Abstract
Plant alkaloid tetrandrine (Tet), purified from Chinese herb Han-Fang Chi, is a potent immunomodulator used to treat rheumatic disorders, silicosis and hypertension in mainland China.
We previously demonstrated that Tet effectively suppresses cytokine production and proliferation of CD28-costimulated T cells. In the present study, we investigated the possible involvement of nuclear factor kappa B (NF-κB) transcription factors, critical in CD28 costimulation, in Tet-mediated immunosuppression in human peripheral blood T cells.
We showed that Tet inhibited NF-κB DNA-binding activities induced by various stimuli, including CD28 costimulation. At equal molar concentrations, Tet was as strong as methotrexate in suppressing CD28-costimulated NF-κB activities. Since Tet itself did not affect NF-κB binding to its corresponding DNA sequence, the results suggested that Tet might regulate NF-κB upstream signaling molecules.
Further studies demonstrated that Tet could prevent the degradation of IκBα and inhibit nuclear translocation of p65 by blocking IκBα kinases α and β activities. In addition, the activation of mitogen-activated protein kinases such as c-jun N-terminal kinase, p38 and extracellular signal-regulated kinase and activator protein-1 DNA-binding activity were all downregulated by Tet. Transfection assays performed in purified human peripheral blood T cells also confirmed the inhibition of NF-κB transcriptional activity by Tet.
When four Tet analogues were readily compared, dauricine appeared to preserve the most potent inhibition on CD28-costimulated but not on H2O2-induced NF-κB DNA-binding activities.
Our results provide the molecular basis of immunomodulation of Tet for being a potential disease-modifying antirheumatic drug in the therapy of autoimmune disorders like rheumatoid arthritis.
Keywords: Nuclear factor kappaB, tetrandrine, immunomodulation, T lymphocyte
Introduction
Autoimmune diseases are a group of disorders presenting with the destruction of multiple organs. While the etiologies of these disorders remain largely unknown, the activation of pathogenic and autoreactive T cells has been considered to play a critical role in initiating and modulating immune responses (Fox, 1997; Panayi et al., 2001). The cytokines secreted from these activated T cells act as triggers to exaggerate the inflammatory responses. In order to control or to reduce the inflammatory response in autoimmune disorders, it is therefore reasonable to suppress T-cell activation. Over the past few decades, many different approaches were taken towards eliminating T cells or inhibiting T-cell activation to achieve suppression of immune responses. In contrast to elimination of T cells, which leads to limited success yet with great side effects, pharmacological treatment with drugs like cyclosporin A that inhibits T-cell activation has been shown to be more promising (Cranney and Tugwell, 1998). Current treatment of autoimmune disorders, especially rheumatoid arthritis (RA), recommends using the combination of several disease-modifying antirheumatic drugs (DMARDs) preserving different immunomodulatory mechanisms (O'Dell, 1998; Pincus et al., 1999).
Differing from Western societies taking medications with scientific basis, a great proportion of Chinese people still choose to take traditional medicine nowadays. One of the most commonly used antirheumatic Chinese herbs for rheumatic disorders is an active purified compound called tetrandrine (Tet) from the dried tuberous root of the creeper Stephania tetrandra S Moore. Besides antirheumatic purposes, Tet has also been used for diseases like silicosis and hypertension (Li et al., 1981; Feng and Chen, 1985; Kwan, 1996). Our previous work demonstrated that Tet inhibits cellular proliferation, cytokine production, as well as activation marker expression in CD28-costimulated human peripheral blood T cells (Lai et al., 1999). In addition, at higher concentrations, Tet induces T-cell apoptosis utilizing a mechanism different from that of a Western DMARD, hydroxychloroquine (Lai et al., 2001).
Nuclear factor kappa B (NF-κB) transcription factors are a group of proteins comprising of homo- and heterodimers of Rel-domain-containing family proteins. In resting cells, through masking the nuclear localization signal, NF-κB transcription factors are retained in the cytosol by inhibitory proteins, including IκBα, IκBβ, IκBɛ and IκBγ. After receiving a stimulatory signal, the IκBα inhibitory protein is phosphorylated at both 32 and 36 serine residues by IκBα kinases (IKKs), resulting in its ubiquitination and subsequent degradation by proteosome (Mercurio et al., 1997; Zandi et al., 1997). These sequential yet highly regulated signal transduction events then cause nuclear translocation of NF-κB transcription factors from cytosol to nucleus and induce the activation of the corresponding genes, including those of cytokines and growth factors. Given the importance of NF-κB signaling pathway in CD28 costimulation (Kane et al., 2002) as well as the broad inhibition of CD28-costimulated T-cell activities by Tet (Lai et al., 1999), it is highly possible that NF-κB transcription factors could be the potential targets of this drug.
In this study, we examined whether Tet could block the activation of NF-κB transcription factors. Our results provide evidence showing that the Tet-mediated immunosuppression may involve the inhibition of IKK-IκBα-NF-κB signaling pathway in human peripheral blood T cells. In addition, among Tet and its analogues examined, dauricine appeared to be the most potent inhibitor of CD28-costimulated NF-κB activation.
Methods
Preparation of Tet, its analogues and DMARDs
The preparation of Tet and its analogues has been described previously (Lai et al., 1999). In brief, the powder of Tet with purity of more than 98% was obtained from Yichang Pharmaceutical Company, Hubei Province, the People's Republic of China, and dissolved in 0.1 N HCl. The Tet analogues, hernandezine, berbamine and dauricine (kindly provided by Dr C.-Y. Kwan, McMaster University, Ontario, Canada), were dissolved in 0.1 N HCl (Lai et al., 1999). Methotrexate was purchased from Calbiochem (La Jolla, CA, U.S.A.) and dissolved in H2O. Sulfasalazine was purchased from Sigma (St Louis, MO, U.S.A.) and dissolved in ethanol. For stimulation, the required concentrations of Tet, its analogues and DMARDs were made by further dilution of the concentrated stock solution with culture medium. Within concentrations used in this study for Tet, its analogues and DMARDs alone, there was no detectable cytotoxicity by trypan blue exclusion assays (data not shown).
Preparation of peripheral blood T cells
Human peripheral blood T cells were purified from whole blood with negative selection according to our previous report (Lai et al., 2001). Briefly, buffy coat from blood bank (Taipei, Taiwan) was mixed with Ficoll-Hypaque, after centrifugation, and a layer of mononuclear cells was collected. After lysis of red blood cells, the peripheral blood mononuclear cells were incubated with antibodies, including L243 (anti-DR; American Type Culture Collection (ATCC), Rockville, MD, U.S.A.), OKM1 (anti-CD11b; ATCC) and LM2 (anti-Mac1; ATCC) for 30 min at 4°C. The cells were then washed with medium containing 10% fetal bovine serum and incubated with magnetic beads conjugated with goat anti-mouse IgG (R&D, Minneapolis, MN, U.S.A.). The antibody-stained cells were then removed with a magnet. Following a repeat of the above procedures, the T cells were obtained with purity of more than 98% as determined by the percentage of CD3+ cells in flow cytometry (Beckton Dickinson, Mountain View, CA, U.S.A.).
Cell stimulation
For cell activation, the following stimuli and concentrations were used: phorbol 12-myristate 13-acetate (PMA, Sigma) at 10 ng ml−1; ionomycin (Sigma) at 1 μM; PHA (Sigma) and ConA (Calbiochem) at 10 μg ml−1; immobilized anti-CD3 mAb (OKT3, ATCC) at 10 μg ml−1 and soluble anti-CD28 mAb (Beckton Dickinson) at 1 μg ml−1 concentrations. The cells were incubated with a series of stimuli for variable time points and the cell pellets or supernatants were collected for analysis.
Nuclear extract preparation
Nuclear extracts were prepared according to our published work (Yang et al., 2003). Briefly, the treated cells (2 × 106 cells) were left at 4°C in 50 μl of buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol (DTT), 1 mM PMSF, and 3.3 μg ml−1 aprotinin) for 15 min with occasional gentle vortexing. The swollen cells were centrifuged at 15,000 r.p.m. for 3 min. After removal of the supernatants (cytoplasmic extracts), the pelleted nuclei were washed with 50 μl buffer A and subsequently, the cell pellets were resuspended in 30 μl buffer C (20 mM HEPES, pH 7.9, 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 25% glycerol, 1 mM DTT, 0.5 mM PMSF and 3.3 μg ml−1 aprotinin) and incubated at 4°C for 30 min with occasional vigorous vortexing. Then the mixtures were centrifuged at 15,000 r.p.m. for 20 min, and the supernatants were used as nuclear extracts.
Electrophoretic mobility shift assay (EMSA)
The EMSA was performed as detailed in our previous report (Yang et al., 2003). The oligonucleotides containing NF-κB-binding site (5′-AGT TGA GGG GAC TTT CCC AGG C- 3′) and activator protein 1 (AP-1)-binding site (5′-CGC TTG ATG AGT CAG CCG GAA- 3′) were purchased and used as DNA probes (Promega, Mandison, WI, U.S.A.). The DNA probes were radiolabeled with [γ-32p]ATP using the T4 kinase according to the manufacturer's instructions (Promega). For the binding reaction, the radiolabeled NF-κB or AP-1 probe was incubated with 5 μg of nuclear extracts. The binding buffer contained 10 mM Tris-HCl (pH 7.5), 50 mM NaCl, 0.5 mM EDTA, 1 mM DTT, 1 mM MgCl2, 4% glycerol and 2 μg poly(dI-dC). The reaction mixture was left at room temperature to preceed with the binding reaction for 20 min. If unradiolabeled competitive oligonucleotides or antibodies for supershift assays were added, they were preincubated with nuclear extracts for 10 min before the addition of the radiolabeled probes. The final reaction mixture was analyzed in a 6% nondenaturing polyacrylamide gel with 0.25 × Tris-borate/EDTA (TBE) as an electrophoresis buffer.
Western blotting
ECL Western blotting (Amersham, Arlington Heights, IL, U.S.A.) was performed as described (Lai et al., 2001). Briefly, after extensive wash, the cells were pelleted and resuspended in lysis buffer. After periodic vortexing, the mixture was centrifuged and the supernatant was collected and the protein concentration measured. Equal amounts of whole cellular extracts were analyzed on 10% SDS–polyacrylamide gel electrophoresis (SDS–PAGE) and transferred to the nitrocellulose filter. For immunoblotting, the nitrocellulose filter was incubated with TBS-T containing 5% nonfat milk (milk buffer) for 2 h, and then blotted with antisera against IκBα, p65, IκBα kinase alpha (IKKα) or IκBα kinase beta (IKKβ) (Santa Cruz Biotechnology) or β-actin for overnight at 4°C. After washing with milk buffer twice, the filter was incubated with donkey anti-mouse IgG conjugated to horseradish peroxidase at a concentration of 1 : 5000 for 30 min. The filter was then incubated with the substrate and exposed to X-ray film.
Immunoprecipitation kinase assay
The construction of plasmid pGEX-4T-2-IκBα containing sequences of N-terminal 4–54 amino acids of IκBα fused in-frame to the expression plasmid pGEX-4T-2 has been detailed (Lai, 1996). The GST-IκBα fusion protein induced in Escherichia coli transfected with pGEX-4T-2-IκBα was used as a substrate for IKKα and IKKβ. The c-Jun N-terminal kinase (JNK) substrate, GST-c-Jun fusion protein, was a kind gift from Dr S.-F. Yang (Academia Sinica, Taiwan). Myelin basic protein used as a substrate for extracellular signal-regulated protein kinase (ERK) and p38 were purchased from Sigma. The antibodies for kinase assays were purchased from Cell Signaling (Beverly, MA, U.S.A. for both anti-JNK and anti-p38 polyclonal antibodies) or purchased from Santa Cruz Biotechnology (anti-ERK, anti-IKKα and anti-IKKβ polyclonal antibodies). To perform immunoprecipitation kinase assay, the whole cellular extract 50–100 μg was incubated with 5 μl of specific antibody in incubation buffer containing 25 mM HEPES (pH 7.7), 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton-X-100, 20 mM β-glycerophosphate, 0.1 mM Na3VO4, 2 μM leupeptin and 400 μM PMSF overnight. The mixture was then immunoprecipitated by addition of protein A beads and rotated at 4°C for 2 h. After extensive wash, twice with HEPES washing buffer containing 20 mM HEPES (pH 7.7), 50 mM NaCl, 2.5 mM MgCl2, 0.1 mM EDTA and 0.05% Triton X-100, twice with LiCl washing buffer containing 500 mM LiCl, 100 mM Tris (pH 7.6), 0.1% Triton X-100 and 1 mM DTT and twice with kinase buffer containing 20 mM MOPS (pH 7.2), 2 mM EDTA, 10 mM MgCl2, 0.1% Triton X-100 and 1 mM DTT, the beads were resuspended in 40 μl kinase buffer with addition of cold ATP (30 μM), substrates and 10 μCi of [γ-32P]ATP. The mixture was incubated at 30°C with occasional gentle mixing for 30 min. The reaction was then terminated by resuspending in 1% SDS solubilizing buffer and boiled for 5 min and analyzed in SDS–PAGE.
Transfection assays in purified human peripheral blood T cells
The transfection assays were performed by electroporation with an Amaxa Nucleofector apparatus according to the manufacturer's instructions (Amaxa, Cologne, Germany). In brief, primary T cells were mixed with 5 μg of the reporter plasmid pNF-kB-Luc (Stratagene, La Jolla, CA, U.S.A.) in 100 μl of the provided electroporation buffer. After electroporation, the cells were transferred to 2 ml of prewarmed RPMI medium. The cells were then equally distributed for individual conditions as described in the figure legends. For drug treatment, the drugs were added 2 h before the addition of stimuli. After another 24 h, the cell pellets were collected, the total cell lysates prepared and the luciferase activities, after normalization to the total protein amounts, were determined according to the manufacturer's instructions (Promega).
Statistics
The results are expressed as means±s.d. A paired or unpaired Student's t-test was used to determine the significance of differences; a value of P<0.05 was considered statistically significant.
Results
Tet downregulated NF-κB DNA-binding activity in various stimuli-activated T cells
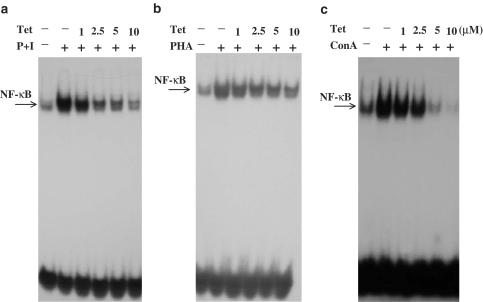
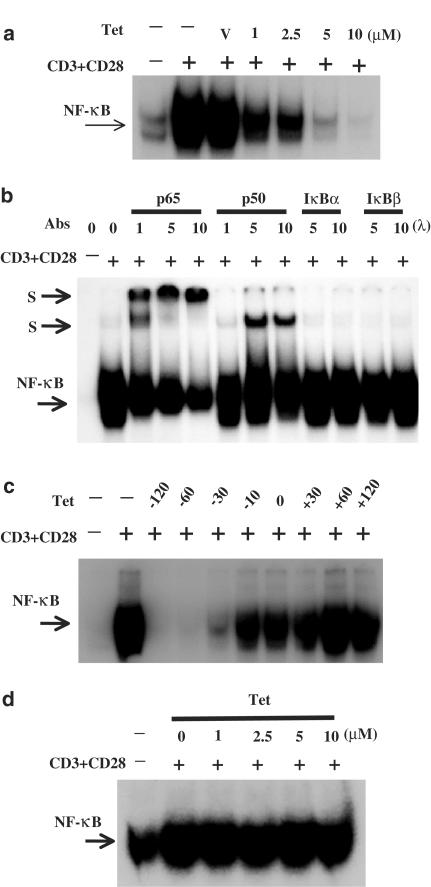
The extensive inhibition of T-cell cytokine production by Tet (Lai et al., 1999) raises a possibility that Tet may target certain signaling molecules that are commonly involved in T-cell activation. We therefore examined the effect of Tet on the activation of NF-κB transcription factors, a family of proteins extensively involved in a variety of signaling pathways of T-cell activation. After stimulation with PMA+ionomycin, PHA or ConA for various time points as indicated in the figure legends, peripheral blood T cells were collected and the nuclear extracts were prepared for analysis by EMSA. As shown in Figure 1, these stimuli induced strong NF-κB DNA-binding activities that could only be competed by wild-type but not mutant κB oligonucleotides (data not shown). In the presence of Tet, the induced NF-κB DNA-binding activities were suppressed in a dose-dependent manner (Figure 1a–c). Such effects were not due to cytotoxicities because coincubation of both Tet at 10 μM concentration and individual stimuli with T cells for 8 h caused less than 15% of cell death (data not shown). Under the same conditions, Tet at equal to or less than 5 μM concentrations in combination with individual stimuli did not induce significant cell death (data not shown). To be more physiological, we chose anti-CD3+anti-CD28 mAbs to stimulate T cells. Consistently, Tet effectively blocked NF-κB DNA-binding activities induced by CD28 costimulation (Figure 2a, upper panel). By EMSA supershift assays, we showed that the CD28 costimulation-induced NF-κB DNA-binding complex contained at least p65 and p50 (Figure 2b). In addition, when Tet was present before the addition of stimuli, it effectively blocked NF-κB DNA-binding activity (Figure 2c). However, the inhibitory effect was totally abolished when Tet was added after the addition of stimuli (Figure 2c). Furthermore, Tet itself did not directly interfere in the binding of NF-κB to κB oligonucleotides (Figure 2d).
Figure 1.
Tet dose-dependently inhibited various stimuli-induced NF-κB DNA-binding activities. Human peripheral blood T cells at 2 × 106 ml−1 were pretreated with various concentrations of Tet for 2 h and then stimulated or not with PMA + ionomycin (a) for 2 h, PHA (b) for 3 h or ConA (c) for 6 h. The nuclear extracts were prepared and analyzed by EMSA as described in Methods. The results shown are representative of at least three independent experiments using different donor T cells.
Figure 2.
Tet inhibited CD28-costimulated NF-κB DNA-binding activities but had no effect on direct binding of NF-κB to its consensus sequence. In (a), the purified T cells were treated or not with Tet at indicated concentrations for 2 h and then stimulated with anti-CD3+anti-CD28 mAbs for 6 h and the EMSA was performed as in Figure 1. In (b), after stimulated or not with anti-CD3+anti-CD28 mAbs, the T-cell nuclear extracts were preincubated in the presence or absence of anti-p65, anti-p50, anti-IκBα or anti-IκBβ polyclonal antibodies for 30 min. Then, the radiolabeled κB oligonucleotides were added into the mixture and the samples were analyzed by EMSA. S stands for supershifted bands. In (c), the T cells were treated with Tet for various time points before (−) or after (+) addition of anti-CD3+anti-CD28 mAbs. The nuclear extracts were analyzed by EMSA. In (d), after treatment, the CD28-costimulated T-cell nuclear extracts were incubated or not with various concentrations of Tet and then analyzed with EMSA.
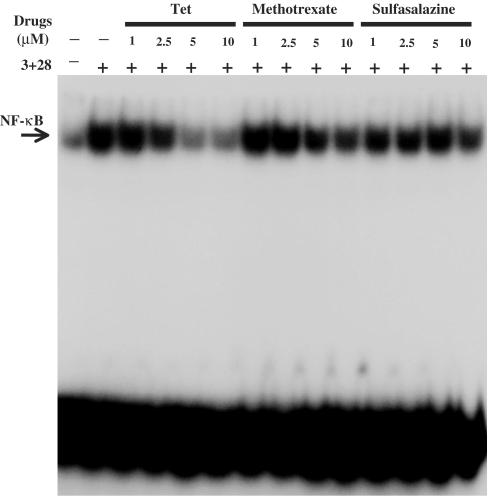
Inhibition of CD28-costimulated NF-κB activities by Tet and Western DMARDs, sulfasalazine and methotrexate
The inhibitory potency of Tet and Western DMARDs, methotrexate and sulfasalazine, was readily examined and compared side by side at equal molar concentrations. Figure 3 shows that the CD28-inhibitory potency of Tet was no less or relatively stronger than methotrexate. Given a therapeutic concentration of less than 700 nM in antirheumatic purposes for methotrexate, these results added additional support for Tet to be considered as part of DMARDs for RA treatment. At the examined concentrations, sulfasalazine did not show significantly detectable effects on CD28-costimulated NF-κB activities.
Figure 3.
Suppressive potency of Tet and Western DMARDs, methotrexate and sulfasalazine. The purified T cells were treated in the presence or absence of Tet, methotrexate or sulfasalazine at the indicated concentrations for 2 h and then stimulated with anti-CD3+anti-CD28 mAbs for 6 h. The EMSA analysis was performed as described in Methods.
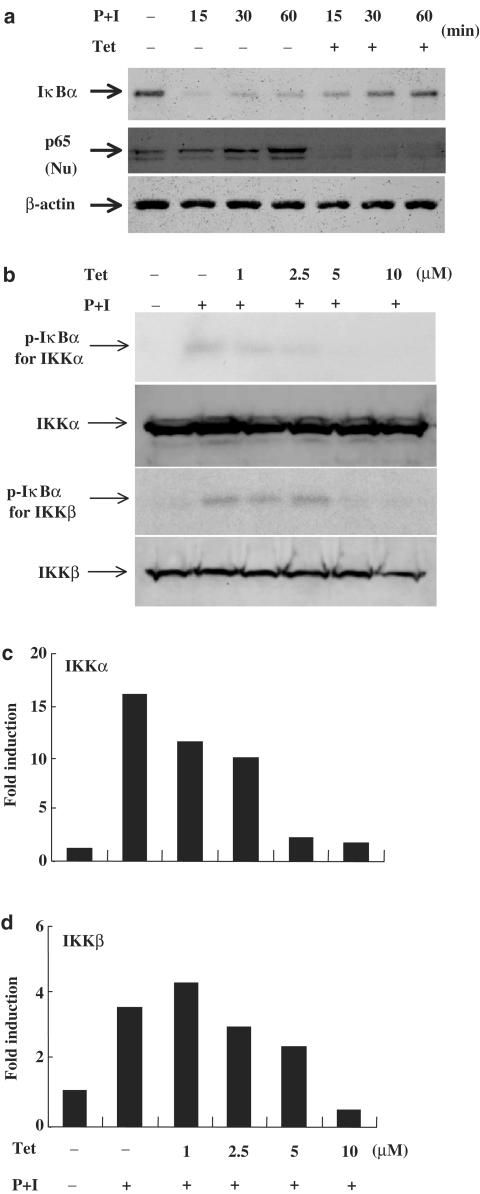
Tet blocked IκBα degradation through inhibiting IKKα and IKKβ activities
The inhibition of NF-κB activity by Tet suggests that this drug might have some effects on the inhibitory protein IκBα that retains NF-κB in an inactive status in the cytosol of resting T cells. We showed that Tet successfully prevented IκBα degradation and nuclear translocation of p65 in activated T cells (Figure 4a). As a comparison, Tet had no effect on β-actin levels. As the regulation of IκBα protein level depends on the activity of IκBα kinases, which through phosphorylating IκBα brings IκBα ubiquitination and degradation, we determined if Tet could suppress IKK activities. The experiments demonstrated that Tet effectively blocked kinase activities of IKKα and IKKβ while it did not affect the protein amounts of both IKKs (Figure 4b and c).
Figure 4.
Tet prevented IκBα degradation, nuclear translocation of p65 and blocked IKKα and IKKβ kinases activities. In (a), human peripheral blood T cells at 2 × 106 ml−1 were pretreated or not with Tet for various time points and then stimulated with PMA+ionomycin for various time points. After washing, cell pellets were collected and the total cell lysates or nuclear extracts were prepared and analyzed for determining the protein levels of IκBα and p65, respectively, in Western blotting assays as described in Methods. In (b), T cells were pretreated or not with various concentrations of Tet and then stimulated with PMA+ionomycin. After washing, cell pellets were collected and the total cell lysates were immunoprecipitated with anti-IKKα or anti-IKKβ antibodies. After sequential washing, GST-IκBα and 10 μCi of [γ-32P]ATP were added. After kinase reaction, the reaction mixture was analyzed. For determination of the IKKα and IKKβ protein level, Western blotting assays were performed. In (c), the fold induction was presented as a comparison with the intensity determined in the medium control.
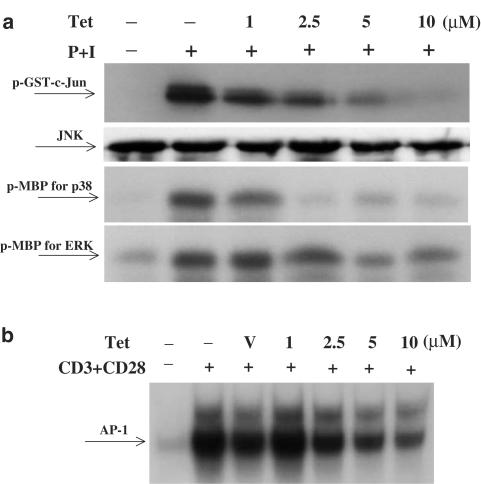
Inhibition of MAP kinase activities by Tet
Aside from IKKs, MAP kinases also play important roles in T-cell receptor and CD28-induced NF-κB activation (Tuosto et al., 2000), and the effect of Tet on MAP kinase activation was examined. The results showed that Tet could block MAP kinases, including JNK, p38 and ERK activities in a dose-dependent manner (Figure 5a). In parallel, the DNA-binding activities of MAP kinase downstream transcription factors, AP-1, were reduced by Tet (Figure 5b).
Figure 5.
Tet downregulated MAP kinase activities as well as AP-1 DNA-binding activity. In (a), human peripheral blood T cells were pretreated or not with various concentrations of Tet for 2 h and then stimulated with PMA+ionomycin for 30 min. After washing, cell pellets were collected and the total cell lysates were immunoprecipitated with anti-JNK, anti-p38 or anti-ERK antibodies. After sequential washing, the substrates (GST-c-Jun for JNK and myelin basic protein for both p38 and ERK) and 10 μCi of [γ-32P]ATP were added. After the kinase reaction, the reaction mixture was analyzed. For determination of the JNK protein level, Western blotting assays were performed. In (b), T cells at 2 × 106 ml−1 were pretreated with various concentrations of Tet for 2 h and then stimulated or not with anti-CD3+anti-CD28 mAbs for 6 h. The nuclear extracts were prepared and analyzed by EMSA as described to determine the AP-1 DNA-binding activities.
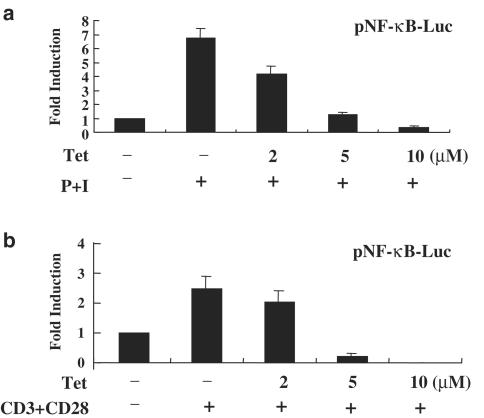
Tet inhibited NF-κB transcriptional activity in peripheral blood T cells
To further investigate whether the inhibition of NF-κB activity by Tet in vitro could also be seen in vivo, we transiently transfected NF-κB luciferase reporter plasmid into purified human peripheral blood T cells. At 48 h after transfection, the cells were equally distributed and treated or not with Tet at various dosages and then stimulated with PMA+ionomycin or anti-CD3+anti-CD28 mAbs to induce NF-κB transcriptional activities. Consistent with in vitro observations, Tet significantly inhibited transcriptional activity of NF-κB induced by these two different stimuli (Figure 6a and b).
Figure 6.
Tet suppressed transcriptional activity of NF-κB in human peripheral blood T cells. Human peripheral blood T cells at 1 × 106 ml−1 were mixed together with pNF-κB-Luc reporter plasmids and the transfection reagent. The transfection was performed using an Amaxa Nucleofector according to the manufacturer's instructions. After electroporation, the cells were equally divided and pretreated in the presence or absence of Tet at various dosages for 2 h. After stimulation with PMA+ionomycin (a) or anti-CD3+anti-CD28 mAbs (b) for another 22 h, cells were collected and the total cell lysates were analyzed for luciferase activities. The representative data out of at least three independent experiments with similar results are shown.
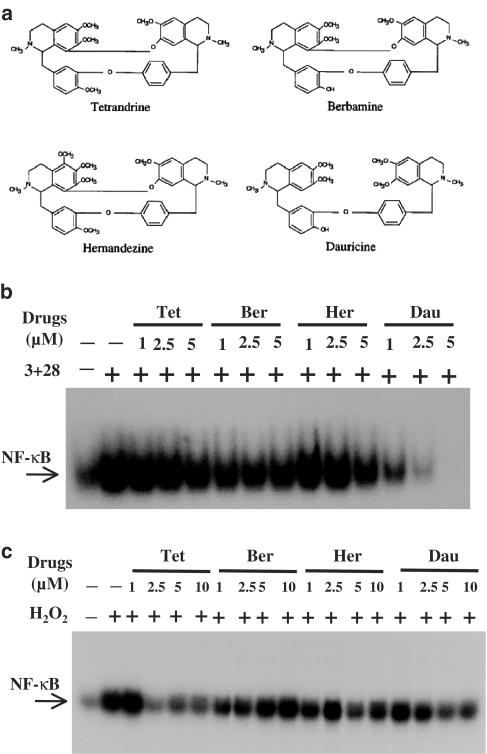
Dauricine, among Tet analogues, preserved the most potent inhibition on CD28-costimulated NF-κB DNA-binding activities
Aside from Tet, there are at least three Tet analogues, namely hernandezine, berbamine and dauricine, that preserve very similar structures with one another (Figure 7a). Previously we showed that dauricine is specifically potent on suppressing CD28-costimulated T-cell proliferation and shares equal potency in inhibiting mixed lymphocyte reaction with other Tet analogues (Lai et al., 1999). The NF-κB-suppressive potencies of these Tet analogues were readily compared. As shown in Figure 7b, at similar concentrations, dauricine was the strongest inhibitor of CD28-costimulated NF-κB DNA-binding activities. Since it has been demonstrated that Tet itself preserves anti-oxidative property, we examined and compared the suppressive potency of H2O2-induced NF-κB DNA-binding activities by Tet analogues. As shown in Figure 7c, although certain variation existed, dauricine appeared not to preserve stronger antioxidative potency compared to other Tet analogues. In this context, berbamine appeared to have the least effect on H2O2-induced NF-κB DNA-binding activity (Figure 7c). These results also suggest that the Tet analogues-mediated CD28 suppression might not be simply due to their antioxidative properties.
Figure 7.
Dauricine, among Tet analogues, preserved the most potent capacity in inhibiting CD28-costimulated NF-κB DNA-binding activities. The structures of Tet analogues are shown in (a). In (b), T cells were pretreated with various concentrations of Tet and its analogues, including hernandezine (Her), berbamine (Ber) and dauricine (Dau) for 2 h, and then stimulated with anti-CD3+anti-CD28 mAbs for 6 h. The NF-κB DNA-binding activities were performed as described in Figure 1. In (c), the experiments were performed exactly as in (b), except that the stimuli anti-CD3+anti-CD28 mAbs was replaced with an oxidant H2O2. The representative results of at least three independent experiments are shown.
Discussion
In the present study, we took molecular approaches to examine the mechanisms of Tet-mediated immunosuppression in human peripheral blood T cells. In light of the significance of NF-κB transcription factors in T-cell activation and cytokine gene expression, we investigated whether Tet could inhibit NF-κB activation in T cells. We observed that Tet dose-dependently blocked NF-κB DNA-binding activities induced by several different stimuli, including PMA+ionomycin, PHA, ConA and CD28 costimulation (Figures 1 and 2). When equal molar concentrations of Tet and DMARDs were examined, Tet preserved equal or relatively stronger potency as methotrexate in suppressing CD28-costimulated NF-κB activities (Figure 3). The Tet-mediated inhibition of NF-κB activation might be due to suppression of IκBα degradation (Figure 4a), an event following the reduction of IκBα kinase activities by Tet (Figure 4b and c). Meanwhile, Tet also effectively blocked MAP kinase activities as well as AP-1 DNA-binding activity (Figure 5a and b). The results from transfection assays further support this conclusion (Figure 6). Moreover, when several Tet analogues were examined, dauricine appeared to be the most potent one in inhibiting CD28-costimulated NF-κB DNA-binding activities (Figure 7).
The family of NF-κB transcription factors has been demonstrated to be involved in regulation of a wide variety of immunologically relevant genes that are critical in mediating immune and inflammatory responses (Tak and Firestein, 2001). The activation of IKK-IκBα-NF-κB signaling pathway also contributes significantly to the pathogenesis of rheumatoid arthritis (Makarov, 2001). Use anti-p50 and anti-p65 antibodies to perform immunohistochemical stainings reveals nuclear distribution of NF-κB in vascular endothelium and synovial lining cells of RA patients (Handel et al., 1995; Marok et al., 1996). The presence of activated NF-κB transcription factors is considered to provide protection against TNF-α or Fas ligand-mediated cellular apoptosis in RA synovium (Miagkov et al., 1998). Treatment with inhibitors that block NF-κB activation leads to loss of this protection and causes cellular apoptosis (Miagkov et al., 1998). In addition, the introduction of NF-κB decoy oligodeoxynucleotides that bind NF-κB with high affinity and block their binding to κB elements in cytokine genes also suppresses arthritis symptoms in collagen-induced arthritis of rats (Tomita et al., 1999). Consistent with the results using inhibitors targeting NF-κB molecules, intra-articular gene transfer of adenoviral construct encoding dominant active IKKβ into the joints of normal rats results in joint swelling and histological evidence of synovial inflammation (Tak et al., 2001). In contrast, the delivery of an adenoviral construct encoding dominant negative IKKβ into joints leads to reduction of arthritis symptoms (Tak et al., 2001). Although both IKKα and IKKβ could phosphorylate IκBα and lead IκBα to degradation and activate NF-κB transcription factors, there are still subtle differences between these two kinases. Infection of fibroblast-like synoviocytes with adenoviral constructs encoding dominant negative IKKβ but not dominant negative IKKα downregulates cytokine-induced IκBα degradation and NF-κB activation (Aupperle et al., 2001). The results seem to indicate that IKKβ rather than IKKα play a more critical role in cytokine-mediated synovitis. Nevertheless, the equal susceptibility of both IKKα and IKKβ to Tet inhibition has provided this drug a broad application in pathologies mediated by NF-κB activation.
Given the importance of NF-κB activation in immunopathogenesis of autoimmune disorders, the pharmacological approaches to block it have been one of the mainstreams for drug development (Karin et al., 2004). In this context, many drugs such as corticosteroid, aspirin, sulfasalazine, leflunomide and methotrexate have been demonstrated to mediate their protection against RA in part through inhibition of NF-κB signaling pathway in T cells (Wahl et al., 1998; Yin et al., 1998; Manna et al., 2000; Majumdar and Aggarwal, 2001). When two of these agents, sulfasalazine and methotrexate, were compared with Tet at equal molar concentrations on CD28-induced NF-κB activities, Tet showed relatively stronger suppressive potency among them (Figure 3). In addition, the inhibition of NF-κB was also observed in several Tet analogues. In particular, we demonstrated in this and other reports that, among Tet anlogues, dauricine preserved the strongest CD28-suppressive potencies. This conclusion was made basing upon the measurement of CD28-costimulated cytokine production, activation marker expression, cellular proliferation as well as NF-κB activation (Lai et al. (1999) and Figure 7b in this report). In contrast, dauricine was not superior to other Tet analogues in inhibition of mixed lymphocyte reaction (Lai et al., 1999) and an oxidant H2O2-induced NF-κB activation (Figure 7c). It is therefore predictable that by analyzing the structural difference between dauricine and other Tet analogues, we may be able to identify more potent and safer dauricine analogues that specifically target CD28 signaling events (Ho and Lai, 2004).
In conclusion, we presented in this study that the IKK-IκBα-NF-κB signaling pathway might play critical roles in Tet-mediated immunosuppression in human peripheral blood T cells. These results may lead to reasonable well-controlled clinical trials on Tet analogues, especially dauricine, as DMARDs in autoimmune disorders such as RA.
Acknowledgments
This work was supported by the National Health Research Institute, the Department of Health (CCMP92-RD-112) and the Tri-Service General Hospital Research Grant (TSGH-C93-04-S05), Taiwan, R.O.C. The kind gifts from Drs S.-F. Yang and C.-Y. Kwan are highly appreciated.
Abbreviations
- ConA
concanavalin A
- EMSA
electrophoresis mobility shift assay
- ERK
extracellular signal-regulated kinase
- IKK
IκBα kinase
- IL
interleukin
- JNK
c-jun N-terminal kinase
- mAb
monoclonal antibody
- MAP kinase
mitogen-activated protein kinase
- NF-κB
nuclear factor kappa B
- PAGE
polyacrylamide gel electrophoresis
- PHA
phytohemagglutinin
- PMA
phorbol 12-myristate 13-acetate
- RA
rheumatoid arthritis
- Tet
tetrandrine
- TNF-α
tumor necrosis factor alpha
References
- AUPPERLE K., BENNETT B., HAN Z., BOYLE D., MANNING A., FIRESTEIN G. NF-kappa B regulation by I kappa B kinase-2 in rheumatoid arthritis synoviocytes. J. Immunol. 2001;166:2705–2711. doi: 10.4049/jimmunol.166.4.2705. [DOI] [PubMed] [Google Scholar]
- CRANNEY A., TUGWELL P. The use of Neoral in rheumatoid arthritis. Rheum. Dis. Clin. North Am. 1998;24:479–488. doi: 10.1016/s0889-857x(05)70021-1. [DOI] [PubMed] [Google Scholar]
- FENG Y.X., CHEN H. Pharmacognostic and chemical identification of Fang Ji (Stephania tetrandra) Chin. J. Pharm. Anal. 1985;5:28–31. [Google Scholar]
- FOX D.A. The role of T cells in the immunopathogenesis of rheumatoid arthritis: new perspectives. Arthritis Rheum. 1997;40:598–609. doi: 10.1002/art.1780400403. [DOI] [PubMed] [Google Scholar]
- HANDEL M.L., MCMORROW L.B., GRAVALLESE E.M. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- HO L.J., LAI J.H. Chinese herbs as immunomodulators and potential disease-modifying antirheumatic drugs in autoimmune disorders. Curr. Drug Metab. 2004;5:181–192. doi: 10.2174/1389200043489081. [DOI] [PubMed] [Google Scholar]
- KANE L.P., LIN J., WEISS A. It's all Rel-ative: NF-kappaB and CD28 costimulation of T-cell activation. Trends Immunol. 2002;23:413–420. doi: 10.1016/s1471-4906(02)02264-0. [DOI] [PubMed] [Google Scholar]
- KARIN M., YAMAMOTO Y., WANG Q.M. The IKK NF-kappa B system: a treasure trove for drug development. Nat. Rev. Drug Discov. 2004;3:17–26. doi: 10.1038/nrd1279. [DOI] [PubMed] [Google Scholar]
- KWAN C.Y.Tetrandrine: an anti-hypertensive drug with Ca2+ antagonistic action: effects on vascular smooth muscle and adrenal gland Adrenal Glands, Vascular System and Hypertension 1996Bristol UK: J Endocrinol Ltd; 103–114.ed. Vinson P & Anderson DC, pp [Google Scholar]
- LAI J.H. The role of Rel/NF-κB and IκBα proteins in CD28 signal transduction 1996. PhD thesis, Baylor College of Medicine, USA, 1996
- LAI J.H., HO L.J., KWAN C.Y., CHANG D.M., LEE T.C. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: potential immunosuppressants in transplantation immunology. Transplantation. 1999;68:1383–1392. doi: 10.1097/00007890-199911150-00027. [DOI] [PubMed] [Google Scholar]
- LAI J.H., HO L.J., LU K.C., CHANG D.M., SHAIO M.F., HAN S.H. Western and Chinese antirheumatic drug-induced T cell apoptotic DNA damage uses different caspase cascades and is independent of Fas/Fas ligand interaction. J. Immunol. 2001;166:6914–6924. doi: 10.4049/jimmunol.166.11.6914. [DOI] [PubMed] [Google Scholar]
- LI Q.L., XU Y.H., ZHON Z.H., CHEN X.W., HUANG X.G., CHEN S.L., ZHAN C.X. The therapeutic effect of tetrandrine on silicosis. Chin. J. Tuberc. Res. Dis. 1981;4:321–328. [PubMed] [Google Scholar]
- MAJUMDAR S., AGGARWAL B.B. Methotrexate suppresses NF-kappaB activation through inhibition of IkappaBalpha phosphorylation and degradation. J. Immunol. 2001;167:2911–2920. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- MAKAROV S.S. NF-kappa B in rheumatoid arthritis: a pivotal regulator of inflammation, hyperplasia, and tissue destruction. Arthritis Res. 2001;3:200–206. doi: 10.1186/ar300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANNA S.K., MUKHOPADHYAY A., AGGARWAL B.B. Leflunomide suppresses TNF-induced cellular responses: effects on NF-kappa B, activator protein-1, c-Jun N-terminal protein kinase, and apoptosis. J. Immunol. 2000;165:5962–5969. doi: 10.4049/jimmunol.165.10.5962. [DOI] [PubMed] [Google Scholar]
- MAROK R., WINYARD P.G., COUMBE A., KUS M.L., GAFFNEY K., BLADES S., MAPP P.I., MORRIS C.J., BLAKE D.R., KALTSCHMIDT C., BAEUERLE P.A. Activation of the transcription factor nuclear factor-kappaB in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- MERCURIO F., ZHU H., MURRAY B.W., SHEVCHENKO A., BENNETT B.L., LI J., YOUNG D.B., BARBOSA M., MANN M., MANNING A., RAO A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation.[comment] Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- MIAGKOV A.V., KOVALENKO D.V., BROWN C.E., DIDSBURY J.R., COGSWELL J.P., STIMPSON S.A., BALDWIN A.S., MAKAROV S.S. NF-kappaB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc. Natl. Acad. Sci., U.S.A. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'DELL J.R. Triple therapy with methotrexate, sulfasalazine, and hydroxychloroquine in patients with rheumatoid arthritis. Rheum. Dis. Clin. North Am. 1998;24:465–477. doi: 10.1016/s0889-857x(05)70020-x. [DOI] [PubMed] [Google Scholar]
- PANAYI G.S., CORRIGALL V.M., PITZALIS C. Pathogenesis of rheumatoid arthritis. The role of T cells and other beasts. Rheum. Dis. Clin. North Am. 2001;27:317–334. doi: 10.1016/s0889-857x(05)70204-0. [DOI] [PubMed] [Google Scholar]
- PINCUS T., O'DELL J.R., KREMER J.M. Combination therapy with multiple disease-modifying antirheumatic drugs in rheumatoid arthritis: a preventive strategy. Ann. Intern. Med. 1999;131:768–774. doi: 10.7326/0003-4819-131-10-199911160-00009. [DOI] [PubMed] [Google Scholar]
- TAK P.P., FIRESTEIN G.S. NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. 2001;107:7–11. doi: 10.1172/JCI11830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAK P.P., GERLAG D.M., AUPPERLE K.R., VAN DE GEEST D.A., OVERBEEK M., BENNETT B.L., BOYLE D.L., MANNING A.M., FIRESTEIN G.S. Inhibitor of nuclear factor kappaB kinase beta is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897–1907. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- TOMITA T., TAKEUCHI E., TOMITA N., MORISHITA R., KANEKO M., YAMAMOTO K., NAKASE T., SEKI H., KATO K., KANEDA Y., OCHI T. Suppressed severity of collagen-induced arthritis by in vivo transfection of nuclear factor kappaB decoy oligodeoxynucleotides as a gene therapy. Arthritis Rheum. 1999;42:2532–2542. doi: 10.1002/1529-0131(199912)42:12<2532::AID-ANR5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- TUOSTO L., COSTANZO A., GUIDO F., MARINARI B., VOSSIO S., MORETTI F., LEVRERO M., PICCOLELLA E. Mitogen-activated kinase kinase kinase 1 regulates T cell receptor- and CD28-mediated signaling events which lead to NF-kappaB activation. Eur. J. Immunol. 2000;30:2445–2454. doi: 10.1002/1521-4141(200009)30:9<2445::AID-IMMU2445>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- WAHL C., LIPTAY S., ADLER G., SCHMID R.M. Sulfasalazine: a potent and specific inhibitor of nuclear factor kappa B. J. Clin. Invest. 1998;101:1163–1174. doi: 10.1172/JCI992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANG S.P., HO L.J., LIN Y.L., CHENG S.M., TSAO T.P., CHANG D.M., HSU Y.L., SHIH C.Y., JUAN T.Y., LAI J.H. Carvedilol, a new anti-oxidative beta-blocker, blocks in-vitro human peripheral blood T cell activation via down-regulating NF-kappaB activity. Cardiovasc. Res. 2003;59:776–787. doi: 10.1016/s0008-6363(03)00459-0. [DOI] [PubMed] [Google Scholar]
- YIN M.J., YAMAMOTO Y., GAYNOR R.B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta [see comments] Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- ZANDI E., ROTHWARF D.M., DELHASE M., HAYAKAWA M., KARIN M. The IkappaB kinase complex (IKK) contains two kinase subunits, IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91:243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]