Abstract
The antihyperalgesic effect of pentoxifylline was investigated in three experimental pain models.
Pentoxifylline (0.5–1.6 mg kg−1) given 30 min before the stimulus significantly inhibited the writhing response induced by the intraperitoneal (i.p.) administration of either acetic acid (−90%) or zymosan (−83%), but not that of iloprost, in mice, as well as the zymosan-induced articular hyperalgesia in the zymosan arthritis in rats (−50%).
Pentoxifylline also inhibited the mechanical hypernociception in rats induced by the intraplantar injection of either carrageenin (−81%), bradykinin (−56%) or tumor necrosis factor α (TNF-α; −46%), but not that induced by interleukin-1β (IL-1β) or prostaglandin E2 (PGE2).
Pentoxifylline did not inhibit the nociceptive response in the hot plate test in mice. Further, the antinociceptive effect of pentoxifylline in the writhing test in mice and the zymosan-induced articular hyperalgesia were not reversed by the coadministration of the opioid receptor antagonist naloxone. Thus, pentoxifylline antinociceptive effect is probably not mediated at a central level.
Pentoxifylline significantly reduced TNF-α (−43%) and IL-1β (−42%) concentrations in the joint exudates of rats stimulated by intra-articular injection of zymosan and the production of both cytokines (−66 and −86%, respectively) by mouse peritoneal macrophages stimulated in vivo with zymosan as well as the expression of TNF-α at the tissue level in carrageenin-injected rat paws.
In conclusion, the antinociceptive activity of pentoxifylline is associated with the inhibition of the release of both TNF-α and IL-1β.
Keywords: Pentoxifylline, pain, cytokines, hyperalgesia, tumor necrosis factor, interleukin, inflammation, zymosan
Introduction
Either through direct effects or by inducing the release of other mediators, proinflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin-1 beta (IL-1β) are involved in many aspects of inflammation, including cell migration, oedema development, fever and hyperalgesia (Faccioli et al., 1990; Dinarello, 1996; 2000; Rouveix, 1997; Boraschi et al., 1998; Oppenheim, 2001). Resident cells such as macrophages, mast cells and lymphocytes are able to release large amounts of TNF-α and IL-1β after stimulation by exogenous inflammatory stimuli and/or endogenous mediators, such as lipopolysaccharide (LPS), enterotoxins, viruses, antigens from fungi and parasites, interferon-γ (IFN-γ), IL-1β, granulocyte macrophage-colony stimulating factor (GM-CSF), TNF-α, the complement activation product – C5a, Concavalin A, phorbol ester and chemokines (Old, 1985; Philip & Epstein, 1986; Beutler & Cerami, 1988; Gordon et al., 1990).
Regarding the involvement of TNF-α and IL-1β in inflammatory pain, we have suggested that after administration of inflammatory stimuli, for example, carrageenin or LPS, TNF-α is produced and triggers the release of a cascade of cytokines, which mediate the release of prostaglandins and sympathomimetic amines, the (final) mediators involved in the sensitization of nociceptors. Indeed, TNF-α stimulates the production of IL-1β and IL-6, which in turn stimulate the production of cyclooxygenase products (Ferreira et al., 1988; Cunha et al., 1992), and IL-8/neutrophil chemoattractant-1 (CINC-1), thereby enhancing the production of sympathomimetic amines (Cunha et al., 1991; Lorenzetti et al., 2002). Other authors have also reported the hypernociceptive effects of TNF-α and IL-1β and their participation in nociceptive responses induced by various stimuli (Perkins & Kelly, 1994; Woolf et al., 1997; Safieh-Garabedian et al., 2002; Schafers et al., 2003). Based on these data, we proposed that TNF is a central cytokine in these events, triggering the release of other mediators and hypernociception. Consequently, strategies aimed at blocking TNF-α and IL-1 may become effective analgesics in clinical practice. Actually, the administration of anti-TNF-α and IL-1β monoclonal antibodies provided analgesia in rheumatoid arthritis patients, besides reducing structural joint lesions (Furst et al., 2003).
Apart from the so-called biologic therapies (anticytokine antibodies and soluble receptors) that were recently introduced into clinical practice, other anticytokine therapies may also provide benefit in relieving inflammation. Thalidomide and chlorpromazine are able to block TNF activity in vitro (Moreira et al., 1993; Zinetti et al., 1995) and in vivo (Bertini et al., 1991; Gadina et al., 1991; Sampaio et al., 1991; Aarestrup et al., 1995; Ghezzi et al., 1996). These compounds were also shown to be antihyperalgesic both in animals (Jakoubek, 1984; Sommer et al., 1998; George et al., 2000) and in man (Gorin et al., 1990; Merskey, 1997; Mehl-Madrona, 1999; Peuckmann et al., 2003). We have shown that thalidomide also has an antinociceptive effect on inflammatory pain. It inhibited carrageenin and LPS-induced mechanical hyperalgesia in rats and zymosan and acetic acid-induced writhing responses in mice. These effects were associated with the inhibition of TNF-α production (Ribeiro et al., 2000b).
Pentoxifylline, a haemorheological drug, has been used in the clinic as a treatment for intermittent claudication (Accetto, 1982; Porter et al., 1982), with minor side effects (Ward & Clissold, 1987). Pentoxifylline was shown to be beneficial in immunologically based disorders where TNF-α, among other cytokines, appears to play a role, for example, allergic contact dermatitis, leprosy, rheumatoid arthritis, cancer, and cerebral malaria (Graninger et al., 1991; Dezube et al., 1993; Schwarz et al., 1993; Huizinga et al., 1996; Sampaio et al., 1998). Recently, it was shown that the local injection of either pentoxifylline or propentoxifylline inhibited the formalin-induced pain in rats. This effect was associated with a decreased TNF-α mRNA expression in the rat paws (Dorazil-Dudzik et al., 2004). Although pentoxifylline may have direct effects through its ability to inhibit the transcription of TNF-α mRNA (Doherty et al., 1991; Schmidt-Choudhury et al., 1996), it does also decrease IL-6 and IL-1 release (Schandné et al., 1992; Weinberg et al., 1992). Additionally, pentoxifylline may stimulate IL-10 production, thereby blocking TNF production/activity (D'Hellencourt et al., 1996; van Furth et al., 1997).
The effect of pentoxifylline upon cytokine production seems to be due, at least in part, to an increase in intracellular cAMP levels (Semmler et al., 1993). In contrast to what happens in nociceptive neuron terminals, where the increase of intracellular cAMP levels causes nociception (Ferreira & Nakamura, 1979; Aley & Levine, 1999; Cunha et al., 1999; Aley et al., 2000), an increase in the intracellular level of cAMP in inflammatory cells has anti-inflammatory and antinociceptive effects mediated by reducing the release of cytokines, histamine and leukotrienes (Marone et al., 1987; Renz et al., 1988; Francischi et al., 2000; Brito et al., 2001).
In the present study, we provide additional evidence that pentoxifylline is antihyperalgesic in three different pain models and that this effect is associated with a decreased TNF-α production.
Methods
Animals
Male Wistar rats (180–200 g) and male Swiss mice (25–30 g) were housed in a temperature-controlled room, with access to water and food ad libitum and 12 h of dark–light cycles until use. All experiments were conducted in accordance with National Institute of Health guidelines and Ethical Guidelines for Investigations of Experimental Pain in Conscious Animals (Zimmermann, 1983) on the welfare of experimental animals and with the approval of the Ethics Committee of the School of Medicine of Ribeirão Preto (University of São Paulo) and of the School of Medicine of the Federal University of Ceará.
Nociceptive tests
Writhing test
Nociceptive activity was tested in mice using the writhing model (Koster et al., 1959; Collier et al., 1968). The nociceptive stimuli were injected into the peritoneal cavities of mice, which were placed in a large glass cylinder and the intensity of nociception was quantified by counting the total number of writhes occurring between 0 and 30 min after stimulus injection. The writhing response consists of the contraction of the abdominal muscle together with a stretching of the hind limbs. The doses of the nociceptive stimuli were: zymosan (1 mg mouse−1; 0.2 ml), acetic acid (0.1 ml 10 g body weight−1of a 0.6% solution, v v−1) or iloprost (0.5 μg mouse−1; 0.2 ml).
Rat knee joint incapacitation test
The rat knee joint incapacitation test is described in detail elsewhere (Tonussi & Ferreira, 1992). In this test, a computer-assisted device measures the time that a specific hind paw fails to touch the surface of a rotating cylinder over a 1-min period (paw elevation time). Control (normal) paw elevation time is approximately 10–15 s. In our experiment, articular incapacitation was quantified as the increase in paw elevation time after injection of zymosan (1 mg cavity−1; 50 μl) into the knee joint, and the period during which the hind paw failed to touch the rotating cylinder was interpreted as being proportional to the pain felt by the animal. In this model, articular incapacitation is used as a measure of articular inflammatory hyperalgesia. Paw elevation time was measured before (control time, T0) and 1, 2, 3 and 4 h (T1, T2, T3 and T4) after zymosan administration.
Mechanical hypernociception
The mechanical nociceptive method used was the constant pressure nociceptive test, which is a modification of the classical Randall–Sellito test (Ferreira et al., 1978a). In this method, a constant pressure of 20 mmHg (measured using a sphygmomanometer) is applied (via a syringe piston moved by compressed air) to a 15-mm2 area on the dorsal surface of the hind paw, and discontinued when the rat presents a typical ‘freezing reaction.' This reaction is comprised of brief apnoea, concomitant with retraction of the head and forepaws and reduction in the escape movements that animals normally make to free themselves from the position imposed by the experimental situation. Usually, the apnoea is associated with successive waves of muscular tremor. For each animal, the latency to the onset of the freezing reaction was measured before administration (zero time) and at different times after administration of the hyperalgesic agents. The intensity of mechanical hypernociception was quantified as the reduction in the reaction time, calculated by subtracting the value of the second measurement from the first (Ferreira et al., 1978a). Reaction times were typically 32–34 s (s.e.m. of 0.5–1.0 s) before injection of the hyperalgesic agents. Mechanical hypernociception was measured after intraplantar injection of hyperalgesic stimuli into the hind paw of rats. Multiple paw treatments with PBS did not alter basal reaction times. Different individuals prepared solutions to be injected, performed the injections and measured the reaction times. The hyperalgesic stimuli used in this method were carrageenin (100 μg paw−1), bradykinin (500 ng paw−1), TNF-α (2.5 pg paw−1), IL-1β (1 pg paw−1) or PGE2 (100 ng paw−1).
Hot plate test
The hot plate test was conducted according to the low-temperature (51.5±2°C) hot plate method described by Eddy & Leimbach (1953), where each mouse received two trials on the hot plate, separated by a 30-min interval. The first trial allowed the animal to be familiarized with the test procedure, and the second trial served as the control reaction time (licking of hind feet or jumping) for the animal. Male Swiss mice were preselected; any showing a reaction time greater than 10 s were excluded. The reaction time for each mouse was determined on the hot plate surface at 30-min intervals after drug administration for a total of 90 min. To avoid possible injury, there was a cutoff period of 40 s while measuring reaction time.
Detection of TNF-α and IL-1β produced by peritoneal cells harvested from peritoneal cavity stimulated with zymosan or present in articular cavity exudate of joints injected with zymosan
Vehicle (saline; 0.2 ml) or zymosan (1 mg mouse−1; 0.2 ml) was injected intraperitoneally into mice. After 15 min, the peritoneal cavity was washed with RPMI culture medium (1 ml cavity−1), and the exudate was centrifuged at 300 × g for 10 min. The pelleted cells were resuspended in 500 μl of RPMI with 10% fetal calf serum and counted, after which 5 × 105 cells were plated in 48-well plastic tissue culture plates. The concentrations of TNF-α and IL-1β in the supernatant after culture for 12 h were determined by enzyme-linked immunosorbent assay (ELISA), as described previously (Cunha et al., 1993). TNF-α and IL-1β concentrations were determined in rat knee joint exudate 2 h after intra-articular injection of vehicle (saline; 50 μl) or zymosan (1 mg cavity−1; 50 μl). The articular cavity was washed twice with 200 μl of heparinized saline and the pooled washes then centrifuged. The supernatant was used to determine TNF-α and IL-1β levels. Briefly, microtiter plates were coated overnight at 4°C with antibody against murine TNF-α or IL-1β (10 μg ml−1). After blocking the plates, the samples and standard at various dilutions were added in duplicate and incubated at 4°C for 24 h. The plates were washed three times with buffer, and a second biotinylated polyclonal antibody against TNF-α or IL-1β diluted 1 : 1000 was added (100 μl well−1). After further incubation at room temperature for 1 h, the plates were washed and 100 μl of avidin-horseradish peroxidase (HRP) diluted 1 : 5000 were added. The color reagent o-phenylenediamine (OPD; 100 μl) was added 15 min later and the plates were incubated in the dark at 37°C for 15–20 min. The enzyme reaction was stopped with H2SO4 and absorbance was measured at 490 nm. The results are reported as means±s.e.m. for four animals.
Immunohistochemical reaction to TNF
Immunohistochemistry for TNF-α was performed on skin tissues of rat paws using the streptavidin–biotin–peroxidase method (Bayer & Wilchek, 1980) in formalin-fixed, paraffin-embedded tissue sections (4 μm thick), mounted on poly-L-lysine-coated microscope slides. The sections were deparaffinized and rehydrated through xylene and graded alcohols. After antigen retrieval, endogenous peroxidase was blocked (15 min) with 3% (v v−1) hydrogen peroxide and washed in phosphate-buffered saline solution (PBS). Sections were incubated overnight (4°C) with primary rabbit anti-rat TNF-α antibody diluted 1 : 100 in PBS plus bovine serum albumin (PBS-BSA). The slides were then incubated with biotinylated goat anti-rabbit; diluted 1 : 400 in PBS-BSA. After washing, the slides were incubated with avidin-biotin–HRP conjugate (Strep ABC complex by Vectastain® ABC Reagent and peroxidase substrate solution) for 30 min, according to the Vectastain protocol. TNF-α was visualized with the chromogen 3,3′diaminobenzidine (DAB). Negative control sections were processed simultaneously as described above but with the first antibody being replaced by PBS-BSA 5%. None of the negative controls showed TNF-α Immunoreactivity. Slides were counterstained with Harry's haematoxylin, dehydrated in a graded alcohol series, cleared in xylene and coverslipped.
Experimental protocols
Evaluation of the pretreatment with pentoxifylline on the writhing response induced by acetic acid, zymosan or iloprost in mice
Mice were treated with vehicle (saline; S) or pentoxifylline (0.1–1.6 mg kg−1) and 30 min later, zymosan (1 mg mouse−1; 0.2 ml), acetic acid (0.1 ml 10 g body weight−1of a 0.6% solution, v v−1) or iloprost (0.5 μg mouse−1; 0.2 ml) was injected i.p. (Ribeiro et al., 2000a). The number of writhes was counted as described above.
Evaluation of the pretreatment with pentoxifylline in the rat knee joint incapacitation test induced by zymosan
Rats were pretreated with pentoxifylline (0.5–5 mg kg−1) or the vehicle (saline) and 30 min after zymosan (1 mg cavity−1) was injected intra-articularly in a volume of 50 μl. Incapacitation was measured as described above.
Evaluation of the pretreatment of animals with pentoxifylline upon mechanical hypernociception induced by carrageenin, bradykinin, TNF-α, IL-1β or prostaglandin E2 (PGE2)
Hypernociception was measured 1, 3 and 5 h after injection of carrageenin (100 μg paw−1; 0.1 ml) into the hind paws (intraplantar, i.pl.) of rats. Vehicle (saline) or pentoxifylline (1–9 mg paw−1) was injected intraplantarly 30 min before or 2 h after i.pl. administration of carrageenin. The hypernociception was also measured 3 h after i.pl. injection of bradykinin (500 ng paw−1), TNF-α (2.5 pg paw−1), IL-1β (1 pg paw−1) or PGE2 (100 ng paw−1) in rats pretreated 30 min before with intraplantar injection of vehicle or pentoxifylline (9 mg paw−1). The carrageenin, bradykinin, TNF-α, IL-1β and PGE2 doses injected were the smallest doses that evoked maximum acute mechanical hypernociception (Ferreira et al., 1978a, 1978b; 1988; 1993; Cunha et al., 1992).
Evaluation of the pentoxifylline pretreatment in the hot plate test
Immediately after the determination of control reaction time (see above), groups of six mice were treated i.p. with saline, pentoxifylline (1.6 mg kg−1), morphine (5 mg kg−1) or indomethacin (2 mg kg−1) in a volume of 0.2 ml. The reaction time was measured 30, 60 and 90 min after the treatments. Controls for indomethacin received the vehicle.
Evaluation of the pretreatment with naloxone on the antinociceptive activity of pentoxifylline in zymosan-induced writhing response in mice and knee joint incapacitation in rats
Mice or rats were pretreated, s.c., with vehicle (saline) or naloxone (2 mg kg−1). After 15 min, animals were injected i.p. with vehicle (saline), pentoxifylline (1.6 mg kg−1) or morphine (5 mg kg−1). After 30 min, zymosan (1 mg mouse−1) was injected i.p. and the number of writhes (in mice) or knee joint incapacitation (in rats) was determined, as described above.
Evaluation of the pentoxifylline pretreatment on TNF-α and IL-1β production by peritoneal cells harvested from peritoneal cavity after stimulation with zymosan and on TNF-α and IL-1β present in articular exudate from rat knee joints stimulated with zymosan
Mice were pretreated i.p. with vehicle (saline) or pentoxifylline (0.5–1.6 mg kg−1) 30 min before the i.p. administration of zymosan (1 mg mouse−1; 0.2 ml). At 15 min after zymosan injection, the total resident peritoneal cells were harvested with RPMI culture medium, plated (5 × 105 cells well−1) and incubated in a CO2 incubator. TNF-α and IL-1β levels were measured in the supernatants after 12 h of culture. Rats were pretreated i.p. with vehicle (saline) or pentoxifylline (1.6 mg kg−1) 30 min before the intra-articular administration of zymosan (1 mg cavity−1). The control group received only an intra-articular injection of the vehicle (saline). After 2 h the joints were washed with PBS plus heparin, and the collected fluids were centrifuged. The concentrations of TNF-α and IL-1β in the supernatant were determined by ELISA as described above.
Evaluation of the pentoxifylline pretreatment on immunohistochemical detection of TNF
Rats were pretreated intraplantarly with vehicle (saline) or pentoxifylline (9 mg paw−1) and 30 min later, carrageenin was injected i.pl. into the same paw(s) (100 μg paw−1). After 2 h, areas of the plantar skin of the injected paws were removed and the immunohistochemical protocol applied to these samples as described above.
Drugs, cytokines and antibodies
The following drugs were used: Zymosan A (Sigma-Aldrich, St Louis, MO, U.S.A.), glacial acetic acid (Merck, São Paulo, Brazil), carrageenin (FMC Corporation; Philadelphia, PA, U.S.A.), bradykinin (Sigma-Aldrich, St Louis, MO, U.S.A.), PGE2, iloprost, pentoxifylline (Sigma-Aldrich, St Louis, MO, U.S.A.) indometacin (Merck, Sharp and Dohme-MSD, São Paulo, Brazil), morphine (Cristália – São Paulo, Brazil), naloxone (Rhodia Farma – São Paulo, Brazil). Rabitt anti-rat TNF-α primary antibody, biotinylated anti-goat IgG antibody (NIBSC; National Institute for Biological Standards and Control, U.K.). The human recombinant cytokines IL-1β, and TNF-α were NIBSC preparations. The specific activities of these materials were: IL-1β, 100,000 IU 1 mg−1 ampoule−1 and TNF-α, 40,000 IU 1 mg−1 ampoule−1. Biotinylated anti-goat IgG antibody used in immunohistochemistry (Vector Laboratories, Burlingame, CA, U.S.A.) Vectastatin ABC detection system (Vector Laboratories, Burlingame, CA, U.S.A.), VIP substrate kit (Vector Laboratories, Burlingame, CA, U.S.A.).
Zymosan, morphine, naloxone and cytokines were diluted in a 0.9% NaCl solution. Indomethacin was diluted in a 5% NaHCO3 solution and pH was adjusted to 7.3 using 0.1 N HCl. Glacial acetic acid was diluted in deionized water.
Statistical analysis
Results are presented as means±s.e.m. of measurements made on at least 4–6 animals in each group. Differences between responses were evaluated by analysis of variance (ANOVA) followed by Tukey's test. Statistical differences were considered to be significant at P<0.05.
Results
Effect of pretreatment with pentoxifylline on the writhing response induced by acetic acid, zymosan or iloprost
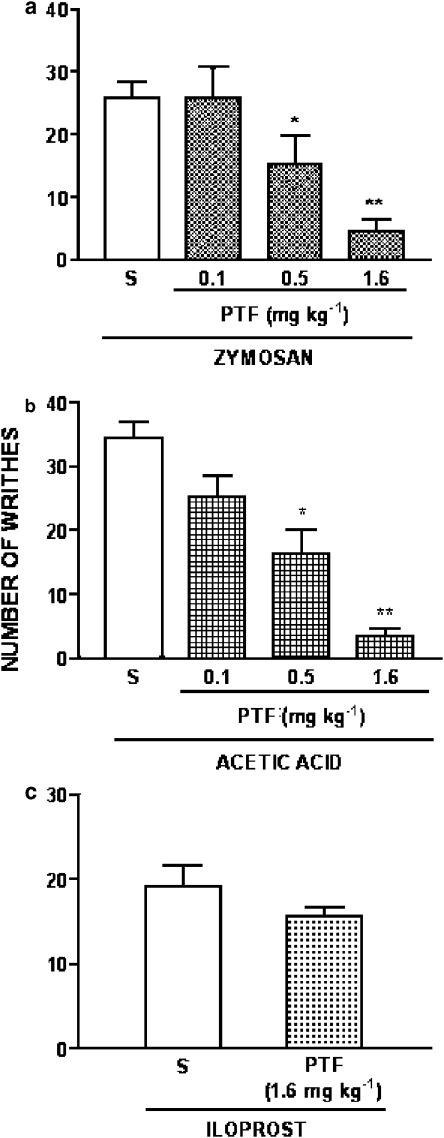
The intraperitoneal injection of 0.1 ml 10 g body weight−1 of a 0.6% (v v−1) of acetic acid solution, zymosan (1 mg mouse−1) or iloprost (0.5 μg mouse−1) in mice induced a writhing response that was determined during the 30 min following injection of the stimulus. The pretreatment of the mice with pentoxifylline (0.1–1.6 mg kg−1, 30 min before stimuli) inhibited in a dose-dependent manner the zymosan- (Figure 1a) and acetic acid- (Figure 1b) induced writhing responses. The dose of 1.6 mg kg−1 inhibited the zymosan and acetic acid writhing responses in 83 and 90%, respectively. In contrast, this dose of pentoxifylline was ineffective on iloprost-induced writhing response (Figure 1c). The administration of the active compound pentoxifylline i.p. to naïve animals did not promote a writhing response (data not shown).
Figure 1.
Effect of systemic administration of pentoxifylline on the writhing response induced by zymosan, acetic acid or iloprost in mice. The number of writhes was determined for the interval of 0–30 min, after i.p. injection of zymosan (1 mg mouse−1, panel a), acetic acid (0.1 ml 10 g body weight−1of a 0.6% solution, v v−1) (panel b) or iloprost (0.5 μg mouse−1; panel c). Pentoxifylline (PTF; 0.1–1.6 mg kg−1; i.p.) or vehicle (saline; S) was given 30 min before stimulus administration. In panel c, the mice were pretreated with vehicle or pentoxifylline (PTF; 1.6 mg kg−1) 30 min before iloprost. Results are expressed as means±s.e.m. for groups of six mice. Asterisks indicate statistically significant differences between groups and respective controls (*P<0.05; **P<0.001).
Effect of pretreatment with pentoxifylline in the rat knee joint incapacitation test induced by zymosan
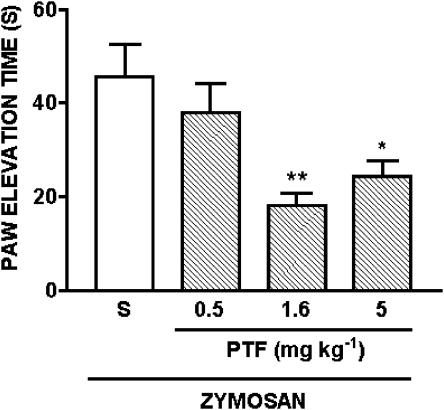
The intra-articular injection of zymosan (1 mg cavity−1) induces articular incapacitation (Rocha et al., 1999, Vale et al., 2003). Figure 2 shows articular incapacitation determined between 3 and 4 h after zymosan injection, which is the interval of maximal incapacitation response (Rocha et al., 1999, Vale et al., 2003). Pentoxifylline at doses of 1.6 and 5 mg kg−1, but not at a dose of 0.5 mg kg−1, significantly inhibited zymosan-evoked articular hyperalgesia.
Figure 2.
Effect of systemic administration of pentoxifylline on zymosan-evoked articular incapacitation in rats. Vehicle (saline; S) or pentoxifylline (PTF; 0.5–5 mg kg−1) was injected i.p. and, 30 min later, zymosan (1 mg cavity−1; 50 μl) was injected intra-articularly into the right knee joint. Paw elevation time was measured before and after zymosan administration, over a 60-min period, until the 4th h. Bars show means of maximal values obtained between 3rd and 4th hour after zymosan injection. Results are expressed as means±s.e.m. of the paw elevation time (s) for groups of six rats. Asterisks indicate statistically significant differences between groups and respective controls (*P<0.05; **P<0.01).
Effect of pentoxifylline pretreatment on mechanical hypernociception induced by carrageenin
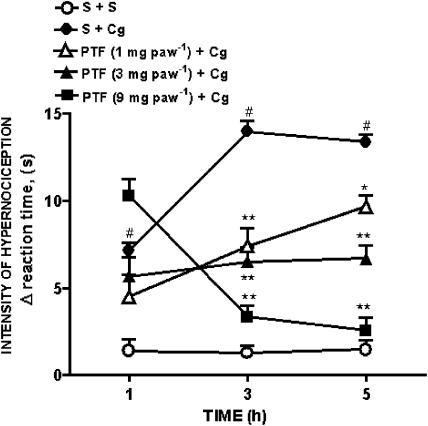
Compared with vehicle, the injection of carrageenin (100 μg) into the hind paw of rats evoked a significant hypernociceptive effect, measured 1, 3 or 5 h after carrageenin injection. The pretreatment of the paws 30 min before carrageenin injection with pentoxifylline (1–9 mg paw−1) inhibited in a dose-dependent manner, the carrageenin-evoked hypernociception measured 3 and 5 h after i.pl. injection of carrageenin (Figure 3). The inhibition of the carrageenin-evoked hypernociception by pentoxifylline was observed only when the drug was injected before the carrageenin (also given i.pl.). The administration of pentoxifylline at 9 mg paw−1 (2 h after carrageenin) did not affect the evoked hypernociception (data not shown). Also, pentoxifylline did not inhibit the hypernociception evoked by intraplantar injection of carrageenin in contralateral paws (data not shown).
Figure 3.
Effect of pretreatment of animals with pentoxifylline on mechanical hypernociception induced by carrageenin. Hypernociception was measured before and 1, 3 and 5 h after injection of vehicle (saline; S) or carrageenin (Cg; 100 μg) into the hind paws (intraplantar i.pl.) of rats. Vehicle (saline, S) or pentoxifylline (PTF; 1–9 mg paw−1) was injected i.pl. 30 min before carrageenin administration. Results are expressed as means±s.e.m. of the intensity of hypernociception (Δ reaction time, s) for groups of 5–6 rats. Asterisks indicates statistically significant differences between groups pretreated with different doses pentoxifylline and group injected with carrageenin and pretreated with saline (S+Cg; *P<0.01; **P<0.001). #Symbols indicates statistically significant differences between saline treated group (S+S) and control (S+Cg) group (#P<0.001).
Effect of pentoxifylline treatment on mechanical hypernociception induced by bradykinin, TNF-α, IL-1β and PGE2
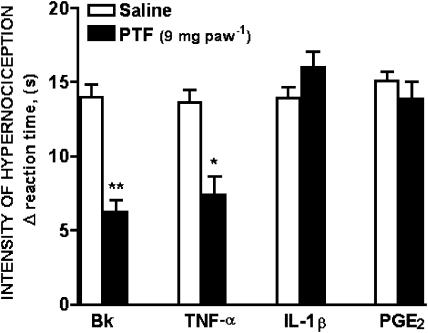
The injection of bradykinin (500 ng paw−1), TNF-α (2.5 pg paw−1), IL-1β (1 pg paw−1), or PGE2 (100 ng paw−1) into the hind paws (intraplantar, i.pl.) of rats evoked hypernociceptive responses measured 3 h after injections. The simultaneous injection of pentoxifylline (9 mg paw−1; i.pl.) inhibited the bradykinin and TNF-α induced-hypernociception by 56 and 46%, respectively, but not the hypernociception evoked by IL-1β and PGE2 (Figure 4).
Figure 4.
Effect of pentoxifylline pretreatment on mechanical hypernociception induced by bradykinin, TNF-α, IL-1β and PGE2. Hypernociception was measured before and 3 h after injection of bradykinin (Bk, 500 ng), tumor necrosis factor-α (TNF-α; 2.5 pg), interleukin-1β (IL-1β; 1 pg) or prostaglandin E2 (PGE2; 100 ng) into the hind paws (intraplantar, i.pl.) of rats. Vehicle (saline) or pentoxifylline (PTF; 9 mg paw−1) was injected intraplantar 30 min before hypernociceptive stimulus administration. Results are expressed as means±s.e.m. of the intensity of hypernociception (Δ reaction time, s) for groups of 5–6 rats. Asterisks indicate statistically significant differences between groups and respective controls (*P<0.01, **P<0.001).
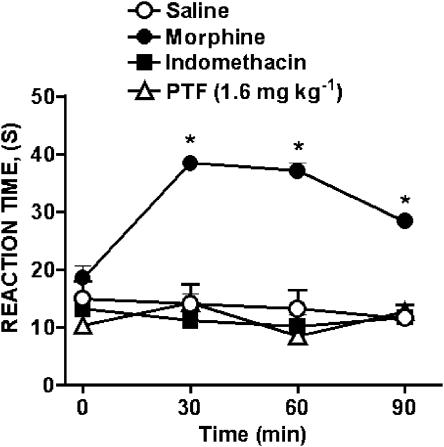
Effect of pentoxifylline treatment upon the hot plate response
Pentoxifylline (1.6 mg kg−1) or indomethacin (2 mg kg−1), administrated intraperitoneally did not change the hot plate reaction time during the 90 min of observation that followed their injection. In contrast, morphine (5 mg kg−1, i.p.), used as a positive control, caused a significant elevation of the reaction time of the mice during this period (Figure 5).
Figure 5.
Effect of treatment of mice with pentoxifylline, indomethacin or morphine on the course of the reaction times to a thermal stimulus (hot plate). Mice were treated i.p. with vehicle (5% NaHCO3 plus HCl; control), morphine (5 mg kg−1), indomethacin (2 mg kg−1) or pentoxifylline (PTF; 1.6 mg kg−1). Reaction times were measured before injection of the above substances (control time) and 30, 60, and 90 min afterwards. Results are expressed as means±s.e.m. for groups of 5–6 mice. Asterisks indicate statistically significant differences between treated groups and control (*P<0.001).
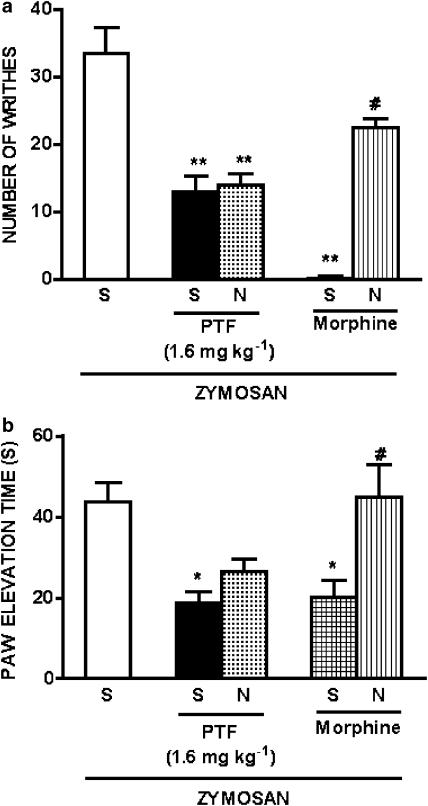
Effect of pretreatment with naloxone on the antinociceptive activity of pentoxifylline on zymosan-induced writhing response in mice and articular incapacitation in rats
The pretreatment with naloxone at dose of 2 mg kg−1, 15 min before pentoxifylline (1.6 mg kg−1) did not affect the antinociceptive activity of this drug on zymosan-induced writhing in mice or articular incapacitation in rats. However, at this dose naloxone blocked the antinociceptive activity of morphine (5 mg kg−1) on zymosan-induced writhing and articular incapacitation (Figure 6). The injection of naloxone (2 mg kg−1) to naïve animals did not modify the nociceptive response (data not shown).
Figure 6.
Effect of pretreatment with naloxone on the antinociceptive action of pentoxifylline or morphine in zymosan-induced writhing response in mice and knee joint incapacitation in rats. Mice or rats were pretreated with vehicle (saline, S) or naloxone (N; 2 mg kg−1). After 15 min, they were pretreated with pentoxifylline (PTF; 1.6 mg kg−1) or morphine (5 mg kg−1) i.p., and 30 min after that, zymosan (1 mg mouse−1) was injected i.p. into mice, or intra-articularly into rats (1 mg cavity−1). The number of writhes in mice was determined during the following 30 min (panel a), and paw elevation times were measured before and after zymosan administration, over a 60-min period, until the 4th h (panel b). Bars on panel b show means of maximal values obtained between 3rd and 4th hour after zymosan intra-articular injection. Results are expressed as means±s.e.m. for groups of 6 mice and 5–6 rats. Asterisks indicates statistically significant differences comparing the group injected with zymosan and pretreated with pentoxifylline and saline or morphine and saline with the group injected with zymosan and pretreated with saline (*P<0.05; **P<001). #Symbols indicates statistically significant differences comparing groups injected with zymosan and pretreated with morphine and naloxone with the group injected with zymosan and pretreated with morphine and saline (#P<0.001).
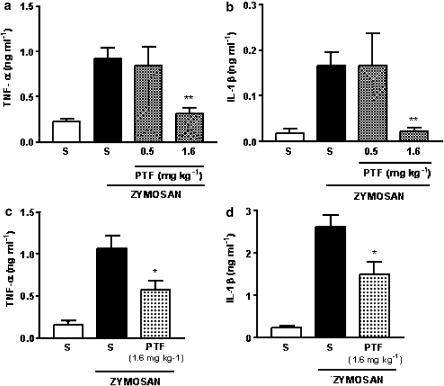
Effect of pentoxifylline pretreatment on TNF-α and IL-1β production by peritoneal cells harvested from peritoneal cavities stimulated with zymosan
Mouse peritoneal cells harvested from cavities injected 15 min earlier with zymosan (1 mg mouse−1) and incubated for 12 h in vitro released into the supernatants significant amounts of TNF-α and IL-1β, compared to the release of cells harvested from cavities injected with vehicle (saline). The pretreatment of the mice with pentoxifylline at dose of 1.6 mg kg−1, but not at dose of 0.5 mg kg−1, caused a significant decrease in TNF-α (−66%) and IL-1β (−86%) release (Figure 7a and b, respectively).
Figure 7.
Effect of pentoxifylline pretreatment on TNF-α and IL-1β production by cells harvested from peritoneal cavities and knee joints injected with zymosan. In panels a and b, the mice were injected with saline (S; 0.2 ml mice−1; i.p.) or zymosan (1 mg mouse−1; i.p.), and 15 min later, the peritoneal cells were harvested and incubated in vitro for 12 h. The concentrations of TNF-α (panel a) and IL-1β (panel b) in the supernatants were determined by ELISA. Pentoxifylline (PTF; 0.5 and 1.6 mg kg−1) or vehicle (saline) was given i.p. 30 min prior to zymosan injection. In panels c and d, the animals were injected intra-articular with saline (S; 50 μl cavity−1) or zymosan (1 mg cavity−1; 50 μl) and 2 h later, the articular cavities were washed and the concentrations of TNF-α (panel c) and IL-1β (panel d) in the exudates were determined by ELISA. Pentoxifylline (PTF; 1.6 mg kg−1) or vehicle (saline) was given 30 min before the intra-articular administration of zymosan. Results are reported as means±s.e.m. of four wells and are representative of two different experiments. Asterisks indicate statistically significant differences between groups and respective controls (*P<0.05; **P<0.001).
Effect of pretreatment on TNF-α and IL-1β release into articular exudates from of rat knee joint stimulated with zymosan
The exudates harvested from rat articular joints stimulated with zymosan (1 mg cavity−1) showed significant amounts of TNF-α and IL-1β, compared with the fluid harvest from articular joints injected with saline. The pretreatment of the animals with pentoxifylline (1.6 mg kg−1; i.p.) caused a significant decrease of TNF-α (−43%) and IL-1β (−42%) release, when compared with the zymosan-treated group pretreated with vehicle (saline) (Figure 7c and d, respectively).
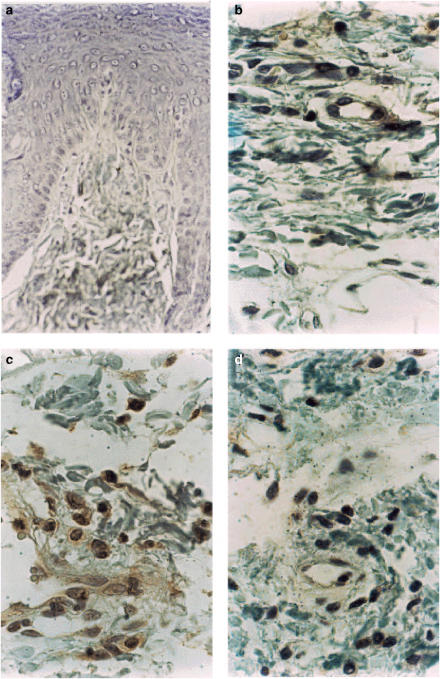
Immunohistochemistry
Immunohistochemical staining of TNF-α was seen in inflammatory cells present in the hind paw tissue of rats pretreated with saline and stimulated with carrageenin (Figure 8, panel c). Immunostaining of TNF-α in the paw tissue inflammatory cells was clearly decreased by the pretreatment of the animals with pentoxifylline (Figure 8, panel d) No immunostaining of TNF-α was found in paw tissue of animals injected with saline (Figure 8, panel b) or negative control of paw-tissue incubated in the absence of anti-rabbit TNF-α antibody (Figure 8, panel a).
Figure 8.
Effect of pentoxifylline pretreatment on TNF-α expression in skin tissue of hind paw of rats injected with carrageenin. Panel a: Negative control for immunostaining of TNF-α. The skin tissue slice from a rat paw pretreated with saline and injected with carrageenin was incubated in the absence of first anti-rat TNF-α and no staining was obtained. Panel b: absence of immunostaining of TNF-α in skin tissue of rat paw injected with vehicle (saline) alone. Panel c: intense immunostaining of TNF-α in inflammatory cells of rat paw pretreated with vehicle (saline) and stimulated with carrageenin (100 μg paw−1). Panel d: reduction of immunostaining of TNF-α in inflammatory cells of rat paw pretreated with pentoxifylline (9 mg paw−1) and stimulated with carrageenin (100 μg paw−1). DAB and Mayer's hematoxylin.
Discussion
In the present study, we investigated the antihyperalgesic effect of pentoxifylline in different pain models. Our data show that pentoxifylline dose-dependently inhibited the writhing response in mice induced by both zymosan and acetic acid, while not altering the writhes induced by the stable prostacyclin analog iloprost. Also, joint hyperalgesia, measured as the articular incapacitation in the zymosan-induced arthritis, was inhibited by the prophylactic administration of pentoxifylline.
We have shown that, in the writhings test, specific antibodies against the cytokines TNF-α, IL-1β or IL-8 reduce the hyperalgesia by more than 50%. The administration of a combination of these antibodies is even more effective in preventing the hyperalgesia, virtually abolishing it (Thomazzi et al., 1997; Ribeiro et al., 2000a). These data suggest a synergism of these cytokines in promoting hyperalgesia and that strategies to collectively block them might be more effective as an antinociceptive strategy. Compounds such as thalidomide, chlorpromazine, as well as pentoxifylline, that inhibit a variety of cytokines, may achieve this goal.
Coupled to the antihyperalgesia, we obtained, in the present study, that pentoxifylline administration significantly reduced TNF-α and IL-1β concentrations in the joint exudates of rats stimulated by zymosan intra-articular injection. In addition, the production of these cytokines by mouse peritoneal macrophages was also reduced by pentoxifylline and the expression of TNF-α at the tissue level in carrageenin-injected rat paws was also inhibited. These data clearly indicate that pentoxifylline antihyperalgesic effect is not due to the blockade of a specific cytokine. Moreover, our data also indicate that the pentoxifylline effect is not restricted to specific tissue or animals' species. Other groups reported similar results, showing that pentoxifylline inhibits TNF-α, IL-1β and IL-6 production (Schandné et al., 1992; Weinberg et al., 1992; Ohtsuka et al., 1997).
The fact that pentoxifylline did not alter the nociceptive effect of the stable prostacyclin analog iloprost indicate an indirect mechanism for the antihyperalgesic effect of pentoxifylline. Indeed, iloprost-induced nociception occurs directly after its coupling to specific IP receptors, thereby activating intracellular pathways (Smith et al., 1998). Therefore, our data reinforce the notion that pentoxifylline exerts its effects at an earlier and indirect stage in the inflammatory response, by reducing proinflammatory cytokines release.
The participation of cytokines, such as TNF-α, on the induction of knee joint incapacitation has been shown previously (Tonussi & Ferreira, 1999). Moreover, the participation of cytokines in arthritic pain has been shown to occur in other experimental models and also in man (Feldmann et al., 1990; 1996; Davis & Perkins, 1994; Shafer et al., 1994; Koch et al., 1995; Smith et al., 1997; Francischi et al., 2000; Hayashida et al., 2004). Pentoxifylline reduced the articular incapacitation only when given prior to the zymosan injection (prophylactic intervention). Similar data were reported in the formalin-induced nociception in rats, where pre-emptive pentoxifylline, but not therapeutic administration, reduced the pain behaviour. That effect was associated to a decreased TNF-α mRNA transcription (Dorazil-Dudzik et al., 2004).
In the present study, pentoxifylline pretreatment did also inhibit the hypernociception induced by carrageenin in the rat paw. This was dose and time-dependent effect. Additionally, pentoxifylline inhibited the bradykinin and TNF-α-induced hypenociception in the rat paw, but not that induced by IL-1β. These results reinforce the proposal that pentoxifylline antihyperalgesic effect is mostly due to a reduction in TNF-α and IL-1 production, since the TNF-induced mechanical hyperalgesia is IL-1 dependent and bradykinin-induced mechanical hyperalgesia is TNF-α dependent, whereas IL-1β-induced hyperalgesia is associated with local eicosanoids release (Cunha et al., 1991; 1992; Ferreira et al., 1993; Poole et al., 1999). The fact that the pretreatment with pentoxifylline fails to inhibit IL-1 and PGE2-induced hypernociception corroborates with the idea of a preventive effect of this drug upon IL-1 production. So does the fact that pentoxifylline when injected 2 h after carrageenin (data not shown) fails to inhibit the hyperalgesic effect of this stimulus, suggesting that pentoxifylline possibly is acting inhibiting TNF production which seems to be triggered by carrageenin.
In order to evaluate a possible central component involved in the antihyperalgesia of pentoxifylline, we investigated its activity in the hot plate test. Our data show that pentoxifylline was clearly ineffective in this test. As an internal control of this experiment, indomethacin, as expected, also did not inhibit the reaction time, whereas morphine was clearly antinociceptive. Additionally, the coadministration of naloxone, that significantly reversed the morphine antihyperalgesia, was unable to alter pentoxifylline antihyperalgesia in both the articular incapacitation test and in the writhing test. Although speculative, these data propose that pentoxifylline antihyperalgesia is not linked to a centrally derived pathway, and that a peripheral endogenous opioid release mechanism seems unlikely. However, additional studies are still needed to clarify this assumption.
As alluded to above, the introduction of anti-TNF therapies in the rheumatology clinical practice has provided substantial pain relief and appears to significantly alter disease outcome (Furst et al., 2003). However, there are concerns about the emergence of neutralizing antibodies against these compounds, thereby limiting their effectiveness. Not to mention their high cost and the reported increased occurrence of opportunistic infections and a fear for an increased risk for neoplasias, that may also limit their use in the long term. Therefore, it could well be that, at least in some patients, alternative strategies to block TNF, perhaps associated with the so-called ‘biologic agents', may be beneficial in clinical practice either by enhancing their effectiveness or tolerance, and also by reducing their high costs.
A previous study has shown that pentoxifylline administration to human patients prior to cholecistectomy significantly reduced the opioid dose required to alleviated pain postoperatively. However, no difference was perceived using patient response to a visual analogue scale as a pain measurement (Wordliczek et al., 2000). The above data, showing that pentoxifylline is antihyperalgesic in various pain models and that this effect is associated with a reduction of TNF-α release clearly indicate that human studies to further explore these results merit consideration.
Acknowledgments
We gratefully acknowledge the technical assistance of Giuliana B. Francisco, Sergio R. Rosa, Ieda R.S. Schivo and Maria Silvandira Pinheiro. This work was supported by the grants from the following Brazilian foundations: FAPESP, CNPq (Pronex), and CAPES (Procad). We also thank Dr Albert Leyva for reading the manuscript.
Abbreviations
- ANOVA
one-way analysis of variance
- Bk
bradykinin
- cAMP
adenosine 3′5′ cyclic monophosphate
- CINC-1
neutrophil chemoattractant-1
- ELISA
enzyme-linked immunosorbent assay
- GM–CSF
granulocyte macrophage-colony stimulating factor
- IFN-γ
interferon-gamma
- IL
interleukin
- i.p.
intraperitoneal
- i.pl.
intraplantar
- LPS
lipopolysaccharide
- mRNA
messenger ribonucleic acid
- PGE2
prostaglandin E2
- PTF
pentoxifylline
- s.c.
subcutaneous
- TNF
tumor necrosis factor
References
- AARESTRUP F.M., GONCALVES-DA-COSTA S.C., SARNO E.N. The effect of thalidomide on BCG-induced granulomas in mice. Braz. J. Med. Biol. Res. 1995;28:1069–1076. [PubMed] [Google Scholar]
- ACCETTO B. Beneficial hemorheologic therapy of chronic peripheral arterial disorders with pentoxifylline: results of double-blind study versus vasodilator-nylidrin. Am. Heart J. 1982;103:864–869. doi: 10.1016/0002-8703(82)90401-x. [DOI] [PubMed] [Google Scholar]
- ALEY K.O., LEVINE J.D. Role of protein kinase A in the maintenance of inflammatory pain. J. Neurosci. 1999;19:2181–2186. doi: 10.1523/JNEUROSCI.19-06-02181.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEY K.O., MESSING R.O., MOCHLY-ROSEN D., LEVINE J.D. Chronic hypersensitivity for inflammatory nociceptor sensitization mediated by the epsilon isozyme of protein kinase C. J. Neurosci. 2000;20:4680–4685. doi: 10.1523/JNEUROSCI.20-12-04680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAYER E.A., WILCHEK W. The use of the avidin–biotin complex as a tool in molecular biology. Methods Biochem. Anal. 1980;26:1–45. doi: 10.1002/9780470110461.ch1. [DOI] [PubMed] [Google Scholar]
- BERTINI R., MENGOZZI M., BIANCHI M., SIPE J.D., GHEZZI P. Chlorpromazine protection against interleukin-1 and tumor necrosis factor-mediated activities in vivo. Int. J. Immunopharmacol. 1991;13:1085–1090. doi: 10.1016/0192-0561(91)90159-5. [DOI] [PubMed] [Google Scholar]
- BEUTLER B., CERAMI A. Cachectin (tumor necrosis factor): a macrophage hormone governing cellular metabolism and inflammatory response. Endocr. Rev. 1988;9:57–66. doi: 10.1210/edrv-9-1-57. [DOI] [PubMed] [Google Scholar]
- BORASCHI D., CIFONE M.G., FALK W., FLAD H.D., TAGLIABUE A., MARTIN M.U. Cytokines in inflammation. Eur. Cytokine Netw. 1998;9:205–212. [PubMed] [Google Scholar]
- BRITO G.A., SARAIVA S.N., FALCAO J.L., VALE M.L., LIMA A.A., CUNHA F.Q., RIBEIRO R.A. Dual effect of cAMP on the writhing response in mice. Eur. J. Pharmacol. 2001;416:223–230. doi: 10.1016/s0014-2999(01)00813-5. [DOI] [PubMed] [Google Scholar]
- COLLIER H.O.J., DINNEEN L.C., CHISTINE A., JOHNSON A., SCHNEIDER C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968;32:295–310. doi: 10.1111/j.1476-5381.1968.tb00973.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., BOUKILI M.A., MOTTA J.I.B., VARGAFTIG B.B., FERREIRA S.H. Blockade by fenspiride of endotoxin-induced neutrophil migration in the rat. Eur. J. Pharmacol. 1993;238:47–52. doi: 10.1016/0014-2999(93)90503-a. [DOI] [PubMed] [Google Scholar]
- CUNHA F.Q., LORENZETTI B.B., POOLE S., FERREIRA S.H. Interleukin-8 as a mediator of sympathetic pain. Br. J. Pharmacol. 1991;104:765–767. doi: 10.1111/j.1476-5381.1991.tb12502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., POOLE S., LOREZETTI B.B., FERREIRA S.H. The pivotal role of tumor necrosis factor α in the development of inflammatory hyperalgesia. Br. J. Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUNHA F.Q., TEIXEIRA M.M., FERREIRA S.H. Pharmacological modulation of secondary mediator systems-cyclic AMP and cyclic GMP – on inflammatory hyperalgesia. Br. J. Pharmacol. 1999;127:671–678. doi: 10.1038/sj.bjp.0702601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS A.J., PERKINS M.N. The involvement of bradykinin B1 and B2 receptor mechanisms in cytokine-induced mechanical hyperalgesia in the rat. Br. J. Pharmacol. 1994;113:63–68. doi: 10.1111/j.1476-5381.1994.tb16174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEZUBE B.J., SHERMAN M.L., FRIDOVICH-KEIL J.L., ALLEN-RYAN J., PARDEE A.B. Down-regulation of tumor necrosis factor expression by pentoxifylline in cancer patients: a pilot study. Cancer Immunol. Immunother. 1993;36:57–60. doi: 10.1007/BF01789132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'HELLENCOURT C.L., DIAW L., CORNILLET P., GUENOUNOU M. Differential regulation of TNF alpha, IL-1 beta, IL-6, IL-8, TNF beta, and IL-10 by pentoxifylline. Int. J. Immunopharmacol. 1996;18:739–748. doi: 10.1016/s0192-0561(97)85556-7. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. Thermoregulation and the pathogenesis of fever. Infect. Dis. Clin. North. Am. 1996;10:433–449. doi: 10.1016/s0891-5520(05)70306-8. [DOI] [PubMed] [Google Scholar]
- DINARELLO C.A. Pro-inflammatory cytokines. Chest. 2000;118:503–508. doi: 10.1378/chest.118.2.503. [DOI] [PubMed] [Google Scholar]
- DOHERTY G.M., JENSEN J.C., ALEXANDER H.R., BURESH C.M., NORTON J.A. Pentoxifylline suppression of tumor necrosis factor gene transcription. Surgery. 1991;110:192–198. [PubMed] [Google Scholar]
- DORAZIL-DUDZIK M., MIKA J., SCHAFER M.K., LI Y., OBARA I., WORDLICZEK J., PRZEWLOCKA B. The effects of local pentoxifylline and propentofylline treatment on formalin-induced pain and tumor necrosis factor-alpha messenger RNA levels in the inflamed tissue of the rat paw. Anesth. Analg. 2004;98:1566–1573. doi: 10.1213/01.ANE.0000113235.88534.48. [DOI] [PubMed] [Google Scholar]
- EDDY N.B., LEIMBACH D. Synthetic analgesics. II. Dithienylbutenyl- and dithienylbutylamines. J. Pharmacol. Exp. Ther. 1953;107:385–393. [PubMed] [Google Scholar]
- FACCIOLI L.H., SOUZA G.E., CUNHA F.Q., POOLE S., FERREIRA S.H. Recombinant interleukin-1 and tumor necrosis factor induce neutrophil migration ‘in vivo' by indirect mechanisms. Agents Actions. 1990;30:344–349. doi: 10.1007/BF01966298. [DOI] [PubMed] [Google Scholar]
- FELDMANN M., BRENNAN F.M., CHANTRY D., HAWORTH C., TURNER M., ABNEY E., BUCHAN G., BARRETT K., BARKLEY D., CHU A. Cytokine production in the rheumatoid joint: implications for treatment. Ann. Rheum. Dis. 1990;49:480–486. [PubMed] [Google Scholar]
- FELDMANN M., BRENNAN F.M., MAINI R.N. Role of cytokines in rheumatoid arthritis. Annu. Rev. Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., BRISTOW A.F., POOLE S. Interleukin-1 beta as a potent hyperalgesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., CORREA F.M. Central and peripheral antialgesic action of aspirin-like drugs. Eur. J. Pharmacol. 1978a;53:39–49. doi: 10.1016/0014-2999(78)90265-0. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., LORENZETTI B.B., POOLE S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br. J. Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERREIRA S.H., NAKAMURA M. I – Prostaglandin hyperalgesia, a cAMP/Ca2+ dependent process. Prostaglandins. 1979;18:179–190. doi: 10.1016/0090-6980(79)90103-5. [DOI] [PubMed] [Google Scholar]
- FERREIRA S.H., NAKAMURA M., CASTRO M.S.A. The hyperalgesic effects of prostacyclin and prostaglandin E2. Prostaglandins. 1978b;16:31–37. doi: 10.1016/0090-6980(78)90199-5. [DOI] [PubMed] [Google Scholar]
- FRANCISCHI J.N., YOKORO C.M., POOLE S., TAFURI W.L., CUNHA F.Q., TEIXEIRA M.M. Anti-inflammatory and analgesic effects of the phosphodiesterase 4 inhibitor rolipram in a rat model of arthritis. Eur. J. Pharmacol. 2000;399:243–249. doi: 10.1016/s0014-2999(00)00330-7. [DOI] [PubMed] [Google Scholar]
- FURST D.E., SCHIFF M.H., FLEISCHMANN R.M., STRAND V., BIRBARA C.A., COMPAGNONE D., FISCHKOFF S.A., CHARTASH E.K. Adalimumab, a fully human anti tumor necrosis factor-alpha monoclonal antibody, and concomitant standard antirheumatic therapy for the treatment of rheumatoid arthritis: results of STAR (Safety Trial of Adalimumab in Rheumatoid Arthritis) J. Rheumatol. 2003;30:2563–2571. [PubMed] [Google Scholar]
- GADINA M., BERTINI R., MENGOZZI M., ZANDALASINI M., MANTOVANI A., GHEZZI P. Protective effect of chlorpromazine on endotoxin toxicity and TNF production in glucocorticoid-sensitive and glucocorticoid-resistant models of endotoxic shock. J. Exp. Med. 1991;173:1305–1310. doi: 10.1084/jem.173.6.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE A., MARZINIAK M., SCHAFERS M., TOYKA K.V., SOMMER C. Thalidomide treatment in chronic constrictive neuropathy decreases endoneurial tumor necrosis factor-alpha, increases interleukin-10 and has long-term effects on spinal cord dorsal horn met-enkephalin. Pain. 2000;88:267–275. doi: 10.1016/S0304-3959(00)00333-X. [DOI] [PubMed] [Google Scholar]
- GHEZZI P., GARATTINI S., MENNINI T., BERTINI R., DELGADO HERNANDEZ R., BENIGNI F., SACCO S., SKORUPSKA M., MENGOZZI M., LATINI R., KUROSAKI M., LOMBET A., FRADIN A., BONNET J., ROLLAND Y., BRION J.D. Mechanism of inhibition of tumor necrosis factor production by chlorpromazine and its derivatives in mice. Eur. J. Pharmacol. 1996;317:369–376. doi: 10.1016/s0014-2999(96)00728-5. [DOI] [PubMed] [Google Scholar]
- GORDON J.R., BURD P.R., GALLI S.J. Mast cell as source of multifunctional cytokines. Immunol. Today. 1990;11:458–464. doi: 10.1016/0167-5699(90)90176-a. [DOI] [PubMed] [Google Scholar]
- GORIN I., VILETTE B., GEHANNO P., ESCANDE J.P. Thalidomide in hyperalgic pharyngeal ulceration of AIDS. Lancet. 1990;335:1343. doi: 10.1016/0140-6736(90)91223-w. [DOI] [PubMed] [Google Scholar]
- GRANINGER W., THALHAMMER F., LOCKER G. Pentoxifylline in cerebral malaria. J. Infect. Dis. 1991;164:829. doi: 10.1093/infdis/164.4.829. [DOI] [PubMed] [Google Scholar]
- HAYASHIDA K., KANEKO T., TAKEUCHI T., SHIMIZU H., ANDO K., HARADA E. Oral administration of lactoferrin inhibits inflammation and nociception in rat adjuvant-induced arthritis. J. Vet. Med. Sci. 2004;66:149–154. doi: 10.1292/jvms.66.149. [DOI] [PubMed] [Google Scholar]
- HUIZINGA T.W., DIJKMANS B.A., VAN DER VELDE E.A., VAN DE POUW KRAAN T.C., VERWEIJ C.L., BREEDVELD F.C. An open study of pentoxyfylline and thalidomide as adjuvant therapy in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 1996;55:833–836. doi: 10.1136/ard.55.11.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOUBEK B. Analgesia induced by painful stimulation and/or anticipation of pain; different mechanisms are operating. Physiol. Bohemoslov. 1984;33:171–178. [PubMed] [Google Scholar]
- KOCH A.E., KUNKEL S.L., STRIETER R.M. Cytokines in rheumatoid arthritis. J. Invest. Med. 1995;1995;43:28–38. [PubMed] [Google Scholar]
- KOSTER R., ANDERSON M., DE BEER E.J. Acetic acid for analgesic screening. Fed. Proc. 1959;18:412. [Google Scholar]
- LORENZETTI B.B., VEIGA F.H., CANETTI C.A., POOLE S., CUNHA F.Q., FERREIRA S.H. Cytokine-induced neutrophil chemoattractant 1 (CINC-1) mediates the sympathetic component of inflammatory mechanical hypersensitivity in rats. Eur. Cytokine. Netw. 2002;13:456–461. [PubMed] [Google Scholar]
- MARONE G., COLUMBO M., TRIGGIANI M., CIRILLO R., GENOVESE A., FORMISANO S. Inhibition of IgE-mediated release of histamine and peptide leukotriene from human basophils and mast cells by forskolin. Biochem. Pharmacol. 1987;36:13–20. doi: 10.1016/0006-2952(87)90377-7. [DOI] [PubMed] [Google Scholar]
- MEHL-MADRONA L.E. Comparison of ketorolac-chlorpromazine with meperidine-promethazine for treatment of exacerbations of chronic pain. J. Am. Board. Fam. Pract. 1999;12:188–194. doi: 10.3122/jabfm.12.3.188. [DOI] [PubMed] [Google Scholar]
- MERSKEY H. Pharmacological approaches other than opioids in chronic non-cancer pain management. Acta Anaesthesiol. Scand. 1997;41:187–190. doi: 10.1111/j.1399-6576.1997.tb04636.x. [DOI] [PubMed] [Google Scholar]
- MOREIRA A.L., SAMPAIO E.P., ZMUIDZINAS A., FRINDT P., SMITH K.A., KAPLAN G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J. Exp. Med. 1993;177:1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OHTSUKA H., HIGUCHI T., MATSUZAWA H., SATO H., TAKAHASHI K., TAKAHASHI J., YOSHINO T.O. Inhibitory effect on LPS-induced tumor necrosis factor in calves treated with chlorpromazine or pentoxifylline. J. Vet. Med. Sci. 1997;59:1075–1077. doi: 10.1292/jvms.59.1075. [DOI] [PubMed] [Google Scholar]
- OLD L.J. Tumor necrosis factor (TNF) Science. 1985;230:630. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- OPPENHEIM J.J. Cytokines: past, present and future. Int. J. Hematol. 2001;74:3–8. doi: 10.1007/BF02982543. [DOI] [PubMed] [Google Scholar]
- PERKINS M.N., KELLY D. Interleukin-1 beta induced-desArg9bradykinin-mediated thermal hyperalgesia in the rat. Neuropharmacology. 1994;33:657–660. doi: 10.1016/0028-3908(94)90171-6. [DOI] [PubMed] [Google Scholar]
- PEUCKMANN V., STRUMPF M., ZENZ M., BRUERA E. [Novel potential uses of thalidomide in the management of pain? A review of the literature] Schmerz. 2003;17:204–210. doi: 10.1007/s00482-003-0216-z. [DOI] [PubMed] [Google Scholar]
- PHILIP R., EPSTEIN L.B. Tumour necrosis factor as immunomodulator and mediator of monocyte cytotoxicity induced by itself, gamma-interferon and interleukin-1. Nature. 1986;323:86–89. doi: 10.1038/323086a0. [DOI] [PubMed] [Google Scholar]
- POOLE S., LORENZETTI B.B., CUNHA J.M., CUNHA F.Q., FERREIRA S.H.Bradykinin B1 and B2 receptors, tumour necrosis factor alpha and inflammatory hyperalgesia Br. J. Pharmacol. Br. J. Pharmacol. 19991999126127649, 314–656.Erratum in [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER J.M., CUTLER B.S., LEE B.Y., REICH T., REICHLE F.A., SCOGIN J.T., STRANDNESS D.E. Pentoxifylline efficacy in the treatment of intermittent claudication: multicenter controlled double-blind trial with objective assessment of chronic occlusive arterial disease patients. Am. Heart J. 1982;104:66–72. doi: 10.1016/0002-8703(82)90642-1. [DOI] [PubMed] [Google Scholar]
- RENZ H., GONG J.H., SCHMIDT A., NAIN M., GEMSA D. Release of tumor necrosis factor-alpha from macrophages. Enhancement and suppression are dose-dependently regulated by prostaglandin E2 and cyclic nucleotides. J. Immunol. 1988;141:2388–2393. [PubMed] [Google Scholar]
- RIBEIRO R.A., VALE M.L., THOMAZZI S.M., PASCHOALATO A.B., POOLE S FERREIRA S.H., CUNHA F.Q. Involvement of resident macrophages and mast cells in the writhing nociceptive response induced by zymosan and acetic acid in mice. Eur. J. Pharmacol. 2000a;387:111–118. doi: 10.1016/s0014-2999(99)00790-6. [DOI] [PubMed] [Google Scholar]
- RIBEIRO R.A., VALE M.L., FERREIRA S.H., CUNHA F.Q. Analgesic effect of thalidomide on inflammatory pain. Eur. J. Pharmacol. 2000b;391:97–103. doi: 10.1016/s0014-2999(99)00918-8. [DOI] [PubMed] [Google Scholar]
- ROCHA F.A., ARAGAO A.G., JR, OLIVEIRA R.C., POMPEU M.M., VALE M.R., RIBEIRO R.A. Periarthritis promotes gait disturbance in zymosan-induced arthritis in rats. Inflamm. Res. 1999;48:485–490. doi: 10.1007/s000110050491. [DOI] [PubMed] [Google Scholar]
- ROUVEIX B. Clinical pharmacology of cytokines. Eur. Cytokine Netw. 1997;8:291–293. [PubMed] [Google Scholar]
- SAFIEH-GARABEDIAN B., POOLE S., HADDAD J.J., MASSAAD C.A., JABBUR S.J., SAADE N.E. The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology. 2002;42:864–872. doi: 10.1016/s0028-3908(02)00028-x. [DOI] [PubMed] [Google Scholar]
- SAMPAIO E.P., MORAES M.O., NERY J.A., SANTOS A.R., MATOS H.C., SARNO E.N. Pentoxifylline decreases in vivo and in vitro tumour necrosis factor-alpha (TNF-alpha) production in lepromatous leprosy patients with erythema nodosum leprosum (ENL) Clin. Exp. Immunol. 1998;111:300–308. doi: 10.1046/j.1365-2249.1998.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMPAIO E.P., SARNO E.N., GALILLY R., COHN Z.A., KAPLAN G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J. Exp. Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHAFERS M., GEIS C., SVENSSON C.I., LUO Z.D., SOMMER C. Selective increase of tumour necrosis factor-alpha in injured and spared myelinated primary afferents after chronic constrictive injury of rat sciatic nerve. Eur. J. Neurosci. 2003;17:791–804. doi: 10.1046/j.1460-9568.2003.02504.x. [DOI] [PubMed] [Google Scholar]
- SCHANDNÉ L., VANDENBUSSCHE P., CRUSIAUX A., ALEGRE M.L., ABRAMOWICZ D., DUPONT E., CONTENT J., GOLDMAN M. Differential effects of pentoxifylline on the production of tumour necrosis factor-alpha (TNF-alpha) and interleukin-6 (IL-6) by monocytes and T cells. Immunology. 1992;76:30–34. [PMC free article] [PubMed] [Google Scholar]
- SCHMIDT-CHOUDHURY A., FURUTA G.T., LAVIGNE J.A., GALLI S.J., WERSHIL B.K. The regulation of tumor necrosis factor-alpha production in murine mast cells: pentoxifylline or dexamethasone inhibits IgE-dependent production of TNF-alpha by distinct mechanisms. Cell Immunol. 1996;171:140–146. doi: 10.1006/cimm.1996.0184. [DOI] [PubMed] [Google Scholar]
- SCHWARZ T., SCHWARZ A., KRONE C., LUGER T.A. Pentoxifylline suppresses allergic patch test reactions in humans. Arch. Dermatol. 1993;129:513–514. [PubMed] [Google Scholar]
- SEMMLER J., GEBERT U., EISENHUT T., MOELLER J., SCHONHARTING M.M., ALLERA A., ENDRES S. Xanthine derivatives: comparison between suppression of tumour necrosis factor-alpha production and inhibition of cAMP phosphodiesterase activity. Immunology. 1993;78:520–525. [PMC free article] [PubMed] [Google Scholar]
- SHAFER D.M., ASSAEL L., WHITE L.B., ROSSOMANDO E.F.Tumor necrosis factor-alpha as a biochemical marker of pain and outcome in temporomandibular joints with internal derangements J. Oral. Maxillofac. Surg. 199452786–791.discussion 791–792 [DOI] [PubMed] [Google Scholar]
- SMITH M.D., TRIANTAFILLOU S., PARKER A., YOUSSEF P.P., COLEMAN M. Synovial membrane inflammation and cytokine production in patients with early osteoarthritis. J. Rheumatol. 1997;24:365–371. [PubMed] [Google Scholar]
- SMITH J.A., AMAGASU S.M., EGLEN R.M., HUNTER J.C., BLEY K.R. Characterization of prostanoid receptor-evoked responses in rat sensory neurones. Br. J. Pharmacol. 1998;124:513–523. doi: 10.1038/sj.bjp.0701853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMMER C., MARZINIAK M., MYERS R.R. The effect of thalidomide treatment on vascular pathology and hyperalgesia caused by chronic constriction injury of rat nerve. Pain. 1998;74:83–91. doi: 10.1016/S0304-3959(97)00154-1. [DOI] [PubMed] [Google Scholar]
- THOMAZZI S.M., RIBEIRO R.A., CAMPOS D.I., CUNHA F.Q., FERREIRA S.H. Tumor necrosis factor, interleukin-1 and interleukin-8 mediates the nociceptive activity of the supernatant of LPS-stimulated macrophages. Mediat. Inflamm. 1997;6:195–200. doi: 10.1080/09629359791686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TONUSSI C.R., FERREIRA S.H. Rat knee-joint carrageenin incapacitation test: an objective screen for central and peripheral analgesics. Pain. 1992;48:421–427. doi: 10.1016/0304-3959(92)90095-S. [DOI] [PubMed] [Google Scholar]
- TONUSSI C.R., FERREIRA S.H. Tumour necrosis factor-alpha mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain. 1999;82:81–87. doi: 10.1016/S0304-3959(99)00035-4. [DOI] [PubMed] [Google Scholar]
- VALE M.L., MARQUES J.B., MOREIRA C.A., ROCHA F.A., FERREIRA S.H., POOLE S., CUNHA F.Q., RIBEIRO R.A. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J. Pharmacol. Exp. Ther. 2003;304:102–108. doi: 10.1124/jpet.102.038703. [DOI] [PubMed] [Google Scholar]
- VAN FURTH A.M., VERHARD-SEIJMONSBERGEN E.M., VAN FURTH R., LANGERMANS J.A. Effect of lisofylline and pentoxifylline on the bacterial-stimulated production of TNF-alpha, IL-1 beta IL-10 by human leucocytes. Immunology. 1997;91:193–196. doi: 10.1046/j.1365-2567.1997.00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARD A., CLISSOLD S.P. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987;34:50–97. doi: 10.2165/00003495-198734010-00003. [DOI] [PubMed] [Google Scholar]
- WEINBERG J.B., MASON S.N., WORTHAM T.S. Inhibition of tumor necrosis factor-alpha (TNF-alpha) and interleukin-1 beta (IL-1 beta) messenger RNA (mRNA) expression in HL-60 leukemia cells by pentoxifylline and dexamethasone: dissociation of acivicin-induced TNF-alpha and IL-1 beta mRNA expression from acivicin-induced monocytoid differentiation. Blood. 1992;79:3337–3343. [PubMed] [Google Scholar]
- WOOLF C.J., ALLCHORNE A., SAFIEH-GARABEDIAN B., POOLE S. Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br. J. Pharmacol. 1997;121:417–424. doi: 10.1038/sj.bjp.0701148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORDLICZEK J., SZCZEPANIK A.M., BANACH M., TURCHAN J., ZEMBALA M., SIEDLAR M., PRZEWLOCKI R., SEREDNICKI W., PRZEWLOCKA B. The effect of pentoxifiline on post-injury hyperalgesia in rats and postoperative pain in patients. Life Sci. 2000;66:1155–1164. doi: 10.1016/s0024-3205(00)00419-7. [DOI] [PubMed] [Google Scholar]
- ZIMMERMANN M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- ZINETTI M., GALLI G., DEMITRI M.T., FANTUZZI G., MINTO M., GHEZZI P., ALZANI R., COZZI E., FRATELLI M. Chlorpromazine inhibits tumour necrosis factor synthesis and cytotoxicity in vitro. Immunology. 1995;86:416–421. [PMC free article] [PubMed] [Google Scholar]