Abstract
Renin–angiotensin and endothelin systems are involved in the cardiovascular effects produced by treatment with ouabain. We recently demonstrated that the contractile response to phenylephrine is decreased in ouabain-treated rats. The present study investigated whether endothelin-1 (ET-1) and angiotensin II (Ang II) contributes to the vascular changes observed in rats chronically treated with ouabain.
Wistar rats were treated with ouabain (8.0 μg day−1, s.c. pellets for 5 weeks) alone or in combination with an endothelin type A receptor (ETA) antagonist, BMS182874 (40 mg kg−1 day−1, per gavage) or an angiotensin type 1 (AT1) receptor antagonist, losartan (15 mg kg−1 day−1, p.o.).
Treatment with ouabain increased systolic blood pressure and treatment with either losartan or BMS182874 prevented the development of ouabain-induced hypertension.
The sensitivity and maximal response for phenylephrine were reduced in aortic rings from ouabain-treated rats. Removal of the endothelium or in vitro exposure to an inhibitor of nitric oxide synthase (NOS), N-nitro-L-arginine methyl ester (L-NAME, 100 μM) increased the responses to phenylephrine, an effect that was more pronounced in aortas from ouabain-treated rats. Endothelial NOS protein (eNOS) expression was increased after ouabain treatment. Treatment with BMS182874, but not with losartan, prevented the effects of ouabain on the reactivity of phenylephrine and in eNOS protein expression.
Gene expression of pre–pro-ET-1 and ETA receptors was increased in aortic rings from ouabain-treated rats. ETB receptor gene expression was not altered by ouabain treatment.
In conclusion, our results suggest that endothelin and angiotensin systems play an important role in the development of ouabain-induced hypertension. However, ET-1, by activation of ETA receptors, but not Ang II, contributes to changes in vascular reactivity to phenylephrine induced by chronic treatment with ouabain.
Keywords: Ouabain, hypertension, endothelium, nitric oxide, renin–angiotensin system, endothelin
Introduction
There is extensive experimental evidence supporting an important role for the endogenous cardiac glycoside ouabain on the pathogenesis of hypertension (Hamlyn & Manunta, 1992; Hamlyn et al., 1996). One of the most important observations that support this affirmation is that plasma ouabain levels are increased in some forms of hypertension, as well as in human essential hypertension (Overbeck et al., 1976; Hamlyn et al., 1982; 1996). In addition, the acute or chronic administration of ouabain or its related isomers to normotensive rats increases blood pressure (Huang et al., 1994; Manunta et al., 1994; 2001; Kimura et al., 2000; Rossoni et al., 2001; 2002a, 2002b; D'Amico et al., 2003; Di Filippo et al., 2003; Xavier et al., 2004a, 2004b). However, conflicting results showing that hypertension is accompanied by normal plasma ouabain levels (Doris, 1994; Gonick et al., 1998; Priyadarshi et al., 2003; Wang et al., 2003) or that ouabain is ineffective to produce hypertension (Li et al., 1995; Pidgeon et al., 1996; Cargnelli et al., 2000) have also been published.
Both central and peripheral mechanisms are involved in the production of hypertension by ouabain. Chronic treatment with ouabain increases the content of ouabain in some brain areas related to cardiovascular regulation (Huang et al., 1994). The interplay of the cardiac glycoside with some excitatory components present in these areas is one of the mechanisms that explain the genesis of hypertension in this model (Huang et al., 1994; Huang & Leenen, 1999; Zhang & Leenen, 2001; Di Filippo et al., 2003). The central renin–angiotensin and endothelin systems are examples of these sympathoexcitatory components involved in the hypertensinogenic effect of ouabain, since both the sympathetic hyperactivity and hypertension induced by chronic treatment with ouabain are prevented by angiotensin type 1 (AT1) and endothelin-1 (ET-1) type A (ETA) receptors blockade (Zhang & Leenen, 2001; Di Filippo et al., 2003).
In addition to central mechanisms, peripheral mechanisms may contribute to the elevated arterial blood pressure induced by chronic ouabain treatment (Kimura et al., 2000; Di Filippo et al., 2003). However, it is possible that changes in peripheral mechanisms may play a compensatory role or may counteract ouabain-induced hypertension (Rossoni et al., 2002a, 2002b; Xavier et al., 2004a, 2004b). Cargnelli et al. (2000) demonstrated that phenylephrine-induced contraction was reduced while ET-1-induced contraction was increased in rat aortae after long-term treatment with ouabain. We have also demonstrated that in this hypertension model the vascular reactivity to phenylephrine is decreased and that this is accompanied by an increased activity and expression of the sodium–potassium ATPase pump (Na+, K+-ATPase) (Rossoni et al., 2002a). These alterations are also associated with increased endothelium-derived nitric oxide (NO) production and increased expression of the endothelial nitric oxide synthase (eNOS) and neuronal isoforms of NOS (Rossoni et al., 2002b).
Based on these observations, the purpose of the present work was to investigate if the endothelin and/or renin–angiotensin systems contribute to the vascular changes in rats chronically treated with ouabain. Therefore, we evaluated if treatment with losartan, an AT1–angiotensin-receptor antagonist, or BMS182874, a selective ETA-receptor antagonist, would prevent the changes in aortic reactivity produced by chronic treatment with ouabain. Expression of the genes for pre–pro-ET-1, ETA and ETB receptors in aorta were also evaluated.
Methods
Male Wistar rats (6 weeks old) were obtained from colonies maintained at the Animal Quarters of the Institute of Biomedical Sciences of the University of Sao Paulo and Facultad de Medicina of the Universidad Autónoma de Madrid. Rats were housed at a constant room temperature, humidity and 12 h light/dark cycle and had free access to tap water and standard rat chow. All experiments comply with the guidelines for biomedical research as stated by the Brazilian Societies of Experimental Biology and with the current Spanish and European laws (RD 223/88 MAPA and 609/86).
Rats were anaesthetized with diethyl ether and a small incision was made in the back of the neck to implant a subcutaneous controlled time-release pellet (Innovative Research of America, U.S.A.) containing ouabain (0.5 mg pellet−1) or vehicle (placebo). Animals were divided into six groups: vehicle, ouabain (approximately 8.0 μg day−1), Vehicle plus losartan (15 mg kg−1 day−1, p.o.), Ouabain plus losartan, Vehicle plus BMS182874 (40 mg kg−1 day−1, p.o. per gavage) and Ouabain plus BMS182874. Losartan, administered from the time the pellets were implanted, was dissolved in tap water at a final concentration calculated as a function of body weight and the volume of water consumed. The dose of losartan was chosen in order to produce a reduction of approximately 40 mmHg in the systolic blood pressure (SBP) of ouabain-treated rats (Bravo et al., 2001). The dose of the ETA antagonist BMS 182874 was chosen based on previous works from our group (Callera et al., 2003). SBP was measured weekly in awake animals by the tail-cuff method (Buñag, 1973) before the beginning of treatment and once a week during treatment until week 5.
Vascular reactivity study
After 5 weeks, animals were anaesthetized with diethyl ether and killed by exsanguination. The thoracic aorta was removed and placed in cold oxygenated Krebs–Henseleit bicarbonate buffer (KHB). The buffer consisting of (in mM): NaCl 118; KCl 4.7; NaHCO3 25; CaCl2·2H2O 2.5; KH2PO4 1.2; MgSO4·7H2O 1.2; glucose 11 and ethylenediamine-tetraacetic acid 0.01. Segments of thoracic aorta (3 mm in length), free of fat and connective tissue, were mounted between two steel hooks in isolated tissue chambers containing gassed (95% O2 and 5% CO2) KHB, at 37°C, under a resting tension of 1 g. Isometric tension was recorded by using an isometric force displacement transducer connected to an acquisition system (PowerLab 8/S, ADInstruments Pty Ltd, Castle Hill, Australia).
After a 45 min equilibration period, each aortic ring was exposed twice to KCl (75 mM), to assess its maximum contractility. After 30 min, rings were contracted with a concentration of phenylephrine that induced approximately 50% of the KCl contraction, and then acetylcholine (10 μM) was added to assess the integrity of the endothelium. A relaxation equal to or greater than 90% was considered as evidence of the functional integrity of the endothelium.
After 60 min, cumulative concentration–response curves for phenylephrine (0.1 nM–30 μM) were generated. In some experiments, the endothelium was removed by gently rubbing the intimal surface with a stainless-steel rod. The effectiveness of endothelium removal was confirmed by the absence of relaxation induced by acetylcholine (10 μM) in rings precontracted with phenylephrine. Additionally, the effect of the nonselective NOS inhibitor N-nitro-L-arginine methyl ester (L-NAME, 100 μM) on concentration–response curves for phenylephrine was investigated. L-NAME was added 30 min before generating the concentration–response curve.
Western blot analysis of eNOS protein expression
Proteins from homogenized aortae (30 μg per lane) were separated by 7.5% sodium lauryl sulphate–polyacrylamide gel (SDS–PAGE) and transferred to polyvinyl difluoride membranes overnight. Next, the membranes were incubated for 1 h at room temperature with mouse monoclonal antibody for eNOS (1 : 2500, Transduction Laboratories, Lexington, U.K.), and after washing were incubated with antimouse IgG antibody conjugated to horseradish peroxidase (1 : 2000, Transduction Laboratories, U.K.). The immunocomplexes were detected using an enhanced horseradish peroxidase/luminol chemiluminiscence system (ECL Plus, Amersham International plc, Little Chalfont, U.K.) and subjected to autoradiography (Hyperfilm ECL, Amersham International plc, U.S.A.). Signals on the immunoblot were quantified using a computer program (NIH Image V1.56, U.S.A.). The same membranes were used to determine α-actin expression using a mouse monoclonal antibody (1 : 3,000,000, Boehringer Mannheim, Mannheim, Germany). Homogenates from human endothelial cells were used for eNOS-positive control.
Reverse transcriptase–polymerase chain reaction (RT–PCR)
Total cell RNA was isolated from aortae using Trizol Reagent (Gibco BRL, Life Technologies, Rockville, MD, U.S.A.). Total RNA (20 ng per sample) was used for RT in the presence of an RNAase inhibitor 20 U (RNAase OUT, Invitrogen Life Technologies), 200 U of Superscript II RT (Invitrogen, Carlsbad, CA, U.S.A.) and 0.5 μg of oligo-(dT) 12–18 primer at 42°C for 50 min, according to the manufacturer's specifications. The cDNA was stored at −20°C until required for the PCR. PCR primers were designed as follows: pre–pro-ET-1, antisense primer CTCGCTCTATGTAAGTCATGG, sense primer GCTCCTGCTCCTCCTTGATG (471-bp fragment); ETA receptor, antisense primer CTGTGCTGCTCGCCCTTGTA, sense primer GAAGTCGTCCGTGGGCATCA (216-bp fragment); ETB receptor, antisense primer CACGATGAGGACAATGAGAT, sense primer TTACAAGACAGCCAAAGACT (565-bp fragment); and GAPDH, antisense primer CACCACCCTGTTGCTGTA, sense primer TATGATGACATCAAGAAGGTGG (219-bp fragment). GAPDH was used as an internal control for the coamplification. The following conditions were selected for PCR in a volume of 25 μl: RT products from 20 ng of RNA, 1 U of Platinum® Taq DNA polymerase (Invitrogen), 28 cycles of amplification for pre–pro-ET-1, 32 cycles for ETA and 30 for ETB receptor genes, and 24 cycles for the GAPDH gene. Amplification was carried out using an initial denaturing cycle at 94°C for 5 min and the subsequent cycles as follows: denaturation, 30 s at 94°C; annealing, 30 s at 64°C for pre–pro-ET-1, 58°C for ETB, 66°C for ETA or 62°C for GAPDH; and extension, 45 s at 72°C. PCR products (10 μl per lane) were electrophoresed using 1% agarose gel containing ethidium bromide (0.5 μg ml−1). The gel was subjected to ultraviolet light and photographed. The band intensities were measured using a software package (Kodak Digital Science, Eastman Kodak Company, New Haven, CT, U.S.A.).
Solutions, drugs and statistical analysis
Phenylephrine hydrochloride, acetylcholine chloride and L-NAME dihydrochloride were purchased from Sigma (St Louis, MO, U.S.A.). Stock solutions (10 mM) of drugs were made in distilled water and were kept at −20°C, and appropriate dilutions were made on the day of the experiment. Losartan and BMS182874 were a gift of Merck Sharp & Dohme and of Bristol Myers Squibb Co., respectively.
All values are expressed as means±s.e.m. Contractile responses are expressed as a percentage of the maximum response produced by 75 mM KCl. For each concentration–response curve, the maximum effect (Emax) and the concentration of agonist that produced half of Emax (log EC50) were estimated using nonlinear regression analysis (GraphPad Prism Software, San Diego, CA, U.S.A.). The sensitivity of the agonist is expressed as negative logarithm of concentrations producing 50% of maximum response (pD2) (−log EC50). In order to compare the magnitude of the effects of endothelium denudation and NOS blockade on the responses to phenylephrine, some results were expressed as ‘differences' of area under the concentration–response curves (dAUC) in control and experimental situations. AUCs were calculated from the individual concentration–response curve plot and the differences were expressed as a percentage of the difference to AUC of the corresponding control situation. Results of eNOS expression are expressed as the ratio between optical density for NOS and for α-actin. Results of pre–pro-ET-1, ETA and ETB mRNA expressions are expressed relative to the intensity of GAPDH.
Results were analysed using Student's t-test and by completely randomized two-way ANOVA for comparison between groups. When ANOVA showed a significant treatment effect, Bonferroni's post hoc test was used to compare individual means. Differences were considered statistically significant at P<0.05.
Results
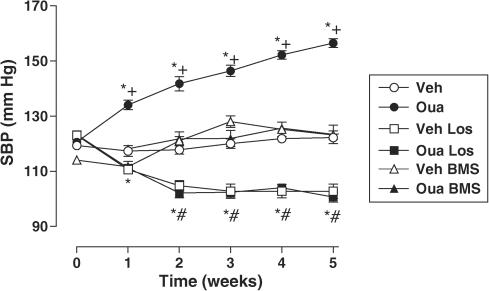
Figure 1 shows the SBP for all groups over the course of treatment. An increase, from the first week of treatment, was observed in ouabain-treated rats compared to vehicle group. In ouabain and vehicle groups, treatment with losartan significantly reduced SBP to similar levels. Treatment with BMS182874 did not change SBP in the vehicle group but prevented the development of hypertension in ouabain-treated animals. Body weight gain during the 5 weeks of treatment was similar in all groups (data not shown).
Figure 1.
SBP in Wistar rats treated with vehicle (Veh, N=13), ouabain (Oua, N=21), Vehicle plus losartan (Veh Los, N=7), Ouabain plus Losartan (Oua Los, N=8), Vehicle plus BMS182874 (Veh BMS, N=7) or Ouabain plus BMS182874 (Oua BMS, N=8). Results are expressed as mean±s.e.m. *P<0.05 vs 0 week; # vs 0 week Los group; +P<0.05 vs vehicle.
Vascular reactivity study
To assess the maximum contraction for each preparation, rings were exposed to 75 mM KCl. Vasoconstrictor responses to this depolarizing solution were similar in aortae from ouabain-treated rats compared to vehicle group (ouabain: 24.9±1.6 vs vehicle: 24.7±1.5 mN, N=9 and 12, respectively, t-test, P>0.05). This response was not altered by treatment with losartan (ouabain losartan: 25.4±1.5 vs vehicle losartan: 24.7±1.2 mN, N=8, t-test, P>0.05) or BMS182874 (ouabain BMS: 21.0±0.9 vs vehicle BMS: 21.2±1.4 mN, N=5, t-test, P>0.05).
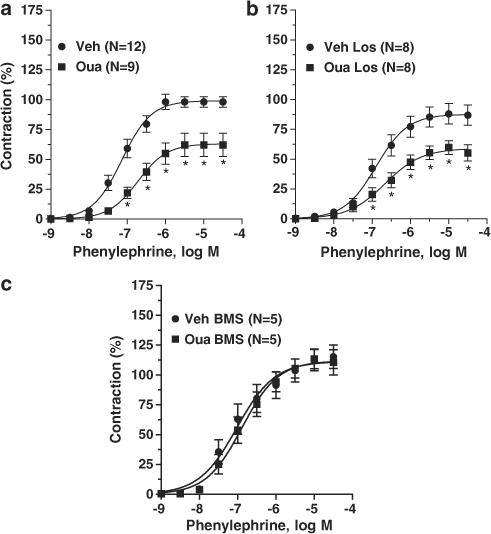
As previously demonstrated, both the Emax and pD2 for phenylephrine were reduced in intact aortic rings obtained from animals treated for 5 weeks with ouabain (Figure 2a and Table 1). Treatment with losartan did not change the concentration–response curve for phenylephrine and did not alter the effects of ouabain treatment on the phenylephrine response (Figure 2b and Table 1). In contrast to losartan, treatment with BMS182874 prevented the effects of ouabain on the actions of phenylephrine (Figure 2c and Table 1).
Figure 2.
Contractile responses to phenylephrine in thoracic aortic rings from vehicle (Veh) and ouabain-treated (Oua) rats (a); vehicle losartan-treated (Veh Los) and Ouabain plus losartan-treated rats (Oua Los) (b); vehicle BMS184874-treated rats (Veh BMS) and Ouabain plus BMS184874-treated rats (Oua BMS) (c). Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): *P<0.05 vs vehicle. Number of animals used is indicated within parentheses.
Table 1.
Changes in Emax (expressed as a percentage of the response to 75 mM KCl) and pD2 to phenylephrine in thoracic aorta with (E+) and without (E−) endothelium and after L-NAME treatment from Wistar rats treated with vehicle (N=5–9), ouabain (N=5–12), Vehicle plus losartan (N=8–9), Ouabain plus Losartan (N=8–12), vehicle plus BMS182874 (N=4–5) or Ouabain plus BMS182874 (N=4–5)
| Emax (%) | pD2 | |
|---|---|---|
| Vehicle | ||
| E+ | 93.0±4.7 | 7.17±0.1 |
| E− | 119±7.7+ | 7.79±0.1+ |
| L-NAME | 136±1.3+ | 7.48±0.1+ |
| Ouabain | ||
| E+ | 62.3±9.8* | 6.65±0.1* |
| E− | 126±7.8+ | 7.78±0.1+ |
| L-NAME | 140±7.0+ | 7.46±0.1+ |
| Vehicle+losartan | ||
| E+ | 87.5±8.1 | 6.90±0.1 |
| E− | 108±4.5+ | 7.45±0.1+ |
| L-NAME | 116±3.4+ | 7.34±0.1+ |
| Ouabain+losartan | ||
| E+ | 58.6±6.2* | 6.50±0.1* |
| E− | 107±5.0+ | 7.36±0.1+ |
| L-NAME | 115±2.7+ | 7.17±0.1+ |
| Vehicle+BMS182874 | ||
| E+ | 111±7.8 | 6.82±0.1 |
| E− | 142±1.5+ | 7.23±0.1+ |
| L-NAME | 143±10.3+ | 7.43±0.1+ |
| Ouabain+BMS182874 | ||
| E+ | 110±7.8 | 6.82±0.1 |
| E− | 148±4.6+ | 7.37±0.1+ |
| L-NAME | 154±15.4+ | 7.50±0.1+ |
t-Test,
P<0.05 vs vehicle and +P<0.05 vs E+;.
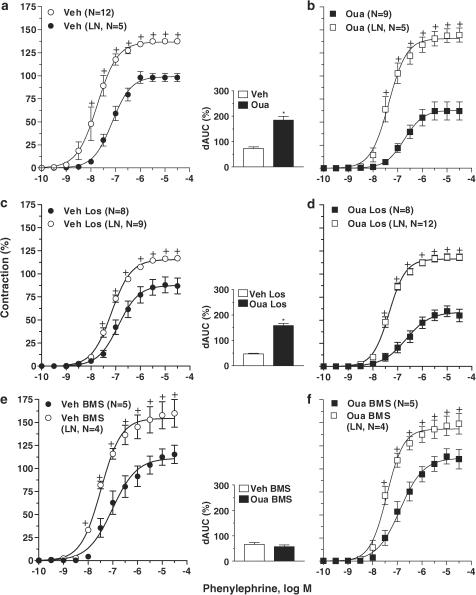
Effect of endothelium removal or NO synthesis blockade on phenylephrine responses
Endothelial denudation or treatment with L-NAME increased the maximal response and sensitivity to phenylephrine in aorta obtained from both ouabain and vehicle groups (Figures 3a, b and 4a, b). In both cases, the increase was larger in ouabain-treated rats, as shown by the values for dAUC (see inset graph in Figures 3a, b and 4a, b). The concentration–response curves for phenylephrine in aorta from the ouabain group and the vehicle group in the presence of L-NAME or following endothelium removal were not different (Table 1).
Figure 3.
Effect of endothelium denudation on the concentration-dependent response curves to phenylephrine in thoracic aortic rings from vehicle (Veh) and ouabain-treated (Oua) rats (a and b); vehicle losartan-treated (Veh Los) and Ouabain plus losartan-treated rats (Oua Los) (c and d); vehicle BMS184874-treated rats (Veh BMS) and Ouabain plus BMS184874-treated (Oua BMS) rats (e and f). Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): +P<0.05 vs E+. Inset graph shows dAUC to phenylephrine in endothelium-intact (E+) and -denuded (E−) arteries. dAUC values (means±s.e.m.) are expressed as a percentage of the difference of the corresponding AUC for segments with intact endothelium (unpaired t-test, *P<0.05 vs vehicle). Number of animals used is indicated within parentheses.
Figure 4.
Effect of L-NAME (LN, 100 μM) on the concentration-dependent response curves to phenylephrine performed in thoracic aortic rings from vehicle (Veh) and ouabain-treated (Oua) rats (a and b); vehicle losartan-treated (Veh Los) and Ouabain plus losartan-treated rats (Oua Los) (c and d); vehicle BMS184874-treated rats (Veh BMS) and Ouabain plus BMS184874-treated rats (Oua BMS) (e and f). Results (means±s.e.m.) are expressed as a percentage of response elicited by KCl. ANOVA (two-way): +P<0.05 vs without L-NAME. Inset graph shows dAUC to phenylephrine in segments in the absence and presence of L-NAME. dAUC values (means±s.e.m.) are expressed as a percentage of the differences of the corresponding AUC for segments in the absence of L-NAME (unpaired t-test, *P<0.05 vs vehicle). Number of animals used is indicated within parentheses.
Similar changes were observed in aortae from the losartan groups (Figures 3c, d and 4c, d). Namely, the shifts in concentration–response curves for phenylephrine were greater in aorta from ouabain-treated animals in the presence of L-NAME or following endothelial denudation (see inset graph in Figures 3c, d and 4c, d). In aortae from rats treated with losartan, there were no differences in the concentration–response curves for phenylephrine in the presence of L-NAME or after endothelium damage (Table 1).
However, treatment with BMS182874 prevented the effects of ouabain on the actions of phenylephrine and abolished the differential effect of ouabain on the modulation of the endothelium and NO on the actions of phenylephrine (see dAUC graph in Figures 3e, f and 4e, f). In endothelium-denuded or L-NAME-treated aortae from rats that received BMS182874, the responses to this α1-adrenergic agonist were similar in ouabain and vehicle groups (Table 1).
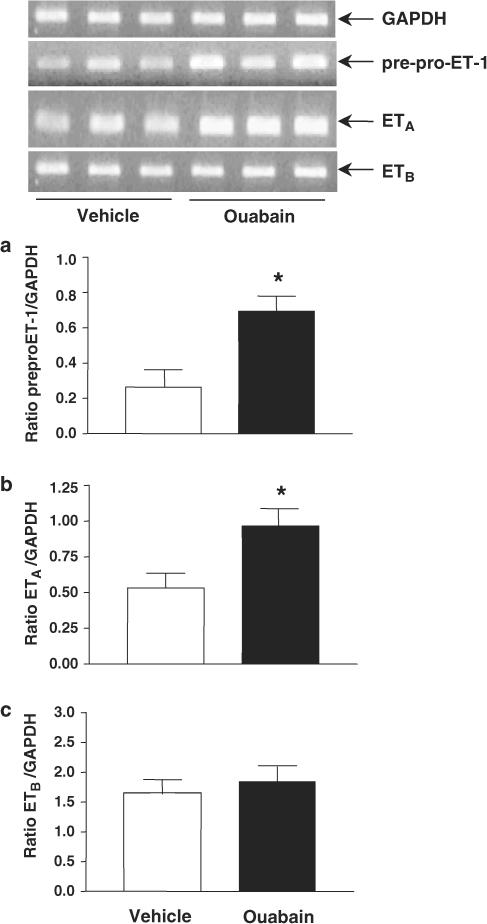
Pre–pro-ET-1, ETA and ETB mRNA expression
Figure 5 shows results of mRNA expression, obtained by RT–PCR, for pre–pro-ET-1, ETA and ETB receptors in aortae from ouabain and vehicle groups. Expression of pre–pro-ET-1 mRNA was significantly increased in aortae from ouabain-treated rats. Similarly, increased ETA receptor mRNA expression was observed in aortae from ouabain-treated rats compared to vehicle rats. ETB receptor mRNA expression remained unchanged after chronic treatment with ouabain.
Figure 5.
Representative RT–PCR products of 3 μg total RNA extracted from aortae of vehicle and ouabain-treated rats. Bar graphs show the densitometric values for gene expression of pre–pro-ET-1 (a), ETA (b) and ETB (c) subtype receptors. Values were normalized by the corresponding RT–PCR products for GAPDH (N=4–6). *P<0.05 vs vehicle.
eNOS protein expression
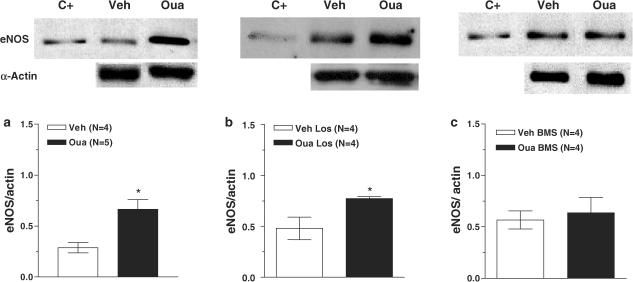
eNOS expression was evaluated in homogenates of aortae obtained from all experimental groups (Figure 6). In arteries from ouabain and Ouabain plus losartan groups, eNOS expression was increased compared to that in their respective control groups (Figure 6a and b). However, no differences were observed in eNOS expression in the aortae from vehicle and ouabain rats treated with BMS182874 (Figure 6c).
Figure 6.
Upper panel: Representative blot for eNOS protein expression in aortic arteries from vehicle (Veh) and ouabain-treated (Oua) (a); or vehicle losartan-treated (Veh Los) and Ouabain plus losartan-treated rats (Oua Los) (b); or vehicle BMS184874-treated rats (Veh BMS) and Ouabain plus BMS184874-treated rats (Oua BMS) (c). The first lane shows the positive control (C+, human endothelial cells) for eNOS. Lower panel: Densitometric analysis of the Western blot for eNOS protein expression. Results (means±s.e.m.) are expressed as the ratio between the signal for the eNOS protein and the signal for α-actin. Unpaired t-test, *P<0.05 vs vehicle. Number of animals used is indicated within parentheses.
Discussion
The present study shows that although ET-1 and angiotensin II (Ang II) contribute to the hypertension induced by chronic ouabain treatment (Huang & Leenen, 1999; Zhang & Leenen, 2001; Di Filippo et al., 2003), only ET-1 contributes to changes in vascular reactivity in aortae from ouabain hypertensive rats. Our data reinforce earlier observations on the effects of ouabain increasing blood pressure in rats, reducing the contractile actions of phenylephrine and producing an increase in the negative modulation of vascular reactivity by the endothelium (Huang et al., 1994; Manunta et al., 1994; 2001; Kimura et al., 2000; Rossoni et al., 2002a, 2002b; Di Filippo et al., 2003; Xavier et al., 2004a, 2004b).
It has been reported that acutely central administration of ouabain causes sympathoexcitatory and pressor effects in rats (Huang & Leenen, 1996; D'Amico et al., 2003). In addition, following its chronic administration, ouabain accumulates in brain areas related to cardiovascular regulation (Huang et al., 1994; Manunta et al., 1994) where it might induce activation of sympathoexcitatory outflow (Huang et al., 1994; Huang & Leenen, 1999; Zhang & Leenen, 2001) and by this way increases peripheral vascular resistance and induces hypertension (Di Filippo et al., 2003). On the other hand, this hypertension induced by ouabain is also accompanied by vascular compensatory mechanisms (Rossoni et al., 2002a, 2002b; Xavier et al., 2004a, 2004b), which seems to be independent of elevated arterial blood pressure (Xavier et al., 2004a). This compensatory mechanism could be a consequence of a direct vascular action of ouabain and/or an adaptive response to the increased sympathetic outflow.
The central renin–angiotensin system is one of the sympathoexcitatory components involved in the hypertensinogenic actions of ouabain. This is shown by the demonstration that treatment with an AT1-receptor antagonist prevents the increase in central sympathetic outflow and hypertension produced by the long-term administration of ouabain (Huang & Leenen, 1999; Zhang & Leenen, 2001). In addition, the acute hypertensinogenic effect of ouabain is attenuated in transgenic rats that are deficient in brain angiotensinogen (Huang et al., 2001). In agreement with these observations, the results presented here show that AT1 receptor blockade with losartan prevented the development of hypertension in ouabain-treated rats.
Although treatment with losartan prevented the development of hypertension produced by ouabain, it did not prevent the ability of ouabain to alter either the vascular actions of phenylephrine, the increased negative endothelial modulation of vascular reactivity or the increased eNOS expression. Similar results were recently obtained with the effects of treatment with losartan on the vascular reactivity of conductance and resistance mesenteric arteries obtained from ouabain hypertensive rats (Xavier et al., 2004a). These results suggest that Ang II via AT1 receptors activation is involved in the genesis of hypertension but not in vascular reactivity changes caused by chronic treatment with ouabain. These two actions appear to be mediated by different pathways.
In addition, treatment with BMS182874, an ETA-receptor antagonist, also prevented the development of hypertension in chronically ouabain-treated rats. This result is in agreement with that of Di Filippo et al. (2003), showing that treatment with a selective ETA-receptor antagonist prevented the increase on renal vascular resistance and blood pressure in chronically ouabain-treated rats. Similarly, results from D'Amico et al. (2003) demonstrated that acute ouabain administration on intraperiaqueductal grey area increases total peripheral resistance and blood pressure, probably through an activation of brain ET-1. Altogether, these results suggest an important role of endothelin system on the hypertensinogenic effect of ouabain.
The endothelin system also contributes to the genesis of hypertension and to the cardiovascular changes in volume expansion-dependent models of hypertension, such as deoxycorticosterone acetate (DOCA)-salt hypertension (Tostes et al., 2000; Callera et al., 2003; Iglarz & Schiffrin, 2003). Salt retention and plasma volume expansion may stimulate the release of endogenous ouabain that, in turn, would stimulate the release of ET-1 from endothelial cells (Overbeck et al., 1976; Yamada et al., 1990; Hamlyn & Manunta, 1992; Hamlyn et al., 1996; Saunders & Scheiner-Bobis, 2004).
The results presented here showed, for the first time, that the ETA receptor blockade prevents the chronic actions of ouabain on the contractile response of phenylephrine in rat aortae. Here and in previous studies (Rossoni et al., 2002a, 2002b), we have reported that in aorta from ouabain hypertensive rats, the enhanced negative endothelial modulation on the contractile responses to phenylephrine is probably mediated by an increase in eNOS expression and consequent increase of synthesis and NO release. ETA receptor blockade completely prevented the increased expression of eNOS and enhanced negative modulatory action of the endothelium caused by treatment with ouabain. The effects of both endothelium removal and NOS inhibition on the actions of phenylephrine in aortic rings from rats treated with Ouabain plus BMS182874 were similar to those obtained in arteries from rats treated with Vehicle plus BMS182874. These results suggest a link between ET-1-activated ETA receptors and the increased negative modulatory actions of the endothelium observed in aortic rings from ouabain-treated rats.
To further evaluate the vascular endothelin system in aortae from ouabain-treated rats, we investigated gene expression of pre–pro-ET-1 and its receptors. The expression of mRNAs for pre–pro-ET-1 and ETA receptors were increased in aortic rings from ouabain-treated rats. Interestingly, in brain areas of rats associated with cardiovascular regulation, Di Filippo et al. (2003) observed about a three-fold increase in ET-1 content and a decrease in the expression of mRNA for ETA receptors following treatment with ouabain. Moreover, it was demonstrated that ouabain stimulates ET-1 release and gene expression in endothelial cells (Yamada et al., 1990; Saunders & Scheiner-Bobis, 2004). The mechanisms by which ouabain induces gene expression and release of ET-1 include both genomic and nongenomic effects that are independent of its ability to inhibit the sodium pump (Saunders & Scheiner-Bobis, 2004).
ET-1 effects are mediated by activation of both ETA and ETB receptors (Masaki, 2004). In the vascular wall, ETA receptors are predominantly expressed in smooth muscle cells, whereas the ETB subtype is expressed in both endothelial and smooth muscle cells (Masaki, 2004). The activation of either smooth muscle receptor produces vasoconstriction. In contrast, the activation of ETB receptors present on the endothelium produces vasodilation, an effect mediated mainly by synthesis and release of NO (Warner et al., 1989). However, there is evidence suggesting that activation of ETA receptors also stimulates NO release. Marsen et al. (1999) showed that ET-1 acting on ETA receptors induces eNOS gene expression via tyrosine kinase- and protein kinase-dependent pathways. We observed an increase in eNOS protein expression in aortae from ouabain-treated rats that was prevented by BMS182874 treatment. These results suggest that increased expression of mRNA for pre–pro-ET-1 and ETA receptors observed in rat aorta after treatment with ouabain might be responsible for the increased negative endothelial modulation of the contractile actions of phenylephrine in this artery following treatment with ouabain.
In conclusion, we have shown that the changes in phenylephrine vascular reactivity observed in aortae from ouabain hypertensive rats are dependent on the activation of ETA receptors by ET-1 and independent of the elevated arterial blood pressure or the renin–angiotensin system. Blockade of ETA receptors with BMS182874 prevented both the hypertension and the negative endothelial modulation of the contractile actions of phenylephrine observed after treatment with ouabain. Our results also suggest that chronic treatment with ouabain in rats activates a local endothelin system that, in turn, stimulates eNOS protein expression.
Acknowledgments
We gratefully acknowledge Professor Louis A. Barker for his helpful discussion and suggestions in the preparation of the article. This work was supported by FAPESP, CNPq, DGICYT (SAF 2003 00633) and Banco Santander Central Hispano. Fabiano E. Xavier was supported by CAPES (PDEE-BEX0339/02-4, Brazil) and by FISS (C03/01, Spain).
Abbreviations
- Ang II
angiotensin II
- AT1
angiotensin type 1 receptor
- dAUC
difference of area under the concentration–response curves
- Emax
maximum response
- eNOS
endothelial nitric oxide synthase
- ET-1
endothelin-1
- ETA
endothelin type A receptor
- ETB
endothelin type B receptor
- KHB
Krebs–Henseleit bicarbonate buffer
- L-NAME
N-nitro-L-arginine methyl ester
- NO
nitric oxide
- PAGE
polyacrylamide gel
- pD2
negative logarithm of concentrations producing 50% of maximum response
- RT–PCR
reverse transcriptase–polymerase chain reaction
- SBP
systolic blood pressure
- SDS
sodium lauryl sulphate
References
- BRAVO R., SOMOZA B., RUIZ-GAYO M., GONZALEZ C., RUILOPE L.M., FERNANDEZ-ALFONSO M.S. Differential effect of chronic antihypertensive treatment on vascular smooth muscle cell phenotype in spontaneously hypertensive rats. Hypertension. 2001;37:E4–E10. doi: 10.1161/01.hyp.37.5.e4. [DOI] [PubMed] [Google Scholar]
- BUÑAG R.D. Validation in awake rats of a tail-cuff method for measuring systolic pressure. J. Appl. Physiol. 1973;34:279–282. doi: 10.1152/jappl.1973.34.2.279. [DOI] [PubMed] [Google Scholar]
- CALLERA G.E., TOUYZ R.M., TEIXEIRA S.A., MUSCARA M.N., CARVALHO M.H., FORTES Z.B., NIGRO D., SCHIFFRIN E.L., TOSTES R.C. ETA receptor blockade decreases vascular superoxide generation in DOCA-salt hypertension. Hypertension. 2003;42:811–817. doi: 10.1161/01.HYP.0000088363.65943.6C. [DOI] [PubMed] [Google Scholar]
- CARGNELLI G., TREVISI L., DEBETTO P., LUCIANI S., BOVA S. Effect of long-term ouabain treatment on contractile responses of rat aortae. J. Cardiovasc. Pharmacol. 2000;35:538–542. doi: 10.1097/00005344-200004000-00004. [DOI] [PubMed] [Google Scholar]
- D'AMICO M., DI FILIPPO C., PIEGARI E., RINALDI B., ROSSI F., FILIPPELLI A. ETA endothelin receptors are involved in the ouabain-induced haemodynamic effects in the periaqueductal gray area of rats. Life Sci. 2003;72:2211–2218. doi: 10.1016/s0024-3205(03)00099-7. [DOI] [PubMed] [Google Scholar]
- DI FILIPPO C., FILIPPELLI A., RINALDI B., PIEGARI E., ESPOSITO F., ROSSI F., D'AMICO M. Chronic peripheral ouabain treatment affects the brain endothelin system of rats. J. Hypertens. 2003;21:747–753. doi: 10.1097/00004872-200304000-00018. [DOI] [PubMed] [Google Scholar]
- DORIS P.A. Ouabain in plasma from spontaneously hypertensive rats. Am. J. Physiol. 1994;266:H360–H364. doi: 10.1152/ajpheart.1994.266.1.H360. [DOI] [PubMed] [Google Scholar]
- GONICK H.C., DING Y., VAZIRI N.D., BAGROV A.Y., FEDOROVA O.V. Simultaneous measurement of marinobufagenin, ouabain, and hypertension-associated protein in various disease states. Clin. Exp. Hypertens. 1998;20:617–627. doi: 10.3109/10641969809053240. [DOI] [PubMed] [Google Scholar]
- HAMLYN J.M., MANUNTA P. Ouabain, digitalis-like factors and hypertension. J. Hypertens. 1992;10 Suppl 7:S99–S111. [PubMed] [Google Scholar]
- HAMLYN J.M., HAMILTON B.P., MANUNTA P. Endogenous ouabain, sodium balance and blood pressure: a review and a hypothesis. J. Hypertens. 1996;14:151–171. doi: 10.1097/00004872-199602000-00002. [DOI] [PubMed] [Google Scholar]
- HAMLYN J.M., RINGEL R., SCHAEFFER J., LEVINSON P.D., HAMILTON B.P., KOWEARSKI A.A., BLAUSTEIN M.P. A circulating inhibitor of (Na+, K+) ATPase associated with essential hypertension. Nature. 1982;300:650–652. doi: 10.1038/300650a0. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., GANTEN D., LEENEN F.H. Responses to central Na(+) and ouabain are attenuated in transgenic rats deficient in brain angiotensinogen. Hypertension. 2001;37:683–686. doi: 10.1161/01.hyp.37.2.683. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., HUANG X., HARMSEN E., LEENEN F.H.H. Chronic central versus peripheral ouabain, blood pressure, and sympathetic activity in rats. Hypertension. 1994;23:1087–1090. doi: 10.1161/01.hyp.23.6.1087. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., LEENEN F.H.H. Sympathoexcitatory and pressor responses to increased brain sodium and ouabain are mediated via brain ANG II. Am. J. Physiol. 1996;270:H275–H280. doi: 10.1152/ajpheart.1996.270.1.H275. [DOI] [PubMed] [Google Scholar]
- HUANG B.S., LEENEN F.H.H. Brain renin–angiotensin system and ouabain-induced sympathetic hyperactivity and hypertension in Wistar rats. Hypertension. 1999;34:107–112. doi: 10.1161/01.hyp.34.1.107. [DOI] [PubMed] [Google Scholar]
- IGLARZ M., SCHIFFRIN E.L. Role of endothelin-1 in hypertension. Curr. Hypertens. Reports. 2003;5:144–148. doi: 10.1007/s11906-003-0071-4. [DOI] [PubMed] [Google Scholar]
- KIMURA K., MANUNTA P., HAMILTON B.P., HAMLYN J.M. Different effects of in vivo ouabain and digoxin on renal artery function and blood pressure in the rat. Hypertens. Res. 2000;23:S67–S76. doi: 10.1291/hypres.23.supplement_s67. [DOI] [PubMed] [Google Scholar]
- LI M., MARTIN A., WEN C., TURNER S.W., LEWIS L.K., WHITWORTH J.Á. Long-term ouabain administration does not alter blood pressure in conscious Sprague–Dawley rats. Clin. Exp. Pharmacol. Physiolol. 1995;22:919–923. doi: 10.1111/j.1440-1681.1995.tb02327.x. [DOI] [PubMed] [Google Scholar]
- MANUNTA P., HAMILTON B.P., HAMLYN J.M. Structure–activity relationship for the hypertensinogenic activity of ouabain: role of the sugar and lactone ring. Hypertension. 2001;37:471–477. doi: 10.1161/01.hyp.37.2.472. [DOI] [PubMed] [Google Scholar]
- MANUNTA P., ROGOWSKI A.C., HAMILTON B.P., HAMLYN J.M. Ouabain-induced hypertension in the rat: relationships among plasma and tissue ouabain and blood pressure. J. Hypertens. 1994;12:549–560. [PubMed] [Google Scholar]
- MARSEN T.A., EGINK G., SUCKAU G., BALDAMUS C.A. Tyrosine-kinase-dependent regulation of the nitric oxide synthase gene by endothelin-1 in human endothelial cells. Eur. J. Physiol. 1999;438:538–544. doi: 10.1007/s004249900079. [DOI] [PubMed] [Google Scholar]
- MASAKI T. Historical review: endothelin. Trends in Pharmacol. Sci. 2004;25:219–224. doi: 10.1016/j.tips.2004.02.008. [DOI] [PubMed] [Google Scholar]
- OVERBECK H.W., PAMNANI M.B., AKERA T., BRODY T.M., HADDY F.J. Depressed function of a ouabain-sensitive sodium–potassium pump in blood vessels from renal hypertensive dogs. Circ. Res. 1976;38:48–52. doi: 10.1161/01.res.38.6.48. [DOI] [PubMed] [Google Scholar]
- PIDGEON G.B., RICHARDS A.M., NICHOLLS M.G., CHARLES C.J., RADEMAKER M.T., LYNN K.L., BAILEY R.R., LEWIS L.K., YANDLE T.G. Chronic ouabain infusion does not cause hypertension in sheep. Am. J. Physiol. 1996;270:E386–E392. doi: 10.1152/ajpendo.1996.270.3.E386. [DOI] [PubMed] [Google Scholar]
- PRIYADARSHI S., VALENTINE B., HAN C., FEDOROVA O.V., BAGROV A.Y., LIU J., PERIYASAMY S.M., KENNEDY D., MALHOTRA D., XIE Z., SHAPIRO J.I. Effect of green tea extract on cardiac hypertrophy following 5/6 nephrectomy in the rat. Kidney Int. 2003;63:1785–1790. doi: 10.1046/j.1523-1755.2003.00914.x. [DOI] [PubMed] [Google Scholar]
- ROSSONI L.V., PINTO V.D., VASSALLO D.V. Effects of small doses of ouabain on arterial blood pressure of anesthetized hypertensive and normotensive rats. Braz. J. Med. Biol. Res. 2001;34:1065–1077. doi: 10.1590/s0100-879x2001000800014. [DOI] [PubMed] [Google Scholar]
- ROSSONI L.V., SALAICES M., MARÍN J., VASSALLO D.V., ALONSO M.J. Alterations on vascular reactivity to phenylephrine and Na+, K+-ATPase activity and expression in hypertension induced by chronic administration of ouabain. Br. J. Pharmacol. 2002a;135:771–781. doi: 10.1038/sj.bjp.0704501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSSONI L.V., SALAICES M., MIGUEL M., BRIONES A.M., BARKER L.A., VASSALLO D.V., ALONSO M.J. Ouabain-induced hypertension is accompanied by increases in endothelial vasodilator factors. Am. J. Physiol. 2002b;283:H2110–H2118. doi: 10.1152/ajpheart.00454.2002. [DOI] [PubMed] [Google Scholar]
- SAUNDERS R., SCHEINER-BOBIS G. Ouabain stimulates endothelin release and expression in human endothelial cells without inhibiting the sodium pump. Eur. J. Biochem. 2004;271:1054–1062. doi: 10.1111/j.1432-1033.2004.04012.x. [DOI] [PubMed] [Google Scholar]
- TOSTES R.C., DAVID F.L., CARVALHO M.H., NIGRO D., SCIVOLETTO R., FORTES Z.B. Gender differences in vascular reactivity to endothelin-1 in deoxycorticosterone-salt hypertensive rats. J. Cardiovasc. Pharmacol. 2000;36 Suppl 1:S99–S101. doi: 10.1097/00005344-200036051-00032. [DOI] [PubMed] [Google Scholar]
- WANG J.G., STAESSEN J.A., MESSAGGIO E., NAWROT T., FAGARD R., HAMLYN J.M., BIANCHI G., MANUNTA P. Salt, endogenous ouabain and blood pressure interactions in the general population. J. Hypertens. 2003;21:1475–1481. doi: 10.1097/00004872-200308000-00010. [DOI] [PubMed] [Google Scholar]
- WARNER T.D., DE NUCCI G., VANE J.R. Rat endothelin is a vasodilator in the isolated mesentery of the rat. Eur. J. Pharmacol. 1989;159:325–326. doi: 10.1016/0014-2999(89)90167-2. [DOI] [PubMed] [Google Scholar]
- XAVIER F.E., ROSSONI L.V., ALONSO M.J., BALFAGÓN G., VASSALLO D.V., SALAICES M. Ouabain-induced hypertension alters the participation of endothelial factors in alpha-adrenergic responses differently in rat resistance and conductance mesenteric arteries. Br. J. Pharmacol. 2004a;143:215–225. doi: 10.1038/sj.bjp.0705919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XAVIER F.E., SALAICES M., MARQUEZ-RODAS I., ALONSO M.J., ROSSONI L.V., VASSALLO D.V., BALFAGÓN G. Neurogenic nitric oxide release increases in mesenteric arteries from ouabain hypertensive rats. J. Hypertens. 2004b;22:949–957. doi: 10.1097/00004872-200405000-00017. [DOI] [PubMed] [Google Scholar]
- YAMADA K., GOTO A., HUI C., SUGIMOTO T. Endogenous digitalis-like factor as a stimulator of endothelin secretion from endothelial cells. Biochem. Biophys. Res. Commun. 1990;172:178–183. doi: 10.1016/s0006-291x(05)80190-1. [DOI] [PubMed] [Google Scholar]
- ZHANG J., LEENEN F.H.H. AT(1) receptor blockers prevent sympathetic hyperactivity and hypertension by chronic ouabain and hypertonic saline. Am. J. Physiol. 2001;280:H1318–H1323. doi: 10.1152/ajpheart.2001.280.3.H1318. [DOI] [PubMed] [Google Scholar]