Abstract
As pretreatment with intraperitoneal capsaicin (8-methyl-N-vanillyl-6-nonenamide, CAP), an agonist of the vanilloid receptor known as VR1 or transient receptor potential channel-vanilloid receptor subtype 1 (TRPV-1), has been shown to block the first phase of lipopolysaccharide (LPS) fever in rats, this phase is thought to depend on the TRPV-1-bearing sensory nerve fibers originating in the abdominal cavity. However, our recent studies suggest that CAP blocks the first phase via a non-neural mechanism. In the present work, we studied whether this mechanism involves the TRPV-1.
Adult Long–Evans rats implanted with chronic jugular catheters were used.
Pretreatment with CAP (5 mg kg−1, i.p.) 10 days before administration of LPS (10 μg kg−1, i.v.) resulted in the loss of the entire first phase and a part of the second phase of LPS fever.
Pretreatment with the ultrapotent TRPV-1 agonist resiniferatoxin (RTX; 2, 20, or 200 μg kg−1, i.p.) 10 days before administration of LPS had no effect on the first and second phases of LPS fever, but it exaggerated the third phase at the highest dose. The latter effect was presumably due to the known ability of high doses of TRPV-1 agonists to cause a loss of warm sensitivity, thus leading to uncontrolled, hyperpyretic responses.
Pretreatment with the selective competitive TRPV-1 antagonist capsazepine (N-[2-(4-chlorophenyl)ethyl]-1,3,4,5-tetrahydro-7,8-dihydroxy-2H-2-benzazepine-2-carbothioamidem, CPZ; 40 mg kg−1, i.p.) 90 min before administration of LPS (10 μg kg−1, i.v.) or CAP (1 mg kg−1, i.p.) did not affect LPS fever, but blocked the immediate hypothermic response to acute administration of CAP.
It is concluded that LPS fever is initiated via a non-neural mechanism, which is CAP-sensitive but RTX- and CPZ-insensitive. The action of CAP on this mechanism is likely TRPV-1-independent. It is speculated that this mechanism may be the production of prostaglandin E2 by macrophages in LPS-processing organs.
Keywords: TRPV-1, VR1, capsazepine, iodo-resiniferatoxin, endotoxin, body temperature, thermoregulation, satiety, cholecystokinin, eye-wiping response
Introduction
There are several contradictions regarding the role of the vagus nerve in fever (for a review see Romanovsky, 2004). One of them concerns the mediation of the so-called first febrile phase. In the rat, moderate intravenous doses of bacterial lipopolysaccharide (LPS) induce a polyphasic fever consisting of at least three consecutive body temperature rises – three phases (Romanovsky et al., 1998a, 1998b). Although its magnitude is small (often just a few tenths of a degree), the first febrile phase is highly reproducible (Székely & Szelényi, 1979; Romanovsky et al., 1996; 1997; 1998a, 1998b; Székely et al., 2000; Ivanov et al., 2002; 2003a; Dogan et al., 2003; Steiner et al., 2004) and reliably detectable by a separate burst of thermoeffector activity (i.e. a decrease in tail skin blood flow) or as a separate loop in a phase–plane plot (either the first time derivative of body temperature or thermoeffector activity plotted against body temperature; see Romanovsky et al., 1998a, 1998b). The first phase possesses several remarkable features. Owing to its short latency (20–30 min), it represents the earliest thermoregulatory event occurring in response to LPS and an important end point measure for studying febrigenic signaling. It is also the only febrile phase coupled with hyperalgesia (Romanovsky et al., 1996) and other so-called early sickness symptoms (i.e. those symptoms that alarm the body about the forthcoming inflammation or infection and prepare it for active defense). Finally, it is the only phase of LPS fever that was proposed (Morimoto et al., 1987) and is currently thought (for a review see Romanovsky, 2004) to be triggered by afferent nerve fibers.
Febrigenic signals likely originate in macrophages of the LPS-processing organs, the liver and lung (Ivanov & Romanovsky, 2004). These cells are crucial for LPS uptake (Mathison & Ulevitch, 1979; Freudenberg et al., 1982) and for generation of the febrile (Sehic et al., 1997) and hyperalgesic (Watkins et al., 1994b) responses to LPS. Within minutes, systemic administration of LPS induces prostaglandin (PG)E2-synthesizing enzymes, including cyclo-oxygenase (COX)-2, in the liver and lung (Ivanov et al., 2002). Peripherally originated PGE2 can then be delivered to the brain by albumin (Romanovsky et al., 1999), or it can act locally on afferent nerve fibers, possibly the hepatic vagal fibers. Hepatic fibers bear PG receptors of the EP3 type (Ek et al., 1998), which is one of the two types mediating the first phase of LPS fever (Ushikubi et al., 1998; Oka et al., 2003). Furthermore, activation of vagal afferents by pyrogenic cytokines is at least partially PG-mediated (Niijima, 1996; Ek et al., 1998). Although the effect of selective transection of the hepatic vagal branch on the first phase of LPS fever has not been studied, hepatic vagotomy has been shown to prevent both the monophasic febrile response to a very small, near-threshold dose of LPS (Simons et al., 1998) and the hyperalgesic response to a higher dose of LPS (Watkins et al., 1994a).
Although the circumstantial evidence for an involvement of the hepatic vagus is strong, the direct evidence is lacking, and which sensory nerves mediate the first febrile phase is unclear. We have shown that the first phase of LPS fever in Wistar rats is blocked by pretreatment with small intraperitoneal doses of capsaicin (8-methyl-N-vanillyl-6-nonenamide, CAP; Székely et al., 1997; 2000), which affects the unmyelinated C and thin myelinated Aδ afferent fibers running within both the abdominal vagus and the splanchnic nerve (Jänig & Morrison, 1986; Holzer, 1998). The loss of many functions of sensory fibers in response to this excitotoxin is well documented, and CAP pretreatment is widely used in functional studies (Holzer, 1991). Whereas CAP pretreatment blocked the first phase of LPS fever in Wistar rats, bilateral subdiaphragmatic truncal vagotomy had no effect on this phase in the same rat strain (Romanovsky et al., 1997; Székely et al., 2000), and bilateral splanchnicotomy did not change the first phase of LPS fever in Long–Evans rats (Dogan et al., 2003). Hence, the robust effect of small intraperitoneal doses of CAP on the first febrile phase observed in Wistar rats has no identified anatomical substrate; whether this effect takes place in other strains is unknown; and the mechanisms of this effect are speculative.
CAP is generally accepted to exert its actions via the vanilloid receptor (VR) of subtype 1 (VR1), a ligand-gated nonselective cation channel expressed predominantly on sensory neurons (Holzer, 1998; Szallasi & Blumberg, 1999). According to the new nomenclature (Montell et al., 2002), the VR1 is termed TRPV-1, because it is the first identified member of the family of the so-called transient receptor potential channels-VRs (TRPV). CAP and other TRPV-1 agonists cause a transient excitation of the receptor followed by ‘desensitization', whereas antagonists of the TRPV-1 inhibit the receptor function by preventing its excitation (Kwak et al., 1998; Szallasi & Blumberg, 1999). According to Szallasi (2002), the term desensitization is traditionally used to describe a lasting refractory state, that is, the state when a TPRV-1-mediated response to a stimulus of interest is decreased. The mechanisms of such a decrease are largely unknown, they include the loss of TPRV-1, neuropeptide depletion in sensory nerve terminals, and at least some ultrastructural changes in nerve fibers (see, for example, Farkas-Szallasi et al., 1996; Avelino & Cruz, 2000).
The present study was designed to determine (by either desensitizing or blocking the TRPV-1) whether this receptor is involved in triggering LPS fever in Long–Evans rats. In addition to CAP, the ultrapotent TRPV-1 agonist resiniferatoxin (RTX; see Szallasi & Blumberg, 1989; Szolcsányi et al., 1990) and the selective competitive TRPV-1 antagonist capsazepine (N-[2-(4-chlorophenyl)ethyl]-1,3,4,5-tetrahydro-7,8-dihydroxy-2H-2-benzazepine-2-carbothioamide, CPZ; Bevan et al., 1992) were used. Although recent data show that CPZ is a more potent antagonist of the human TRPV-1 than of the rat TRPV-1 (McIntyre et al., 2001), this drug effectively blocks a variety of TRPV-1-mediated effects in rats, including CAP-induced inhibition of thermogenesis in the brown fat (Osaka et al., 1998) and CAP-induced pain (as evidenced by Fos expression in the brain; Kwak et al., 1998).
Methods
Animals
Whereas our previous studies of CAP desensitization were conducted in Wistar rats (Székely et al., 1997; 2000), the present study was performed in Long–Evans rats (Charles River, Wilmington, MA, U.S.A.). The first phase of LPS fever, the phenomenon of interest, has a 50–100% higher magnitude in the Long–Evans and Long–Evans-derived strains than in the Wistar strain (Romanovsky et al., 1998b; Ivanov & Romanovsky, 2002; Ivanov et al., 2003a). In all, 145 male rats weighing 300–320 g at the time of surgery were used. The animals were housed individually in standard ‘shoe box' cages kept in a rack equipped with a Smart Bio-Pack ventilation system (model SB4100) and Thermo-Pak temperature control system (model TP2000; Allentown Caging Equipment, Allentown, NJ, U.S.A.). The temperature of the incoming air was maintained at 28°C. Standard rat chow (Teklad Rodent Diet ‘W' 8604; Harlan Teklad, Madison, WI, U.S.A.) and tap water were available ad libitum. The room was on a 12 : 12 h light–dark cycle (lights on at 07 : 00). The cage space was enriched with artificial ‘rat holes' (cylindrical confiners made of stainless-steel wire). In addition to spending time in the confiners voluntarily, the rats were systematically habituated to them (eight daily training sessions, 4 h each). The same confiners were used later in the fever test. When well-adapted rats are confined, they exhibit no stress fever (Romanovsky et al., 1998a). Each rat was used in only one experiment. At the end of the study, the rats were euthanized with an overdose of sodium pentobarbital (100 mg kg−1, i.v.). The protocols of the five experiments performed (Exps. 1–5) were approved by the St Joseph's Hospital Animal Care and Use Committee.
Timeline of the procedures and tests
Exp. 1: Effect of CAP pretreatment on LPS fever. The rats were pretreated with a small intraperitoneal dose of CAP or its vehicle on Day 0. The extent of desensitization of afferent nerve fibers was verified by measuring the wiping response to eye irritation on Day 7 and by performing a two-measurement satiety test on ∼Day 15 (first measurement) and Day 19 (second measurement). On Day 8, jugular catheterization was performed. On Day 11, the rats were subjected to the fever test: their body temperature response to LPS or saline was studied. This timeline was chosen based on the fact that the effects attributed to CAP desensitization (impaired satiety response to postdeprivational feeding and impaired first phase of LPS fever) in the model used reach their maxima between 1 and 2 weeks after CAP administration and last for at least 1 month after administration (Székely & Romanovsky, 1997; Székely et al., 2000). The dynamics of CAP and RTX desensitization in other models is remarkably similar (Craft et al., 1995; Xu et al., 1997).
Exps. 2–4: Effect of RTX pretreatment on LPS fever. The protocols and time schedule of these experiments were the same as of Exp. 1, except that the rats were pretreated with one of three doses of RTX instead of CAP. Different doses of RTX were used to achieve different degrees of afferent nerve desensitization. Whereas the two smallest doses (Exps. 2 and 3) were administered in one injection, the largest dose (Exp. 4) was administered in three increments on 3 consecutive days. In Exp. 4, the day of the last injection of RTX (or vehicle) was considered Day 0.
Exp. 5: Effect of CPZ on LPS fever. The rats were catheterized on Day 0 and subjected to the fever test on Day 3. The effect of CPZ or its vehicle on the body temperature response to LPS or saline was studied. In a separate group, the effectiveness of the dose of CPZ used in the fever tests was verified by its ability to antagonize the immediate hypothermic response to acute administration of CAP. In this group, the rats were catheterized on Day 0 and subjected to the CPZ effectiveness test on Day 3.
Pretreatment with CAP or RTX
Unless stated otherwise, all drugs and reagents were purchased from Sigma-Aldrich (St Louis, MO, U.S.A.). Both CAP and RTX (from Euphorbia poisonii) were dissolved in a 10% ethanol, 10% Tween 80 solution in saline. The total dose of CAP (5 mg kg−1, Exp. 1) was administered in one intraperitoneal injection. The two smallest doses of RTX (2 and 20 μg kg−1, Exps. 2 and 3, respectively) were also administered in one injection. The largest dose of RTX (200 μg kg−1, Exp. 4) was administered in three injections (20, 50, and 130 μg kg−1, respectively) on 3 consecutive days. The first (Exp. 4) or the only (Exps. 1–3) administration of CAP or RTX was performed under sedation with ketamine–xylazine–acepromazine (5.6, 0.6, and 1.2 mg kg−1, i.p., respectively). As the first dose of RTX was sufficient to desensitize intra-abdominal nerves (see Results), the subsequent doses of RTX were administered without sedation. Control (‘sham-desensitized') rats were injected with the vehicle according to the same protocols.
Wiping response to eye irritation
Both the eye wiping test and the satiety test (see below) were performed according to Kelly et al. (2000). When the rat was in its home cage, a small (20 μl) amount of a 1% NH4OH solution in saline was dropped in its right eye to elicit defensive wiping. The number of wipes during the first 30 s was counted. If desensitization of afferent nerve fibers extended to locations outside the abdominal cavity and resulted in the abolition of the chemosensitivity of the cornea, the test was expected to reveal a suppression of the wiping defense (Szallasi & Blumberg, 1989).
Satiety test
The test is based on the ability of cholecystokinin (CCK) to cause satiety by acting on sensory vagal fibers (Ritter & Ladenheim, 1985; South, 1992; Schwartz et al., 1999). Each rat was tested on two separate days, each time after a 24-h food deprivation. The rat was injected with CCK-8 sulfate (Tyr(SO3H)27-fragment 26–33 amide, 6 μg kg−1, i.p.) on one day and with saline (1 ml kg−1, i.p.) on the other day; the order of the injections was randomized. After the injection, the rat was returned to its home cage. An excessive, preweighed amount of regular chow was introduced in the cage 5 min after the injection, and the rat was allowed to eat for 30 min. The remaining food was weighed, and the consumed amount was calculated. For each rat, the difference in food consumption (CCK test minus saline test) was expressed as percentage of the amount consumed in the saline test. A large negative percentage value would mean that CCK was effective in decreasing food consumption, as it was expected to do in vehicle-pretreated (sham-desensitized) rats. A near-zero or positive value would mean that CAP had no satiety effect; such a value could be expected in animals with functionally impaired (desensitized) intra-abdominal vagal afferents.
Jugular catheterization
Under ketamine–xylazine–acepromazine (55.6, 5.5, and 1.1 mg kg−1, i.p., respectively) anesthesia and antibiotic (enrofloxacin, 12 mg kg−1, s.c.) protection, each rat was implanted with a jugular catheter. The rat was placed on an operating board, and a 1-cm longitudinal incision was made on the ventral surface of the neck, 1 cm left of the trachea. The left jugular vein was exposed, freed from its surrounding connective tissue, and ligated. A silicone catheter (ID 0.5 mm, OD 0.9 mm) filled with heparinized (50 U ml−1) pyrogen-free saline was passed into the superior vena cava through the jugular vein and secured in place with ligatures. The 10-cm free end of the silicone catheter was knotted, tunneled under the skin, and exteriorized at the nape. The surgical wound was sutured. The catheter was flushed with heparinized saline every other day.
Fever test
Each rat was placed in a confiner and equipped with a copper–constantan thermocouple for recording colonic temperature (Tc). The thermocouple was inserted 10 cm beyond the anal sphincter and fixed to the base of the tail with adhesive tape. It was plugged into a data logger (Dianachart, Rockaway, NJ, U.S.A.), which was connected to a computer. The rat was transferred to a climatic chamber (Forma Scientific, Marietta, OH, U.S.A.) set to 30.0°C, which is within the thermoneutral zone for male adult Long–Evans rats in this experimental setup (Romanovsky et al., 2002). The jugular catheter was extended with a length of PE-50 tubing filled with saline, and the extension was passed through a wall port and connected to a syringe filled with the drug of interest. This setup permitted intravenous drug administration from outside the chamber, without disturbing the animal. To induce fever, Escherichia coli 0111:B4 LPS (10 μg kg−1) suspended in saline (10 μg ml−1) was injected over 1 min; control rats received saline (1 ml kg−1). In Exp. 5, each rat was pretreated with CPZ (Tocris Cookson, Ellisville, MO, U.S.A.; 40 mg kg−1, i.p.) or its vehicle (20% ethanol, 10% Tween 80 in saline; 1 ml kg−1) 90 min before the injection of LPS or saline. Tc was recorded every 2 min for at least 60 min before and 420 min after the injection.
CPZ effectiveness test
This test was based on the ability of TRPV-1 agonists to cause hypothermia and inhibit thermogenesis in the brown fat when administered peripherally (Jancsó-Gábor et al., 1970; Woods et al., 1994; Osaka et al., 1998) and on the ability of CPZ to block this effect (Osaka et al., 1998). The rats were instrumented as for the fever test. CPZ (40 mg kg−1, i.p.) or its vehicle was injected 90 min before administration of CAP (1 mg kg−1, i.p.). Tc was recorded for 240 min postinjection.
Data processing and analysis
The absolute value of Tc was used to evaluate deep body temperature responses. The Tc curves were compared across treatments and time points by two-way ANOVA for repeated measures using Statistica AX'99 (StatSoft, Tulsa, OK, U.S.A.). For the purpose of analysis, the febrile phases were defined based on the following timing: 20–70 min post-LPS for the first phase, 80–160 min for the second phase, and 170–420 min for the third phase. The results of the wiping test (number of eye wipes) and satiety test (relative difference in food consumption; see Satiety test above) were compared by Student's t-test. Student's t-test was also applied to compare the maximal decreases in Tc after acute administration of CAP to the CPZ-pretreated and vehicle-pretreated rats. The maximal decrease in Tc was calculated as a difference between the nadir of the hypothermic response and the Tc immediately before the injection of CAP. The data are reported as means±s.e.
Results
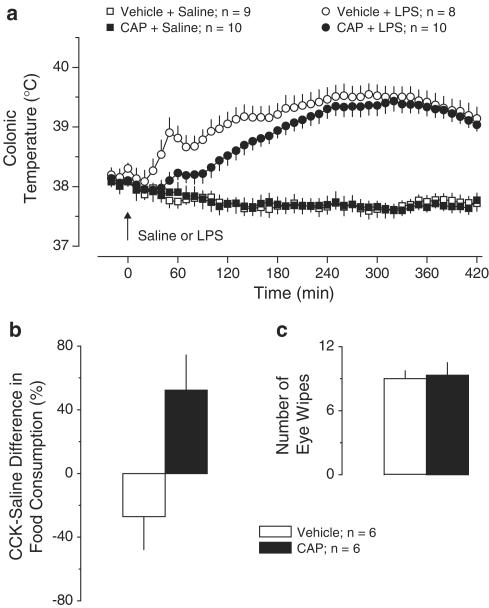
Exp. 1: Effect of CAP (5 mg kg−1, i.p.) pretreatment on LPS fever. The Tc responses of the vehicle-pretreated and CAP-pretreated Long–Evans rats are shown in Figure 1a. In the vehicle-pretreated rats, LPS (10 μg kg−1, i.v.) caused a triphasic febrile response. The onset of the response (the entire first phase and a part of the second phase) was strongly attenuated in the CAP-pretreated rats (P<2.5 × 10−2). At 50 min post-LPS, Tc in the CAP-pretreated group was only 38.1±0.1°C, whereas in the controls it was 38.9±0.2°C. Saline had no thermal effect in either group. The CCK-induced satiety (a 27±21% decrease in food intake) seen in the vehicle-pretreated rats was reversed in the CAP-pretreated rats: they exhibited a 52±22% increase in food intake in response to CCK (P<1.7 × 10−2, Figure 1b). This finding indicates that the afferent fibers involved in food intake (presumably vagal fibers of intra-abdominal origin) were clearly affected by CAP pretreatment. However, CAP desensitization was not generalized as evident by the unaffected chemosensitivity of the cornea (Figure 1c).
Figure 1.
Effects of pretreatment with CAP (5 mg kg−1, i.p.) or vehicle on the following responses: the body temperature response to LPS (10 μg kg−1, i.v.) or saline (a); the relative difference in the amount of food consumed by food-deprived rats during a 30-min period after administration of CCK-8 sulfate (6 μg kg−1, i.p.) or saline (b); and the number of eye wipes during a 30-s period after intraocular administration of a drop of 1% NH4OH (c).
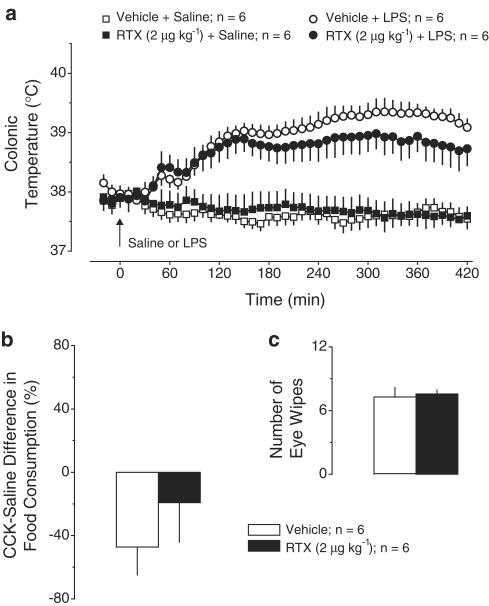
Exp. 2: Effect of RTX (2 μg kg−1, i.p.) pretreatment on LPS fever. Pretreatment with this low dose of RTX did not affect the thermal response to LPS or saline (Figure 2a). This pretreatment did not cause desensitization of nerve fibers either inside the abdominal cavity (no effect on the CCK-induced satiety; Figure 2b) or in extra-abdominal locations (no effect on the eye-wiping response to irritation; Figure 2c).
Figure 2.
Effects of pretreatment with RTX (2 μg kg−1, i.p.) or vehicle on the following responses: the body temperature response to LPS (10 μg kg−1, i.v.) or saline (a); the relative difference in the amount of food consumed by food-deprived rats during a 30-min period after administration of CCK-8 sulfate (6 μg kg−1, i.p.) or saline (b); and the number of eye wipes during a 30-s period after intraocular administration of a drop of 1% NH4OH (c).
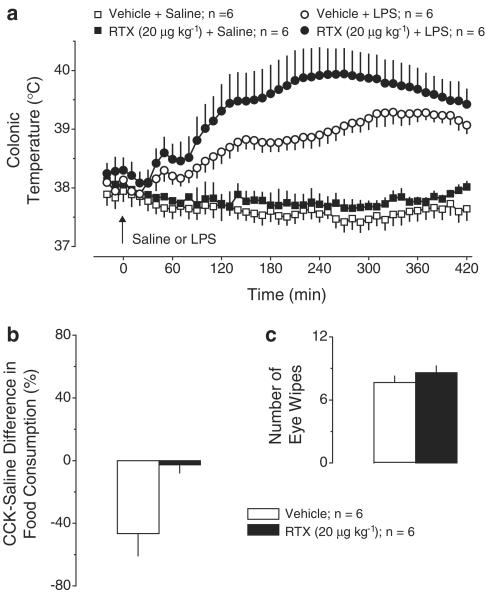
Exp. 3: Effect of RTX (20 μg kg−1, i.p.) pretreatment on LPS fever. Pretreatment with this moderate dose of RTX did not affect the thermal response to saline but tended to change LPS fever (Figure 3a). The first phase was not attenuated, but the second and third febrile phases tended to be higher than in the vehicle-pretreated rats. Pretreatment with RTX affected the intra-abdominal nerve afferents. Indeed, CCK (as compared to saline) decreased the food intake by 47±14% in the vehicle-pretreated rats, but only by 6±5% in the RTX-pretreated rats (P<1.8 × 10−2, Figure 3b). The same pretreatment failed to affect the chemosensitivity of the cornea (Figure 3c).
Figure 3.
Effects of pretreatment with RTX (20 μg kg−1, i.p.) or vehicle on the following responses: the body temperature response to LPS (10 μg kg−1, i.v.) or saline (a); the relative difference in the amount of food consumed by food-deprived rats during a 30-min period after administration of CCK-8 sulfate (6 μg kg−1, i.p.) or saline (b); and the number of eye wipes during a 30-s period after intraocular administration of a drop of 1% NH4OH (c).
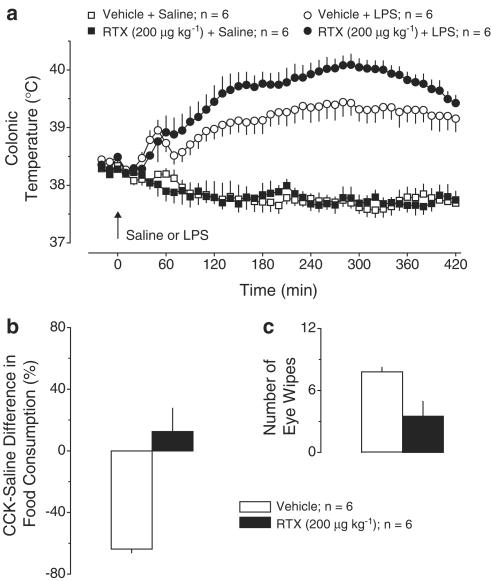
Exp. 4: Effect of RTX (200 μg kg−1, i.p.) pretreatment on LPS fever. Pretreatment with this high dose of RTX affected neither the Tc response to saline nor the first phase of LPS fever (Figure 4a). However, the second phase of fever showed a tendency to be increased in the RTX-pretreated rats, whereas the third phase was significantly (P<1.7 × 10−2) exaggerated. At 280 min post-LPS, Tc in the RTX-treated rats was 40.1±0.2°C, whereas it was only 39.4±0.2°C in the vehicle-pretreated rats. Pretreatment with this high dose of RTX affected sensory nerve fibers originated both inside and outside the abdominal cavity. In response to CCK, food intake was decreased by 64±7% in the vehicle-pretreated rats but increased by 13±15% in the RTX-pretreated rats (P<2.4 × 10−3, Figure 4b). The wiping response to eye irritation was decreased in the RTX-pretreated rats as compared to the vehicle-pretreated controls (4±1 vs 8±1 wipes, P<2.0 × 10−2, Figure 4c).
Figure 4.
Effects of pretreatment with RTX (200 μg kg−1, i.p.) or vehicle on the following responses: the body temperature response to LPS (10 μg kg−1, i.v.) or saline (a); the relative difference in the amount of food consumed by food-deprived rats during a 30-min period after administration of CCK-8 sulfate (6 μg kg−1, i.p.) or saline (b); and the number of eye wipes during a 30-s period after intraocular administration of a drop of 1% NH4OH (c).
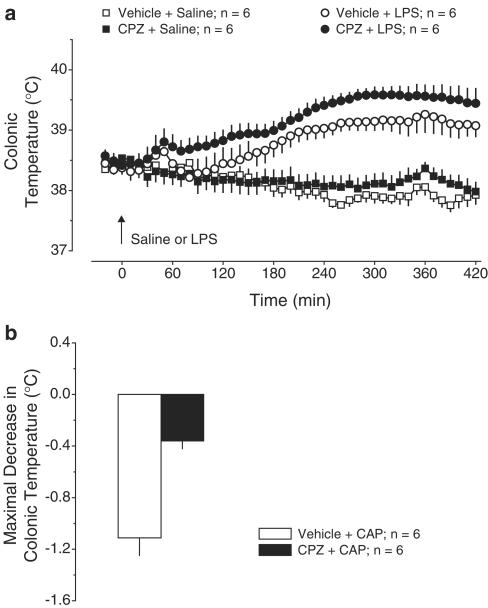
Exp. 5: Effects of CPZ (40 mg kg−1, i.p.) on LPS fever. In agreement with how ethanol-containing vehicles affect LPS fever (Ivanov et al., 2003b), the entire febrile response of the vehicle-pretreated rats was slightly suppressed, but it still had a distinct first phase (Figure 5a). Pretreatment with CPZ affected neither the febrile response to LPS nor the Tc response to saline. If anything, the first febrile phase tended to be increased in the CPZ-pretreated rats. However, the same dose of CPZ was effective in blocking the hypothermic response to acute administration of CAP (1 mg kg−1, i.p.): the maximal decrease of Tc in response to CAP was 1.1±0.1°C in the vehicle pretreated rats but only 0.3±0.1°C in the CPZ-pretreated rats (P<2.6 × 10−3, Figure 5b).
Figure 5.
Effects of pretreatment with CPZ (40 mg kg−1, i.p.) or vehicle on the following responses: the body temperature response to LPS (10 μg kg−1, i.v.) or saline (a) and CAP (1 mg kg−1, i.p.)-induced hypothermia (b). CPZ was administered 90 min before LPS or CAP.
Discussion
Effect of CAP on LPS fever: a reproducible phenomenon
The present study shows that pretreatment of Long–Evans rats with a small intraperitoneal dose of CAP results in a blockade of the first phase of LPS fever. Other effects of the same pretreatment suggest that it affects sensory nerve fibers locally (i.e. fibers in the abdominal cavity involved in the satiety response) but does not impair sensory nerves outside the abdominal cavity (i.e. chemosensitive fibers involved in the response to irritation of the cornea). These results closely reproduce our earlier observations in Wistar rats. Indeed, pretreatment of Wistar rats with low doses of CAP (5–25 mg kg−1, i.p.) blocks the first phase of LPS fever (Székely et al., 1997; 2000) and affects the satiety response (Székely et al., 1997; 2000) but does not cause generalized desensitization (Székely & Romanovsky, 1997) that follows administration of high doses (50–400 mg kg−1, s.c.) to newborn (Gourine et al., 2001) or adult (Szolcsányi, 1982) animals. High doses of CAP cause thermoregulatory impairments compatible with a loss of warm sensors, for example, an exaggeration of the entire course of LPS fever (Székely & Szolcsányi, 1979; Szolcsányi, 1982) or a pronounced increase in the threshold Tc for tail skin vasodilation (Székely & Romanovsky, 1997).
Blockade of the first febrile phase by CAP: a non-neural effect
Mechanisms of the blockade of the first phase of LPS fever by low doses of CAP are unknown. This febrile phase has been repeatedly found to be completely insensitive to bilateral subdiaphragmatic truncal vagotomy (Romanovsky et al., 1997; Székely et al., 2000) or local desensitization of vagal fibers by direct application of CAP on vagal trunks (M. Székely, & E. Pétervári, unpublished observation). The first phase has also been found to be insensitive to bilateral transection of the major splanchnic nerve (Dogan et al., 2003). Furthermore, LPS-induced hyperalgesia, which occurs at the first febrile phase (Romanovsky et al., 1996), is also insensitive to splanchnic denervation of the abdominal viscera by combined celiac and superior mesenteric ganglionectomy (Watkins et al., 1994a). Cumulatively, these data suggest that neither the vagus nor the splanchnic nerve is responsible for the blockade of the first phase of LPS fever by low doses of CAP. As the same doses of CAP that result in the loss of the first phase do not cause desensitization of nerve fibers outside the abdominal cavity, an involvement of extra-abdominal neural targets is also unlikely. Hence, it is plausible that CAP affects the first phase of LPS fever by acting on non-neural targets.
That CAP can affect a variety of non-neural cells, including immune cells, is well known. This TRPV-1 agonist binds to mast cells and causes release of proinflammatory cytokines (Bíró et al., 1998). It has also been shown to inhibit cytokine-induced activation of NF-κB transcription factor (by blocking the degradation of its inhibitory protein IκB-α) in myeloid cells (Singh et al., 1996), to enhance the release of substance P from lymphocytes (Lai et al., 1998), and to stimulate the migration of polymorphonuclear leukocytes (Partsch & Matucci-Cerinic, 1993). Even more relevant to pathogenesis of fever is that CAP has profound effects on macrophages. Being responsible for LPS uptake and for hepatic and pulmonary production of the ultimate mediator of fever, PGE2, macrophages are thought to mediate the first phase of LPS fever (see Introduction). It has been shown that CAP impairs mitochondrial functions in macrophages (Garle et al., 2000) and modulates the production of inflammatory mediators (Ho et al., 1997), including PGs (Joe & Lokesh, 1994; Joe et al., 1997), by these cells. Recently, Kim et al. (2003) have demonstrated that CAP inhibits LPS-induced production of PGE2 by peritoneal macrophages. The mechanisms of the latter effect include inhibition of LPS-induced activation of NF-κB by blocking the degradation of IκB-α and direct inhibition of the enzymatic activity of COX-2 (Kim et al., 2003). It is also important that at least some non-neural effects of CAP, such as a nephrotoxic action (Creppy et al., 2000), impairment of mitochondria in monocytes (Garle et al., 2000), and decrease in the number of mast cells in the jejunal mucosa (Gottwald et al., 1997), are likely to have long-lasting functional consequences.
Blockade of the first febrile phase by CAP: a non-TRPV-1-mediated effect
The failure to identify a neural substrate of CAP effect of the first phase of fever caused us to think about ‘nonspecific' (non-TRPV-1-mediated) effects. Indeed, there is plenty of pharmacological evidence for heterogeneity of VRs, including the existence of a CPZ-insensitive VR (Liu et al., 1998). After the identification of TRPV-1 (Caterina et al., 1997), several related family members were cloned (Harteneck et al., 2000; Gunthorpe et al., 2002). The second family member isolated, which was termed VR-like protein-1 (VRL-1) and is now known as TRPV-2, is expressed on a variety of non-neural cells throughout the body (Caterina et al., 1999). Human and mouse TRPV-2 orthologs (the latter termed growth-factor-regulated channel) have been isolated from myeloid cells (Kanzaki et al., 1999). At least two different splice variants of the TRPV-1 have also been identified in non-neural tissues (Suzuki et al., 1999; Schumacher et al., 2000).
Importantly, macrophages express VRs that are different from the TRPV-1. Murine peritoneal macrophages profoundly express the TRPV-2 but do not express the TRPV-1 (Kim et al., 2003). Although rat peripheral blood mononuclear cells express the TRPV-1, they also express its 5′-splice variant with a drastically different pharmacological profile (Schumacher et al., 2000). In macrophages, CAP causes mitochondrial damage via a non-TRPV-1 mechanism (Garle et al., 2000). The ability of CAP to block PGE2 synthesis in macrophages has also been shown to be CPZ-insensitive and TRPV-1-independent (Kim et al., 2003).
As RTX is generally a much more (in some cases, 10,000-fold) potent agonist of the TRPV-1 than CAP (Szallasi & Blumberg, 1989; Szolcsányi et al., 1990), the spectrum of its effects is likely to be shifted towards more TRPV-1-mediated and less non-TRPV-1-mediated effects. In the present study, pretreatment with RTX at any dose tested was ineffective in attenuating the first phase of LPS fever, although different doses of RTX exerted several different (and fully expected) effects. For example, the use of the highest dose resulted in an abolition of the chemosensitivity of the cornea and in an exaggeration of the third febrile phase; both of these effects are symptoms of generalized desensitization of afferent nerves (Székely & Szolcsányi, 1979; Szolcsányi, 1982; Szallasi & Blumberg, 1989). In addition to being RTX-insensitive, the first phase of LPS fever was also found to be CPZ-insensitive, even though pretreatment with the same dose of CPZ effectively eliminated the immediate hypothermic response to acute administration of CAP. These data suggest that CAP-induced blockade of the first phase of LPS fever likely occurs via a TRPV-1-independent mechanism.
A more definite conclusion would require obtaining additional evidence of noninvolvement of the TRPV-1 in the first febrile phase. Unfortunately, such evidence cannot be produced with the best tools currently available: the TRPV-1 null mutant (knockout) mouse (Caterina et al., 2000) and the high-affinity, selective TRPV-1 antagonist 5′-iodo-RTX (I-RTX; see Wahl et al., 2001; Undem & Kollarik, 2002; Rigoni et al., 2003). Indeed, noninvolvement of the TRPV-1 cannot be confirmed in an experiment in the receptor knockout mouse, because negative results obtained in knockouts are inconclusive. They can mean either that the receptor of interest plays no role in the response studied or that the receptor normally plays a role in the response, but that this role is assumed by other players (compensatory mechanisms) when the receptor is genetically deleted. Furthermore, data obtained in the knockout mouse may not tell much about the role of the TRPV-1 in the rat, the species used in the present study, because the bodily distribution of the TRPV-1 differs substantially between the two species (Woodbury et al., 2004).
As for I-RTX, there is a single study reporting its ability to antagonize TRPV-1-mediated effects upon systemic administration in vivo; the doses found effective in this study were 300 ng kg−1 and 1.0 μmol kg−1, i.v. (Rigoni et al., 2003). We have tested similar doses of I-RTX (100 ng kg−1–1.0 μmol kg−1, i.p.) and found that they cause marked hypothermia (up to 3°C) at a neutral ambient temperature and even larger hypothermia (∼5°C) at a subneutral temperature (S. Patel, A.A. Steiner & A.A. Romanovsky, unpublished observations). As the magnitude of this hypothermia is several fold larger than the magnitude of the first febrile phase, these doses cannot be used to study LPS fever. At a lower dose of 20 nmol kg−1, I-RTX was thermally ineffective, but it was also ineffective in blocking CAP-induced, TRPV-1-mediated hypothermia.
The hypothermic action is not the only undesired in vivo effect of I-RTX. Symanowicz et al. (2004) have recently reported that intranasal I-RTX produces what they call dramatic changes in basal breathing pattern. The fact that both noniodinated RTX and RTX iodinated in position 2 (instead of 5) are agonists of the TRPV-1 (McDonnell et al., 2002) makes it plausible to think about an intrinsic TRPV-1 agonistic activity of I-RTX. However, several experiments aimed at finding a TRPV-1 agonistic activity of I-RTX in vitro have failed (Wahl et al., 2001). Substantial contamination of I-RTX preparation with TRPV-1 agonists is also unlikely. Indeed, most preparations of I-RTX (Wahl et al., 2001; Seabrook et al., 2002), including Sigma's preparation we tested, are 97–99% pure. It is more likely that I-RTX activates a different, presently unidentified receptor. In support of such a proposition, Seabrook et al. (2002) have found that intraplantar I-RTX causes aversive behavior both in wild-type and TRPV-1 knockout mice. The authors have also found that intraplantar I-RTX is ineffective in blocking the TRPV-1 in their in vivo model, even when it is administered at a dose shown to block TRPV-1-mediated responses upon intrathecal administration (Wahl et al., 2001). Seabrook et al. (2002) concluded that I-RTX has limited utility for behavioral studies, and that antagonists with better pharmaceutical properties have to be developed to fully explore functional relevance of the TRPV-1 in vivo. According to our experience, I-RTX is unsuitable for studying thermoregulatory responses in vivo, at least in the rat.
Conclusions
By summarizing our present and past results, we conclude that LPS fever is initiated via a non-neural mechanism, which is CAP-sensitive, but RTX- and CPZ-insensitive. The action of CAP on this mechanism is likely TRPV-1-independent. It is speculated that this mechanism may involve the production of PGE2 by macrophages in LPS-processing organs such as the liver and lung, and that pretreatment with CAP may suppress the release of PGE2 by these cells in response to LPS. That peripherally originated PGE2 is crucial only for the first phase of LPS fever, whereas the subsequent phases are mediated by both peripheral and central PGE2 (Ivanov & Romanovsky, 2004) agrees well with the fact that CAP affects mainly the first phase. The possibility of using CAP for targeted inhibition of PGE2 production in peripheral tissues should be evaluated.
Acknowledgments
We thank F.E. Farmer and J.W. Liu for graphic assistance and Dr A.I. Ivanov for reading the manuscript and providing important feedback. The work was funded in part by a National Institute of Neurological Disorders and Stroke Grant NS-41233 and Arizona Disease Control Research Commission category II Grant 8016 (A.A. Romanovsky).
Abbreviations
- CAP
capsaicin
- CCK
cholecystokinin
- COX
cyclo-oxygenase
- CPZ
capsazepine
- Exp(s).
experiment(s)
- I-RTX
iodo-resiniferatoxin
- LPS
lipopolysaccharide
- PG(s)
prostaglandin(s)
- RTX
resiniferatoxin
- Tc
colonic temperature
- TRPV
transient receptor potential channel-vanilloid receptor
- VR(s)
vanilloid receptor(s)
- VR1
subtype 1 VR (a.k.a. TRPV-1)
- VRL-1
VR-like protein-1 (a.k.a. TRPV-2)
References
- AVELINO A., CRUZ F. Peptide immunoreactivity and ultrastructure of rat urinary bladder nerve fibers after topical desensitization by capsaicin or resiniferatoxin. Auton. Neurosci. 2000;86:37–46. doi: 10.1016/S1566-0702(00)00204-6. [DOI] [PubMed] [Google Scholar]
- BEVAN S., HOTHI S., HUGHES G., JAMES I.F., RANG H.P., SHAH K., WALPOLE C.S.J., YEATS J.C. Capsazepine: a competitive antagonist of the sensory neuron excitant capsaicin. Br. J. Pharmacol. 1992;107:544–552. doi: 10.1111/j.1476-5381.1992.tb12781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BÍRÓ T., MAURER M., MODARRES S., LEWIN N.E., BRODIE C., ÁCS G., ÁCS P., PAUS R., BLUMBERG P.M. Characterization of functional vanilloid receptors expressed by mast cells. Blood. 1998;91:1332–1340. [PubMed] [Google Scholar]
- CATERINA M.J., LEFFLER A., MALMBERG A.B., MARTIN W.J., TRAFTON J., PETERSEN-ZEITZ K.R., KOLTZENBURG M., BASBAUM A.I., JULIUS D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., ROSEN T.A., TOMINAGA M., BRAKE A.J., JULIUS D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- CATERINA M.J., SCHUMACHER M.A., TOMINAGA M., ROSEN T.A., LEVINE J.D., JULIUS D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- CRAFT R.M., COHEN S.M., PORRECA F. Long-lasting desensitization of bladder afferents following intravesical resiniferatoxin and capsaicin in the rat. Pain. 1995;61:317–323. doi: 10.1016/0304-3959(94)00193-I. [DOI] [PubMed] [Google Scholar]
- CREPPY E.E., RICHEUX F., CARRATÚ M.-R., CUOMO V., COCHEREAU C., ENNAMANY R., SABOUREAU D. Cytotoxicity of capsaicin in monkey kidney cells: lack of antagonistic effects of capsazepine and Ruthenium red. Arch. Toxicol. 2000;73:40–47. doi: 10.1007/s002040050650. [DOI] [PubMed] [Google Scholar]
- DOGAN M.D., KULCHITSKY V.A., PATEL S., PÉTERVÁRI E., SZÉKELY M., ROMANOVSKY A.A. Bilateral splanchnicotomy does not affect lipopolysaccharide-induced fever in rats. Brain Res. 2003;993:227–229. doi: 10.1016/j.brainres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- EK M., KUROSAWA M., LUNDEBERG T., ERICSSON A. Activation of vagal afferents after intravenous injection of interleukin-1β: role of endogenous prostaglandins. J. Neurosci. 1998;18:9471–9479. doi: 10.1523/JNEUROSCI.18-22-09471.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FARKAS-SZALLASI T., BENNETT G.J., BLUMBERG P.M., HÖKFELT T., LUNDBERG J.M., SZALLASI A. Vanilloid receptor loss is independent of the messenger plasticity that follows systemic resiniferatoxin administration. Brain Res. 1996;719:213–218. doi: 10.1016/0006-8993(96)00065-0. [DOI] [PubMed] [Google Scholar]
- FREUDENBERG M.A., FREUDENBERG N., GALANOS C. Time course and cellular distribution of endotoxin in liver, lungs, and kidneys of rats. Br. J. Exp. Pathol. 1982;63:56–65. [PMC free article] [PubMed] [Google Scholar]
- GARLE M.J., KNIGHT A., DOWNING A.T., JASSI K.L., CLOTHIER R.H., FRY J.R. Stimulation of dichlorofluorescein oxidation by capsaicin and analogues in RAW 264 monocyte/macrophages: lack of involvement of the vanilloid receptor. Biochem. Pharmacol. 2000;59:563–572. doi: 10.1016/s0006-2952(99)00370-6. [DOI] [PubMed] [Google Scholar]
- GOTTWALD T., LHOTÁK Š., STEAD R.H. Effect of truncal vagotomy and capsaicin on mast cells and IgA-positive plasma cells in rat jejunal mucosa. Neurogastroenterol. Motil. 1997;9:25–32. doi: 10.1046/j.1365-2982.1997.d01-4.x. [DOI] [PubMed] [Google Scholar]
- GOURINE A.V., RUDOLPH K., KORSAK A.S., KUBATKO J., TESFAIGZI J., KOZAK W., KLUGER M.J. Role of capsaicin-sensitive afferents in fever and cytokine responses during systemic and local inflammation in rats. Neuroimmunomodulation. 2001;9:13–22. doi: 10.1159/000049003. [DOI] [PubMed] [Google Scholar]
- GUNTHORPE M.J., BENHAM C.D., RANDALL A., DAVIS J.B. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol. Sci. 2002;23:183–191. doi: 10.1016/s0165-6147(02)01999-5. [DOI] [PubMed] [Google Scholar]
- HARTENECK C., PLANT T.D., SCHULTZ G. From worm to man: three subfamilies of TRP channels. Trends Neurosci. 2000;23:159–166. doi: 10.1016/s0166-2236(99)01532-5. [DOI] [PubMed] [Google Scholar]
- HO W.-Z., LAI J.-P., ZHU X.H., UVAYDOVA M., DOUGLAS S.D. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 1997;159:5654–5660. [PubMed] [Google Scholar]
- HOLZER P. Capsaicin: cellular targets, mechanism of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991;43:143–201. [PubMed] [Google Scholar]
- HOLZER P. Neural injury, repair, and adaptation in the GI tract. II. The elusive action of capsaicin on the vagus nerve. Am. J. Physiol. 1998;275:G8–G13. doi: 10.1152/ajpgi.1998.275.1.G8. [DOI] [PubMed] [Google Scholar]
- IVANOV A.I., KULCHITSKY V.A., ROMANOVSKY A.A. Role for the cholecystokinin-A receptor in fever: a study of a mutant rat strain and a pharmacological analysis. J. Physiol. 2003a;547:941–949. doi: 10.1113/jphysiol.2002.033183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOV A.I., PATEL S., KULCHITSKY V.A., ROMANOVSKY A.A. Platelet-activating factor: a previously unrecognized mediator of fever. J. Physiol. 2003b;553:221–228. doi: 10.1113/jphysiol.2003.055616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVANOV A.I., PERO R.S., SHECK A.C., ROMANOVSKY A.A. Prostaglandin E2-synthesizing enzymes in fever: differential transcriptional regulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;283:R1104–R1117. doi: 10.1152/ajpregu.00347.2002. [DOI] [PubMed] [Google Scholar]
- IVANOV A.I., ROMANOVSKY A.A. Fever responses of Zucker rats with and without fatty mutation of the leptin receptor. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002;282:R311–R316. doi: 10.1152/ajpregu.00376.2001. [DOI] [PubMed] [Google Scholar]
- IVANOV A.I., ROMANOVSKY A.A. Prostaglandin E2 as a mediator of fever: synthesis and catabolism. Front. Biosci. 2004;9:1977–1993. doi: 10.2741/1383. [DOI] [PubMed] [Google Scholar]
- JANCSÓ-GÁBOR A., SZOLCSÁNYI J., JANCSÓ N. Stimulation and desensitization of the hypothalamic heat-sensitive structures by capsaicin in rats. J. Physiol. 1970;208:449–459. doi: 10.1113/jphysiol.1970.sp009130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JÄNIG W., MORRISON J.F.B. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on nociception. Progr. Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- JOE B., LOKESH B.R. Role of capsaicin, curcumin and dietary n-3 fatty acids in lowering the generation of reactive oxygen species in rat peritoneal macrophages. Biochim. Biophys. Acta. 1994;1224:255–263. doi: 10.1016/0167-4889(94)90198-8. [DOI] [PubMed] [Google Scholar]
- JOE B., RAO U.J., LOKESH B.R. Presence of an acidic glycoprotein in the serum of arthritic rats: modulation by capsaicin and curcumin. Mol. Cell. Biochem. 1997;169:125–134. doi: 10.1023/a:1006877928703. [DOI] [PubMed] [Google Scholar]
- KANZAKI M., ZHANG Y.-Q., MASHIMA H., LI L., SHIBATA H., KOJIMA I. Translocation of a calcium-permeable cation channel induced by insulin-like growth factor-I. Nat. Cell Biol. 1999;1:165–170. doi: 10.1038/11086. [DOI] [PubMed] [Google Scholar]
- KELLY L., MORALES S., SMITH B.K., BERTHOUD H.-R. Capsaicin-treated rats permanently overingest low- but not high-concentration sucrose solutions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000;279:R1805–R1812. doi: 10.1152/ajpregu.2000.279.5.R1805. [DOI] [PubMed] [Google Scholar]
- KIM C.-S., KAWADA T., KIM B.-S., HAN I.-S., CHOE S.-Y., KURATA T., YU R. Capsaicin exhibits anti-inflammatory property by inhibiting IκB-a degradation in LPS-stimulated peritoneal macrophages. Cell Signal. 2003;15:299–306. doi: 10.1016/s0898-6568(02)00086-4. [DOI] [PubMed] [Google Scholar]
- KWAK J.Y., JUNG J.Y., HWANG S.W., LEE W.T., OH U. A capsaicin-receptor antagonist, capsazepine, reduces inflammation-induced hyperalgesic responses in rat: evidence for an endogenous capsaicin-like substance. Neuroscience. 1998;86:619–626. doi: 10.1016/s0306-4522(98)00012-8. [DOI] [PubMed] [Google Scholar]
- LAI J.-P., DOUGLAS S.D., HO W.-Z. Human lymphocytes express substance P and its receptor. J. Neuroimmunol. 1998;86:80–86. doi: 10.1016/s0165-5728(98)00025-3. [DOI] [PubMed] [Google Scholar]
- LIU L., SZALLASI A., SIMON S.A. A non-pungent resiniferatoxin analogue, phorbol 12-phenylacetate 13 acetate 20-homovanillate, reveals vanilloid receptor subtypes on rat trigeminal ganglion neurons. Neuroscience. 1998;84:569–581. doi: 10.1016/s0306-4522(97)00523-x. [DOI] [PubMed] [Google Scholar]
- MATHISON J.C., ULEVITCH R.J. The clearance, tissue distribution, and cellular localization of intravenously injected lipopolysaccharide in rabbits. J. Immunol. 1979;123:2133–2143. [PubMed] [Google Scholar]
- MCDONNELL M.E., ZHANG S.P., DUBIN A.E., DAX S.L. Synthesis and in vitro evaluation of a novel iodinated resiniferatoxin derivative that is an agonist at the human vanilloid VR1 receptor. Bioorg. Med. Chem. Lett. 2002;22:1189–1192. doi: 10.1016/s0960-894x(02)00127-0. [DOI] [PubMed] [Google Scholar]
- MCINTYRE P., MCLATCHIE L.M., CHAMBERS A., PHILLIPS E., CLARKE M., SAVIDGE J., TOMS C., PEACOCK M., SHAH K., WINTER J., WEERASAKERA N., WEBB M., RANG H.P., BEVAN S., JAMES I.F. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br. J. Pharmacol. 2001;132:1084–1094. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONTELL C., BIRNBAUMER L., FLOCKERZI V., BINDELS R.J., BRUFORD E.A., CATERINA M.J., CLAPHAM D.E., HARTENECK C., HELLER S., JULIUS D., KOJIMA I., MORI Y., PENNER R., PRAWITT D., SCHARENBERG A.M., SCHULTZ G., SHIMIZU N., ZHU M.X. A unified nomenclature for the superfamily of TRP cation channels. Mol. Cell. 2002;9:229–231. doi: 10.1016/s1097-2765(02)00448-3. [DOI] [PubMed] [Google Scholar]
- MORIMOTO A., MURAKAMI N., NAKAMORI T., WATANABE T. Evidence for separate mechanisms of induction of biphasic fever inside and outside the blood–brain barrier in rabbits. J. Physiol. 1987;383:629–637. doi: 10.1113/jphysiol.1987.sp016433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIIJIMA A. The afferent discharges from sensors for interleukin-1β in the hepatoportal system in the anesthetized rat. J. Auton. Nerv. Syst. 1996;61:287–291. doi: 10.1016/s0165-1838(96)00098-7. [DOI] [PubMed] [Google Scholar]
- OKA T., OKA K., KOBAYASHI T., SUGIMOTO Y., ICHIKAWA A., USHIKUBI F., NARUMIYA S., SAPER C.B. Characteristics of thermoregulatory and febrile responses in mice deficient in prostaglandin EP1 and EP3 receptors. J. Physiol. 2003;551:945–954. doi: 10.1113/jphysiol.2003.048140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSAKA T., KOBAYASHI A., NAMBA Y., EZAKI O., INOUE S., KIMURA S., LEE T.H. Temperature- and capsaicin-sensitive nerve fibers in brown adipose tissue attenuate thermogenesis in the rat. Pflügers Arch. 1998;437:36–42. doi: 10.1007/s004240050743. [DOI] [PubMed] [Google Scholar]
- PARTSCH G., MATUCCI-CERINIC M. Capsaicin stimulates the migration of human polymorphonuclear cells (PMN) in vitro. Life Sci. 1993;53:PL309–PL314. doi: 10.1016/0024-3205(93)90625-d. [DOI] [PubMed] [Google Scholar]
- RIGONI M., TREVISANI M., GAZZIERI D., NADALETTO R., TOGNETTO M., CREMINON C., DAVIS J.B., CAMPI B., AMADESI S., GEPPETTI P., HARRISON S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br. J. Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RITTER R.C., LADENHEIM E.E. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am. J. Physiol. 1985;248:R501–R504. doi: 10.1152/ajpregu.1985.248.4.R501. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A. Signaling the brain in the early sickness syndrome: are sensory nerves involved. Front. Biosci. 2004;9:494–504. doi: 10.2741/1247. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., IVANOV A.I., KARMAN E.K. Blood-borne, albumin-bound prostaglandin E2 may be involved in fever. Am. J. Physiol. Regul. Integr. Comp. Physiol. 1999;276:R1840–R1844. doi: 10.1152/ajpregu.1999.276.6.R1840. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., IVANOV A.I., SCHIMANSKY Y.P. Ambient temperature for experiments in rats: a new method for determining the zone of thermal neutrality. J. Appl. Physiol. 2002;92:2667–2679. doi: 10.1152/japplphysiol.01173.2001. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., KULCHITSKY V.A., AKULICH N.V., KOULCHITSKY S.V., SIMONS C.T., SESSLER D.I., GOURINE V.N. First and second phases of biphasic fever: two sequential stages of the sickness syndrome. Am. J. Physiol. 1996;271:R244–R253. doi: 10.1152/ajpregu.1996.271.1.R244. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., KULCHITSKY V.A., SIMONS C.T., SUGIMOTO N. Methodology of fever research: why are polyphasic fevers often thought to be biphasic. Am. J. Physiol. 1998a;275:R332–R338. doi: 10.1152/ajpregu.1998.275.1.R332. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SIMONS C.T., KULCHITSKY V.A. ‘Biphasic' fevers often consist of more than two phases. Am. J. Physiol. 1998b;275:R323–R331. doi: 10.1152/ajpregu.1998.275.1.R323. [DOI] [PubMed] [Google Scholar]
- ROMANOVSKY A.A., SIMONS C.T., SZÉKELY M., KULCHITSKY V.A. The vagus nerve in the thermoregulatory response to systemic inflammation. Am. J. Physiol. 1997;273:R407–R413. doi: 10.1152/ajpregu.1997.273.1.R407. [DOI] [PubMed] [Google Scholar]
- SCHUMACHER M.A., MOFF I., SUDANAGUNTA S.P., LEVINE J.D. Molecular cloning of an N-terminal splice variant of the capsaicin receptor. Loss of N-terminal domain suggests functional divergence among capsaicin receptor subtypes. J. Biol. Chem. 2000;275:2756–2762. doi: 10.1074/jbc.275.4.2756. [DOI] [PubMed] [Google Scholar]
- SCHWARTZ G.J., SALORIO C.F., SKOGLUND C., MORAN T.H. Gut vagal afferent lesions increase meal size but do not block gastric preload-induced feeding suppression. Am. J. Physiol. 1999;276:R1623–R1629. doi: 10.1152/ajpregu.1999.276.6.R1623. [DOI] [PubMed] [Google Scholar]
- SEABROOK G.R., SUTTON K.G., JAROLIMEK W., HOLLINGWORTH G.J., TEAGUE S., WEBB J., CLARK N., BOYCE S., KERBY J., ALI Z., CHOU M., MIDDLETON R., KACZOROWSKI G., JONES A.B. Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin. J. Pharmacol. Exp. Ther. 2002;303:1052–1060. doi: 10.1124/jpet.102.040394. [DOI] [PubMed] [Google Scholar]
- SEHIC E., HUNTER W.S., UNGAR A.L., BLATTEIS C.M. Blockade of Kupffer cells prevents the febrile and preoptic prostaglandin E2 responses to intravenous lipopolysaccharide in guinea pigs. Ann. NY Acad. Sci. 1997;813:448–452. doi: 10.1111/j.1749-6632.1997.tb51732.x. [DOI] [PubMed] [Google Scholar]
- SIMONS C.T., KULCHITSKY V.A., SUGIMOTO N., HOMER L.D., SZÉKELY M., ROMANOVSKY A.A. Signaling the brain in systemic inflammation: which vagal branch is involved in fever genesis. Am. J. Physiol. 1998;275:R63–R68. doi: 10.1152/ajpregu.1998.275.1.R63. [DOI] [PubMed] [Google Scholar]
- SINGH S., NATARAJAN K., AGGARWAL B.B. Capsaicin (8-methyl-N-vanillyl-6-nonenamide) is a potent inhibitor of nuclear transcription factor-kB activation by diverse agents. J. Immunol. 1996;157:4412–4420. [PubMed] [Google Scholar]
- SOUTH E. Cholecystokinin reduces body temperature in vehicle- but not in capsaicin-pretreated rats. Am. J. Physiol. 1992;263:R1215–R1221. doi: 10.1152/ajpregu.1992.263.6.R1215. [DOI] [PubMed] [Google Scholar]
- STEINER A.A., RUDAYA A.Y., IVANOV A.I., ROMANOVSKY A.A. Febrigenic signaling to the brain does not involve nitric oxide. Br. J. Pharmacol. 2004;141:1204–1213. doi: 10.1038/sj.bjp.0705713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUZUKI M., SATO J., KUTSUWADA K., OOKI G., IMAI M. Cloning of a stretch-inhibitable nonselective cation channel. J. Biol. Chem. 1999;274:6330–6335. doi: 10.1074/jbc.274.10.6330. [DOI] [PubMed] [Google Scholar]
- SYMANOWICZ P.T., GIANUTSOS G., MORRIS J.B. Lack of role for the vanilloid receptor in response to several inspired irritant air pollutants in the C57Bl/6J mouse. Neurosci. Lett. 2004;362:150–153. doi: 10.1016/j.neulet.2004.03.016. [DOI] [PubMed] [Google Scholar]
- SZALLASI A. Vanilloid (capsaicin) receptors in health and disease. Am. J. Clin. Pathol. 2002;118:110–121. doi: 10.1309/7AYY-VVH1-GQT5-J4R2. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience. 1989;30:515–520. doi: 10.1016/0306-4522(89)90269-8. [DOI] [PubMed] [Google Scholar]
- SZALLASI A., BLUMBERG P.M. Vanilloid (capsaicin) receptors and mechanisms. Pharmacol. Rev. 1999;51:159–211. [PubMed] [Google Scholar]
- SZÉKELY M., BALASKÓ M., KULCHITSKY V.A., SIMONS C.T., IVANOV A.I., ROMANOVSKY A.A. Multiple neural mechanisms of fever. Auton. Neurosci. 2000;85:78–82. doi: 10.1016/S1566-0702(00)00223-X. [DOI] [PubMed] [Google Scholar]
- SZÉKELY M., BALASKÓ M., ROMANOVSKY A.A. Peripheral neural inputs: their role in fever development. Ann. NY Acad. Sci. 1997;813:427–434. doi: 10.1111/j.1749-6632.1997.tb51728.x. [DOI] [PubMed] [Google Scholar]
- SZÉKELY M., ROMANOVSKY A.A. Thermoregulatory reactions of capsaicin pretreated rats (Abstract) FASEB J. 1997;11:A528. [Google Scholar]
- SZÉKELY M., SZELÉNYI Z. Endotoxin fever in the rat. Acta Physiol. Acad. Sci. Hung. 1979;53:265–277. [PubMed] [Google Scholar]
- SZÉKELY M., SZOLCSÁNYI J. Endotoxin fever in capsaicin treated rats. Acta Physiol. Acad. Sci. Hung. 1979;53:469–477. [PubMed] [Google Scholar]
- SZOLCSÁNYI J.Capsaicin type pungent agents producing pyrexia Pyretics and Antipyretics. Handbook of Experimental Pharmacology 1982Berlin: Springer-Verlag; 437–478.ed. Milton, A.S. Vol. 60, pp [Google Scholar]
- SZOLCSÁNYI J., SZALLASI A., SZALLASI Z., JOÓ F., BLUMBERG P.M. Resiniferatoxin, an ultrapotent selective modulator of capsaicin-sensitive primary afferent neurons. J. Pharmacol. Exp. Ther. 1990;255:923–928. [PubMed] [Google Scholar]
- UNDEM B.J., KOLLARIK M. Characterization of the vanilloid receptor 1 antagonist iodo-resiniferatoxin on the afferent and efferent function of vagal sensory C-fibers. J. Pharmacol. Exp. Ther. 2002;303:716–722. doi: 10.1124/jpet.102.039727. [DOI] [PubMed] [Google Scholar]
- USHIKUBI F., SEGI E., SUGIMOTO Y., MURATA T., MATSUOKA T., KOBAYASHI T., HIZAKI H., TUBOI K., KATSUYAMA M., ICHIKAWA A., TANAKA T., YOSHIDA N., NARUMIYA S. Impaired febrile response in mice lacking the prostaglandin E receptor subtype EP3. Nature. 1998;395:281–284. doi: 10.1038/26233. [DOI] [PubMed] [Google Scholar]
- WAHL P., FOGED C., TULLIO S., THOMSEN C. Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist. Mol. Pharmacol. 2001;59:9–15. doi: 10.1124/mol.59.1.9. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., WIERTELAK E.P., GOEHLER L.E., MOONEY-HEIBERGER K., MARTINEZ J., FURNESS L., SMITH K.P., MAIER S.F. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994a;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- WATKINS L.R., WIERTELAK E.P., GOEHLER L.E., SMITH K.P., MARTIN D., MAIER S.F. Characterization of cytokine-induced hyperalgesia. Brain Res. 1994b;654:15–26. doi: 10.1016/0006-8993(94)91566-0. [DOI] [PubMed] [Google Scholar]
- WOODBURY C.J., ZWICK M., WANG S., LAWSON J.J., CATERINA M.J., KOLTZENBURG M., ALBERS K.M., KOERBER H.R., DAVIS B.M. Nociceptors lacking TRPV1 and TRPV2 have normal heat responses. J. Neurosci. 2004;24:6410–6415. doi: 10.1523/JNEUROSCI.1421-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOODS A.J., STOCK M.J., GUPTA A.N., WONG T.T.L., ANDREWS P.L.R. Thermoregulatory effects of resiniferatoxin in the rat. Eur. J. Pharmacol. 1994;264:125–133. doi: 10.1016/0014-2999(94)00445-5. [DOI] [PubMed] [Google Scholar]
- XU X.-J., FARKAS-SZALLASI T., LUNDBERG J.M., HÖKFELT T., WIESENFELD-HALLIN Z., SZALLASI A. Effects of the capsaicin analogue resiniferatoxin on spinal nociceptive mechanisms in the rat: behavioral, electrophysiological and in situ hybridization studies. Brain Res. 1997;752:52–60. doi: 10.1016/s0006-8993(96)01444-8. [DOI] [PubMed] [Google Scholar]