Abstract
Voltage-gated Na+ channel blockers have been widely used as local anaesthetics and antiarrhythmic agents. It has recently been proposed that Na+ channel agonists can be used as inotropic agents. Here, we report the identification of a natural substance that acts as a Na+ channel agonist.
Using the patch-clamp technique in isolated rat ventricular myocytes, we investigated the electrophysiological effects of the substances isolated from the root extract of Salvia miltiorrhiza, which is known as ‘Danshen' in Asian traditional medicine. By the intensive activity-guided fractionation, we identified dimethyl lithospermate B (dmLSB) as the most active component, while LSB, which is the major component of the extract, showed negligible electrophysiological effect. Action potential duration (APD90) was increased by 20 μM dmLSB from 58.8±12.1 to 202.3±9.5 ms. In spite of the prolonged APD, no early after-depolarization (EAD) was observed.
dmLSB had no noticeable effect on K+ or Ca2+ currents, but selectively affected Na+ currents (INa). dmLSB slowed the inactivation kinetics of INa by increasing the proportion of slowly inactivating component without inducing any persistent INa. The relative amplitude of slow component compared to the peak fast INa was increased dose dependently by dmLSB (EC50=20 μM). Voltage dependence of inactivation was not affected by dmLSB, while voltage dependence of activation shifted by 5 mV to the depolarised direction.
Since the APD prolongation by dmLSB did not provoke EAD, which is thought as a possible mechanism for the proarrhythmia seen in other Na+ channel agonists, dmLSB might be an excellent candidate for a Na+ channel agonist.
Keywords: Cardiac myocyte, lithospermate B, Na+ channel agonist, inotropic agent, electrophysiology
Introduction
The voltage-gated Na+ channel (VGSC) is the major determinant underlying the upstroke phase of the action potential (AP) in most excitable cells. The gating of VGSC is modulated by various intracellular signal transduction mechanisms and by drugs. In addition, mutations of VGSC are known to be responsible for a variety of conditions, such as cardiac arrhythmia and epilepsy. Long QT-3 and Brugada syndromes are well-characterized examples of gain-of-function and loss-of-function mutations of VGSC, respectively (Veldkamp et al., 2000; Grant et al., 2002).
Drugs targeting VGSCs are widely used clinically as local anaesthetics, muscle relaxants, and antiarrhythmic and antiepileptic agents. They are commonly Na+ channel inhibitors. Recently, synthetic Na+ channel agonists have been proposed as a possible new pharmacological tool for improving cardiac contractility in congestive heart failure patients (Muller-Ehmsen et al., 1998). These agents are characterized by the slowing of the inactivation phase of the Na+ current (INa) and the prolongation of action potential duration (APD). These effects are expected to increase Na+ influx and intracellular Na+ load, which in turn leads to a positive inotropic effect mediated by the Na+/Ca2+ exchange activities of ventricular myocytes. Investigations of the therapeutic potential of Na+ channel agonists for the treatment of Brugada syndrome may also be interesting, particularly because no therapeutic agent is yet available for this life-threatening disease. However, synthetic Na+ channel agonists have a critical drawback to the clinical application because of the proarrhythmic effect. Most synthetic Na+ channel agonists induce a slowing of INa inactivation and develop a persistent INa (Yuill et al., 2000), causing the generation of early after-depolarization (EAD; Ruegg & Nuesch, 1995; Yuill et al., 2000). Such proarrhythmic risk makes these drugs less suitable for clinical application.
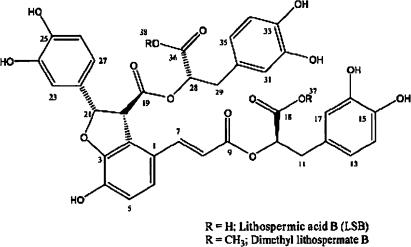
Here, we report the identification of a natural substance from the root extract of Salvia miltiorrhiza (Labiatae), which functionally resembles synthetic Na+ channel agonists. The root of this plant, known as ‘Danshen', is used in an oriental medicinal medicine to improve blood circulation. It has been reported to contain lithospermate B (LSB) as a major active constituent (Tanaka et al., 1989), which reportedly enhances endothelium-dependent vasodilatation (Kamata et al., 1993), and shows beneficial effects on renal injury (Lee et al., 2003). We examined the effect of the root extract of S. miltiorrhiza on cardiac AP, and found that it increases the AP duration in isolated rat ventricular myocytes. Interestingly, when active component of the extract was purified by repeated activity-guided fractionation, it was not LSB, but finally identified as dimethyl LSB (dmLSB), which was present as a minor component (Figure 1). We further performed detailed electrophysiological investigations to identify the target channel of dmLSB.
Figure 1.
Chemical structure of dmLSB.
Methods
Isolation of rat ventricular myocytes
Ventricular myocytes were isolated from the hearts of 3-week or 6- to 7-week-old Sprague–Dawley rats of either sex. Rats were anaesthetized with pentobarbitone sodium (i.p. 200 mg kg−1). Isolated hearts were mounted on a Langendorff perfusion apparatus, washed at 37°C for 5 min with a modified Tyrode solution containing (mM): 143 NaCl, 5.4 KCl, 5 HEPES, 0.5 MgCl2, 0.5 NaH2PO4, 1.8 CaCl2, 10 glucose, adjusted pH to 7.4 with NaOH and then perfused with Ca2+-free Tyrode solution for 5 min. After the hearts had stopped beating, Ca2+-free Tyrode solution containing collagenase (0.5 mg ml−1, Type II, Worthington) was perfused for 30 min. Finally, this enzyme containing solution was washed out for 5 min with a high K+, low Cl− KB solution containing (in mM): 70 K-glutamate, 55 KCl, 10 HEPES, 3 MgCl2, 20 taurine, 20 KH2PO4, 0.5 K-EGTA adjusted pH to 7.3 with KOH. A portion of the left ventricle was then dissected out and gently stirred with a forceps in KB solution. The isolated ventricular myocytes were kept in KB solution at 5°C before use.
Electrophysiological recordings
Patch pipettes (1∼2 MΩ when filled with experimental solutions) were pulled from borosilicate glass capillaries (Harvard Apparatus Ltd, U.K.). We used conventional whole-cell patch-clamp technique to record membrane current or voltage from single ventricular myocytes. In current-clamp mode, APs were evoked by a brief suprathreshold current pulse. In voltage-clamp mode, access resistance was monitored throughout the experiment and data were accepted only when the access resistance was kept below 6 MΩ. Filtered signals (10 KHz) from a patch-clamp amplifier (Biologic RK 300, France) were fed into AD/DA converter (PCI-MIO-16E-4, National Instrument, U.S.A.), digitized at 20 KHz and stored in PC for later analysis. The flow rate of the perfusion solution was 0.5–1 ml min−1. All electrophysiological experiments were performed at room temperature (23–25°C). The statistical results in the text and in figures were presented as means±s.e.m. (n=number of cells studied). Statistical significance was tested with Student's t-test and accepted for P-value <0.05.
Solutions and drugs
The K-rich pipette solution containing (in mM): 90 K-aspartate, 30 KCl, 2 MgCl2, 5 Mg-ATP, 10 HEPES, 5 K-EGTA, 5 diTris-creatine phosphate, 2.5 Na2-creatine phosphate was used. Cs-aspartate internal solution contained (in mM): 90 Cs-aspartate, 20 CsCl, 2 MgCl2, 5 Mg-ATP, 10 HEPES, 2.5 Na2-creatine phosphate, 10 tetraethyl-ammonium chloride (TEA-Cl), 5 Cs-EGTA with pH adjusted to 7.3 using CsOH. When Cs-aspartate internal solution was used in combination with normal Tyrode (NT) bathing solution, the holding current level was inward at –80 mV, probably due to K+ influx via inward rectifier K channels. To prevent this inward holding current, KCl in the NT solution was substituted with equimolar CsCl.
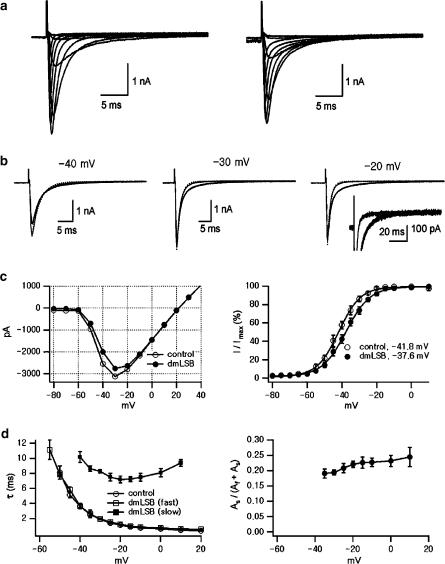
As INa in ventricular myocytes is so fast and large that membrane potential is prone to escape from the command voltage, it is necessary to reduce INa for a quantitative analysis. For this purpose, we used small ventricular myocytes isolated from young (3-week-old) rats, and recorded currents in a modified Cs+-based low Na+ bath solution (Yuill et al., 2000), which contained (in mM): 130 CsCl, 10 NaCl, 2.5 MgCl2, 0.5 CaCl2, 20 HEPES, 11 glucose, in experiments shown in Figure 5. It was reported that the kinetics of INa in the standard solution (145 mM Na+) and in Cs-based solution (10 mM Na+) were similar.
Figure 5.
Effects of dmLSB on cardiac INa in the condition of low [Na+] (a). Whole-cell INa was elicited from a Vh of −120 mV to test potentials ranging from −80 to +10 mV in 10 mV step increments before (left) and after (right) the application of 10 μM dmLSB to the ventricular myocyte from the young rat (3 weeks old). (b) A pair of INa was superimposed in control (grey line) and in dmLSB (black line) at the same test potential for comparison. Inset, the same INa at −20 mV in the expanded vertical scale. Notice the absence of persistent INa in dmLSB. (c) Left, shows the I–V relationships, which represent the peak INa values before (open circle) and after (closed circle) the application of dmLSB. Right, the activation curves were estimated from I–V curves of four different cells. The half-activation voltage was significantly shifted to the positive direction by dmLSB (n=4, paired t-test; P<0.05). (d) Left, time constants estimated from fitting inactivation phases with mono- or biexponential function. Only a single time constant for each test potential was plotted when monoexponential function was sufficient for fitting (for VT=−60, −50 and −40 mV). Right, the plot of relative amplitude of slow component induced by dmLSB as a function of test potential.
To prepare a stock solution of dmLSB, it was dissolved in 50% ethanol at 40 mM and stored at −20°C. Since the pharmacological effects of dmLSB decayed with time, stock solutions were used within 48 h of make-up. Tetrodotoxin (TTX) was purchased from Wako (Japan). Unless mentioned separately, all chemicals were purchased from Sigma-Aldrich Chemical Co. (St Louis, U.S.A.).
Isolation of dmLSB from the root extract of S. miltiorrhiza
Dried roots of S. miltiorrhiza (6 kg) were soaked in MeOH for 7 days at room temperature. After filtration, the extract was concentrated under the reduced pressure to give 470 g of a dark syrupy MeOH extract. This was suspended in H2O and sequentially partitioned with n-hexane, EtOAc and BuOH. This process yielded 69 g in the n-hexane fraction, 52 g in the EtOAc fraction, 69 g in the BuOH fraction and a water-soluble residue. Upon the assessment of each part individually, only the EtOAc-soluble part produced an effect of increasing AP duration in the isolated rat ventricular myocytes. Thus, a half of the EtOAc fraction (26 g) was subjected to octadecyl silica gel column (∅6.0 × h 60 cm) chromatography. The column was eluted in a stepwise gradient manner with 300 ml aliquots of MeOH in H2O (0–100%), which delivered four fractions, that is, Fr. 1 (3.2 g), Fr. 2 (13 g), Fr. 3 (2.4 g) and Fr. 4 (7.0 g). Among these fractions (Fr. 1–Fr. 4), Fr. 2 was most potent, and was further purified by the Sephadex LH-20 column chromatography using 20% MeOH in CH2Cl2, which finally delivered 110 mg of dmLSB and 2.4 g of LSB. Moreover, LSB was easily converted to dmLSB by simple methylation of LSB in MeOH using p-toluenesulphonic acid as catalyst. The chemical structure of dmLSB was elucidated using 1H-NMR and 13C-NMR data (Kohda et al., 1989).
Results
dmLSB prolonged AP in rat ventricular myocytes
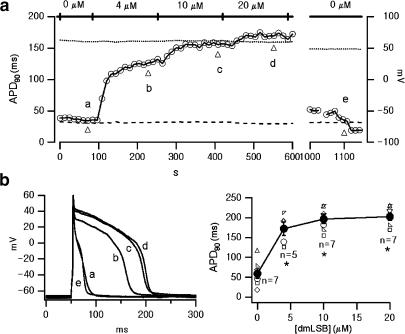
To investigate the effects of dmLSB on the electrical activity of the rat ventricular myocytes, we observed changes in AP shapes during the application of dmLSB to the bath solution. APs were evoked by applying 50–70 pA depolarizing current pulses (5 ms in duration) every 500 ms through a patch pipette in current clamp mode with a whole-cell configuration. The effects of dmLSB on APD were quantified in terms of APD90, defined as the APD measured at the voltage at which repolarization process is 90% complete. Time profile of the change in APD90 values averaged from five sequential APs selected every 12 s was plotted in Figure 2a as a function of whole-cell recording (WCR) time. Single APs recorded in control conditions and those recorded in the presence of dmLSB at various concentrations were superimposed in Figure 2b (left). dmLSB markedly inhibited the initial rapid repolarization phase and prolonged the plateau phase. In contrast to the effect of other lipid-soluble Na+ channel agonists, such as pyrethroids (Spencer et al., 2001), no secondary upward voltage deflection (referred to as EAD) was observed during the prolonged plateau phases. No changes either in resting membrane potential (RMP) or in the overshoot potentials of the APs were observed during the dmLSB superfusion (RMP: dashed line; overshoot potential: dotted line in Figure 2a). The mean values for the RMP and the overshoot potential were −68.46±2.6 mV (n=7) and 53.53±8.8 mV (n=7), respectively. The steady-state APD90 values obtained from seven myocytes at various concentrations of dmLSB are summarized in Figure 2b (right). APD prolongation induced by dmLSB was maximal at 20 μM. At higher concentrations up to 100 μM, APD did not increase further, but slightly decreased (data not shown) with no EAD observed. The mean value of APD90 under control condition was 58.8±12.1 ms and that in the presence of 20 μM dmLSB was 202.3±9.5 ms, indicating that dmLSB significantly prolonged APDs (paired t-test; n=7, P<0.01).
Figure 2.
Effects of dmLSB on cardiac AP. (a) Change in AP duration during the bath application of dmLSB. All data points were obtained from the same cell. Horizontal bars above the plot indicate the duration of the bath application of dmLSB. APs were evoked by depolarizing current pulse (55 pA, 5 ms, 2 Hz). APD90 (AP duration at 90% repolarization) was obtained from the average of five sequential APs selected every 12 s. (b) Left, exemplary AP records at various concentration of dmLSB (a: control; b: 4 μM; c: 10 μM; d: 20 μM; e: wash out of dmLSB). The time point of each AP record was indicated by a triangle in (a). Right, APD90 as a function of the concentration of dmLSB. APD values obtained from seven different cells were superimposed (smaller open symbols). At each concentration of dmLSB, a mean value for APD90 was also superimposed (larger closed circles; error bars, s.e.m.). Asterisks indicate statistical significance (paired t-test; P<0.01).
dmLSB affects the initial transient component of the current
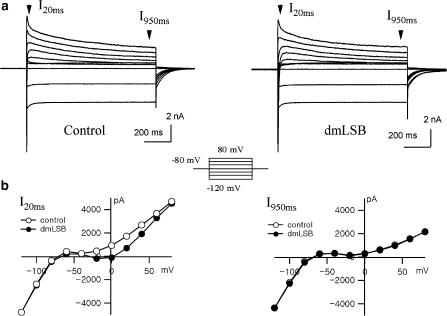
To elucidate the mechanism underlying APD change by dmLSB, we performed voltage-clamp experiments and investigated the effects of dmLSB on the ionic currents in rat ventricular myocytes. Membrane currents were recorded in a voltage-clamp mode using a patch pipette containing a K+-rich (140 mM) internal solution. Figure 3a shows the current responses to a set of 1 s depolarization pulses from a holding potential (Vh) of −80 mV to various voltages (inset) under control conditions and those in the presence of 10 μM dmLSB. Hyperpolarizing pulses induced large inward currents that showed a characteristic of inward rectifier K+ currents. Depolarizing pulses induced complex current responses, each of which was probably composed of a fast inward INa, an L-type Ca2+ current (ICa,L) and a transient and a delayed outward K+ currents.
Figure 3.
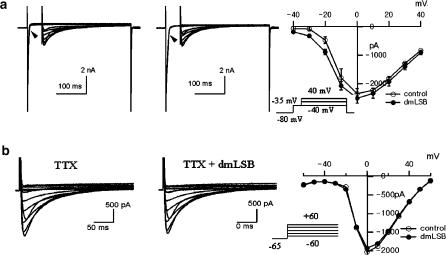
Effects of dmLSB on the whole-cell current. (a) Current responses to step voltage pulses (inset, −120 to +80 mV) from Vh of −80 mV were recorded in whole-cell mode using K+-rich pipette solution. Left: control condition; right: in the presence of 10 μM dmLSB. (b) Voltage dependence of current amplitude measured at 20 ms (left) and at 950 ms (right) of the step pulses. The I–V relationships before (open circle) and after (closed circle) the dmLSB application were superimposed in each plot.
Steady-state amplitudes of the currents which were measured 950 ms after the start of the square pulses (I950 ms) showed no significant difference with or without 10 μM dmLSB, indicating that neither inward rectifier K+ currents nor delayed rectifier K+ currents were affected by dmLSB (Figure 3b, right). However, initial amplitudes measured at 20 ms (I20 ms) in the presence of dmLSB differed from those measured under control conditions, and the current–voltage (I–V) relationship deviated inwardly by dmLSB. The deviation was prominent in the voltage range from −20 to +20 mV, resulting in a bell-shaped I–V relationship in dmLSB (Figure 3b, left). The inward deviation of I–V relationship in the presence of dmLSB indicates that dmLSB might decrease a transient outward current or increase a transient inward current. However, this bell-shaped I–V relationship implies that the involvement of inward currents such as Ca2+ channels or Na+ channels is more likely.
Enhancement of the fast inward INa by dmLSB
To examine the effect of dmLSB on inward currents, we used a Cs+-rich pipette solution containing 10 mM EGTA. To distinguish ICa,L from INa, a two-step pulse protocol was used (inset of Figure 4a). From the holding potential of −80 mV, a ventricular myocyte was depolarized to −35 mV for 50 ms to inactivate INa, and this was followed by test pulses to various depolarization to record ICa,L. The superimposed current traces under the control condition showed the rapid activation and inactivation of large INa at −35 mV followed by the slower activation and inactivation of ICa,L at test pulses (Figure 4a). Although the peak amplitude of INa was unaffected by dmLSB, significant broadening of inactivation phase was observed. In contrast, ICa,L was hardly affected by dmLSB (10 μM).
Figure 4.
Effects of dmLSB on cardiac inward current (a). Current in response to the double step depolarizing pulses (inset). Sets of current traces before (left) and after (middle) the application of 10 μM dmLSB. Notice the slowing of the fast inward current in the presence of dmLSB (arrow heads). Right, voltage dependence of peak amplitude of the current evoked by the second step pulses. The I–V relationships before (open circle) and after (closed circle) the dmLSB application were superimposed (n=4; error bars, s.e.m.). (b) Effects of dmLSB on the inward current in the presence of 100 μM TTX. Sets of current traces in response to the depolarization pulses (inset) before and after bath-applying dmLSB (10 μM).
The effect of dmLSB on ICa,L was further studied in the presence of 100 μM TTX to block INa. Depolarizing test pulses (from −60 to +50 mV) for 200 ms from Vh of −65 mV were applied to activate ICa,L. Figure 4b shows representative current traces before (left) and after (middle) dmLSB (10 μM) superfusion in the presence of TTX, from which the I–V curves of the peak amplitudes of ICa,L were obtained (Figure 4b, right). No significant difference was observed in I–V curves between control and dmLSB. These results strongly indicate that dmLSB has little effect on ICa,L, but that it affects TTX-sensitive INa.
dmLSB induces a slow component of INa inactivation
To avoid the problem of voltage escape and to investigate the effects of dmLSB on INa in a quantitative manner, INa was recorded in low external [Na+] (10 mM; see Methods). It is well known that the steady-state inactivation shifted spontaneously during whole-cell mode recording. To activate INa from the potential at which inactivation is fully recovered, depolarizing test pulses were applied following a 500 ms prepulse to −120 mV. To eliminate the contamination of ICa,L and to isolate pure INa, current response to the same test pulse from holding potential (Vh) of −40 mV, which was regarded as ICa,L, was subtracted from that from Vh of −120 mV. The subtract traces obtained in this way were not inhibited by 40 μM Ni2+, a T-type Ca2+ current (ICa,T) blocker, indicating that ICa,T is not contaminated (Hagiwara et al., 1988).
Figure 5a shows representative results of INa under control conditions and those in the presence of 10 μM dmLSB recorded from the same ventricular myocyte. For comparison, a pair of INa traces recorded before and after the application of dmLSB is superimposed at various test potentials (VT) in Figure 5b.
As was noticed in Figure 4, the most prominent effect of dmLSB on INa was a slowing of the inactivation kinetics, which is well demonstrated in the superimposed current traces obtained at –20 mV (Figure 5b, right). It is noted that the initial phase of fast inactivation was not affected, and the late phase was selectively slowed by dmLSB. Although dmLSB slowed the inactivation, it was completed within 50 ms without leaving a persistent inward current (inset; the same set of traces in an expanded scale), indicating that dmLSB does not enhance the persistent sodium current, but slows down the inactivation process of the fast INa.
It is noted that amplitude of INa decreased slightly by dmLSB. The voltage dependence of peak INa amplitude is further analysed in the I–V relationships (Figure 5c). The reversal potential (Erev) was close to the expected equilibrium potential for Na+ (+20 mV). The peak amplitude of INa was reduced slightly by dmLSB in the hyperpolarized range below −20 mV, while they were similar in the more depolarized range where Na+ channels are fully activated. Accordingly, the activation curve that was obtained by dividing the current amplitude by the electromotive force, V−Erev, shifted to the right direction by 5 mV in the presence of dmLSB (Figure 5c, right). The mean voltage for half-maximal activation in the presence of dmLSB was –37.28±0.8 (n=4), which is significantly more depolarized than that in control condition (−42.35±1.1; paired t-test, P<0.05, n=4).
For further investigation of the effect of dmLSB on the inactivation kinetics, the decay phase of INa was fitted with an exponential function. Under control conditions, a monoexponential curve was sufficient to fit the decay phase of INa (dotted line in Figure 5b). In the presence of dmLSB, however, the decay phase clearly deviated from the monoexponential function, and a slow component emerged. The discrepancy became more pronounced as VT was more depolarized. When the decay phase was fitted with a biexponential function, the amplitude of the slow component (AS) was estimated to be 22.1±1.02% (n=4) of the peak INa at −20 mV, and it increased as VT became more depolarized (Figure 5d, right). In contrast, the time constant of the fast component (τf) in the presence of dmLSB was similar to that of the monoexponential time constant under control conditions (Figure 5d, left). These results indicate that dmLSB induced a slow component of inactivation at the expense of the normal fast component of INa.
dmLSB has no effect on the steady-state inactivation of INa
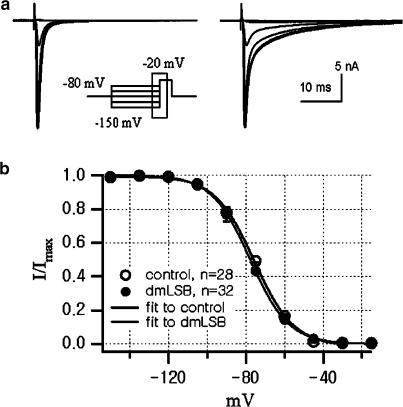
The effects of dmLSB on the steady-state inactivation of INa were studied using the pulse protocol shown in Figure 6 (inset). Prepulses of 500 ms duration at variable potentials (from −150 to −15 mV in 15 mV steps) preceded a 50 ms test pulse to −20 mV. The superimposed current responses to the test pulse (−20 mV) are shown in Figure 6a (left, control; right, 20 μM dmLSB). The slow inactivating INa in the presence of dmLSB indicates that dmLSB exerts its pharmacological effect (Figure 6a, right). The relative peak amplitudes of INa are plotted against prepulse voltages in Figure 6b. No differences in the steady-state inactivation curves were found before and after the dmLSB application, indicating that dmLSB has no effect on the steady-state inactivation of INa. The voltages for half-inactivation were −75.7±0.61 mV (n=28) in control and −77.8±1.05 mV (n=32) in the presence of dmLSB (t-test, P>0.05).
Figure 6.
No effect of dmLSB on the steady-state inactivation of INa. (a) INa elicited by step pulses to −20 mV after 500 ms prepulses of various levels in control condition (left) and in the presence of 20 μM dmLSB (right). (b) Summary of mean availability of Na+ channel as a function of prepulse voltages in the absence (open circle, n=28) and in the presence (closed circle, n=32) of 20 μM dmLSB. Data were fitted by 1−(1/(1+exp((V1/2−Vm)/k))). V1/2 and k were −75.7 and 10.20 for control, and −77.8 and 9.99 for dmLSB, respectively. Error bars, s.e.m.
Dose–response relationship
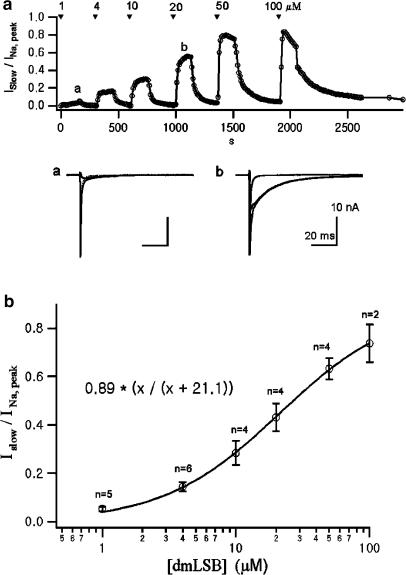
The effects of dmLSB on INa can be well demonstrated by subtracting the INa evoked by depolarization to −20 mV under control conditions from that in the presence of dmLSB. Since dmLSB specifically affected the slow phase of inactivation, the difference current represented the slow inactivating INa (ISlow) induced by dmLSB. To quantify the magnitude of the effect of dmLSB on INa, the relative amplitude of ISlow to the peak INa was plotted. Figure 7a shows the time course of change in the relative amplitude of ISlow during the bath application of sequentially higher concentration of dmLSB.
Figure 7.
Dose–response relationship. (a) Upper, the change in the relative amplitude of ISlow to the peak INa as a function of WCR time. A pair of step pulse to −20 mV from the prepulse of −130 mV and from that of −40 mV were applied every 10 s to obtain INa (see text for details). The dmLSB-induced ISlow was obtained by subtracting INa in control condition from INa in the presence of dmLSB. Lower, INa (grey) traces in control condition and in the presence of dmLSB and ISlow (black) were superimposed. The time when each set of current records was obtained is marked in the upper plot with ‘a' and ‘b'. (b) Relative amplitude of slow components induced by dmLSB to the peak INa was plotted as a function of the concentration of dmLSB. The relationship was fitted to a Hill equation. The half-maximum effective concentration and Hill coefficient were 21.1 μM and 1.02, respectively.
The maximal effect of the drug at each concentration reached in ca. 150 s. Effects of dmLSB were reversible, although a longer time was required to wash out the drug effect at the higher concentration. From a set of similar experiments, dose–response relationship was obtained. The relative amplitude of ISlow to the peak INa was plotted as a function of dmLSB concentrations in Figure 7b. The relationship was well fitted to the Hill equation with an EC50 of 21.1 μM and a Hill coefficient of 1.02.
Intracellular application of dmLSB has no effect on INa inactivation
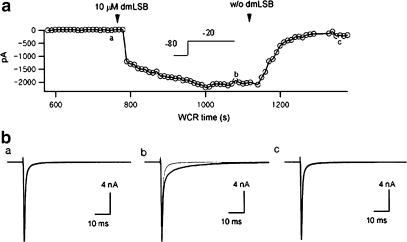
Since dmLSB is lipophilic, it is possible that it permeates the plasma membrane and binds to intracellular receptors or to Na channel protein itself. It is not clear whether its effects were mediated via a cell surface binding site, an intracellular site or both. To test this possibility, a ventricular myocyte was perfused intracellularly with the patch pipette solution containing 20 μM dmLSB, and INa was monitored while patch pipette solution diffused in. Meanwhile, the series resistance (RS) was kept below 7 MΩ. As soon as the whole-cell patch was established, INa was recorded at a test potential of −20 mV, and this current trace was subtracted from subsequent INa records. The amplitudes of the subtracted currents were plotted as a function of WCR time (Figure 8a). Intracellular dmLSB exerted essentially no effect on INa (n=4), suggesting that the dmLSB binding site(s) is located on the surface of ventricular myocytes. No change in the inactivation kinetics of INa was observed until dmLSB was applied externally. When 5 μM dmLSB was bath applied at ca. 13 min of WCR time to the same cell, Islow appeared, indicative of the dmLSB effect.
Figure 8.
Effects of intra- and extracellular dmLSB on INa. The whole-cell mode was obtained with the pipette solution containing 20 μM dmLSB. INa was evoked by test pulse to −20 mV from Vh of −80 mV. (a) The time course of peak ISlow (INa, dmLSB–INa, control) during bath application of 5 μM dmLSB. The arrow heads indicate the start and the end of the solution changes. (b) Current traces obtained from before (left) and after (middle) the bath application of dmLSB to the same cell. Extracellular dmLSB slowed the INa inactivation. The dmLSB effect was completely reversed after the wash out of external dmLSB (right). In each plot, the initial INa recorded just after break-in was superimposed for comparison (grey traces).
Discussion
LSB is a caffeic acid tetramer and was originally isolated from the root of S. miltiorrhiza (also called as Dansham or Danshen), an important herb in oriental medicine, which has been used to treat cardiovascular disease. LSB, the main component of S. miltiorrhiza, has been shown to induce endothelium-dependent vasodilation, which was inhibited by NG-monomethyl-L-arginine (Kamata et al., 1993), and to inhibit the norepinephrine-induced contraction of the aortic strips by reducing Ca2+ mobilization (Nagai et al., 1996). The effect of LSB on the heart has also been reported. LSB was found to reduce the myocardial damage induced in a rabbit ischaemia–reperfusion model (Fung et al., 1993). However, no report has been issued on the effects of LSB on cardiac electrical activity. In the present study, we found that LSB itself has no effect on cardiac AP, but dimethyl ester form of LSB (dmLSB) increases the APD of the rat ventricular myocytes. A voltage-clamp study revealed that the slowing of INa inactivation underlies the APD prolongation, and that no current system except fast INa was affected by dmLSB. The prolongation of APD induced by dmLSB was not associated with the generation of EAD. Consistent with the absence of the EAD generation, dmLSB developed no persistent INa. These pharmacological properties render dmLSB a promising candidate for clinical application.
Comparison of the effect of dmLSB on INa with other lipid-soluble Na channel agonists
The gating parameters of Na+ channels are highly susceptible to a number of lipid-soluble alkaloids, insecticides and toxins from tropical frog. In spite of their structural diversity, these lipid-soluble toxins share a common mechanism of action, which suggests the presence of a receptor in a strongly hydrophobic region of Na+ channel. The most thoroughly studied compounds are veratridine, bartrachotoxin, aconitine and pyrethroids (Caterall, 1980; Narahashi, 1996; Wang & Wang, 2003). Lipid-soluble alkaloids (also called as Na+ channel agonist) shift the voltage dependence of activation to more negative potentials and inhibit or completely block inactivation. These two effects combine to cause persistent activation of a fraction of Na+ channels at resting Vm.
A recent study reported that pyrethroids also increase the window region of cardiac INa: a potential range over which activation and inactivation curves overlap (Spencer et al., 2001). Moreover, type II pyrethroids, fenpropathrin, produced leftward shifts in both the activation and the inactivation curves of INa, resulting in the shift of window region to a more negative potential. These changes might be involved in the generation of EADs on a prolonged plateau phase in cardiac ventricular myocytes (Spencer et al., 2001).
The effect of dmLSB on INa is distinct from that of other Na+ channel agonists in that it shifts the activation curve to the positive direction and has no effect on the steady-state inactivation curve of INa. In addition, while dmLSB slowed down the inactivation kinetics of INa, it produced no sustained component of INa. These characteristics may be related to the change of APD caused by dmLSB. It prolonged the APD without provoking EADs. Considering that generation of EAD is responsible for generation of arrhythmias by these alkaloids, the characteristics of the dmLSB effect are of particular interest. It remains to be elucidated whether the lack of EAD in the presence of dmLSB indicates that dmLSB does not have arrhythmogenic potentials.
Potential target for the clinical use of dmLSB: comparison of the effect of dmLSB on INa with other newly developed inotropic agents
DPI 201-106, BDF 9148, BDF 9198 and its related compounds have been reported to exert a positive inotropic effect on cardiac myocardium (Muller-Ehmsen et al., 1998). Subsequent studies revealed that the slowing of INa inactivation is responsible for the inotropic effects (Yuill et al., 2000). In addition to the slowing of INa inactivation, these drugs were found to induce a persistent INa, causing the increase in net depolarizing current during AP. Accordingly, they induced extensive prolongation of APD and of the QT interval in ECG, which is associated with risk of proarrhythmia mediated by EAD (Ruegg & Nuesch, 1995; Yuill et al., 2000). Such proarrhythmic risk makes these drugs less suitable for clinical application. Moreover, the effects of DPI 201-106 and BDF 9148 are not restricted to Na+ channels, but they inhibit the L-type calcium current and inward rectifier K+ current (Raven et al., 1995).
Recently, it was reported that KB130015, a newly developed amiodarone derivative, slowed INa inactivation without developing persistent INa (Macianskiene et al., 2003b). From these properties, KB130015 is expected to be less proarrhythmic than other synthetic Na+ channel modulators. However, it reduces ICa and consequently reduces APD, making it less useful as an inotropic agent (Macianskiene et al., 2003a). In contrast, dmLSB did not affect any current system other than fast INa in cardiac myocytes, while its effect on the inactivation kinetics of cardiac INa was similar to those of KB130015.
At this stage, it is not possible to conclude anything about clinical utility of dmLSB, but Brugada syndrome may be considered as a potential target of dmLSB. Brugada syndrome is a genetic disorder in which mutations are identified in SCN5A, the gene encoding cardiac Na+ channel, Nav1.5. Multiple mutations have been identified in virtually all regions of Nav1.5, and it has been shown that many of the Brugada syndrome mutations alter Na+ channels gating function in a manner that reduces the Na+ channel availability. Delayed recovery of the Na+ channel from slow inactivation state has been proposed as the main functional defect found in Brugada syndrome (Wang et al., 2000). A reduced Na+ channel function causes the loss of AP dome, preferentially in epicardial cells, which leads to early epicardial repolarization. The consequent heterogeneity of repolarization in the right ventricular wall increases the risk of re-entrant arrhythmia. Prolongation of APD by Na+ channel enhancement is a plausible therapeutic strategy for the rectification of APD shortening found in Brugada syndrome. However, it should be determined whether dmLSB has also similar effects on mutated Na+ channels before we move forward to consider the possibility of therapeutic potential of dmLSB in Brugada syndrome.
Acknowledgments
This work was supported by a grant from the Ministry of Health and Welfare (00-PJ9-PG1-CO03-0001). J.Y. Yoon is a postgraduate student supported by BK21 program from the Ministry of Education.
Abbreviations
- AP
action potential
- APD
action potential duration
- dmLSB
dimethyl lithospermate B
- EAD
early after-depolarization
- ICa,L
L-type Ca2+ current
- ICa,T
T-type Ca2+ current
- INa
Na+ current
- ISlow
slowly inactivating Na+ current
- RMP
resting membrane potential
- TTX
tetrodotoxin
- VGSC
voltage-gated Na+ channel
- WCR
whole-cell recording
References
- CATERALL W.A. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Ann Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- FUNG K.P., WU J., ZENG L.H., WONG H.N., LEE C.M., HON P.M., CHANG H.M., WU T.W. Lithospermic acid B as an antioxidant-based protector of cultured ventricular myocytes and aortic endothelial cells of rabbits. Life Sci. 1993;53:PL189–PL193. doi: 10.1016/0024-3205(93)90129-q. [DOI] [PubMed] [Google Scholar]
- GRANT A.O., CARBONI M.P., NEPLIOUEVA V., STARMER C.F., MEMMI M., NAPOLITANO C., PRIORI S. Long QT syndrome, Brugada syndrome, and conduction system disease are linked to a single sodium channel mutation. J Clin Invest. 2002;110:1201–1209. doi: 10.1172/JCI15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGIWARA N., IRISAWA H., KAMEYAMA M. Contribution of two types of calcium currents to the pacemaker potentials of rabbit sino-atrial node cells. J. Physiol. 1988;395:233–253. doi: 10.1113/jphysiol.1988.sp016916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., IIZUKA T., NAGAI M., KASUYA Y. Endothelium-dependent vasodilator effects of the extract from Salviae miltiorrhizae radix. A study on the identification of lithospermic acid B in the extracts. Gen Pharmacol. 1993;24:977–981. doi: 10.1016/0306-3623(93)90176-x. [DOI] [PubMed] [Google Scholar]
- KOHDA H., TAKEDA O., TANAKA S., YAMASAKI K., YAMASHITA A., KUROKAWA T., ISHIBASHI S. Isolation of inhibitors of adenylate cyclase from dan-shen, the root of Salvia miltiorrhiza. Chem. Pharm. Bull. (Tokyo) 1989;37:1287–1290. doi: 10.1248/cpb.37.1287. [DOI] [PubMed] [Google Scholar]
- LEE G.T., HA H., JUNG M., LI H., HONG S.W., CHA B.S., LEE H.C., CHO Y.D. Delayed treatment with lithospermate B attenuates experimental diabetic renal injury. J. Am. Soc. Nephrol. 2003;14:709–720. doi: 10.1097/01.asn.0000051660.82593.19. [DOI] [PubMed] [Google Scholar]
- MACIANSKIENE R., BITO V., RAEYMAEKERS L., BRANDTS B., SIPIDO K.R., MUBAGWA K. Action potential changes associated with a slowed inactivation of cardiac voltage-gated sodium channels by KB130015. Br. J. Pharmacol. 2003a;139:1469–1479. doi: 10.1038/sj.bjp.0705379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACIANSKIENE R., VIAPPIANI S., SIPIDO K.R., MUBAGWA K. Slowing of the inactivation of cardiac voltage-dependent sodium channels by the amiodarone derivative 2-methyl-3-(3,5-diiodo-4-carboxymethoxybenzyl)benzofuran ( KB130015) JPET. 2003b;304:130–138. doi: 10.1124/jpet.102.042218. [DOI] [PubMed] [Google Scholar]
- MULLER-EHMSEN J., BRIXIUS K., SCHWINGER R.H.G. Positive inotropic effects of the novel Na+ channel modulator BDF 9198 in human failing and non-failing myocardium. J. Cardiovasc. Pharm. 1998;31:684–689. doi: 10.1097/00005344-199805000-00006. [DOI] [PubMed] [Google Scholar]
- NAGAI M., NOGUCHI M., IIZUKA T., OTANI K., KAMATA K. Vasodilator effects of des(alpha-carboxy-3,4-dihydroxyphenethyl)lithospermic acid (8-epiblechnic acid), a derivative of lithospermic acids in Salviae miltiorrhizae radix. Biol. Pharm. Bull. 1996;19:228–232. doi: 10.1248/bpb.19.228. [DOI] [PubMed] [Google Scholar]
- NARAHASHI T. Neuronal ion channels as the target sites of insecticides. Pharmacol. Toxicol. 1996;78:1–14. doi: 10.1111/j.1600-0773.1996.tb00234.x. [DOI] [PubMed] [Google Scholar]
- RAVEN U., AMOS G.J., EHRING T., HEUSCH G. BDF 9148 – a sodium channel modulator with positive inotropic action. Cardiol. Drug Rev. 1995;3:260–274. [Google Scholar]
- RUEGG P.C., NUESCH E. The effect of a new inotropic agent, DPI 201-106 on systolic time intervals and the electrocardiogram in healthy subjects. Br. J. Clin. Pharmacol. 1995;24:453–458. doi: 10.1111/j.1365-2125.1987.tb03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER C.I., YUILL K.H., BORG J.J., HANCOX J.C., KOZLOWSKI R.Z. Actions of pyrethroid insecticides on sodium currents, action potentials, and contractile rhythm in isolated mammalian ventricular myocytes and perfused hearts. J. Pharmacol. Exp. Therap. 2001;298:1067–1082. [PubMed] [Google Scholar]
- TANAKA T., MORIMOTO S., NONAKA G., NISHIOKA I., YOKOZAWA T., CHUNG H.Y., OURA H. Magnesium and ammonium–potassium Lithospermates B, the active principles having a uremia-preventive effect from Salvia miltiorrhiza. Chem. Pharm. Bull. 1989;37:340–344. [Google Scholar]
- VELDKAMP M.W., VISWANATHAN P.C., BEZZINA C., BAARTSCHEER A., WILDE A.A.M., BALSER J.R. Two distinct congenital arrhythmias evoked by a multidysfunctional Na1 channel. Circ. Res. 2000;86:e91–e97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- WANG D.W., MAKITA N., KITABATAK A., BALSER J.R., GEORGE A. Enhanced Na+ channel intermediate inactivation in Brugada syndrome. Circ. Res. 2000;87:e37–e43. doi: 10.1161/01.res.87.8.e37. [DOI] [PubMed] [Google Scholar]
- WANG S.Y., WANG G.K. Voltage-gated sodium channels as primary targets of diverse lipid-soluble neurotoxins. Cell. Signal. 2003;15:151–159. doi: 10.1016/s0898-6568(02)00085-2. [DOI] [PubMed] [Google Scholar]
- YUILL K.H., CONVERY M.K., DOOLEY P.C., DOGGRELL S.A., HANCOX J.C. Effects of BDF 9198 on action potentials and ionic currents from guinea-pig isolated ventricular myocytes. Br. J. Pharmacol. 2000;130:1753–1766. doi: 10.1038/sj.bjp.0703476. [DOI] [PMC free article] [PubMed] [Google Scholar]