Abstract
The protease inhibitor ritonavir is an integral part of current antiretroviral therapy targeting human immunodeficiency virus. Recent studies demonstrate that ritonavir induces apoptotic cell death with high efficiency in lymphoblastoid cell lines. Moreover, ritonavir can suppress activation of the transcription factor nuclear factor-κB and is an inhibitor of interleukin-1β and tumor necrosis factor-α production in peripheral blood mononuclear cells. Thus, ritonavir appears to have anti-inflammatory properties.
In the present study, we investigated in DLD-1 colon carcinoma cell effects of ritonavir on apoptotic cell death and expression of heme oxygenase-1 (HO-1), an anti-inflammatory enzyme that may be critically involved in the modulation of colonic inflammation.
Compared to unstimulated control, ritonavir resulted in a moderate increase in the rate of apoptotic cell death as observed after 20 h of incubation. Notably, ritonavir potently synergized with the short-chain fatty acid butyrate for induction of caspase-3-dependent apoptosis in DLD-1 cells.
Ritonavir enhanced mRNA and protein expression of HO-1 in DLD-1 cells. Ritonavir-induced HO-1 protein was suppressed by SB203580 or SB202190 and preceded by immediate upregulation of cellular c-Fos and c-Jun protein levels. This process was associated with induction of activator protein-1 as detected by electrophoretic mobility shift analysis.
The present data suggest that ritonavir has the potential to curb colon carcinogenesis by reducing cell growth via mechanisms that include apoptosis and by simultaneously modulating colonic inflammation via induction of anti-inflammatory HO-1.
Keywords: Apoptosis, butyrate, colon carcinoma cells, heme oxygenase-1, ritonavir
Introduction
Ritonavir is a potent inhibitor of human immunodeficiency virus (HIV) protease and is successfully used in current highly active antiretroviral therapy. Besides inhibition of HIV protease, cellular proteasome activity is modulated by ritonavir (Andre et al., 1998). Inhibition of the chymotrypsin-like activity of the 20S proteasome with subsequently hindered degradation of inhibitor-κBα likely mediates suppression of nuclear factor-κB (NF-κB) observed in cells exposed to ritonavir (Andre et al., 1998; Pati et al., 2002). Since the transcription factor NF-κB is a pivotal component of the proinflammatory signal transduction machinery (Ardite et al., 1998), these observations concur with the ability of ritonavir to inhibit the release of interleukin (IL)-1β and tumor necrosis factor-α (TNFα) from activated human peripheral blood mononuclear cells (Pati et al., 2002). In addition, ritonavir mediates antiproliferative effects and induces apoptosis in human lymphoblastoid cell lines. In the study, apoptosis was not observed in nontransformed 3T3 fibroblasts or terminally differentiated bone marrow-derived macrophages, suggesting that proapoptotic effects of ritonavir may primarily affect cancerous cells (Gaedicke et al., 2002).
Heme oxygenase-1 (HO-1), also called heat-shock protein-32, is an enzyme that is critically involved in responses toward diverse types of cellular stress (Immenschuh & Ramadori, 2000). We have recently reported on induction of HO-1 in DLD-1 colon carcinoma cells by nitric oxide and by the activator protein-1 (AP-1)-inducing agent pyrrolidine dithiocarbamate (PDTC) (Hellmuth et al., 2002). Induction of HO-1 via this PDTC-inducible pathway has been recently demonstrated in vivo in a rat model (Hata et al., 2003). Protective functions of HO-1 are evident in models of diseases that are associated with immunoactivation such as experimental autoimmune encephalomyelitis (Liu et al., 2001) and experimental colitis (Wang et al., 2001). The beneficial role of HO-1 observed in these disorders may be mediated by anti-inflammatory actions of this enzyme and its reaction products, biliverdin/bilirubin and carbon monoxide. These are illustrated by HO-1-mediated control of TNFα production in macrophages (Otterbein et al., 2000; Lee & Chau, 2002). Interestingly, HO-1 is also inducible by IL-10 (Lee & Chau, 2002) and transforming growth factor-β (Ning et al., 2002), which may contribute to anti-inflammatory properties of these cytokines.
Since anti-inflammatory and proapoptotic functions have been associated with ritonavir and both of these properties potentially affect colon carcinogenesis, cellular actions of this compound on human epithelial-like DLD-1 colon carcinoma cells were investigated.
Methods
Materials
Pure ritonavir (R) was kindly provided by Abbott GmbH (Wiesbaden, Germany). Clasto-Lactacystin, PDTC, SB202190, and SB203580 were purchased by Calbiochem-Novabiochem (Bad Soden, Germany). Sodium butyrate was from Sigma (Deisenhofen, Germany). z-VAD-fmk was purchased from Alexis Biochemicals (Grünberg, Germany).
Cell culture of DLD-1 colon carcinoma cells
Human DLD-1 carcinoma/epithelial cells were obtained from the Centre for Applied Microbiology and Research (Salisbury, U.K.). Cells were maintained in DMEM supplemented with 100 U ml−1 penicillin, 100 μg ml−1 streptomycin, and 10% heat-inactivated FCS (GIBCO-BRL, Eggenstein, Germany). For the experiments, confluent cells grown on polystyrene plates (Greiner, Frickenhausen, Germany) were washed with PBS and incubated with the indicated agents in the aforementioned medium. Unless otherwise indicated, unstimulated control (C) denotes vehicle control that is 0.1% DMSO for ritonavir at 60 μM.
Determination of CK18-Asp396 by ELISA
Cell-free culture supernatants were analyzed for cytokeratin-18 neoantigen CK18-Asp396 content by ELISA using a commercial kit (Alexis Biochemicals, Grünberg, Germany) according to the manufacturer's instructions.
Detection of HO-1, caspase-3, c-Fos, and c-Jun protein by immunoblotting
After stimulation, total cell lysates were prepared using lysis-buffer (300 mM NaCl, 50 mM Tris-HCl (pH 7.6), 0.5% Triton X-100) supplemented with protease inhibitor cocktail (Roche, Mannheim, Germany). Unless otherwise stated, 70 μg of total cellular proteins were separated by 10 or 12% SDS–polyacrylamide gel electrophoresis and transferred onto Immobilon membrane (Millipore, Bedford, U.K.). HO-1 (Stressgene, Hamburg, Germany), caspase-3, c-Fos, and c-Jun (Santa Cruz Biotechnology Inc., Heidelberg, Germany) were detected using rabbit polyclonal antibodies as primary antibodies, horseradish peroxidase labeled secondary antibodies (Biorad, Munich, Germany), and a chemiluminescence detection kit (Amersham Pharmacia Biotech, Freiburg, Germany) according to the manufacturer's instructions.
Determination of mRNA for HO-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) by reverse transcriptase-PCR (RT–PCR)
Total RNA was isolated using Tri-Reagent according to the manufacturer's instructions (Sigma). RNA (1 μg) was used for RT–PCR (GeneAmp RNA PCR kit using Amplitaq Gold, Perkin-Elmer Corp., Weiterstadt, Germany). The following sequences were performed for PCR: 94°C for 10 min (one cycle); 94°C for 45 s, 60°C (GAPDH) or 58°C (HO-1) for 45 s, and 72°C for 60 s (numbers of cyles see below); the final extension was at 72°C for 10 min. Sequences of the primers, numbers of cycles performed, and length of resulting amplicons were as follows: HO-1 (F): 5′-cagcatgccccaggatttg-3′, HO-1 (R): 5′-agctggatgttgagcagga-3′, 29 cycles, 616 bp; GAPDH (F): 5′-accacagtccatgccatcac-3′, GAPDH (R): 5′-tccaccaccctgttgctgta-3′, 23 cycles, 452 bp (Hellmuth et al., 2002). PCR products were run on a 1.5% agarose gel containing 0.5 μg ml−1 ethidium bromide. The identity of amplicons was confirmed by sequencing (310 Genetic Analyzer, Perkin-Elmer Corp.).
RNase protection assay for analysis of HO-1 and GAPDH mRNA accumulation
RNA was isolated using Trizol-Reagent (GIBCO-BRL) according to the manufacturer's instructions. Total RNAs (10 μg) were used for RNase protection assay, performed as described recently (Hellmuth et al., 2002). Briefly, DNA probes were cloned into the transcription vector pBluescript II KS (+) (Stratagene, Heidelberg, Germany). After linearization, an antisense transcript was synthesized in vitro with T3 or T7 RNA polymerase and [α-32P]UTP (800 Ci mmol−1). RNA samples were hybridized at 42°C overnight with 100,000 c.p.m. of the labeled antisense transcript. Hybrids were digested with RNase A and T1 for 1 h at 30°C. Under these conditions, every single mismatch was recognized by the RNases. Protected fragments were separated on 5% (w v−1) polyacrylamide/8 M urea gels and analyzed using a PhosphoImager (Fuji, Straubenhardt, Germany). The individual gene expression of HO-1 was evaluated on the basis of the GAPDH housekeeping gene expression. The cDNA probes were cloned by RT–PCR, and correspond to nucleotides (nt) 148–302 (for GAPDH) and nt 706–949 (for HO-1) of the published sequences (Hellmuth et al., 2002).
Electrophoretic mobility shift assay (EMSA)
Preparation of crude extracts from DLD-1 cells was performed as described previously (Hellmuth et al., 2002). Consensus oligonucleotides used in the binding reactions were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, U.S.A.). Sequences of the double-stranded oligonucleotides used for analysis of specific binding of AP-1 to DNA are as follows: AP-1, wild type 5′-cgc ttg atg act cag ccg gaa-3′; AP-1, mutated 5′-cgc ttg atg act tgg ccg gaa-3′. Complementary oligonucleotides were end labeled by T4 polynucleotide kinase (MBI Fermentas, St Leon-Roth, Germany) using [γ-32P]ATP (3000 Ci mmol−1, Amersham Pharmacia Biotech, Braunschweig, Germany). Binding reactions were performed for 2 h on ice with 7.7 μg of protein in 20 μl of binding buffer containing 4% Ficoll, 20 mM HEPES (pH 7.9), 50 mM KCl, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, 0.25 mg ml−1 BSA, 2 μg of poly(dI–dC), and 10,000 c.p.m. of 32P-labeled oligonucleotide. For AP-1 super-shift analysis, nuclear proteins were preincubated for 20 min at room temperature with a polyclonal anti-c-Jun antibody (Santa Cruz, CA, U.S.A.) before the binding reaction was performed. DNA–protein complexes were separated from unbound oligonucleotide by electrophoresis through a 4% polyacrylamide gel using 0.5 × TBE buffer. Thereafter, gels were fixed and analyzed by PhosphoImager analysis (Fuji). Competition experiments were performed by coincubation with a 100-fold excess (20 pmol) of unlabeled double-stranded oligonucleotide in the DNA–protein binding reaction.
Detection of apoptotic cell death by analysis of cytosolic oligosome-bound DNA
Cytosolic oligonucleosome-bound DNA was quantified using an ELISA according to the manufacturer's instructions (Boehringer Mannheim, Mannheim, Germany). Absorbance values (A405 nm) give a semiquantitative measure of ongoing DNA fragmentation, a common marker for cell death by apoptosis. Data are expressed as an enrichment factor calculated as a ratio of absorbances from treated versus untreated cells.
Quantification of DNA fragmentation by the diphenylamine assay
DNA fragmentation was assayed as described previously (Mühl et al., 1996). Following incubations, cells were centrifuged, resuspended in 250 μl TE buffer (10 mM Tris-HCl, 1 mM EDTA (pH 8.0)), and lysed by adding 250 μl of cold lysis buffer, containing 2 mM EDTA, 0.5% (v v−1) Triton X-100, and 5 mM Tris-HCl (pH 8.0). Samples were allowed to lyse for 30 min on ice prior to centrifugation (15 min, 13,000 r.p.m.) to separate intact chromation (pellet) from DNA fragments (supernatant). Pellets were resuspended in 500 μl TE buffer and the DNA content of pellets and supernatants was determined using the diphenylamine reagent.
Quantification of viable cells via 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) reduction
The viability of DLD-1 cells cultured in 96-well plates was quantified by measuring the amount of soluble formazan produced by cellular reduction of MTS according to the manufacturer's instructions. After 20 h of incubation, 20 μl of CellTiter 96@AQueus One solution-MTS Reagent (Promega, Mannheim, Germany) were added to each well. After 1 h at 37°C, absorbance was measured at 490 nm. In each experiment, increasing cell numbers were used to ascertain the linearity of the assay.
Statistics
For experiments using DLD-1 cells, data are shown as mean absorbance±s.d. or as the percentage of control±s.d. and were analyzed by unpaired Student's t-test on raw data using Sigma Plot (Jandel Scientific, Munich, Germany).
Results
Ritonavir mediates apoptotic cell death of DLD-1 colon carcinoma cells
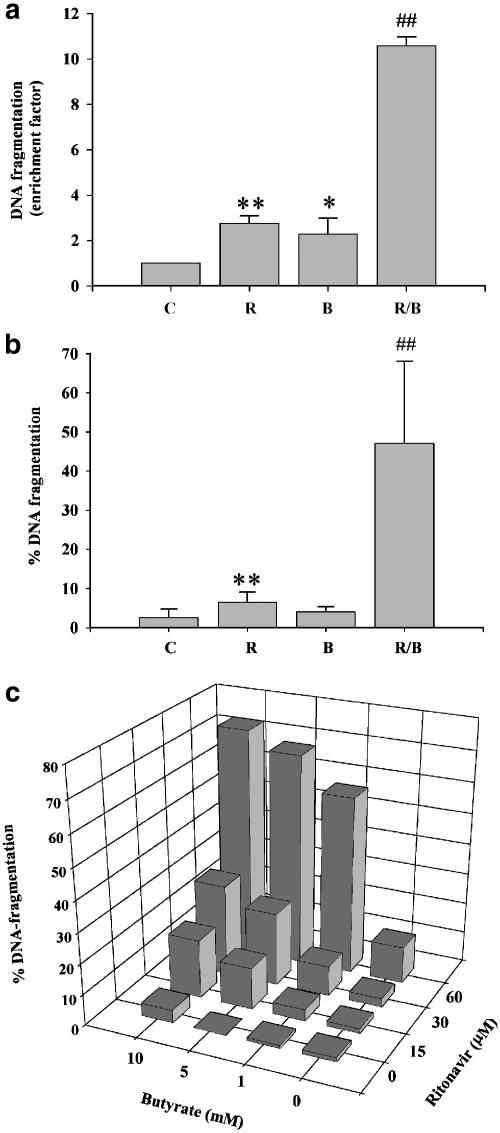
Previous studies revealed that ritonavir induces apoptotic cell death in lymphoblastoid cells (Gaedicke et al., 2002) and in Kaposi sarcoma-derived cell lines (Pati et al., 2002). Therefore, we initially analyzed effects of ritonavir on viability of DLD-1 colon carcinoma cells. As shown in Figure 1a and b, ritonavir alone was able to significantly increase the rate of apoptosis in these cells during a 20 h incubation period. Induction of DNA fragmentation was associated with a reduction of cell viability within the 20 h of incubation to 56.5±8.4% compared to vehicle control (0.1% DMSO) set as 100% (P<0.05; n=4). Ritonavir at 30 μM was the lowest concentration able to induce significant DNA fragmentation in DLD-1 cells (170% for ritonavir versus vehicle control set at 100% (P<0.05; n=4).
Figure 1.
Ritonavir synergizes with butyrate for induction of apoptosis in DLD-1 cells. (a) Analysis of apoptotic DNA fragmentation in DLD-1 cells exposed to ritonavir (R, 60 μM), butyrate (B, 10 mM), or ritonavir plus butyrate (R/B). DLD-1 cells were kept as vehicle control (0.1% DMSO) or were stimulated as indicated. After 20 h, cells were harvested and DNA fragmentation was semiquantitively analyzed by an ELISA based on the detection of cytosolic histone/DNA complexes. Data are shown as mean enrichment factor±s.d. (n=3). **P<0.01 compared to unstimulated controls; *P<0.05 compared to unstimulated controls; ##P<0.01 compared to ritonavir or butyrate alone. (b) Diphenylamine-based analysis of apoptotic DNA fragmentation in DLD-1 cells exposed to ritonavir (R, 60 μM), butyrate (B, 5 mM), or ritonavir plus butyrate (R/B). DLD-1 cells were kept as vehicle control (0.1% DMSO) or stimulated as indicated. DNA fragmentation after 20 h of incubation was quantified using the diphenylamine reaction. Data are shown as the mean percentage of DNA fragmentation±s.d. (n=3). **P<0.01 compared to unstimulated control; ##P<0.01 compared to ritonavir or butyrate alone. (c) Dose–response curves of synergistic induction of apoptosis in DLD-1 cells exposed to combinations of ritonavir plus butyrate. Cells were either kept as vehicle control or exposed to the indicated concentrations of ritonavir and/or butyrate. After 20 h of stimulation, DNA fragmentation was quantified using the diphenylamine reaction. Data are shown as the percentage of DNA fragmentation. One representative of two independently performed experimental series is shown.
Butyrate is a short-chain fatty acid that is produced by intestinal bacteria and is supposed to be an important regulator of colonic epithelial cell biology (Wächtershauser & Stein, 2000). Since butyrate is known to sensitize colon carcinoma cells for induction of apoptosis (Bonnotte et al., 1998; Luhrs et al., 2002), DLD-1 cells were treated with the combination ritonavir plus butyrate. These experiments revealed a strong synergism between ritonavir and butyrate with regard to induction of apoptotic cell death (Figure 1a and b). Under these conditions and after a 20 h incubation period, viability was significantly reduced to 31.3±8.0% compared to vehicle control (0.1% DMSO) set at 100% (ritonavir at 60 μM, butyrate at 10 mM; P<0.05; n=4). Detailed dose–response curves demonstrating synergistic induction of apoptosis by ritonavir plus butyrate in DLD-1 cells are shown in Figure 1c.
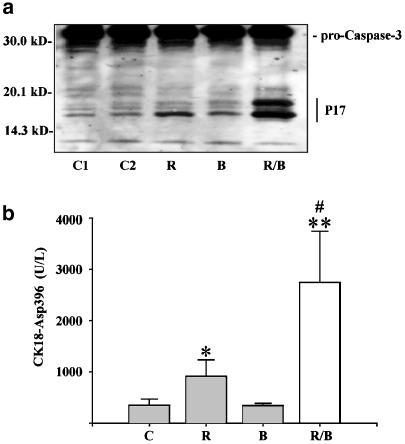
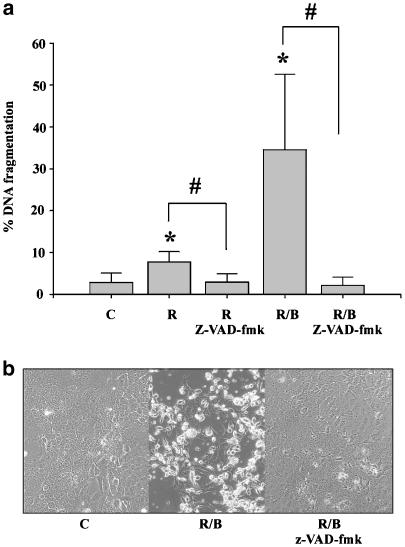
Induction of apoptosis in DLD-1 cells was associated with processing of procaspase-3 and generation of the large p17 subunit of the active protease (Figure 2a). Moreover, enhanced caspase activity was detected under the influence of ritonavir, particularly in combination with butyrate (Figure 2b). In these experiments, upregulation of caspase activity was quantified by detection of a caspase-specific cytokeratin-18 neoantigen (CK18-Asp396) that is released from apoptotic cells (Biven et al., 2003). Butyrate alone did not enhance caspase activity in DLD-1 cells. By use of the general caspase inhibitor z-VAD-fmk, ritonavir/butyrate-induced apoptosis was determined to be strongly dependent on cellular caspase activity (Figure 3a and b).
Figure 2.
Activation of caspase-3 by ritonavir plus butyrate in DLD-1 cells. (a) DLD-1 cells were kept as unstimulated control (C1), as vehicle control (C2, 0.1% DMSO), or were stimulated with ritonavir (R, 60 μM), butyrate (B, 5 mM), or ritonavir plus butyrate (R/B). After 20 h of stimulation, DLD-1 cells were harvested and homogenates were assayed for caspase-3 protein by immunoblot analysis. One representative blot of two independently performed experiments is shown. Total protein (100 μg) was applied per lane. (b) After 20 h of stimulation, cell-free culture supernatants were assayed for the presence of the cytokeratin-18 neoantigen CK18-Asp396 by ELISA. Data are shown as mean CK18-Asp396 concentrations±s.d. (n=4). Vehicle control (0.1% DMSO). *P<0.05 compared to unstimulated control; **P<0.01 compared to unstimulated control; #P<0.05 compared to ritonavir alone.
Figure 3.
Induction of apoptosis by ritonavir plus butyrate in DLD-1 cells is potently suppressed by coincubation with z-VAD-fmk. (a) DLD-1 cells were either kept as unstimulated control (C, 0.1% DMSO), or were stimulated with ritonavir (R, 60 μM), with ritonavir plus z-VAD-fmk (30 μM), with ritonavir/butyrate (B, 5 mM), or with with ritonavir/butyrate plus z-VAD-fmk. After 20 h, DNA fragmentation was quantified using the diphenylamine reaction. Data are shown as the mean percentage of DNA fragmentation±s.d. (n=4). *P<0.05 compared to unstimulated control; #P<0.05 compared to ritonavir or ritonavir/butyrate in the absence of z-VAD-fmk. (b) Protective effects of z-VAD-fmk in the same experimental setting as shown by light microscopy.
Ritonavir induces expression of HO-1 in DLD-1 colon carcinoma cells
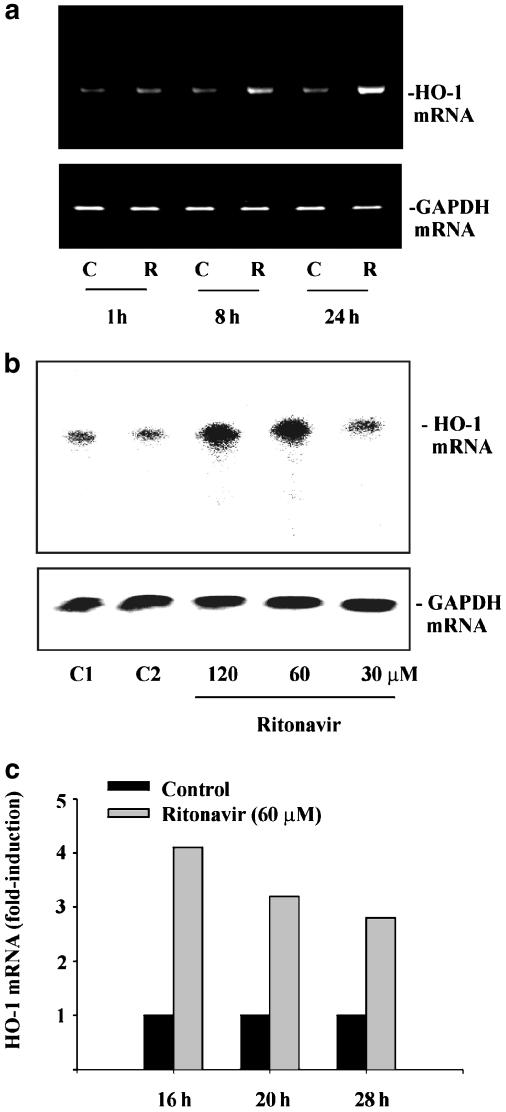
Since ritonavir exhibits anti-inflammatory properties, we investigated expression of HO-1 in DLD-1 cells exposed to ritonavir. RT–PCR analysis and RNase protection assay (Figure 4a–c) revealed that ritonavir increases steady-state levels of HO-1 mRNA. Ritonavir-induced HO-1 mRNA translated into enhanced expression of HO-1 protein as detected by immunoblot analysis (Figure 5a and b). To investigate whether inhibition of proteasome function may mediate HO-1 expression in DLD-1 cells, these were incubated with clasto-Lactacystin or sodium butyrate. Both agents have been associated with inhibition of proteasome activity (Yin et al., 2001; Almond & Cohen, 2002). As shown in Figure 6, clasto-Lactacystin (Figure 6a) and sodium butyrate (Figure 6b) both mediated HO-1 expression. Combined treatment with ritonavir plus sodium butyrate resulted in efficient induction of apoptotic cell death (Figure 1). Compared to unstimulated control, this condition was likewise associated with enhanced levels of HO-1 protein (Figure 6c). In some experiments, the combination of ritonavir plus butyrate resulted in additive effects with regard to HO-1 expression. However, this observation was not consistent within the set of experiments performed, which likely is due to massive cell death under these conditions (data not shown). Previous data revealed that the p38 mitogen-activated protein (MAP) kinase pathway can mediate expression of HO-1 in response to several stimuli, for example, nitric oxide (Chen & Maines, 2000). In accordance with these reports, we observed that coincubation with the p38 MAP kinase inhibitors SB203580 (Figure 7a) or SB202190 (Figure 7c) suppressed ritonavir-induced HO-1. In contrast, blockage of p38 MAP kinase activity did not significantly affect apoptosis of DLD-1 cells in response to ritonavir or ritonavir plus butyrate (Figure 7b). Moreover, inhibition of apoptosis by coincubation with z-VAD-fmk did not significantly modulate expression of HO-1 in DLD-1 cells exposed to ritonavir plus butyrate (n=3; data not shown).
Figure 4.
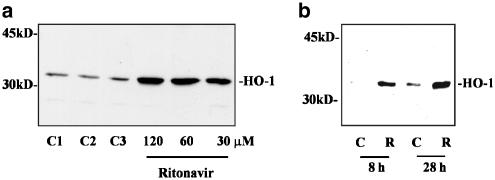
Ritonavir induces HO-1 mRNA in DLD-1 cells. (a) DLD-1 cells were either kept as unstimulated control (C, 0.1% DMSO) or were stimulated with ritonavir at 60 μM (R). After the indicated time periods, cells were harvested, total RNA was isolated, and mRNA expression of HO-1 and GAPDH was determined by semiquantitative RT–PCR analysis. One representative of three independent experiments evaluating HO-1 expression by RT–PCR is shown. (b and c) Ritonavir-induced HO-1 mRNA induction was assessed by RNase protection assay. DLD-1 cells were stimulated in three independent experiments with the indicated concentrations of ritonavir for either 16, 20, or 28 h. One representative of these RNase protection assays is shown in (b); cells were stimulated for 20 h. (c) The degree of HO-1 mRNA induction by ritonavir (60 μM) relative to GAPDH mRNA was quantified by PhosphoImager (Fuji) analysis of the radiolabeled gels. C1 (0.1% DMSO) and C2 (0.2% DMSO) represent vehicle control for ritonavir at 60 and 120 μM, respectively.
Figure 5.
Ritonavir induces expression of HO-1 protein. (a) Dose–response curve of ritonavir-induced HO-1 protein. DLD-1 cells were kept as unstimulated control or were stimulated for 16 h with the indicated concentrations of ritonavir. Thereafter, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. C1 denotes medium alone; C2 (0.1% DMSO) and C3 (0.2% DMSO) represent vehicle control for ritonavir at 60 and 120 μM, respectively. One representative of four independently performed experiments is shown. (b) Kinetic analysis of ritonavir-induced HO-1 protein. DLD-1 cells were stimulated with ritonavir (R, 60 μM) for the indicated periods of time. Thereafter, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. One representative of three independently performed kinetics is shown.
Figure 6.
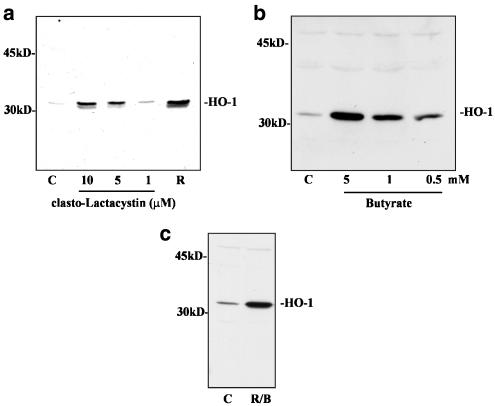
Induction of HO-1 by clasto-Lactacystin, butyrate, and ritonavir plus butyrate. (a) DLD-1 cells were kept as unstimulated vehicle control (C, 0.1% DMSO) or were stimulated with the indicated concentrations of clasto-Lactacystin or with ritonavir (R) at 60 μM. After 24 h, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. (b) DLD-1 cells were kept as unstimulated control (C, without DMSO) or were stimulated with the indicated concentrations of sodium butyrate (B). After 24 h, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. One representative of four independent experiments is shown. (c) DLD-1 cells were kept as unstimulated control (C, 0.1% DMSO) or were stimulated with sodium butyrate (B, 10 mM) plus ritonavir (R, 60 μM). After 20 h, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. One representative of five independent experiments is shown.
Figure 7.
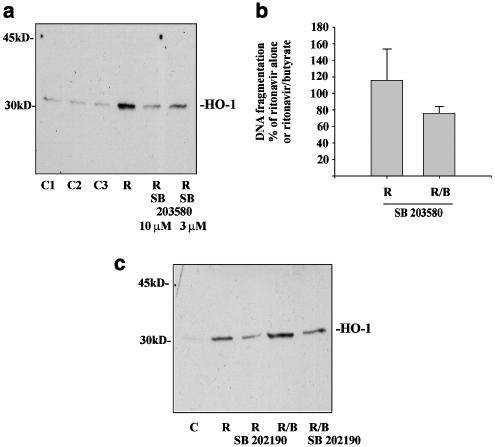
Ritonavir-induced expression of HO-1 is inhibited by SB203580 (a) or SB202190 (c). (a) DLD-1 cells were kept as unstimulated control or were stimulated with ritonavir (R, 60 μM) alone or in combination with the indicated concentrations of SB203580. After 20 h, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. C1 denotes medium alone; C2 (0.1% DMSO) and C3 (0.2% DMSO) representing vehicle control for ritonavir and ritonavir plus SB203580 (10 μM), respectively. One representative of four independent experiments is shown. (b) DLD-1 cells were stimulated with ritonavir (R, 60 μM) or with ritonavir plus butyrate (B, 5 mM) in the presence or absence of SB203580 (10 μM). After 20 h of stimulation, DNA fragmentation was quantified using the diphenylamine reaction. Data are shown as mean DNA fragmentation (percentage of ritonavir alone or ritonavir/butyrate alone) ±s.d. (n=3). (c) DLD-1 cells were kept as unstimulated vehicle control or stimulated with ritonavir (R, 60 μM), or ritonavir/butyrate (B, 5 mM) in the presence or absence of SB202190 (20 μM). After 20 h of stimulation, cells were harvested and homogenates were assayed for HO-1 protein expression by immunoblot analysis. One representative of four independently performed experiments is shown.
Ritonavir activates the AP-1 signaling pathway in DLD-1 colon carcinoma cells
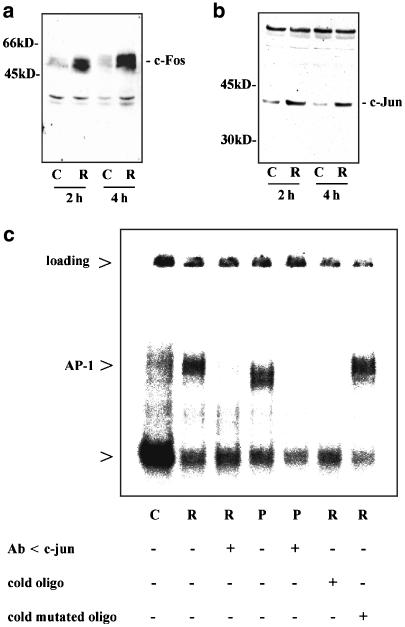
Since AP-1 is a key regulator of HO-1 transcription (Alam & Den, 1992), and basal expression of c-Fos and c-Jun can be augmented by appropriate stimuli in colon carcinoma cells (Wilson et al., 1999; Yao & O'Dwyer, 2003), we investigated the effects of ritonavir on the AP-1 system. Immunoblot analysis of total cell lysates revealed that within 2 h of incubation expression of both c-Jun and c-Fos is upregulated in DLD-1 cells exposed to ritonavir (Figure 8a and b). Activation of the AP-1 pathway was furthermore investigated by EMSA analysis. Compared to background AP-1 binding activity in unstimulated cells, we observed enhanced specific binding of AP-1 to the respective wild-type oligonucleotides when nuclear extracts of DLD-1 cells were analyzed that had been exposed to ritonavir (Figure 8c). Appearance of the shifted band was prevented by coincubation with an anti-c-Jun antibody. In contrast, a constitutive band, which was apparent on the EMSA gel was not diminished when the anti-c-Jun antibody was added to nuclear extracts derived from ritonavir-activated DLD-1 cells (lane 2 versus lane 3). The band that was induced by ritonavir comigrated with a signal that was inducible by PDTC, a known activator of AP-1 in various cell types, among them DLD-1 cells (Hellmuth et al., 2002). Appearance of this PDTC-inducible complex was likewise prevented by cotreatment with the anti-c-Jun antibody (lane 4 versus lane 5). The specificity of the ritonavir-induced complex was furthermore characterized by the observation that the signal could be quenched by excess unlabeled wild-type oligonucleotides but not by excess unlabeled mutated oligonucleotides (lane 2 versus lanes 6 and 7). In contrast, the constitutive band was not modulated by coincubation with excess unlabeled wild-type oligonucleotides (lane 2 versus lane 6).
Figure 8.
Ritonavir activates the AP-1 signaling pathway in DLD-1 cells. (a and b) ritonavir induces expression of c-Fos and c-Jun in DLD-1 cells. DLD-1 cells were kept as unstimulated control (C, 0.1% DMSO) or were stimulated with ritonavir (R, 60 μM). After the indicated time periods, cells were harvested and homogenates (140 μg total protein/lane) were assayed for c-Fos (a) or c-Jun (b) protein expression by immunoblot analysis. One representative of three independent experiments performed analyzing ritonavir-induced c-Fos and c-Jun expression is shown. (c) Detection of ritonavir-induced AP-1 DNA binding activity in DLD-1 cells by EMSA analysis. DLD-1 cells were incubated as unstimulated control (C, 0.1% DMSO), or stimulated with ritonavir (R, 60 μM), or with PDTC (PDTC, 200 μM) for 2 h. Thereafter, nuclear extracts were prepared and EMSA analysis was performed using 32P-labeled AP-1 consensus oligonucleotide. As indicated, experiments were performed in the presence or absence of an anti-c-Jun antibody, or of excess cold wild-type or mutated oligonucleotide.
Discussion
HO-1 is an anti-inflammatory enzyme, the deficiency of which is associated with inflammation in mice and humans (Poss & Tonegawa, 1997; Yachie et al., 1999). Upregulation of HO-1 in intestinal tissues is associated with reduction of proinflammatory parameters such as TNFα and inducible nitric oxide synthase and is protective in models of experimental colitis (Wang et al., 2001; Fujii et al., 2003). Accordingly, augmentation of HO-1 expression by pharmacological means is likely a promising therapeutic strategy for the treatment of inflammatory bowel diseases. Since colonic inflammation can be regarded a precancerogenic condition, HO-1 induction may have the potential to interfere with the process of colon carcinogenesis at an early stage. Here, we report that exposure to ritonavir mediates expression of HO-1 in DLD-1 colon carcinoma cells. Induction by ritonavir was apparent at concentrations of 30 μM or higher. Common treatment regimes for ritonavir in HIV therapy consist of 600 mg orally twice a day and peak concentrations in sera of patients can reach up to 46 μM (Gaedicke et al., 2002). Thus, it is reasonable to assume that local concentrations of ritonavir, particularly at the colonic epithelium, may actually reach higher levels. Upregulation of HO-1 in DLD-1 cells by clasto-Lactacystin suggests that the action of ritonavir described herein may be related to modulation of cellular proteasome activity. Similarly, HO-1 was inducible by butyrate, which also has been shown to diminish proteasome activity in colon carcinoma cells (Yin et al., 2001). In accordance with these observations, it has recently been described that the proteasome inhibitor MG132 mediates gene induction of HO-1 in rat cardiomyocytes (Lüss et al., 2002). Suppression of HO-1 induction by coincubation with SB203580 or SB202190 suggests that activation of the p38 MAP kinase is an essential component of the signal transduction pathway leading to ritonavir-induced HO-1. Interestingly, MG132-induced HO-1 in cardiomyocytes is similarly dependent on activation of p38 MAP kinase. Indeed, inhibition of proteasome function can mediate activation of this kinase (Lüss et al., 2002), again implying that modulation of proteasomal activity may be related to ritonavir-induced HO-1.
The transcription factor AP-1 is a major trigger for gene expression of HO-1 (Alam & Den, 1992). Therefore, the effects of ritonavir on the AP-1 signaling system were investigated. Here, we demonstrate that ritonavir can efficiently augment cellular levels of c-Fos and c-Jun protein. Furthermore, EMSA analysis revealed upregulation of AP-1-specific binding to the corresponding oligonucleotides, indicative of activation of the AP-1 signal transduction machinery under the influence of ritonavir. In the present study, we did not further investigate molecular mechanisms of HO-1 induction by butyrate. However, it is of interest to note that a previous report demonstrates activation of AP-1-dependent gene transcription in colon carcinoma cells exposed to this short-chain fatty acid (Glinghammar et al., 1999). Altogether, present and previous data suggest that ritonavir, like butyrate, has the capacity to inhibit cellular proteasome function and to activate AP-1. Expression of HO-1 appears to be a consequence of this profile of activation and may mediate protective functions (Liu et al., 2001) of ritonavir in chronic inflammation as seen in experimental autoimmune encephalomyelitis (Hosseini et al., 2001).
Induction of apoptotic cell death in cancerous cells is a fundamental principle in anticancer pharmacotherapy. In fact, colon carcinogenesis is associated with reduction of tumor cell sensitivity toward apoptosis (Bedi et al., 1995). Here, we report that exposure of DLD-1 cells to ritonavir resulted in augmented cell death and apoptotic DNA fragmentation. Induction of apoptosis by ritonavir has been described before in Kaposi sarcoma-derived cells, lymphoblastoid cells, and multiple myeloma cells (Gaedicke et al., 2002; Pati et al., 2002; Ikezoe et al., 2004) and may likewise be related to modulation of proteasome activity (Almond & Cohen, 2002). To further evaluate its potential as an inducer of cell death, DLD-1 cells were coincubated with the combination ritonavir plus sodium butyrate. Butyrate is a short-chain fatty acid, which is primarily provided by intestinal bacteria during fermentation of dietary fiber. Cellular actions of butyrate are diverse and include inhibition of histone deacetylase (Archer & Hodin, 1999; Ishihara et al., 2004), inhibition of proteasome activity (Yin et al., 2001), suppression of hypoxia-inducible factor-1α bioactivity (Zgouras et al., 2003), and activation of AP-1 (Glinghammar et al., 1999). In the context of colorectal cancer, it is of particular interest that butyrate is able to enhance the rate of apoptosis in colon carcinoma cells exposed to the Fas ligand (Bonnotte et al., 1998) or to TNFα (Luhrs et al., 2002). Moreover, local bioactivity of the supposedly tumor suppressive cytokine IL-18 may be augmented by butyrate (Paulukat et al., 2001; Kalina et al., 2002). A low-fiber diet has been considered a risk factor for colorectal cancer (Burkitt, 1971). Actually, dietary supplementation of fiber or application of butyrate enemas can suppress colon carcinogenesis in rat models of disease (D'Argenio et al., 1996). Here, we demonstrate that butyrate (⩾1 mM) potently enhanced ritonavir-induced cell death. Notably, peak concentrations of butyrate in the human colon can reach up to 20 mM (Wächtershauser & Stein, 2000). Cell death was associated with activation of caspase-3 and with accumulation of the caspase-dependent neoantigen CK18-Asp396. Furthermore, cell death was potently suppressed by the general caspase inhibitor z-VAD-fmk. These observations further confirm that apoptosis is the prime mode of cell death that is responsible for loss of viability in DLD-1 cell cultures exposed to ritonavir or ritonavir plus butyrate. In accordence with the present data, it has been shown that butyrate synergizes with the proteasome inhibitors MG132 (Giuliano et al., 1999) or bortezomib (Pei et al., 2004) for induction of apoptosis in human Y79 retinoblastoma cells or multiple myeloma cells, respectively. Experiments with z-VAD-fmk also revealed that apoptotic cell death is not the driving force behind HO-1 expression in response to ritonavir plus butyrate. Taking into account our aforementioned data concerning the effects of p38 MAP kinase inhibitors on HO-1 expression and apoptosis, we propose that HO-1 induction and apoptosis are parallel but independent cellular responses toward ritonavir or ritonavir plus butyrate. Altogether, the present observations suggest that increasing the colonic butyrate content by high-fiber diet or by butyrate enemas may dramatically potentiate proapoptotic actions of ritonavir on carcinoma cells in patients with colorectal cancer.
Animal models demonstrate that anti-inflammatory properties of HO-1 can suppress leukocytic infiltration into immunologically active tissues (Ishikawa et al., 1997; Poss and Tonegawa, 1997; Busssolati et al., 2004). Leukocytic infiltration into tumor tissues consists mainly of macrophages and can make up to 50% of the tumor biomass. Since angiogenesis and poor prognosis correlate with the degree of macrophage infiltration in several human malignancies (Bingle et al., 2002) including colorectal cancer (Etoh et al., 2000), induction of HO-1 by ritonavir appears to be of advantage and agrees with tumor suppressive properties of this drug. Moreover, HO-1 activity has been shown to reduce production of proinflammatory factors associated with growth and progression of colon cancer, such as IL-8 (Fox et al., 1998; Brew et al., 2000; Bulger et al., 2003) and NO (Cavicchi et al., 2000; Lagares-Garcia et al., 2001; Wang et al., 2001). Current data suggest an anticancer potential of ritonavir. In fact, ritonavir showed protective effects in a murine model of Kaposi sarcoma (Pati et al., 2002) and in a thymoma model (Gaedicke et al., 2002). The potent synergism between ritonavir and butyrate with regard to induction of colon carcinoma cell death by apoptosis may indicate the potential of ritonavir for the treatment of colorectal cancer.
Acknowledgments
This work was supported by a grant from the August Scheidel Stiftung to H.M.
Abbreviations
- AP-1
activator protein-1
- EMSA
electrophoretic mobility shift assay
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- HIV
human immunodeficiency virus
- HO-1
heme oxygenase-1
- IL
interleukin
- MAP
mitogen-activated protein
- NF-κB
nuclear factor-κB
- TNFα
tumor necrosis factor-α
References
- ALAM J., DEN Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J. Biol. Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- ALMOND J.B., COHEN G.M. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433–443. doi: 10.1038/sj.leu.2402417. [DOI] [PubMed] [Google Scholar]
- ANDRE P., GROETTRUP M., KLENERMAN P., DE GIULI R., BOOTH B.L., JR, CERUNDOLO V., BONNEVILLE M., JOTEREAU F., ZINKERNAGEL R.M., LOTTEAU V. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc. Natl. Acad. Sci. U.S.A. 1998;95:13120–13124. doi: 10.1073/pnas.95.22.13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ARCHER S.Y., HODIN R.A. Histone acetylation and cancer. Curr. Opin. Genet. Dev. 1999;9:171–174. doi: 10.1016/s0959-437x(99)80026-4. [DOI] [PubMed] [Google Scholar]
- ARDITE E., PANES J., MIRANDA M., SALAS A., ELIZALDE J.I., SANS M., ARCE Y., BORDAS J.M., FERNANDEZ-CHECA J.C., PIQUE J.M. Effects of steroid treatment on activation of nuclear factor kappaB in patients with inflammatory bowel disease. Br. J. Pharmacol. 1998;124:431–433. doi: 10.1038/sj.bjp.0701887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEDI A., PASRICHA P.J., AKHTAR A.J., BARBER J.P., BEDI G.C., GIARDIELLO F.M., ZEHNBAUER B.A., HAMILTON S.R., JONES R.J. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–1816. [PubMed] [Google Scholar]
- BINGLE L., BROWN N.J., LEWIS C.E. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J. Pathol. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- BIVEN K., ERDAL H., HAGG M., UENO T., ZHOU R., LYNCH M., ROWLEY B., WOOD J., ZHANG C., TOI M., SHOSHAN M.C., LINDER S. A novel assay for discovery and characterization of pro-apoptotic drugs and for monitoring apoptosis in patient sera. Apoptosis. 2003;8:263–268. doi: 10.1023/a:1023672805949. [DOI] [PubMed] [Google Scholar]
- BONNOTTE B., FAVRE N., REVENEAU S., MICHEAU O., DROIN N., GARRIDO C., FONTANA A., CHAUFFERT B., SOLARY E., MARTIN F. Cancer cell sensitization to fas-mediated apoptosis by sodium butyrate. Cell Death Differ. 1998;5:480–487. doi: 10.1038/sj.cdd.4400371. [DOI] [PubMed] [Google Scholar]
- BREW R., ERIKSON J.S., WEST D.C., KINSELLA A.R., SLAVIN J., CHRISTMAS S.E. Interleukin-8 as an autocrine growth factor for human colon carcinoma cells in vitro. Cytokine. 2000;12:78–85. doi: 10.1006/cyto.1999.0518. [DOI] [PubMed] [Google Scholar]
- BURKITT D.P. Epidemiology of cancer of the colon and rectum. Cancer. 1971;28:3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- BULGER E.M., GARCIA I., MAIER R.V. Induction of heme-oxygenase-1 inhibits endothelial cell activation by endotoxin and oxidant stress. Surgery. 2003;134:146–152. doi: 10.1067/msy.2003.215. [DOI] [PubMed] [Google Scholar]
- BUSSSOLATI B., AHMED A., PEMBERTON H., LANDIS R.C., DI CARLO F., HASKARD D.O., MASON J.C. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- CAVICCHI M., GIBBS L., WHITTLE B.J. Inhibition of inducible nitric oxide synthase in the human intestinal epithelial cell line, DLD-1, by the inducers of heme oxygenase 1, bismuth salts, heme, and nitric oxide donors. Gut. 2000;47:771–778. doi: 10.1136/gut.47.6.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN K., MAINES M.D. Nitric oxide induces heme oxygenase-1 via mitogen-activated protein kinases ERK and p38. Cell. Mol. Biol. 2000;46:609–617. [PubMed] [Google Scholar]
- D'ARGENIO G., COSENZA V., DELLE CAVE M., IOVINO P., DELLE VALLE N., LOMBARDI G., MAZZACCA G. Butyrate enemas in experimental colitis and protection against large bowel cancer in a rat model. Gastroenterology. 1996;110:1727–1734. doi: 10.1053/gast.1996.v110.pm8964397. [DOI] [PubMed] [Google Scholar]
- ETOH T., SHIBUTA K., BARNARD G.F., KITANO S., MORI M. Angiogenin expression in human colorectal cancer: the role of focal macrophage infiltration. Clin. Cancer Res. 2000;6:3545–3551. [PubMed] [Google Scholar]
- FOX S.H., WHALEN G.F., SANDERS M.M., BURLESON J.A., JENNINGS K., KURTZMAN S., KREUTZER D. Angiogenesis in normal tissue adjacent to colon cancer. J. Surg. Oncol. 1998;69:230–234. doi: 10.1002/(sici)1096-9098(199812)69:4<230::aid-jso7>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- FUJII H., TAKAHASHI T., NAKAHIRA K., UEHARA K., SHIMIZU H., MATSUMI M., MORITA K., HIRAKAWA M., AKAGI R., SASSA S. Protective role of heme oxygenase-1 in the intestinal tissue injury in an experimental model of sepsis. Crit. Care Med. 2003;31:893–902. doi: 10.1097/01.CCM.0000050442.54044.06. [DOI] [PubMed] [Google Scholar]
- GAEDICKE S., FIRAT-GEIER E., CONSTANTINIU O., LUCCHIARI-HARTZ M., FREUDENBERG M., GALANOS C., NIEDERMANN G. Antitumour effect of the human immunodeficiency virus protease inhibitor ritonavir: induction of tumour-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002;62:6901–6908. [PubMed] [Google Scholar]
- GIULIANO M., LAURICELLA M., CALVARUSO G., CARABILLO M., EMANUELE S., VENTO R., TESORIERE G. The apoptotic effects and synergistic interaction of sodium butyrate and MG132 in human retinoblastoma Y79 cells. Cancer Res. 1999;59:5586–5595. [PubMed] [Google Scholar]
- GLINGHAMMAR B., HOLMBERG K., RAFTER J. Effects of colonic lumenal components on AP-1-dependent gene transcription in cultured human colon carcinoma cells. Carcinogenesis. 1999;20:969–976. doi: 10.1093/carcin/20.6.969. [DOI] [PubMed] [Google Scholar]
- HATA K., YAMAMOTO Y., NAKAJIMA A., TAURA K., YONEZAWA K., UCHINAMI H., IKEDA F., YAMAOKA Y. Induction of heme oxygenase-1 and dilatation of hepatic sinusoids by an administration of pyrrolidine dithiocarbamate in rat livers. J. Surg. Res. 2003;115:310–317. doi: 10.1016/j.jss.2003.08.240. [DOI] [PubMed] [Google Scholar]
- HELLMUTH M., WETZLER C., NOLD M., CHANG J.H., FRANK S., PFEILSCHIFTER J., MÜHL H. Expression of interleukin-8, heme oxygenase-1 and vascular endothelial growth factor in DLD-1 colon carcinoma cells exposed to pyrrolidine dithiocarbamate. Carcinogenesis. 2002;23:1273–1279. doi: 10.1093/carcin/23.8.1273. [DOI] [PubMed] [Google Scholar]
- HOSSEINI H., ANDRE P., LEFEVRE N., VIALA L., WALZER T., PESCHANSKI M., LOTTEAU V. Protection against experimental autoimmune encephalomyelitis by a proteasome modulator. J. Neuroimmunol. 2001;118:233–244. doi: 10.1016/s0165-5728(01)00352-6. [DOI] [PubMed] [Google Scholar]
- IKEZOE T., SAITO T., BANDOBASHI K., YANG Y., KOEFFLER H.P., TAGUCHI H. HIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2. Mol. Cancer Ther. 2004;4:473–479. [PubMed] [Google Scholar]
- IMMENSCHUH S., RAMADORI G. Gene regulation of heme oxygenase-1 as a therapeutic target. Biochem. Pharmacol. 2000;60:1121–1128. doi: 10.1016/s0006-2952(00)00443-3. [DOI] [PubMed] [Google Scholar]
- ISHIHARA K., HONG J., ZEE O., OHUCHI K. Possible mechanism of action of the histone deacetylase inhibitors for the induction of differentiation of HL-60 clone 15 cells into eosinophils. Br. J. Pharmacol. 2004;142:1020–1030. doi: 10.1038/sj.bjp.0705869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIKAWA K., NAVAB M., LEITINGER N., FOGELMAN A.M., LUSIS A.J. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J. Clin. Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALINA U., KOYAMA N., HOSODA T., NUERNBERGER H., SATO K., HOELZER D., HERWECK F., MANIGOLD T., SINGER M.V., ROSSOL S., BOCKER U. Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur. J. Immunol. 2002;32:2635–2643. doi: 10.1002/1521-4141(200209)32:9<2635::AID-IMMU2635>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- LAGARES-GARCIA J.A., MOORE R.A., COLLIER B., HEGGERE M., DIAZ F., QIAN F. Nitric oxide synthase as a marker in colorectal carcinoma. Am. Surg. 2001;67:709–713. [PubMed] [Google Scholar]
- LEE T.S., CHAU L.Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- LIU Y., ZHU B., LUO L., LI P., PATY D.W., CYNADER M.S. Heme oxygenase-1 plays an important protective role in experimental autoimmune encephalomyelitis. Neuroreport. 2001;12:1841–1845. doi: 10.1097/00001756-200107030-00016. [DOI] [PubMed] [Google Scholar]
- LUHRS H., KUDLICH T., NEUMANN M., SCHAUBER J., MELCHER R., GOSTNER A., SCHEPPACH W., MENZEL T.P. Butyrate-enhanced TNFα-induced apoptosis is associated with inhibition of NF-κB. Anticancer Res. 2002;22:1561–1568. [PubMed] [Google Scholar]
- LÜSS H., SCHMITZ W., NEUMANN J. A proteasome inhibitor confers cardioprotection. Cardiovasc. Res. 2002;54:140–151. doi: 10.1016/s0008-6363(02)00232-8. [DOI] [PubMed] [Google Scholar]
- MÜHL H., SANDAU K., BRÜNE B., BRINER V.A., PFEILSCHIFTER J. Nitric oxide donors induce apoptosis in glomerular mesangial cells, epithelial cells and endothelial cells. Eur. J. Pharmacol. 1996;317:137–149. doi: 10.1016/s0014-2999(96)00701-7. [DOI] [PubMed] [Google Scholar]
- NING W., SONG R., LI C., PARK E., MOHSENIN A., CHOI A.M., CHOI M.E. TGF-β1 stimulates HO-1 via the p38 mitogen-activated protein kinase in A549 pulmonary epithelial cells. Am. J. Physiol. 2002;283:L1094–L1102. doi: 10.1152/ajplung.00151.2002. [DOI] [PubMed] [Google Scholar]
- OTTERBEIN L.E., BACH F.H., ALAM J., SOARES M., TAO LU H., WYSK M., DAVIS R.J., FLAVELL R.A., CHOI A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- PATI S., PELSER C.B., DUFRAINE J., BRYANT J.L., REITZ M.S., JR, WEICHOLD F.F. Antitumourigenic effects of HIV protease inhibitor ritonavir: inhibition of Kaposi sarcoma. Blood. 2002;99:3771–3779. doi: 10.1182/blood.v99.10.3771. [DOI] [PubMed] [Google Scholar]
- PAULUKAT J., BOSMANN M., NOLD M., GARKISCH S., KÄMPFER H., FRANK S., RAEDLE J., ZEUZEM S., PFEILSCHIFTER J., MÜHL H. Expression and release of IL-18 binding protein in response to IFN-γ. J. Immunol. 2001;167:7038–7043. doi: 10.4049/jimmunol.167.12.7038. [DOI] [PubMed] [Google Scholar]
- PEI X.Y., DAI Y., GRANT S. Synergistic induction of oxidative injury and apoptosis in human multiple myeloma cells by the proteasome inhibitor bortezomib and histone deacetylase inhibitors. Clin. Cancer Res. 2004;10:3839–3852. doi: 10.1158/1078-0432.CCR-03-0561. [DOI] [PubMed] [Google Scholar]
- POSS K.D., TONEGAWA S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc. Natl. Acad. Sci. U.S.A. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WÄCHTERSHAUSER A., STEIN J. Rationale for the luminal provision of butyrate in intestinal diseases. Eur. J. Nutr. 2000;39:164–171. doi: 10.1007/s003940070020. [DOI] [PubMed] [Google Scholar]
- WANG W.P., GUO X., KOO M.W., WONG B.C., LAM S.K., YE Y.N., CHO C.H. Protective role of heme oxygenase-1 on trinitrobenzene sulfonic acid-induced colitis in rats. Am. J. Physiol. 2001;281:G586–G594. doi: 10.1152/ajpgi.2001.281.2.G586. [DOI] [PubMed] [Google Scholar]
- WILSON L., SZABO C., SALZMAN A.L. Protein kinase C-dependent activation of NF-κB in enterocytes is independent of IkappaB degradation. Gastroenterology. 1999;117:106–114. doi: 10.1016/s0016-5085(99)70556-1. [DOI] [PubMed] [Google Scholar]
- YACHIE A., NIIDA Y., WADA T., IGARASHI N., KANEDA H., TOMA T., OHTA K., KASAHARA Y., KOIZUMI S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J. Clin. Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YAO K.-S., O'DWYER P.J. Role of the AP-1 element and redox factor-1 (Ref-1) in mediating transcriptional induction of DT-diaphorase gene expression by oltipraz: a target for chemoprevention. Biochem. Pharmacol. 2003;66:15–23. doi: 10.1016/s0006-2952(03)00163-1. [DOI] [PubMed] [Google Scholar]
- YIN L., LAEVSKY G., GIARDINA C. Butyrate suppression of colonocyte NF-κB activation and cellular proteasome activity. J. Biol. Chem. 2001;276:44641–44646. doi: 10.1074/jbc.M105170200. [DOI] [PubMed] [Google Scholar]
- ZGOURAS D., WACHTERSHAUSER A., FRINGS D., STEIN J. Butyrate impairs intestinal tumor cell-induced angiogenesis by inhibiting HIF-1α nuclear translocation. Biochem. Biophys. Res. Commun. 2003;300:832–838. doi: 10.1016/s0006-291x(02)02916-9. [DOI] [PubMed] [Google Scholar]