Abstract
The immunomodulator Linomide has been shown to protect against septic liver injury by reducing hepatic accumulation of leukocytes although the detailed anti-inflammatory mechanisms remain elusive. This study examined the effect of Linomide on the production of CXC chemokines, including macrophage inflammatory protein-2 (MIP-2) and cytokine-induced neutrophil chemoattractant (KC) and interleukin-10 (IL-10) in lipopolysaccharide (LPS)/D-galactosamine (Gal)-induced liver injury in mice.
It was found that pretreatment with 300 mg kg−1 of Linomide markedly suppressed leukocyte recruitment, perfusion failure, and hepatocellular damage and apoptosis in the liver of endotoxemic mice.
Administration of Linomide inhibited endotoxin-induced gene expression of MIP-2 and KC and significantly reduced the hepatic production of MIP-2 and KC by 63 and 80%, respectively. Moreover, it was found that Linomide increased the liver content of IL-10 by more than three-fold in endotoxemic mice.
The protective effect of Linomide against endotoxin-induced inflammation and liver injury was abolished in IL-10-deficient mice, suggesting that the beneficial effect of Linomide is dependent on the function of IL-10.
Taken together, these novel findings suggest that the protective effect of Linomide is mediated via local upregulation of IL-10, which in turn decreases the generation of CXC chemokines and pathological recruitment of leukocytes in the liver of endotoxemic mice.
Keywords: Adhesion, chemokines, endotoxin, intravital microscopy, linomide, sepsis
Introduction
The immunomodulator Linomide has been shown to protect against septic liver injury (Klintman et al., 2002). It is widely held that leukocyte recruitment is a rate-limiting step in endotoxin-induced liver damage (Hewett et al., 1993; Klintman et al., 2004). Indeed, it has previously been demonstrated that the protective effect of Linomide is related to its inhibitory impact on hepatic accumulation of leukocytes (Klintman et al., 2002) although the detailed anti-inflammatory mechanisms of Linomide remain elusive. Tissue recruitment of leukocytes is coordinated by secreted chemokines, a family of small peptides subdivided into two main groups (CC and CXC) based on structural properties (Bacon & Oppenheim, 1998; Zlotnik & Yoshie, 2000). The CXC chemokines are considered to predominately attract neutrophils (Bacon & Oppenheim 1998; Zlotnik & Yoshie, 2000). A recent study showed that endotoxin-induced extravascular infiltration of leukocytes into the liver is critically dependent on the generation and action of CXC chemokines (MIP-2 and KC) (Li et al., 2004). Thus, it may be possible that Linomide can interfere with the production of MIP-2 and/or KC in endotoxemia.
Tissue homeostasis is dependent on a fine-tuned balance between pro- and anti-inflammatory cytokines, including transforming growth factor-β and IL-10. For example, it has been reported that endotoxin-induced organ damage and lethality are increased in IL-10-deficient mice (Standiford et al., 1995) and administration of exogenous IL-10 has been shown to ameliorate liver injury in endotoxemic mice (Santucci et al., 1996; Louis et al., 1997). Numerous studies have shown that IL-10 has the capacity to inhibit the inflammatory process at multiple levels, such as cytokine and chemokine secretion, and adhesion molecule expression (Fiorentino et al., 1991; Kasama et al., 1994; Hickey et al., 1998; Kopydlowski et al., 1999). Interestingly, in models of encephalomyelitis (Diab et al., 1998; Zhu et al., 1998) and diabetes (Gross et al., 1994), Linomide has been reported to upregulate IL-10 production locally and in peripheral leukocytes. However, it is not known whether Linomide may modulate the expression of IL-10 in septic liver injury and whether such a mechanism may help explain the protective effect exerted by Linomide in endotoxin-induced liver injury.
Based on the considerations above, the hypothesis of this study was that Linomide induces the local generation of IL-10 in the liver, which in turn is related to downstream modulation of CXC chemokine production and inflammatory cell recruitment.
Methods
Animals
Adult male C57/Bl6 and IL-10-deficient (B6.129P2-Il10tm1Cgn/J, The Jackson Laboratory, Bar Harbor, MA, U.S.A.) mice weighing 21–26 g were kept on a 12–12 h light–dark cycle with free access to food and tap water. Animals were anesthetized by intraperitoneal (i.p.) administration of 7.5 mg ketamine hydrochloride and 2.5 mg xylazine per 100 mg body weight. The right jugular vein was cannulated with a polyethylene catheter for intravenous (i.v.) administration of test substances, fluorescent dyes and additional anesthesia. The local ethics committee approved all the experiments of this study.
Experimental protocol
Linomide was administered subcutaneously (s.c.) at 30 and 300 mg kg day−1 dissolved in 0.2 ml phosphate-buffered saline (PBS) for 3 days prior to experimentation. The protective effect of 4 h pretreatment of Linomide was also evaluated in separate experiments. Mice were challenged i.p. with 0.25 ml PBS (control animals) or a combination of LPS (10 μg per mouse) and D-galactosamine (18 mg mouse−1) dissolved in PBS to a total volume of 0.25 ml. A transverse subcostal incision was performed in anesthetized mice and the ligamentous attachments from the liver to the diaphragm and the abdominal wall were gently released. The animals were positioned on their left side and the left liver lobe was carefully exteriorized onto an adjustable stage for analysis of hepatic microcirculation by intravital fluorescence microscopy. The liver surface was covered with a circular glass to avoid tissue drying and exposure to ambient oxygen. An equilibration period of 10 min was allowed before starting microscopical observation. Animals were killed by exsanguination, and blood drawn from the heart was immediately centrifuged and used for analysis of plasma enzyme markers of hepatocellular injury (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) using standard spectrophotometric procedures. Briefly, 30 μl of blood was applied onto reagent strips (Reflotron® Test Strip, Roche Diagnostics Scandinavia AB, Bromma, Sweden) designed for the specific quantitative determination of ALT and AST. These strips incorporate a plasma-separating system, which makes it possible to use whole blood. Systemic leukocyte counts, including polymorphonuclear leukocytes (PMNL) and mononuclear leukocytes (MNL) were determined using a hematocytometer.
Intravital microscopy
For observations of the liver microcirculation, we used a modified Olympus microscope (BX50WI, Olympus Optical Co. GmbH, Hamburg, Germany) equipped with different water immersion lenses ( × 40 NA 0.75/ × 63 NA 0.9). The image was televised (Sony Trinitron) using a charge-coupled device video camera (FK 6990 Cohu, Pieper GmbH, Schwerte, Germany) and recorded on videotape (Panasonic SVT-S3000 S-VHS recorder) for subsequent off-line evaluation. Blood perfusion within individual microvessels was studied after contrast enhancement by FITC-dextran (0.1 ml, 0.1 mg ml−1, molecular weight 150,000). In vivo labeling of leukocytes with rhodamine-6G (0.1 ml, 0.05 mg ml−1) enabled quantitative analysis of leukocyte flow behavior in both sinusoids and postsinusoidal venules. Quantification of microcirculatory parameters was performed off-line by frame-to-frame analysis of the videotaped images. Five postsinusoidal venules with connecting sinusoids were evaluated in each animal. Microcirculatory analysis included determination of the number of perfused sinusoids given as a percentage of the total number of sinusoids observed (i.e. sinusoidal perfusion). Leukocyte sequestration in the sinusoids was evaluated off-line by counting the number of trapped leukocytes in 15–30 high-power fields (HPF, 300 × 220 μm) per animal, and is given as leukocytes per 10 HPF. Within postsinusoidal venules, leukocyte rolling was measured by counting the number of cells rolling in each venule during 30 s, and is expressed as cells/min. Leukocyte adhesion was measured by counting the number of cells that adhered along the venular endothelium and remained stationary during the observation period of 30 s, and is expressed as cells/mm venule length. The diameter of the venules was not different between the experimental groups. Blood flow velocities were measured using CapImage software (Zeintl, Heidelberg, Germany). Hepatocyte apoptosis was measured in the same microscopic setup as above. For this purpose, the fluorochrome Hoechst 33342 (Hoechst, 0.02 ml, 0.2 μg ml−1) was topically applied onto the liver surface for staining of hepatocyte DNA. Hoechst is a fluorescent dye that has been widely used for analysis of nuclear morphology (Kroemer et al., 1995), for example, nuclear condensation and fragmentation in cultured hepatocytes and endothelial cells (Rauen et al., 1999). After exsanguination and 10 min of incubation, six microscopical fields (using a × 63 lens) were recorded for off-line quantification of hepatocyte nuclei showing signs of apoptosis (chromatin condensation and fragmentation). Hepatocyte apoptosis is given as the percentage of the number of hepatocyte nuclei showing apoptotic features from the total number of hepatocyte nuclei observed. The results of this method correlate well with measurements of caspase-3 protease activity (Klintman et al., 2004).
RT–PCR
Total RNA was extracted from mouse livers using an acid guanidinium–phenol–chloroform method (Trizol Reagent; GIBCO-BRL Life Technologies, Grand Island, NY, U.S.A.) and treated with RNase-free DNase (DNase 1; Amersham Pharmacia Biotech Sollentuna, Sweden) in order to remove potential genomic DNA contaminants according to the manufacturer's protocol. RNA concentrations were determined by measuring the absorbance at 260 nm spectrophotometrically. RT–PCR was performed with SuperScrip One-Step RT–PCR system (GIBCO-BRL Life Technologies). Each reaction contained 500 ng of liver total RNA as template and 0.2 μM of each primer in a final volume of 50 μl. Mouse β-actin served as an internal control gene. The RT–PCR profile was 1 cycle of cDNA synthesis at 50°C for 30 min, followed by 40 cycles of denaturation at 94°C for 30 s, annealing at 55°C and extension at 72°C for 1 min, 1 cycle of final extension at 72°C for 10 min. After RT–PCR, aliquots of the RT–PCR products were separated on 2% agarose gel containing ethidium bromide and photographed. The primer sequences of MIP-2, KC and β-actin were as follows: MIP-2 (f) 5′-GCT TCC TCG GGC ACT CCA GAC-3′, MIP-2(r) 5′-TTA GCC TTG CCT TTG TTC AGT AT-3′; KC (f) 5′-GCC AAT GAG CTG CGC TGT CAA TGC-3′, KC(r) 5′-CTT GGG GAC ACC TTT TAG CAT CTT-3′; β-actin (f) 5′-ATG TTT GAG ACC TTC AAC ACC-3′, β-actin (r) 5′-TCT CCA GGG AGG AAG AGG AT-3′.
ELISA
The right liver lobe was weighed and washed in PBS containing penicillin, streptomycin and fungizon (100 U ml−1) and then kept cool in cold serum-free Dulbecco's minimal essential medium. The liver was then incubated in 1 ml of Dulbecco's minimal essential medium solution containing 10% fetal calf serum and 1% penicillin and streptomycin at 37°C in a 12-well plate for 24 h as described previously (Riaz et al., 2003). The cultured medium was harvested and stored in −20°C until analysis of MIP-2, KC and IL-10 by use of double antibody Quantikine ELISA kit using recombinant murine MIP-2, KC and IL-10 as standards. The minimal detectable protein concentrations are less than 0.5 pg ml−1.
Materials
FITC-dextran, D-galactosamine, lipopolysaccharide from Escherichia coli, and rhodamine-6G were purchased from Sigma Chemical Co., St Louis, MO, U.S.A. Ketamine hydrochloride was from Hoffman-La Roche, Basel, Switzerland. Xylazine was from Janssen Pharmaceutica, Beerse, Belgium. Hoechst 33342 was purchased from Molecular Probes, Leiden, the Netherlands. Linomide was generously provided by Active Biotech Research, Lund, Sweden.
Statistical analyses
Data are presented as mean values±s.e.m. Statistical evaluations were performed using Kruskal–Wallis one-way analysis of variance on ranks followed by multiple comparisons versus control group (Dunn's method). P<0.05 was considered significant and n represents the number of animals.
Results
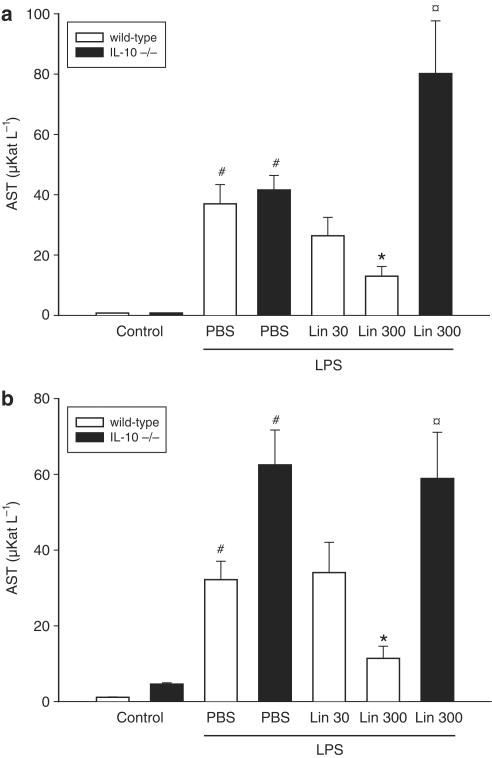
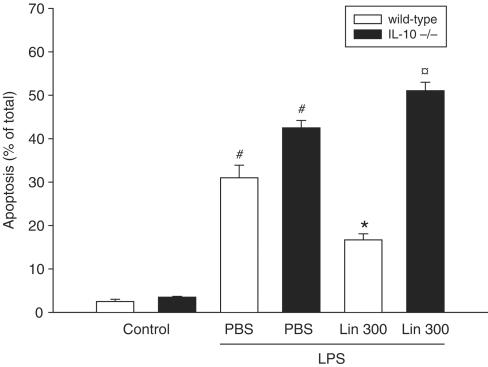
Challenge with LPS provoked massive damage to the liver parenchyma, as indicated by elevated liver enzymes: AST increased from 0.8±0.1 to 37.0±6.4 μKat l−1 and ALT increased from 1.1±0.1 to 32.2±4.9 μKat l−1 (Figure 1a and b, P<0.05 vs PBS, n=4–5). It was found that pretreatment with 300 mg kg day−1 of Linomide, but not 30 mg kg day−1, for 3 days prior to LPS challenge reduced AST and ALT by more than 65% (Figure 1a and b, P<0.05 vs LPS alone, n=5–12). In separate experiments, we found that pretreatment with Linomide for only 4 h prior to LPS exerted no protective effect, that is, ALT was 41.6±4.8 μKat l−1 and AST was 62.5±9.2 μKat l−1 (P>0.05 vs LPS alone, n=5). Based on these experiments, we evaluated the anti-inflammatory mechanisms of Linomide administered at 300 mg kg−1day−1 for 3 days. Hepatocyte apoptosis constitutes an integral part of the pathogenesis in septic liver injury (Klintman et al., 2004). Apoptosis was quantified by applying the DNA-binding fluorochrome Hoechst 33342, which stains the nuclei of hepatocytes and allows quantification of the percentage of cells with nuclear condensation and fragmentation (Rauen et al., 1999). This approach correlates very well to quantitative measurements of caspase-3 in this model (Klintman et al., 2004). In PBS-treated controls, the baseline level of apoptosis was 2.5±0.5%, which increased to 31.0±2.9% in endotoxemic mice (Figure 2, P<0.05 vs PBS, n=4–5). Linomide decreased the percentage of apoptotic hepatocytes down to 16.7±1.4%, corresponding to a 46% reduction in LPS-treated animals (Figure 2, P<0.05 vs LPS alone, n=5–12). Indeed, hepatic injury is not only regulated by proinflammatory cytokines but is also under inhibitory influence exerted by counter-regulatory cytokines, such as IL-10 (Hickey et al., 1998). It was found that Linomide exerted no beneficial effect on endotoxin-induced liver injury in IL-10 gene-targeted mice, that is, AST (Figure 1a), ALT (Figure 1b) and apoptosis (Figure 2) increased significantly in response to LPS in IL-10-deficient mice pretreated with Linomide as compared to wild-type mice (P<0.05 vs wild type, n=4–5).
Figure 1.
Effect of Linomide on levels of (a) AST and (b) ALT 6 h after treatment with PBS alone (Control) or with lipopolysaccharide (LPS 10 μg)/D-galactosamine (1.1 g kg−1) in wild-type and IL-10-deficient (−/−) mice. Linomide pretreatment (30 and 300 mg kg day−1) was started 3 days prior to LPS challenge. Liver enzymes were measured spectrophotometrically. Data represent mean±s.e.m. and n=4–12. #P<0.05 vs control and *P<0.05 vs PBS + LPS (wild-type mice).  P<0.05 vs Lin 300 (wild-type mice).
P<0.05 vs Lin 300 (wild-type mice).
Figure 2.
Effect of Linomide on apoptosis of hepatocytes 6 h after treatment with PBS alone (control) or with lipopolysaccharide (LPS 10 μg)/D-galactosamine (1.1 g kg−1) wild-type and IL-10-deficient (−/−) mice. Linomide pretreatment (300 mg kg day−1) was started 3 days prior to LPS challenge. Hepatocyte apoptosis is given as the percentage of observed hepatocyte nuclei with morphological signs of apoptosis, that is, chromatin condensation and fragmentation, after administration of the fluorochrome Hoechst 33342. Data represent mean±s.e.m. and n=4–12. #P<0.05 vs control and *P<0.05 vs PBS+LPS (wild-type mice). P<0.05 vs Lin 300 (wild-type mice).
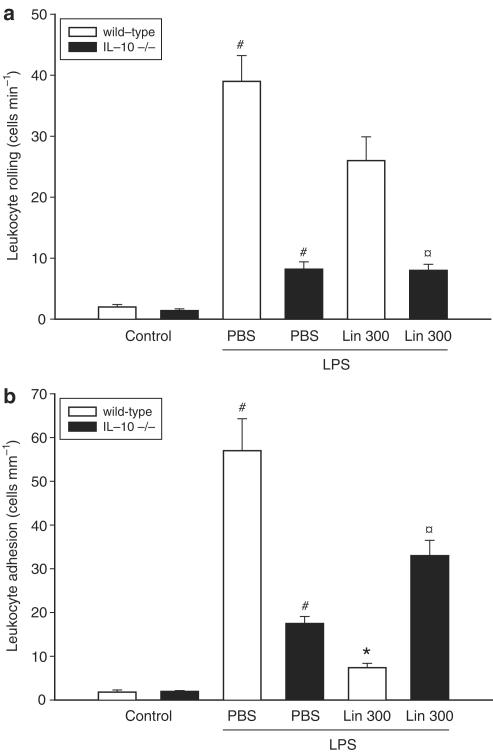
Next, we used intravital microscopy to determine the microvascular effects of Linomide in endotoxemic mice. We observed that the number of rolling and firmly adherent leukocytes was 3.3±0.5 cells mm−1 and 1.8±0.5 cells mm−1 venule length, respectively, in PBS-treated control animals (Figure 3a and b, n=4). LPS treatment increased leukocyte rolling to 39.0±4.2 cells min−1 and firm adhesion to 57.0±7.3 cells mm−1 (Figure 3a and b, P<0.05 vs PBS, n=4–5). Pretreatment with Linomide had no effect on endotoxin-induced leukocyte rolling (Figure 3a, n=5–12). As expected, LPS challenge caused a marked increase in leukocyte adhesion (Figure 3b, P<0.05 vs wild type, n=4–5). Interestingly, Linomide pretreatment significantly reduced LPS-induced leukocyte adhesion in wild-type (87% reduction) but not in IL-10-deficient animals (Figure 3b, n=5–12). In fact, LPS-induced leukocyte adhesion was significantly higher in IL-10-deficient mice compared to wild types (Figure 3b, P<0.05 vs wild type, n=4–5).
Figure 3.
Effect of Linomide on leukocyte (a) rolling and (b) adhesion 6 h after treatment with PBS alone (control) or with lipopolysaccharide (LPS 10 μg)/D-galactosamine (1.1 g kg−1) wild-type and IL-10-deficient (−/−) mice. Linomide pretreatment (300 mg kg day−1) was started 3 days prior to LPS challenge. Data represent mean±s.e.m. and n=4–12. #P<0.05 vs control and *P<0.05 vs PBS+LPS (wild-type mice). P<0.05 vs Lin 300 (wild-type mice).
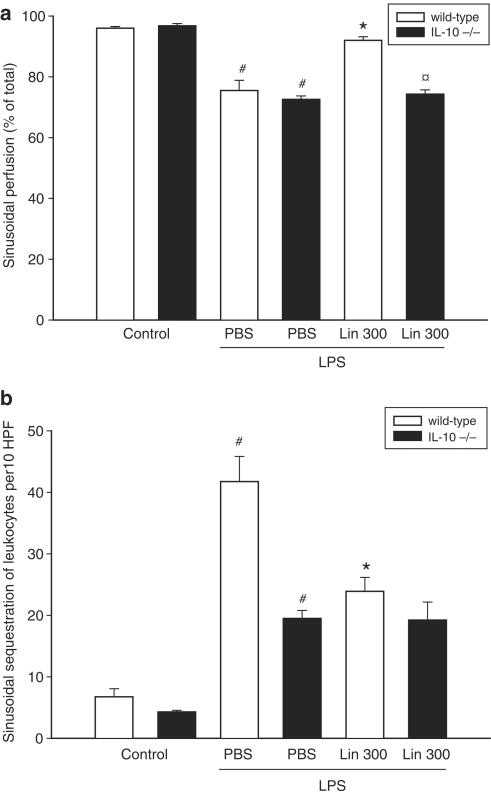
The hepatic injury associated endotoxemia is also characterized by decreased perfusion and increased sequestration of leukocytes in the sinusoids (Klintman et al., 2004). Indeed, we found that LPS challenge decreased sinusoidal perfusion by 21% and increased sinusoidal trapping of leukocytes by more than five-fold (Figure 4a and b, P<0.05 vs PBS, n=4–5). It was found that Linomide significantly improved microvascular perfusion and reduced sinusoidal sequestration of leukocytes (Figure 4a, b, P<0.05 vs LPS alone, n=5–12). In contrast, Linomide had no effect on the number of sequestered leukocytes in sinusoids provoked by LPS in IL-10-deficient mice (Figure 4b, n=5–12). Importantly, pretreatment with Linomide did not change systemic leukocyte counts (data not shown).
Figure 4.
Effect of Linomide on sinusoidal (a) perfusion and (b) leukocyte sequestration 6 h after treatment with PBS alone (control) or with lipopolysaccharide (LPS 10 μg)/D-galactosamine (1.1 g kg−1) wild-type and IL-10-deficient (−/−) mice. Linomide pretreatment (300 mg kg day−1) was started 3 days prior to LPS challenge. Perfusion rates are given as perfused sinusoids as percentage of all sinusoids observed. Sinusoidal sequestration of leukocytes was determined in 10 HPF. Data represent mean±s.e.m. and n=4–12. #P<0.05 vs control and *P<0.05 vs PBS + LPS (wild-type mice). P<0.05 vs Lin 300 (wild-type mice).
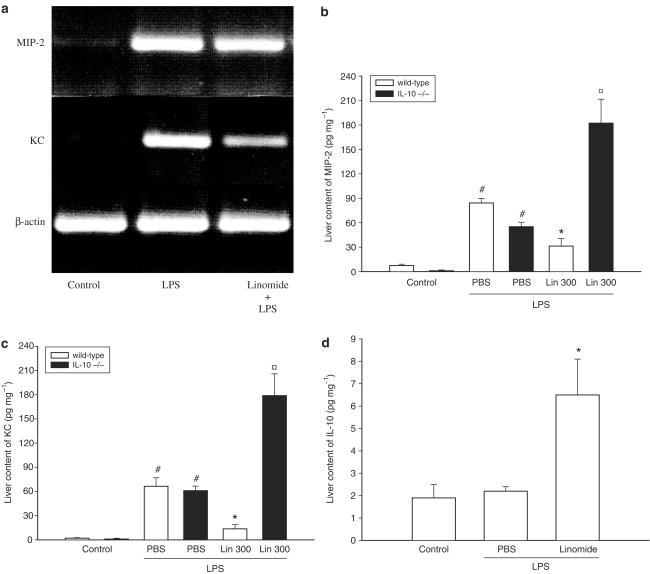
Recent findings have shown that CXC chemokines are important regulators of leukocyte recruitment in endotoxin-induced liver damage (Li et al., 2004). Herein, we first examined the mRNA expression of MIP-2 and KC. Total RNA was isolated from the liver, reverse transcribed into cDNA and PCR amplificated with specific primer for MIP-2 and KC. The results showed no gene expression of MIP-2 and KC in PBS-treated controls (Figure 5a). Notably, after LPS challenge the mRNA expression of both MIP-2 and KC was markedly increased (Figure 5a), suggesting that both MIP-2 and KC are expressed in the liver of endotoxemic mice. Interestingly, it was found that pretreatment with Linomide decreased endotoxin-induced expression of CXC chemokine mRNA, especially KC (Figure 5a). Next, the protein levels of MIP-2 and KC were examined. Indeed, we observed that the hepatic levels of MIP-2 and KC increased by more than 10- and 32-fold, respectively, in response to LPS exposure (Figure 5b and c, P<0.05 vs PBS, n=4–5). Pretreatment with Linomide decreased LPS-induced expression of MIP-2 by 63% (from 84.2±5.7 down to 31.3±9.2 pg mg−1) and KC by 80% (from 66.4±10.6 down to 13.6±5.2 pg mg−1) (Figure 5b and c, P<0.05 vs LPS alone, n=4–5). However, Linomide pretreatment did not reduce CXC chemokine levels in IL-10-deficient mice (Figure 5b and c). In fact, administration of endotoxin significantly increased the hepatic levels of MIP-2 and KC in IL-10-deficient mice pretreated with Linomide (Figure 5b and c, P<0.05 vs wild type, n=4–5) as compared to wild-type animals. Interestingly, we found that Linomide increased the production of IL-10 by more than three-fold in the liver (from 2.2±0.2 to 6.5±1.6 pg mg−1) (Figure 5c and d, P<0.05 vs LPS alone, n=4–5).
Figure 5.
Effect of Linomide on the (a) gene expression of MIP-2 and KC and on the protein levels of (b) MIP-2 (c) KC and (d) IL-10 in the liver 6 h after treatment with PBS alone (control) or with lipopolysaccharide (LPS 10 μg)/D-galactosamine (1.1 g kg−1) wild-type and IL-10-deficient (−/−) mice. Linomide pretreatment (300 mg kg day−1) was started 3 days prior to LPS challenge. Levels of MIP-2, KC and IL-10 were determined by use of ELISA. Data represent mean±s.e.m. and n=4–5. #P<0.05 vs control and *P<0.05 vs PBS + LPS (wild-type mice). P<0.05 vs Lin 300 (wild-type mice).
Discussion
Linomide has been shown to exert protective effects against septic liver injury. This study not only confirms the protective effect of Linomide in the liver but also demonstrates that Linomide inhibits endotoxin-induced expression of CXC chemokines via local upregulation of IL-10. Considering the important role of CXC chemokines in the pathological recruitment of leukocytes, this Linomide-mediated downregulation of CXC chemokines may help explain the anti-inflammatory mechanisms of this immunomodulator in endotoxin-induced liver damage.
The immunomodulator Linomide is known to protect against a broad spectrum of conditions, including inflammatory and autoimmune diseases (Björck & Kleinau, 1989; Gonzalo et al., 1993; Gross et al., 1994; Hortelano et al., 1997; Diab et al., 1998; Zhu et al., 1998; Liu et al., 2003). We have previously shown that Linomide protects against tumor necrosis factor-α (TNF-α)-induced leukocyte recruitment and liver damage (Zhang et al., 2000; Klintman et al., 2002). We now extend these observations by showing that Linomide also protects against LPS-induced liver injury. This is compatible with the known downstream role of TNF-α in mediating the harmful effects of endotoxemia in the liver (Hishinuma et al., 1990). Recent studies have shown that CXC chemokines are key mediators in endotoxin-induced liver injury (Li et al., 2004) by promoting the extravasation of leukocytes into the liver. In fact, there is evidence in the literature supporting the concept that intravascular adhesion of leukocytes is not sufficient to cause liver injury but that actual extravasation of leukocytes is required to significantly damage the liver (Chosay et al., 1997). We observed in the present investigation that Linomide significantly reduced local production of MIP-2 and KC by more than 63% in livers of endotoxemic mice. This Linomide-induced suppression of MIP-2 and KC correlated very well with the attenuation of liver damage as evidenced by decreased liver enzymes, leukocyte adhesion, hepatocyte apoptosis and increased sinusoidal perfusion as observed herein. In light of the critical role played by the CXC chemokines in leukocyte extravasation in this model (Li et al., 2004), these findings suggest that inhibition of MIP-2 and KC is an important anti-inflammatory mechanism exerted by Linomide. This is the first study showing that Linomide can negatively regulate the expression of chemokines, although considering the potent effect of Linomide against leukocyte activation and recruitment reported in numerous and diverse models of pathological inflammation, downregulation of chemokine production may not be limited to models of endotoxemia.
An accumulating body of evidence indicates the importance of a delicate balance between pro- and anti-inflammatory mediators in tissue homeostasis (Netea et al., 2003). We have shown that Linomide inhibits the expression and function of proinflammatory mediators, such as TNF-α and CXC chemokines (this study, Klintman et al., 2002). Interestingly, we found that Linomide increased the liver content of IL-10 by more than three-fold in endotoxemic mice in the present study. Thus, our novel data demonstrate that Linomide favors an anti-inflammatory profile by simultaneously antagonizing proinflammatory substances, including MIP-2 and KC, and inducing counter-regulatory cytokines (i.e. IL-10). This notion is also supported by our finding that IL-10-deficient mice pretreated with Linomide are not protected against liver inflammation and hepatocellular damage and apoptosis after challenge with endotoxin. In this context, knowing that Hogaboam et al. (1998) have shown that nitric oxide inhibits IL-10 production in an experimental model of sepsis, it is interesting to note that Linomide attenuates LPS-mediated induction of nitric oxide synthase (Hortelano et al., 1997). Thus, it may be speculated that Linomide may inhibit nitric oxide synthesis, which in turn leads to increased levels of IL-10. However, the establishment of such an anti-inflammatory mechanism of Linomide requires further studies.
In conclusion, our novel findings demonstrate that Linomide protects against septic liver injury by locally upregulating IL-10, which in turn inhibits CXC chemokine production. Our findings help explain the anti-inflammatory mechanisms of Linomide in endotoxin-provoked liver damage and lends further support to the concept that Linomide may be a candidate drug to protect the liver in sepsis.
Acknowledgments
This work was supported by grants from the Swedish Medical Research Council (2001-6576, 2002-955, 2002-8012, 2003-4661), Crafoordska stiftelsen, Blanceflors stiftelse, Einar och Inga Nilssons stiftelse, Harald och Greta Jaenssons stiftelse, Greta och Johan Kocks stiftelser, Fröken Agnes Nilssons stiftelse, Franke och Margareta Bergqvists stiftelse för främjande av cancerforskning, Magnus Bergvalls stiftelse, Mossfelts stiftelse, Nanna Svartz stiftelse, Ruth och Richard Julins stiftelse, Svenska Läkaresällskapet (2001-907), Teggers stiftelse, Allmäna sjukhusets i Malmö stiftelse för bekämpande av cancer, MAS fonder, Malmö University Hospital and Lund University.
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- ELISA
enzyme-linked immunosorbent assay
- FITC
fluorescein isothiocyanate
- Gal
D-galactosamine
- HPF
high-power fields
- IL
interleukin
- i.p.
intraperitoneally
- i.v.
intravenously
- KC
cytokine-induced neutrophil chemoattractant
- LPS
lipopolysaccharide
- MIP-2
macrophage inflammatory protein-2
- MNL
monomorphonuclear leukocytes
- PBS
phosphate-buffered saline
- PMNL
polymorphonuclear leukocytes
- RT–PCR
reverse transcriptase polymerase chain reaction
- s
second(s)
- TNF-α
tumor necrosis factor-α
References
- BACON K.B., OPPENHEIM J.J. Chemokines in disease models and pathogenesis. Cytokine Growth Factor Rev. 1998;9:167–173. doi: 10.1016/s1359-6101(98)00005-7. [DOI] [PubMed] [Google Scholar]
- BJÖRCK J., KLEINAU S. Paradoxical effects of LS-2616 (Linomide) treatment in the type (II) collagen arthritis model in mice. Agents Actions. 1989;27:319–321. doi: 10.1007/BF01972810. [DOI] [PubMed] [Google Scholar]
- CHOSAY J.G., ESSANI N.A., DUNN C.J., JAESCHKE H. Neutrophil margination and extravasation in sinusoids and venules of liver during endotoxin-induced injury. Am. J. Physiol. 1997;272:G1195–G1200. doi: 10.1152/ajpgi.1997.272.5.G1195. [DOI] [PubMed] [Google Scholar]
- DIAB A., MICHAEL L., WAHREN B., DENG G.M., BJÖRK J., HEDLUND G., ZHU J. Linomide suppresses acute experimental autoimmune encephalomyelitis in Lewis rats by counteracting the imbalance of pro-inflammatory versus anti-inflammatory cytokines. J. Neuroimmunol. 1998;85:146–154. doi: 10.1016/s0165-5728(98)00023-x. [DOI] [PubMed] [Google Scholar]
- FIORENTINO D.F., ZLOTNIK A., VIEIRA P., MOSMANN T.R., HOWARD M., MOORE K.W., O'GARRA A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J. Immunol. 1991;146:3444–3451. [PubMed] [Google Scholar]
- GONZALO J.A., GONZÁLEZ-GARCIA A., KALLAND T., HEDLUND G., MARTINEZ A.C., KROEMER G. Linomide, a novel immunomodulator that prevents death in four models of septic shock. Eur. J. Immunol. 1993;23:2372–2374. doi: 10.1002/eji.1830230949. [DOI] [PubMed] [Google Scholar]
- GROSS D.J., SIDI H., WEISS L., KALLAND T., ROSENMANN E., SLAVIN S. Prevention of diabetes mellitus in non-obese diabetic mice by Linomide, a novel immunomodulating drug. Diabetologica. 1994;37:1195–1201. doi: 10.1007/BF00399792. [DOI] [PubMed] [Google Scholar]
- HEWETT J.A., JEAN P.A., KUNKEL S.L., ROTH R.A. Relationship between tumor necrosis factor-alpha and neutrophils in endotoxin-induced liver injury. Am. J. Physiol. 1993;265:G1011–G1015. doi: 10.1152/ajpgi.1993.265.6.G1011. [DOI] [PubMed] [Google Scholar]
- HICKEY M.J., ISSEKUTZ A.C., REINHARDT P.H., FEDORAK R.N., KUBES P. Endogenous interleukin-10 regulates hemodynamic parameters, leukocyte–endothelial cell interactions, and microvascular permeability during endotoxemia. Circ. Res. 1998;83:1124–1131. doi: 10.1161/01.res.83.11.1124. [DOI] [PubMed] [Google Scholar]
- HISHINUMA I., NAGAKAWA J., HIROTA K., MIYAMOTO K., TSUKIDATE K., YAMANAKA T., KATAYAMA K., YAMATSU I. Involvement of tumor necrosis factor-alpha in development of hepatic injury in galactosamine-sensitized mice. Hepatology. 1990;12:1187–1191. doi: 10.1002/hep.1840120518. [DOI] [PubMed] [Google Scholar]
- HOGABOAM C.M., STEINHAUSER M.L., SCHOCK H., LUKACS N., STRIETER R.M., STANDIFORD T., KUNKEL S.L. Therapeutic effects of nitric oxide inhibition during experimental fecal peritonitis: role of interleukin-10 and monocyte chemoattractant protein 1. Infect. Immun. 1998;66:650–655. doi: 10.1128/iai.66.2.650-655.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HORTELANO S., DÍAZ-GUERRA M.J.M., GONZÁLEZ-GARCIA A., LEONARDO E., GAMALLO C., BOSCÁ L., MARTINEZ A.C. Linomide administration to mice attenuates the induction of nitric oxide synthase elicited by lipopolysaccharide-activated macrophages and prevents nephritis in MRL/Mp-lpr/lpr mice. J. Immunol. 1997;158:1402–1408. [PubMed] [Google Scholar]
- KASAMA T., STRIETER R.M., LUKACS N.W., BURDICK M.D., KUNKEL S.L. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 1994;152:3559–3569. [PubMed] [Google Scholar]
- KLINTMAN D., HEDLUND G., THORLACIUS H. Protective effect of Linomide on TNF-α-provoked liver damage. J. Hepatol. 2002;36:226–232. doi: 10.1016/s0168-8278(01)00261-6. [DOI] [PubMed] [Google Scholar]
- KLINTMAN D., XIANG L., THORLACIUS H. Important role of P-selectin in leukocyte recruitment, hepatocellular injury and apoptosis in endotoxemic mice. Clin. Diagn. Lab. Immunol. 2004;11:56–62. doi: 10.1128/CDLI.11.1.56-62.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOPYDLOWSKI K.M., SALKOWSKI C.A., CODY M.J., VAN ROOIJEN N., MAJOR J., HAMILTON T.A., VOGEL S.N. Regulation of macrophage chemokine expression by lipopolysaccharide in vitro and in vivo. J. Immunol. 1999;163:1537–1544. [PubMed] [Google Scholar]
- KROEMER G., PETIT P., ZAMZAMI N., VAYSSIERE J.L., MIGNOTTE B. The biochemistry of programmed cell death. FASEB J. 1995;9:1277–1287. doi: 10.1096/fasebj.9.13.7557017. [DOI] [PubMed] [Google Scholar]
- LI X., KLINTMAN D., WANG Y., SATO T., JEPPSSON B., THORLACIUS H. Critical role of CXC chemokines in the extravasation process of leukocytes in endotoxemia. J. Leukocyte Biol. 2004;75:443–452. doi: 10.1189/jlb.0603297. [DOI] [PubMed] [Google Scholar]
- LIU Q., WANG Y., ZHANG X.W., ANDERSSON G., HEDLUND G., THORLACIUS H. Linomide prevents dextran sodium sulfate-induced colitis in mice. Inflam. Res. 2003;52:64–68. doi: 10.1007/s000110300002. [DOI] [PubMed] [Google Scholar]
- LOUIS H., LE MOINE O., PENY M.O., GULBIS B., NISOL F., GOLDMAN M., DEVIERE J. Hepatoprotective role of interleukin 10 in galactosamine/lipopolysaccharide mouse liver injury. Gastroenterology. 1997;112:935–942. doi: 10.1053/gast.1997.v112.pm9041256. [DOI] [PubMed] [Google Scholar]
- NETEA M.G., VAN DER MEER J.W., VAN DEUREN M., KULLBERG B.J. Proinflammatory cytokines and sepsis syndrome: not enough, or too much of a good thing. Trends Immunol. 2003;24:254–258. doi: 10.1016/s1471-4906(03)00079-6. [DOI] [PubMed] [Google Scholar]
- RAUEN U., POLZAR B., STEPHAN H., MANNHERZ H.G., DE GROOT H. Cold-induced apoptosis in cultured hepatocytes and liver endothelial cells: mediation by reactive oxygen species. FASEB J. 1999;13:155–168. doi: 10.1096/fasebj.13.1.155. [DOI] [PubMed] [Google Scholar]
- RIAZ A.A., SCHRAMM R., SATO T., MENGER M.D., JEPPSSON B., THORLACIUS H. Oxygen radical-dependent expression of CXC chemokines regulate ischemia/reperfusion-induced leukocyte adhesion in the mouse colon. Free Radic. Biol. Med. 2003;35:782–789. doi: 10.1016/s0891-5849(03)00405-2. [DOI] [PubMed] [Google Scholar]
- SANTUCCI L., FIORUCCI S., CHIOREAN M., BRUNORI P.M., DI MATTEO F.M., SIDONI A., MIGLIORATI G., MORELLI A. Interleukin 10 reduces lethality and hepatic injury induced by lipopolysaccharide in galactosamine-sensitized mice. Gastroenterology. 1996;111:736–744. doi: 10.1053/gast.1996.v111.pm8780580. [DOI] [PubMed] [Google Scholar]
- STANDIFORD T.J., STRIETER R.M., LUKACS N.W., KUNKEL S.L. Neutralization of IL-10 increases lethality in endotoxemia. Cooperative effects of macrophage inflammatory protein-2 and tumor necrosis factor. J. Immunol. 1995;155:2222–2229. [PubMed] [Google Scholar]
- ZHANG X.W., HEDLUND G., BORGSTRÖM P., ARFORS K.E., THORLACIUS H. Linomide abolishes leukocyte adhesion and extravascular recruitment induced by tumor necrosis factor-αin vivo. J. Leukocyte Biol. 2000;68:621–626. [PubMed] [Google Scholar]
- ZHU J., DIAB A., MUSTAFA M., LEVI M., WAHREN B., BJÖRK J., HEDLUND G. Linomide suppresses chronic-relapsing experimental autoimmune encephalomyelitis in DA rats. J. Neurol. Sci. 1998;160:113–120. doi: 10.1016/s0022-510x(98)00244-5. [DOI] [PubMed] [Google Scholar]
- ZLOTNIK A., YOSHIE O. Chemokines. A new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]