al., 1999; Kimura et al., 2000). The S1P-induced proliferation was PTX-sensitive, indicating a receptor-dependent mechanism (Lee et al., 1999b; Kimura et al., 2000). In addition, Kimura et al. (2000) showed that the S1P-induced 3H-thymidine incorporation into human aortic endothelial cells was blocked by the selective MAPK kinase inhibitor PD98059 but not by the MAPK p38 inhibitor SB203580.
Although S1P inhibits cell migration in most cell types, it is now clear that it stimulates migration in endothelial cells (Lee et al., 1999b; Wang et al., 1999; English et al., 2000; Kimura et al., 2000; Panetti et al., 2000; Liu et al., 2001; Morales-Ruiz et al., 2001; Paik et al., 2001). Also, the migratory effects of S1P seem to be mediated via Gi-coupled cell surface receptors since these effects could be blocked by PTX in all the before-mentioned studies. S1P-induced human umbilical vein endothelial cell migration is potently inhibited by antisense phosphothioate oligonucleotides against S1P1 as well as S1P3 receptors (Liu et al., 2001; Paik et al., 2001). Interestingly, both S1P1 and S1P3 receptors were required for Rho activation. In addition, C3 exotoxin blocked the S1P-induced migration process, suggesting that the Rho pathway is critical for S1P-induced cell migration (Paik et al., 2001). Also, in the study by Liu et al. (2001), various strategies to interrupt Rho family signalling, including C(3) exotoxin, dominant/negative Rho, or the addition of Y27632 (a cell-permeable Rho-kinase inhibitor), significantly attenuated S1P- but not VEGF-induced migration. Also, a study by Morales-Ruiz et al. (2001) showed that the Gi-coupled S1P1 receptor is critical for S1P-induced endothelial cell migration. As discussed previously, this study also showed that inhibition of NO synthesis diminished VEGF-induced migration, but not that induced by S1P. Exposure of endothelial cells to S1P leads to a PTX-sensitive activation of both ERK and p38 (Lee et al., 1999b; Kimura et al., 2000). However, conflicting results have been reported regarding their respective roles in S1P-induced endothelial cell migration. For instance, in the report by Panetti et al. (2000), MAPK kinase inhibition with PD98059 caused a loss of phosphorylation of ERK, but resulted in only a minimal decrease in migration. This is in accordance with the findings of Lee et al. (1999b), who showed that S1P-induced migration was not affected by inhibition of ERK with U0126 and p38 MAPK with SB203580. Also, Kimura et al. (2000) have reported that MAPK kinase inhibition with PD98059 was ineffective in this respect. However, in this same study, SB203580 could inhibit S1P-induced endothelial cell migration, as has been shown by Liu et al. (2001). In a later report of the same group similar results were reported (Kimura et al., 2003). Thus, although both ERK and p38 are activated upon S1P stimulation, there is most likely only a role for p38 MAPK in S1P-induced migration.
Tube formation
Subsequent to migration and proliferation, the new outgrowth of endothelial cells needs to reorganize into a patent three-dimensionally tubular structure. Among many others, S1P might be a factor that has a regulatory role in this process. S1P induces tube formation in umbilical vein endothelial cells on Matrigel (a matrix-rich product prepared from Engelbreth–Holm–Swarm tumour cells whose primary component is laminin) (Lee et al., 1999b) and bovine aortic endothelial cells grown on collagen gel (Wang et al., 1999). This S1P-induced tube formation was PTX-sensitive and was markedly inhibited by the MAPK kinase inhibitor U0126 in contrast to S1P-induced migration. Similar results were later reported by Miura et al. (2003), who demonstrated that a Ras/Raf1-dependent ERK activation, mediated by PTX-sensitive G-protein-coupled receptors, mediates S1P-induced human coronary endothelial cell tube formation. Also, the PI3K, Akt, eNOS pathway may play a role in S1P-induced tube formation, since inhibition of either of these three components inhibited tube formation on Matrigel (Rikitake et al., 2002). In a recent paper, Bayless & Davis (2003) have shown that S1P-induced endothelial cell invasion and tube formation in collagen and fibrin three-dimensional matrices require metalloproteinases. Future research is necessary to elucidate the exact role of S1P and other factors in the complex regulation of tube formation.
Vascular maturation
The now newly formed tube has to be stabilized by recruiting mural cells (pericytes and vascular smooth muscle cells) and the production of an extracellular matrix. Besides platelet-derived growth factor B, angiopoietin 1 and transforming growth factor-β, S1P is a key player in this process. First evidence for the role of S1P and its S1P1 receptor in vascular maturation came from a study by Liu et al. (2000), in which they globally disrupted the S1P1 gene in mice.
While this did not appear to affect vasculogenesis or angiogenesis, it led to embryonic haemorrhage and thereby to intrauterine death between E12.5 and E14.5. This was linked to incomplete vessel maturation, which was due to a defect in the recruitment of mural cells to the vessel wall. Later studies from these investigators using conditional endothelium-specific and smooth muscle-specific S1P1 receptor knockout animals demonstrated that the above effects were attributable to an endothelial S1P1 receptor (Allende et al., 2003). Although disruption of the platelet-derived growth factor B or its receptor β gene leads to a similar phenotype as disruption of the S1P1 receptor gene (Leveen et al., 1994; Soriano, 1994; Lindahl et al., 1997) and although platelet-derived growth factor can stimulate S1P formation by smooth muscle cells (Olivera & Spiegel, 1993; Hobson et al., 2001), the involvement of an endothelial S1P1 receptor questions the proposed role of S1P in platelet-derived growth factor-induced vascular maturation.
Although there is a very clear important role for S1P in vessel maturation, further research is warranted to elucidate the mechanisms involved.
In vivo angiogenesis
Owing to all the aforementioned characteristics of S1P, being involved in almost every aspect of the angiogenic process, one would expect S1P to be a potent in vivo angiogenic factor. Indeed, S1P proved to be very potent in promoting angiogenesis in the developing chick embryo chorioallantoic membrane (English et al., 2001). However, in the matrigel plug implant model, S1P significantly potentiated the basic fibroblast growth factor and VEGF-induced angiogenesis, but S1P alone proved ineffective. Similar results were obtained in the mouse cornea micro pocket assay, where also S1P alone was not very potent but the angiogenic responses to basic fibroblast growth factor were markedly potentiated by the addition of S1P (English et al., 2000). Basic fibroblast growth factor and S1P treatment increased the number of neovessels with well-developed adherens junctions and basement membrane-like structures (Lee et al., 1999a).
Other SMM in angiogenesis
Most studies concerning the role of SMM in angiogenesis have focused on the role of S1P in this process, as discussed in previous paragraphs. Only a few studies have investigated the role of other SMM such as SPC and sphingosine. Both SPC and sphingosine induce chemotactic migration of human and bovine endothelial cells, as potent as VEGF (Boguslawski et al., 2000). In addition, both compounds induced in vitro tube formation on matrigel. Interestingly, in the same study, the authors showed that the response to sphingosine and SPC was associated with a downregulation of S1P1 receptor mRNA. In analogy with S1P, SPC at low concentrations stimulates vascular smooth muscle cell migration, whereas higher concentrations (1 μM or higher) have inhibitory effects (Boguslawski et al., 2002).
It is unclear whether the effects of SPC and sphingosine are direct effects of these compounds or that they are mediated through the active conversion to either S1P or ceramide. As stated earlier, it is probably the actual balance between S1P and ceramide that determines cell fate, since most of the actions of S1P can be counteracted by ceramide. Ceramide has been shown to be growth inhibitory to endothelial and vascular smooth muscle cells and may induce apoptosis in these cell types (Lopez-Marure et al., 2000; Johns et al., 2001; Erdreich-Epstein et al., 2002; Radin, 2003). For this reason, ceramide or its analogues are potential candidates to inhibit tumour angiogenesis (Hayakawa et al., 2002) or to function as anticancer agents in general, since ceramide is also involved in radiation or drug-induced apoptosis of tumour cells (Radin, 2003).
Increased knowledge of the mechanisms by which growth factors are involved in the angiogenic process may provide therapeutic targets to influence wanted or unwanted blood vessel formation. Although S1P is at least theoretically an interesting candidate for therapeutic angiogenesis because it is involved in so many aspects of angiogenesis, we have to realize that, because of the complexity of the angiogenic process, simply adding growth factors to an ischaemic area will not be sufficient to achieve therapeutic angiogenesis. However, the development of specific agonists or antagonists for the different S1P receptors may provide tools to influence at least endothelial or smooth muscle cell proliferation and migration, which also play important roles in other (patho)physiological processes.
Chronic effects upon cardiac function
Two studies have assessed the growth effects of SMM using neonatal rat isolated cardiomyocytes as the model system. Both studies report SPC to induce hypertrophy in this model, whereas the effects of S1P were prominent in one study (Robert et al., 2001) but not the other (Sekiguchi et al., 1999). Moreover, dihydro-S1P was found to enhance hypertrophy in the neonatal rat cadiomyocytes (Robert et al., 2001), whereas sphingosine lacked such effects (Sekiguchi et al., 1999). The latter finding is in good agreements with reports that both ceramide (Bielawska et al., 1997) and sphingosine (Krown et al., 1996) can induce apoptosis in these cells. When SMM induce neonatral rat cardiomyocyte hypertrophy, this appears to involve activation of various protein kinases including ERK, p38, jun-N-terminal kinase, Akt and p70S6K (Sekiguchi et al., 1999; Robert et al., 2001).
Perspective
Based upon the above, it is evident that SMM can act on all major cells of the cardiovascular system. Their ubiquitous formation and their presence in plasma at high concentrations make them an interesting potential physiological regulator of cardiovascular function acutely and chronically. In our opinion, three main questions should be addressed by future research in this area. Firstly, the vast majority of studies until now has focused on cells and tissues from healthy animals, whereas, with the exception of cardiac ischaemia, only few studies have addressed SMM responses in pathophysiological settings. Such studies will be necessary to determine their role as a valid drug target in cardiovascular pharmacology. Secondly, it is one of the most fascinating aspects of SMM that several SMM, particularly S1P and SPC, can act potentially act as intra- and inter-cellular messengers. This is a biologically fairly unique role. Thus, identification of SMM targets, including their receptor subtypes, will be a major challenge. While considerable progress has been made in the identification of S1P receptor subtypes, molecular targets for other SMM, particularly the identity of possible SPC receptors, remain to be identified. Finally, it is obvious from the above that ceramide frequently has effects opposite to those of S1P and SPC, with sphingosine sometimes mimicking one and sometimes the other. This applies to the question of apoptosis/cell growth, vasodilatation/vasoconstriction and ischaemic heart damage. Since the various SMM can largely be metabolized to each other and since only few enzymes are required in this process, it appears that regulation of the activity of these enzymes could play a key role in cardiovascular pharmacology. Beyond this, opposing effects of such closely related messenger molecules is also a fairly unique situation in biology and hence its further study should also yield fundamental insight into homeostasis.
Abbreviations
- ERK
extracellular signal-regulated kinase
- MAPK
mitogen-activated protein kinase
- NOS
NO synthase
- PI3K
phosphatidyl-inositol 3′-kinase
- PTX
pertussis toxin
- S1P
sphingosine-1-phosphate
- SMM
sphingomyelin metabolites
- SPC
sphingosylphosphorylcholine
- TNF
tumour necrosis factor
- VEGF
vascular endothelial growth factor
References
- ALLENDE M.L., PROIA R.L. Sphingosine-1-phosphate receptors and the development of the vascular system. Biochem. Biophys. Acta. 2002;1582:222–227. doi: 10.1016/s1388-1981(02)00175-0. [DOI] [PubMed] [Google Scholar]
- ALLENDE M.L., YAMASHITA T., PROIA R.L. G-protein-coupled receptor S1P1 acts within endothelial cells to regulate vascular maturation. Blood. 2003;102:3665–3667. doi: 10.1182/blood-2003-02-0460. [DOI] [PubMed] [Google Scholar]
- ALTMANN C., FETSCHER C., BÖYÜKBAS D., MICHEL M.C. Contraction of mesenteric resistance vessels by sphingosine-1-phosphate and sphingosylphosphorylcholine in aged rats. Naunyn-Schmiedeberg's Arch. Pharmacol. 2003a;367 Suppl:R57. [Google Scholar]
- ALTMANN C., FETSCHER C., BÖYÜKBAS D., MICHEL M.C. Effects of sphingosine-1-phosphate, sphingosylphosphorylcholine and ceramide on mesenteric artery contraction and relaxation in spontaneously hypertensive rats. Br. J. Pharmacol. 2003b;138 Suppl:157. [Google Scholar]
- ALTMANN C., FETSCHER C., BÖYÜKBAS D., MICHEL M.C. Rat microvessel relaxation by ceramide, isoprenaline and nitroprusside sodium: differential regulation in genetic hypertension and aging. FASEB J. 2003c;17:397.3. [Google Scholar]
- ALTMANN C., STEENPAß V., CZYBORRA P., HEIN P., MICHEL M.C. Comparison of signalling mechanisms involved in rat mesenteric microvessel contraction by noradrenaline and sphingosylphosphorylcholine. Br. J. Pharmacol. 2003d;138:261–271. doi: 10.1038/sj.bjp.0705028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AMADOU A., NAWROCKI A., BEST-BELPOMME M., PAVOINE C., PECKER F. Arachidonic acid mediates dual effect of TNF-alpha on Ca2+ transients and contraction of adult rat cardiomyocytes. Am. J. Physiol. 2002;282:C1339–C1347. doi: 10.1152/ajpcell.00471.2001. [DOI] [PubMed] [Google Scholar]
- ANCELLIN N., HLA T. Differential pharmacological properties and signal transduction of the sphingosine 1-phosphate receptors EDG-1, EDG-3, and EDG-5. J. Biol. Chem. 1999;274:18997–19002. doi: 10.1074/jbc.274.27.18997. [DOI] [PubMed] [Google Scholar]
- BAI J., HEEMANN U., MICHEL M.C. Comparison of renal effects of systemic and intrarenal administration of sphingosylphosphorylcholine. Br. J. Pharmacol. 2002;135 Suppl:245. [Google Scholar]
- BAYLESS K.J., DAVIS G.E. Sphingosine-1-phosphate markedly induces matrix metalloproteinase and integrin-dependent human endothelial cell invasion and lumen formation in three-dimensional collagen and fibrin matrices. Biochem. Biophys. Res. Commun. 2003;312:903–913. doi: 10.1016/j.bbrc.2003.11.017. [DOI] [PubMed] [Google Scholar]
- BEKTAS M., BARAK L.S., JOLLY P.S., LIU H., LYNCH K.R., LACANA E., SUHR K.B., MILSTIEN S., SPIEGEL S. The G protein-coupled receptor GPR4 suppresses ERK activation in a ligand-independent manner. Biochemistry. 2003;42:12181–12191. doi: 10.1021/bi035051y. [DOI] [PubMed] [Google Scholar]
- BENEDIKTSDOTTIR V.E., JONSDOTTIR A.M., SKULADOTTIR B.H., GRUNBERG A., SKARPHEDINSSON J.O., HELGASON J., GUDBJARNASON S. Sphingosine modulation of cAMP levels and beating rate in rat heart. Fundam. Clin. Pharmacol. 2002;16:495–502. doi: 10.1046/j.1472-8206.2002.00105.x. [DOI] [PubMed] [Google Scholar]
- BETTO R., TERESI A., TURCATO F., SALVIATI G., SABBADINI R.A., KROWN K., GLEMBOTSKI C.C., KINDMAN L.A., PEREON Y., YASUI K., PALADE P.T. Sphingosylphosphorylcholine modulates the ryanodine receptor/calcium-release channel of cardiac sarcoplasmic reticulum membranes. Biochem. J. 1997;322:327–333. doi: 10.1042/bj3220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIELAWSKA A.E., SHAPIRO J.P., JIANG L., MELKONYAN H.S., PIOT C., WOLFE C.L., TOMEI L.D., HANNUN Y.A., UMANSKY S.R. Ceramide is involved in triggering of cardiomyocyte apoptosis induced by ischaemia and reperfusion. Am. J. Pathol. 1997;151:1257–1263. [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., FETSCHER C., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine-1-phosphate and sphingosylphosphorylcholine constrict renal and mesenteric microvessels in vitro. Br. J. Pharmacol. 2000a;130:1871–1877. doi: 10.1038/sj.bjp.0703515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., CZYBORRA P., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Sphingosine-1-phosphate reduces rat renal and mesenteric blood flow in vivo in a pertussis toxin-sensitive manner. Br. J. Pharmacol. 2000b;130:1878–1883. doi: 10.1038/sj.bjp.0703516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BISCHOFF A., FINGER J., MICHEL M.C. Nifedipine inhibits sphingosine-1-phosphate-induced renovascular contraction in vitro and in vivo. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001a;364:179–182. doi: 10.1007/s002100100446. [DOI] [PubMed] [Google Scholar]
- BISCHOFF A., MEYER ZU HERINGDORF D., JAKOBS K.H., MICHEL M.C. Lysosphingolipid receptor-mediated diuresis and natriuresis in anaesthetised rats. Br. J. Pharmacol. 2001b;132:1925–1933. doi: 10.1038/sj.bjp.0703969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOGUSLAWSKI G., GROGG J.R., WELCH Z., CIECHANOWICZ S., SLIVA D., KOVALA A.T., MCGLYNN P., BRINDLEY D.N., RHOADES R.A., ENGLISH D. Migration of vascular smooth muscle cells induced by sphingosine 1-phosphate and related lipids: potential role in the angiogenic response. Exp. Cell Res. 2002;274:264–274. doi: 10.1006/excr.2002.5472. [DOI] [PubMed] [Google Scholar]
- BOGUSLAWSKI G., LYONS D., HARVEY K.A., KOVALA A.T., ENGLISH D. Sphingosylphosphorylcholine induces endothelial cell migration and morphogenesis. Biochem. Biophys. Res. Commun. 2000;272:603–609. doi: 10.1006/bbrc.2000.2822. [DOI] [PubMed] [Google Scholar]
- BOLZ S.S., VOGEL L., SOLLINGER D., DERWAND R., BOER C., PITSON S.M., SPIEGEL S., POHL U. Sphingosine kinase modulates microvascular tone and myogenic responses through activation of RhoA/Rho kinase. Circulation. 2003a;108:342–347. doi: 10.1161/01.CIR.0000080324.12530.0D. [DOI] [PubMed] [Google Scholar]
- BOLZ S.S., VOGEL L., SOLLINGER D., DERWAND R., DE WIT C., LOIRAND G., POHL U. Nitric oxide-induced decrease in calcium sensitivity of resistance arteries is attributable to activation of the myosin light chain phosphatase and antagonized by the RhoA/Rho kinase pathway. Circulation. 2003b;107:3081–3087. doi: 10.1161/01.CIR.0000074202.19612.8C. [DOI] [PubMed] [Google Scholar]
- BÜNEMANN M., BRANDTS B., MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H., POTT L. Activation of muscarinic K+ current in guinea-pig atrial myocytes by sphingosine-1-phosphate. J. Physiol. (London) 1995;489:701–707. doi: 10.1113/jphysiol.1995.sp021084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÜNEMANN M., LILIOM K., BRANDTS B.K., POTT L., TSENG J.-L., DESIDERIO D.M., SUN G., MILLER D., TIGYI G. A novel membrane receptor with high affinity for lysosphingomyelin and sphingosine 1-phosphate in atrial myocytes. EMBO J. 1996;15:5527–5534. [PMC free article] [PubMed] [Google Scholar]
- CARMELIET P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- CAVALLI A.L., LIGUTTI J.A., GELLINGS N.M., CASTRO E., PAGE M.T., KLEPPER R.E., PALADE P.T., MCNUTT W.T., SABBADINI R.A. The role of TNFα and sphingolipid signaling in cardiac hypoxia: evidence that cardiomyocytes release TNFα and sphingosine. Basic Appl. Myol. 2002;12:167–175. [Google Scholar]
- CHIN T.-Y., CHUEH S.-H. Sphingosylphosphorylcholine stimulates mitogen-activated protein kinase via a Ca2+-dependent pathway. Am. J. Physiol. 1998;275:C1255–C1263. doi: 10.1152/ajpcell.1998.275.5.C1255. [DOI] [PubMed] [Google Scholar]
- CHIN T.-Y., CHUEH S.-H. Distinct Ca2+ signalling mechanisms induced by ATP and sphingosylphosphorylcholine in porcine aortic smooth muscle cells. Br. J. Pharmacol. 2000;129:1365–1374. doi: 10.1038/sj.bjp.0703190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHUN J., GOETZL E.J., HLA T., IGARASHI Y., LYNCH K.R., MOOLENAAR W., PYNE S., TIGYI G. International Union of Pharmacology. XXXIV. Lysophospholipid receptor nomenclature. Pharmacol. Rev. 2002;54:265–269. doi: 10.1124/pr.54.2.265. [DOI] [PubMed] [Google Scholar]
- CLAIR T., AOKI J., KOH E., BANDLE R.W., NAM S.W., PTASZYNSKA M.M., MILLS G.B., SCHIFFMANN E., LIOTTA L.A., STRACKE M.L. Autotaxin hydrolyzes sphingosylphosphorylcholine to produce the regulator of migration, sphingosine-1-phosphate. Cancer Res. 2003;63:5446–5453. [PubMed] [Google Scholar]
- COUSSIN F., MACREZ N., MOREL J.L., MIRONNEAU J. Requirement of ryanodine receptor subtypes 1 and 2 for Ca2+-induced Ca2+ release in vascular myocytes. J. Biol. Chem. 2000;275:9596–9603. doi: 10.1074/jbc.275.13.9596. [DOI] [PubMed] [Google Scholar]
- COUSSIN F., SCOTT R.H., NIXON G.F. Sphingosine 1-phosphate induces CREB activation in rat cerebral artery via a protein kinase C-mediated inhibition of voltage-gated K+ channels. Biochem. Pharmacol. 2003;66:1861–1870. doi: 10.1016/s0006-2952(03)00546-x. [DOI] [PubMed] [Google Scholar]
- COUSSIN F., SCOTT R.H., WISE A., NIXON G.F. Comparison of sphingosine 1-phosphate-induced intracellular signaling pathways in vascular smooth muscles: differential role in vasoconstriction. Circ. Res. 2002;91:151–157. doi: 10.1161/01.res.0000028150.51130.36. [DOI] [PubMed] [Google Scholar]
- COWLEY A.W., JR Long-term control of aterial blood pressure. Physiol. Rev. 1992;72:231–300. doi: 10.1152/physrev.1992.72.1.231. [DOI] [PubMed] [Google Scholar]
- CZYBORRA P., SAXE M., FETSCHER C., MEYER ZU HERINGDORF D., HERZIG S., JAKOBS K.H., MICHEL M.C., BISCHOFF A. Transient relaxation of rat mesenteric microvessels by ceramides. Br. J. Pharmacol. 2002;135:417–426. doi: 10.1038/sj.bjp.0704498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANTAS A.P.V., IGARASHI J., MICHEL T. Sphingosine 1-phosphate and control of vascular tone. Am. J. Physiol. 2003;284:H2045–H2052. doi: 10.1152/ajpheart.01089.2002. [DOI] [PubMed] [Google Scholar]
- DETTBARN C.S., BETTO R., SALVIATI G., PALADE P., JENKINS G.M., SABBADINI R.A. Modulation of cardiac sarcoplasmic reticulum ryanodine receptor by sphingosine. J. Mol. Cell. Cardiol. 1994;26:229–242. doi: 10.1006/jmcc.1994.1026. [DOI] [PubMed] [Google Scholar]
- ENDO A., NAGASHIMA K., KUROSE H., MOCHIZUKI S., MATSUDA M., MOCHIZUKI N. Sphingosine 1-phosphate induces membrane ruffling and increases motility of human umbilical vein endothelial cells via vascular endothelial growth factor receptor and CrkII. J. Biol. Chem. 2002;277:23747–23754. doi: 10.1074/jbc.M111794200. [DOI] [PubMed] [Google Scholar]
- ENGLISH D., BRINDLEY D.N., SPIEGEL S., GARCIA J.G. Lipid mediators of angiogenesis and the signalling pathways they initiate. Biochim. Biophys. Acta. 2002;1582:228–239. doi: 10.1016/s1388-1981(02)00176-2. [DOI] [PubMed] [Google Scholar]
- ENGLISH D., GARCIA J.G., BRINDLEY D.N. Platelet-released phospholipids link haemostasis and angiogenesis. Cardiovasc. Res. 2001;49:588–599. doi: 10.1016/s0008-6363(00)00230-3. [DOI] [PubMed] [Google Scholar]
- ENGLISH D., WELCH Z., KOVALA A.T., HARVEY K., VOLPERT O.V., BRINDLEY D.N., GARCIA J.G. Sphingosine 1-phosphate released from platelets during clotting accounts for the potent endothelial cell chemotactic activity of blood serum and provides a novel link between hemostasis and angiogenesis. FASEB J. 2000;14:2255–2265. doi: 10.1096/fj.00-0134com. [DOI] [PubMed] [Google Scholar]
- ERDREICH-EPSTEIN A., TRAN L.B., BOWMAN N.N., WANG H., CABOT M.C., DURDEN D.L., VLCKOVA J., REYNOLDS C.P., STINS M.F., GROSHEN S., MILLARD M. Ceramide signaling in fenretinide-induced endothelial cell apoptosis. J. Biol. Chem. 2002;277:49531–49537. doi: 10.1074/jbc.M209962200. [DOI] [PubMed] [Google Scholar]
- FORREST M., SUN S.-Y., HAJDU R., BERGSTROM J., CARD D., DOHERTY G., HALE J., KEOHANE C., MEYERS C., MILLIGAN J., MILLS S., NOMURA N., ROSEN H., ROSENBACH M., SHEI G., SINGER I.I., TIAN M., WEST S., WHITE V., XIE J., PROIA R., MANDALA S. Immune cell regulation and cardiovascular effects of sphingosine 1-phosphate receptor agonists in rodents are mediated via distinct receptor sub-types. J. Pharmacol. Exp. Ther. 2004;309:758–768. doi: 10.1124/jpet.103.062828. [DOI] [PubMed] [Google Scholar]
- FRIEDRICHS G.S., SWILLO R.E., JOW B., BRIDAL T., NUMANN R., WARNER L.M., KILLAR L.M., SIDEK K. Sphingosine modulates myocyte electrophysiology, induces negative inotropy, and decreases survival after myocardial ischaemia. J. Cardiovasc. Pharmacol. 2002;39:18–28. doi: 10.1097/00005344-200201000-00003. [DOI] [PubMed] [Google Scholar]
- FULTON D., GRATTON J.P., MCCABE T.J., FONTANA J., FUJIO Y., WALSH K., FRANKE T.F., PAPAPETROPOULOS A., SESSA W.C. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GONDA K., OKAMOTO H., TAKUWA N., YATOMI Y., OKAZAKI H., SAKURAI T., KIMURA S., SILLARD R., HARII K., TAKUWA Y. The novel sphingosine 1-phosphate receptor AGR16 is coupled via pertussis toxin-sensitive and -insensitive G-proteins to multiple signalling pathways. Biochem. J. 1999;337:67–75. [PMC free article] [PubMed] [Google Scholar]
- GRALER M.H., BERNHARDT G., LIPP M. EDG6, a novel G-protein-coupled receptor related to receptors for bioactive lysophospholipids, is specifically expressed in lymphoid tissue. Genomics. 1998;53:164–169. doi: 10.1006/geno.1998.5491. [DOI] [PubMed] [Google Scholar]
- GUO J., MACDONELL K.L., GILES W.R. Effects of sphingosine 1-phosphate on pacemaker activity in rabbit sino-atrial cells. Pflügers Arch. 1999;438:642–648. doi: 10.1007/s004249900067. [DOI] [PubMed] [Google Scholar]
- HANNUN Y.A., LUBERTO C., ARGRAVES K.M. Enzymes of sphingolipid metabolism: from modular to integrative signaling. Biochemistry. 2001;40:4893–4903. doi: 10.1021/bi002836k. [DOI] [PubMed] [Google Scholar]
- HAYAKAWA Y., TAKEDA K., YAGITA H., SMYTH M.J., VAN KAER L., OKUMURA K., SAIKI I. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood. 2002;100:1728–1733. [PubMed] [Google Scholar]
- HEDEMANN J., PETERS S.L., MICHEL M.C. Comparison of vascular lysosphingolipid responses in rats and mice. Fundam. Clin. Pharmacol. 2004;18 Suppl. 1:60. [Google Scholar]
- HERNANDEZ O.M., DISCHER D.J., BISHOPRIC N.H., WEBSTER K.A. Rapid activation of neutral sphingomyelinase by hypoxia-reoxygenation of cardiac myocytes. Biochem. Biophys. Res. Commun. 2000;86:198–204. doi: 10.1161/01.res.86.2.198. [DOI] [PubMed] [Google Scholar]
- HIGUCHI K., HARA J., OKAMOTO R., KAWASHIMA M., IMOKAWA G. The skin of atopic dermatitis patients contains a novel enzyme, glucosylceramide sphingomyelin deacylase, which cleaves the N-acyl linkage of sphingomyelin and glucosylceramide. Biochem. J. 2000;350:747–756. [PMC free article] [PubMed] [Google Scholar]
- HIMMEL H.M., MEYER ZU HERINGDORF D., GRAF E., DOBREV D., KORTNER A., SCHULER S., JAKOBS K.H., RAVENS U. Evidence for Edg-3 receptor-mediated activation of I(K.ACh) by sphingosine-1-phosphate in human atrial cardiomyocytes. Mol. Pharmacol. 2000;58:449–454. doi: 10.1124/mol.58.2.449. [DOI] [PubMed] [Google Scholar]
- HLA T., MACIAG T. An abundant transcript induced in differentiating human endothelial cells encodes a polypeptide with structural similarities to G-protein-coupled receptors. J. Biol. Chem. 1990a;265:9308–9313. [PubMed] [Google Scholar]
- HLA T., MACIAG T. Isolation of immediate-early differentiation mRNAs by enzymatic amplification of subtracted cDNA from human endothelial cells. Biochem. Biophys. Res. Commun. 1990b;167:637–643. doi: 10.1016/0006-291x(90)92072-8. [DOI] [PubMed] [Google Scholar]
- HOBSON J.P., ROSENFELDT H.M., BARAK L.S., OLIVERA A., POULTON S., CARON M.G., MILSTIEN S., SPIEGEL S. Role of the sphingosine-1-phosphate receptor EDG-1 in PDGF-induced cell motility. Science. 2001;291:1800–1803. doi: 10.1126/science.1057559. [DOI] [PubMed] [Google Scholar]
- HOFMANN U., DOMEIER E., FRANTZ S., LASER M., WECKLER B., KUHLENCORDT P., HEUER S., KEWELOH B., ERTL G., BONZ A.W. Increased myocardial oxygen consumption by TNF-alpha is mediated by a sphingosine signaling pathway. Am. J. Physiol. 2003;284:H2100–H2105. doi: 10.1152/ajpheart.00888.2002. [DOI] [PubMed] [Google Scholar]
- IGARASHI J., BERNIER S.G., MICHEL T. Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase. Differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 2001;276:12420–12426. doi: 10.1074/jbc.M008375200. [DOI] [PubMed] [Google Scholar]
- IGARASHI J., ERWIN P.A., DANTAS A.P., CHEN H., MICHEL T. VEGF induces S1P1 receptors in endothelial cells: implications for cross-talk between sphingolipid and growth factor receptors. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10664–10669. doi: 10.1073/pnas.1934494100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IM D.S., HEISE C.E., ANCELLIN N., O'DOWD B.F., SHEI G.J., HEAVENS R.P., RIGBY M.R., HLA T., MANDALA S., MCALLISTER G., GEORGE S.R., LYNCH K.R. Characterization of a novel sphingosine 1-phosphate receptor, Edg-8. J. Biol. Chem. 2000;275:14281–14286. doi: 10.1074/jbc.275.19.14281. [DOI] [PubMed] [Google Scholar]
- ISHII I., FRIEDMAN B., YE X., KAWAMURA S., MCGIFFERT C., CONTOS J.J., KINGSBURY M.A., ZHANG G., BROWN J.H., CHUN J. Selective loss of sphingosine 1-phosphate signaling with no obvious phenotypic abnormality in mice lacking its G protein-coupled receptor, LP(B3)/EDG-3. J. Biol. Chem. 2001;276:33697–33704. doi: 10.1074/jbc.M104441200. [DOI] [PubMed] [Google Scholar]
- JIN Z.-Q., ZHOU H.-Z., ZHU P., HONBO N., MOCHLY-ROSEN D., MESSING R.O., GOETZL E.J., KARLINER J.S., GRAY M.O. Cardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKCɛ knockout mouse hearts. Am. J. Physiol. 2002;282:H1970–H1977. doi: 10.1152/ajpheart.01029.2001. [DOI] [PubMed] [Google Scholar]
- JOHNS D.G., JIN J.-S., WEBB R.C. The role of the endothelium in ceramide-induced vasodilation. Eur. J. Pharmacol. 1998;349:R9–R10. doi: 10.1016/s0014-2999(98)00299-4. [DOI] [PubMed] [Google Scholar]
- JOHNS D.G., OSBORN H., WEBB R.C. Ceramide: a novel cell signaling mechanism for vasodilation. Biochem. Biophys. Res. Commun. 1997;237:95–97. doi: 10.1006/bbrc.1997.7084. [DOI] [PubMed] [Google Scholar]
- JOHNS D.G., WEBB R.C., CHARPIE J.R. Impaired ceramide signalling in spontaneously hypertensive rat vascular smooth muscle: a possible mechanism for augmented cell proliferation. J. Hypertens. 2001;19:63–70. doi: 10.1097/00004872-200101000-00009. [DOI] [PubMed] [Google Scholar]
- KARLINER J.S., HONBO N., SUMMERS K., GRAY M.O., GOETZL E.J. The lysophospholipids sphingosine-1-phosphate and lysophosphatidic acid enhance survival during hypoxia in neonatal rat cardiac myocytes. J. Mol. Cell. Cardiol. 2001;33:1713–1717. doi: 10.1006/jmcc.2001.1429. [DOI] [PubMed] [Google Scholar]
- KIMURA T., SATO K., MALCHINKHUU E., TOMURA H., TAMAMA K., KUWABARA A., MURAKAMI M., OKAJIMA F. High-density lipoprotein stimulates endothelial cell migration and survival through sphingosine 1-phosphate and its receptors. Arterioscler. Thromb. Vasc. Biol. 2003;23:1283–1288. doi: 10.1161/01.ATV.0000079011.67194.5A. [DOI] [PubMed] [Google Scholar]
- KIMURA T., WATANABE T., SATO K., KON J., TOMURA H., TAMAMA K., KUWABARA A., KANDA T., KOBAYASHI I., OHTA H., UI M., OKAJIMA F. Sphingosine 1-phosphate stimulates proliferation and migration of human endothelial cells possibly through the lipid receptors, Edg-1 and Edg-3. Biochem. J. 2000;348:71–76. [PMC free article] [PubMed] [Google Scholar]
- KLUK M.J., HLA T. Role of the sphingosine 1-phosphate receptor EDG-1 in vascular smooth muscle cell proliferation and migration. Circ. Res. 2001;89:496–502. doi: 10.1161/hh1801.096338. [DOI] [PubMed] [Google Scholar]
- KOLESNICK R. The therapeutic potential of modulating the ceramide/sphingomyelin pathway. J. Clin. Invest. 2002;110:3–8. doi: 10.1172/JCI16127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KROWN K.A., PAGE M.T., NGUYEN C., ZECHNER D., GUTIERREZ V., COMSTOCK K.L., GLEMBOTSKI C.C., QUINTANA P.J.E., SABBADINI R.A. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Invest. 1996;98:2854–2865. doi: 10.1172/JCI119114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWON Y.G., MIN J.K., KIM K.M., LEE D.J., BILLIAR T.R., KIM Y.M. Sphingosine 1-phosphate protects human umbilical vein endothelial cells from serum-deprived apoptosis by nitric oxide production. J. Biol. Chem. 2001;276:10627–10633. doi: 10.1074/jbc.M011449200. [DOI] [PubMed] [Google Scholar]
- LANGLOIS S., GINGRAS D., BELIVEAU R. Membrane type 1-matrix metalloproteinase (MT1-MMP) cooperates with sphingosine 1-phosphate to induce endothelial cell migration and morphogenic differentiation. Blood. 2004;103:3020–3028. doi: 10.1182/blood-2003-08-2968. [DOI] [PubMed] [Google Scholar]
- LEE M.J., THANGADA S., CLAFFEY K.P., ANCELLIN N., LIU C.H., KLUK M., VOLPI M., SHA'AFI R.I., HLA T. Vascular endothelial cell adherens junction assembly and morphogenesis induced by sphingosine-1-phosphate. Cell. 1999a;99:301–312. doi: 10.1016/s0092-8674(00)81661-x. [DOI] [PubMed] [Google Scholar]
- LEE M.J., VAN BROCKLYN J.R., THANGADA S., LIU C.H., HAND A.R., MENZELEEV R., SPIEGEL S., HLA T. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279:1552–1555. doi: 10.1126/science.279.5356.1552. [DOI] [PubMed] [Google Scholar]
- LEE O.H., KIM Y.M., LEE Y.M., MOON E.J., LEE D.J., KIM J.H., KIM K.W., KWON Y.G. Sphingosine 1-phosphate induces angiogenesis: its angiogenic action and signaling mechanism in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 1999b;264:743–750. doi: 10.1006/bbrc.1999.1586. [DOI] [PubMed] [Google Scholar]
- LEE P.C., SALYAPONGSE A.N., BRAGDON G.A., SHEARS L.L., II, WATKINS S.C., EDINGTON H.D., BILLIAR T.R. Impaired wound healing and angiogenesis in eNOS-deficient mice. Am. J. Physiol. 1999c;277:H1600–H1608. doi: 10.1152/ajpheart.1999.277.4.H1600. [DOI] [PubMed] [Google Scholar]
- LEVEEN P., PEKNY M., GEBRE-MEDHIN S., SWOLIN B., LARSSON E., BETSHOLTZ C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- LI P.L., ZHANG D.X., ZOU A.P., CAMPBELL W.B. Effect of ceramide on KCa channel activity and vascular tone in coronary arteries. Hypertension. 1999;33:1441–1446. doi: 10.1161/01.hyp.33.6.1441. [DOI] [PubMed] [Google Scholar]
- LILIOM K., SUN G., BUNEMANN M., VIRAG T., NUSSER N., BAKER D.L., WANG D.A., FABIAN M.J., BRANDTS B., BENDER K., EICKEL A., MALIK K.U., MILLER D.D., DESIDERIO D.M., TIGYI G., POTT L. Sphingosylphosphocholine is a naturally occurring lipid mediator in blood plasma: a possible role in regulating cardiac function via sphingolipid receptors. Biochem. J. 2001;355:189–197. doi: 10.1042/0264-6021:3550189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDAHL P., JOHANSSON B.R., LEVEEN P., BETSHOLTZ C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- LIU F., VERIN A.D., WANG P., DAY R., WERSTO R.P., CHREST F.J., ENGLISH D.K., GARCIA J.G. Differential regulation of sphingosine-1-phosphate- and VEGF-induced endothelial cell chemotaxis. Involvement of G(ialpha2)-linked Rho kinase activity. Am. J. Respir. Cell. Mol. Biol. 2001;24:711–719. doi: 10.1165/ajrcmb.24.6.4323. [DOI] [PubMed] [Google Scholar]
- LIU S.J., KENNEDY R.H. Positive inotropic effect of ceramide in adult ventricular myocytes: mechanisms dissociated from its reduction in Ca2+ influx. Am. J. Physiol. 2003;285:H735–H744. doi: 10.1152/ajpheart.01098.2002. [DOI] [PubMed] [Google Scholar]
- LIU Y., WADA R., YAMASHITA T., MI Y., DENG C.X., HOBSON J.P., ROSENFELDT H.M., NAVA V.E., CHAE S.S., LEE M.J., LIU C.H., HLA T., SPIEGEL S., PROIA R.L. Edg-1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J. Clin. Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOPEZ-MARURE R., VENTURA J.L., SANCHEZ L., MONTANO L.F., ZENTELLA A. Ceramide mimics tumour necrosis factor-alpha in the induction of cell cycle arrest in endothelial cells. Induction of the tumour suppressor p53 with decrease in retinoblastoma/protein levels. Eur. J. Biochem. 2000;267:4325–4333. doi: 10.1046/j.1432-1327.2000.01436.x. [DOI] [PubMed] [Google Scholar]
- MACDONELL K.L., SEVERSON D.L., GILES W.R. Depression of excitability by sphingosine 1-phosphate in rat ventricular myocytes. Am. J. Physiol. 1998;275:H2291–H2299. doi: 10.1152/ajpheart.1998.275.6.H2291. [DOI] [PubMed] [Google Scholar]
- MAZURAIS D., ROBERT P., GOUT B., BERREBI-BERTRAND I., LAVILLE M.P., CALMELS T. Cell type-specific localization of human cardiac S1P receptors. J. Histochem. Cytochem. 2002;50:661–670. doi: 10.1177/002215540205000507. [DOI] [PubMed] [Google Scholar]
- MCDONOUGH P.M., YASUI K., BETTO R., SALVIATI G., GLEMBOTSKI C.C., PALADE P.T., SABBADINI R.A. Control of cardiac Ca2+ levels. Inhibitory actions of sphingosine on Ca2+ transients and L-type Ca2+ channel conductance. Circ. Res. 1994;75:981–989. doi: 10.1161/01.res.75.6.981. [DOI] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., HIMMEL H.M., JAKOBS K.H. Sphingosylphosphorylcholine – biological functions and mechanisms of action. Biochim. Biophys. Acta. 2002;1582:178–189. doi: 10.1016/s1388-1981(02)00154-3. [DOI] [PubMed] [Google Scholar]
- MEYER ZU HERINGDORF D., VAN KOPPEN C.J., JAKOBS K.H. Molecular diversity of sphingolipid signalling. FEBS Lett. 1997;410:34–38. doi: 10.1016/s0014-5793(97)00320-7. [DOI] [PubMed] [Google Scholar]
- MIURA S., TANIGAWA H., MATSUO Y., FUJINO M., KAWAMURA A., SAKU K. Ras/Raf1-dependent signal in sphingosine-1-phosphate-induced tube formation in human coronary artery endothelial cells. Biochem. Biophys. Res. Commun. 2003;306:924–929. doi: 10.1016/s0006-291x(03)01065-9. [DOI] [PubMed] [Google Scholar]
- MOGAMI K., MIZUKAMI Y., TODOROKI-IKEDA N., OHMURA M., YOSHIDA K., MIWA S., MATSUZAKI M., MATSUDA M., KOBAYASHI S. Sphingosylphosphorylcholine induces cytosolic Ca2+ elevation in endothelial cells in situ and causes endothelium-dependent relaxation through nitric oxide production in bovine coronary artery. FEBS Lett. 1999;457:375–380. doi: 10.1016/s0014-5793(99)01076-5. [DOI] [PubMed] [Google Scholar]
- MORALES-RUIZ M., LEE M.J., ZOLLNER S., GRATTON J.P., SCOTLAND R., SHIOJIMA I., WALSH K., HLA T., SESSA W.C. Sphingosine 1-phosphate activates Akt, nitric oxide production, and chemotaxis through a Gi protein/phosphoinositide 3-kinase pathway in endothelial cells. J. Biol. Chem. 2001;276:19672–19677. doi: 10.1074/jbc.M009993200. [DOI] [PubMed] [Google Scholar]
- MUROHARA T., ASAHARA T., SILVER M., BAUTERS C., MASUDA H., KALKA C., KEARNEY M., CHEN D., SYMES J.F., FISHMAN M.C., HUANG P.L., ISNER J.M. Nitric oxide synthase modulates angiogenesis in response to tissue ischaemia. J. Clin. Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MUROHARA T., KUGIYAMA K., OHGUSHI M., SUGIYAMA S., OHTA Y., YASUE H. Effects of sphingomyelinase and sphingosine on arterial vasomotor regulation. J. Lipid Res. 1996;37:1601–1608. [PubMed] [Google Scholar]
- NAKAJIMA N., CAVALLI A.L., BIRAL D., GLEMBOTSKI C.C., MCDONOUGH P.M., HO P.D., BETTO R., SANDONA D., PALADE P.T., DETTBARN C.A., KLEPPER R.E., SABBADINI R.A. Expression and characterization of Edg-1 receptors in rat cardiomyocytes: calcium deregulation in response to sphingosine 1-phosphate. Eur. J. Biochem. 2000;267:5679–5686. doi: 10.1046/j.1432-1327.2000.01656.x. [DOI] [PubMed] [Google Scholar]
- NAKAO F., KOBAYASHI S., MOGAMI K., MIZUKAMI Y., SHIRAO S., MIWA S., TODOROKI-IKEDA N., ITO M., MATSUZAKI M. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ. Res. 2002;91:953–960. doi: 10.1161/01.res.0000042702.04920.bf. [DOI] [PubMed] [Google Scholar]
- NIEDERNBERG A., TUNARU S., BLAUKAT A., ARDATI A., KOSTENIS E. Sphingosine 1-phosphate and dioleoylphosphatidic acid are low affinity agonists for the orphan receptor GPR63. Cell. Signal. 2003;15:435–446. doi: 10.1016/s0898-6568(02)00119-5. [DOI] [PubMed] [Google Scholar]
- OHMORI T., YATOMI Y., OSADA M., KAZAMA F., TAKAFUTA T., IKEDA H., OZAKI Y. Sphingosine 1-phosphate induces contraction of coronary artery smooth muscle cells via S1P2. Cardiovasc. Res. 2003;58:170–177. doi: 10.1016/s0008-6363(03)00260-8. [DOI] [PubMed] [Google Scholar]
- OKAJIMA F. Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator. Biochim. Biophys. Acta. 2002;1582:132–137. doi: 10.1016/s1388-1981(02)00147-6. [DOI] [PubMed] [Google Scholar]
- OLIVERA A., SPIEGEL S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- ORAL H., DORN G.W., II, MANN D.L. Sphingosine mediates the immediate negative inotropic effects of tumor necrosis factor-α in the adult mammalian cardiac myocyte. J. Biol. Chem. 1997;272:4836–4842. doi: 10.1074/jbc.272.8.4836. [DOI] [PubMed] [Google Scholar]
- PAIK J.H., CHAE S., LEE M.J., THANGADA S., HLA T. Sphingosine 1-phosphate-induced endothelial cell migration requires the expression of EDG-1 and EDG-3 receptors and Rho-dependent activation of alpha vbeta3- and beta1-containing integrins. J. Biol. Chem. 2001;276:11830–11837. doi: 10.1074/jbc.M009422200. [DOI] [PubMed] [Google Scholar]
- PANETTI T.S., NOWLEN J., MOSHER D.F. Sphingosine-1-phosphate and lysophosphatidic acid stimulate endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 2000;20:1013–1019. doi: 10.1161/01.atv.20.4.1013. [DOI] [PubMed] [Google Scholar]
- PAYNE S.G., MILSTIEN S., SPIEGEL S. Sphingosine-1-phosphate: dual messenger functions. FEBS Lett. 2002;531:54–57. doi: 10.1016/s0014-5793(02)03480-4. [DOI] [PubMed] [Google Scholar]
- PYNE S., PYNE N.J. Sphingosine 1-phosphate signalling in mammalian cells. Biochem. J. 2000;349:385–402. doi: 10.1042/0264-6021:3490385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADIN N.S. Killing tumours by ceramide-induced apoptosis: a critique of available drugs. Biochem. J. 2003;371:243–256. doi: 10.1042/BJ20021878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RELLING D.P., HINTZ K.K., REN J. Acute exposure of ceramide enhances cardiac contractile function in isolated ventricular myocytes. Br. J. Pharmacol. 2003;140:1163–1168. doi: 10.1038/sj.bjp.0705510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIKITAKE Y., HIRATA K., KAWASHIMA S., OZAKI M., TAKAHASHI T., OGAWA W., INOUE N., YOKOYAMA M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- ROBERT P., TSUI P., LAVILLE M.P., LIVI G.P., SARAU H.M., BRIL A., BERREBI-BERTRAND I. EDG1 receptor stimulation leads to cardiac hypertrophy in rat neonatal myocytes. J. Mol. Cell. Cardiol. 2001;33:1589–1606. doi: 10.1006/jmcc.2001.1433. [DOI] [PubMed] [Google Scholar]
- RUVOLO P.P. Intracellular signal transduction pathways activated by ceramide and its metabolites. Pharmacol. Res. 2003;47:383–392. doi: 10.1016/s1043-6618(03)00050-1. [DOI] [PubMed] [Google Scholar]
- RYU Y., TAKUWA N., SUGIMOTO N., SAKURADA S., USUI S., OKAMOTO H., MATSUI O., TAKUWA Y. Sphingosine-1-phosphate, a platelet-derived lysophospholipid mediator, negatively regulates cellular Rac activity and cell migration in vascular smooth muscle cells. Circ. Res. 2002;90:325–332. doi: 10.1161/hh0302.104455. [DOI] [PubMed] [Google Scholar]
- SALOMONE S., YOSHIMURA S., REUTER U., FOLEY M., THOMAS S.S., MOSKOWITZ M.A., WAEBER C. S1P3 receptors mediate the potent constriction of cerebral arteries by sphingosine-1-phosphate. Eur. J. Pharmacol. 2003;469:125–134. doi: 10.1016/s0014-2999(03)01731-x. [DOI] [PubMed] [Google Scholar]
- SANNA M.G., LIAO J., JO E., ALFONSO C., AHN M.-Y., PETERSON M.S., WEBB B., LEFEBVRE S., CHUN J., GRAY N., ROSEN H. Distinct S1P receptor subtypes S1P1 and S1P3 respectively regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004;279:13839–13848. doi: 10.1074/jbc.M311743200. [DOI] [PubMed] [Google Scholar]
- SEKIGUCHI K., YOKOYAMA T., KURABAYASHI M., OKAJIMA F., NAGAI R. Sphingosylphosphorylcholine induces a hypertrophic growth response through the mitogen-activated protein kinase signaling cascade in rat neonatal cardiac myocytes. Circ. Res. 1999;85:1000–1008. doi: 10.1161/01.res.85.11.1000. [DOI] [PubMed] [Google Scholar]
- SHIRAO S., KASHIWAGI S., SATO M., MIWA S., NAKAO F., KUROKAWA T., TODOROKI-IKEDA N., MOGAMI K., MIZUKAMI Y., KURIYAMA S., HAZE K., SUZUKI M., KOBAYASHI S. Sphingosylphosphorylcholine is a novel messenger for rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery. Unimportant role for protein kinase C. Circ. Res. 2002;91:112–119. doi: 10.1161/01.res.0000026057.13161.42. [DOI] [PubMed] [Google Scholar]
- SHU X., WU W., MOSTELLER R.D., BROEK D. Sphingosine kinase mediates vascular endothelial growth factor-induced activation of ras and mitogen-activated protein kinases. Mol. Cell. Biol. 2002;22:7758–7768. doi: 10.1128/MCB.22.22.7758-7768.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SORIANO P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MILSTIEN S. Functions of a new family of sphingosine-1-phosphate receptors. Biochim. Biophys. Acta. 2000;1484:107–116. doi: 10.1016/s1388-1981(00)00010-x. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MILSTIEN S. Sphingosine 1-phosphate, a key cell signaling molecule. J. Biol. Chem. 2002;277:25851–25854. doi: 10.1074/jbc.R200007200. [DOI] [PubMed] [Google Scholar]
- SPIEGEL S., MILSTIEN S. Exogenous and intracellularly generated sphingosine 1-phosphate can regulate cellular processes by divergent pathways. Biochem. Soc. Trans. 2003;31:1216–1219. doi: 10.1042/bst0311216. [DOI] [PubMed] [Google Scholar]
- SUGISHITA K., KINUGAWA K., SHIMIZU T., HARADA K., MATSUI H., TAKAHASHI T., SERIZAWA T., KOHMOTO O. Cellular basis for the acute inhibitory effects of IL-6 and TNF-α on excitation–contraction coupling. J. Mol. Cell. Cardiol. 1999;31:1457–1467. doi: 10.1006/jmcc.1999.0989. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., AYE N.N., YATOMI Y., OZAKI Y., HASHIMOTO K. Effects of sphingosine 1-phosphate, a naturally occurring biologically active lysophospholipid, on the rat cardiovascular system. Jpn. J. Pharmacol. 2000a;82:338–342. doi: 10.1254/jjp.82.338. [DOI] [PubMed] [Google Scholar]
- SUGIYAMA A., YATOMI Y., OZAKI Y., HASHIMOTO K. Sphingosine 1-phosphate induces sinus tachycardia and coronary vasoconstriction in the canine heart. Cardiovasc. Res. 2000b;46:119–125. doi: 10.1016/s0008-6363(00)00013-4. [DOI] [PubMed] [Google Scholar]
- TAKUWA Y. Subtype-specific differential regulation of Rho family G proteins and cell migration by the Edg family sphingosine-1-phosphate receptors. Biochim. Biophys. Acta. 2002;1582:112–120. doi: 10.1016/s1388-1981(02)00145-2. [DOI] [PubMed] [Google Scholar]
- TAKUWA Y., OKAMOTO H., TAKUWA N., GONDA K., SUGIMOTO N., SAKURADA S. Subtype-specific, differential activities of the EDG family receptors for sphingosine-1-phosphate, a novel lysophospholipid mediator. Mol. Cell. Endocrinol. 2001;177:3–11. doi: 10.1016/s0303-7207(01)00441-5. [DOI] [PubMed] [Google Scholar]
- TAMAMA K., KON J., SATO K., TOMURA H., KUWABARA A., KIMURA T., KANDA T., OHTA H., UI M., KOBAYASHI I., OKAJIMA F. Extracellular mechanism through the Edg family of receptors might be responsible for sphingosine-1-phosphate-induced regulation of DNA synthesis and migration of rat aortic smooth-muscle cells. Biochem. J. 2001;353:139–146. [PMC free article] [PubMed] [Google Scholar]
- TANIMOTO T., JIN Z.G., BERK B.C. Transactivation of vascular endothelial growth factor (VEGF) receptor Flk-1/KDR is involved in sphingosine 1-phosphate-stimulated phosphorylation of Akt and endothelial nitric-oxide synthase (eNOS) J. Biol. Chem. 2002;277:42997–43001. doi: 10.1074/jbc.M204764200. [DOI] [PubMed] [Google Scholar]
- THIELMANN M., DÖRGE H., MARTIN C., BELOSJOROW S., SCHWANKE U., VAN DE SAND A., KONIETZKA I., BÜCHERT A., KRÜGER A., SCHULZ R., HEUSCH G. Myocardial dysfunction with coronary microembolization. Signal transduction through a sequence of nitric oxide, tumor necrosis factor-α, and sphingosine. Circ. Res. 2002;90:807–813. doi: 10.1161/01.res.0000014451.75415.36. [DOI] [PubMed] [Google Scholar]
- TODOROKI-IKEDA N., MIZUKAMI Y., MOGAMI K., KUSUDA T., YAMAMOTO K., MIYAKE T., SATO M., SUZUKI S., YAMAGATA H., HOKAZONO Y., KOBAYASHI S. Sphingosylphosphorylcholine induces Ca2+-sensitization of vascular smooth muscle contraction: possible involvement of rho-kinase. FEBS Lett. 2000;482:85–90. doi: 10.1016/s0014-5793(00)02046-9. [DOI] [PubMed] [Google Scholar]
- TOSAKA M., OKAJIMA F., HASHIBA Y., SAITO N., NAGANO T., WATANABE T., KIMURA T., SASAKI T. Sphingosine 1-phosphate contracts canine basilar arteries in vitro and in vivo: possible role in pathogenesis of cerebral vasospasm. Stroke. 2001;32:2913–2919. doi: 10.1161/hs1201.099525. [DOI] [PubMed] [Google Scholar]
- UEHARA A., YASUKOCHI M., IMANAGA I., BERLIN J.R. Effect of sphingosylphosphorylcholine on the single channel gating properties of the cardiac ryanodine receptor. FEBS Lett. 1999;460:467–471. doi: 10.1016/s0014-5793(99)01385-x. [DOI] [PubMed] [Google Scholar]
- UHLENBROCK K., GASSENHUBER H., KOSTENIS E. Sphingosine 1-phosphate is a ligand of the human gpr3, gpr6 and gpr12 family of constitutively active G protein-coupled receptors. Cell. Signal. 2002;14:941–953. doi: 10.1016/s0898-6568(02)00041-4. [DOI] [PubMed] [Google Scholar]
- UHLENBROCK K., HUBER J., ARDATI A., BUSCH A.E., KOSTENIS E. Fluid shear stress differentially regulates gpr3, gpr6, and gpr12 expression in human umbilical vein endothelial cells. Cell. Physiol. Biochem. 2003;13:75–84. doi: 10.1159/000070251. [DOI] [PubMed] [Google Scholar]
- VAN KOPPEN C.J., MEYER ZU HERINGDORF D., LASER K.T., ZHANG C., JAKOBS K.H., BÜNEMANN M., POTT L. Activation of a high affinity Gi protein-coupled plasma membrane receptor by sphingosine-1-phosphate. J. Biol. Chem. 1996;271:2082–2087. doi: 10.1074/jbc.271.4.2082. [DOI] [PubMed] [Google Scholar]
- WANG F., VAN BROCKLYN J.R., HOBSON J.P., MOVAFAGH S., ZUKOWSKA-GROJEC Z., MILSTIEN S., SPIEGEL S. Sphingosine 1-phosphate stimulates cell migration through a G(i)-coupled cell surface receptor. Potential involvement in angiogenesis. J. Biol. Chem. 1999;274:35343–35350. doi: 10.1074/jbc.274.50.35343. [DOI] [PubMed] [Google Scholar]
- WEBSTER R.J., SABBADINI R.A., DETTBARN C.A., PAOLINI P.J. Sphingosine effects on the contractile behavior of skinned cardiac myocytes. J. Mol. Cell. Cardiol. 1994;26:1273–1290. doi: 10.1006/jmcc.1994.1147. [DOI] [PubMed] [Google Scholar]
- WECKING C., MICHEL M.C., BISCHOFF A. Effects of indomethacin on sphingolipid-induced diuresis and natriuresis. Naunyn-Schmiedeberg's Arch. Pharmacol. 2001;363 Suppl:R113. [Google Scholar]
- XU Y., ZHU K., HONG G., WU W., BAUDHUIN L.M., XIAO Y., DAMRON D.S. Sphingosylphosphorylcholine is a ligand for ovarian cancer G-protein-coupled receptor 1. Nat. Cell Biol. 2000;2:261–267. doi: 10.1038/35010529. [DOI] [PubMed] [Google Scholar]
- YASUI K., PALADE P. Sphingolipid actions on sodium and calcium currents of rat ventricular myocytes. Am. J. Physiol. 1996;270:C645–C649. doi: 10.1152/ajpcell.1996.270.2.C645. [DOI] [PubMed] [Google Scholar]
- YASUKOCHI M., UEHARA A., KOBAYASHI S., BERLIN J.R. Ca2+ and voltage dependence of cardiac ryanodine receptor channel block by sphingosylphosphorylcholine. Pflügers Arch. 2003;445:665–673. doi: 10.1007/s00424-002-0945-3. [DOI] [PubMed] [Google Scholar]
- YATOMI Y., IGARASHI Y., YANG L., HISANO N., QI R., ASAZUMA N., SATOH K., OZAKI Y., KUME S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J. Biochem. (Tokyo) 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- ZHANG D.X., ZOU A.-P., LI P.-L. Ceramide reduces endothelium-dependent vasodilation by increasing superoxide production in small bovine coronary arteries. Circ. Res. 2001;88:824–831. doi: 10.1161/hh0801.089604. [DOI] [PubMed] [Google Scholar]
- ZHANG G., CONTOS J.J., WEINER J.A., FUKUSHIMA N., CHUN J. Comparative analysis of three murine G-protein coupled receptors activated by sphingosine-1-phosphate. Gene. 1999;227:89–99. doi: 10.1016/s0378-1119(98)00589-7. [DOI] [PubMed] [Google Scholar]
- ZHENG T., LI W., WANG J., ALTURA B.T., ALTURA B.M. C2-Ceramide attenuates phenylephrine-induced vasoconstriction and elevation in [Ca2+]i in rat aortic smooth muscle. Lipids. 1999;34:689–695. doi: 10.1007/s11745-999-0414-4. [DOI] [PubMed] [Google Scholar]