Abstract
The sodium–calcium exchanger (NCX) was considered to play an important role in arrhythmogenesis under certain conditions such as heart failure or calcium overload. In the present study, the effect of SEA-0400, a selective inhibitor of the NCX, was investigated on early and delayed afterdepolarizations in canine ventricular papillary muscles and Purkinje fibres by applying conventional microelectrode techniques at 37°C. The amplitude of both early and delayed afterdepolarizations was markedly decreased by 1 μM SEA-0400 from 26.6±2.5 to 14.8±1.8 mV (n=9, P<0.05) and from 12.5±1.7 to 5.9±1.4 mV (n=3, P<0.05), respectively. In enzymatically isolated canine ventricular myocytes, SEA-0400 did not change significantly the L-type calcium current and the intracellular calcium transient, studied using the whole-cell configuration of the patch-clamp technique and Fura-2 ratiometric fluorometry. It is concluded that, through the reduction of calcium overload, specific inhibition of the NCX current by SEA-0400 may abolish triggered arrhythmias.
Keywords: Sodium/calcium exchanger, early and delayed afterdepolarizations, SEA-0400, papillary muscle, Purkinje fibres, calcium transient, L-type calcium current
Introduction
The sodium–calcium exchanger (NCX) is considered a major regulator to maintain the Ca2+ homeostasis of the myocardium (Bers, 2000; 2002). It is known that NCX, in the forward mode, extrudes Ca2+ from the cell to the extracellular space during diastole, at relatively low free cytoplasmic Ca2+ concentrations and negative transmembrane potentials. Since the extrusion of each Ca2+ is coupled with the entry of 3 Na+, a net inward current is carried during the forward mode of the NCX, which can cause substantial depolarization, leading to early (EAD) and delayed (DAD) afterdepolarizations when intracellular Ca2+ is elevated. EAD and DAD are generally thought to play an important role in arrhythmogenesis (Volders et al., 2000; Pogwizd & Bers, 2002), especially under conditions when potassium conductance is decreased, such as in heart failure (Pogwizd et al., 2001). One may speculate, therefore, that specific blockers of NCX can be potentially antiarrhythmic in dysrhythmias related to Ca2+ overload (Pogwizd & Bers, 2002; Pogwizd, 2003). This hypothesis could not be directly tested so far, since the available NCX inhibitors also decreased the L-type calcium current (ICa) which, in turn, is known to decrease intracellular Ca2+ load, thereby indirectly changing the magnitude of NCX. Recently, it was found that KB-R7943, an effective inhibitor of NCX primarly in the reverse mode but not in the forward mode, reduced the incidence of ischaemia/reperfusion-related arrhythmias induced by calcium overload (Elias et al., 2001). KB-R7943, however, also inhibits the L-type calcium current (Tanaka et al., 2002), which makes the interpretation of its antiarrhythmic effect quite uncertain. More recently, it was reported that SEA-0400 selectively inhibited NCX without altering various potassium and L-type calcium currents (Tanaka et al., 2002). In the present study, therefore, we could directly test the above-mentioned hypothesis by investigating the effect of SEA-0400, as a selective NCX inhibitor devoid of ICa-blocking property, on the formation of EAD and DAD in the canine ventricular muscle and Purkinje fibre preparations.
Methods
Conventional microelectrode measurements
Adult mongrel dogs of either sex weighing 8–16 kg were used. Following anaesthesia, induced by sodium pentobarbital (30 mg kg−1 i.v.), the heart was rapidly removed through a right lateral thoracotomy and immediately rinsed in oxygenated modified Locke's solution containing (in mM): NaCl 128.3, NaHCO3 21.4, KCl 5.0, D-glucose 10.0, CaCl2 1.8, MgCl2 0.42. The pH of the solution ranged from 7.35 to 7.45 when gassed with 95% O2 plus 5% CO2 at 37°C. Purkinje strands obtained from either ventricle and right ventricular papillary muscle tips were mounted individually in a tissue chamber (volume∼40 ml). The preparation was stimulated (stimulator type 215/II, Hugo Sachs Elektronik, March-Hugstetten, Germany) initially at a constant cycle length of 1000 ms using rectangular constant current pulses of 2 ms. The current pulses were isolated from ground and delivered through bipolar platinum electrodes in contact with the preparations. At least 1 h was allowed for each preparation to equilibrate while continously superfused with modified Locke's solution warmed to 37°C before the experimental measurements commenced. Transmembrane potentials were recorded using conventional glass micro-electrodes, having resistances of 5–20 MΩ when filled with 3 M KCl, connected to the input of a high-impedance electrometer (Biologic Amplifier VF 102, Claixe, France). The records were continuously monitored on a dual-beam storage oscilloscope (model 2230, Tektronix, Beaverton, OR, U.S.A.) and were sampled at 40 kHz using an ADA 3300 type analogue-to-digital data acquisition board (Real Time Devices Inc., State College, PA, U.S.A.). The signals were digitally stored and automatically analysed by means of a software developed in our laboratory. Attempts were made to maintain the same impalement throughout each experiment. However, if an impalement was dislodged, adjustment of the electrode was attempted, and when the action potential characteristics of the re-established impalement deviated by more than 5% from the previous measurement, the experiment was discarded.
Cardiac cell isolation
Myocytes were isolated from adult canine hearts. Dogs were heparinized (1200 U kg−1 i.v.) and anaesthetized with thiopental (30 mg kg−1, i.v.). The left ventricular segment was excised and perfused using a Langendorff apparatus for 5 min with MEM solution (M0518) at 37°C containing (in mM) CaCl2 1.25, HEPES 10 and NaHCO3 4.4. The pH of this solution was 7.2 when equilibrated with 100% O2. Perfusion was continued with Ca-free solution for 10 min, then collagenase (0.03%, type I), protease (0.004%, type XIV) and CaCl2 (33 μM) were added and the heart was perfused for an additional 40 min. The heart then was minced and gently agitated, the isolated cells were washed three times and stored at 10°C in MEM solution containing 1.25 mM CaCl2.
Calcium transient measurements
Changes in intracellular free calcium concentration ([Ca2+]I) were assessed during repetitive contraction–relaxation cycles (‘calcium transients') by the ‘ratiometric' fluorescence technique using Fura-2 AM. The cells were incubated for 30 min in HEPES buffered Tyrode solution (composition in mM: NaCl 144, NaH2PO4 0.33, KCl 4.0, CaCl2 1.8, MgCl2 0.53, Glucose 5.5 and HEPES 5.0 at pH=7.4) containing also 2 μM dye and then washed. Fluorescence measurements were carried out in modified, UV transparent, temperature-controlled and perfused cell chamber (Cell MicroControls, VA, U.S.A.) with a pair of platinum electrodes added for field stimulation. The chamber was attached to the stage of an inverted fluorescence microscope (Diaphot 200, Nikon, Japan). Cells were selected using a red-sensitive video camera system, and were paced at 1 Hz. A 75 W xenon arc lamp (Optosource, Cairn, U.K.) was used for excitation. Excitatory wavelengths (340 and 380 nm) were selected via a rapid switching galvanometric monochromator (Optoscan, Cairn, U.K.). The output of the monochromator was connected to the epifluorescence input of the microscope by a fused silica light guide. Excitatory wavelengths were switched at 100 Hz. Cells were excited through a Fluor 40/0.70 type Nikon) objective. Optical signals from the cells were filtered with a band pass filter (425±17.5 nm) and directed into a photomultiplier tube (PMT). An adjustable window was used to restrict the light reaching the PMT in order to minimize background fluorescence from the remainder of the field. The output of the PMT was sampled and demultiplexed at 200 Hz with the Optoscan. Data acquisition and basic processing were performed by a software (Acquisition Engine) supplied with the Optoscan system. Changes in intracellular free calcium levels were approximated by the ratio of the demultiplexed optical signals (340/380) previously corrected against nonspecific background fluorescence.
Calcium current measurements
The L-type calcium current (ICa) was recorded in three dogs by the whole-cell configuration of the patch-clamp technique in HEPES buffered Tyrode solution supplemented with 3 mM 4-aminopyridine in order to block transient outward current. A special solution was used for filling the micropipettes (composition in mM: KCl 110, KOH 40, EGTA 10, HEPES 10, TEACl 20, MgATP 5, GTP 0.25, the pH was adjusted to 7.2 with KOH). ICa was evoked by 400 ms depolarizing test pulses to 0 mV arising from the holding potential of −40 mV. The amplitude of ICa was defined as the difference between the peak inward current measured at the beginning of the pulse and the current found at the end of the pulse. All experiments were carried out at 37°C. The drug incubation time was 5 min.
All chemicals, except SEA-0400 (Orion Pharma, Espoo, Finland) and Fura-2 AM (Molecular Probes Inc., Eugene, OR, U.S.A.), were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.).
All data are expressed as mean±s.e.m. Statistical analysis was performed using Student's test for paired data. The results were considered significant when P was <0.05.
Results
Effect of SEA-0400 on the early and delayed afterdepolarizations
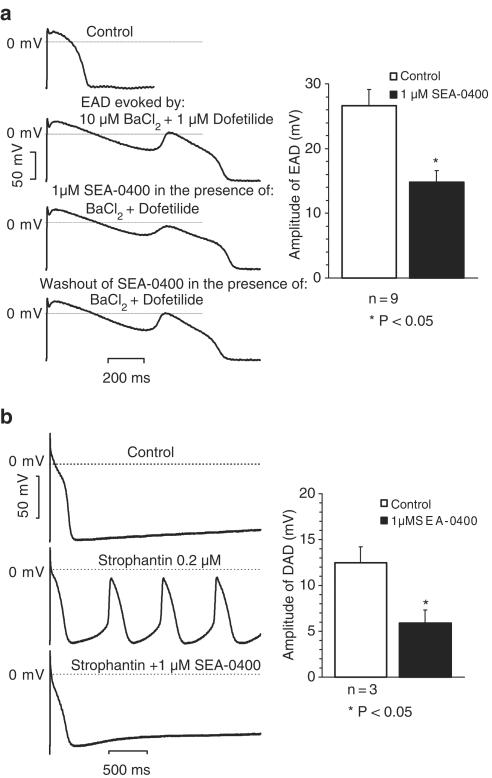
Using the conventional microelectrode technique, the effects of SEA-0400 on early (EAD) and delayed (DAD) afterdepolarizations were studied in canine right ventricular papillary muscles and Purkinje fibres, respectively. EAD was evoked in papillary muscle preparations, stimulated at slow cycle lengths (1500–3000 ms), in the presence of 1 μM dofetilide plus 10 μM BaCl2. The amplitude of the EAD was determined as the difference between the most negative voltage before the appearence of EAD and the peak of the EAD. As Figure 1a shows, 1 μM SEA-0400 decreased the amplitude of EAD. This effect was reversible upon washout of the compound from the tissue bath with solution containing dofetilide and BaCl2. Similar results were obtained in eight additional experiments: amplitude of the EADs was decreased by SEA-0400 from 26.6±2.5 to 14.8±1.8 mV in average (n=9; P<0.05). DADs were evoked in Purkinje fibre preparations superfused with 0.2 μM strophantin for 40 min. In these experiments a train of 40 stimuli was applied at a cycle length of 400 ms. The train was then followed by a 20 s long stimulation-free period in order to generate DADs. These strophantin-induced DADs evoked extrasystoles and automaticity in all six fibres investigated (Figure 1b). In three out of the six fibres, DADs were fully abolished by 1 μM SEA-0400. In the three fibres the amplitude of DADs was decreased by SEA-0400 from 12.5±1.7 to 5.9±1.4 mV (P<0.05).
Figure 1.
In panel a, the effect of 1 μM SEA-0400 on the EAD in canine cardiac right ventricular papillary muscles is summarized. On the left, the results of a representative experiment are shown; on the right, the average values of the amplitude of EADs are presented before (open bar) and after (filled bar) the administration of SEA-0400. In panel b, the effect of SEA-0400 on the DAD in canine cardiac Purkinje fibres is summarized. On the left, results of a representative experiment with triggered activity are shown; on the right, average values of the amplitude of DADs are given before (open bar) and after (filled bar) the application of 1 μM SEA-0400.
Effect of SEA-0400 on L-type Ca2+ current and Ca2+ transients
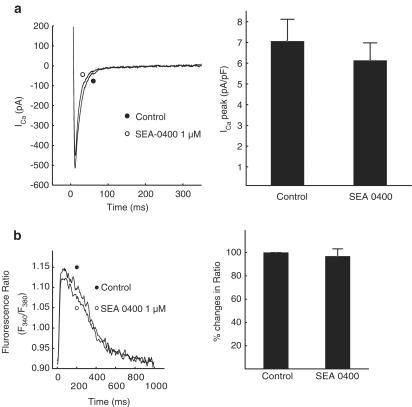
Since the selectivity of SEA-0400 on the NCX is an important issue regarding Ca2+ handling in this work, we investigated the effects of 1 μM SEA-0400 on ICa and [Ca2+]I in canine myocytes. As summarized in Figure 2a, 1 μM SEA-0400 did not change significantly the amplitude of ICa. The slight nonsignificant decrease of the peak Ca2+ current, shown in Figure 2a, can be attributed to the run-down of ICa, since a similar magnitude of decrease was observed in time-matched control measurements.
Figure 2.
In panel a, the effect of 1 μM SEA-0400 on the L-type Ca2+ current in canine cardiac myocytes experiment is shown in a representative experiment and summarized in five cells. In panel b, ratios of fluorescence emission (excitation at 340 and 380 nm, respectively) approximating Ca2+ transients before and 5 min following the application of 1 μM SEA-0400 are shown in a representative experiment and the statistics for four cells are given.
Using the Fura-2-based ratiometric fluorescence technique, no significant changes in the calcium transients could be found. As shown in Figure 2b, application of 1 μM SEA-0400 for 5 min changed neither the shape nor the magnitude of the calcium transient. The slight, nonsignificant decrease in the peak values (to 96.9±6.4% of the control) can well be attributed to slow run-down of the cells.
Discussion
The major finding of this study was that SEA-0400 effectively decreased the amplitude of EADs and DADs evoked in canine ventricular papillary muscles and Purkinje fibres, respectively. The only study which has recently described specific inhibition of the NCX current by a new compound, SEA-0400 (Tanaka et al., 2002), neither included the examination of the effect of this compound on arrhythmogenesis, EAD and DAD formation, nor contained measurements on action potentials and contractility. In that study, performed in isolated guinea-pig ventricular myocytes, submicromolar concentrations of SEA-0400 were shown to reduce the NCX current markedly without affecting other transmembrane currents up to a concentration of 10 μM (Tanaka et al., 2002). On the other hand, in experiments performed in heart tubes from NCX−/− (NCX1 knockout) mouse embryos (Reuter et al., 2002), the amplitude of the Ca2+ transient was decreased by SEA-0400, and therefore, the selectivity of SEA-0400 on NCX was questioned.
Our results obtained in canine myocytes indicate that SEA-0400 at a concentration of 1 μM fails to change significantly either the L-type calcium current, or the calcium transient. This is in agreement with the results of Tanaka et al. (2002), but is in sharp contrast with those of Reuter et al. (2002). We do not have an explanation why Reuter et al. found profound effect on the Ca2+ transient with SEA-0400 in knockout mice; therefore, it is possible that an influence of SEA-0400 exists on Ca2+ transients in embryonic mice tubes.
KB-R7943 was reported to decrease NCX (Kimura et al., 1999) and abolish experimental arrhythmias (Watano et al., 1999; Elias et al., 2001). This compound, however, cannot be considered as a specific inhibitor of NCX, since it depresses the L-type calcium current as well (Tanaka et al., 2002). Therefore, the present investigation is the first one directly addressing the question whether specific NCX inhibition may result in suppression of triggered arrhythmias in in vitro cardiac preparations.
It is noteworthy to mention that 1 μM SEA-0400 did not completely abolish EAD and DAD in our experiments, which can be interpreted that either EAD or DAD does not entirely depend on the NCX function and/or 1 μM SEA-0400 does not inhibit NCX more than about 80%. NCX reduction most likely decreases EAD and DAD amplitude by decreasing the enhanced turnover of forward mode of NCX due to spontaneous Ca2+ release from the SR.
The possible therapeutic implication of our study appears to be rather complex. It is tempting to speculate that suppression of EAD and DAD may be antiarrhythmic both in the ventricles and atria (Chen et al., 2000) during Ca2+ overload, as is the case in heart failure or at the beginning of atrial flutter and fibrillation, especially when potassium currents likely are downregulated (Yue et al., 1997; van Wagoner & Nerbonne, 2000) and the NCX current is upregulated (Studer et al., 1994). In other experiments in chronic atrial fibrillation in the goat where IK1 is upregulated, inhibition of Na+/H+ and NCX was not beneficial (Blaauw et al., 2004). Also, it was considered that on reperfusion following myocardial ischaemia, Ca2+ influx may involve the reverse mode operation of the NCX, resulting in Ca2+ overload and release of Ca2+ from the sarcoplasmic reticulum and thereby can also cause cardiac arrhythmias (Levi et al., 1993). Thus, blocking the reverse portion of the NCX current can also be beneficial. In addition, the positive inotropic effect of the inhibition of the forward mode of the NCX current can improve myocardial perfusion and, as such, it can be also useful therapeutically.
On the other hand, elevated intracellular Ca2+ concentration and enhanced cardiac force development may increase myocardial oxygen consumption, and if the Ca2+ extrusion systems other than NCX (Bassani et al., 1995; Choi & Eisner, 1999) cannot properly compensate for, the resulting excessive Ca2+ overload may impair mitochondrial function and eventually cause irreversible myocardial damage.
Conclusions
In the present study, evidence has been obtained that specific NCX inhibitory activity can suppress elementary arrhythmogenic phenomena, such as EAD and DAD. Considering the pros and cons, further research is needed with both in vitro and in vivo methods to elucidate the potential therapeutic targets and, in a broader sense, the possible beneficial effect of specific NCX inhibition.
Acknowledgments
This work was supported by grants from the Hungarian National Research Foundation OTKA T-035018, T-037520 and T-038121), Hungarian Ministry of Health (ETT 144/2001 and 188/2003), Hungarian Ministry of Education (FKFP 0064/2001), National Research and Development Programmes (NKFP 1A/0011/2002), by the Hungarian Academy of Sciences, and by János Bolyai Research Scholarship (for L.V.).
Abbreviations
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- ICa
L-type calcium current
- NCX
sodium–calcium exchanger
References
- BASSANI R.A., BASSANI J.W., BERS D.M. Relaxation in ferret ventricular myocytes: role of the sarcolemmal Ca-ATPase. Pflügers Arch. 1995;430:573–578. doi: 10.1007/BF00373894. [DOI] [PubMed] [Google Scholar]
- BERS D.M. Calcium fluxes involved in control of cardiac myocyte contraction. Circ. Res. 2000;87:275–281. doi: 10.1161/01.res.87.4.275. [DOI] [PubMed] [Google Scholar]
- BERS D.M. Cardiac excitation–contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- BLAAUW Y., BEIER N., VAN DER VOORT P., VAN HUNNIK A., SCHOTTEN U., ALLESSIE M.A. Inhibitors of the Na+/H+ exchanger cannot prevent atrial electrical remodeling in the goat. J. Cardiovasc. Electrophysiol. 2004;15:440–446. doi: 10.1046/j.1540-8167.2004.03498.x. [DOI] [PubMed] [Google Scholar]
- CHEN Y.J., CHEN S.A., CHANG M.S., LIN C.I. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc. Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- CHOI H.S., EISNER D.A. The effects of inhibition of the sarlocemmal Ca-ATPase on systolic calcium fluxes and intracellular calcium concentration in rat ventricular myocytes. Pflügers Arch. 1999;437:966–971. doi: 10.1007/s004240050868. [DOI] [PubMed] [Google Scholar]
- ELIAS C.L., LUKAS A., SHURRAW S., SCOTT J., OMELCHENKO A., GROSS G.J., HNATOWICH M., HRYSHKO L.V. Inhibition of Na+/Ca2+ exchange by KB-R7943: transport mode selectivity and antiarrhythmic consequences. Am. J. Physiol., Heart. Circ. Physiol. 2001;281:H1334–H1345. doi: 10.1152/ajpheart.2001.281.3.H1334. [DOI] [PubMed] [Google Scholar]
- KIMURA J., WATANO T., KAWAHARA M., SAKAI E., YATABE J. Direction-independent block of bi-directional Na+/Ca2+ exchange current by KB-R7943 in guinea-pig cardiac myocytes. Br. J. Pharmacol. 1999;128:969–974. doi: 10.1038/sj.bjp.0702869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVI A., BROOKSBY P., HANCOX J.C. One hump or two? The triggering of calcium release from the sarcoplasmic reticulum and the voltage dependence of contraction in mammalian cardiac muscle. Cardiovasc. Res. 1993;27:1743–1757. doi: 10.1093/cvr/27.10.1743. [DOI] [PubMed] [Google Scholar]
- POGWIZD S.M. Clinical potential of sodium–calcium exchanger inhibitors as antiarrhythmic agents. drugs. 2003;63:439–452. doi: 10.2165/00003495-200363050-00001. [DOI] [PubMed] [Google Scholar]
- POGWIZD S.M., BERS D.M. Calcium cycling in heart failure: the arrhythmia connection. J. Cardiovasc. Electrophysiol. 2002;13:88–91. doi: 10.1046/j.1540-8167.2002.00088.x. [DOI] [PubMed] [Google Scholar]
- POGWIZD S.M., SCHLOTTHAUER K., LI L., YUAN W., BERS D.M. Arrhythmogenesis and contractile dysfunction in heart failure. Roles of sodium–calcium exchange, inward rectifier potassium current, and residual β-adrenergic responsiveness. Circ. Res. 2001;88:1159–1167. doi: 10.1161/hh1101.091193. [DOI] [PubMed] [Google Scholar]
- REUTER H., HENDERSON S.A., HAN T., MATSUDA T., BABA A., ROSS R.S., GOLDHABER J.I., PHILIPSON K.D. Knockout mice for pharmacological screening. Testing the specificity of Na+–Ca2+ exchange inhibitors. Circ. Res. 2002;91:90–92. doi: 10.1161/01.res.0000027529.37429.38. [DOI] [PubMed] [Google Scholar]
- STUDER R., REINECKE H., BILGER J., ESCHENHANGEN T., BOHM M., HASENFUSS G., JUST H., HOLTZ J., DREXLER H. Gene expression of the cardiac Na+–Ca2+ exchanger in end-stage human heart failure. Circ. Res. 1994;75:443–453. doi: 10.1161/01.res.75.3.443. [DOI] [PubMed] [Google Scholar]
- TANAKA H., NISHIMARU K., AIKAWA T., HIRAYAMA W., TANAKA Y., SHIGENOBU K. Effect of SEA0400, a novel inhibitor of sodium–calcium exchanger, on myocardial ionic currents. Br. J. Pharmacol. 2002;135:1096–1100. doi: 10.1038/sj.bjp.0704574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLDERS P.G.A., VOS M.A., SZABO B., SIPIDO K.R., MARIEKE D.E., GROOT S.H., GORGELS A.P.M., WELLENS H.J.J., LAZZARA R. Progress in the understanding of cardiac early afterdepolarizations and torsades de pointes: time to revise current concepts. Cardiovasc. Res. 2000;46:376–392. doi: 10.1016/s0008-6363(00)00022-5. [DOI] [PubMed] [Google Scholar]
- VAN WAGONER D.R., NERBONNE J.M. Molecular basis of electrical remodeling in atrial fibrillation. J. Mol. Cell. Cardiol. 2000;32:1101–1117. doi: 10.1006/jmcc.2000.1147. [DOI] [PubMed] [Google Scholar]
- WATANO T., HARADA Y., HARADA K., NISHIMURA N. Effect of Na+/Ca2+ exchange inhibitor, KB-R7943 on ouabain-induced arrhythmias in guinea-pigs. Br. J. Pharmacol. 1999;127:1846–1850. doi: 10.1038/sj.bjp.0702740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YUE L., FENG J., GASPO R., LI G.R., WANG Z., NATTEL S. Ionic remodeling underlying action potential changes in a canine model of atrial fibrillation. Circ. Res. 1997;81:512–525. doi: 10.1161/01.res.81.4.512. [DOI] [PubMed] [Google Scholar]