Abstract
Calcitonin gene-related peptide (CGRP) is believed to play a pivotal role in the pathogenesis of migraine via activation of CGRP receptors in the trigeminovascular system. The CGRP receptor antagonist, BIBN4096BS, has proven efficacy in the acute treatment of migraine attacks and represents a new therapeutic principle.
We used an improved closed cranial window model to measure changes of the middle meningeal artery (MMA) and cortical pial artery/arteriole diameter (PA) and changes in local cortical cerebral blood flow (LCBFFlux) in anaesthetised artificially ventilated rats.
The ability of BIBN4096BS (i.v.) to prevent the vasodilatatory actions of rat-αCGRP, βCGRP and endogenously released CGRP following transcranial electrical stimulation (TES) was investigated.
BIBN4096BS was per se without vasoactive effect on any of the measured variables and significantly inhibited the hypotension induced by both types of CGRP (P<0.001).
The αCGRP induced MMA dilatation was reduced from 97.4±14 to 2.1±1.3% (P<0.001) and the βCGRP induced dilatation was fully blocked by BIBN4096BS. ID50 was 5.4±1.6 μg kg−1 for αCGRP and 16.3±1.6 μg kg−1 for βCGRP.
Transcranial electrical stimulation induced a 119.1±6.9% increase in MMA diameter. BIBN4096BS (333 μg kg−1) attenuated this increase (19.8±2.1%) (P<0.001).
Systemic CGRP and TES induced an increase in PA diameter that was not significantly inhibited by BIBN4096BS. The CGRP induced increase in LCBFFlux was similar not prevented by the antagonist.
We suggest that systemic BIBN4096BS exerts its inhibitory action mainly on large dural blood vessels (MMA).
Keywords: Migraine, CGRP and CGRP receptor antagonism, BIBN4096BS, middle meningeal artery, cortical pial arterioles and cortical cerebral blood flow
Introduction
Trigeminal sensory C-fibres innervating the cranial vessels contain several vasoactive peptides such as calcitonin gene-related peptide (CGRP), neurokinin A, substance P and pituitary adenylate cyclase activating peptide (PACAP) (Gulbenkian et al., 2001). Electrical stimulation of the trigeminal ganglion liberates vasoactive peptides into the perivascular space. CGRP is a potent dilator of cerebral and dural blood vessels (Williamson et al., 1997a; Jansen-Olesen et al., 2003) and may interact not only with smooth muscle cells, but also with presynaptic receptors on the perivascular nerves (Williamson et al., 1997b). CGRP plasma levels are increased during acute attacks of migraine and cluster headache (Goadsby et al., 1990; Goadsby & Edvinsson, 1994) and infusion of CGRP induces headache and migraine attacks in migraine patients (Lassen et al., 2002).
The peptide fragment of CGRP (CGRP8–37) and compounds such as ‘compound 1' and SB-273779 are effective in antagonising CGRP-induced responses in vitro and in vivo (Williamson et al., 1997b; Aiyar et al., 2001; Edvinsson et al., 2001b). These antagonists are, however, not suitable for human use. BIBN4096BS is a well-characterised antagonist, which is highly selective and effective in animal and human experiments (Doods et al., 2000; Wu et al., 2000; Edvinsson et al., 2002; Petersen et al., 2003). It is safe for human administration and in a proof of concept phase-II study, it was found to be effective in aborting acute migraine attacks (Olesen et al., 2004).
In the present study, we used a closed cranial window model often applied in migraine research (Williamson et al., 1997a; Akerman et al., 2002). The experimental set-up allows investigation of cerebrovascular responses to transcranial electrical stimulation (TES) and systemic administration of CGRP (Williamson et al., 1997a). In previous studies using this method, only data on the reactivity of the middle meningeal artery (MMA) was reported (Williamson et al., 1997a; Akerman et al., 2002). We have verified the method and further demonstrated the possibility of measuring diameter changes of cortical pial arteries (PA) and local cortical cerebral blood flow (LCBFFlux) by laser Doppler flowmetry (LDF) (Petersen et al., 2004a).
We have currently used this technique to investigate the ability of BIBN4096BS to inhibit the effect of αCGRP, βCGRP and TES on MMA, PA, LCBFFlux and mean arterial blood pressure (MABP). The study contributes to a further understanding of the antimigraine effect of BIBN4096BS.
Methods
Surgical preparation
All experiments were performed in accordance with national guidelines and regulations for animal care and treatment. The study protocol was approved by the Danish Animal Experimental Inspectorate (file: 2001/561–390).
Male Sprague–Dawley rats (300–400 g) were anaesthetised throughout the study with pentobarbital (Mebumal®, 60 mg kg−1, i.p. for induction and 20 mg kg−1 h−1 i.v. for maintenance). A tracheotomy was performed and the animals were artificially ventilated (Abovent 7025, Ugo Basil, Italy) with a 30/70% air mixture of O2/N2O (stroke rate 60–65 min−1 and a stroke volume of 3.5–4.0 ml). The body temperature was kept at 37.0–37.5°C using an automatic regulated heating plate (Letica HB101, Panlab, Spain). The femoral artery and vein were cannulated bilateral with polythene catheters (Portex® polyethylene catheters ID 0.4, Astratech, Denmark) for measurement of MABP (Transducer TCM4-7, World Precision Instruments Inc., U.S.A.), arterial blood samples, infusion of anaesthetics and test substances. All data were continuously displayed on a computer monitor by the data acquisition and analysis software Perisoft® (Perisoft® for Windows 2.0, Perimed AB, Sweden). Arterial blood analysis (ABL520, Radiometer A/S, Denmark) was performed at least three times during each study. PaCO2, PaO2 and pH were kept within normal limits (pH 7.40–7.42, PaCO2 36.5–38.7 mmHg and PaO2 112.5–121.9 mmHg). The inspiratory and expiratory air was continually monitored by a capnograph (Capnomac AGM103, Datex-Ohmeda, Finland). The animal was placed in a stereotactic frame. Skin and connective tissue were removed from the dorsal side of the skull. The right parietal bone was thinned until transparency using a dental drill. During the drilling, cooling was obtained by local application of ice-cold isotonic saline.
Video microscopy
The cranial window was covered with mineral oil (37°C), a branch of the MMA and a PA were visualised using a video-microscope (Sony DSP digital camera, MS50 objective, Japan). The real-time image was displayed on a TV-screen. The diameter of the vessels was continuously measured by a video dimension analyser (V94, Living Systems Instrumentation Inc., U.S.A.). LCBFFlux was measured continuously using LDF (Perimed PF4001, Perimed AB, Sweden). The probe (Perimed 410, fibre separation 0.25 mm, Perimed AB, Sweden) was placed using a micromanipulator and microscope in an area with no or few observable vessels. The probe was placed free of the bone, but in touch with the mineral oil, which enhances the accuracy of the LDF measurement (Gerrits et al., 1998).
Experimental protocols
Dose–effect relationship of r-αCGRP and βCGRP
After preparation, the rat rested for 1–1½ h before the effect of the two forms of CGRP on MMA, PA, LCBFFlux and MABP was determined by cumulative infusions of semilogarithmic increasing doses. The CGRP (0.01 ng kg−1–3 μg kg−1) was administered as a bolus injection of 100 μl succeeded by a 150 μl isotonic saline (0.9% NaCl) flush, at 10 min intervals. Only one dose–effect curve was obtained in each animal.
Repeated infusions of αCGRP and βCGRP
Previously repeated exposure to CGRP (four to seven times) did not induce tolerance or tachyphylaxia (Foulkes et al., 1991; Wu, 1999; Akerman et al., 2002). To study, if tolerance or tachyphylaxia developed with αCGRP or βCGRP (0.3 μg kg−1), infusions of the peptides were repeated seven times with a 15 min interval between infusions, as in the agonist–antagonist experiments described below.
Effect of BIBN4096BS
BIBN4096BS (1–333 μg kg−1) was infused in cumulative semilogarithmic increasing doses with a 15 min interval to investigate a possible vasoactivity.
Electrical stimulation parameters
To investigate whether neurogenically induced vasodilatation was inhibited by BIBN4096BS, a bipolar stimulation electrode (NE-200x, Harvard Instruments, U.K.) was placed on the surface of the cranial window approximately 200 μm from the investigated arteries. Stimulations at 5 Hz, 1 ms pulse width and of 10 s duration were applied (Grass Stimulator S48, Grass Instruments, U.S.A.). Stimulations were done with increasing voltage until maximum dilation was observed. The same voltage was used for the subsequent TES in the same animal (Williamson et al., 1997a).
A control experiment was performed to elucidate the reproducibility of seven consecutive electrically evoked responses in each animal. The seven stimulations were applied with 25 min intervals, as in the pharmacological experiments.
Effect of BIBN4096BS on αCGRP and βCGRP-induced hypotension and vasodilatation
In each rat, a control response to αCGRP or βCGRP (0.3 μg kg−1) was first recorded. The first dose of BIBN4096BS (1 μg kg−1) was administered 10 min later and followed by a second bolus of CGRP 5 min later. An increased dose of BIBN4096BS (3.3 μg kg−1) was given after another 10 min, and again followed by a CGRP bolus after 5 min This sequence was repeated six times in all in each animal with increasing doses of BIBN4096BS until 333 μg kg−1.
Effect of BIBN4096BS on neurogenically induced vasodilatation
The same protocol as for the CGRP application was used. However, the interval between the TES was 25 min, while the interval from administration of the antagonist to renewed stimulation was 5 min. In this protocol, LCBFFlux was not measured due to technical reasons.
Data analysis
The maximum effect (peak) of CGRP or applied TES on MMA, PA and LCBFFlux was calculated as the percentage increase or decrease from the baseline. The prestimulation baseline was defined as the average of the 60 s preceding the stimulation. The MMA and PA diameter were measured in arbitrary units, since the experimental set-up was dependent on the individual course of the arteries chosen for each experiment and a different magnification was used to optimise the image and analytic possibilities of the video dimension analyser. A standardised artery measurement would therefore be im-practical. Laser Doppler flowmetry was measured in tissue perfusion units (arbitrary). The unit of the mean arterial blood pressure was mmHg and changes expressed as Δ mmHg. All listed data are mean±s.e.m. and a significance level of P<0.05 was applied. Statistical analysis was performed using ANOVA. In the analytic design, the dose and animal were included as factors. If significance was found, a post hoc analysis was performed using Dunnett's multiple comparison tests with the initial stimulation or infusion as control. Comparison between the different groups of experiments with the different agonists was performed using an unpaired t-test. All statistical analyses were performed using SPSS version 10.0 (Chicago, IL, U.S.A.). Graphs and estimation of ID50-values were done using GraphPrism©, version 3 (GraphPad, U.S.A.).
Drugs
Rat-α and β-calcitonin gene-related peptide (Neosystem, France) was dissolved in distilled water. BIBN4096BS was synthesised by Boehringer Ingelheim Pharma GmbH & Co. KG Germany, and provided as a gift. BIBN4096BS was dissolved in a small volume (20 μl) of 1 N HCl, further diluted with saline (0.9% NaCl), and finally adjusted to pH 6.5–7.0 by 1 N NaOH. Stock solutions of each compound were aliquotted and frozen at −20°C. Before infusion all solutions were diluted to the final concentration using isotonic saline.
Results
Dose–effect relationship of αCGRP and βCGRP
Figure 1a–d illustrates the dose–effect curves obtained for MABP, MMA, PA and LCBFFlux (n=6). There were no significant difference in maximal responses to αCGRP and βCGRP on MABP (P=0.4), MMA diameter (P=0.06) and PA diameter (P=0.5). Data on MABP and PA have been published previously (Petersen et al., 2004b) LCBFFlux increased more after αCGRP than βCGRP at doses above 10 ng kg−1.
Figure 1.
(a–d) Dose–effect curves of semilogarithmic increasing dose of αCGRP (○, n=6) and βCGRP (•, n=6). The dose–effect curves are displayed for (a) mean arterial blood pressure, (b) middle meningeal artery, (c) pial arteriole and (d) local cortical cerebral blood flow. Values are mean±s.e.m. (a) and (c) with permission Petersen et al., 2004b.
Control of repeated infusions or TES
Both forms of CGRP resulted in consistent and reproducible responses in all measured parameters, when CGRP was administered seven times as an intravenous bolus at 15 min intervals (Table 1). After an initial voltage determination, it was possible to evoke the same dilatatory response to TES of both MMA and PA diameter seven times without any significant differences (Table 1).
Table 1.
Repeated CGRP infusion and TES
| Infusion or TES no. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | P= |
|---|---|---|---|---|---|---|---|---|
| r-αCGRP (n=4) | ||||||||
| ΔMABP (mmHg) | −25.6±4.4 | −23.1±4.6 | −22.5±4 | −23.9±0.7 | −26.8±4.1 | −28.5±3.9 | −29.6±6 | 0.3 |
| ΔDura (%) | 88.5±21.7 | 91.6±22.8 | 86.6±20.4 | 83.2±18 | 82.3±15.5 | 90.7±21.5 | 89.1±21.3 | 0.5 |
| ΔPia (%) | 28.6±11.6 | 32.6±15.2 | 30.4±12.6 | 32.4±17.8 | 29.3±9.8 | 26.8±8.7 | 34.3±17.9 | 0.8 |
| ΔLCBFFlux(%) | 30.5±8.5 | 31.5±7.9 | 28.6±9.3 | 29.9±8.9 | 28±5.7 | 26.7±6.6 | 27.9±6.5 | 0.5 |
| r-βCGRP (n=6) | ||||||||
| ΔMABP (mmHg) | −15.4±1.6 | −18.6±3.2 | −17.8±2.8 | −21.5±2.7 | −17.3±2.3 | −20.4±3.2 | −20.8±3 | 0.2 |
| ΔDura (%) | 105.9±16.8 | 105.5±19.5 | 108.7±18.1 | 104.5±13.6 | 109.7±13.5 | 113.9±18.7 | 112.3±14.9 | 0.9 |
| ΔPia (%) | 16.7±2.7 | 18.9±3 | 15.4±2.5 | 21.1±4.3 | 20±4.1 | 17.3±2.7 | 22±5.2 | 0.4 |
| ΔLCBFFlux(%) | 17.4±1.5 | 19.4±4.1 | 20.1±2.8 | 25±3.1 | 20.1±2.5 | 20.5±1.7 | 20.3±1.6 | 0.2 |
| TES (n=6) | ||||||||
| ΔMABP (mmHg) | −3.6±2.4 | −4.9±2.3 | −2.5±1.9 | −3.3±2 | −4.9±2.2 | −3.2±2.6 | −2.6±2.5 | 0.2 |
| ΔDura (%) | 131.8±13.3 | 125.2±12 | 130.9±14.1 | 140.5±10.9 | 137.5±13.4 | 130.5±9.4 | 133.5±11.1 | 0.5 |
| ΔPia (%) | 69±13.7 | 66.4±13.5 | 72.9±16.9 | 68.9±13.5 | 64.4±14.2 | 57±16.6 | 56±14.4 | 0.2 |
Percentual or mmHg change from baseline of the seven consecutive infusions of αCGRP, βCGRP or stimulations. Data are listed as mean±s.e.m. The P-values are the statistical comparison between the different infusions and stimulations (ANOVA).
Effect of BIBN4096BS per se
In the dose–effect studies of BIBN4096BS (n=3, Table 2), doses up to the maximum tested (1–333 μg kg−1) had no effect on MABP (P=0.12), MMA (P=0.8), PA (P=0.5) or LCBFFlux (P=0.6).
Table 2.
Effect of BIBN4096BS per se on the measured variables compared to preinfusion baseline, (n=3)
| Dose (μg kg−1) | MABP (mmHg) | MMA (%) | PA (%) | LCBFFlux (%) |
|---|---|---|---|---|
| 1 | 1.1±0.7 | 0 | 0 | 1.8a |
| 3.3 | 2.9±1.6 | 0 | 0 | 0 |
| 10 | 3.7±0.6 | 1.3a | 0 | 2.9a |
| 33 | 2.7±1.5 | 0 | 0 | 6.5±4.3 |
| 100 | −2.2±2.6 | 6.3a | 2.5a | 7.4±3.7 |
| 333 | −2.4±3.5 | 6.9±10.9 | 0 | 8.6±4.8 |
Denotes changes only seen in one animal.
Effect of BIBN4096BS on αCGRP-induced hypotension and vasodilatation
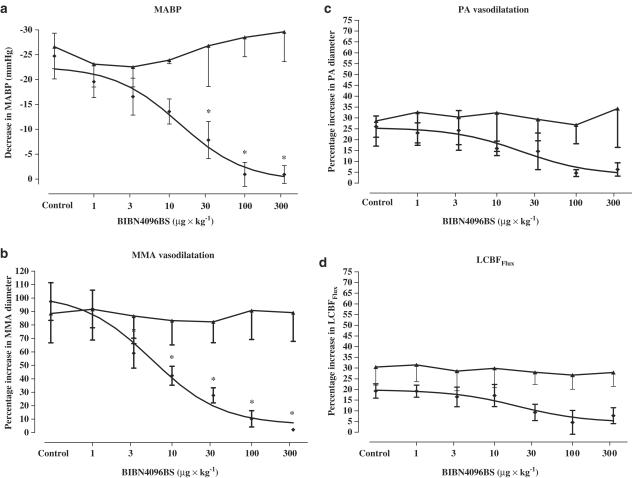
The maximum hypotensive response to αCGRP was −24.7±4.6 mmHg and the decrease in MABP after pre-treatment with the highest dose of the antagonist was −0.9±1.8 mmHg, P<0.001. The ID50 of BIBN4096BS was 21.3±1.4 μg kg−1. αCGRP caused a relaxation of the MMA (97.4±14%). BIBN4096BS inhibited this dilatation (2.1±1.3%, P<0.001). The ID50 of BIBN4096BS was 5.4±1.6 μg kg−1. αCGRP also induced a dilatation of the PA and an increase in LCBFFlux. The PA and LCBFFlux responses were attenuated dose-dependently, but not significantly by the antagonist. The control response of the PA was 26±4.9% and after the maximum dose of BIBN4096BS this was reduced to 6.3±3.1% (P=0.08). The increase in LCBFFlux was reduced from 19.3±3.4 to 7.7±3.7% (P=0.08, Figure 2a–d).
Figure 2.
(a–d) Effect of semilogarithmic increasing doses of BIBN4096BS on αCGRP induced changes (n=6, (⧫)). *=P<0.05 (Dunnett's test). In addition, the challenge of the seven repeated infusions of αCGRP (0.3 μg kg−1, n=6) from the control experiment are displayed as the upper line in the figure (▴), data shown in detail in Table 1.
Effect of BIBN4096BS on βCGRP-induced hypotension and vasodilatation
The βCGRP-induced hypotension and MMA vasodilatation were significantly inhibited by BIBN4096BS (P<0.001). The hypotensive response to βCGRP was −15.5±1.7 mmHg and the decrease in MABP after pretreatment with the maximum dose of BIBN4096BS was completely blocked (P<0.001). The ID50 was 24.7±1.3 μg kg−1. βCGRP relaxed the MMA by 74.6±13.6%, a dilatation fully blocked by the maximum dose of BIBN4096BS (P<0.001). The ID50 was 16.3±1.6 μg kg−1 (Figure 3a and b). The βCGRP-induced PA dilatation (11.8±3.2) was not affected by BIBN4096BS (333 μg kg−1, 8.8±2.3%, P=0.4). The βCGRP induced increase in LCBFFlux (13.8±1.6%) was not altered by the maximum dose of BIBN4096BS (8.2±4.1%, P=0.7).
Figure 3.
(a–b) Effect of semilogarithmic increasing doses of BIBN4096BS on βCGRP-induced changes (n=6, (⧫)). *=P<0.05 (Dunnett's test). In addition, the challenge of the seven repeated infusions of βCGRP (0.3 μg kg−1, n=6) from the control experiment are displayed as the upper line in the figure (▴), data shown in detail in Table 1.
Effect of BIBN4096BS on neurogenic vasodilatation
TES increased MMA diameter by 119.1±6.9%, which was reduced to 19.8±2.1% after BIBN4096BS (P<0.001, n=6, Figure 4a). The ID50 of BIBN4096BS on neurogenic dural vasodilatation was 48.3±1.9 μg kg−1. TES increased PA diameter by 96.4±14.2%, however was not significantly reduced by BIBN4096BS (P=0.2, Figure 4b).
Figure 4.
(a–b) Effect of semilogarithmic increasing doses of BIBN4096BS on neurogenic TES-induced vasodilatation (n=6, (⧫)). *=P<0.05 (Dunnett's test). In addition, the result of the seven repeated neurogenic vasodilatations from the control experiment are displayed as the second line in the figure (▴), data shown in detail in Table 1.
Discussion
The present series of experiments provide data on the in vivo effectiveness of the CGRP receptor antagonist BIBN4096BS and illustrates its possible site of action. The importance of BIBN4096BS resides in the fact that it is the first CGRP receptor antagonist proven to be effective in the treatment of acute migraine and thus represents a new principle in migraine therapy (Olesen et al., 2004).
Effects of αCGRP and βCGRP on the cranial circulation
We studied whether rat-αCGRP and βCGRP elicited the same effect on the peripheral and cerebral hemodynamics and investigated the ability of BIBN4096BS to prevent their vasodilatatory properties. A dose of 0.3 μg kg−1 of CGRP was chosen for the inhibition studies. The choice was based on the dose–effect relationship data obtained in this study (Figure 1) and existing knowledge of a submaximal and reproducible effect on the MMA of this dose (Williamson et al., 1997b; Honey et al., 2002). In the present study, αCGRP and βCGRP were equally potent and dose-dependently dilated both the MMA and the PA. This in agreement with previous findings showing that there are no major differences in the response of cranial arteries (human or animal) to the different subtypes of CGRP (Jansen-Olesen et al., 2003). The somewhat less-pronounced relaxation of the PAs by CGRP may be explained by the concomitant hypotension, since we and others have documented a correlation between PA diameter and hemorrhagic-induced hypotension (Kontos et al., 1978; Petersen et al., 2004a).
Studies investigating the effect of systemic CGRP on CBF have revealed conflicting results (Beattie et al., 1993; Baskaya et al., 1995). In most species including the rat, the peptide may increase CBF in response to i.v. administration (Suzuki et al., 1989; Baskaya et al., 1995). Our data supported this and showed that infusion of αCGRP or βCGRP, the former being more potent, dose-dependently increased LCBFFlux.
Mechanism and site of action of BIBN4096BS
BIBN4096BS is a relatively large hydrophilic compound and is therefore unlikely to pass the blood–brain barrier (BBB) in acute experiments. However, no direct data exist to support this. In contrast to cerebral vessels, the meningeal arteries have no BBB and BIBN4096BS is likely to diffuse freely into the wall of the MMA (Knudsen et al., 1988; Faraci et al., 1989). BIBN4096BS has been shown to have a high affinity for the CGRP1 receptor in vitro (Doods et al., 2000; Edvinsson et al., 2001b; 2002; Schindler & Doods, 2002). Since receptors with a CGRP1 pharmacological profile are primarily situated on the smooth muscle cells of cerebral arteries (Edvinsson et al., 2002; Oliver et al., 2002; Petersen et al., 2004b), it is not clear whether BIBN4096BS has any effect on cerebral arteries in vivo. However, an endothelial CGRP receptor may exist, since CRLR, RAMP1, RAMP2 and receptor component protein are expressed in microvascular endothelial cells and in human cerebral arteries, in addition RAMP3 (Edvinsson et al., 2002; Moreno et al., 2002; Oliver et al., 2002; Jansen-Olesen et al., 2003), but there are no functional data to support this.
We found that the MMA dilatation in response to systemically infused CGRP was blocked and the vasodilatation induced by TES (endogenously released CGRP) was markedly inhibited. Activation of the trigeminal ganglion by electrical stimulation releases primarily CGRP, but also, to a minor extent, substance P and PACAP and other peptides to the abluminal side of the vessels (Goadsby et al., 1988; Zagami et al., 1990; Williamson et al., 1997b). These co-released peptides may account for the remaining 20% of the dilatation not inhibited by BIBN4096BS. A higher local CGRP concentration and unspecific effects of TES are alternative explanations for the lack of complete inhibition. The inhibitory effect of BIBN4096BS was equally potent towards the αCGRP and βCGRP response, this in disagreement with previous in vivo findings (Wu et al., 2000), however in agreement with the larger part of existing in vitro data (Jansen-Olesen et al., 2003).
The PA investigated did not exceed a size of 100 μm and can be defined as second-order arterioles (Harper et al., 1984). The αCGRP-induced PA vasodilatation was only nonsignificantly reduced after the highest doses of BIBN4096BS. The antagonist did not inhibit βCGRP and the neurogenically evoked PA dilatation. Furthermore, BIBN4096BS did not significantly inhibit the increase in LCBFFlux seen after intravenously administration of CGRP.
The difference in inhibitory effect of BIBN4096BS on MMA and PA raises an interesting subject for further discussion. As outlined previously, the two types of arteries express a different vessel wall structure. The PA directly investigated and the smaller cerebral vessels indirectly measured by LDF are believed to posses a BBB. In theory, this would mean that BIBN4096BS could not pass the BBB and hence could not bind to the cerebrovascular CGRP receptors (Petersen et al., 2004b). The influence of the BBB was supported by the inability of BIBN4096BS to block the effect of TES on PA vasodilatation. With increasing doses of BIBN4096BS, the αCGRP-induced PA dilatation decreased. The reduction in PA dilatation is most likely to be secondary to the prevention of the induced hypotension. It is unlikely to be a direct effect of the antagonist. Since βCGRP induced a less-pronounced degree of hypotension (approximately 10 mmHg), a similar reduction in PA dilatation was not seen for βCGRP. The origin of the minor dilatation seen in PA after infusion of the lower doses of CGRP with only limited effect on the blood pressure is not readily explained. The application of TES induced a dilatation of PA without any effect on the MABP.
It has been proposed (Markowitz et al., 1988; Williamson et al., 1997b) that the dilatation of MMA induced by electrical stimulation mainly is mediated by CGRP. This was further supported by the use of BIBN4096BS in this study. Electrical stimulation of the superior sagital sinus in the cat (Goadsby et al., 1991) and the dura mater in rats (Kurosawa et al., 1995) leads to an increase in cerebral blood flow, an increase found to be mediated by CGRP. Based on existing data, it is known that PAs are innervated by CGRP containing C-fibres originating in the trigeminal ganglion (Edvinsson et al., 2001a) and furthermore that PA dilatation is mediated through CGRP receptors (McCulloch et al., 1986; Wei et al., 1992; Hong et al., 1994). It is therefore most likely that the TES-induced PA dilatation in this study was caused by CGRP release, however no direct evidence is obtained due to the ineffectiveness of BIBN4096BS.
The results from these experiments contribute to the understanding of the site of action for BIBN4096BS. It seems that BIBN4096BS does not pass the BBB in the rat, but is very effective in preventing CGRP-induced vasodilatation in vessels without a BBB.
The present study strongly suggest that the clinically effective migraine drug BIBN4096BS (Olesen et al., 2004) does not cross the BBB. With the caution of species differences in BBB function or the possible occurrence of transient BBB changes during the migraine attack, this indicates that dural arteries may play an important role in migraine pathogenesis.
Acknowledgments
The Danish Research Council, Danish Headache Society supported the study and the ABL used was supplied by Radiometer, Denmark.
Abbreviations
- BIBN4096BS
[R-(R*, S*)]-N-[2-[[5-amino-1-[[4-pyridinyl)-1-piperazinyl] carbonyl]pentyl]amino]-1-(3,5-dibromo-4-hydroxyphenyl)methyl]-2-oxoethyl]-4-(1,4-dihydro-2-oxo-3(2H)-quinazolinyl)-,1-Piperidinecarboxamide
- r-αCGRP
rat-alphaCGRP
- r-βCGRP
rat-betaCGRP
- MMA
middle meningeal artery
- PA
pial arteriole
- LCBFFlux
local cortical cerebral blood flow
- TES
transcranial electrical stimulation
References
- AIYAR N., DAINES R.A., DISA J., CHAMBERS P.A., SAUERMELCH C.F., QUINIOU M., KHANDOUDI N., GOUT B., DOUGLAS S.A., WILLETTE R.N. Pharmacology of SB-273779, a nonpeptide calcitonin gene-related peptide 1 receptor antagonist. J. Pharmacol. Exp. Ther. 2001;296:768–775. [PubMed] [Google Scholar]
- AKERMAN S., WILLIAMSON D.J., KAUBE H., GOADSBY P.J. Nitric oxide synthase inhibitors can antagonize neurogenic and calcitonin gene-related peptide induced dilation of dural meningeal vessels. Br. J. Pharmacol. 2002;137:62–68. doi: 10.1038/sj.bjp.0704842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BASKAYA M.K., SUZUKI Y., ANZAI M., SEKI Y., SAITO K., TAKAYASU M., SHIBUYA M., SUGITA K. Effects of adrenomedullin, calcitonin gene-related peptide, and amylin on cerebral circulation in dogs. J. Cereb. Blood Flow Metab. 1995;15:827–834. doi: 10.1038/jcbfm.1995.103. [DOI] [PubMed] [Google Scholar]
- BEATTIE D.T., MCNEIL D.K., CONNOR H.E. The influence of neurokinins and calcitonin gene-related peptide on cerebral blood flow in anaesthetized guinea-pigs. Neuropeptides. 1993;24:343–349. doi: 10.1016/0143-4179(93)90005-u. [DOI] [PubMed] [Google Scholar]
- DOODS H., HALLERMAYER G., DONGMEI W., ENTZEROT M., RUDOLF K., ENGEL W., EBERLEIN W. Pharmacological profile of BIBN4096BS, the first selective small molecule CGRP antagonist. Br. J. Pharmacol. 2000;129:420–423. doi: 10.1038/sj.bjp.0703110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDVINSSON L., ALM R., SHAW D., RUTLEDGE R.Z., KOBLAN K.S., LONGMORE J., KANE S.A. Effect of the CGRP receptor antagonist BIBN4096BS in human cerebral, coronary and omental arteries and in SK-N-MC cells. Eur. J. Pharmacol. 2002;434:49–53. doi: 10.1016/s0014-2999(01)01532-1. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., ELSAS T., SUZUKI N., SHIMIZU T., LEE T.J. Origin and co-localization of nitric oxide synthase, CGRP, PACAP, and VIP in the cerebral circulation of the rat. Microsc. Res. Tech. 2001a;53:221–228. doi: 10.1002/jemt.1086. [DOI] [PubMed] [Google Scholar]
- EDVINSSON L., SAMS A., JANSEN-OLESEN I., TAJTI J., KANE S.A., RUTLEDGE R.Z., KOBLAN K.S., HILL R.G., LONGMORE J. Characterisation of the effects of a non-peptide CGRP receptor antagonist in SK-N-MC cells and isolated human cerebral arteries. Eur. J. Pharmacol. 2001b;415:39–44. doi: 10.1016/s0014-2999(00)00934-1. [DOI] [PubMed] [Google Scholar]
- FARACI F.M., KADEL K.A., HEISTAD D.D. Vascular responses of dura mater. Am. J. Physiol. 1989;257:H157–H161. doi: 10.1152/ajpheart.1989.257.1.H157. [DOI] [PubMed] [Google Scholar]
- FOULKES R., SHAW N., BOSE C., HUGHES B. Differential vasodilator profile of calcitonin gene-related peptide in porcine large and small diameter coronary artery rings. Eur. J. Pharmacol. 1991;201:143–149. doi: 10.1016/0014-2999(91)90337-p. [DOI] [PubMed] [Google Scholar]
- GERRITS R.J., STEIN E.A., GREENE A.S. Laser-Doppler flowmetry utilizing a thinned skull cranial window preparation and automated stimulation. Brain Res. Brain Res. Protoc. 1998;3:14–21. doi: 10.1016/s1385-299x(98)00016-6. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain. 1994;117:427–434. doi: 10.1093/brain/117.3.427. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988;23:193–196. doi: 10.1002/ana.410230214. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., EDVINSSON L., EKMAN R. Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- GOADSBY P.J., ZAGAMI A.S., LAMBERT G.A. Neural processing of craniovascular pain: a synthesis of the central structures involved in migraine. Headache. 1991;31:365–371. doi: 10.1111/j.1526-4610.1991.hed3106365.x. [DOI] [PubMed] [Google Scholar]
- GULBENKIAN S., UDDMAN R., EDVINSSON L. Neuronal messengers in the human cerebral circulation. Peptides. 2001;22:995–1007. doi: 10.1016/s0196-9781(01)00408-9. [DOI] [PubMed] [Google Scholar]
- HARPER S.L., BOHLEN H.G., RUBIN M.J. Arterial and microvascular contributions to cerebral cortical autoregulation in rats. Am. J. Physiol. 1984;246:H17–H24. doi: 10.1152/ajpheart.1984.246.1.H17. [DOI] [PubMed] [Google Scholar]
- HONEY A.C., BLAND-WARD P.A., CONNOR H.E., FENIUK W., HUMPHREY P.P. Study of an adenosine A1 receptor agonist on trigeminally evoked dural blood vessel dilation in the anaesthetized rat. Cephalalgia. 2002;22:260–264. doi: 10.1046/j.1468-2982.2002.00345.x. [DOI] [PubMed] [Google Scholar]
- HONG K.W., PYO K.M., LEE W.S., YU S.S., RHIM B.Y. Pharmacological evidence that calcitonin gene-related peptide is implicated in cerebral autoregulation. Am. J. Physiol. 1994;266:H11–H16. doi: 10.1152/ajpheart.1994.266.1.H11. [DOI] [PubMed] [Google Scholar]
- JANSEN-OLESEN I., JORGENSEN L., ENGEL U., EDVINSSON L. In-depth characterization of CGRP receptors in human intracranial arteries. Eur. J. Pharmacol. 2003;481:207–216. doi: 10.1016/j.ejphar.2003.09.021. [DOI] [PubMed] [Google Scholar]
- KNUDSEN G., JUHLER M., PAULSEN O.Morphology, physiology and pathophysiology of the blood brain barrier Basic Mechanisms of Migraine 1988Amsterdam: Elsevier Science Publisher; 49–60.ed. Olesen, J. & Edvinsson, L. pp [Google Scholar]
- KONTOS H.A., WEI E.P., NAVARI R.M., LEVASSEUR J.E., ROSENBLUM W.I., PATTERSON J.L., JR Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am. J. Physiol. 1978;234:H371–H383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- KUROSAWA M., MESSLINGER K., PAWLAK M., SCHMIDT R.F. Increase of meningeal blood flow after electrical stimulation of rat dura mater encephali: mediation by calcitonin gene-related peptide. Br. J. Pharmacol. 1995;114:1397–1402. doi: 10.1111/j.1476-5381.1995.tb13361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LASSEN L.H., HADERSLEV P.A., JACOBSEN V.B., IVERSEN H.K., SPERLING B., OLESEN J. CGRP may play a causative role in migraine. Cephalalgia. 2002;22:54–61. doi: 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- MARKOWITZ S., SAITO K., MOSKOWITZ M.A. Neurogenically mediated plasma extravasation in dura mater: effect of ergot alkaloids. A possible mechanism of action in vascular headache. Cephalalgia. 1988;8:83–91. doi: 10.1046/j.1468-2982.1988.0802083.x. [DOI] [PubMed] [Google Scholar]
- MCCULLOCH J., UDDMAN R., KINGMAN T.A., EDVINSSON L. Calcitonin gene-related peptide: functional role in cerebrovascular regulation. Proc. Natl. Acad. Sci. U.S.A. 1986;83:5731–5735. doi: 10.1073/pnas.83.15.5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORENO M.J., TERRON J.A., STANIMIROVIC D.B., DOODS H., HAMEL E. Characterization of calcitonin gene-related peptide (CGRP) receptors and their receptor-activity-modifying proteins (RAMPs) in human brain microvascular and astroglial cells in culture. Neuropharmacology. 2002;42:270–280. doi: 10.1016/s0028-3908(01)00176-9. [DOI] [PubMed] [Google Scholar]
- OLESEN J., DIENER H.C., HUSSTEDT I.W., GOADSBY P.J., HALL D., MEIER U., POLLENTIER S., LESKO L.M. Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 2004;350:1104–1110. doi: 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- OLIVER K.R., WAINWRIGHT A., EDVINSSON L., PICKARD J.D., HILL R.G. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J. Cereb. Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- PETERSEN K., LASSEN L.H., BIRK S., OLESEN J. The effect of the CGRP-antagonist, BIBN4096BS on human-alphaCGRP induced headache and hemodynamics in healthy volunteers. Cephalalgia. 2003;23:725. doi: 10.1111/j.1468-2982.2004.00830.x. [DOI] [PubMed] [Google Scholar]
- PETERSEN K., DYRBY L., WILLIAMSON D.J., EDVINSSON L., OLESEN J.Effect of hypotension and carbon dioxide changes in an improved genuine closed cranial window rat model Cephalalgia 2004a. in press [DOI] [PubMed]
- PETERSEN K., NILSSON E., OLESEN J., EDVINSSON L.Presence and function of the CGRP receptor on rat pial arteries investigated in vitro and in vivo Cephalalgia 2004b. in press [DOI] [PubMed]
- SCHINDLER M., DOODS H.N. Binding properties of the novel, non-peptide CGRP receptor antagonist radioligand, [(3)H]BIBN4096BS. Eur. J. Pharmacol. 2002;442:187–193. doi: 10.1016/s0014-2999(02)01544-3. [DOI] [PubMed] [Google Scholar]
- SUZUKI Y., SATOH S., IKEGAKI I., OKADA T., SHIBUYA M., SUGITA K., ASANO T. Effects of neuropeptide Y and calcitonin gene-related peptide on local cerebral blood flow in rat striatum. J. Cereb. Blood Flow Metab. 1989;9:268–270. doi: 10.1038/jcbfm.1989.44. [DOI] [PubMed] [Google Scholar]
- WEI E.P., MOSKOWITZ M.A., BOCCALINI P., KONTOS H.A. Calcitonin gene-related peptide mediates nitroglycerin and sodium nitroprusside-induced vasodilation in feline cerebral arterioles. Circ. Res. 1992;70:1313–1319. doi: 10.1161/01.res.70.6.1313. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Intravital microscope studies on the effects of neurokinin agonists and calcitonin gene-related peptide on dural vessel diameter in the anaesthetized rat. Cephalalgia. 1997a;17:518–524. doi: 10.1046/j.1468-2982.1997.1704518.x. [DOI] [PubMed] [Google Scholar]
- WILLIAMSON D.J., HARGREAVES R.J., HILL R.G., SHEPHEARD S.L. Sumatriptan inhibits neurogenic vasodilation of dural blood vessels in the anaesthetized rat – intravital microscope studies. Cephalalgia. 1997b;17:525–531. doi: 10.1046/j.1468-2982.1997.1704525.x. [DOI] [PubMed] [Google Scholar]
- WU D. The Netherlands: Doctural, University of Amsterdam; 1999. Pharmacological characterization of calcitonin gene-related peptide receptors and BIBN4096BS – a novel CGRP receptor antagonist. [Google Scholar]
- WU D., EBERLEIN W., RUDOLF K., ENGEL W., HALLERMAYER G., DOODS H. Characterisation of calcitonin gene-related peptide receptors in rat atrium and vas deferens: evidence for a [Cys(Et)(2, 7)]hCGRP-preferring receptor. Eur. J. Pharmacol. 2000;400:313–319. doi: 10.1016/s0014-2999(00)00407-6. [DOI] [PubMed] [Google Scholar]
- ZAGAMI A.S., GOADSBY P.J., EDVINSSON L. Stimulation of the superior sagittal sinus in the cat causes release of vasoactive peptides. Neuropeptides. 1990;16:69–75. doi: 10.1016/0143-4179(90)90114-e. [DOI] [PubMed] [Google Scholar]