Abstract
We have examined the mechanisms of cAMP-induced gallbladder relaxation by recording isometric tension and membrane potential in the intact tissue, and global intracellular calcium concentrations ([Ca2+]i) and F-actin content in isolated myocytes.
Both the phosphodiesterase (PDE) inhibitor, IBMX (100 μM) and the adenylate cyclase activator, forskolin (2 μM) caused decreases in basal tone that exhibited similar kinetics. IBMX and forskolin both caused concentration dependent, right-downward shifts in the concentration–response curves of KCl and cholecystokinin (CCK).
IBMX and forskolin elicited a membrane hyperpolarization that was almost completely inhibited by the ATP-sensitive K+ channel (KATP) channel blocker, glibenclamide (10 μM). IBMX also induced an increase in large-conductance Ca2+-dependent K+ (BK) channel currents, although the simultaneous blockade of BK and KATP channels did not block IBMX- and forskolin-induced relaxations.
Ca2+ influx activated by L-type Ca2+ channel activation or store depletion was also impaired by IBMX and forskolin, indicating a general impairment in Ca2+ entry mechanisms.
IBMX also decreases [Ca2+]i transients activated by CCK and 3,6-Di-O-Bt-IP4-PM, a membrane permeable analog of inositol triphosphate, indicating an impairment in Ca2+ release through IP3 receptors.
Ionomycin-induced [Ca2+]i transients were not altered by IBMX, but the contractile effects of the Ca2+ ionophore were reduced in the presence of IBMX, suggesting that cAMP can decrease Ca2+ sensitivity of the contractile apparatus. A depolymerization of the thin filament could be reason for this change, as forskolin induced a decrease in F-actin content.
In conclusion, these findings suggest that multiple, redundant intracellular processes are affected by cAMP to induce gallbladder relaxation.
Keywords: cAMP, gallbladder smooth muscle, relaxation, L-type Ca2+ channel, ATP-sensitive K+ channel, large-conductance K+ channel, Ca2+ release, Ca2+ sensitivity, IBMX, forskolin, F-actin content
Introduction
The storage and concentration of the bile during the interdigestive phase is essential to the gallbladder's role in fat digestion and absorption (Shaffer et al., 1980). Although it is unclear if gallbladder filling involves passive and/or active mechanisms, the existence of inhibitory innervation (Davison et al., 1978; McKirdy et al., 1994; Chen et al., 1998; Alcon et al., 2001c) provides evidence to support a neurally mediated relaxation in the gallbladder. Cyclic nucleotides are known to act as intracellular mediators of relaxation in gastrointestinal smooth muscle (Jin et al., 1993; Bayguinov et al., 1999). In the gallbladder, cAMP plays a critical role in neurotransmitter-mediated relaxation. For example, increases in cAMP levels have been associated to VIP, CGRP and β-adrenergic relaxation (Chen et al., 1997; Kline & Pang, 1997) and activation of protein kinase A (PKA) mediates activation of KATP channels and an associated hyperpolarization in response to CGRP (Zhang et al., 1994a, 1994b). Consistent with these findings, cAMP, its derivatives such as 8-Br-cAMP, and agents such as forskolin that elevate cAMP levels can relax gallbladder muscle strips that have been precontracted with cholecystokinin (CCK) (Andersson et al., 1972; Kline & Pang, 1997; Kline et al., 1997) or diacylglycerol (DAG) (Chen et al., 1997). However, the precise mechanisms through which cAMP mediates its relaxing effects are not known.
It is clear from other vascular and visceral smooth muscle preparations that cAMP-mediated signal transduction is complicated and tissue-specific in terms of the different targets and intracellular mechanisms involved. For example, increasing intracellular cAMP stimulates Ca2+ uptake into the sarcoplasmic reticulum (SR) (Mueller & van Breemen, 1979), inhibits inositol phosphate (IP) hydrolysis and inositol triphosphate (IP3)-dependent Ca2+ release (Xuan et al., 1991), all of which result in the lowering of [Ca2+]i and relaxation. Alternatively, cAMP may reduce [Ca2+]i by enhancing Ca2+ extrusion to the extracellular space through the activation of the sarcolemmal Ca2+ pump (Bulbring & Tomita, 1987) and/or the increase in the Na+–Ca2+ exchanger activity (Yamanaka et al., 2003). In addition, inhibitory agonists that activate adenylate cyclase hyperpolarize the membrane via the activation of K+ channels (Sadoshima et al., 1988), which could indirectly inhibit Ca2+ influx, although direct regulation of L-type Ca2+ channels by cAMP increasing agents has also been reported (Koh & Sanders, 1996). Furthermore, Ca2+-independent mechanisms, such as the phosphorylation of the myosin light-chain kinase may result in a net dephosphorylation of the myosin light chains and a concomitant inhibition of the contractile response (Adelstein et al., 1978; Nishimura & van Breemen, 1989).
The goal of the present investigation was to gain insight into the intracellular mechanisms responsible for cAMP-induced relaxation in gallbladder smooth muscle (GBSM) and to explore new pathways mediating this relaxation. The results reported here suggest that a number of mechanisms contribute to cAMP-mediated relaxation responses, including the following: smooth muscle hyperpolarization, resulting from activation of KATP and BK conductances; reduction of [Ca2+]i due to the inhibition of both Ca2+ influx through L-type and capacitative Ca2+ entry (CCE) channels and Ca2+ release from SR; and desensitization of the contractile machinery to Ca2+ due, at least in part, to an increase in actin depolymerization.
Methods
Tissue preparation
Gallbladders, isolated from 300- to 500-g male guinea-pigs, after deep halothane anesthesia and cervical dislocation, were immediately placed in cold Krebs–Henseleit solution (K–HS; for composition see Solutions and drugs) at pH 7.35. The gallbladder was opened from the end of the cystic duct to the base, and trimmed of any adherent liver tissue. After the preparation was washed with the nutrient solution to remove any biliary component, the mucosa was carefully dissected away. All the experiments were carried out according to the guidelines of Animal Care and Use Committees of the University of Extremadura and the University of Vermont.
Contraction recording of guinea-pig GBSM strips
On average, four strips (measuring ∼3 × 10 mm) were obtained from a single guinea-pig gallbladder. Each strip was placed vertically in a 10 ml organ bath filled with K–HS maintained at 37°C and gassed with 95% O2–5% CO2. Isometric contractions were measured using force–displacement transducers that were interfaced with a Macintosh computer using a MacLab hardware unit and software (AD Instruments, Colorado Spring, CO, U.S.A.). The muscle strips were placed under an initial resting tension equivalent to 1.5 g load and allowed to equilibrate for 60 min, with solution changes every 20 min. Every strip coming from the same animal was used in a different experimental protocol.
The direct effects of forskolin and IBMX, agents that indirectly increase intracellular cAMP levels as the result of activation of adenylate cyclase or inhibition of phosphodiesterases (PDEs), respectively, on gallbladder tone were studied by addition of these agents, at known concentrations, to the organ bath. We also studied the effects of forskolin and IBMX on CCK-induced contractions. Preparations were first precontracted with 1 μM CCK. Once the steady state of contraction was reached, cumulative doses of forskolin (1–32 μM) or IBMX (1–500 μM) were added. The effects of forskolin and IBMX on CCK and KCl concentration–response curves were also tested. For CCK, a cumulative protocol was designed (over the range of 10 pM–1 μM). After the maximal response was reached (about 40 min), the tissue was bathed in K–HS for 1 h for recovery and return to resting tension. The muscle strips were then incubated for 20 min with forskolin or IBMX (during which forskolin and IBMX effects were assessed) and the concentration–response curve was repeated. For KCl, noncumulative concentration–response curves were done by exposition of the tissue to single doses of KCl (over the range of 10–80 mM). To test whether the relaxing effects of forskolin or IBMX involved a decrease in the Ca2+ sensitivity of the contractile apparatus, the Ca2+ ionophore ionomycin (1 μM) was applied in the absence or presence of both drugs. The strips could only be exposed to a single administration of ionomycin.
Intracellular recording from smooth muscle
The methods to be used for intracellular electrophysiological recording were similar to those previously described (Zhang et al., 1993). The gallbladder whole-mount preparation was pinned, serosal side up, in a 3 ml tissue chamber and placed on the stage of an inverted microscope (Diaphot T300, Nikon). Smooth muscle bundles were visualized at × 200 with Hoffman Modulation Contrast optics (Modulation Optics, Inc., Greenvale, NY, U.S.A.). The preparations were continuously perfused at a rate of 10–12 ml min−1 with modified Krebs solution (for composition see Solutions and drugs) aerated with 95% O2–5% CO2. Temperature was maintained between 36 and 37°C at the recording site.
Glass microelectrodes were filled with 2.0 M KCl and had resistances in the range of 50–110 MΩ. A negative-capacity compensation amplifier (Axoclamp 2A; Axon Instruments, Foster City, CA, U.S.A.) with bridge circuit was used to record membrane potentials, and outputs were displayed on an oscilloscope (Hitachi VC-6050). Electrical signals were recorded using the computer program, MacLab (CB Sciences, Milford, MA, U.S.A.). Wortmannin (100–400 nM), which inhibits myosin light-chain kinase activity without altering electrical properties or calcium transients in smooth muscle (Burdyga & Wray, 1998), was added to the Krebs solution to inhibit tissue contractions so that intracellular impalements could be maintained. Experimental compounds were applied by addition to the superfusing solution.
Cell isolation
GBSM cells were dissociated enzymatically using a previously described method (Pozo et al., 2002). Briefly, after preparing the tissue as indicated above, the gallbladder was cut into small pieces and incubated for 35 min at 37°C in enzyme solution (ES, for composition see Solutions and drugs) supplemented with 1 mg ml−1 bovine serum albumin (BSA), 1 mg ml−1 papain and 1 mg ml−1 dithioerythritol. Then the tissue was transferred to fresh ES containing 1 mg ml−1 BSA, 1 mg ml−1 collagenase and 100 μM CaCl2, and incubated for 9 min at 37°C. The tissue was then washed three times using ES, and the single smooth muscle cells were isolated by several passages of the tissue pieces through the tip of a fire-polished glass Pasteur pipette. The resultant cell suspension was kept in ES at 4°C until use, generally within 6 h. All experiments involving isolated cells were performed at room temperature (22°C).
Cell loading and [Ca2+]i determination
[Ca2+]i was determined by epifluorescence microscopy using the fluorescent ratiometric Ca2+ indicator, fura-2. Isolated cells were loaded with 4 μM fura-2 AM at room temperature for 25 min. An aliquot of cell suspension was placed in an experimental chamber made with a glass poly-D-lysine-treated coverslip (0.17 mm thick) filled with Na+-HEPES solution (for composition see Solutions and drugs), and mounted on the stage of an inverted microscope (Diaphot T200, Nikon). After the cell sedimentation, a gravity-fed system was used to perfuse the chamber with Na+-HEPES solution in absence or presence of experimental agents. For de-esterification of the dye, ⩾20 min were allowed to elapse before Ca2+ measurements were started.
Cells were illuminated at 340 and 380 nm by a computer-controlled filter wheel (Lambda-2, Sutter Instruments) at 0.3–1 cycles s−1 and the emitted fluorescence was selected by a 500-nm band-pass filter. The emitted fluorescence images were captured with a cooled digital charge-coupled device camera (model C-4880-91, Hamamatsu Photonics) and recorded using dedicated software (Argus-HisCa, Hamamatsu Photonics). The ratio of fluorescence at 340 nm to fluorescence at 380 nm (F340/F380) was calculated pixel by pixel and used to indicate the changes in [Ca2+]i. A calibration of the ratio for [Ca2+] was not performed in view of the many uncertainties related to the binding properties of fura-2 with Ca2+ inside of smooth muscle cells.
Patch–clamp electrophysiology
Ionic currents were measured in isolated muscle cells using the whole cell, perforated-patch configuration of the patch–clamp technique. A small amount of the cell suspension was transferred to the experimental chamber mounted on the stage of an inverted microscope (Nikon). After the cells settled, the chamber was continuously gravity-superfused with bath solution (for composition see Solutions and drugs). The pipette solution (for composition see Solutions and drugs) also contained amphotericin B (100 μg ml−1).
For cells used in this study cell capacitance was 39.3±4.5 pF and series resistance was 15.4±1.2 MΩ. Currents were recorded using an Axopatch 200A amplifier (Axon instruments, Foster City, CA, U.S.A.) filtered a 1 kHz and digitized at 4 kHz. Characterization of IBMX effects was performed at a holding potential of −20 mV.
F-actin content measurement
The F-actin content of resting and stimulated GBSM cells was determined according to a previously published procedure (Rosado & Sage, 2000). Briefly, samples of GBSM cell suspensions (200 μl) were challeged in Na+-HEPES solution and quickly transferred to 200 μl ice-cold 3% (w v−1) formaldehyde in phosphate-buffered saline solution (PBS; for composition see Solution and drugs) for 10 min. Fixed cells were permeabilized by incubation for 10 min with 0.025% (v v−1) Nonidet P-40 detergent dissolved in PBS. Cells were then incubated for 30 min with fluorescein isothiocyanate-labeled phalloidin (FITC-phalloidin; 1 μM) in PBS solution supplemented with 0.5% (w v−1) BSA. After incubation, the cells were collected by centrifugation for 2 min at 10,000 × g and resuspended in PBS solution. Staining of actin filaments was measured using a Shimadzu fluorescence spectrofluorimeter. Samples were excited at 496 nm and emission was recorded at 516 nm.
Solutions and drugs
The K–HS contained (in mM): NaCl 113, KCl 4.7, CaCl2 2.5, KH2PO4 1.2, MgSO4 1.2, NaHCO3 25 and D-glucose 11.5. This solution had a final pH of 7.35 after equilibration with 95% O2–5% CO2. The high K+ K–HS was prepared by isosmotic replacement of NaCl by KCl. The composition (mM) of the modified Krebs solution used in the intracellular recordings was: NaCl 121, KCl 5.9, CaCl2 2.5, NaH2PO4 1.2, MgCl 1.2, NaHCO3 25 and D-glucose 8. The ES used to disperse cells was made up of (in mM): N-2-hydroxyethylpiperazine-N′-2-sulfonic acid (HEPES) 10, NaCl 55, KCl 5.6, Na-glutamate 80, MgCl2 2, and D-glucose 10, with pH adjusted to 7.3 with NaOH. The Na+-HEPES solution contained (in mM): HEPES 10, NaCl 140, KCl 4.7, CaCl2 2, MgCl2 2 and D-glucose 10, with pH adjusted to 7.3 with NaOH. The Ca2+ free Na+-HEPES solution was prepared by substituting EGTA (1 mM) for CaCl2. The bath solution used in patch–clamp studies contained (in mM): NaCl 134, KCl 6, MgCl2 1, CaCl2 2, D-glucose 10 and HEPES 10, with pH adjusted to 7.4 with NaOH. The pipette solution used in patch–clamp experiments contained (in mM): potassium aspartate 110, KCl 30, NaCl 10, MgCl2 1, HEPES 10 and EGTA 0.05, with pH adjusted to 7.2 with NaOH. The PBS solution used in F-actin studies contained (in mM): NaCl 137, KCl 2.7, Na2HPO4 5.62, NaH2PO4 1.09 and KH2PO4 1.47 with pH adjusted to 7.2.
Drug concentrations are expressed as final bath concentrations of active species. Drugs and chemicals were obtained from the following sources: acetylcholine chloride (ACh), 4-aminopiridine (4-AP), amphotericin B, apamin, cholecystokinin (26–33) (CCK-8) sulfated, 1,4-dithio-DL-threitol (DTT), formaldehyde, forskolin, N-(2-[p-bromocinnamylamino]ethyl)-5-isoquinolinesulfonamine (H-89), ionomycin, cis-N-(2-phenylcyclopentyl)aza-cyclotridec-1-en-2-amine hydrochloride (MDL), nitrendipine, Nonidet P-40, fluorescein isothiocyanate-labeled phalloidin (FITC-phalloidin), N-cyano-N′-4-pyridinyl-N″-[1,2,2-trimethylpropyl]-guanidine (pinacidil), S(-)-BAY K8644, tetraethylammonium chloride (TEA) and wortmannin, were from Sigma Chemical Co. (St Louis, MO, U.S.A.), 3-isobutyl-1-methylxantine (IBMX), 1H-[1,2,4] oxadia-zolo[4,3-a]quinoxalin-1-one (ODQ) were from Calbiochem (La Jolla, CA, U.S.A.), glibenclamide was from Tocris (Bristol, U.K.), 3,6-di-O-butyryl-myo-inositol-1,2,4,5-tetrakisphosphate-octakis(propionoxymethyl) ester (3,6-Di-O-Bt-IP4-PM) was from SiChem (Bremen, Germany), fura-2 AM and jasplakinolide were from Molecular Probes (Molecular Probes Europe, Leiden, Netherlands), collagenase was from Fluka (Madrid, Spain), papain was from Worthington Biochemical Corporation (Lakewood, NJ, U.S.A.). Other chemicals used were of analytical grade from Panreac (Barcelona, Spain).
Stock solutions of forskolin, fura-2 AM, glibenclamide, IBMX, ionomycin, 3,6-Di-O-Bt-IP4, ODQ, pinacidil, and wortmannin were prepared in dimethylsulfoxide (DMSO) and stocks solutions of Bay K8644 and FITC-phalloidin were prepared in ethanol. The solutions were diluted such that the final concentration of DMSO or ethanol was ⩽0.1% v v−1. These concentrations of solvents did not themselves affect the mechanical activity of the tissue nor interfere with fura-2 fluorescence.
Quantification and statistics
Results are expressed as mean±the standard error of the mean of n cells or gallbladder strips. Gallbladder tension is given in milliNewtons (mN). CCK- and KCl-induced contractions were expressed as a percentage of the maximal response. Each concentration–response curve was analyzed to evaluate the concentration producing a half-maximal response (EC50) by using GraphPad Prism v. 3.00 software (GradPad Software, Inc.). Half-maximal inhibition effect (IC50) values were also determined using GraphPad Prism. For characterization of transient outward currents, a 4-min period elapsed before adding the drug and a 4-min period within the steady state of the effect were used for analysis as control and data. Transient outward currents were analyzed using Mini Analysis (Synaptosoft) with an amplitude threshold of three times the unitary BK channel current for guinea-pig GBSM cells at the −20 mV holding potential (Pozo et al., 2002). Statistical differences between means were determined by Student's t-test. Differences were considered significant when P<0.05.
Results
IBMX and forskolin induce GBSM relaxation
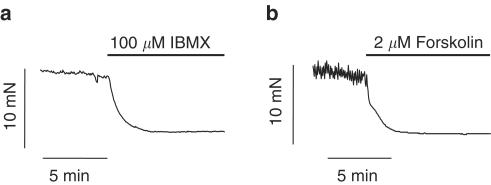
To characterize the intracellular mechanisms responsible for cAMP-induced relaxation two different cAMP-elevating agents, IBMX and forskolin, were used. When guinea-pig gallbladder strips were exposed to 100 μM IBMX a general inhibitor of PDEs, under resting tone conditions, a relaxation of 6.4±1.1 mN was recorded (from 12.1±1.6 to 5.6±0.9 mN, n=22, P<0.01). The time course of the effect of IBMX (100 μM) on GBSM basal tone is illustrated in Figure 1a. IBMX-induced relaxation was insensitive to pretreatment with the guanylyl cyclase inhibitor, ODQ (IBMX, 9.0±2.0 mN; IBMX plus 20 μM ODQ, 7.0±1.3 mN, n=6, P>0.05), which indicates that there was not a cGMP-dependent component in the IBMX-induced relaxation.
Figure 1.
cAMP increase induces gallbladder relaxation. Original traces of isometric tension recordings from intact GBSM preparations, under basal conditions, showing the relaxing effects of the nonspecific inhibitor of phosphodiesterases, IBMX (a) and the activator of adenylate cyclase forskolin (b) added to the organ bath when indicated by the bar. Traces are typical of 22 and 23 experiments for IBMX and forskolin, respectively.
Similar to the results described above, when cAMP levels were elevated by application of the adenylate cyclase activator, forskolin (2 μM), a 7.2±1.3 mN decrease in basal tone was detected (from 13.0±1.5 to 5.8±0.8 mN, n=23, P<0.001, Figure 1b). The kinetics of the forskolin-induced relaxation were similar to those of the IBMX response (half time of decay (t1/2)=84.7±16.4 and 105.3±7.8 s for IBMX and forskolin, respectively, P>0.05).
IBMX and forskolin reduce KCl- and CCK-induced contractions
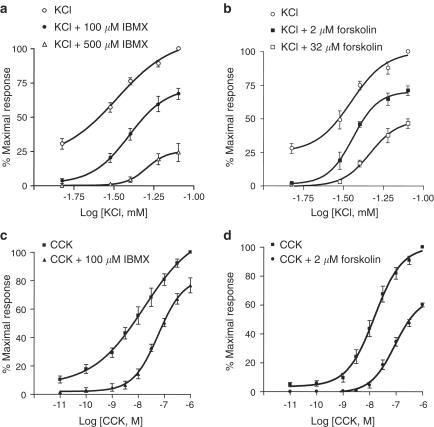
To assess whether cAMP elevating agents were able to antagonize gallbladder contractions that involved two main excitatory mechanisms in smooth muscle, Ca2+ influx through L-type Ca2+ channels and Ca2+ release from intracellular stores, the effects of IBMX and forskolin on KCl- and CCK-induced contractions were tested. Figure 2 shows that both IBMX and forskolin caused a concentration-dependent, right-downward shift in the KCl concentration–response curve (Table 1 ). The IC50 of IBMX and forskolin could not be determined because the KCl-induced response decreased progressively after reaching the maximum with a kinetic that was variable both within and between preparations.
Figure 2.
cAMP inhibits KCl- and CCK-induced contractions in the gallbladder. Effects of IBMX and forskolin on KCl and CCK concentration–response curves. For KCl, noncumulative concentration–response curves were obtained by exposing the tissue to single doses of KCl (over the range of 10–80 mM). For CCK, a cumulative protocol was used (over the range of 10 pM–1 μM). To assess the effects of IBMX or forskolin, the tissue was exposed to the drugs at least for 20 min. Data points indicate means from six to eight experiments and vertical lines show s.e. of the mean.
Table 1.
Effects of IBMX and forskolin in the concentration–response curves to KCl and CCK
| Control | 100 μM IBMX | 500 μM IBMX | Control | 2 μM forskolin | 32 μM forskolin | |
|---|---|---|---|---|---|---|
| Maximal responses to KCl (mN) | 31.9±2.2 | 21.2±1.4*** | 6.7±1.7*** | 28.3±3.5 | 21.0±3.2** | 14.7±1.6*** |
| KCl pD2 | 1.6±0.1 | 1.4±0.02 | 1.3±0.04*** | 1.45±0.04 | 1.47±0.06 | 1.30±0.02** |
| Maximal responses to CCK (mN) | 29.6±3.5 | 22.0±2.3** | 19.1±1.4 | 11.4±1.05*** | ||
| CCK pD2 | 8.1±0.2 | 7.1±0.2** | 7.9±0.1 | 7.0±0.1*** |
P<0.01 vs control;
P<0.001 vs control.
Addition of IBMX (1–500 μM) or forskolin (1–32 μM) to muscle strips that were precontracted with 1 μM CCK produced a concentration-dependent relaxation. When applied during CCK-induced maximal contractions, IBMX and forskolin caused maximum relaxations of 93.9±6.1 and 95.7±8.1% relative to the maximal contractile response to CCK (n=6), with IC50's of 86.5±15.4 and 2.1±0.5 μM, respectively. Concentration–response curves for CCK were determined in the absence or presence of 100 μM IBMX and 2 μM forskolin, concentrations that were very close to IC50 for both agents. As demonstrated in Figure 2c and d, both IBMX and forskolin induced a right-downward shift in the CCK concentration–response curve (see Table 1) suggesting that cAMP can impair the contractile response to [Ca2+]i release.
IBMX and forskolin induce GBSM hyperpolarization through KATP and BK channel activation
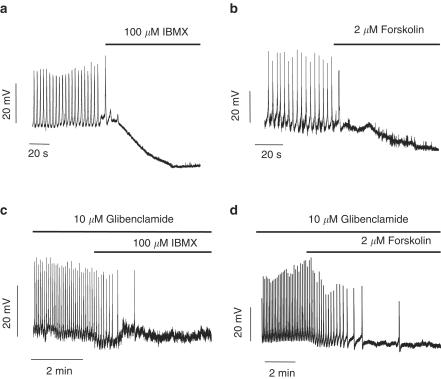
As smooth muscle relaxation is usually associated with a membrane hyperpolarization, intracellular recordings techniques were used to test whether IBMX and forskolin altered the membrane potential of guinea-pig GBSM. A total of 30 impalements were done in 12 different gallbladders, with a mean of membrane potential of −56.7±3.4 mV. Spontaneous action potentials were observed in all the impalements, with a mean frequency of 0.3±0.02 Hz. As demonstrated in Figure 3a and b, addition of either 100 μM IBMX or 2 μM forskolin to the bathing solution caused hyperpolarization of GBSM (IBMX, 16.1±2.9 mV; forskolin, 22.9±3.4 mV; n=6 and 5, P<0.001) that was associated with a loss of the spontaneous action potentials.
Figure 3.
cAMP hyperpolarizes GBSM cells. Representative membrane potential (Vm) recordings showing that both IBMX (a) and forskolin (b) elicited a prolonged membrane hyperpolarization that was associated with a loss of spontaneous action potentials. In the presence of glibenclamide, the hyperpolarization induced by either IBMX (c) or forskolin (d) was almost abolished, but the effects of the drugs on spontaneous action potential persisted. Traces are representative of five to six experiments.
To determine whether the IBMX- and forskolin-induced hyperpolarizations involve KATP channel activation, the KATP channel blocker, glibenclamide (10 μM) was used. As seen in Figure 3c and d, glibenclamide almost abolished the hyperpolarizations induced by both cAMP elevating agents (IBMX, 16.1±2.9 mV, IBMX plus glibenclamide, 1.7±0.8 mV, 94.2% reduction; forskolin, 22.9±3.4 mV, forskolin plus glibenclamide, 1.8±0.9 mV, 92.0% reduction; n=6 for IBMX and 5 for forskolin, respectively, P<0.001 for both). It is worth noting that in most preparations (13 out of 18), while glibenclamide inhibited the IBMX- or forskolin-induced hyperpolarizations, the interruption of action potential generation persisted upon application of IBMX or forskolin in the presence of glibenclamide (Figure 3c and d). In those strips where spontaneous action potentials were recorded in the presence of glibenclamine plus IBMX or forskolin there was a reduction in the plateau area of these action potentials (from 8.3±0.8 to 3.9±0.3 mV s, n=5, P<0.001). These results indicate that activation of KATP channels is probably not the only target of cAMP that mediates relaxation. Consistent with this concept, 10 μM glibenclamide was able to reverse 100 μM IBMX-induced relaxation only by 19% (from 7.3±0.6 to 6.3±0.8 mN, n=14, P<0.01) and 2 μM forskolin-induced relaxation by 24% (from 7.2±1.7 to 5.2±0.7 mN, n=4, P<0.05). This concentration of glibenclamide was effective on blocking the relaxation caused by 5 μM pinacidil, an activator of KATP channels (from 17.4±4.3 to 2.4±4.3 mN, 83% inhibition, n=6, P<0.001).
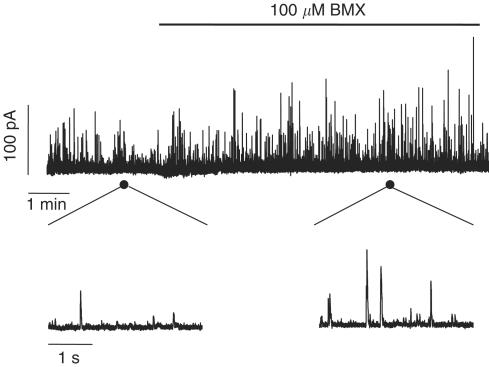
To assess whether cAMP elevating agents also caused large-conductance Ca2+-dependent K+ (BK) channel activation, we evaluated spontaneous transient outward currents (STOCs) in gallbladder isolated cells. Figure 4 shows an original recording of BK currents from a GBSM cell that was held at −20 mV before and after treatment with 100 μM IBMX. IBMX increased the BK current frequency by 45.5% (from 0.8±0.08 to 1.5±0.3 Hz, n=5, P<0.05) and the amplitude of the transients by 12.7% (from 34.6±3.5 to 38.6±2.7 pA, n=5, P<0.05), indicating that BK channels are activated by IBMX and could contribute to the membrane hyperpolarization. When gallbladder strips were pretreated with 1 mM TEA to block BK channels, IBMX- and forskolin-induced relaxations were only slightly reduced (IBMX: 4.5% reduction, from 4.6±0.9 to 4.4±0.9 mN, n=5, P<0.05; forskolin: 9.7% reduction, from 7.50±1.0 to 6.8±1.0 mN, n=5, P<0.05) and when both TEA and glibenclamide were used in combination IBMX-induced relaxation was reversed almost to the same extent that in the presence of glibenclamide alone (25.6% reduction, from 4.6±0.8 to 3.5±0.7 mN, n=5, P<0.01). Similar results were obtained when forskolin was used as cAMP elevating agent (33.6% reduction, from 7.5±1.0 to 4.8±0.6 mN, n=5, P<0.01), suggesting that other intracellular targets are still affected by the increase in cAMP. Application of 5 mM 4-AP, an inhibitor of voltage-dependent K+ (Kv) channels and 1 μM apamin, an inhibitor of small conductance Ca2+-activated K+ (SK) channels had no effects of IBMX-induced relaxations (4-AP: from 7.6±1.7 to 7.5±1.8 mN, n=5; apamin: from 7.2±1.5 to 6.9±1.6 mN, n=5).
Figure 4.
cAMP activates BK channels. Effects of IBMX on spontaneous transient BK currents recorded in isolated gallbladder myocytes using the perforated patch configuration of the patch–clamp technique. IBMX administration caused an increase in amplitude and frequency of the outward currents. The bottom traces represent regions of the patch recording displayed on an expanded time scale. Traces are representative of five experiments.
cAMP elevating agents reduce Ca2+ influx
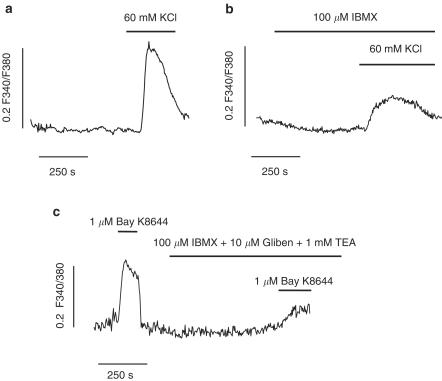
The finding that spontaneous action potentials were frequently still lacking when the IBMX- and forskolin-induced hyperpolarizations were reversed by glibenclamide suggests that cAMP may lead to inhibition of L-type Ca2+ channels. To test this hypothesis, we measured changes in [Ca2+]i in isolated GBSM cells loaded with fura-2 in response to agents that cause activation of these channels. Thus, as represented in Figure 5a, exposure of GBSM cells to 60 mM KCl induced a transient increase in the F340/F380 ratio (0.24±0.02, n=14) that was significantly reduced (Figure 5b) by pretreatment with 100 μM IBMX (0.14±0.02, 41% reduction vs control, n=13, P<0.01). The reduction in KCl-induced Ca2+ influx could be due to the hyperpolarization caused by IBMX, which could negate the depolarization induced by KCl, and diminish the activation of L-type Ca2+ channels. Indeed, alterations in the K+ driving force in these experiments could mask the effects of IBMX. In order to get around this, we used Bay K8644 to activate L-type Ca2+ channels in the presence of 10 μM glibenclamide and 1 mM TEA. Under these conditions, as represented in Figure 5c, 1 μM Bay K8644 caused an increase in the F340/F380 ratio that was reduced by 72% in the presence of 100 μM IBMX (Bay K8644, 0.16±0.03; Bay K8644 plus IBMX, 0.034±0.006, n=10, P<0.001).
Figure 5.
cAMP inhibits Ca2+ transients elicited by high K+ and Bay K8644. (a and b) Representative recordings of increases in [Ca2+]i induced by 60 mM KCl in the absence (a) and presence (b) of IBMX. (c) Representative trace of Ca2+ transients induced by the Ca2+ channel opener Bay K8644 in the absence and presence of IBMX. Glibenclamide and TEA were applied to prevent the hyperpolarizing effect of IBMX. [Ca2+]i data are expressed as changes in absolute fluorescence ratio F340/F380 (ΔF340/F380). Traces are representative of 10–14 experiments.
Another Ca2+ influx pathway that contributes to the CCK-induced gallbladder contraction is the activation of CCE mechanisms (Morales et al., 2004). To test whether cAMP could affect this pathway, CCE was activated by depletion of the stores with thapsigargin in a free Ca2+ medium and then Ca2+ was restored in the extracellular medium in the presence of 1 μM nitrendipine to block non-CCE. Forskolin (2 μM) significantly reduced the sustained [Ca2+]i increase indicative of CCE (0.065±0.009 vs 0.04±0.009F340/F380, 57% reduction, n=9, P<0.001). On the other hand, both the adenylate cyclase inhibitor, MDL (10 μM), and the protein kinase A inhibitor, H89 (10 μM), caused an increase in CCE (control: 0.058±0.007 F340/F380, MDL: 0.088±0.010 F340/F380, 54% increase, n=9, P<0.01; control: 0.057±0.008 F340/F380, H89: 0.0848±0.010 F340/F380, 51% increase, n=10, P<0.001), suggesting a role of endogenous cAMP in regulating CCE mechanisms.
IBMX reduces Ca2+ release through IP3 receptors
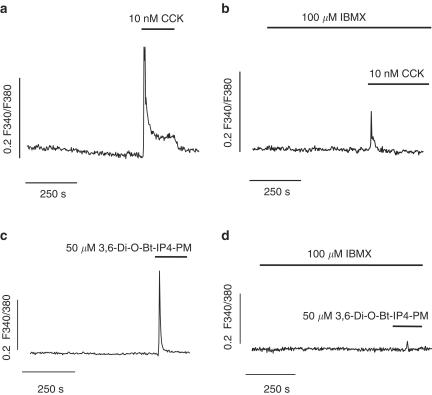
As noted above, the contractile response to CCK was attenuated by cAMP elevating agents, suggesting that cAMP may reduce Ca2+ mobilization from intracellular stores. To assess this Ca2+ levels were quantified in response to CCK in the absence and presence of IBMX. As shown in Figure 6a and b, the time course of CCK-changes in [Ca2+]i shows the typical pattern for SR depleting agents as the response consisted of a transient elevation followed by a steady state level slightly above the resting level. Pretreatment of the cells with 100 μM IBMX (Figure 6b) reduced the peak response to CCK by 88% (CCK, 0.22±0.03 F340/F380; CCK plus IBMX, 0.03±0.008 F340/F380, n=14 and 18, P<0.001), suggesting that cAMP impairs Ca2+ release from SR. To more directly test whether Ca2+ release through IP3 receptor was impaired by IBMX, we used 50 μM 3,6-Di-O-Bt-IP4-PM, a membrane permeable analog of IP3 (Schultz, 2003), which caused a Ca2+ transient similar to that reached after CCK treatment (0.18±0.02 F340/F380, n=11, Figure 6c). When cells were pretreated with 100 μM IBMX, the 3,6-Di-O-Bt-IP4-PM-induced Ca2+ transient was almost abolished (0.005±0.003 F340/F380, 97% inhibition respect to control, n=11, P<0.001, Figure 6d) indicating that the ability of IP3 to initiate Ca2+ release was reduced in the presence of increased levels of cAMP.
Figure 6.
cAMP inhibits Ca2+ release through IP3 channels. (a and b) Representative recordings of the increase in [Ca2+]i induced by administration of CCK in the absence (a) and presence (b) of IBMX. (c and d) Representative recordings of Ca2+ transients induced by the membrane permeable IP3 analog, 3,6-Di-O-Bt-IP4-PM, in the absence (c) and presence (d) of IBMX. Results are expressed as changes in absolute fluorescence ratio F340/F380 (ΔF340/F380). Traces are representative of 11–18 experiments.
IBMX decreases contractile response to [Ca2+]i elevation
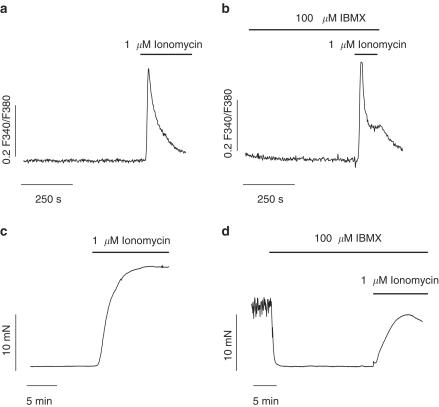
There are numerous lines of evidence suggesting that cAMP decreases the Ca2+ sensitivity of contractile elements (Adelstein et al., 1978; Nishimura & van Breemen, 1989). To test this in our model, we used the Ca2+ ionophore, ionomycin to elevate [Ca2+]i without activating intracellular pathways that could interfere with our results. A measure of 1 μM ionomycin caused a transient increase in F340/F340 reflecting an increase in [Ca2+]i, which was similar to that obtained in cells pretreated with 100 μM IBMX for 15 min (ionomycin: 0.38±0.05F340/F380, n=28; ionomycin+IBMX: 0.37±0.07 F340/F380, n=14, P>0.05, Figure 7a and b). The isometric contractile response of gallbladder strips to ionomycin was recorded in the presence and absence of IBMX. As shown in Figure 7c ionomycin induced a stable contraction that was reduced by IBMX (20.3±3.5 vs 9.74±1.18 mN, n=8, P<0.05) indicating a reduction in the coupling between [Ca2+]i and contraction.
Figure 7.
cAMP decreases Ca2+ sensitivity of contractile machinery. (a and b) Representative Ca2+ transients induced by the Ca2+ ionophore, ionomycin in the absence (a) and presence (b) of IBMX. (c and d) Original traces of isometric tension recordings from gallbladder muscle strips showing the contractile effects of ionomycin in absence (c) and presence (d) of IBMX. Traces are representative of 14–28 experiments for the Ca2+ imaging and 8–12 for tension recordings. [Ca2+]i results are expressed as changes in absolute fluorescence ratio F340/F380 (ΔF340/F380).
To gain insight into the mechanisms responsible for this uncoupling, we evaluated the F-actin content in a suspension of FITC-phalloidin-stained GBSM cells by spectrofluorometry. Forskolin treatment (for 10 min) caused a reduction in F-actin content (from 12.9±1.1 to 9.3±0.9 arbitrary units, 28.1% reduction, n=6, P<0.01). To our knowledge, this is the first experimental evidence of cAMP-induced changes in the thin filament of smooth muscle cells. Consistent with these results, stabilization of F-actin by 10 μM jasplakinolide treatment significantly reduced the forskolin-induced relaxation (20.1±3.0% reduction, n=3, P<0.05).
Discussion
The present study was conducted to elucidate the intracellular mechanisms through which the second messenger, cAMP causes gallbladder relaxation. The data presented here suggest that cAMP-mediated gallbladder relaxation involves at least three mechanisms: (1) activation of K+ currents leading to membrane hyperpolarization; (2) inhibition of Ca2+ entry and release from intracellular stores; and (3) decreasing the sensitivity of contractile machinery to Ca2+, at least in part, through activation of actin depolymerization.
In this study, we have used two different experimental approaches to elevate cAMP: application of the nonselective PDE inhibitor, IBMX, and the activator of the adenylate cyclase, forskolin. Both of these compounds induced a significant decrease in gallbladder basal tone. Whereas, the later approach induces only elevations in cAMP (Seamon & Daly, 1986) the use of IBMX could induce increases in both cAMP and cGMP depending on the type(s) of PDE expressed in the tissue, and the levels of basal activity of cyclic nucleotide cyclases. In our study, it is unlikely that IBMX-induced gallbladder relaxation involved an elevation in cGMP, as demonstrated by the lack of effect of the guanylate cyclase inhibitor, ODQ, on the IBMX-induced relaxation. The dose of ODQ used in the current study is known to block the NO-induced relaxation in GBSM (Alcon et al., 2001a). Finally, the fact that forskolin-induced relaxation has the same kinetics as IBMX-induced response is consistent with the hypothesis that IBMX mediates its effects in the gallbladder primarily through increases in cAMP.
cAMP-induced relaxation of precontracted smooth muscle varies according to the contractile stimulant that is used. For example, the relaxant effects of cAMP are more pronounced on noradrenaline, carbachol or tromboxane A2 analogue-induced contractions than against high K+-induced contraction (Ahn et al., 1988; Yamagishi et al., 1994; Kato et al., 2000). In these previous studies, the cAMP-induced relaxation coincided with a decrease in [Ca2+]i for agonist-induced contractions, but not high K+-induced responses, which could be responsible for the different efficacies of cAMP. In the current study, cAMP elevating agents exhibited comparable efficacies when tested on high K+ and agonist-induced GBSM contraction. This is consistent with the finding that in GBSM, [Ca2+]i elevations elicited by agonist (CCK) application, as well as by high K+, were reduced in response to elevated cAMP.
In our study, both forskolin and IBMX-induced relaxations are related to hyperpolarization of GBSM. Potassium channel activity is a major determinant of membrane potential in smooth muscle, and K+ efflux causes hyperpolarization, which, in turn, inhibits voltage-gated Ca2+ channel activation and promotes relaxation (Nelson & Quayle, 1995). In GBSM, KATP channels mediate CGRP hyperpolarization (Zhang et al., 1994a, 1994b), and KATP channel activity is dependent on a balance between phosphorylation by PKA and PKC and dephosphorylation of their respective sites by phosphatases (Firth et al., 2000). Data from the current study support the concept that cAMP elevation causes a hyperpolarization that is largely reversed by the KATP channel inhibitor, glibenclamide. However, glibenclamide did not completely reverse the membrane hyperpolarization, and the loss of spontaneous action potentials following application of IBMX or forskolin persisted in the presence of glibenclamide. Furthermore, glibenclamide did not block cAMP-induced relaxation, suggesting that the activation of KATP channels is not the only process involved in the cAMP-mediated relaxation.
Another potential contributor to the cAMP-mediated membrane hyperpolarization is the opening of BK channels. These channels are responsible for spontaneous transient hyperpolarizing outward currents (STOCs) that are activated in response to spontaneous Ca2+ sparks (transient localized Ca2+ signals) arising from intracellular store Ca2+ release. We have previously suggested a role of this process in the regulation of gallbladder excitability (Pozo et al., 2002). In addition, previous reports suggest that BK channels may be modulated by cyclic nucleotide second messengers in various tissues, including vascular and visceral smooth muscle (Wellman et al., 2001; Ise et al., 2003). The increase in STOC activity by IBMX, reported here, supports this hypothesis. However, BK and KATP channel blockers did not completely block the cAMP-induced relaxation, indicating that these channels may not be the only mediators of cAMP-induced relaxation.
Calcium influx through L-type Ca2+ channels is a crucial step for maintaining basal tone and for agonist-mediated contraction in GBSM (Shaffer et al., 1992; Alcon et al., 2001b). In smooth muscle cells, the activity of these channels can be modulated by cAMP-dependent mechanisms, but this modulation is highly tissue specific. For example, in rabbit portal vein and rat mesenteric artery (Taguchi et al., 1997; Ruiz-Velasco et al., 1998) cAMP facilitates Ca2+ entry through L-type Ca2+ channels, and this is also the case for cardiac muscle (Kamp & Hell, 2000). On the other hand, in rat artery tail and guinea-pig detrusor smooth muscle (Chik et al., 1996; Kobayashi et al., 2003) cAMP exerts negative modulation. It has also been suggested that low concentrations of cAMP enhances Ca2+ currents via cAMP-dependent kinases, whereas higher concentrations of the nucleotide may inhibit Ca2+ currents through activation of PKG (Koh & Sanders, 1996; Ruiz-Velasco et al., 1998). Several lines of evidence presented in the current study suggest that increased cAMP inhibits L-type Ca2+ channels in GBSM: the lack of spontaneous action potentials in the presence of glibenclamide, the IBMX-induced reduction in the high KCl-induced Ca2+ transients, and the reduction in Bay K-induced Ca2+ transient in the presence of both glibenclamide and TEA (added to inhibit the IBMX-induced hyperpolarization).
Recently, CCE, which has been shown to be essential in nonexcitable cells (Putney, 1986), is gaining attention in the area of smooth muscle physiology (Gibson et al., 1998) where it participates in contraction (Morales et al., 2004). However, the possible role of cAMP controlling this process remains unknown. The present study demonstrates for the first time that cAMP exerts a negative effect in Ca2+ entry mediated by store depletion in smooth muscle, as forskolin diminished CCE. In addition, our study demonstrates that this is not an artifact of experimental manipulation of cAMP levels, since endogenous activation of adenylate cyclase and protein kinase A are exerting negative influences in this response. Activation of Ca2+-dependent adenylate cyclase by Ca2+ entry through CCE pathways has been shown (for revision see Cooper, 2003), but there are no data on the role of the concomitant increase of cAMP on CCE. Our results provide a new example of negative feedback in cell physiology: activation of CCE induces an increase in cAMP output, which could limit CCE.
In addition to attenuating Ca2+ entry into GBSM cells, data reported here suggest that cAMP interferes with Ca2+ release from intracellular stores. It is known that cAMP can regulate [Ca2+]i mobilization from sarcoplasmic reticulum in a number of ways, including phosphorylation of IP3 and ryanodine receptors (RyR), and modulating the accumulation of Ca2+ in the SR. To date, there is little evidence for an influence of cAMP on RyRs in smooth muscle, as it is difficult to separate the direct effects of cAMP on RyR channel activity from indirect changes due to alterations in SR load. In cerebral arteries, PKA phosphorylates phospholamban to augment SR Ca2+-ATPase activity and SR Ca2+ load, which leads to enhanced Ca2+ spark frequency and BK channel activity (Wellman et al., 2001) but results of this study also suggest that PKA may also directly activate RyRs. In GBSM, we have shown the presence of Ca2+ sparks and functional coupling between Ca2+ sparks and BK channel activation (Pozo et al., 2002). In the current investigation, we found that elevated cAMP leads to an increase in BK channel activity that could be due to an increase in SR Ca2+ content and/or RyR channel open probability. Our data indicate that the potential increase in SR Ca2+ load did not correspond to an indiscriminate increase in SR depletion through all types of SR channels. In the current study, IBMX reduced Ca2+ transient activated by CCK as well as the IP3 precursor, 3,6-Di-O-Bt-IP4, suggesting an inhibition of IP3 receptor-mediated Ca2+ release in agreement with Murthy et al. (1993), who found a decrease in IP3-dependent Ca2+ mobilization in gastric muscle cells.
Smooth muscle tone is regulated not only by [Ca2+]i levels but also by the Ca2+ sensitivity of the contractile apparatus (Somlyo et al., 1999). In the current study, ionomycin was used to supply Ca2+ directly to the contractile proteins and induce GBSM contraction. Ionomycin elevates [Ca2+]i by the creating Ca2+ permeable pores in the plasma membrane. We found that cAMP elevation did not change ionomycin-induced [Ca2+]i increases, but its contractile action was diminished in the presence of IBMX. In addition, forskolin induced a decrease in the F-actin content of GBSM cells, which could contribute to the cAMP-induced relaxation. In fact, when cytochalasin D is used to depolymerizate F-actin, a decrease in gallbladder tone and an impairment in the contractile responses to agonists are achieved (S. Morales, P.J. Camello and M.J. Pozo, unpublished observations), suggesting that depolymerization of the thin filament could be a mechanism to induce relaxation. In agreement with this, in the present study, a reduction in forskolin-induced relaxation was observed during treatment with jasplakinolide, which favors actin polymerization (Bubb et al., 2000) and could counteract cAMP-induced depolymerization.
In conclusion, the findings of this investigation suggest that a broad range of intracellular processes are affected by cAMP to decrease GBSM excitability and induce relaxation. According to functional studies, these mechanisms probably do not operate simultaneously, and their relative contributions may depend on the stimuli to increase cAMP levels and/or the functional status of the smooth muscle cells. Simultaneous recordings of multiple parameters in intact tissue will be necessary to resolve which of these mechanisms operates in a particular situation.
Acknowledgments
We thank Dr Edward Parr for assistance with the intracellular electrophysiological studies and M.P. Delgado for her technical assistance. This work was supported by the Spanish MCyT Grant SAF-2001-0295 (MJP) and NIH Grant NS26995 (GMM). S. Morales is supported by Ministry of Education Predoctoral Research Grant.
Abbreviations
- ES
enzyme solution
- GBSM
gallbladder smooth muscle
- K–HS
Krebs–Henseleit solution
- STOC
spontaneous transient outward current
References
- ADELSTEIN R.S., CONTI M.A., HATHAWAY D.R., KLEE C.B. Phosphorylation of smooth muscle myosin light chain kinase by the catalytic subunit of adenosine 3′: 5′-monophosphate-dependent protein kinase. J. Biol. Chem. 1978;253:8347–8350. [PubMed] [Google Scholar]
- AHN H.Y., KARAKI H., URAKAWA N. Inhibitory effects of caffeine on contractions and calcium movement in vascular and intestinal smooth muscle. Br. J. Pharmacol. 1988;93:267–274. doi: 10.1111/j.1476-5381.1988.tb11430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALCON S., MORALES S., CAMELLO P.J., HEMMING J.M., JENNINGS L., MAWE G.M., POZO M.J. A redox-based mechanism for the contractile and relaxing effects of NO in the guinea-pig gall bladder. J. Physiol. 2001a;532:793–810. doi: 10.1111/j.1469-7793.2001.0793e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALCON S., MORALES S., CAMELLO P.J., POZO M.J. Tyrosine kinases regulate basal tone and responses to agonists in the guinea pig gallbladder through modulation of Ca2+ mobilization. Anal. Pharmacol. 2001b;2:48–56. [Google Scholar]
- ALCON S., MORALES S., CAMELLO P.J., SALIDO G.M., MILLER S.M., POZO M.J. Relaxation of canine gallbladder to nerve stimulation involves adrenergic and non-adrenergic non-cholinergic mechanisms. Neurogastroenterol. Motil. 2001c;13:555–566. doi: 10.1046/j.1365-2982.2001.00286.x. [DOI] [PubMed] [Google Scholar]
- ANDERSSON K.E., ANDERSSON R., HEDNER P. Cholecystokinetic effect and concentration of cyclic AMP in gall-bladder muscle in vitro. Acta Physiol. Scand. 1972;85:511–516. doi: 10.1111/j.1748-1716.1971.tb05289.x. [DOI] [PubMed] [Google Scholar]
- BAYGUINOV O., KEEF K.D., HAGEN B., SANDERS K.M. Parallel pathways mediate inhibitory effects of vasoactive intestinal polypeptide and nitric oxide in canine fundus. Br. J. Pharmacol. 1999;126:1543–1552. doi: 10.1038/sj.bjp.0702450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUBB M.R., SPECTOR I., BEYER B.B., FOSEN K.M. Effects of jasplakinolide on the kinetics of actin polymerisation. An explanation for certain in vivo observations. J. Biol. Chem. 2000;275:5163–5170. doi: 10.1074/jbc.275.7.5163. [DOI] [PubMed] [Google Scholar]
- BULBRING E., TOMITA T. Catecholamine action on smooth muscle. Pharmacol. Rev. 1987;39:49–96. [PubMed] [Google Scholar]
- BURDYGA T.V., WRAY S. The effect of inhibition of myosin light chain kinase by Wortmannin on intracellular [Ca2+], electrical activity and force in phasic smooth muscle. Pflugers Arch. 1998;436:801–803. doi: 10.1007/s004240050705. [DOI] [PubMed] [Google Scholar]
- CHEN Q., AMARAL J., OH S., BIANCANI P., BEHAR J. Gallbladder relaxation in patients with pigment and cholesterol stones. Gastroenterology. 1997;113:930–937. doi: 10.1016/s0016-5085(97)70189-6. [DOI] [PubMed] [Google Scholar]
- CHEN Q., LEE K., XIAO Z., BIANCANI P., BEHAR J. Mechanism of gallbladder relaxation in the cat: role of norepinephrine. J. Pharmacol. Exp. Ther. 1998;285:475–479. [PubMed] [Google Scholar]
- CHIK C.L., LI B., OGIWARA T., HO A.K., KARPINSKI E. PACAP modulates L-type Ca2+ channel currents in vascular smooth muscle cells: involvement of PKC and PKA. FASEB J. 1996;10:1310–1317. doi: 10.1096/fasebj.10.11.8836045. [DOI] [PubMed] [Google Scholar]
- COOPER D.M. Molecular and cellular requirements for the regulation of adenylate cyclases by calcium. Biochem. Soc. Trans. 2003;31:912–915. doi: 10.1042/bst0310912. [DOI] [PubMed] [Google Scholar]
- DAVISON J.S., AL HASSANI M., CROWE R., BURNSTOCK G. The non-adrenergic, inhibitory innervation of the guinea-pig gallbladder. Pflugers Arch. 1978;377:43–49. doi: 10.1007/BF00584372. [DOI] [PubMed] [Google Scholar]
- FIRTH T.A., MAWE G.M., NELSON M.T. Pharmacology and modulation of K(ATP) channels by protein kinase C and phosphatases in gallbladder smooth muscle. Am. J. Physiol. Cell Physiol. 2000;278:C1031–C1037. doi: 10.1152/ajpcell.2000.278.5.C1031. [DOI] [PubMed] [Google Scholar]
- GIBSON A., MCFADZEAN I., WALLACE P., WAYMAN C.P. Capacitative Ca2+ entry and the regulation of smooth muscle tone. Trends Pharmacol. Sci. 1998;19:266–269. doi: 10.1016/s0165-6147(98)01222-x. [DOI] [PubMed] [Google Scholar]
- ISE S., NISHIMURA J., HIRANO K., HARA N., KANAIDE H. Theophylline attenuates Ca2+ sensitivity and modulates BK channels in porcine tracheal smooth muscle. Br. J. Pharmacol. 2003;140:939–947. doi: 10.1038/sj.bjp.0705508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J.G., MURTHY K.S., GRIDER J.R., MAKHLOUF G.M. Activation of distinct cAMP- and cGMP-dependent pathways by relaxant agents in isolated gastric muscle cells. Am. J. Physiol. 1993;264:G470–G477. doi: 10.1152/ajpgi.1993.264.3.G470. [DOI] [PubMed] [Google Scholar]
- KAMP T.J., HELL J.W. Regulation of cardiac L-type calcium channels by protein kinase A and protein kinase C. Circ. Res. 2000;87:1095–1102. doi: 10.1161/01.res.87.12.1095. [DOI] [PubMed] [Google Scholar]
- KATO K., FURUYA K., TSUTSUI I., OZAKI T., YAMAGISHI S. Cyclic AMP-mediated inhibition of noradrenaline-induced contraction and Ca2+ influx in guinea-pig vas deferens. Exp. Physiol. 2000;85:387–398. [PubMed] [Google Scholar]
- KLINE L.W., PANG P.K. Cyclic AMP modulates part of the relaxant action of calcitonin gene-related peptide in guinea pig gallbladder strips. Regul. Pept. 1997;72:55–59. doi: 10.1016/s0167-0115(97)01036-7. [DOI] [PubMed] [Google Scholar]
- KLINE L.W., ZHANG M.L., PANG P.K. Cyclic AMP induces a relaxation response in the bullfrog Rana catesbeiana, but nitric oxide does not. J. Exp. Biol. 1997;200:2669–2674. doi: 10.1242/jeb.200.20.2669. [DOI] [PubMed] [Google Scholar]
- KOBAYASHI H., MIWA T., NAGAO T., ADACHI-AKAHANE S. Negative modulation of L-type Ca2+ channels via beta-adrenoceptor stimulation in guinea-pig detrusor smooth muscle cells. Eur. J. Pharmacol. 2003;470:9–15. doi: 10.1016/s0014-2999(03)01762-x. [DOI] [PubMed] [Google Scholar]
- KOH S.D., SANDERS K.M. Modulation of Ca2+ current in canine colonic myocytes by cyclic nucleotide-dependent mechanisms. Am. J. Physiol. 1996;271:C794–C803. doi: 10.1152/ajpcell.1996.271.3.C794. [DOI] [PubMed] [Google Scholar]
- MCKIRDY M.L., MCKIRDY H.C., JOHNSON C.D. Non-adrenergic non-cholinergic inhibitory innervation shown by electrical field stimulation of isolated strips of human gall bladder muscle. Gut. 1994;35:412–416. doi: 10.1136/gut.35.3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORALES S., CAMELLO P.J., ALCON S., SALIDO G.M., MAWE G., POZO M.J. Coactivation of capacitative calcium entry and L-type calcium channels in guinea pig gallbladder. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;286:G1090–G1100. doi: 10.1152/ajpgi.00260.2003. [DOI] [PubMed] [Google Scholar]
- MUELLER E., VAN BREEMEN C. Role of intracellular Ca2+ sequestration in beta-adrenergic relaxation of a smooth muscle. Nature. 1979;281:682–683. doi: 10.1038/281682a0. [DOI] [PubMed] [Google Scholar]
- MURTHY K.S., SEVERI C., GRIDER J.R., MAKHLOUF G.M. Inhibition of IP3 and IP3-dependent Ca2+ mobilization by cyclic nucleotides in isolated gastric muscle cells. Am. J. Physiol. 1993;264:G967–G974. doi: 10.1152/ajpgi.1993.264.5.G967. [DOI] [PubMed] [Google Scholar]
- NELSON M.T., QUAYLE J.M. Physiological roles and properties of potassium channels in arterial smooth muscle. Am. J. Physiol. 1995;268:C799–C822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- NISHIMURA J., VAN BREEMEN C. Direct regulation of smooth muscle contractile elements by second messengers. Biochem. Biophys. Res. Commun. 1989;163:929–935. doi: 10.1016/0006-291x(89)92311-5. [DOI] [PubMed] [Google Scholar]
- POZO M.J., PEREZ G.J., NELSON M.T., MAWE G.M. Ca(2+) sparks and BK currents in gallbladder myocytes: role in CCK-induced response. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G165–G174. doi: 10.1152/ajpgi.00326.2001. [DOI] [PubMed] [Google Scholar]
- PUTNEY J.W., JR A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- ROSADO J.A., SAGE S.O. Farnesylcysteine analogues inhibit store-regulated Ca2+ entry in human platelets: evidence for involvement of small GTP-binding proteins and actin cytoskeleton. Biochem. J. 2000;347 Part 1:183–192. [PMC free article] [PubMed] [Google Scholar]
- RUIZ-VELASCO V., ZHONG J., HUME J.R., KEEF K.D. Modulation of Ca2+ channels by cyclic nucleotide cross activation of opposing protein kinases in rabbit portal vein. Circ. Res. 1998;82:557–565. doi: 10.1161/01.res.82.5.557. [DOI] [PubMed] [Google Scholar]
- SADOSHIMA J., AKAIKE N., KANAIDE H., NAKAMURA M. Cyclic AMP modulates Ca-activated K channel in cultured smooth muscle cells of rat aortas. Am. J. Physiol. 1988;255:H754–H759. doi: 10.1152/ajpheart.1988.255.4.H754. [DOI] [PubMed] [Google Scholar]
- SCHULTZ C. Prodrugs of biologically active phosphate esters. Bioorg. Med. Chem. 2003;11:885–898. doi: 10.1016/s0968-0896(02)00552-7. [DOI] [PubMed] [Google Scholar]
- SEAMON K.B., DALY J.W. Forskolin: its biological and chemical properties. Adv. Cyclic. Nucleotide Protein Phosphoryl. Res. 1986;20:1–150. [PubMed] [Google Scholar]
- SHAFFER E.A., BOMZON A., LAX H., DAVISON J.S. The source of calcium for CCK-induced contraction of the guinea-pig gall bladder. Regul. Pept. 1992;37:15–26. doi: 10.1016/0167-0115(92)90060-8. [DOI] [PubMed] [Google Scholar]
- SHAFFER E.A., MCORMOND P., DUGGAN H. Quantitative cholescintigraphy: assessment of gallbladder filling and emptying and duodenogastric reflux. Gastroenterology. 1980;79:899–906. [PubMed] [Google Scholar]
- SOMLYO A.P., WU X., WALKER L.A., SOMLYO A.V. Pharmacomechanical coupling: the role of calcium, G-proteins, kinases and phosphatases. Rev. Physiol. Biochem. Pharmacol. 1999;134:201–234. doi: 10.1007/3-540-64753-8_5. [DOI] [PubMed] [Google Scholar]
- TAGUCHI K., UEDA M., KUBO T. Effects of cAMP and cGMP on L-type calcium channel currents in rat mesenteric artery cells. Jpn. J. Pharmacol. 1997;74:179–186. doi: 10.1254/jjp.74.179. [DOI] [PubMed] [Google Scholar]
- WELLMAN G.C., SANTANA L.F., BONEV A.D., NELSON M.T. Role of phospholamban in the modulation of arterial Ca(2+) sparks and Ca(2+)-activated K(+) channels by cAMP. Am. J. Physiol. Cell Physiol. 2001;281:C1029–C1037. doi: 10.1152/ajpcell.2001.281.3.C1029. [DOI] [PubMed] [Google Scholar]
- XUAN Y.T., WATKINS W.D., WHORTON A.R. Regulation of endothelin-mediated calcium mobilization in vascular smooth muscle cells by isoproterenol. Am. J. Physiol. 1991;260:C492–C502. doi: 10.1152/ajpcell.1991.260.3.C492. [DOI] [PubMed] [Google Scholar]
- YAMAGISHI T., YANAGISAWA T., SATOH K., TAIRA N. Relaxant mechanisms of cyclic AMP-increasing agents in porcine coronary artery. Eur. J. Pharmacol. 1994;251:253–262. doi: 10.1016/0014-2999(94)90407-3. [DOI] [PubMed] [Google Scholar]
- YAMANAKA J., NISHIMURA J., HIRANO K., KANAIDE H. An important role for the Na+–Ca2+ exchanger in the decrease in cytosolic Ca2+ concentration induced by isoprenaline in the porcine coronary artery. J. Physiol. 2003;549:553–562. doi: 10.1113/jphysiol.2002.037135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG L., BONEV A.D., MAWE G.M., NELSON M.T. Protein kinase A mediates activation of ATP-sensitive K+ currents by CGRP in gallbladder smooth muscle. Am. J. Physiol. 1994a;267:G494–G499. doi: 10.1152/ajpgi.1994.267.3.G494. [DOI] [PubMed] [Google Scholar]
- ZHANG L., BONEV A.D., NELSON M.T., MAWE G.M. Ionic basis of the action potential of guinea pig gallbladder smooth muscle cells. Am. J. Physiol. 1993;265:C1552–C1561. doi: 10.1152/ajpcell.1993.265.6.C1552. [DOI] [PubMed] [Google Scholar]
- ZHANG L., BONEV A.D., NELSON M.T., MAWE G.M. Activation of ATP-sensitive potassium currents in guinea-pig gall-bladder smooth muscle by the neuropeptide CGRP. J. Physiol. 1994b;478:483–491. doi: 10.1113/jphysiol.1994.sp020267. [DOI] [PMC free article] [PubMed] [Google Scholar]