Abstract
Asthma is associated with abnormal airway smooth muscle (ASM) growth that may contribute to airway narrowing and hyperresponsiveness. We investigated the role of mitogen-activated protein kinase (MAPK) pathway in IL-1β induced ASM proliferation in the rat.
Rat tracheal ASM cells were dissociated and maintained in culture. We examined the effect of selective MAPK inhibitors, SB239063 (a p38 MAPK inhibitor), U0126 (a mitogen-activated and extracellular regulated kinase kinase, MEK-1, inhibitor which inhibits downstream extracellular regulated kinase, ERK, activity), and SP600125 (a c-jun N-terminal kinase, JNK, inhibitor) on IL-1β-induced proliferation.
Proliferation of ASM cells was significantly increased following exposure to IL-1β in a dose-dependent manner. p38, JNK and ERK MAPKs were activated by IL-1β in a time-dependent manner, with peak activation time at 30, 60 min and at 6 h, respectively. This activation was inhibited by their respective inhibitors. SP600125 (20 μM) had no effect on IL-1β-induced ERK and p38 phosphorylation.
SB239063, U0126 and SP600125 dose-dependently inhibited IL-1β-dependent proliferation at doses that inhibit the activities of p38, ERK and JNK MAPKs, respectively. No additive or synergistic effects were observed on proliferative responses with any combination of these compounds.
In conclusion, the three major MAPK pathways, ERK as well as the p38 MAPK and JNK pathways, are independent regulators of IL-1β-dependent proliferation of rat ASM.
Keywords: Mitogen-activated protein kinase (MAPK), c-jun N-terminal kinase, extra-cellular regulated kinase, p38 MAPK, airway smooth muscle proliferation
Introduction
Airway smooth muscle (ASM) proliferation is a central feature of asthmatic airways since it is increased in patients with mild to moderate asthma (Johnson et al., 2001). ASM mass in airways of patients who have died of asthma is increased both in the large and small airways (Ebina et al., 1993). Although numerous factors are known to contribute to excessive narrowing, an increase in ASM mass is regarded as an important abnormality responsible for the increased airway narrowing observed in response to bronchoconstrictor agents in asthma (James et al., 1989). Therefore, the characterization of stimuli and associated intracellular mechanisms that regulate ASM proliferation remain of considerable interest (Panettieri, 1998).
The mitogen-activated protein kinases (MAPKs) constitute a family of serine/threonine kinases that mediate the transduction of external stimuli from the cell surface to the nucleus, usually resulting in the phosphorylation and subsequent activation of transcriptional factors, producing altered gene transcription (Johnson & Lapadat, 2002). Three MAPK families that differ in their substrate specificity and responses to stress have been identified in vertebrates: c-Jun amino-terminal kinase (JNK) or stress-activated protein kinase (SAPK), extracellular-regulating kinase (ERK), also referred to as p42/p44 MAPK, and p38 MAPK (Kyriakis & Avruch, 2001). The ERK signalling module is a vital mediator of a number of cellular fates including growth, proliferation and survival. There are two ERK isoforms that are ubiquitously expressed, ERK 1 and ERK 2, also referred to as p42/p44 MAP kinases, which phosphorylate members of the AP-1 and ELK-1 family of transcriptional factors (Davis, 2000). MEK1 and MEK2, which are MAPK/ERK kinase function upstream of ERK, function as MAPKK (MAP kinase kinase) or MEK, and the Raf protein as MAPKKK (MAP kinase kinase kinase) or MEKK.
The JNK signalling pathway is a cell stress-activated pathway involved in regulation of cell proliferation and apoptosis. It consists of three alternatively spliced JNK protein kinase isoforms JNK-1, JNK-2 and JNK-3. The two primary AP-1 transcription components that are phosphorylated by JNK are c-jun and ATF-2. The p38 MAPK pathway consists of four isoforms, and is stimulated by a variety of cytokines (IL-1, TNF-α, TGF-β) and a number of pathogens and environmental factors such as osmotic and heat shock. p38 MAPK, in addition to being involved in cell proliferation and apoptosis, is important in regulation of a wide range of immunological responses, but there is a considerable overlap between the signalling pathways of MAPK such as ERK and JNK. These highly homologous MAPK cascades are subject to regulation at numerous levels and exhibit cross-talk among themselves and other pathways for the purpose of integrating intracellular signals into discrete physiological responses (Hershenson et al., 1997). The MAPKs therefore form a highly integrated network required to achieve specialized cell functions controlling cell differentiation, cell proliferation and cell death (Kyriakis & Avruch, 2001).
Although the role of the ERK MAPK pathway in mitogen-induced ASM proliferation appears to be well established, the contributions of JNK and p38 MAPK pathways remain relatively unclear. In the present study, we examined the concomitant role of the ERK, JNK and p38 MAPK in ASM proliferation. We used selective inhibitors of the MEK1, MEK2, and of ERK, U0126 (Favata et al., 1998), JNK, SP600125 (Bennett et al., 2001) and of p38 MAPK, SB239063 (Underwood et al., 2000a) to investigate the role of these pathways in IL-1β-induced ASM proliferation in the rat. We found that these pathways participate in IL-1β induced ASM proliferation, without evidence of apparent interaction between these pathways.
Methods
ASM cell culture
Adult Brown-Norway rats (250–300 g) were anesthetized with pentobarbital sodium, and the trachea was aseptically removed and cleared of connective tissue. Using a dissecting microscope, ASM strips were dissected from the surrounding parenchyma. The epithelium was removed from the luminal surface, and bands of ASM were gently separated from the underlying connective tissue. The ASM strips were washed three times with DMEM containing 50 U ml−1 of penicillin, 20 μg ml−1 of streptomycin, and 10 μg ml−1 of amphotericin B. The muscle was finely chopped into small (1 mm2) pieces and digested in DMEM containing 0.1% type I collagenase (1 mg ml−1; Worthington, U.K.) for a 3-h period at 37°C, under 5% CO2. Enzyme digests were centrifuged at 500 × g, and the pellet was resuspended and cultured in DMEM supplemented with 10% foetal bovine serum (FBS), nonessential amino acids, gentamicin (50 μg ml−1) and amphotericin B (1 μg ml−1) in a humidified atmosphere of 5% CO2 in 95% air at 37°C for 4 days. Fresh medium was changed and cells were then subcultured until they reached confluence (usually by 2 weeks). The identification of smooth muscle cells was confirmed by the presence of positive staining for α-smooth muscle actin, calponin and myosin, and this yielded 99% pure smooth muscle cells. For experimentation, third- to fifth-passaged cells were plated at a density of 2 × 104 cm−2 in either six- or 96-well plates in the above FBS-supplemented medium. After 5 days later, cells were growth-arrested by re-feeding cells with FBS-free DMEM containing 0.1% BSA, 5 μg ml−1 each of transferrin and insulin for 24 h.
ASM cell proliferation assay
ASM cell proliferation was quantified using a colorimetric method based on the cellular conversion of the yellow tetrazolium salt 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) into a soluble purple formazan product by the action of mitochondrial succinyl dehydrogenase (CellTiter 96 AQ nonradioactive cell proliferation kit, Promega). The absorbance measured in this assay is directly proportional to the number of living cells in culture. For proliferation studies, cells were seeded in 96-well plates at 1 × 104 per well and cultured at 5% CO2, 37°C about 3–4 days until they almost reached confluence. Cells were then growth-arrested in FBS-free medium for 24 h, and treated with either different concentrations of recombinant human IL-1β or a mixture of IL-1β (10 ng ml−1) and increasing concentrations of the inhibitors of MAPK pathway: U0126, SB239063 or SP600125. A minimum of six wells was studied for each treatment condition. A preliminary study for determining the optimal concentration and incubation time at which the rate of conversion was linear and proportional to the number of cell was performed with 10–30 μl per well of MTS incubated for 1–5 h. In all studies, proliferation was assessed on day 3 after the treatment by adding 20 μl of MTS into 100 μl of culture medium. After 3 h of additional incubation, the absorbance of soluble formazan dye was measured on an automated wavelength spectrophotometer (Anthos, Austria) against a reagent blank (i.e. no cells) at a test wavelength of 490 nm. To verify the correlation between absorbance reading (A490) and ASM cell numbers, direct cell counts were also performed with a hemocytometer in some experiments. Briefly, cells were trypsinized with 0.5% trypsin-EDTA solution and centrifuged at 1500 r.p.m. for 5 min. The supernatants were removed, and the pellets were washed with DPBS and resuspended with 200 μl DMEM. Cells were counted with hemocytometer under inverted microscope (Olympus, Japan).
Analysis of MAPK phosphorylation
ASM cells were seeded in six-well plates as described above. After cells have reached confluence, they were equilibrated in 0.1% BSA and DMEM for 24 h. The cells were stimulated with IL-1β (10 ng ml−1) in FBS-free medium for 0–24 h. At the indicated time points, cells were washed twice with cold Hank's solution and lysed on ice for 30 min with 300 μl of cold lysis buffer (20 mM Tris-HCl, 150 mM NaCl, 0.5% SDS, 1% NP40, 1 mM sodium orthovandate, 2 mM EDTA, 2 μg ml−1 aprotinin, 1 μM pepstatin, 1% leupeptin and 1mM phenylmethylsulfonyl fluoride, pH 7.4). Cells were then scraped into 1.5 ml polypropylene tubes, briefly sonicated for 5 s and centrifuged at 12 000 r.p.m. for 15 min at 4°C. The supernatants were collected and protein concentration was determined using Bio-Rad protein microassay kit (Bio-Rad Laboratories, U.S.A.). Total cell protein (40 μg) was fractionated by electrophoresis on a 4–20% SDS–polyacrylamide gradient gel. After electrophoresis, proteins were transferred onto nitrocellulose membrane, blocked with 5% milk in TBS-T buffer (20 mM Tris-CHl, pH 7.4, 137 mM NaCl and 0.1% Tween 20), and probed with different antibodies targeting the ERK, p38 and JNK signalling pathways. These antibodies specifically recognize the phosphorylated amino acid Thr202/Tyr204 of p42/p44, Thr180/Tyr182 of p38, or Ser63/Ser73 of c-Jun (Cell Signalling Technology, U.S.A.) or Thr183/Tyr185 of JNK (New England BioLabs, Hertfordshire, U.K.), respectively. Following incubation with primary antibodies (1 : 1000 diluted in 5% milk TBS-T) overnight at 4°C, the membrane was washed three times with TBS-T and incubated with horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG antibody (1 : 4000 in TBS-T and 5% nonfat milk) for 1 h at room temperature. The blot was then washed three times with TBS-T and visualized by enhanced chemiluminescence. The bands of interest were quantified by computerized densitometry (Molecular Analyst).
Effect of inhibitors SB 239063, SP60012 and U0126
In the experiments investigating the inhibition of IL-1β-induced MAPK phosphorylation on ASM cells by specific inhibitors of ERK, p38 and JNK, cells were seeded in six-well plates at a density of 2 × 104 cells cm−2, grown for 4 days and arrested with 0.1% BSA DMEM for 24 h, then pretreated with SB239063 (Smith-Kline Beecham, Philadelphia, U.S.A.), SP600125 (Celgene Inc., San Diego, U.S.A.) or U0126 (Cell Signalling Technology, MA, U.S.A.) at 10 μM for 30 min. Cells were then stimulated with either IL-1β (10 ng ml−1) or a combination of IL-1β (10 ng ml−1) and increasing concentrations of SB239063, or SP600125 or U0126 at 0–50 μM for 30–60 min. These inhibitors were dissolved in DMSO and then stock solutions (10, 50 and 20mM, respectively) were diluted in FCS-free DMEM to required concentrations. Cells exposed to diluent alone were used as control. The cell extracts were then subjected to Western blot analysis.
In order to determine the selectivity of SP600125 (20 μM) on the MAPK pathway, we examined its effects on IL-1β-induced phosphorylation of ERK and p38.
In the experiments investigating the effect of MAPK-specific inhibitors on ASM cell proliferation induced by IL-1β, cells were seeded on 96-well plates at 2 × 104 cells cm−2, grown for about 3–4 days (cells about 90% confluent), starved in 0.1% BSA DMEM for 24 h, then pretreated with different MAPK inhibitors at 10 μM for 30 min. Cells were then stimulated with IL-1β (10 ng ml−1) or a mixture of IL-1β (10 ng ml−1) and increasing concentrations of MAPK inhibitors (0–30 μM). At least six wells were used at each concentration. Cells were then incubated at 37°C for 72 h and analyzed by the ASM proliferation assay described above.
Statistical analysis
All experiments were performed on cells from at least three different rats. A mean value for the density of the protein band in the Western blots was calculated from all experiments. The density of the control was taken as 100%. Results were expressed as mean±s.e.m. Statistical significance was determined by one-way ANOVA with Bonferroni correction. Differences were considered significant at P⩽0.05.
Results
ASM proliferation induced by IL-1β
We found that IL-1β dose-dependently induced rat ASM cell proliferation with a significant increase in cell numbers measured at 3 days at a concentration of 0.6 ng ml−1, reaching a plateau response at 2.5 ng ml−1 (Figure 1). The effect of IL-1β on ASM proliferation was similar at cell passages two and six. In both unstimulated cells or in cells exposed to 10% FCS which induced proliferation, the absorbance of the MTS formazan product at 490 nm correlated very well with cell numbers directly counted using a hemocytometer (r2=0.98 and 0.77 for unstimulated cells and proliferative cells, respectively; data not shown).
Figure 1.
Effect of IL-1β on rat airway smooth muscle (RASM) mitogenesis. Growth-arrested RASM cells were stimulated with increasing concentrations of IL-1β (0–10 ng ml−1). Data are presented as mean±s.e.m. from three experiments performed 72 h following incubation with IL-1β. *P<0.05, **P<0.01, ***P<0.001 versus unstimulated cells.
Activation of p42/p44, p38 and c-Jun/JNK kinases
Examination of p38, ERK (p42/p44) and JNK (Figure 2) activation in rat ASM cultures revealed that IL-1β activated these kinases in a time-dependent manner (n=4). IL-1β transiently activated p38, which was apparent by 10 min, reaching significance at 30 min (∼seven-fold of basal at 30 min) and returning towards basal levels by 6 h (Figure 2a). Similarly, IL-1β activated ERK to an appreciable extent (Figure 2b). ERK activation was elevated at 1 h, peaking at 6 h and still increased but nonsignificantly at 24 h (∼six-fold of basal at 6 h). Similar to p38 activation, JNK activation measured by phosphorylation of c-jun was maximal between 30 and 60 min (∼five-fold of basal at 60 min), returning towards basal level by 6 h (Figure 2c). In RASM cell lines cultured from three rats, phosphorylation of JNK used as a measure of JNK activation also showed activation at 15 and 30 min (∼five-fold of basal at 30 min), returning to basal level at 6 h after IL-1β (Figure 2d).
Figure 2.
Time-course of IL-1β-induced p38 activation (Panel a), p42/p44 ERK activation (Panel b), and JNK activation with c-jun (Panel c) or with JNK phosphorylation (Panel d). Growth-arrested rat airway smooth muscle cells were harvested at various time points following stimulation with IL-1β (10 ng ml−1). Lysates were subjected to immunoblotting using antisera directed against phosphorylated p42/p44, p38, c-jun or JNK, as described in Methods. Autoradiographs generated were quantitated by densitometry. For each panel is shown a representative Western blot; mean±s.e.m. densitometric measurements from four experiments performed 72 h following incubation with IL-1β are shown in Panels a–c. In Panel d, one representative blot of three experiments is shown. *P<0.05, **P<0.01, ***P<0.001 versus cells at time 0.
Effect of specific inhibitors of MAPK on ASM proliferation
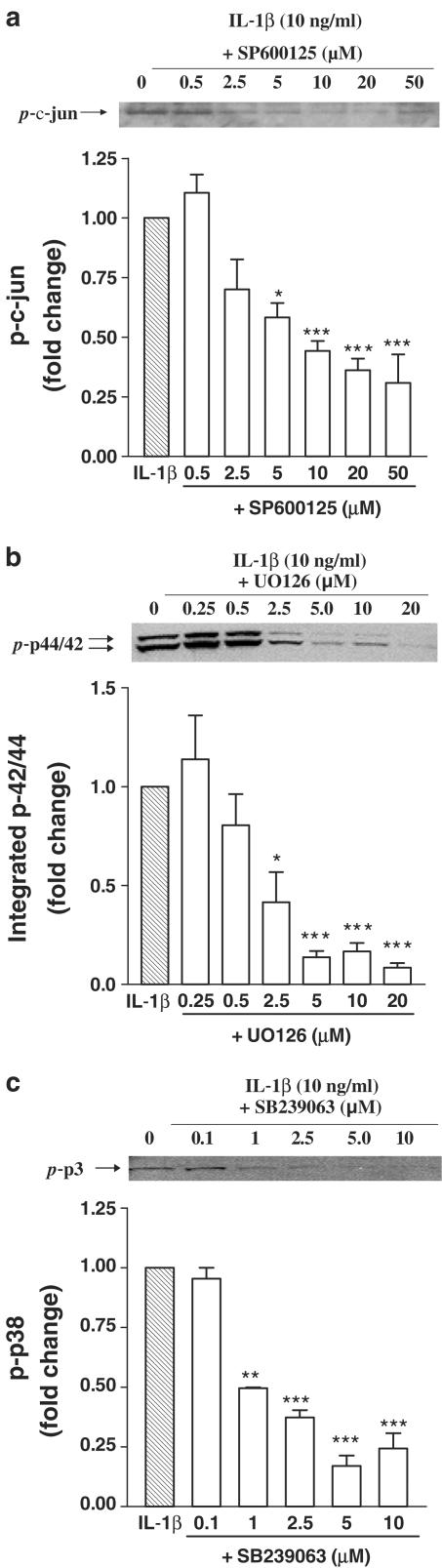
To further explore the relationship between ERK, JNK/SAPK and p38 MAPK activation and rat ASM proliferation, we utilized their pharmacological inhibitors U0126, SP600125 and SB239063, respectively. We first tested whether these inhibitors could block the activation of ERK, JNK/SAPK and p38 MAPK induced by IL-1β. Pretreatment with increasing concentrations of SP600125 (range 0.5–50 μM) dose-dependently inhibited IL-1β-mediated activation of c-jun (IC50=3.69 μM; Figure 3a), and the maximum inhibition achieved was approximately 70%. Pretreatment for 30 min with increasing concentrations of U0126 (range 0.25–20 μM) dose-dependently inhibited IL-1β-mediated activation of p42/p44 (IC50=1.67 μM) (Figure 3b). Increasing concentrations of SB239063 (0.1–10 μM) dose-dependently inhibited IL-1β-mediated activation of p38 MAPK (IC50=0.95 μM; Figure 3c).
Figure 3.
Effect of SP600125 (Panel a), U0126 (Panel b) or SB239063 (Panel c) on JNK, p42/p44 (or ERK) and p38 MAPK activation. Growth-arrested rat airway smooth muscle cells were pretreated with either vehicle or increasing concentrations of SP600125 (0.5–50 μM), U0126 (0.25–20 μM) or SB239063 (0.1–20 μM) followed by stimulation with 10 ng ml−1 of IL-1β. Lysates were subjected to immunoblotting with the use of antisera directed against phosphorylated p42/p44, p38 or c-jun, as described in Methods. Autoradiographs generated were quantitated by densitometry. For each panel is shown a representative Western blot, together with the mean±s.e.m. densitometric measurements from four experiments. *P<0.05, **P<0.01, ***P<0.001 versus IL-1β alone stimulated cells.
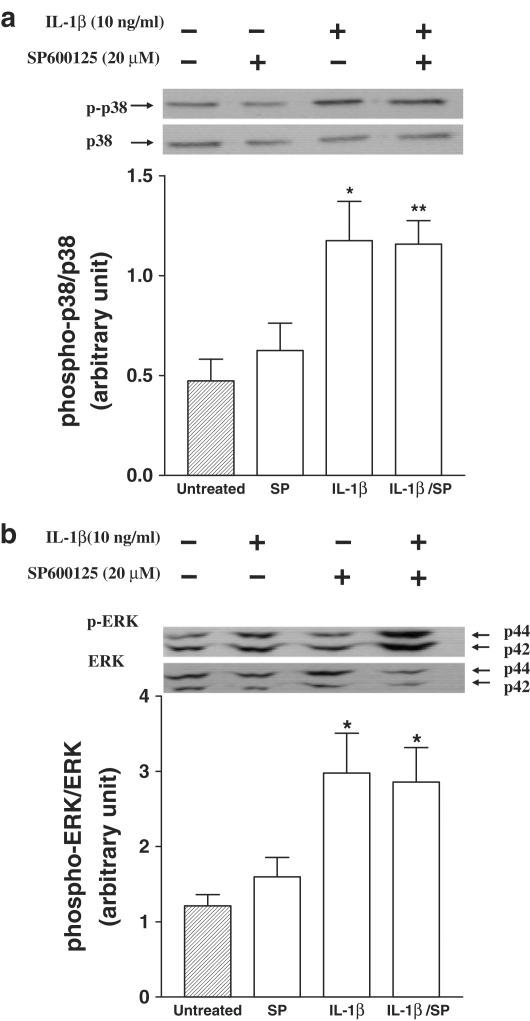
SP600125, at a concentration of 20 μM that induced most significant inhibition of JNK activity, had no significant effect on IL-1β-induced ERK and p38 phosphorylation at 60 and 30 min, respectively (n=3; Figure 4). This indicates the relative selectivity of this compound for JNK, without affecting ERK and p38 phosphorylation.
Figure 4.
Effects of SP600125 (20 μM) on IL-1β-induced phosphorylation of p38 (Panel a) and of ERK (Panel b). Upper portion of each panel shows a representative Western blot and the lower portion mean±s.e.m. of densitometric measurements from three experiments. There was no significant effect of SP600125. *P<0.05; **P<0.01 compared to untreated.
U0126, SP600125 and SB239063 had similar effects on IL-1β−induced rat ASM proliferation commensurate with their respective abilities to inhibit ERK, JNK/SAPK and p38 MAPK activation (Figure 5a–c).
Figure 5.
Effect of SP600125 (Panel a), U0126 (Panel b) or SB239063 (Panel c) on rat airway smooth muscle proliferation induced by IL-1β. Growth-arrested airway smooth muscle cells were pretreated with either vehicle or increasing concentrations of SP600125 (0.5–50 μM), U0126 (0.25–20 μM) or SB239063 (0.1–20 μM) followed by stimulation with 10 ng ml−1 IL-1β. Values for MAPK activation were determined by densitometry. Data are presented as mean±s.e.m. from three experiments. *P<0.05, **P<0.01, ***P<0.001 versus stimulated cells in the absence of inhibitor.
Interaction of selective inhibitors of MAPK
To explore the potential interaction between ERK, JNK/SAPK and p38 MAPK activation and ASM proliferation, we tested the combined effects of U0126, SP600125 or SB239063. We chose a concentration of each inhibitor (5 μM) that caused approximately 50% inhibition, and examined the effects of combination of these concentrations of each inhibitor. On its own, each inhibitor (U0126, SP600125 and SB239063, 5 μM each) reduced proliferation stimulated by IL-1β. The combination of SB239063 and SP600125, of SB239063 and U0126, and of SP600125 and U0126 caused a reduction in proliferation of a similar extent to that of either agent alone, indicating that a lack of either additive or synergistic effects of these inhibitors (Figure 6). U0126, SP600125 and SB239063 (5 μM each) when given in combination was not significantly more effective in inhibiting mitogen-induced RASM proliferation than when each was given alone (Figure 6). Inhibition of one of the intracellular MAPK pathways was sufficient to inhibit IL-1β-induced ASM cell proliferation and simultaneous inhibition did not lead to further reduction in IL-1β-induced ASM proliferation.
Figure 6.
Effect of SP600125, U0126 or SB239063 alone or in combination on rat airway smooth muscle proliferation induced by IL-1β. Growth-arrested airway smooth muscle cells were pretreated with either vehicle (CTL) or SP600125 (SP), U0126 (U) or SB239063 (SB) alone or with combination of each inhibitor, followed by stimulation with 10 ng ml−1 of IL-1β. Data are presented as mean±s.e.m. from three experiments. **P<0.01, ***P<0.001 versus control (CTL) cells.
Discussion
In this study, we demonstrate that IL-1β is an effective inducer of rat airway smooth muscle (RASM) proliferation and that IL-1β produced transient activation of ERK, p38 and JNK MAPK. U0126, a selective MEK1 & MEK2 inhibitor (Favata et al., 1998), SB239063, a selective inhibitor of p38 MAP kinase (Underwood et al., 2000b) and SP600125, a selective inhibitor JNK (Bennett et al., 2001), inhibited of IL-1β-induced rat tracheal ASM cell proliferation. In all three cases, the inhibitory effect occurred at IC50 values that were within similar range to the IC50 value of inhibition of their respective kinase activities in vitro in ASM, indicating that these compounds are readily taken up by the cells and that access to the relevant target enzyme was unhindered. At the concentrations required for almost complete inhibition of ASM cell proliferation (∼16 μM for U0126, ∼16 μM for SB239063 and ∼8 μM for SP600125), comparable effects was observed on IL-1β-induced MAPK activity. These data indicate that activation of MEK1 and the downstream kinases ERK1 and ERK2, the p38 MAPK and JNK MAPK play a major role in the induction of ASM cell proliferation by the proinflammatory cytokine, IL-1β. All three MAPKs are positive regulators of rat ASM cell proliferation, but there was no evidence for synergy between the MAPK signalling pathways. Our results are similar to those reported in rat aortic vascular smooth muscle cells where the proliferation induced by PDGF was inhibited by inhibitors of ERK, JNK and p38 MAPK (Kavurma & Khachigian, 2003).
ERK MAPK activation has been known to play a crucial role in the proliferation of ASM cells induced by various mitogens. U0126 previously identified as an inhibitor of the MAPK cascade leading to ERK1 and ERK2 inhibition (Favata et al., 1998) blocks the phosphorylation and activation of ERK in RASM cells, together with IL-1β induced ASM proliferation. In human ASM, foetal bovine serum, EGF, PDGF, thrombin and phorbol esters produce a very sustained activation of p42/p44/ERK MAPK activity, and inhibition of ERK MAPK by the MEK inhibitors, PD98059 and U0126, blocked proliferation induced by these growth factors, consistent with their ability to inhibit p42/p44 activation (Orsini et al., 1999; Lee et al., 2001). PDGF-stimulated bovine ASM proliferation is also blocked by an ERK inhibitor, indicating ERK pathway involvement (Karpova et al., 1997). On the other hand, IL-1β does not cause proliferation of human ASM (Orsini et al., 1999), despite being able to activate transiently ERK, as well as JNK and p38 MAPK in human ASM (Orsini et al., 1999). Interestingly, we found that IL-1β caused prolonged activation of ERK in rat ASM lasting up to 6 h, in contrast to the short transient activation reported in human ASM (Laporte et al., 1999; Orsini et al., 1999). The relationship between the duration of ERK activation and mitogenesis indicates that the duration and efficacy of ERK activation correlate with efficacy in ASM cell proliferation (Orsini et al., 1999; Lee et al., 2001).
Other known growth factors can also activate JNK such as endothelin and thrombin (Shapiro et al., 1996). Indeed, endothelin can also stimulate ERK activation in rat ASM and inhibition of such activation by an ERK inhibitor, PD98059, attenuated endothelin-stimulated ASM proliferation (Whelchel et al., 1997). Interestingly, IL-β causes proliferation of ASM from the guinea-pig, through activation of the PDGF receptor (De et al., 1993; 1995). PDGF activates p38 MAPK activity in guinea-pig (Pyne & Pyne, 1997), but it is not known whether IL-1β-induced ASM proliferation in the guinea-pig is dependent on any of the MAPK pathways.
The role of p38 MAPK in ASM is more controversial. Inhibition of p38 MAPK activation increased cyclin D1 promoter transcription and protein in cultured bovine ASM cells, and selective activation of p38 MAPK has been reported to attenuate basal and PDGF-induced transcription from cyclin D1 promoter, indicating that p38 MAPK negatively-regulated cyclin D1 expression (Page et al., 2001). However, there is little data regarding the effect of p38 MAPK inhibition on proliferation. The implication of the study by Page et al. (2001) is that p38 MAPK activation would inhibit mitogen-induced proliferation, perhaps through inhibition of ERK activation, as has been observed in other cell systems regarding apoptotic effects (Xia et al., 1995; Berra et al., 1998). Our data indicate that inhibition of p38 MAPK using the selective inhibitor, SB239063, does inhibit ASM proliferation induced by IL-1β, and that the combination of p38 and ERK MAPK inhibitors did not further increase this inhibition of proliferation.
The involvement of JNK in ASM proliferation has been relatively less well studied. Shapiro et al. (1996) concluded indirectly in studies of rat tracheal ASM cells that both endothelin- or thrombin-induced mitogenesis was regulated by ERK and JNK activation since cyclic AMP inhibited both their activities in addition to mitogen-induced proliferation. We used a selective inhibitor of JNK, SP600125, which is a reversible ATP-competitive inhibitor that exhibits a more than 20-fold selectivity for JNK-1, -2 and -3 against related MAPKs such as ERK and p38 (Bennett et al., 2001). In RASM cells, we showed that at a concentration that maximally inhibited JNK activity (20 μM) SP600125 had no effect on ERK and p38 phosphorylation induced by IL-1β. We have previously shown that the JNK inhibitor, SP600125 inhibits airway smooth proliferation induced by repeated allergen exposure in vivo in the rat (Eynott et al., 2003), supporting our in vitro data. It has been proposed that sustained JNK activation may uncouple ERK activation from MEK in a manner requiring jun-mediated gene transcription (Shen et al., 2003). However, inhibition of JNK with SP600125 in rat ASM cells did not lead to enhancement of proliferation, rather an inhibition.
Activation of ASM cells by IL-1β also leads to the production of mediators and of many cytokines and chemokines (Chung, 2000). These effects are to some extent controlled by MAPK activation. Thus, GM-CSF, IL-8 and RANTES release induced by IL-1β is partly JNK-dependent (Oltmanns et al., 2003), while in response to a cocktail of cytokines containing IL-1β, p38 and ERK MAPK pathways were required for other cytokine expression (Hedges et al., 2000). Thus, many of the functional properties of ASM such as proliferation and cytokine production are influenced by MAPK pathways.
In conclusion, the present study identifies the ERK as well as the p38 MAPK and JNK pathways as major regulators of IL-1β-dependent ASM proliferation. Selective inhibitors of these pathways abolished the induction of ASM cell proliferation and the activity of the relevant MAPK activity. There was no interaction between the different components of the MAPK signalling pathway. Each inhibitor may therefore be a useful therapeutic tool in diseases in which hyperplasia of ASM is an important pathogenetic feature.
Acknowledgments
This work was supported by a Wellcome Trust grant. We thank Dr Brydon Bennett, Celgene Inc., San Diego, U.S.A. for the kind gift of SP600125.
Abbreviations
- ERK
extracellular regulated kinase
- IL-1β
interleukin-1β
- JNK
c-jun N-terminal kinase
- MAPK
mitogen-activated protein kinase
- MAPKK or MEK
MAP kinase kinase or MAPK/ERK kinase
- MAPKKK or MEKK
MAP kinase kinase kinase or MEK kinase
- MEK
MAPK/ERK kinase
- MEKK
MEK kinase
- MKK
MAPK kinase
- RASM
rat airway smooth muscle
- SAPK
stress-activated protein kinase
References
- BENNETT B.L., SASAKI D.T., MURRAY B.W., O'LEARY E.C., SAKATA S.T., XU W., LEISTEN J.C., MOTIWALA A., PIERCE S., SATOH Y., BHAGWAT S.S., MANNING A.M., ANDERSON D.W. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. U.S.A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERRA E., DIAZ-MECO M.T., MOSCAT J. The activation of p38 and apoptosis by the inhibition of Erk is antagonized by the phosphoinositide 3-kinase/Akt pathway. J. Biol. Chem. 1998;273:10792–10797. doi: 10.1074/jbc.273.17.10792. [DOI] [PubMed] [Google Scholar]
- CHUNG K.F. Airway smooth muscle cells: contributing to and regulating airway mucosal inflammation. Eur. Respir. J. 2000;15:961–968. doi: 10.1034/j.1399-3003.2000.15e26.x. [DOI] [PubMed] [Google Scholar]
- DAVIS R.J. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- DE S., ZELAZNY E.T., SOUHRADA J.F., SOUHRADA M. Interleukin-1 beta stimulates the proliferation of cultured airway smooth muscle cells via platelet-derived growth factor. Am. J. Respir. Cell. Mol. Biol. 1993;9:645–651. doi: 10.1165/ajrcmb/9.6.645. [DOI] [PubMed] [Google Scholar]
- DE S., ZELAZNY E.T., SOUHRADA J.F., SOUHRADA M. IL-1 beta and IL-6 induce hyperplasia and hypertrophy of cultured guinea pig airway smooth muscle cells. J. Appl. Physiol. 1995;78:1555–1563. doi: 10.1152/jappl.1995.78.4.1555. [DOI] [PubMed] [Google Scholar]
- EBINA M., TAKAHASHI T., CHIBA T., MOTOMIYA M. Cellular hypertrophy and hyperplasia of airway smooth muscle underlying bronchial asthma. Am. Rev. Respir. Dis. 1993;148:720–726. doi: 10.1164/ajrccm/148.3.720. [DOI] [PubMed] [Google Scholar]
- EYNOTT P.R., NATH P., LEUNG S.Y., ADCOCK I.M., BENNETT B.L., CHUNG K.F. Allergen-induced inflammation and airway epithelial and smooth muscle cell proliferation: role of Jun N-terminal kinase. Br. J. Pharmacol. 2003;140:1373–1380. doi: 10.1038/sj.bjp.0705569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAVATA M.F., HORIUCHI K.Y., MANOS E.J., DAULERIO A.J., STRADLEY D.A., FEESER W.S., VAN DYK D.E., PITTS W.J., EARL R.A., HOBBS F., COPELAND R.A., MAGOLDA R.L., SCHERLE P.A., TRZASKOS J.M. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J. Biol. Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- HEDGES J.C., SINGER C.A., GERTHOFFER W.T. Mitogen-activated protein kinases regulate cytokine gene expression in human airway myocytes. Am. J. Respir. Cell. Mol. Biol. 2000;23:86–94. doi: 10.1165/ajrcmb.23.1.4014. [DOI] [PubMed] [Google Scholar]
- HERSHENSON M.B., NAURECKAS E.T., LI J. Mitogen-activated signaling in cultured airway smooth muscle cells. Can. J. Physiol. Pharmacol. 1997;75:898–910. [PubMed] [Google Scholar]
- JAMES A.L., PARE P.D., HOGG J.C. The mechanics of airway narrowing in asthma. Am. Rev. Respir. Dis. 1989;139:242–246. doi: 10.1164/ajrccm/139.1.242. [DOI] [PubMed] [Google Scholar]
- JOHNSON G.L., LAPADAT R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- JOHNSON P.R., ROTH M., TAMM M., HUGHES M., GE Q., KING G., BURGESS J.K., BLACK J.L. Airway smooth muscle cell proliferation is increased in asthma. Am. J. Respir. Crit. Care Med. 2001;164:474–477. doi: 10.1164/ajrccm.164.3.2010109. [DOI] [PubMed] [Google Scholar]
- KARPOVA A.Y., ABE M.K., LI J., LIU P.T., RHEE J.M., KUO W.L., HERSHENSON M.B. MEK1 is required for PDGF-induced ERK activation and DNA synthesis in tracheal myocytes. Am. J. Physiol. 1997;272:L558–L565. doi: 10.1152/ajplung.1997.272.3.L558. [DOI] [PubMed] [Google Scholar]
- KAVURMA M.M., KHACHIGIAN L.M. ERK, JNK, and p38 MAP kinases differentially regulate proliferation and migration of phenotypically distinct smooth muscle cell subtypes. J. Cell. Biochem. 2003;89:289–300. doi: 10.1002/jcb.10497. [DOI] [PubMed] [Google Scholar]
- KYRIAKIS J.M., AVRUCH J. Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol. Rev. 2001;81:807–869. doi: 10.1152/physrev.2001.81.2.807. [DOI] [PubMed] [Google Scholar]
- LAPORTE J.D., MOORE P.E., ABRAHAM J.H., MAKSYM G.N., FABRY B., PANETTIERI R.A., JR, SHORE S.A. Role of ERK MAP kinases in responses of cultured human airway smooth muscle cells to IL-1β. Am. J. Physiol. Lung Cell. Mol. Physiol. 1999;277:L943–L951. doi: 10.1152/ajplung.1999.277.5.L943. [DOI] [PubMed] [Google Scholar]
- LEE J.H., JOHNSON P.R., ROTH M., HUNT N.H., BLACK J.L. ERK activation and mitogenesis in human airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L1019–L1029. doi: 10.1152/ajplung.2001.280.5.L1019. [DOI] [PubMed] [Google Scholar]
- OLTMANNS U., ISSA R., SUKKAR M.B., JOHN M., CHUNG K.F. Role of c-jun N-terminal kinase in the induced release of GM-CSF, RANTES and IL-8 from human airway smooth muscle cells. Br. J. Pharmacol. 2003;139:1228–1234. doi: 10.1038/sj.bjp.0705345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORSINI M.J., KRYMSKAYA V.P., ESZTERHAS A.J., BENOVIC J.L., PANETTIERI R.A., JR, PENN R.B. MAPK superfamily activation in human airway smooth muscle: mitogenesis requires prolonged p42/p44 activation. Am. J. Physiol. 1999;277:L479–L488. doi: 10.1152/ajplung.1999.277.3.L479. [DOI] [PubMed] [Google Scholar]
- PAGE K., LI J., HERSHENSON M.B. p38 MAP kinase negatively regulates cyclin D1 expression in airway smooth muscle cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001;280:L955–L964. doi: 10.1152/ajplung.2001.280.5.L955. [DOI] [PubMed] [Google Scholar]
- PANETTIERI R.A.J. Cellular and molecular mechanisms regulating airway smooth muscle proliferation and cell adhesion molecule expression. Am. J. Respir. Crit. Care Med. 1998;158:S133–S140. doi: 10.1164/ajrccm.158.supplement_2.13tac900. [DOI] [PubMed] [Google Scholar]
- PYNE N.J., PYNE S. Platelet-derived growth factor activates a mammalian Ste20 coupled mitogen-activated protein kinase in airway smooth muscle. Cell Signal. 1997;9:311–317. doi: 10.1016/s0898-6568(96)00190-8. [DOI] [PubMed] [Google Scholar]
- SHAPIRO P.S., EVANS J.N., DAVIS R.J., POSADA J.A. The seven-transmembrane-spanning receptors for endothelin and thrombin cause proliferation of airway smooth muscle cells and activation of the extracellular regulated kinase and c-Jun NH2-terminal kinase groups of mitogen-activated protein kinases. J. Biol. Chem. 1996;271:5750–5754. doi: 10.1074/jbc.271.10.5750. [DOI] [PubMed] [Google Scholar]
- SHEN Y.H., GODLEWSKI J., ZHU J., SATHYANARAYANA P., LEANER V., BIRRER M.J., RANA A., TZIVION G. Cross-talk between JNK/SAPK and ERK/MAPK pathways: sustained activation of JNK blocks ERK activation by mitogenic factors. J. Biol. Chem. 2003;278:26715–26721. doi: 10.1074/jbc.M303264200. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD D.C., OSBORN R.R., BOCHNOWICZ S., WEBB E.F., RIEMAN D.J., LEE J.C., ROMANIC A.M., ADAMS J.L., HAY D.W., GRISWOLD D.E. SB 239063, a p38 MAPK inhibitor, reduces neutrophilia, inflammatory cytokines, MMP-9, and fibrosis in lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000a;279:L895–L902. doi: 10.1152/ajplung.2000.279.5.L895. [DOI] [PubMed] [Google Scholar]
- UNDERWOOD D.C., OSBORN R.R., KOTZER C.J., ADAMS J.L., LEE J.C., WEBB E.F., CARPENTER D.C., BOCHNOWICZ S., THOMAS H.C., HAY D.W., GRISWOLD D.E. SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence. J. Pharmacol. Exp. Ther. 2000b;293:281–288. [PubMed] [Google Scholar]
- WHELCHEL A., EVANS J., POSADA J. Inhibition of ERK activation attenuates endothelin-stimulated airway smooth muscle cell proliferation. Am. J. Respir. Cell. Mol. Biol. 1997;16:589–596. doi: 10.1165/ajrcmb.16.5.9160841. [DOI] [PubMed] [Google Scholar]
- XIA Z., DICKENS M., RAINGEAUD J., DAVIS R.J., GREENBERG M.E. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]