Abstract
Quercitrin, 3-rhamnosylquercetin, is a bioflavonoid with antioxidant properties, which exerts anti-inflammatory activity in experimental colitis. In the present study, different in vivo experiments were performed in order to evaluate the mechanisms of action involved in this effect, with special attention to its effects on proinflammatory mediators, including nitric oxide (NO).
Experimental colitis was induced in female Wistar rats by incorporation of dextran sodium sulfate (DSS) in drinking water. Oral treatment of quercitrin (1 or 5 mg kg−1 day−1) to colitic rats ameliorated the evolution of the inflammatory process induced when administered in a preventative dosing protocol. When quercitrin (1 mg kg−1 day−1) was administered on established colitis, it facilitated the recovery of the inflamed mucosa.
The beneficial effects exerted by quercitrin were evidenced both histologically and biochemically, and were associated with an improvement in the colonic oxidative status, altered as a consequence of the colonic insult induced by DSS. In addition, a reduction of colonic NO synthase activity was observed, probably related to a decreased expression in the inducible form of the enzyme via downregulation in the colonic activity of the nuclear factor-κB.
Immunohistochemical studies showed that quercitrin treatment reduced macrophage and granulocyte infiltration in the inflamed tissue.
Keywords: Antioxidant activity, dextran sodium sulfate colitis, intestinal anti-inflammatory activity, nuclear factor-κB, nitric oxide synthase, quercitrin
Introduction
Chronic inflammatory bowel disease (IBD), mainly ulcerative colitis and Crohn's disease, is a naturally remitting and recurring condition of the digestive tract. Although its precise etiology is unknown, it is probably related to an abnormal exacerbated immune response to otherwise innocuous stimuli, which is not properly abrogated by the feedback system that normally downregulates the mucosal response to luminal factors (Fiocchi, 1998). As other inflammatory processes, IBD is characterized by an upregulation of the synthesis and release of a variety of proinflammatory mediators, such as eicosanoids, platelet-activating factor, reactive oxygen and nitrogen metabolites and cytokines, thus influencing mucosal integrity and leading to excessive tissue injury (Katz et al., 1999; Podolsky & Fiocchi, 2000). Moreover, most of these mediators can induce the biosynthesis and release of other such compounds, generating a ‘vicious cycle' that may result in the propagation and perpetuation of the inflammatory response. Nowadays, a specific causal treatment of IBD is still not available, and the best strategy to effectively downregulate the exacerbated immune response that characterizes IBD may be to interfere with multiple stages of the inflammatory cascade, preferably with a single drug treatment (Kho et al., 2001). In fact, the drugs currently used for the management of human IBD, that is, 5-aminosalicylic acid derivatives and systemic or local glucocorticoids, exert their beneficial effects through a combination of different mechanisms (Travis & Jewel, 1994; Bratts & Linden, 1996). Unfortunately, these drugs are not devoid of potentially serious side effects, thus limiting their use (Enns & Sutherland, 1998; Stein & Hanauer, 2000). For this reason, the development of new drug treatments that combine efficacy and safety is an important goal in IBD therapy.
This could be the case of flavonoids, natural products presenting several biological activities, mainly related to their ability to inhibit enzymes and/or their antioxidant properties, and which have also been described to downregulate the immune response (Middleton et al., 2000). These activities could make their consideration as valid drugs in the pharmacological treatment of IBD. In fact, previous studies have shown the efficacy of some of these compounds, including quercitrin, rutoside, diosmin, hesperidin and morin, in the trinitrobenzene sulphonic acid (TNBS) model of rat colitis (Sanchez de Medina et al., 1996; Cruz et al., 1998; Crespo et al., 1999; Galvez et al., 2001). Although the mechanism of action of these flavonoids is poorly understood, a common feature in all cases is their ability to improve the colonic oxidative stress that characterizes the intestinal inflammatory status, probably related to their well-known antioxidant and/or free radical scavenging properties attributed to flavonoids. However, additional mechanisms might contribute in their beneficial effects. Morin has been shown to downregulate mediators involved in the intestinal inflammatory response, such as cytokines and nitric oxide (NO) (Galvez et al., 2001). Recently, it has been reported that the beneficial effects of quercitrin on TNBS chronic colitis can arise from an early downregulation of the inflammatory cascade, which is associated with amelioration of the disturbances in hydroelectrolytic transport (Sanchez de Medina et al., 2002).
The aim of the present study was to further elucidate the mechanisms involved in the intestinal anti-inflammatory effects of quercitrin. For this purpose, we tested the effects of this flavonoid in the dextran sodium sulfate (DSS) model of rat colitis in two different dosing protocols, that is, preventative and after an initial colonic insult. The DSS model produces inflammation limited to the colonic mucosa that is more closely related to human ulcerative colitis (Kullmann et al., 2001; Gaudio et al., 1999), as opposed to the TNBS model of rat colitis, which induces a transmural lesion with pathological characteristics similar to Crohn's disease (Fuss et al., 1999). Special attention was paid to the effects exerted by the flavonoid on the production of some of the mediators involved in the inflammatory response: leukotriene B4 (LTB4) and NO.
Methods
Animals
Female Wistar rats (170–190 g) obtained from the Laboratory Animal Service of the University of Granada were housed individually in makrolon cages, maintained in air-conditioned animal quarters with a 12 h light–dark cycle, and fed standard rodent chow (Panlab A04, Panlab, Barcelona, Spain) and water ad libitum throughout the experiment. This study was carried out in accordance with the Directive for the Protection of Vertebrate Animals used for Experimental and other Scientific Purposes of the European Union (86/609/EEC).
Induction of colitis and treatment protocols
After a 7-day acclimation period, rats were weighed and randomly distributed in the different experimental groups of eight rats each. Two experimental protocols were followed: (A) Preventative treatment, in which colitis was induced as described previously (Stucchi et al., 2000) by replacing normal drinking water with distilled water containing 5% DSS (w v−1, prepared daily, mol wt 36,000–50,000) for 8 days. The experimental groups were: a noncolitic group that received no flavonoid treatment and distilled water without DSS, a colitic control group that received 5% DSS alone and the treated group that received 5% DSS and quercitrin (at doses of 1 and 5 mg kg−1 day−1). Quercitrin was dissolved in distilled water and administered orally by means of an esophageal catheter, beginning concurrently with DSS administration. All controls received equivalent volumes of water for the duration of the study. All animals were killed 8 days after the beginning of the experiments. (B) Quercitrin treatment on established colitis, in which colitis was induced as described previously, but DSS concentration was 5% (w v−1) for the first 5 days and it was decreased to 2% (w v−1) for the following 10 days. Similarly, three experimental groups of rats were included: noncolitic, control colitic and quercitrin treated; however, quercitrin treatment (at the dose of 1 mg kg−1 day−1) started when the concentration of DSS was reduced. All animals were killed after 15 days of the beginning of the experiments.
Assessment of colonic damage
Animal body weight, the presence of gross blood in the feces and stool consistency were recorded daily for each rat by an observer unaware of the treatment. These parameters were each assigned a score according to the criteria proposed by Cooper et al. (1993) (Table 1), which was used to calculate an average daily disease activity index (DAI) for each animal as these authors described previously. Food and water consumption was also recorded daily throughout the duration of the study. Once rats were killed by cervical dislocation, their colons were immediately removed and rinsed with ice-cold phosphate-buffered saline. The excised colonic segments were placed on an ice-cold plate, cleaned of fat and mesentery, and blotted on filter paper. Each specimen was weighed and its length measured under a constant load (2 g). The colon was longitudinally opened and a cross section from the distal diseased area was immediately fixed in 4% formaldehyde and embedded in paraffin for histological analysis.
Table 1.
Scoring of disease activity index (DAI)
| Score | Weight loss (%) | Stool consistency | Rectal bleeding |
| 0 | None | Normal | Normal |
| 1 | 1–5 | ||
| 2 | 5–10 | Loose stools | |
| 3 | 10–20 | ||
| 4 | >20 | Diarrhea | Gross bleeding |
DAI value is the combined scores of weight loss, stool consistency, and bleeding divided by 3. Adapted from Cooper et al. (1993).
The colon was subsequently sectioned in different fragments to be used for biochemical determinations, which were immediately frozen at −80°C, except for the samples used for glutathione content determination, which was immediately weighed and then frozen in 1 ml of 5% (w v−1) trichloroacetic acid, and for LTB4 synthesis determination, which was immediately processed.
Biochemical determinations in colonic tissue
All biochemical measurements were completed within 1 week from the time of sample collection and were performed in duplicate. Myeloperoxidase (MPO) activity was measured according to the technique described by Krawisz et al. (1984); the results were expressed as MPO units (μmol min−1) per gram of wet tissue. Total glutathione content was quantified with the recycling assay described by Anderson (1985), and the results were expressed as nmol g wet tissue−1. Alkaline phosphatase (AP) activity was determined spectrophotometrically using disodium p-nitrophenylphosphate as substrate (Sanchez de Medina et al., 1996) and the results expressed as mU mg protein−1. Colonic nitric oxide synthase (NOS) activity was determined, in the presence or absence of aminoguanidine (10−4 M), by monitoring the conversion of L-[3H]arginine to L-[3H]citrulline as described previously (Rodriguez-Cabezas et al., 2002), and the results were expressed as pmol L-citrulline 30 min−1 mg protein−1. Colonic samples for LTB4 synthesis were minced on an ice-cold plate and suspended in a tube with 10 mM sodium phosphate buffer (pH 7.4) (1/20 w v−1). The tubes were placed in a shaking water bath (37°C) for 20 min and centrifuged at 9000 × g for 30 s at 4°C. The supernatants were frozen at −80°C until assay, and LTB4 was quantified by enzyme-linked immunosorbent assay (ELISA) (Amersham, Madrid, Spain), and the results expressed as ng g−1 wet tissue.
Microwell colorimetric NF-κB assay
Colonic NF-κB activation was determined in colonic specimens obtained from those rats that received quercitrin once the colonic inflammation was in progress, and this was performed by an ELISA kit (TransAM™ method, Active Motif, Carlsbad, CA, U.S.A.). Colon homogenates were suspended in lysis buffer (20mM HEPES (pH 7.5), 0.35 M NaCl, 20% glycerol, 1% NP-40, 1 mM MgCl2·6H2O, 0.5 mM EDTA, 0.1 mM EGTA) containing protease and phosphatase inhibitors (aprotinin, leupeptin, 1,10-phenanthroline, PMSF, ioadacetamide and sodium orthovanadate). After 10 min on ice, the lysate was centrifuged for 20 min at 12,000 × g, with the supernatants constituting the total protein extract being stored at −80°C until subjected to analysis. In all, 2 μg of each protein extract was incubated in a 96-well plate coated with immobilized oligonucleotide containing the NF-κB consensus site (5′-GGGACTTTCC-3′). The active form of NF-κB contained in cell extracts specifically binds to this oligonucleotide and can be detected by an ELISA technique. The ELISA kit contains a positive control from Jurkat cells stimulated with TPA and calcium ionophore.
Histological analysis
Full-thickness sections of 5 μm were stained with hematoxylin and eosin and graded by three pathologists (Ana Nieto, Angel Concha and Maria Dolores Lorente) blinded to the experimental groups according to the criteria described previously by Stucchi et al. (2000) (Table 2). In the colonic samples obtained from the treatment protocol with quercitrin on established colitis, immunolabelling was also performed on formalin-fixed paraffin wax-embedded samples using the streptavidin–biotin peroxidase complex method and a high-temperature pretreatment as antigen-unmasking protocols. After pretreatment, sections were incubated 15 min with 1% hydrogen peroxidase to block endogenous peroxidase activity, and were preincubated with protein-blocking sera for 30 min to reduce nonspecific reactions. The primary antibody used was a rabbit polyclonal anti-iNOS (Santa Cruz Biotechnology Inc., Santa Cruz, CA, U.S.A.; dilution of 1 : 100, incubated overnight at 4°C). The revealed system (Dako immunoreactivity) was visualized with 3-3′ diaminobenzidine tetrachloride (Sigma Chemical Co., St Louis, U.S.A.) and hydrogen peroxide (0.01%), and slides were counterstained for 2 min with Gill's hematoxylin. Dilutions and washing were made in Tris-buffered saline (TBS). Negative control slides were made by substituting the primary antibody with TBS or with normal swine serum. The indirect immunofluorescence methods were performed according to established procedures. The primary mouse monoclonal antibodies used were anti-rat mononuclear phagocytes (BD Pharmingen, clone 1C7), anti-rat granulocytes (BD Pharmingen, clone RP-1) and anti-rat CD3 (BD Pharmingen, clone G4.18). Primary antibodies were diluted 1 : 40 in TBS and incubated in a humidity chamber overnight at 4°C. Samples were later incubated with appropriate FICT-conjugated secondary antibodies (Dako Co. Inc.) diluted 1 : 30 at 37°C. Sections were then mounted with Aquatex© (Merck and Co. Inc.).
Table 2.
Scoring criteria of full-thickness distal colon sections
| Mucosal epithelium |
| Ulceration: none (0); mild surface (1); moderate (2); extensive-full thickness (3) |
| Crypts |
| Mitotic activity: lower third (0); mild mid-third (1); moderate mid-third (2); upper third (3) |
| Neutrophilic infiltrate |
| Mucus depletion |
| Lamina propria |
| Plasmacytoid infiltrate |
| Neutrophilic infiltrate |
| Vascularity |
| Fibrin deposition: none (0); mucosal (1); submucosal (2); transmural (3) |
| Submucosal |
| Neutrophilic infiltrate |
| Edema |
Scoring scale: 0, none; 1, mild; 2, moderate; 3, severe. Maximum score: 30. Modified from Stucchi et al., (2000).
Protein extraction and Western blot analysis
The colonic samples obtained from rats were homogenized (1/3 w v−1) in PBS supplemented with 0.1% sodium dodecyl sulfate (SDS), 0.1% sodium deoxycholate, 1% Triton X-100 and protease and phosphatase inhibitors. The proteins (100 μg) were boiled at 95°C in Laemmli SDS-loading buffer and separated on 7.5% SDS–PAGE for the detection of iNOS or eNOS. The proteins were then electrotransferred to nitrocellulose membranes (PROTAN©, Schleicher & Schuell, Germany). The membranes were blocked for at least 1 h at room temperature in TBS-0.1% Tween-20 (TBS-T) 5% nonfat dry milk and then incubated with TBS-T containing BSA 5% and the following dilution of each primary antibody: 1 : 2000 iNOS (Transduction Laboratories, Becton Dickinson Biosciences, Madrid, Spain) and 1 : 2000 eNOS (Santa Cruz Biotechnology, Santa Cruz, CA, U.S.A.) overnight at 4°C. After three washes of 5 min each with TBS-T, the membranes were incubated with 5% nonfat dry milk and peroxidase-conjugated anti-rabbit IgG antibody for 1 h (1 : 5000). After three washes of 5 min with TBS-T, ECL detection was performed (NEN Life science Products, Zaventem, Belgium). Control of protein loading and transfer was conducted by detection of the β-actin levels.
Drugs and reagents
Quercitrin was purchased from Extrasynthèse (Genay, France). DSS was provided by ICN Biomedicals (Costa Mesa, CA, U.S.A.), glutathione reductase by Böehringer Mannheim (Barcelona, Spain) and p-nitrophenylphosphate by Merck (Madrid, Spain). All other reagents, unless otherwise stated, were obtained from Sigma (Madrid, Spain).
Statistical analysis
All results are expressed as mean±s.e.m. Differences among means were tested for statistical significance using one-way analysis of variance (ANOVA) and post hoc least significance tests. Statistical significance was set at P<0.05.
Results
Preventative effect of quercitrin in DSS colitis
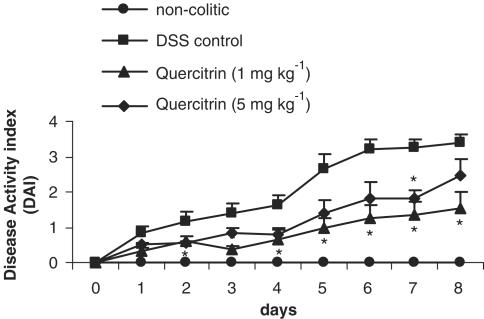
Exposure to DSS in the drinking water at a concentration of 5% (w v−1) for 7 days induced a colonic inflammatory status, with features similar to those described previously (Stucchi et al., 2000; Kullmann et al., 2001). Half of the animals developed loose stools from the first day proceeding from the start of the DSS administration, which turned to diarrhea in the vast majority of them (90–100%) after 4–5 days. By 6–7 days, gross rectal bleeding was evident in 70% of the colitic control group rats. The inflammatory process was also assessed by a progressive loss of weight in all animals associated with anorexia, evident from the first day after the beginning of DSS administration, and that progressively increased during the course of the experiment. Thus, the DAI was increased in control colitic rats throughout the experiment (Figure 1). There were no significant differences in water consumption among all the groups over the period of the experiment, the average volume of daily water consumption being 20.5±0.7 ml per rat for all groups.
Figure 1.
Preventative effects of quercitrin treatment (1 or 5 mg kg−1day−1) on the time-course changes in the DAI over the 8-day experimental period of DSS model of rat colitis, based on the criteria shown in Table 1. Treatment with quercitrin (5 mg kg−1day−1) significantly attenuated the evolution of the colonic inflammatory process from day 2 up to the end of the study. *P<0.05 vs DSS control group.
Macroscopic examination of the colonic specimens after 7 days of DSS treatment revealed bowel wall thickening with a significant increase of the colonic weight/length ratio in comparison with noncolitic rats (112.1±7.1 vs 76.2±1.9 mg cm−1; P<0.01). The histological examination of colonic sections also assessed the intestinal inflammatory status, which showed severe inflammatory changes in the mucosa, including ulceration and loss of normal crypt architecture with necrosis and crypt abscesses. Goblet cells appeared depleted from their mucin content and inflammatory cell infiltration was prominent in all the layers of the intestine. In addition, submucosal edema and vascular hyperplasia were observed in all the samples, whereas fibrin deposition, together with residual proteins, was noted on the necrosed epithelium in 50% of the rats. According to the criteria described in Table 2, control colitic rats showed a tissue damage score of 19.4±1.4. The biochemical determinations are shown in Table 3 , and revealed that DSS administration enhanced colonic MPO and AP activities, increased colonic LTB4 levels, as well as promoted glutathione depletion.
Table 3.
Preventative effects of quercitrin treatment (1 or 5 mg kg−1) on myeloperoxidase (MPO) activity, alkaline phosphatase (AP) activity, glutathione (GSH) content and LTB4 synthesis in the DSS model of rat colitis
| Group | MPO (U g−1 tissue) | AP (mU mg−1 protein) | GSH (nmol g−1 tissue) | LTB4 (ng g−1 tissue) |
| Noncolitic | 5.6±0.3 | 6.7±0.3 | 1665±47 | 2.4±0.3 |
| DSS control | 158.8±22.0 | 15.1±1.6 | 1028±66 | 5.5±0.4 |
| DSS quercitrin | ||||
| 1 mg kg−1 | 61.0±5.2** | 10.5±1.6** | 1310±55** | 4.9±0.4 |
| 5 mg kg−1 | 119.8±10.2 | 14.1±1.8 | 1416±73** | 4.8±0.6 |
Data are expressed as mean±s.e.m.
P<0.01 vs DSS control group. All groups differ significantly from the noncolitic group (P<0.01, not shown), except for glutathione levels in quercitrin-treated groups.
Oral treatment of colitic rats with quercitrin (1 or 5 mg kg−1), when starting the same day as DSS administration, ameliorated the inflammatory process, although it was more patent in the group of colitic rats treated with the lowest dose of flavonoid. During the course of the experiment, colitic animals treated with quercitrin at the dose of 1 mg kg−1 had a significantly attenuated response compared to the nontreated control group, showing a significant decrease in DAI values (P<0.05; Figure 1), which was evident from day 2 to 8, whereas at the dose of 5 mg kg−1 there was only a significant reduction at day 7. Once the rats were killed, macroscopic evaluation of the colonic segments showed that treatment of colitic rats with quercitrin significantly reduced the weight/length ratio in comparison with nontreated colitic animals (84.9±7.1 mg cm−1 at 1 mg kg−1and 92.6±6.9 mg cm−1 at 5 mg kg−1 vs 112.1±7.1 mg cm−1 in control group; P<0.05). Histological examination of the colonic specimens confirmed the intestinal anti-inflammatory effect exerted by the flavonoid. Thus, most of the animals (5/8) from the group of rats that received the lowest dose of quercitrin (1 mg kg−1) showed a reduction in the areas of ulcerated colonic mucosa, affecting only 25% of the surface, without presenting fibrin deposition in the necrosed areas of the colon. The crypts showed a nearly normal architecture and goblet cell component compared with nontreated colitic rats. Although the presence of granulocytes was observed in the lamina propria, this was considered as slight or moderate in the majority of the sections evaluated, whereas macrophages and plasmatic cells were almost absent in lamina propria and submucosa. The administration of quercitrin to DSS colitic animals reduced the tissue damage score by approximately 35% compared to nontreated colitic rats (12.6±2.5 vs 19.4±1.4; P<0.05). On the contrary, the histological analysis of the samples from colitic animals treated with 5 mg kg−1 of quercitrin revealed that the amelioration in crypt architecture was the unique parameter improved, without showing statistical differences in the damage score values in comparison with nontreated colitic rats (18.3±1.8 vs 19.4±1.4; P>0.1).
The preventative intestinal anti-inflammatory effect showed by quercitrin was also assessed biochemically. In comparison with nontreated colitic animals, significant inhibition of both AP and MPO activities by 54 and 57%, respectively, were observed after flavonoid treatment at the dose of 1 mg kg−1 (Table 3). The latter suggests the reduction in the infiltration of neutrophils into the colonic mucosa, since MPO activity is considered as a biochemical marker of neutrophil infiltration (Krawisz et al., 1984). In addition, quercitrin treatment in colitic rats partially counteracted the colonic glutathione depletion (Table 3), thus preserving the colonic tissue from the oxidative damage that characterizes intestinal inflammation. However, quercitrin treatment failed to significantly inhibit colonic LTB4 production (Table 3) in the colonic inflamed tissue at any of the doses assayed.
Effects of quercitrin treatment on DSS colitis
To examine whether the beneficial effect showed by quercitrin might be ascribed to a chemical interaction with DSS rather than to a real anti-inflammatory effect, we tested the effects of quercitrin in the same model of DSS rat colitis, but in a different dosing protocol. For this purpose, rats were given 5% (w v−1) DSS dissolved in the drinking water for 5 days, and then 2% (w v−1) for the following 10 days. Quercitrin, at the dose of 1 mg kg−1 which was shown the most effective in the preventative dosing protocol, was given orally to colitic rats beginning on day 6 to evaluate the effects of the flavonoid on the ongoing colitis.
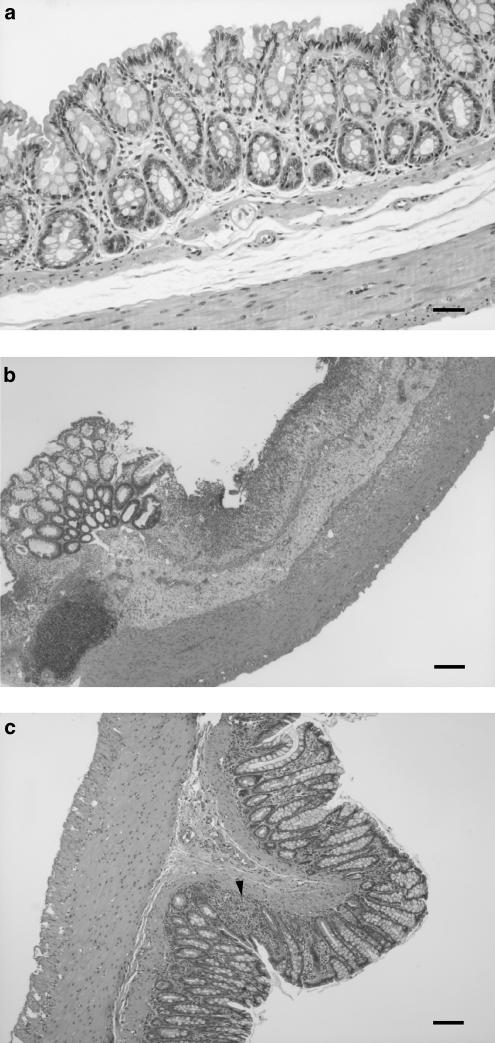
Once the rats from the DSS control group were killed, histological examination of the colonic samples revealed that the inflammatory process showed characteristics similar to those described previously after 7 days of 5% DSS treatment. Thus, bowel wall thickening was evident macroscopically with a significant increase in the colonic weight/length ratio in comparison with noncolitic rats (101.2±5.6 vs 74.5±2.1 mg cm−1; P<0.01). Microscopically, the samples from nontreated colitic rats showed typical inflammatory changes in the colonic architecture: ulceration, crypt dilation, goblet cell depletion as well as mixed cell infiltration, mainly composed by mononuclear cells (macrophages, lymphocytes and plasmatic cells), although granulocytes were also present (Figure 2). According to the criteria in Table 2, the tissue damage score assigned to these rats was 12.4±2.8. As expected, all biochemical parameters studied were significantly modified as a consequence of the inflammatory process, revealing increased colonic MPO and AP activities, higher colonic LTB4 levels and depletion of glutathione content (Table 4).
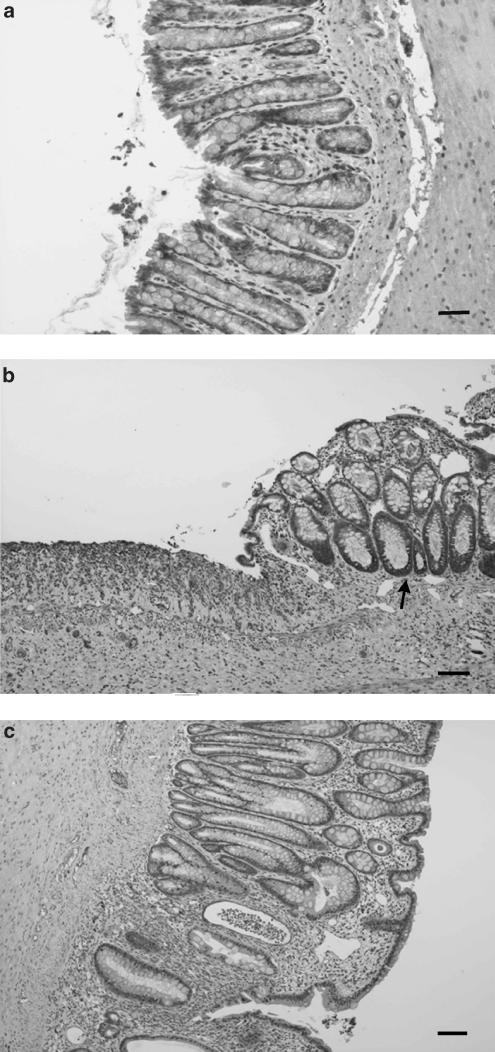
Figure 2.
Histological sections of colonic mucosa from DSS colitic rats stained with hematoxylin and eosin, showing the effects of quercitrin treatment when administered after an initial colonic damage with the offending agent. (a) Noncolitic group showing the normal histology of the rat colon (bar: 60 μm). (b) DSS control group showing extensive intestinal ulceration with a severe inflammatory cell infiltrate in the lamina propria and submucosa (bar: 200 μm). (c) Quercitrin-treated group (1 mg kg−1) showing recovery in the inflammatory process without ulceration of the intestinal mucosa; only a small focus of inflammatory cells can be observed (arrow) (bar: 200 μm).
Table 4.
Effects of quercitrin treatment on established colitis (1 mg kg−1) on myeloperoxidase (MPO) activity, alkaline phosphatase (AP) activity, glutathione (GSH) content and LTB4 synthesis in the DSS model of rat colitis
| Group | MPO (U g−1 tissue) | AP (mU mg−1 protein) | GSH (nmol g−1 tissue) | LTB4 (ng g−1 tissue) |
| Noncolitic | 7.8±0.3 | 7.5±0.3 | 1787±35 | 2.2±0.2 |
| DSS control | 127.2±14.7++ | 14.5±1.4++ | 1517±49++ | 7.3±0.8++ |
| DSS quercitrin | 58.5±8.9++,** | 9.8±0.5++,* | 1762±67** | 5.8±0.3+ |
Data are expressed as mean±s.e.m.
P<0.05
P<0.01 vs DSS control group.
P<0.05
P<0.01 vs noncolitic group.
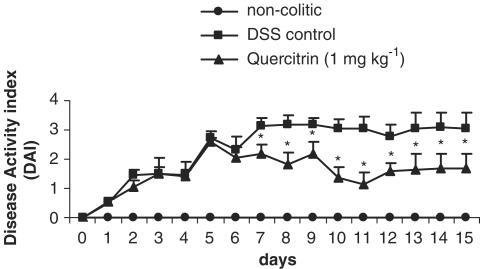
The results obtained after daily administration of quercitrin (1 mg kg−1) in this experimental protocol of chronic colitis revealed that this compound effectively exerted an intestinal anti-inflammatory effect. This was already observed, in comparison with the nontreated colitic group, during the course of the experiment, by a significant reduction in DAI values from day 7 onwards (Figure 3). The macroscopic examination of the colonic segments showed no statistical differences in the weight/length ratio between both colitic groups (101.2±5.6 mg cm−1 in nontreated vs 95.6±4.9 mg cm−1 in quercitrin-treated; P=0.5). However, the histological analysis revealed that quercitrin treatment improved the colonic architecture in comparison with nontreated colitic rats, and they were assigned a tissue damage score of 5.7±1.9 (P<0.05 vs nontreated colitic group). Thus, most of the treated rats showed an almost complete restoration of the damaged colon, displaying a moderate inflammatory infiltrate with a focal distribution, whereas only two of the rats showed signs of gross ulceration with an important inflammatory cell infiltrate. There was also a restoration in the crypt architecture with goblet cell replenishment mucin (Figure 2). Biochemically, quercitrin treatment inhibited 60 and 68% MPO and AP activities, respectively, compared to the DSS control group, and restored glutathione content (Table 4). No significant modifications were seen in LTB4 levels after flavonoid treatment in comparison with the colitic control group (Table 4).
Figure 3.
Effects of quercitrin treatment (1 mg kg−1day−1) on the time-course changes in the DAI over the 15-day experimental period in the DSS model of rat colitis, based on the criteria shown in Table 1. Treatment with quercitrin significantly attenuated the evolution of the colonic inflammatory process from day 7 up to the end of the study. *P<0.05; **P<0.01 vs DSS control group.
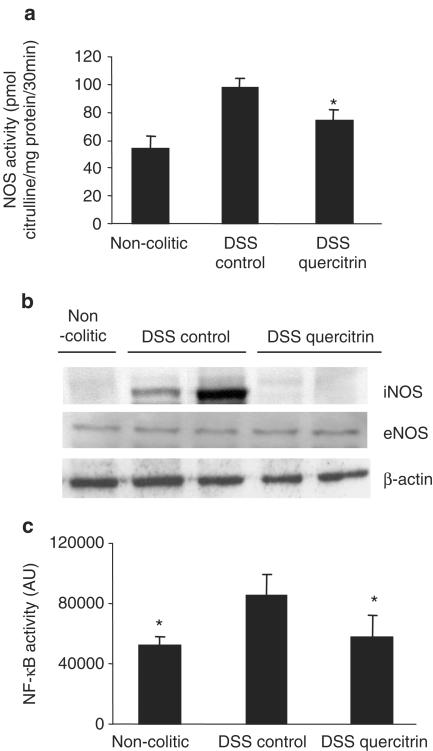
Effect of quercitrin on colonic NOS activity and NF-κB activation
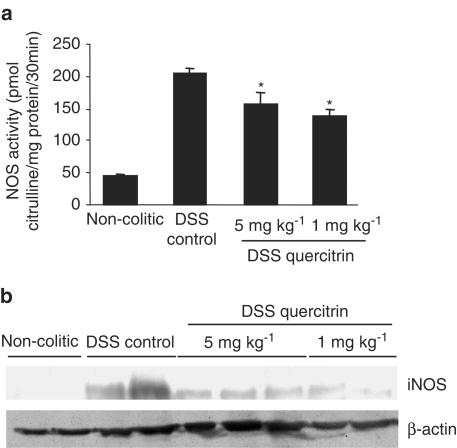
In the recent years, NO has been considered as an important pro-inflammatory mediator playing a key role in the pathogenesis of IBD (Salas et al., 2002). For this reason, we evaluated the effect of quercitrin on colonic NOS activity in the DSS model of experimental colitis, using both treatment protocols. As expected, and according to previous studies (Obermeier et al., 1999), DSS administration to rats, during either 8 or 15 days, produced an increase in colonic NOS activity (Figures 4a and 5a), as well as enhanced expression of iNOS, as detected by Western blot (Figures 4b and 5b). The inflammatory process was not associated with a significant modification in the expression of the constitutive isoform of NOS (eNOS), as evidenced by Western blot (Figure 5b). When the colonic homogenates from control colitic animals were incubated in the presence of the selective iNOS inhibitor aminoguanidine (10−4 M) (Griffiths et al., 1993), a significant inhibition was observed in their enzyme activity (58.3±8.9 vs 97.8±6.1 pmol citrulline mg protein−1; P<0.05), showing similar values to those from noncolitic rats (53.5±8.9 pmol citrulline mg protein−1; P>0.05). The intestinal anti-inflammatory effect exerted by quercitrin, both in the preventative and in the curative dosing protocols, was associated with a significant inhibition of colonic NOS activity (Figures 4a and 5a), and a reduction of iNOS expression (Figures 4b and 5b) when compared to DSS control animals.
Figure 4.
(a) Effect of quercitrin treatment (1 or 5 mg kg−1) on colonic NOS activity in DSS colitic rats in preventative treatment; *P<0.05 vs DSS control rats. All colitic groups differ significantly from the noncolitic group (P<0.01, not shown). (b) Measurement of iNOS expression by Western blotting of colonic protein samples from noncolitic rats, DSS control rats and DSS quercitrin-treated rats (1 or 5 mg kg−1) in the preventative dosing protocol.
Figure 5.
Effects of quercitrin treatment (1 mg kg−1day−1) on established colitis: (a) Colonic NOS activity in DSS colitic rats; *P<0.05 vs DSS control rats; all colitic groups differ significantly from the noncolitic group (P<0.01, not shown). (b) Measurement of iNOS and eNOS expression by Western blotting in DSS colitic rats. (c) NF-κB activity in DSS colitic rats; *P<0.05 vs DSS control rats (Kruskal–Wallis one-way ANOVA, followed by Student–Newman–Keuls test).
According to the well-described role of NF-κB in inflammatory regulation (Schottelius & Baldwin, 1999) and in iNOS expression (Xie et al., 1994), the degree of NF-κB activation was determined in the colonic tissue samples from animals submitted to DSS administration for 15 days. Actually, the inflammatory status induced by DSS was associated with a significant increase of colonic NF-κB activity when compared to noninflamed tissue (Figure 5c). NF-κB activity was downregulated to normal values after quercitrin treatment (Figure 5c).
Involvement of activated macrophages in colonic inflammation
In normal intestinal mucosa from noncolitic rats, we observed minimal immunofluorescence for macrophages (Figure 6a), neutrophils and T lymphocytes (not shown), and a weak immunostaining to iNOS (Figure 7a).
Figure 6.
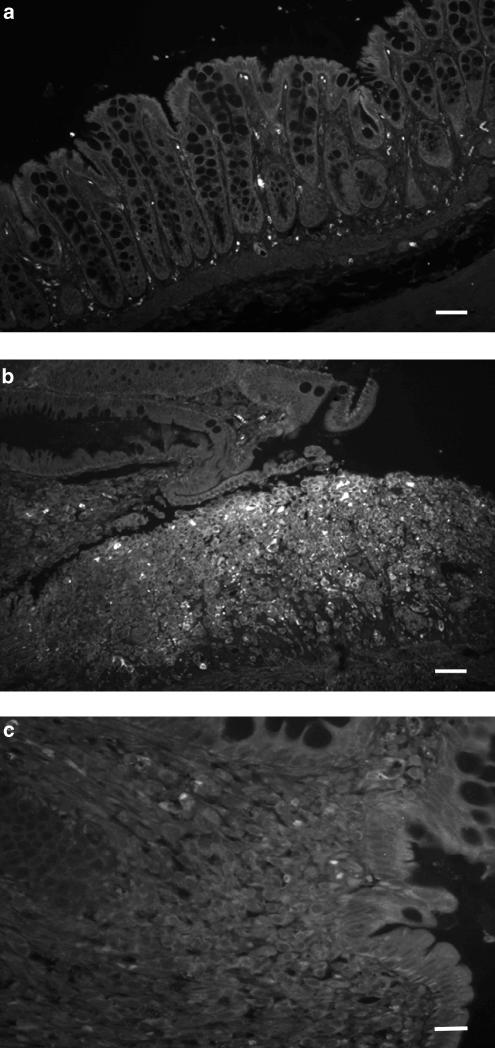
Immunofluorescence of macrophages with clone 1C7 performed in histological sections of colonic mucosa from DSS colitic rats in the treatment protocol with quercitrin on established colitis. (a) Noncolitic group showing scarce expression in the colonic tissue (bar: 60 μm). (b) DSS control group showing greatly increased expression in an ulcerated area (bar: 60 μm). (c) Quercitrin-treated group (1 mg kg−1) showing the decreased in the immunoexpression to macrophages (bar: 30 μm).
Figure 7.
Immunostaining of iNOS performed in histological sections of colonic mucosa from DSS colitic rats in the treatment protocol with quercitrin on established colitis (bar: 200 μm). (a) Noncolitic group; (b) DSS control group showing increased immunoexpression; (c) Quercitrin-treated group (1 mg kg−1) showing the decreased in the immunoexpression to iNOS.
However, immunofluorescence staining of colonic samples from control group of rats that received DSS for 15 days revealed that the predominant cells in the inflammatory areas of the intestine were macrophages, which were mainly located in the lamina propria and the submucosa-adjoining regions of ulceration (Figure 6b). In addition to these cells, neutrophils were also found in crypt abscesses and in the surface of ulcerated epithelium, although in a lesser extent than macrophages (not shown). The presence of T cells in the inflammatory infiltrate was rare (not shown). Moreover, immunostaining to iNOS had a similar distribution in the colonic samples from the nontreated colitic group. Of note, all macrophages, the polymorphonuclear leukocytes infiltrating the lamina propria around the ulcers and the lymphocytes located at the base of the ulcers showed an activated phenotype since immunohistochemistry against iNOS was positive in these cells (Figure 7b). Immunostaining of iNOS was also located at the bases of the epithelial crypts in brush border of the cytoplasm (Figure 7b).
The intestinal anti-inflammatory effect exerted by quercitrin was associated to a decreased number of infiltrated macrophages (Figure 6c) and neutrophils (not shown) in comparison with nontreated colitic rats, as evidenced by immunofluorescence staining of the colonic samples. This was also observed by hematoxylin and eosin staining (Figure 2). T-cell contribution to the inflammatory infiltrate was not modified after quercitrin treatment (not shown). Moreover, the epithelial and inflammatory cells showed a weaker intensity of immunostaining to iNOS (Figure 7c) in quercitrin-treated rats than in the corresponding colitic rats without treatment, and more similar to that found in normal intestinal mucosa.
Discussion
The present study describes that oral administration of quercitrin, at the dose of 1 mg kg−1, significantly prevented the progression of DSS-induced colitis in rats when the compound was administered from the day DSS was added to the drinking water. However, this dosing protocol did not allow us to distinguish between a real anti-inflammatory effect and any possible artefact due to a physical impediment in the damage effect of DSS, or to a delay in the appearance of the colonic damage. These facts led us to undertake additional experiments, in which we assayed the same active dose of quercitrin following a different dosing protocol in which the administration of the flavonoid was started after 5 days of 5% DSS administration in the drinking water when the colonic damage had been established. The results obtained with these assays effectively revealed that quercitrin treatment, during 10 days, facilitated the recovery of the inflammatory process induced by DSS, even when the administration of DSS was maintained to all colitic animals, but at a lower concentration (2%). The beneficial effects observed in both dosing protocols were assessed both clinically, by improvement of weight loss and bloody diarrhea, and histologically by preservation of the intestinal architecture in comparison with the rats from the nontreated colitic group. The biochemical assays performed in the colonic specimens confirmed the anti-inflammatory effect exerted by quercitrin at the dose of 1 mg kg−1, since it was associated to a significant reduction in MPO and AP activities. MPO activity has been widely used to detect and follow intestinal inflammation, and a reduction in the activity of this enzyme can be interpreted as a manifestation of the anti-inflammatory activity of a given compound (Veljaca et al., 1995). Similarly, and based on our previous studies performed in two different models of experimental colitis, AP activity can also be considered as a sensitive marker of inflammation in the intestine, since this enzyme activity is invariably augmented in these experimental conditions (Sanchez de Medina et al., 1996; Galvez et al., 1997). The results obtained in the present study confirm the intestinal anti-inflammatory effect previously demonstrated for this flavonoid in the TNBS model of rat colitis (Sanchez de Medina et al., 1996; 2002), which more closely resembles Crohn's disease (Fuss et al., 1999). On the contrary, the rat model of DSS-induced colitis is a well-characterized model with a predictable disease progression, which shares numerous clinical, biochemical and histological features with human ulcerative colitis (Gaudio et al., 1999; Kullmann et al., 2001). In consequence, the fact that quercitrin has shown beneficial effects in two different models of rat colitis makes this flavonoid a good candidate to be developed in human IBD therapy, even more if we consider the low toxicity associated with the consumption of this type of product, which is present in the normal diet in significant amounts (Middleton et al., 2000).
In addition, the present study gives some clues about the mechanisms involved in the intestinal anti-inflammatory effect of quercitrin. One of these mechanisms could be the inhibition of free radical generation exerted by quercitrin, given the well known antioxidant properties ascribed to this flavonoid, as well as to its aglycone quercetin, both in vitro and in vivo (Galvez et al., 1994; Bors et al., 1990). This activity may play a crucial role in the intestinal anti-inflammatory effect of the flavonoid, because a situation of intense oxidative insult is a common feature in human IBD (Grisham, 1994; McKenzie et al., 1996) and in the different experimental models of rat colitis, such as the TNBS (Sanchez de Medina et al., 1996; Galvez et al., 2001) and the DSS (Stucchi et al., 2000) models, and is an important mechanism for tissue damage during chronic intestinal inflammation. In fact, previous studies have shown that the antioxidant and/or radical scavenging properties of quercitrin may contribute to its beneficial effects when assayed in the initial phase of the TNBS-induced colonic damage (Sanchez de Medina et al., 1996; 2002). In this regard, in the present study, quercitrin treatment of DSS colitic rats counteracted the depletion of colonic glutathione levels that took place in control colitic animals in both experimental treatment protocols. The effect exerted by quercitrin in preserving the colonic mucosa from oxidative insult may collaborate to decrease the neutrophil infiltration that occurs in response to DSS. Free radical generation has been proposed to play an important role early on in the pathogenesis of IBD (Pavlick et al., 2002), and could contribute to the initial neutrophil infiltration in the inflamed colonic mucosa. The recruitment and activation of these cells results in a dramatic increase in free radical production that overwhelms the tissular antioxidant protective mechanisms, resulting in a situation of oxidative stress, which definitively perpetuates colonic inflammation (Grisham, 1994). As a consequence, a rapid inhibition of free radical generation could contribute to a lower level of leukocyte infiltration into the inflamed tissue, thus preventing colonic tissue from inflammation. It is important to note that quercitrin could attenuate neutrophil infiltration via inhibition of other different mediators with chemotactic activity, given the reported ability of this type of natural products to modulate the immune response through downregulation of different pro-inflammatory mediators such as eicosanoids (Middleton et al., 2000). However, no inhibitory effect was observed on colonic LTB4 production, an eicosanoid with chemotactic properties, whose production has been reported to be increased in these intestinal conditions (Sharon & Stenson, 1984). These results are in agreement with previous studies in which no correlation was observed between the ability of quercitrin to downregulate colonic LTB4 production and its anti-inflammatory effect in the acute phase of the TNBS model of rat colitis (Sanchez de Medina et al., 1996).
During the last decade, it has become increasingly clear that NO overproduction by iNOS is deleterious to intestinal function (Hogaboam et al., 1995; Rachmilewitz et al., 1995; Kimura et al., 1997; Salas et al., 2002), thus contributing significantly to gastrointestinal immunopathology during the chronic inflammatory events that take place in IBD (Grisham et al., 2002). The important role attributed to NO in these intestinal conditions prompted us to study whether the beneficial effects of quercitrin on DSS-induced colitis could be related to an effect on colonic NO production. As expected, the results obtained in the present study reveal that colonic inflammation is associated with a higher colonic NOS activity, mainly attributed to an increase in iNOS expression, as evidenced by Western blot analysis. Two experimental findings support this fact: first, when additional experiments were performed in which the colonic homogenates from colitic animals were incubated in the presence of the selective iNOS inhibitor aminoguanidine (10−4 M) (Griffiths et al., 1993), a reduction in NOS activity was obtained, showing similar activity to that obtained in specimens from noncolitic rats; second, colonic eNOS expression was not significantly modified by the inflammatory insult induced with DSS, as evidenced by Western blot analysis (Figure 5b). The increase in iNOS expression in colitic animals in comparison with noncolitic samples was confirmed by the immunohistochemistry analysis (Figure 6). This probably results from the intense activation of macrophages, which took place as a consequence of the inflammatory insult, as also evidenced by the immunofluorescence studies in the intestinal specimens from colitic animals. In fact, macrophages are considered an important source of pro-inflammatory mediators, such as NO and TNFα, playing a key role in the pathophysiology of IBD (Neurath et al., 1996).
Quercitrin treatment to colitic rats effectively inhibited the upregulated colonic NOS activity, being able to reduce iNOS expression as evidenced both by Western blotting and by immunochemistry. The inhibition of NOS activity could also be ascribed to a direct inhibition in this enzyme activity based on the ability of flavonoids to inhibit different enzyme activities, including NOS (Middleton et al., 2000). However, when homogenates from colonic inflamed tissue from colitic rats that received DSS (5% p v−1) for 5 days (as previously described) were used as a NOS activity source, quercitrin or its aglycone quercetin (which is released from quercitrin in vivo) showed a weak inhibitory activity (unpublished results). These results are in agreement with previous studies performed by Kim et al. (1999), who demonstrated a lack of direct inhibitory effect of quercetin or its glycosides on iNOS activity in the macrophage cell line RAW 264.7. In consequence, and in concordance with the results obtained in the present study, the effect showed by quercitrin is most probably related to an inhibition of the expression of this enzyme, which is upregulated as a consequence of the colonic inflammatory process. In fact, the inhibition of iNOS expression by flavonoids has been previously reported in the macrophage cell line RAW 264.7, having been suggested that this occurs by preventing the activation/translocation of NF-κB (Kim et al., 1999; Tsai et al., 1999).
NF-κB, which plays a key role in the activation of genes involved in immune responses (Baldwin, 1996), is an attractive candidate for being involved in IBD pathogenesis. The results obtained in the present study revealed that oral treatment of quercitrin to colitic rats resulted in a significant inhibition of the NF-κB pathway, which was activated as a consequence of the intestinal inflammatory process induced by DSS. The molecular mechanisms involved in the suppressive effects of flavonoids on NF-κB are currently unknown, but several possibilities have been postulated. The first possibility is that flavonoids may inhibit NF-κB by acting as antioxidants, since NF-κB is a redox-sensitive transcription factor and activated by oxidant stress in the inflamed intestinal mucosa (Rogler et al., 1998). The second possibility is that flavonoids inhibit NF-κB via blocking the phosphorylation as well as degradation of IκB protein, a crucial step in the translocation of NF-κB to the nucleus and its subsequent activation, as it has been previously reported in vitro (Tsai et al., 1999).
Finally, it is important to note that increasing doses of the flavonoid resulted in a decreased intestinal anti-inflammatory effect as it has been observed in the preventative dosing protocol (5 mg kg−1), similar to what has been previously described for this compound (Sanchez de Medina et al., 1996), as well as for other flavonoids like hesperidin and diosmin (Crespo et al., 1999) in the TNBS model of rat colitis. The loss of activity may be related to the reported capacity of flavonoids, including quercetin, to inhibit cyclooxygenase when the doses are increased (Kim et al., 1998), an effect described for nonsteroidal anti-inflammatory drugs, which that way can induce an exacerbation of the inflammatory process in experimental colitis (Reuter et al., 1996). We have also to consider the capacity of quercetin to autooxidize in vitro under some circumstances (Canada et al., 1990; Lopez-Lopez et al., 2004), and generate free radicals that could outweigh the beneficial effects of quercitrin at lower doses.
In conclusion, quercitrin treatment ameliorates colonic damage in DSS-induced colitic rats, an effect associated with an improvement in intestinal oxidative stress and a downregulation in colonic NOS activity mediated by the reduction of iNOS protein expression. The iNOS inhibition produced by quercitrin is correlated with the inhibition of NF-κB activity. The inhibition of these intermediates contributes to the resolution of exacerbated inflammation produced by experimental colitis. The antioxidant and/or scavenging properties ascribed to this flavonoid could also contribute to its intestinal anti-inflammatory effect, similarly to other reputed drugs used in the treatment of IBD, such as 5-aminsalycilic derivates (Travis & Jewel, 1994), thus supporting the future application of quercitrin in the treatment of human IBD.
Acknowledgments
We thank E. O'Selmo for the English correction of the manuscript and Puleva Biotech S.A. (Granada, Spain) for the technical assistance in the performance of NF-κB assays and ELISA analysis. This study was supported by grants from Spanish Ministry of Science and Technology (SAF2002-02592) and from Instituto de Salud ‘Carlos III' (PI021732). Mònica Comalada is a recipient of a post-doctoral fellowship from the Spanish Ministry of Science and Technology. Part of these data were presented in the National Meeting of the Spanish Pharmacological Society (2002), in the Falk Symposium No. 133 on Mechanisms of Intestinal Inflammation: Implications for Therapeutic Intervention in IBD (2003) and in the Winter Meeting of the British Pharmacological Society (2003).
Abbreviations
- AP
alkaline phosphatase
- DAI
disease activity index
- DSS
dextran sodium sulfate
- eNOS
endothelial nitric oxide synthase
- IBD
inflammatory bowel disease
- iNOS
inducible nitric oxide synthase
- LTB4
leukotriene B4
- MPO
myeloperoxidase
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- NOS
nitric oxide synthase
- TBS
Tris-buffered saline
- TBSD-T
Tris-buffered saline-Tween-20
- TNBS
trinitrobenzene sulphonic acid
- TNFα
tumor necrosis factor α
References
- ANDERSON M.E. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–555. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- BALDWIN A.S., JR The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu. Rev. Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- BORS W., HELLER W., MICHEL C., SARAN M. Flavonoids as antioxidants: determination of radical-scavenging efficiencies. Methods Enzymol. 1990;186:343–355. doi: 10.1016/0076-6879(90)86128-i. [DOI] [PubMed] [Google Scholar]
- BRATTS R., LINDEN M. Cytokine modulation by glucocorticoids: mechanisms and actions in cellular studies. Aliment. Pharmacol. Ther. 1996;10:81–90. doi: 10.1046/j.1365-2036.1996.22164025.x. [DOI] [PubMed] [Google Scholar]
- CANADA A.T., GIANNELLA E., NGUYEN T.D., MASON R.P. The production of reactive oxygen species by dietary flavonols. Free Radic. Biol. Med. 1990;9:441–449. doi: 10.1016/0891-5849(90)90022-b. [DOI] [PubMed] [Google Scholar]
- COOPER H.S., MURTHY S.N., SHAH R.S., SEDERGRAN D.J. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab. Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- CRESPO M.E., GALVEZ J., CRUZ T., OCETE M.A., ZARZUELO A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999;65:651–653. doi: 10.1055/s-2006-960838. [DOI] [PubMed] [Google Scholar]
- CRUZ T., GALVEZ J., OCETE M.A., CRESPO M.E., SANCHEZ DE MEDINA F., ZARZUELO A. Oral administration of rutoside can ameliorate inflammatory bowel disease in rats. Life Sci. 1998;62:687–695. doi: 10.1016/s0024-3205(97)01164-8. [DOI] [PubMed] [Google Scholar]
- ENNS R., SUTHERLAND L.Adverse events of medical therapy for treatment for inflammatory bowel disease Clinical Challenges in Inflammatory Bowel Disease. Diagnosis, Prognosis and Treatment 1998London: Kluwer Academic Publishers; 113–123.ed. Campieri, M., Bianchi-Porro, G., Fiocchi, C. & Schölmerich, J. pp [Google Scholar]
- FIOCCHI C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- FUSS I.J., MARTH T., NEURATH M.F., PEARLSTEIN G.R., JAIN A., STROBER W. Anti-interleukin 12 treatment regulates apoptosis of Th1T cells in experimental colitis in mice. Gastroenterology. 1999;117:1078–1088. doi: 10.1016/s0016-5085(99)70392-6. [DOI] [PubMed] [Google Scholar]
- GALVEZ J., COELHO G., CRESPO M.E., RODRIGUEZ-CABEZAS M.E., CONCHA A., GONZALEZ M., ZARZUELO A. Intestinal anti-inflammatory activity of morin on chronic experimental colitis in the rat. Aliment. Pharmacol. Ther. 2001;15:2027–2039. doi: 10.1046/j.1365-2036.2001.01133.x. [DOI] [PubMed] [Google Scholar]
- GALVEZ J., CRUZ T., CRESPO E., OCETE M.A., LORENTE M.D., SANCHEZ DE MEDINA F., ZARZUELO A. Rutoside as mucosal protective in acetic acid-induced rat colitis. Planta Med. 1997;63:409–414. doi: 10.1055/s-2006-957723. [DOI] [PubMed] [Google Scholar]
- GALVEZ J., DE LA CRUZ J.P., ZARZUELO A., SANCHEZ DE MEDINA F., JIMENEZ J., SANCHEZ DE LA CUESTA F. Oral administration of quercitrin modifies intestinal oxidative status in rats. Gen. Pharmacol. 1994;25:1237–1243. doi: 10.1016/0306-3623(94)90143-0. [DOI] [PubMed] [Google Scholar]
- GAUDIO E., TADDEI G., VETUSCHI A., SFERRA R., FRIERI G., RICCIARDI G., CAPRILLI R. Dextran sulfate sodium (DSS) colitis in rats: clinical, structural, and ultrastructural aspects. Digest. Dis. Sci. 1999;44:1458–1475. doi: 10.1023/a:1026620322859. [DOI] [PubMed] [Google Scholar]
- GRIFFITHS M.J., MESSENT M., MACALLISTER R.J., EVANS T.W. Aminoguanidine selectively inhibits inducible nitric oxide synthase. Br. J. Pharmacol. 1993;110:963–968. doi: 10.1111/j.1476-5381.1993.tb13907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRISHAM M.B. Oxidants and free radicals in inflammatory bowel disease. Lancet. 1994;344:859–861. doi: 10.1016/s0140-6736(94)92831-2. [DOI] [PubMed] [Google Scholar]
- GRISHAM M.B., PAVLICK K.P., LAROUX F.S., HOFFMAN J., BHARWANI S., WOLF R.E. Nitric oxide and chronic gut inflammation: controversies in inflammatory bowel disease. J. Investig. Med. 2002;50:272–283. doi: 10.2310/6650.2002.33281. [DOI] [PubMed] [Google Scholar]
- HOGABOAM C.M., JACOBSON K., COLLINS S.M., BLENNERHASSETT M.G. The selective beneficial effects of nitric oxide inhibition in experimental colitis. Am. J. Physiol. 1995;268:G673–G684. doi: 10.1152/ajpgi.1995.268.4.G673. [DOI] [PubMed] [Google Scholar]
- KATZ J.A., ITOH J., FIOCCHI C. Pathogenesis of inflammatory bowel disease. Curr. Opin. Gastroenterol. 1999;15:291–297. doi: 10.1097/00001574-199907000-00003. [DOI] [PubMed] [Google Scholar]
- KHO Y.H., POOL M.O., JANSMAN F.G., HARTING J.W. Pharmacotherapeutic options in inflammatory bowel disease: an update. Pharm. World Sci. 2001;23:17–21. doi: 10.1023/a:1011268302386. [DOI] [PubMed] [Google Scholar]
- KIM H.K., CHEON B.S., KIM Y.H., KIM S.Y., KIM H.P. Effects of naturally occurring flavonoids on nitric oxide production in the macrophage cell line RAW 264.7 and their structure–activity relationships. Biochem. Pharmacol. 1999;58:759–765. doi: 10.1016/s0006-2952(99)00160-4. [DOI] [PubMed] [Google Scholar]
- KIM H.P., MANI I., IVERSEN L., ZIBOH V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostagland. Leukotr. Essent. Fatty Acids. 1998;58:17–24. doi: 10.1016/s0952-3278(98)90125-9. [DOI] [PubMed] [Google Scholar]
- KIMURA H., MIURA S., SHIGEMATSU T., OHKUBO N., TSUZUKI Y., KUROSE I., HIGUCHI H., AKIBA Y., HOKARI R., HIROKAWA M., SERIZAWA H., ISHII H. Increased nitric oxide production and inducible nitric oxide synthase activity in colonic mucosa of patients with active ulcerative colitis and Crohn's disease. Digest. Dis. Sci. 1997;42:1047–1054. doi: 10.1023/a:1018849405922. [DOI] [PubMed] [Google Scholar]
- KRAWISZ J.E., SHARON P., STENSON W.F. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- KULLMANN F., MESSMANN H., ALT M., GROSS V., BOCKER T., SCHOLMERICH J., RUSCHOFF J. Clinical and histopathological features of dextran sulfate sodium induced acute and chronic colitis associated with dysplasia in rats. Int. J. Colorect. Dis. 2001;16:238–246. doi: 10.1007/s003840100311. [DOI] [PubMed] [Google Scholar]
- LOPEZ-LOPEZ G., MORENO L., COGOLLUDO A., GALISTEO M., IBARRA M., DUARTE J., LODI F., TAMARGO J., PEREZ-VIZCAINO F. Nitric oxide (NO) scavenging and NO protecting effects of quercetin and their biological significance in vascular smooth muscle. Mol. Pharmacol. 2004;65:851–859. doi: 10.1124/mol.65.4.851. [DOI] [PubMed] [Google Scholar]
- MCKENZIE S.J., BAKER M.S., BUFFINTON G.D., DOE W.F. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J. Clin. Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIDDLETON E., KANDASWAMI C., THEOHARIDES T.C. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- NEURATH M.F., PETTERSSON S., MEYER ZUM BUSCHENFELDE K.H., STROBER W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat. Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]
- OBERMEIER F., KOJOUHAROFF G., HANS W., SCHOLMERICH J., GROSS V., FALK W. Interferon-gamma (IFN-gamma)- and tumour necrosis factor (TNF)-induced nitric oxide as toxic effector molecule in chronic dextran sulphate sodium (DSS)-induced colitis in mice. Clin. Exp. Immunol. 1999;116:238–245. doi: 10.1046/j.1365-2249.1999.00878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAVLICK K.P., LAROUX F.S., FUSELER J., WOLF R.E., GRAY L., HOFFMAN J., GRISHAM M.B. Role of reactive metabolites of oxygen and nitrogen in inflammatory bowel disease. Free Radic. Biol. Med. 2002;33:311–322. doi: 10.1016/s0891-5849(02)00853-5. [DOI] [PubMed] [Google Scholar]
- PODOLSKY D.K., FIOCCHI C.Cytokines, chemokines, growth factors, eicosanoids and other bioactive molecules in inflammatory bowel disease Inflammatory Bowel Disease 2000Philadelphia: W.B. Saunders; 191–207.ed. Kirsner, J.B. pp [Google Scholar]
- RACHMILEWITZ D., KARMELI F., OKON E., BURSZTYN M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–255. doi: 10.1136/gut.37.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REUTER B.K., ASFAHA S., BURET A., SHARKEY K.A., WALLACE J.L. Exacerbation of inflammation-associated colonic injury in rat through inhibition of cyclooxygenase-2. J. Clin. Investig. 1996;98:2076–2085. doi: 10.1172/JCI119013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ-CABEZAS M.E., GALVEZ J., LORENTE M.D., CONCHA A., CAMUESCO D., AZZOUZ S., OSUNA A., REDONDO L., ZARZUELO A. Dietary fiber down-regulates colonic tumor necrosis factor alpha and nitric oxide production in trinitrobenzenesulfonic acid-induced colitic rats. J. Nutr. 2002;132:3263–3271. doi: 10.1093/jn/132.11.3263. [DOI] [PubMed] [Google Scholar]
- ROGLER G., BRAND K., VOGL D., PAGE S., HOFMEISTER R., ANDUS T., KNUECHEL R., BAEUERLE P.A., SCHOLMERICH J., GROSS V. Nuclear factor kappaB is activated in macrophages and epithelial cells of inflamed intestinal mucosa. Gastroenterology. 1998;115:357–369. doi: 10.1016/s0016-5085(98)70202-1. [DOI] [PubMed] [Google Scholar]
- SALAS A.M., GIRONELLA M.M., SALAS A.M., SORIANO A., SANS M., IOVANNA J., PIQUE J.M., PANES J. Nitric oxide supplementation ameliorates dextran sulfate sodium-induced colitis in mice. Lab. Invest. 2002;82:597–607. doi: 10.1038/labinvest.3780454. [DOI] [PubMed] [Google Scholar]
- SANCHEZ DE MEDINA F., GALVEZ J., ROMERO J.A., ZARZUELO A. Effect of quercitrin on acute and chronic experimental colitis in the rat. J. Pharmacol. Exp. Ther. 1996;278:771–779. [PubMed] [Google Scholar]
- SANCHEZ DE MEDINA F., VERA B., GALVEZ J., ZARZUELO A. Effect of quercitrin on the early stages of hapten induced colonic inflammation in the rat. Life Sci. 2002;70:3097–3108. doi: 10.1016/s0024-3205(02)01568-0. [DOI] [PubMed] [Google Scholar]
- SCHOTTELIUS A.J.G., BALDWIN A.S., JR A role for transcription factor NF-κB in intestinal inflammation. Int. J. Colorect. Dis. 1999;14:18–28. doi: 10.1007/s003840050178. [DOI] [PubMed] [Google Scholar]
- SHARON P., STENSON W.F. Enhanced synthesis of leukotriene B4 by colonic mucosa in inflammatory bowel disease. Gastroenterology. 1984;86:453–460. [PubMed] [Google Scholar]
- STEIN R.B., HANAUER S.B. Comparative tolerability of treatments for inflammatory bowel disease. Drug Saf. 2000;23:429–448. doi: 10.2165/00002018-200023050-00006. [DOI] [PubMed] [Google Scholar]
- STUCCHI A.F., SHOFER S., LEEMAN S., MATERNE O., BEER E., MCCLUNG J., SHEBANI K., MOORE F., O'BRIEN M., BECKER J.M. NK-1 antagonist reduces colonic inflammation and oxidative stress in dextran sulfate-induced colitis in rats. Am. J. Physiol. 2000;279:G1298–G1306. doi: 10.1152/ajpgi.2000.279.6.G1298. [DOI] [PubMed] [Google Scholar]
- TRAVIS S.P.L., JEWEL D.P. Salicylates for ulcerative colitis – their mode of action. Pharmacol. Ther. 1994;63:135–161. doi: 10.1016/0163-7258(94)90042-6. [DOI] [PubMed] [Google Scholar]
- TSAI S.H., LIN-SHIAU S.Y., LIN J.K. Suppression of nitric oxide synthase and the down-regulation of the activation of NF kappaB in macrophages by resveratrol. Br. J. Pharmacol. 1999;126:673–680. doi: 10.1038/sj.bjp.0702357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VELJACA M., LESCH C.A., PLLANA R., SANCHEZ B., CHAN K., GUGLIETTA A. BPC-15 reduces trinitrobenzene sulfonic acid-induced colonic damage in rats. J. Pharmacol. Exp. Ther. 1995;272:417–422. [PubMed] [Google Scholar]
- XIE Q.W., KASHIWABARA Y., NATHAN C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J. Biol. Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]