Abstract
The involvement of adenosine triphosphate (ATP) and carbon monoxide (CO) in the non-nitrergic nonpeptidergic component of high-frequency electrical field stimulation (EFS)-induced nonadrenergic noncholinergic (NANC) relaxation of longitudinal muscle strips from the rat gastric fundus was investigated.
Under NANC conditions (1 μM atropine+5 μM guanethidine), NG-nitro-L-arginine methyl ester (L-NAME, 1 mM) slightly reduced the amplitude, but did not affect the area under the curve (AUC) of EFS (13 Hz, 2 min)-induced relaxation of 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U46619, 0.1 μM)-precontracted strips. With L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1), the amplitude and the AUC of relaxation were reduced to approximately two-third and one-third of controls, respectively.
Pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (100 μM), apamin (0.3 μM), desensitization to ATP, suramin (100 μM), zinc protoporphirin IX (300 μM) or ferrous haemoglobin (100 μM) did not inhibit the component of relaxation resistant to L-NAME plus α-chymotrypsin.
L-NAME (1 mM) plus anti-vasoactive intestinal peptide (VIP) serum (1 : 100) reduced the amplitude and the AUC of relaxation to a similar extent as L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1). Adding apamin (0.1 μM) to L-NAME (1 mM) plus anti-VIP serum (1 : 100) further reduced the amplitude and the AUC of relaxation.
These findings suggest that the non-nitrergic nonpeptidergic component of NANC relaxation of the rat gastric fundus induced by high-frequency stimulation is mediated by a neurotransmitter thatacts through apamin-sensitive mechanisms, that is neither ATP nor CO.
Keywords: Nonadrenergic noncholinergic (NANC) relaxation, rat gastric fundus, nitric oxide (NO), vasoactive intestinal polypeptide (VIP), adenosine triphosphate (ATP), carbon monoxide (CO)
Introduction
Nitric oxide (NO) and vasoactive intestinal polypeptide (VIP) are well-established neurotransmitters of the inhibitory motor neurones in the rat gastric fundus (Currò & Preziosi, 1998; Currò et al., 2002). Also, peptide histidine isoleucine (PHI), a peptide co-synthesized, co-stored and co-released with VIP, has been proposed as an inhibitory neurotransmitter in the rat stomach (Currò et al., 2002). NO seems to be the neurotransmitter responsible for the immediate beginning and the initial rapid phase of the nonadrenergic noncholinergic (NANC) relaxation of rat gastric fundus strips induced by in vitro electrical field stimulation (EFS) (Currò & Preziosi, 1998). EFS increases NO production in the rat gastric fundus and this effect does not show any preferential frequency pattern (Currò et al., 1996). On the contrary, a detectable VIP and PHI co-release from the rat gastric fundus can be elicited only by high-frequency (>4 Hz) EFS (D'Amato et al., 1992b; Currò et al., 1994). VIP- and PHI-induced relaxations differ from those induced by EFS in that the former only begin after a latency of a few seconds and are slowly developing (Currò et al., 2002). However, relaxations induced by VIP, PHI and high-frequency EFS are similar in duration (they are all long-lasting) (Currò et al., 2002). These observations and other evidence have led to the suggestion that the involvement of VIP and PHI is limited to the NANC relaxation induced by high-frequency EFS and that these peptides are responsible for most of the duration of this response (Currò et al., 2002).
The combination of pharmacological agents that block NO- and peptide-mediated components does not abolish the EFS-induced relaxation of the rat gastric fundus (Li & Rand, 1990; Boeckxstaens et al., 1992; D'Amato et al., 1992a). Consequently, one or more additional neurotransmitters are very probably involved in this response. Adenosine triphosphate (ATP) has long been thought to be a neurotransmitter of the inhibitory motor neurones in the gut (for a review, see Bennett, 1997). In addition, more recent evidence has been put forward to support a role for carbon monoxide (CO) as a mediator of NANC inhibitory responses in the gastrointestinal tract (Zakhary et al., 1996; 1997; Xue et al., 2000). The aims of the present study were: (1) to quantify the non-nitrergic nonpeptidergic component of NANC relaxation of the rat gastric fundus induced by high-frequency EFS; and (2) to investigate whether ATP and/or CO are responsible for this component.
Methods
Wistar rats of either sex, weighing 180–320 g, were fasted overnight with free access to water, killed afterwards by decapitation and exsanguinated. The gastric fundus was removed and two longitudinal muscle strips (3 × 20 mm) were prepared according to the method of Vane (1957). The strips were mounted between parallel platinum electrodes (20 mm long, 4 mm wide, 5 mm apart) and placed in 5-ml organ baths containing Krebs solution, maintained at 37°C and bubbled with a 95/5 O2/CO2 mixture, of the following composition (mM): NaCl 118.5, KCl 4.8, CaCl2 1.9, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25 and glucose 10.1 (pH 7.4). The strips were connected to isotonic transducers (mod. 7006, Basile Biological Research Apparatus) under a 1-g load. Smooth muscle activity, magnified 10–20 times, was recorded with Rikadenki R-01 or R-02 pen recorders or on a computer using the PowerLab data acquisition system (ADInstruments, Castle Hill, Australia). Isolated EFSs consisting of rectangular and bipolar pulses of constant duration (1 ms) and amplitude (120 mA) were performed by a Palmer Bioscience 6012 Stimulator linked in series with a Basile Biological Research Apparatus constant-current unit. EFS frequency and train duration were always 13 Hz and 2 min, respectively. At these parameters, EFS has been shown to induce a submaximal response in the frequency–response curve based on the area under the curve (AUC) of relaxations (Currò et al., 2002). The Krebs solution contained atropine (1 μM) and guanethidine (5 μM), and 9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α (U46619, 0.1 μM), a thromboxane receptor (TP) agonist (to raise strip tone and so enable recording of relaxant responses). In some experiments, strips were precontracted by 5-hydroxytryptamine (5-HT, 0.3 μM) (see below). Tissues were initially allowed to equilibrate for 60 min; the incubation medium was always changed every 10 min (during the equilibration period and in between drug administration and/or periods of EFS).
In the first series of experiments, the strips were exposed to consecutive 2-min incubations with increasing concentrations of ATP (0.3–100 μM) or CO (50 μM–1 mM). Strips were allowed to recover to basal tone prior to the subsequent ATP or CO concentration. In the strips exposed to ATP, the effects of the P2-purinoceptor antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS, 3–100 μM, pre-incubation time: 20 min) or its vehicle (bidistilled water) on the submaximal relaxation induced by ATP (3 μM) were investigated. This experimental protocol allowed the calculation of the mean pA2 (−log KB) of PPADS, according to the method described by Calderone et al. (1999). In a second series of experiments, the effects of the NOS inhibitor NG-nitro-L-arginine methyl ester (L-NAME, 1 mM, 30 min), the small-conductance Ca2+-dependent K+-channel inhibitor apamin (0.1 μM, 10 min) or the voltage-dependent Na+-channel inhibitor tetrodotoxin (TTX; 10 min) on the submaximal relaxation produced by ATP (3 μM) were evaluated. The effect of apamin (0.1 μM) on the relaxation induced by ATP (3 μM) was evaluated also in the presence of α-chymotrypsin (1 U ml−1, pre-incubation time: 20 min). As far as CO is concerned, the effect of ferrous haemoglobin (Fe2+-Hb, 100 μM) on the submaximal relaxation produced by CO (0.5 mM) was investigated. Studies were then conducted to identify the effects of L-NAME (1 mM, 20 or 40 min), its enantiomer, D-NAME (1 mM, 30 min), apamin (0.1 or 0.3 μM, 30 min), L-NAME (1 mM) plus apamin (0.1 μM), L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1), PPADS (100 μM, 30 min), L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus PPADS (100 μM), desensitization to the relaxant effect of ATP (30 μM), L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus induction of desensitization to ATP, L-NAME (1 mM) plus anti-VIP serum (1 : 100), D-NAME (1 mM) plus normal rabbit serum (NRS, 1 : 100), L-NAME (1 mM) plus anti-VIP serum (1 : 100) plus apamin (0.1 μM), L-NAME (1 mM) plus anti-VIP serum (1 : 100) plus PPADS (100 μM), L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus the haeme oxygenase inhibitor zinc protoporphyrin IX (ZnPPIX, 300 μM) or L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus ferrous Hb (100 μM) on the NANC inhibitory response evoked by 13-Hz EFS for 2 min. Pre-incubation time of drug combinations was 30 min. In these experiments, each strip was subjected to only two (control and post-treatment) stimulations. The strips were exposed to the pharmacological agents, or were subjected to the pharmacological procedure to induce desensitization to ATP, after recovering the basal tone. EFS was repeated in the presence of the pharmacological agents that were maintained in incubation after the cessation of the EFS, until strips recovered again to the pre-EFS tone. In the experiments performed to study the influence of ZnPPIX, a light-sensitive substance, the organ baths were covered with aluminium foil and the light was reduced as much as possible in the lab. A successful procedure to induce desensitization to the relaxant effect of ATP (30 μM) was obtained by 3–5 consecutive 5-min incubations with ATP (1 mM). Relaxant responses to ATP (30 μM) after the desensitization procedure were 9.3±3.7% of controls (n=9) in the experimental series with desensitization to ATP alone, and 7.1±3.5% of controls (n=6) in the experimental series with L-NAME plus α-chymotrypsin plus desensitization to ATP. After each exposure to ATP (1 mM), the bath medium was renewed and strips were allowed to recover to the basal tone before being subjected to the next incubation with ATP (30 μM). Repeated (up to five) 5-min incubations with α, β-methylene ATP (10 and 30 μM) did not induce strip desensitization. The last relaxation produced by α, β-methylene ATP (30 μM) was 97.4±2.2% of the first one (n=4). The P2-purinoceptor antagonists, suramin (100 μM) and reactive blue 2 (cibacron blue F3G-A, 100 μM), each greatly relaxed U46619-precontracted preparations on their own, and hence could not be used as antagonists in these experiments. For this reason, the effects of suramin and reactive blue 2 on 5-HT (0.3 μM)-precontracted strips were investigated. Reactive blue 2 (100 μM) greatly relaxed 5-HT-precontracted strips too. On the contrary, suramin (100 μM) only very slightly relaxed the strips (by 5.7±1.2%, n=9). Thus, the effect of suramin (100 μM) on ATP (3 μM)-induced relaxation was studied. In addition, the effects of suramin (100 μM), L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) or L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus suramin (100 μM) on EFS (13 Hz)-induced relaxations of 5-HT-precontracted strips were evaluated.
EFS-evoked relaxant responses were calculated as both amplitudes and areas under the curves (AUCs). All other relaxations were measured as amplitudes only. For normalization, amplitudes of relaxations and contractions were expressed as percentages of the U46619- (0.1 μM) or 5-HT (0.3 μM)-induced contractions. Similarly, the AUCs (mm min) of the relaxant responses were divided by the amplitude of U46619- (0.1 μM) or 5-HT (0.3 μM)-induced contractions (mm) and expressed as min (Currò et al., 2002).
Drugs
The following drugs were used: ATP disodium salt, atropine sulphate, guanethidine sulphate, 5-HT creatinine sulphate, α, β-methylene ATP lithium salt, PPADS tetrasodium salt (Sigma, St Louis, MO, U.S.A.); L-NAME, D-NAME (Bachem, Bubendorf, Switzerland); α-chymotrypsin (Serva, Heidelberg, Germany); apamin (Calbiochem Novabiochem, La Jolla, CA, U.S.A.); ZnPPIX (Aldrich, Milwakee, WI, U.S.A.); cibacron blue F3G-A (Fluka, Buchs, Switzerland). Suramin (Germanin) was kindly donated by Bayer AG (Leverkusen, Germany). Fe2+-Hb, purified from human blood, was kindly donated by Dr E. Clementi (Institute of Biochemistry and Clinical Biochemistry, Catholic University, Rome, Italy). Substances were dissolved with bidistilled water, except for ZnPPIX that was dissolved in NaOH 0.1 N. Saturated CO solution was prepared by bubbling 99.9% gas in Krebs solution as described by Rattan & Chakder (1993). The concentration of the CO solution was taken to be 2 mM (Colpaert et al., 2002a).
Statistical analysis
The results were evaluated by means of Student's paired and unpaired t test (results within and between tissues, respectively). When more than two groups had to be compared, analysis of variance (ANOVA) followed by Bonferroni's test for multiple comparison was performed. All values are presented as mean±s.e.m. P<0.05 was considered statistically significant. The GraphPad Prism program (GraphPad Software, San Diego, CA, U.S.A.) was used for fitting the concentration–response curves and calculating EC50's and IC50's.
Results
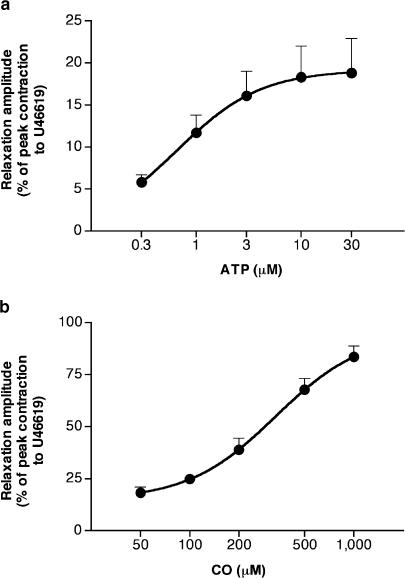
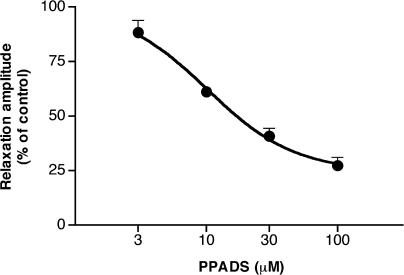
ATP (0.3–1 μM) induced monophasic responses consisting of relaxations. At concentrations >1 μM, ATP induced biphasic responses, consisting of an initial, rapid and short-lasting relaxation followed by a sustained contraction. In the range of 0.3–30 μM, ATP-induced relaxations were concentration-dependent (Figure 1). The mean pD2 (−log EC50) and the maximal peak amplitude of ATP-induced concentration–response curve were 6.25±0.09 and 19.4±4.1%, respectively (n=8). PPADS (3–100 μM) reduced in a concentration-dependent manner the relaxation induced by ATP (3 μM) (Figure 2). The inhibitory effect of PPADS (100 μM) was 27.4±3.7% of controls (n=4). The mean pA2 of PPADS was 5.54±0.25, with a slope of 0.84±0.16, not significantly different from unity (n=4). In the parallel and untreated strips, there were no reductions in ATP (3 μM)-induced relaxations (last relaxation was 99.1±9.0% of controls, n=4). Apamin (0.1 μM) abolished ATP (3 μM)-induced relaxation in four out of six strips and greatly reduced it in the remaining two (mean reduction: 87.8±7.8% of controls, n=6). In the presence of α-chymotrypsin (1 U ml−1), apamin was no longer able to antagonize the relaxant effect of ATP (3 μM), which was 100.5±10.6% of controls (n=4). ATP (3 μM)-induced relaxation was not affected by TTX (1 μM) or L-NAME (1 mM) (96.4±6.4%, n=6, and 98.0±6.5%, n=6, of controls, respectively). CO (50 μM-1 mM) induced concentration-dependent relaxations (Figure 1). The mean pD2 and the maximal peak amplitude of ATP-induced concentration–response curve were 3.5±0.06 and 92.9±3.9%, respectively (n=4). Fe2+-Hb (100 μM) was able to reduce the submaximal relaxation induced by CO (0.5 mM) to 32.7±0.3% of controls (n=3).
Figure 1.
Mean concentration–response curve for relaxations induced by ATP (0.3–30 μM) (a) and CO (50 μM–1 mM) (b) on U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus under NANC conditions. Relaxations were measured as peak amplitudes. Peak amplitudes are expressed as percentages of the maximal contraction induced with U46619 (0.1 μM) in each strip. Each point represents the mean±s.e.m. of responses observed in eight (a) or four (b) strips.
Figure 2.
Mean concentration–response curve for the inhibitory effect of PPADS (3–100 μM) on ATP (3 μM)-induced relaxations of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus under NANC conditions. Relaxations were measured as peak amplitudes and expressed as percentages of the control in each strip. Each point represents the mean±s.e.m. of responses observed in four strips.
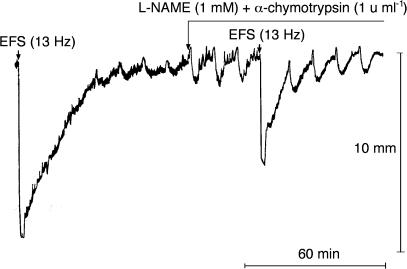
Throughout the experimental series performed to identify the effects of various pharmacological agents on EFS (13 Hz)-induced relaxant response, the relaxation amplitude ranged from 86.3±10.0 to 122.7±5.4% and the relaxation AUC from 15.5±1.6 to 24.1±2.9 min. D-NAME (1 mM) affected neither the amplitude nor the AUC of the EFS (13 Hz)-induced relaxant effects (100.0±2.6 and 99.7±3.1% of controls, respectively, n=8, Table 1). L-NAME (1 mM, 20 and 40 min) slightly, but significantly, reduced the amplitude (by 7.2±3.0%, n=9, P<0.05, and 12.0±2.8, n=5, P<0.05, of control values, respectively), but had no significant effects on the AUC (104.2±4.5 and 101.9±6.0% of control values, respectively) of the relaxant response (Table 1). The attenuation of relaxation amplitude produced by L-NAME at the longer exposure time was not significantly different from that observed with the shorter exposure time (P=0.31). Apamin (0.1 μM) did not affect the amplitude of relaxation (101.3±1.7% of controls, n=8). However, it slightly, but significantly, reduced the AUC of the relaxant response (by 15.2±3.8% of controls, P<0.05, Table 1). Apamin (0.3 μM) did not induce greater reductions (n=4, data not shown). When the strips were stimulated by EFS (13 Hz) in the presence of L-NAME (1 mM) plus apamin (0.1 μM), the relaxation amplitude was not significantly inhibited (93.6±4.2% of controls, n=8, P=0.20) and the reduction in relaxation AUC (by 20.9±5.3% of controls, P<0.01) was not significantly different from that produced by apamin (0.1 μM) (P=0.39, Table 1). In three out of eight strips of this experimental series, a small contraction of 9.0±0.2% preceded the relaxation. L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) was more effective than L-NAME (1 mM) in reducing the amplitude (by 33.9±3.6%, n=9, of controls, P<0.01 vs L-NAME 40 min and P<0.001 vs L-NAME 20 min, ANOVA) and greatly reduced the AUC of relaxation (by 69.7±2.8%, P<0.001, of controls, Figure 3, Table 1). PPADS (100 μM) did not significantly affect either the amplitude or the AUC of relaxation (96.1±2.3 and 92.9±4.1% of controls, respectively, n=6, Table 1). On the contrary, the procedure to induce desensitization to the relaxant effect of ATP (30 μM) produced significant reductions in both amplitude and AUC of EFS-evoked relaxation (by 16.3±2.8%, P<0.05, and 18.2±5.2%, n=9, P<0.05, of control values, respectively, Table 1). However, similar reductions in amplitude and AUC of relaxation (by 15.1±4.1%, P<0.05, and 14.7±4.5%, n=6, P<0.05, of control values, respectively, Table 1) were observed in parallel strips used to evaluate whether the time necessary to induce desensitization to ATP could affect the EFS-induced inhibitory response. The reductions in amplitude and AUC of relaxation produced by L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus PPADS (100 μM) (by 40.3±2.8 and 66.7±4.8%, n=6, of controls, respectively) or L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) after induction of desensitization to the relaxant effect of ATP (30 μM) (by 36.7±4.0 and 65.1±2.1%, n=6, of controls, respectively) were not significantly different from those produced by L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) (P>0.05, ANOVA, Table 1). Similarly, the reductions in amplitude and AUC of relaxation produced by L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus ZnPPIX (300 μM) (by 33.9±4.4 and 66.1±9.3%, n=4, of controls, respectively) or L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus Fe2+-Hb (100 μM) (by 37.2±3.1 and 63.7±3.8%, n=3, of controls, respectively) were not significantly different from those produced by L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) (P>0.05, ANOVA, Table 1).
Table 1.
Effects of various pharmacological agents on EFS (13 Hz, 120 mA, 1 ms, pulse trains of 2 min)-induced relaxant responses of rat gastric fundus strips precontracted with U46619 (0.1 μM) and incubated with atropine (1 μM) and guanethidine (5 μM)
| Test agent | n | Relaxation amplitude (% change from control) | Relaxation AUC (% change from control) |
|---|---|---|---|
| D-NAME (1 mM) | 8 | 0±2.6 | −0.3±3.1 |
| L-NAME (1 mM, 20 min) | 9 | −7.2±3.0* | 4.2±4.5 |
| L-NAME (1 mM, 40 min) | 5 | −12.0±2.8* | 1.9±6.0 |
| Apamin (0.1 μM) | 8 | 1.3±1.7 | −15.2±3.8* |
| L-NAME (1 mM)+Apamin (0.1 μM) | 8 | −6.4±4.2 | −20.9±5.3** |
| L-NAME (1 mM)+α-Chymotrypsin (1 U ml−1) | 9 | −33.9±3.6***, ##, ### | −69.7±2.8*** |
| PPADS (100 μM) | 6 | −3.9±2.3 | −7.1±4.1 |
| Desensitization to ATP | 9 | −16.3±2.8* | −18.2±5.2* |
| Vehicle (time controls for desensitization to ATP) | 6 | −15.1±4.1* | −14.7±4.5* |
| L-NAME (1 mM)+α-chymotrypsin (1 U ml−1)+PPADS (100 μM) | 6 | −40.3±2.8*** | −66.7±4.8*** |
| L-NAME (1 mM)+α-chymotrypsin (1 U ml−1)+desensitization to ATP | 6 | −36.7±4.0** | −65.1±2.1*** |
| L-NAME (1 mM)+α-chymotrypsin (1 U ml−1)+apamin (0.3 μM) | 3 | −23.3±2.9 | −68.8±0.6* |
| L-NAME (1 mM)+α-chymotrypsin (1 U ml−1)+ZnPPIX (300 μM) | 4 | −33.9±4.4** | −66.1±9.3** |
| L-NAME (1 mM)+α-chymotrypsin (1 U ml−1)+ferrous Hb (100 μM) | 3 | −37.2±3.1* | −63.7±3.8* |
| L-NAME (1 mM)+anti-VIP serum (1 : 100) | 7 | −23.3±8.9* | −64.0±3.6***, §§§ |
| D-NAME (1 mM)+normal rabbit serum (1 : 100) | 7 | 1.3±3.4 | −20.1±4.7* |
| L-NAME (1 mM)+anti-VIP serum (1 : 100)+PPADS (100 μM) | 4 | −17.8±5.2 | −57.6±8.9* |
| L-NAME (1 mM)+anti-VIP serum (1 : 100)+apamin (0.1 μM) | 9 | −63.7±4.9***, ††† | −79.9±3.3***, † |
Results are presented as means±s.e.m. of n experiments.
Significant differences between test and control responses: *P<0.05, **P<0.01, ***P<0.001.
P<0.01 vs L-NAME (1 mM, 40 min);
P<0.001 vs L-NAME (1 mM, 20 min);
P<0.001 vs D-NAME (1 mM)+normal rabbit serum (1 : 100);
P<0.05,
P<0.001 vs L-NAME (1 mM)+anti-VIP serum (1 : 100).
Figure 3.
Representative tracings showing the effect of L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) on NANC relaxations induced by EFS (13 Hz, 120 mA, 1 ms, pulse trains of 2 min) of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus.
5-HT (0.3 μM) induced a precontraction level similar to that induced by U46619 (0.1 μM) (data not shown). Also, EFS (13 Hz)-induced amplitude and AUC of relaxations of 5-HT-precontracted strips were similar to those of U46619-precontracted strips (92.5±2.3% and 18.4±1.6 min, respectively, n=17). Suramin (100 μM) reduced ATP (3 μM)-induced relaxation to 38.7±7.7% of controls (n=4). Suramin (100 μM) did not affect the amplitude (96.8±1.8%, n=9, of controls), but significantly increased the AUC of the relaxant response (by 25.3±5.4% of controls, P<0.05). L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) reduced the amplitude and the AUC of relaxation to levels similar to those observed in U46619-precontracted strips (by 34.0±5.1%, P<0.01, and 66.6±2.2%, P<0.05, of controls, respectively, n=4). The reduction in amplitude of relaxation produced by L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus suramin (100 μM) (by 33.8±11.4% of controls, n=4) was not significantly different from that produced by L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) (P=0.98). On the contrary, the reduction in AUC of relaxation observed in the presence of L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus suramin (100 μM) (by 48.1±6.4% of controls, n=4) was significantly smaller than that observed in the presence of L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) (P<0.05).
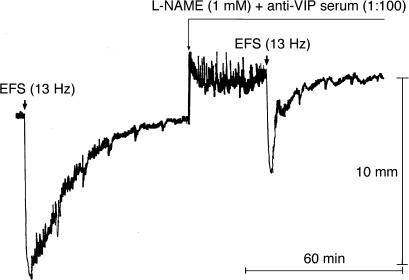
L-NAME (1 mM) plus anti-VIP serum (1 : 100) produced effects similar to those observed with L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) (Figure 4, Table 1). The AUC of the relaxant responses was also significantly reduced by D-NAME (1 mM) plus NRS (1 : 100) (Table 1). However, the reduction in AUC achieved with L-NAME (1 mM) plus anti-VIP serum (1 : 100) was significantly greater (by approximately 44%) than that produced by D-NAME (1 mM) plus NRS (1 : 100) (mean reductions: by 64.0±3.6%, n=7, vs 20.1±4.7%, n=7, of the control AUC, respectively, P<0.001). The reduction in relaxation amplitude achieved with L-NAME (1 mM) plus anti-VIP serum (1 : 100) was 23.3±8.9% of controls (n=7, P<0.05). The addition of PPADS (100 μM) to L-NAME (1 mM) plus anti-VIP serum (1 : 100) did not produce greater reductions in amplitude and AUC of relaxation (by 17.8±5.2 and 57.6±8.9%, n=4, of controls, respectively, P>0.05 vs L-NAME plus anti-VIP serum, ANOVA, Table 1). When the strips were subjected to EFS (13 Hz) in the presence of L-NAME (1 mM) plus anti-VIP serum (1 : 100) plus apamin (0.1 μM), the response became biphasic in seven out of nine strips, a rapid and short-lasting contraction of 22.9±4.9% preceding the relaxation (Figure 5). The reductions in amplitude and AUC of relaxation achieved with L-NAME (1 mM) plus anti-VIP serum (1 : 100) plus apamin (0.1 μM) were significantly greater (by approximately 40 and 16%, respectively) than those produced by L-NAME (1 mM) plus anti-VIP serum (1 : 100) (mean reductions: by 63.7±4.9 and 79.9±3.3%, n=9, of controls, P<0.001 and P<0.05 vs L-NAME plus anti-VIP serum, respectively, Table 1). Apamin, NRS and anti-VIP serum (Figures 4 and 5) contracted the muscle strips (data not shown). We also evaluated the effects of L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus apamin (0.3 μM) on EFS-induced relaxation. The reductions in amplitude and AUC of relaxation achieved with this combination were not significantly different from those observed with L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) (by 23.3±2.9 and 68.8±0.6%, n=3, of the control relaxant responses, P>0.05 vs L-NAME plus α-chymotrypsin, ANOVA).
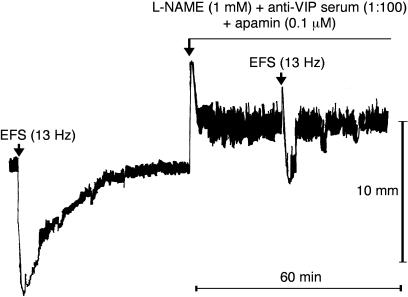
Figure 4.
Representative tracings showing the effect of L-NAME (1 mM) plus anti-VIP serum (1 : 100) on NANC relaxations induced by EFS (13 Hz, 120 mA, 1 ms, pulse trains of 2 min) of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus.
Figure 5.
Representative tracings showing the effect of L-NAME (1 mM) plus α-chymotrypsin (1 U ml−1) plus apamin (0.1 μM) on NANC relaxations induced by EFS (13 Hz, 120 mA, 1 ms, pulse trains of 2 min) of U46619 (0.1 μM)-precontracted longitudinal muscle strips of the rat gastric fundus.
Discussion
The stomach can accommodate large volumes of ingested material, with minimal increases in intraluminal pressure, through the reflex relaxation of the smooth muscle. The relaxation of gastric fundus strips induced by in vitro EFS under NANC conditions is the experimental model most commonly used to study this physiological response. The NANC relaxation of the rat gastric fundus begins immediately, develops rapidly and, for EFS frequency >4 Hz, is long lasting (Currò & Preziosi, 1998). It is now well established that NO and peptides, VIP and PHI, are the neurotransmitters responsible for the initial rapid phase and the long duration of relaxation, respectively (Currò & Preziosi, 1998; Currò et al., 2002). It seems, however, that they are not the only neurotransmitters of the inhibitory motor neurones in the rat gastric fundus, as NANC relaxation is not abolished by the combination of pharmacological agents that block NO- and peptide-mediated components (Li & Rand, 1990; Boeckxstaens et al., 1992; D'Amato et al., 1992a). ATP is a candidate neurotransmitter of this response (Currò & Preziosi, 1998). It relaxes the smooth muscle of the rat gastric fundus (Burnstock et al., 1970; Lefebvre, 1986). In addition, it is released from fundus strips by EFS in a TTX-sensitive manner (Belai et al., 1991). However, conflicting results have been reported concerning the effects on EFS-induced NANC relaxation of drugs or pharmacological procedures that inhibit the relaxant response induced by ATP (Lefebvre, 1986; Belai et al., 1991; Lefebvre et al., 1991; Jenkinson & Reid, 2000a). More recently, also CO has been proposed as an inhibitory neurotransmitter in the gastrointestinal tract (Zakhary et al., 1996; 1997; Xue et al., 2000). CO is produced with biliverdin from haeme through the activity of the enzyme haeme oxygenase (HO). CO relaxes pig gastric fundus strips (Colpaert et al., 2002a). In addition, HO-2, the constitutive neuronal isoform of the enzyme, is localized in submucous and myenteric plexuses and muscular interstitial cells of Cajal of pig and human stomach (Colpaert et al., 2002b). The first objective of the study was to quantify in terms of both amplitude and AUC the non-nitrergic nonpeptidergic component of high-frequency EFS-induced relaxation. The second and main objective was to define whether ATP and/or CO play a role as mediators of this response.
Antagonism of ATP-induced relaxation
At concentrations >1 μM, ATP induced biphasic responses, consisting of a rapid and brief relaxation followed by a sustained contraction. This is the classical response described in many previous studies (Burnstock et al., 1970; Lefebvre, 1986; Lefebvre & Burnstock, 1990; Belai et al., 1991; Lefebvre et al., 1991; Matharu & Hollingsworth, 1992; Jenkinson & Reid, 2000a, 2000b). The EC50 value of ATP-induced concentration–relaxation curve in the present study (0.56 μM) was smaller than that reported in previous studies (3.81–5.5 μM, Lefebvre & Burnstock, 1990; Matharu & Hollingsworth, 1992). The efficacy of ATP in inducing the relaxation was low (19.4% of U46619-induced peak contraction). It was very similar to those reported in two previous studies (Lefebvre, 1986; Lefebvre & Burnstock, 1990), but different from that reported in a third study, in which ATP showed high efficacy (∼80% of carbachol-induced precontraction, Matharu & Hollingsworth, 1992). In the latter study, however, efficacy of ATP was studied in the presence of indomethacin (a cyclooxygenase inhibitor) and 8-sulphophenyltheophylline (a P1-purinoceptor antagonist). Different levels of precontraction could explain the differences in potency and efficacy of ATP among various studies. ATP-induced relaxations were not affected by TTX or L-NAME, indicating that ATP acts directly at the muscular level. This contrasts with the mouse gastric fundus, where exogenous ATP appears to excite enteric neurones that release both NO and ATP (Giaroni et al., 2002).
We then investigated the possibility of inhibiting ATP-induced relaxations by various, commonly used, pharmacological tools. We were unable to desensitize the strips to the relaxant effect of α, β-methylene ATP. Desensitization to α, β-methylene ATP has been reported to occur in the rat gastric fundus (Lefebvre & Burnstock, 1990; Matharu & Hollingsworth, 1992; Jenkinson & Reid, 2000a). We do not have any reasonable explanation for this discrepancy among the present and previous studies. As far as P2-purinoceptor antagonists are concerned, we found that suramin and reactive blue 2 greatly relaxed U46619-precontracted strips. Suramin, at the same concentration (100 μM) used in our study, did not induce any relaxation of rat gastric fundus strips precontracted by 5-HT (Jenkinson & Reid, 2000b). Thus, we studied the effects of these two substances on strips precontracted by 5-HT too. Suramin only very slightly relaxed the strips. On the contrary, reactive blue 2 greatly relaxed also 5-HT-precontracted strips. Thus, only PPADS and suramin could be used to investigate the involvement of ATP in EFS-induced NANC relaxation, in U46619- or 5-HT-precontracted strips, respectively. Suramin- and reactive blue 2-induced relaxations of the rat gastric fundus have not been reported in other studies in which the strips were precontracted by carbachol (Lefebvre & Burnstock, 1990; Matharu & Hollingsworth, 1992). A possible explanation of these findings might be that suramin antagonizes the contracting effect of U46619 on TP receptors. We did not investigate this possibility. A different mechanism seems to be at the basis of reactive blue 2-induced relaxation of the gastric smooth muscle.
Possible role of ATP in non-nitrergic nonpeptidergic NANC relaxation
In the present study, an inhibitor of NO synthase, L-NAME, slightly reduced the amplitude, but did not affect the AUC of relaxation. This evidence indicates that NO plays a minor role, if any, in determining the duration of relaxation. In addition, the other inhibitory neurotransmitters are able to compensate almost completely for the inhibition of the nitrergic component as far as the amplitude of relaxation is concerned. This is true also for the blockade of the peptidergic component: α-chymotrypsin, at a concentration that blocked the relaxations induced by highly effective concentrations of VIP and PHI, only slightly reduced the amplitude of NANC relaxation (Currò et al., 2002). The addition of α-chymotrypsin to L-NAME produced a great reduction in relaxation AUC, similar to that produced by α-chymotrypsin alone (Currò et al., 2002), and a reduction in relaxation amplitude significantly greater than that produced by L-NAME alone. Similar results were obtained with L-NAME plus anti-VIP serum. The reduction in AUC achieved with latter combination, after subtraction of the small reduction observed with D-NAME plus NRS, was smaller than that achieved with L-NAME plus α-chymotrypsin (approximately 44 vs 67%). The relaxant effect induced by PHI can account for the difference in AUC reductions. Indeed, it has been shown that a specific anti-PHI serum reduces the AUC of NANC relaxation (Currò et al., 2002). These results confirm that the prevalent role of VIP and PHI is that of determining the duration of relaxation. Thus, the non-nitrergic nonpeptidergic component of the relaxation has amplitude and AUC equal to approximately two-third and one-third of controls, respectively. This component was not inhibited by PPADS, suramin or desensitization to ATP. This evidence would seem to exclude the involvement of ATP in this response. However, it has been recently proposed that ATP is the third inhibitory neurotransmitter in the rat gastric fundus (Jenkinson & Reid, 2000a). This conclusion was based on the evidence that PPADS and apamin greatly reduced the non-nitrergic nonpeptidergic (L-NAME plus α-chymotrypsin in the bath) component of the NANC relaxation induced by low-frequency (⩽4 Hz), short-duration (30 s) EFS (Jenkinson & Reid, 2000a). In our hands, PPADS was unable to reduce the relaxation induced by high-frequency, long-duration EFS in the presence of L-NAME plus α-chymotrypsin. Also apamin, in the present work, did not affect the component of the relaxation resistant to L-NAME and α-chymotrypsin. This result is, however, not surprising, considering that α-chymotrypsin is a peptidase and apamin a peptide. α-Chymotrypsin cleaves peptide bonds at the level of aromatic (such as phenylalanine and tyrosine) or long-chain ramified amino acids (such as leucine or isoleucine). Leucine is present in the amino-acidic sequence of apamin, making this peptide sensitive to the degradating effect of α-chymotrypsin, as previously shown (Van Rietschoten et al., 1975). Indeed, apamin was no longer able to inhibit the relaxant effect of ATP when α-chymotrypsin was incubated at the same time in the bath medium. PPADS, when used alone, did not influence NANC relaxation. On the contrary, suramin significantly increased the AUC of EFS-induced relaxation. This effect was evident also in experiments performed to study the effects of L-NAME plus α-chymotrypsin plus suramin. It was very surprising, considering that suramin has been reported to be a competitive VIP receptor antagonist in the rat gastric fundus (Jenkinson & Reid, 2000b). We have no certain explanation of this effect. Results may suggest a facilitatory pre- or post-junctional effect of suramin on the neurotransmitter responsible for the non-nitrergic nonpeptidergic component of NANC relaxation. Desensitization to ATP slightly, but significantly, reduced both amplitude and AUC of relaxation. However, similar reductions were observed in experiments performed to evaluate whether the time necessary to induce desensitization to ATP could affect the relaxant response to EFS. The fact that no further reduction was observed in strips treated with L-NAME plus α-chymotrypsin plus desensitization to ATP with respect to those treated with L-NAME plus α-chymotrypsin may suggest that nitrergic and/or peptidergic components are affected by time elapsed between the two EFS. Indeed, the relaxation induced by a second 2-min incubation with VIP (10 nM), performed after a time similar to that necessary to induce desensitization to ATP, was significantly smaller than the control one (data not shown).
Possible role of CO in non-nitrergic nonpeptidergic NANC relaxation
CO induced concentration-dependent relaxant responses of the rat gastric fundus. CO potency and efficacy were much lower and higher, respectively, than those of ATP (Figure 1). In addition, the efficacy of CO in inducing the relaxation (approximately 90% of U46619-induced peak contraction) was much greater than that reported in a previous study performed on porcine gastric fundus (approximately 30–35% of 5-HT-produced precontraction, Colpaert et al., 2002a). ZnPPIX did not affect the component of NANC relaxation resistant to L-NAME plus α-chymotrypsin. However, it has been hypothesized that another haeme oxygenase inhibitor, tin protoporphyrin IX (SnPPIX), may be unable to penetrate in intact tissues (Colpaert et al., 2002a). To exclude the possibility that also ZnPPIX does not penetrate in tissues and is not effective for this reason, we investigated the effects of L-NAME plus α-chymotrypsin plus Fe2+-Hb on NANC relaxation. CO binds to haemoglobin with high affinity (more than 200 times greater than that of oxygen, Giardina & Amiconi, 1981). However, Fe2+-haemoglobin, at a concentration able to greatly reduce the relaxant effect induced by CO (0.5 mM), did not reduce the non-nitrergic nonpeptidergic component of NANC relaxation. These findings would seem to exclude also the involvement of CO in this component. In mice with genomic deletion of HO-2, NANC relaxation of jejunal strips is almost abolished, despite the preservation of NO synthase (Xue et al., 2000). Exogenous CO is able to partially restore NANC relaxation to the wild-type phenotype, indicating that CO is necessary for NO to act (Xue et al., 2000). In the mouse internal anal sphincter, it has been very recently shown that VIP-induced relaxation is mediated by CO (Watkins et al., 2004). Thus, it is possible that CO may have a role as NANC transmitter also in the rat gastric fundus, as a mediator of NO- and/or VIP-induced relaxations. Our experiments do not exclude these possibilities.
Apamin sensitivity of non-nitrergic nonpeptidergic NANC relaxation
Apamin induced a small strip contraction. The contractile effect of apamin in the rat gastric fundus has been reported in many previous studies. The first reports were those of Kamata et al. (1988) and Lefebvre et al. (1991). It also occurs in other preparations such as the guinea-pig taenia coli (Maas & Den Hertog, 1979) and internal anal sphincter (Lim & Muir, 1986), and is thought to be caused by membrane depolarization and spike discharge due to small-conductance Ca2+-activated K+-channel closure. Apamin did not affect the amplitude of EFS-induced relaxation, but slightly reduced relaxation AUC. The effects produced by L-NAME plus apamin were not different from those produced by apamin alone. These data suggest that a neurotransmitter acting through apamin-sensitive mechanisms is involved in NANC relaxation and peptides are able to compensate almost completely for the inhibition of both nitrergic and apamin-sensitive components. The most convincing results indicating that the non-nitrergic nonpeptidergic component of NANC relaxation is mediated by a neurotransmitter acting through apamin-sensitive mechanisms came from experiments with L-NAME plus anti-VIP serum plus apamin. The reductions in amplitude and AUC of relaxation produced by this drug combination, but not those produced by L-NAME plus anti-VIP serum plus PPADS, were significantly greater than those produced by L-NAME plus anti-VIP serum. In addition, in the presence of L-NAME plus anti-VIP serum plus apamin, EFS-induced response became biphasic, a rapid and short-lasting contraction preceding a slowly developing and sustained relaxation. Thus, in our knowledge, these experiments show for the first time the existence of an NANC excitatory response in the rat gastric fundus. Further investigation of this contractile effect was beyond the scope of the present study. The relaxation observed in the presence of L-NAME plus anti-VIP serum plus apamin can be very probably ascribed to PHI that has been shown to be a neurotransmitter of the inhibitory motor neurones in the rat gastric fundus (Currò et al., 2002). As far as the neurotransmitter responsible for the non-nitrergic nonpeptidergic component is concerned, we did not perform any additional experimental work to try to identify it. NRS and anti-VIP serum (Figures 4 and 5) contracted the strips. We already reported this effect in previous works (Currò & Preziosi, 1997; Currò et al., 2002). It is not surprising, considering that many contracting substances are present in the serum, first of all that thromboxane is released during the platelet aggregation process. A higher level of precontraction could theoretically affect both amplitude and AUC of relaxant responses. Indeed, NRS significantly reduced the AUC of the EFS-evoked relaxation, whereas it did not influence relaxation amplitude. Thus, control strips treated with NRS were essential to estimate the actual effect of AUC reduction due to VIP immunoneutralization.
In conclusion, the present data demonstrate that the non-nitrergic nonpeptidergic component of NANC relaxation of the rat gastric fundus induced by high-frequency stimulation is apamin-sensitive. The neurotransmitter responsible for this component does not seem to be ATP. In addition, they also suggest that CO is not involved in this component.
Acknowledgments
This work was supported by MURST ex 60%.
Abbreviations
- ATP
adenosine triphosphate
- AUC
area under the curve
- CO
carbon monoxide
- D-NAME
NG-nitro-D-arginine methyl ester
- EFS
electrical field stimulation
- Fe2+-Hb
ferrous haemoglobin
- HO
haeme oxygenase
- 5-HT
5-hydroxytryptamine
- L-NAME
NG-nitro-L-arginine methyl ester
- NANC
nonadrenergic noncholinergic
- NO
nitric oxide
- NRS
normal rabbit serum
- PHI
peptide histidine isoleucine
- PPADS
pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid
- SnPPIX
tin protoporphirin IX
- TP
thromboxane receptor
- TTX
tetrodotoxin
- U46619
9,11-dideoxy-9α,11α-methanoepoxy prostaglandin F2α
- VIP
vasoactive intestinal polypeptide
- ZnPPIX
zinc protoporphirin IX
References
- BELAI A., LEFEBVRE R.A., BURNSTOCK G. Motor activity and neurotransmitter release in the gastric fundus of streptozotocin-diabetic rats. Eur. J. Pharmacol. 1991;194:225–234. doi: 10.1016/0014-2999(91)90109-4. [DOI] [PubMed] [Google Scholar]
- BENNETT H.R. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Prog. Neurobiol. 1997;52:159–195. doi: 10.1016/s0301-0082(97)00012-9. [DOI] [PubMed] [Google Scholar]
- BOECKXSTAENS G.E., PELCKMANS P.A., DE MAN J.G., BULT H., HERMAN A.G., VAN MAERCKE Y.M. Evidence for a differential release of nitric oxide and vasoactive intestinal polypeptide by nonadrenergic noncholinergic nerves in the rat gastric fundus. Arch. Int. Pharmacodyn. 1992;318:107–115. [PubMed] [Google Scholar]
- BURNSTOCK G., CAMPBELL G., SATCHELL D., SMYTHE A. Evidence that adenosine triphosphate or a related nucleotide is the transmitter substance released by non-adrenergic inhibitory nerves in the gut. Br. J. Pharmacol. 1970;40:668–688. doi: 10.1111/j.1476-5381.1970.tb10646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDERONE V., BARAGATTI B., BRESCHI M.C., MARTINOTTI E. Experimental and theoretical comparisons between the classical Schild analysis and a new alternative method to evaluate the pA2 of competitive antagonists. Naunyn-Schmiedeberg's Arch. Pharmacol. 1999;360:477–487. doi: 10.1007/s002109900082. [DOI] [PubMed] [Google Scholar]
- COLPAERT E.E., TIMMERMANS J.-P., LEFEBVRE R.A. Investigation of the potential modulatory effect of biliverdin, carbon monoxide and bilirubin on nitrergic neurotransmission in the pig gastric fundus. Eur. J. Pharmacol. 2002a;457:177–186. doi: 10.1016/s0014-2999(02)02691-2. [DOI] [PubMed] [Google Scholar]
- COLPAERT E.E., TIMMERMANS J.-P., LEFEBVRE R.A. Immunohistochemical localization of the antioxidant enzymes biliverdin reductase and heme oxygenase-2 in human and pig gastric fundus. Free Radic. Biol. Med. 2002b;32:630–637. doi: 10.1016/s0891-5849(02)00754-2. [DOI] [PubMed] [Google Scholar]
- CURRÒ D., DE MARCO T., PREZIOSI P. Involvement of peptide histidine isoleucine in non-adrenergic non-cholinergic relaxation of the rat gastric fundus induced by high-frequency neuronal firing. Naunyn-Schmiedeberg's Arch. Pharmacol. 2002;366:578–586. doi: 10.1007/s00210-002-0633-z. [DOI] [PubMed] [Google Scholar]
- CURRÒ D., PREZIOSI P. Involvement of vasoactive intestinal polypeptide in nicotine-induced relaxation of the rat gastric fundus. Br. J. Pharmacol. 1997;121:1105–1112. doi: 10.1038/sj.bjp.0701245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRÒ D., PREZIOSI P. Non-adrenergic non-cholinergic relaxation of the rat stomach. Gen. Pharmacol. 1998;31:697–703. doi: 10.1016/s0306-3623(98)00096-2. [DOI] [PubMed] [Google Scholar]
- CURRÒ D., PREZIOSI P., RAGAZZONI E., CIABATTONI G. Peptide histidine isoleucine-like immunoreactivity release from the rat gastric fundus. Br. J. Pharmacol. 1994;113:541–549. doi: 10.1111/j.1476-5381.1994.tb17023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CURRÒ D., VOLPE A.R., PREZIOSI P. Nitric oxide synthase activity and non-adrenergic non-cholinergic relaxation in the rat gastric fundus. Br. J. Pharmacol. 1996;117:717–723. doi: 10.1111/j.1476-5381.1996.tb15249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'AMATO M., CURRÒ D., MONTUSCHI P. Evidence for dual components in the non-adrenergic non-cholinergic relaxation in the rat gastric fundus: role of endogenous nitric oxide and vasoactive intestinal polypeptide. J. Auton. Nerv. Syst. 1992a;37:175–186. doi: 10.1016/0165-1838(92)90039-j. [DOI] [PubMed] [Google Scholar]
- D'AMATO M., CURRÒ D., MONTUSCHI P., CIABATTONI G., RAGAZZONI E., LEFEBVRE R.A. Release of vasoactive intestinal polypeptide from the rat gastric fundus. Br. J. Pharmacol. 1992b;105:691–695. doi: 10.1111/j.1476-5381.1992.tb09040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIARDINA B., AMICONI G. Measurement of binding of gaseous and nongaseous ligands to hemoglobins by conventional spectrophotometric procedures. Methods Enzymol. 1981;76:417–427. doi: 10.1016/0076-6879(81)76133-0. [DOI] [PubMed] [Google Scholar]
- GIARONI C., KNIGHT G.E., RUAN H.-Z., GLASS R., BARDINI M., LECCHINI S., FRIGO G., BURNSTOCK G. P2 receptors in the murine gastrointestinal tract. Neuropharmacology. 2002;43:1313–1323. doi: 10.1016/s0028-3908(02)00294-0. [DOI] [PubMed] [Google Scholar]
- JENKINSON K.M., REID J.J. Evidence that adenosine 5′-triphosphate is the third inhibitory non-adrenergic non-cholinergic neurotransmitter in the rat gastric fundus. Br. J. Pharmacol. 2000a;130:1627–1631. doi: 10.1038/sj.bjp.0703481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENKINSON K.M., REID J.J. The P2-purinoceptor antagonist suramin is a competitive antagonist at vasoactive intestinal peptide receptors in the rat gastric fundus. Br. J. Pharmacol. 2000b;130:1632–1638. doi: 10.1038/sj.bjp.0703482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAMATA K., SAKAMOTO A., KASUYA Y. Similarities between the relaxations induced by vasoactive intestinal peptide and by stimulation of the non-adrenergic non-cholinergic neurons in the rat stomach. Naunyn-Schmiedeberg's Arch. Pharmacol. 1988;338:401–406. doi: 10.1007/BF00172117. [DOI] [PubMed] [Google Scholar]
- LEFEBVRE R.A. Study on the possible neurotransmitter of the non-adrenergic non-cholinergic innervation of the rat gastric fundus. Arch. Int. Pharmacodyn. 1986;280 Suppl:110–136. [PubMed] [Google Scholar]
- LEFEBVRE R.A., BURNSTOCK G. Effect of adenosine triphosphate and related purines in the rat gastric fundus. Arch. Int. Pharmacodyn. 1990;303:199–215. [PubMed] [Google Scholar]
- LEFEBVRE R.A., DE BEURME F.A., SAS S. Effect of apamin on the response to VIP, ATP and NANC neurone stimulation in the rat and cat gastric fundus. J. Auton. Pharmacol. 1991;11:73–83. doi: 10.1111/j.1474-8673.1991.tb00246.x. [DOI] [PubMed] [Google Scholar]
- LI C.G., RAND M.J. Nitric oxide and vasoactive intestinal polypeptide mediate non-adrenergic, non-cholinergic inhibitory transmission to smooth muscle of the rat gastric fundus. Eur. J. Pharmacol. 1990;191:303–309. doi: 10.1016/0014-2999(90)94162-q. [DOI] [PubMed] [Google Scholar]
- LIM S.P., MUIR T.C. Neuroeffector transmission in the guinea-pig internal anal sphincter: an electrical and mechanical study. Eur. J. Pharmacol. 1986;128:17–24. doi: 10.1016/0014-2999(86)90552-2. [DOI] [PubMed] [Google Scholar]
- MAAS A.J., DEN HERTOG A. The effect of apamin on the smooth muscle cells of the guinea-pig taenia coli. Eur. J. Pharmacol. 1979;58:151–156. doi: 10.1016/0014-2999(79)90006-2. [DOI] [PubMed] [Google Scholar]
- MATHARU M.S., HOLLINGSWORTH M. Purinoceptors mediating relaxation and spasm in the rat gastric fundus. Br. J. Pharmacol. 1992;106:395–403. doi: 10.1111/j.1476-5381.1992.tb14346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RATTAN S., CHAKDER S. Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation. Am. J. Physiol. 1993;265:G799–G804. doi: 10.1152/ajpgi.1993.265.4.G799. [DOI] [PubMed] [Google Scholar]
- VAN RIETSCHOTEN J., GRANIER C., ROCHAT H., LISSITZKY S., MIRANDA F. Synthesis of apamin, a neurotoxic peptide from bee venom. Eur. J. Biochem. 1975;56:35–40. doi: 10.1111/j.1432-1033.1975.tb02204.x. [DOI] [PubMed] [Google Scholar]
- VANE J.R. A sensitive method for the assay of 5-hydroxytryptamine. Br. J. Pharmacol. 1957;12:344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATKINS C.C., BOEHNING D., KAPLIN A.I., RAO M., FERRIS C.D., SNYDER S.H. Carbon monoxide mediates vasoactive intestinal polypeptide-associated nonadrenergic/non-cholinergic neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 2004;101:2631–2635. doi: 10.1073/pnas.0308695100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XUE L., FARRUGIA G., MILLER S.M., FERRIS C.D., SNYDER S.H., SZURSZEWSKI J.H. Carbon monoxide and nitric oxide as coneurotransmitters in the enteric nervous system: evidence from genomic deletion of biosynthetic enzymes. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1851–1855. doi: 10.1073/pnas.97.4.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKHARY R., GAINE S.P., DINERMAN J.L., RUAT M., FLAVAHAN N.A., SNYDER S.H. Heme oxygenase 2: endothelial and neuronal localization and role in endothelium-dependent relaxation. Proc. Natl. Acad. Sci. U.S.A. 1996;93:795–798. doi: 10.1073/pnas.93.2.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKHARY R., POSS K.D., JAFFREY S.R., FERRIS C.D., TONEGAWA S., SNYDER S.H. Targeted gene deletion of heme oxygenase 2 reveals neural role for carbon monoxide. Proc. Natl. Acad. Sci. U.S.A. 1997;94:14848–14853. doi: 10.1073/pnas.94.26.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]