Abstract
Previously, we reported that H-2′,6′-dimethyltyrosine [Dmt1]-D-Arg-Phe-Lys-NH2 (DALDA), an analogue of the naturally occurring opioid peptide dermorphin, is a highly potent and selective mu receptor agonist with low cross-tolerance to morphine. In the present study, we investigated the effect of treating mice chronically with [Dmt1]DALDA. The AD50 of [Dmt1]DALDA (s.c.) increased eight-fold in animals given this drug chronically; in contrast, the AD50 increased two-fold in mice chronically treated with morphine. The AD50 of morphine (s.c.) in these [Dmt1]DALDA-treated animals was increased more than 120 times, while that of the more selective μ agonist [D-Ala2-MePhe4-Gly-ol5]enkephalin (DAMGO) given intrathecally was increased more than 240 times. However, the AD50 of DAMGO given intracerebroventricularly was essentially the same in animals treated chronically with [Dmt1]DALDA as in naïve animals. The dose of naloxone required to precipitate withdrawal in [Dmt1]DALDA-treated animals was 20 times lower than that in morphine-tolerant animals.
Using real-time quantitative PCR, we found that expression of the μ opioid receptor, δ opioid receptor, preproenkephalin and preprodynorphin genes was upregulated in the brain by [Dmt1]DALDA treatment. No significant changes in expression of opioid receptor or opioid peptide genes were detected in the spinal cord of [Dmt1]DALDA-treated mice, nor in the brain or spinal cord of morphine-treated mice. We conclude that a high degree of tolerance to [Dmt1]DALDA develops in the spinal cord but not brain, and cannot be accounted for by changes in expression of opioid receptors or opioid peptides in these tissues.
Keywords: Tolerance, gene expression, spinal cord, morphine, DAMGO, [Dmt1]DALDA
Introduction
Morphine is a strong analgesic widely used following major surgery and to alleviate the pain of terminal diseases such as cancer, but its effectiveness is limited by several side effects, particularly the development of tolerance and physical dependence. These limitations have spurred the search for opioids that may retain morphine's analgesic potency but have low tolerance. In previous studies (Schiller et al., 2000; Riba et al., 2002a), we have defined some of the pharmacological characteristics of H-2′,6′-dimethyltyrosine [Dmt1]-D-Arg-Phe-Lys-NH2 (DALDA), an analogue of the naturally occurring opioid peptide dermorphin (Broccardo et al., 1981). [Dmt1]DALDA was a more potent and more selective μ-opioid agonist than morphine, as determined in the in vitro guinea-pig ileum and mouse vas deferens assays, and also showed higher μ-opioid receptor (MOR) affinity and selectivity in receptor-binding assays using rat and guinea-pig brain tissue (Schiller et al., 2000). In vivo, it was 40 times more potent than morphine in producing an antinociceptive effect in the mouse tail-flick test when the drugs were administered subcutaneously (s.c.), and about 10 and 30 times more potent than the MOR-selective agonist [D-Ala2-MePhe4-Gly-ol5]enkephalin (DAMGO) when the drugs were administered intrathecally (i.t.) and intracerebroventricularly (i.c.v.), respectively (Riba et al., 2002a). Most interestingly and promisingly, however, [Dmt1]DALDA showed relatively poor cross-tolerance to s.c. morphine in mice made tolerant by 3-day implantation of a morphine pellet. The AD50 of [Dmt1]DALDA increased insignificantly (Riba et al., 2002a), compared to a 7–8-fold increase in the AD50 of morphine determined under the same conditions.
These results suggest that [Dmt1]DALDA might have low tolerance potential, and thus be an attractive alternative to morphine in some clinical situations. In the present study, we have examined this tolerance potential further, by determining the effect of morphine and the MOR-selective agonist DAMGO in animals chronically treated with [Dmt1]DALDA. We report that a very large degree of tolerance to morphine given s.c. and to DAMGO given i.t. developed under these conditions, but essentially no tolerance developed to DAMGO given i.c.v. In an attempt to understand the basis for these findings, we examined the expression of several opioid receptor genes and peptides in these [Dmt1]DALDA-tolerant animals.
Methods
Animals
Male ICR (Harlan Sprague–Dawley, San Diego, CA, U.S.A.) mice were used for all the pharmacological experiments. All animals weighed 20–25 g, and were housed for at least 24 h before experiments in a temperature- and humidity-controlled environment, and fed ad libitum.
Drug administration
Drugs, including [Dmt1]DALDA, DAMGO, morphine and naloxone, were administered s.c., i.c.v., or i.t. Drugs injected i.c.v. or i.t. (Hylden & Wilcox, 1980) were given in a volume of 5 μl per mouse. A range of doses was used, as indicated in Results.
To induce tolerance to [Dmt1]DALDA, mice were injected s.c. with a dose of 2.5 μmol kg−1 twice daily for 7 days. Morphine tolerance was induced by s.c. injections of morphine at a dose of 300 μmol kg−1 twice daily for 7 days. This dose, more than 20–30 times the morphine AD50, was selected because morphine has a shorter duration of action than [Dmt1]DALDA (Riba et al., 2002a). The AD50's were then determined in response to s.c. injection of each of these drugs.
In the gene expression studies, morphine tolerance was induced by s.c. implantation of one morphine pellet (containing 75 mg morphine free base) for 72 h. This protocol was used because it has been reported to produce a higher degree of tolerance than regular injections (Way et al., 1968; 1969). In these studies, we sought to maximize the degree of tolerance to this drug. Control animals were treated with saline s.c. at a dose of 0.1 ml (10 g−1) twice daily for 7 days, or 3 days, in some of the gene-expression studies. A second set of control animals were implanted with one placebo pellet for 72 h.
Antinociceptive assay
The antinociceptive assay was a modification of the radiant tail-flick test described by Tulunay & Takemori (1974). Measurements were made 30 min after injection of drug, except for s.c. injection of [Dmt1]DALDA, when measurements were made 2 h after injection. The data were made quantal by designating a positive antinociceptive response as one exhibiting an increased latency to tail-flick at least 3 standard deviations above the mean latency of animals not given drug. At least three groups of 10 mice were used to establish dose–response curves and to estimate AD50 values.
Measurement of physical dependence
The naloxone ED50 to precipitate withdrawal jumping has been shown to correlate well with the degree of physical dependence (Way et al., 1969). Mice injected by [Dmt1]DALDA s.c. twice daily for 7 days were injected with variable doses of naloxone s.c. and placed immediately into Plexiglas cylinders (one mouse per cylinder of 30 cm base and 30 cm height). The number of jumps in 15 min was then counted, with greater than four jumps being the criterion for a positive withdrawal response. Three groups of at least 10 mice each were used for each ED50 determination.
Primer design and preparation
Primers for the following genes were prepared: the μ, δ and κ opioid receptors (MOR, DOR and KOR, respectively), and the opioid peptide precursors proenkephalin A (PPE), proenkephalin B (preprodynorphin (PPD)) and pro-opiomelanocortin (POMC). Gene-specific oligonucleotides were designed using Applied Biosystems Primer Express 1.5 Software (Table 1). Primers were designed to have a melting temperature of 58–60°C and to produce an amplicon of 50–150 bp. The last five bases on the 3′ end contained no more than 2 C/G bases in order to reduce the possibility of nonspecific product formation. Primer pairs plus water were subjected to the real-time PCR reaction described below in order to determine the extent of primer dimer formation.
Table 1.
Sequences of primers
| Genes | Forward primers | Reverse primers | Accession number | Nucleotide position |
|---|---|---|---|---|
| β-actin | ACGGCCAGGTCATCACTATTG | TGGATGCCACAGGATTCCAT | X03672 | 811–904 |
| MOR | TGCACCCTCACGTTCTCTCA | TGAGGACCGGCATGATGA | AF347691 | 4120–4210 |
| DOR | AAGGCTGTGCTCTCCATTGAC | TGTAGCGGTCCACGCTCAT | S66181 | 343–421 |
| KOR | TGATCCTGCGCCTGAAGAGT | ATGCGGCGGAGATTTCG | L11065 | 931–1000 |
| PPE | AGAAGCGAACGGAGGAGAGAT | TTCAGCAGATCGGAGGAGTTG | M13227 | 288–389 |
| PPD | GCGTGGTCCAGGCTGATG | AGGCAGTCCGCCATAACATT | U64968 | 4–71 |
| POMC | CCTGCTTCAGACCTCCATAGATG | GGATGCAAGCCAGCAGGTT | NM-008895 | 48–142 |
Primers were designed to amplify only cDNA template and not genomic DNA when possible. The specificities of the primers were demonstrated by the appearance of a single product on polyacrylamide gel and a single dissociation curve of the product. The degree of contamination of genomic DNA was also determined by analysis on 10% gel (Invitrogen).
Tissue collection and cDNA preparation
Mice were killed by cervical dislocation, brain and spinal cord were dissected, frozen on dry ice and stored at −80°C until use. Total RNA was prepared by using Trizol (Gibco-BRL) according to the protocol provided by the manufacturer. cDNA synthesis was performed by following the usual protocol (User Bulletin #2 of ABI prism 7700 sequence detection system, December 11, 1997), modified as follows. RNA (5 μg) for each sample was resuspended to 30 μl with RNAse-free water and treated with a reaction mixture containing 5 × first-strand buffer (Gibco-BRL), 30 U of RNAse-free DNAse (Roche) and 20 U of RNasin ribonuclease inhibitor (Promega) at 37°C for 40 min. RNA was then incubated at 70°C for 10 min in a mixture of oligo (dT) primers (manufactured and purified by Sigma-Genosys) and random hexamers (Gibco-BRL), 1 μg of each per sample. RNA was reverse transcribed using 480 U of Superscript II RNase H-Reverse Transcriptase (Gibco-BRL) in a final reaction mixture containing 0.5 μM dNTPs (Pharmacia) and 20 U of RNasin ribonuclease inhibitor. Samples were adjusted to a final concentration of 10 ng μl−1.
Real-time PCR
cDNA (50 ng) from each sample was used as template for PCR amplification with specific oligonucleotide primers (designed by Applied Biosystems Primer Express 1.5 software as described above, synthesized by Sigma-Genosys) in a 30 μl reaction volume containing 15 μl of Applied Biosystems SyBr Green Master Mix and 330 nM of forward and reverse primer mix. The cycling reactions were performed using the Applied Biosystems 5700 Sequence Detection System. Following a 10 min denaturation step at 95°C, the cycling reactions (35 cycles) were performed as follows: 15 s at 95°C, 1 min at 65°C. Following completion of the PCR reaction, the 5700 Sequence Detection machine then launches a dissociation protocol. Samples are heated gradually to 100°C and the loss of fluorescence when the SyBr green dissociates from the melted amplicon is recorded. The presence of the expected product is verified if the dissociation temperature of the amplicon obtained in the PCR reaction matches the temperature specified by the primer design software for the amplicon. We have validated this protocol by allowing the PCR products to reanneal and subjecting them to polyacrylamide gel analysis (data not shown), and have verified the presence of the correct amplicon.
Data analysis
In the pharmacological assays, AD50 values and their 95% confidence limits were calculated by the method of Litchfield & Wilcoxon (1949).
In the gene-expression studies, the amount of target gene obtained, normalized to the expression of an endogenous reference (β-actin) and relative to control samples, is given by ΔCT (Applied Biosystems, User Bulletin #2, December 11, 1997). Threshold cycle (CT) indicates the fractional cycle number at which the amount of amplified target reaches a fixed threshold. ΔCT is the average of normalized CT. The higher the initial amount of mRNA, the lower the CT value.
Beginning with CT values, we initially used the Wilks lambda criterion for a multivariate analysis of variance (MANOVA) to compare the patterns of expression levels of several genes from [Dmt1]DALDA- or morphine-treated animals with untreated controls. This test takes into account correlations among gene expression levels and controls the false-positive rate (e.g., a P-value that is nominally significant, resulting by chance because of the large number of genes analyzed) by testing the global hypothesis of no differences in gene expressions between treated and normal animals. If the test was significant, then we used univariate t-tests to determine which genes were contributing to the global difference and which were not. All statistical tests were carried out on log (base 2) of the gene expression data, since this transformation is required to achieve normal distribution of values.
Drugs
Morphine pellets and naloxone were provided by the National Institute on Drug Abuse (Rockville, MD, U.S.A.). [Dmt1]DALDA was synthesized as described (Schiller et al., 2000). DAMGO was provided by the National Institute on Drug Abuse and supplied by Multiple Peptide Systems (San Diego, CA, U.S.A.). All drugs were dissolved in saline.
Results
Antinociceptive effect of morphine (s.c.) and [Dmt1]DALDA (s.c.) in [Dmt1]DALDA-treated or morphine-injected mice
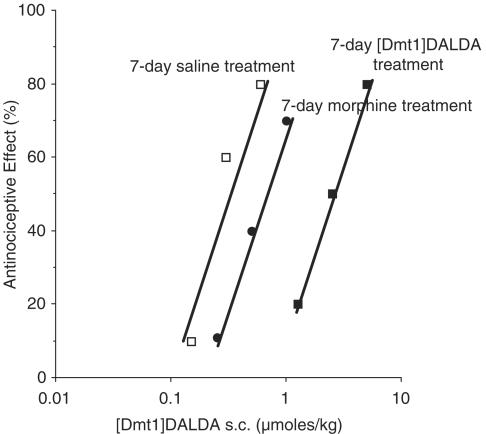
When mice were injected twice daily for 7 days with [Dmt1]DALDA, there was an approximately eight-fold increase in the AD50 of [Dmt1]DALDA (Figure 1) compared to that of saline control mice (2.5(1.4–4.4) versus 0.30(0.18–0.50) μmol kg−1, P<0.05). In contrast, there was only an insignificant two-fold increase (0.62(0.32–1.21) μmol kg−1) in the AD50 of [Dmt1]DALDA in the morphine-injected group (Figure 1). This is in agreement with our previous study (Riba et al., 2002a). In contrast, when tested in the [Dmt1]DALDA-treated group, morphine sulfate at doses up to 1600 μmol kg−1 s.c. did not induce a significant degree of antinociception; thus, no AD50 of morphine could be calculated. However, in morphine-injected animals, there was a slight increase in AD50 to s.c. morphine compared to that of saline control (41.5(20.0–85.1) versus 13.5(5.3–34.4) μmol kg−1, P>0.05; data not shown).
Figure 1.
Effect of chronic opioid treatment on antinociceptive response to [Dmt1]DALDA. Mice were treated for 7 days by twice daily injection (s.c.) with [Dmt1]DALDA (2.5 μmol kg−1); morphine (300 μmol kg−1); or saline (0.1 ml (10 g−1)). The AD50 values of [Dmt1]DALDA (s.c.) were determined by tail flick, using at least 10 animals per group.
Antinociceptive effect of DAMGO (i.t.) and (i.c.v.) in [Dmt1]DALDA-treated mice
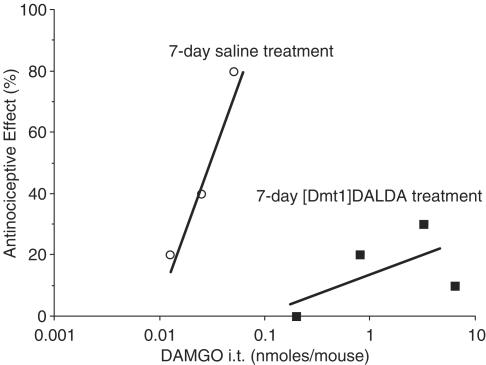
Morphine is a relatively nonselective opioid agonist, interacting with δ as well as μ receptors. Further studies in [Dmt1]DALDA-treated animals were carried out using the μ-selective agonist DAMGO. The [Dmt1]DALDA-treated animals also exhibited a very high degree of tolerance to DAMGO given i.t. (Figure 2). While the AD50 of DAMGO was 0.027(0.015–0.049) nmol per mouse in saline control animals, doses of DAMGO up to 6.4 nmol per mouse, nearly 240 times the AD50 in controls, did not induce antinociception in half the animals (Figure 2). In contrast, in morphine-tolerant animals, tolerance to DAMGO (i.t.) was only 4–5-fold (Riba et al., 2002b).
Figure 2.
Effect of chronic [Dmt1]DALDA treatment on antinoceptive response to DAMGO (i.t.). Mice were treated for 7 days with [Dmt1]DALDA by twice daily injection (s.c.) with 2.5 μmol kg−1 of the peptide, or with saline (0.1 ml (10 g−1)). The AD50 values of DAMGO (i.t.) were determined by tail flick, using at least 10 animals per group.
Finally, when DAMGO was given i.c.v., its AD50 in [Dmt1]DALDA-treated animals was 0.42(0.13–1.30) nmol per mouse, only about 2.5-fold greater than its AD50, 0.17(0.089–0.33) nmol per mouse (P>0.05), in naïve animals, a nonsignificant difference (data not shown).
Naloxone-precipitated withdrawal of [Dmt1]DALDA-treated mice
In animals treated with [Dmt1]DALDA, a dose of naloxone of 3.5(1.1–11.2) μmol kg−1 (s.c.) precipitated withdrawal jumps.
Relative expression of opioid receptor and opioid peptide genes in [Dmt1]DALDA-treated and morphine-treated mice
In an attempt to explore the molecular basis of the very large difference in cross-tolerance to DAMGO at i.t. and i.c.v. sites in [Dmt1]DALDA-treated mice, we used real-time quantitative PCR to analyze the expression of MOR, DOR, KOR, PPE, PPD and POMC. For the [Dmt1]DALDA-treated mice, expression of the six genes analyzed together was significantly different between drug-treated and control mice at P<0.01 in brain and P<0.05 in spinal cord. Accordingly, we then analyzed each of the six genes individually by the univariate test to see which contributed most to this significance. The results are summarized in Tables 2 (brain) and 3 (spinal cord). In brain, the expression levels of MOR and DOR were significantly altered in [Dmt1]DALDA-treated mice relative to controls, at the P<0.01 level, while those of PE and PD were altered significantly at P<0.05. All of these expression levels were upregulated, and, except for PE, this upregulation was essentially complete within 3 days (analysis not shown). In the spinal cord, in contrast, none of the six genes individually analyzed exhibited significantly altered expression (Table 3). That is, each of the genes contributed somewhat to a group-level significance, but none of these individual contributions was itself significant.
Table 2.
The effect of [Dmt1]DALDA chronic treatment on gene expression in brain
| Gene | Saline control | 3-Day treatment | 7-Day treatment | P |
|---|---|---|---|---|
| MOR | 7.42±0.20 | 6.19±0.34 | 6.05±0.17 | <0.01 |
| DOR | 5.68±0.08 | 5.09±0.24 | 5.04±0.21 | <0.01 |
| KOR | 6.71±0.15 | 6.54±0.37 | 6.04±0.26 | 0.15 |
| PPE | 1.01±0.21 | 1.00±0.20 | 0.02±0.09 | <0.05 |
| PPD | 6.12±0.20 | 5.69±0.21 | 5.43±0.28 | <0.05 |
| POMC | 8.04±0.30 | 8.43±0.25 | 7.92±0.06 | 0.41 |
Data are given as mean±s.e.m. of ΔCT values, n=5. P-value, compared with treatments over time and saline control, was determined by one-way ANOVA analysis.
Table 3.
The effect of [Dmt1]DALDA chronic treatment on gene expression in spinal cord
| Gene | Saline control | 3-Day treatment | 7-Day treatment | P |
|---|---|---|---|---|
| MOR | 5.46±0.33 | 5.00±0.23 | 5.64±0.43 | 0.67 |
| DOR | 5.95±0.30 | 5.87±0.26 | 6.78±0.20 | 0.11 |
| KOR | 6.47±0.27 | 6.40±0.19 | 6.64±0.32 | 0.82 |
| PPE | 1.30±0.18 | 1.06±0.07 | 1.76±0.36 | 0.64 |
| PPD | 6.77±0.28 | 6.47±0.19 | 6.95±0.22 | 0.86 |
| POMC | 9.96±0.34 | 10.17±0.21 | 10.16±0.34 | 0.59 |
Data are given as mean±s.e.m. of ΔCT values, n=5. P-value, compared with treatments over time and saline control, was determined by one-way ANOVA analysis.
Finally, in morphine-pelleted animals, MANOVA revealed a significant change in expression of the six genes in brain (P<0.01), but not spinal cord. None of the genes analyzed individually in the brain or spinal cord, however, exhibited significantly altered expression (Table 4). Thus, as with the spinal cord tissue of [Dmt1]DALDA-treated mice, each of the six genes contributed to a group-level significance in the brain tissue of morphine-tolerant mice.
Table 4.
The effect of morphine pellet treatment (72 h) on gene expression in brain and spinal cord
| Brain | Spinal cord | |||||
|---|---|---|---|---|---|---|
| Gene | Placebo | Morphine tolerant | P | Placebo | Morphine tolerant | P |
| MOR | 7.08±0.10 | 6.68±0.32 | 0.33 | 5.85±0.45 | 5.91±0.23 | 0.82 |
| DOR | 6.02±0.23 | 6.13±0.18 | 0.72 | 6.47±0.40 | 6.17±0.25 | 0.62 |
| KOR | 7.10±0.19 | 7.20±0.17 | 0.72 | 6.88±0.22 | 6.96±0.24 | 0.87 |
| PPE | 1.60±0.24 | 1.30±0.16 | 0.32 | 1.64±0.14 | 1.41±0.20 | 0.48 |
| PPD | 6.22±0.19 | 5.97±0.20 | 0.40 | 7.07±0.14 | 6.82±0.20 | 0.43 |
| POMC | 8.58±0.48 | 9.35±0.38 | 0.24 | 10.81±0.17 | 11.18±0.30 | 0.28 |
Data are given as mean±s.e.m. of ΔCT values, n=5. P-values, morphine-tolerant mice compared to placebo mice, were determined by t-test.
Discussion
We have previously shown that [Dmt1]DALDA is a potent and highly selective MOR agonist, both in vitro (Schiller et al., 2000) and in vivo (Riba et al., 2002a). It also exhibits relatively low cross-tolerance to s.c. morphine in morphine-pelleted animals (Neilan et al., 2001; Riba et al., 2002a). However, we report here that animals treated chronically by s.c. injection with [Dmt1]DALDA developed an eight-fold tolerance to the drug when given s.c. (Figure 1), and an even higher degree of tolerance to s.c. morphine, so high in fact that an AD50 could not be determined. In contrast, in animals injected twice daily with morphine, there was a three-fold increase in AD50 to s.c. morphine.
A dose of just 3.5 μmol kg−1 of naloxone was sufficient to induce withdrawal jumping symptoms in [Dmt1]DALDA-treated mice, as compared to a dose of 70 μmol kg−1 in morphine-pelleted mice (Hooke et al., 1995). The dose of naloxone needed to precipitate withdrawal is used to estimate the degree of dependence, with higher dependence levels requiring less naloxone (Way et al., 1968; 1969). Moreover, in the study by Hooke et al. (1995), the animals were made tolerant to morphine by pellet implantation, which results in a greater degree of tolerance than daily injections (Way et al., 1968; 1969). Our results using twice daily injections of [Dmt1]DALDA thus indicate that a very high degree of both tolerance and physical dependence developed to the chronic administration of this drug. Previously, we reported that [Dmt1]DALDA is more potent at interacting with MOR receptors and inducing antinociception than is morphine (Riba et al., 2002a). It appears that it is likewise much more potent at inducing tolerance and physical dependence than morphine. In view of the evidence that the antinociceptive potency of opioids often correlates with potency to induce tolerance (Miglecz et al., 1979), the high potency of [Dmt1]DALDA seems consistent with the much greater cross-tolerance of morphine to [Dmt1]DALDA than of [Dmt1]DALDA to itself, as well as the low cross-tolerance of [Dmt1]DALDA to morphine-treated mice.
[Dmt1]DALDA is highly selective at μ receptors, unlike morphine. Therefore, it was of interest to determine cross-tolerance to [Dmt1]DALDA of another highly selective μ agonist, DAMGO. Given i.t., DAMGO was also highly cross-tolerant to [Dmt1]DALDA (Figure 2). As with morphine (s.c.), an accurate AD50 of DAMGO (i.t.) could not be obtained under these conditions, because even at doses of 6.4 nmol per mouse, more than 200 times the AD50 of DAMGO in saline control animals, there was no antinociceptive effect. This contrasts with a degree of tolerance of less than 10-fold when DAMGO (i.t.) was administered to animals made tolerant to morphine (Riba et al., 2002b). Again, in the latter study, morphine was administered chronically by pellet implantation, which should have maximized the degree of tolerance developed. However, when DAMGO was given i.c.v. to [Dmt1]DALDA-tolerant animals, its AD50 was just 2.5 times greater than the AD50 in naïve animals.
Another group reported a somewhat similar study of chronic treatment with [Dmt1]DALDA (Zhao et al., 2002). They also found that tolerance to spinal drug administration was much greater than to i.c.v. drug (about 44-fold versus three-fold), though they used [Dmt1]DALDA, not DAMGO, as the challenging drug. Their use of [Dmt1]DALDA may account for their observation that tolerance at the spinal level was measurable, whereas it was not in our study using DAMGO. Zhao et al. (2002) also reported a much lower degree of cross-tolerance of morphine (s.c.) than we did to [Dmt1]DALDA, about 15-fold. At least part of the discrepancy may be due to their use of a somewhat weaker dosing regimen. Zhao et al., administered [Dmt1]DALDA at a dose of 1.6 μmol kg−1 twice daily for 2.5 days, whereas we administered twice-daily injections of 2.5 μmol kg−1 for 7 days. However, even the weaker dosing regimen used by Zhao et al. resulted in a much greater tolerance to acute morphine than we observed in animals given morphine twice daily for 7 days (three-fold). Moreover, that longer dosing schedules result in a much greater degree of tolerance is supported by the observation by Zhao et al. that after 7 days of twice-daily administration to rats of [Dmt1]DALDA (i.t.), it was no longer possible to obtain a dose–response curve even using doses of the drug (i.t.) 1000 times the original AD50.
Clearly, our data as well as those of Zhao et al. (2002), show that tolerance develops very differently to [Dmt1]DALDA in the spinal cord and brain. In an attempt to understand these differences, we determined the expression of the three major opioid receptor genes and the three major opioid peptide precursors in brain and spinal cord. Most studies of opioid receptor levels during morphine tolerance have reported no significant changes in either opioid receptor binding (Besse et al., 1992; Polastron et al., 1994), or expression (Kest et al., 1994; Brodsky et al., 1995; Unterwald et al., 1995; Buzas et al., 1996). A similar lack of correlative changes has been reported with studies of opioid peptides at protein levels (Nylander et al., 1995; Trujillo & Akil, 1995) and at mRNA levels (Mocchetti et al., 1989; Gudehithlu & Bhargava, 1995; Tjon et al., 1997; Turchan et al., 1997; Fang et al., 1998). However, the very large degree of tolerance developed when [Dmt1]DALDA was administered systemically or to the spinal cord suggests that this would be a good system in which to re-investigate this question. Zhao et al. (2002) reported that the Bmax of tritiated [Dmt1]DALDA binding was decreased by 30–35%, though this change was observed in both the brain and spinal cord of animals treated chronically with this drug.
Our gene expression analysis of [Dmt1]DALDA-treated mice revealed a significant upregulation of both MOR and DOR, as well as PE and PD, in brain (Tables 2 and 3). Though mean ΔCT values are not very precise, based on them this upregulation ranged from 50 to 60% for DOR and PD, to 100% for PE and 160% for MOR. In contrast, no significant changes in expression of any individual genes were observed in the spinal cord of [Dmt1]DALDA-treated mice, though expression levels of the six genes as a group were significantly increased. No significant changes in the expression level of individual genes were observed in either the brain or spinal cord tissue of morphine-pelleted animals (Table 4), though in the brain expression levels of the group were significantly increased.
The finding of increases in expression levels of MOR, DOR and two opioid peptide precursors restricted to the brain of [Dmt1]DALDA-treated mice is interesting, in that administration to the brain of [Dmt1]DALDA-treated mice resulted in very little tolerance (to DAMGO), whereas there was a very high level of tolerance in the spinal cord. Conceivably, elevated expression of receptor could mask the development of tolerance. However, since we did not measure protein levels of receptor, we cannot conclude that the enhanced expression levels are directly related to the lower degree of tolerance.
In summary, though [Dmt1]DALDA shows little cross-tolerance in morphine-tolerant mice, animals treated chronically with [Dmt1]DALDA show very high tolerance to morphine given s.c. or DAMGO given i.t. The lack of high cross-tolerance when [Dmt1]DALDA is given i.c.v. suggests that [Dmt1]DALDA may have clinical benefits under certain conditions.
Acknowledgments
This work was supported by NIDA 8924.
Abbreviations
- DAMGO
[D-Ala2-MePhe4-Gly-ol5]enkephalin
- [Dmt1]DALDA
H-2′,6′-dimethyltyrosine [Dmt1]-D-Arg-Phe-Lys-NH2
- DOR
δ opioid receptor
- i.c.v.
intracerebroventricular
- i.t.
intrathecal
- KOR
κ opioid receptor
- MOR
μ opioid receptor
- POMC
pro-opiomelanocortin
- PPD
preprodynorphin
- PPE
preproenkephalin
- s.c.
subcutaneous
References
- BESSE D., LOMBARD M.C., BESSON J.M. Up-regulation of [3H]DAMGO and [3H]DTLET opioid binding sites in laminae I–II of the spinal cord in intact and deafferented morphine-tolerant animals. Neurosci. Lett. 1992;136:209–212. doi: 10.1016/0304-3940(92)90050-h. [DOI] [PubMed] [Google Scholar]
- BROCCARDO M., ERSPAMER V., FALCONIERI-ESPAMER G., IMPROTA G., LINARI G., MELCHIORRI P., MONTENUCCHI P.C. Pharmacological data on dermorphins, a new class of potent opioid peptides from amphibian skin. Br. J. Pharmacol. 1981;73:625–631. doi: 10.1111/j.1476-5381.1981.tb16797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRODSKY M., ELLIOTT K., HYANKSY A., INTURRISI C.E. CNS levels of Mu opioid receptor (MOR-1) mRNA during chronic treatment with morphine or naltrexone. Brain Res. Bull. 1995;38:135–141. doi: 10.1016/0361-9230(95)00079-t. [DOI] [PubMed] [Google Scholar]
- BUZAS B., ROSENBERGER J., COX B.M. Mu and delta opioid receptor gene expression after chronic treatment with opioid agonist. Neuroreport. 1996;7:1505–1508. doi: 10.1097/00001756-199606170-00013. [DOI] [PubMed] [Google Scholar]
- FANG Y., KELLY M.J., RONNEKLEIV O.K. Proopiomelanocortin (POMC) mRNA expression: distribution and region-specific down-regulation by chronic morphine in female guinea pig hypothalamus. Brain Res. Mol. Brain Res. 1998;55:1–8. doi: 10.1016/s0169-328x(97)00348-3. [DOI] [PubMed] [Google Scholar]
- GUDEHITHLU K.P., BHARGAVA H.N. Modulation of preproenkephalin mRNA levels in brain regions and spinal cord of rats treated chronically with morphine. Peptides. 1995;16:415–419. doi: 10.1016/0196-9781(94)00199-g. [DOI] [PubMed] [Google Scholar]
- HOOKE L.P., HE L., LEE N.M. Dynorphin A modulates acute and chronic opioid effects. J. Pharmacol. Exp. Ther. 1995;273:292–297. [PubMed] [Google Scholar]
- HYLDEN J.L.K., WILCOX G. Intrathecal morphine in mice: a new technique. Eur. J. Pharmacol. 1980;67:313–316. doi: 10.1016/0014-2999(80)90515-4. [DOI] [PubMed] [Google Scholar]
- KEST B., JENAB S., BRODSKY M., ELLIOTT K., INTURRISI C.E. Supraspinal delta opioid receptor mRNA levels are not altered in [D-Ala2]deltorphin II tolerant mice. J. Neurosci. Res. 1994;39:674–679. doi: 10.1002/jnr.490390608. [DOI] [PubMed] [Google Scholar]
- LITCHFIELD J.T., JR, WILCOXON R. A simplified method of evaluating dose–effect experiments. J. Pharmacol. Exp. Ther. 1949;96:99–113. [PubMed] [Google Scholar]
- MIGLECZ E., SZEKELY J.L., DUNAI-KOVACS Z. Comparison of tolerance development and dependence capacities of morphine, beta-endorphin, and [D-Met2, Pro5]-enkephalinamide. Psychopharmacology. 1979;62:29–34. doi: 10.1007/BF00426031. [DOI] [PubMed] [Google Scholar]
- MOCCHETTI I., RITTER A., COSTA E. Down-regulation of proopiomelanocortin synthesis and beta-endorphin utilization in hypothalamus of morphine-tolerant rats. J. Mol. Neurosci. 1989;1:33–38. doi: 10.1007/BF02896854. [DOI] [PubMed] [Google Scholar]
- NEILAN C.L., NGUYEN T.M., SCHILLER P.W., PASTERNAK G.W. Pharmacological characterization of the dermorphin analog [Dmt1]DALDA, a highly potent and selective mu-opioid peptide. Eur. J. Pharmacol. 2001;419:15–23. doi: 10.1016/s0014-2999(01)00946-3. [DOI] [PubMed] [Google Scholar]
- NYLANDER I., VLASKOVSKA M., TERENIUS L. The effects of morphine treatment and morphine withdrawal on the dynorphin and enkephalin systems in Sprague–Dawley rats. Psychopharmacology. 1995;118:391–400. doi: 10.1007/BF02245939. [DOI] [PubMed] [Google Scholar]
- POLASTRON J., MEUNIER J.C., JAUZAC P. Chronic morphine induces tolerance and desensitization of mu-opioid receptor but not down-regulation in rabbit. Eur. J. Pharmacol. 1994;266:139–146. doi: 10.1016/0922-4106(94)90103-1. [DOI] [PubMed] [Google Scholar]
- RIBA P., BEN Y., NGUYEN T.M.-D., FURST S., SCHILLER P.W., LEE N.M. [Dmt1]DALDA is highly selective and potent at μ opioid receptors, but is not cross-tolerant with systemic morphine. Curr. Med. Chem. 2002a;9:31–39. doi: 10.2174/0929867023371445. [DOI] [PubMed] [Google Scholar]
- RIBA P., BEN Y., SMITH A.P., FURST S., LEE N.M. Morphine tolerance in spinal cord is due to interaction between mu- and delta-receptors. J. Pharmacol. Exp. Ther. 2002b;300:265–272. doi: 10.1124/jpet.300.1.265. [DOI] [PubMed] [Google Scholar]
- SCHILLER P.W., NGUYEN T.M.-D., BEREZOWSKA I., DUPUIS S., WELTROWSKA G., CHUNG N.N., LEMIEUX C. Synthesis and in vitro opioid activity profiles of DALDA analogues. Eur. J. Med. Chem. 2000;35:895–901. doi: 10.1016/s0223-5234(00)01171-5. [DOI] [PubMed] [Google Scholar]
- TJON G.H., VOORN P., VANDERSCHUREN L.J., DE VRIES T.J., MICHIELS N.H., JONKER A.J., KLOP H., NESTBY P., MULDER A.H., SCHOFFELMEER A.N. Delayed occurrence of enhanced striatal preprodynorphin gene expression in behaviorally sensitized rats: differential long-term effects of intermittent and chronic morphine administration. Neuroscience. 1997;76:167–176. doi: 10.1016/s0306-4522(96)00363-6. [DOI] [PubMed] [Google Scholar]
- TRUJILLO K.A., AKIL H. Excitatory amino acids and drugs of abuse: a role for N-methyl-D-aspartate receptors in drug tolerance, sensitization and physical dependence. Drug Alcohol Depend. 1995;38:139–154. doi: 10.1016/0376-8716(95)01119-j. [DOI] [PubMed] [Google Scholar]
- TULUNAY F.C., TAKEMORI A.E. The increased efficacy of narcotic antagonists induced by various anarcotic analgesics. J. Pharmacol. Exp. Ther. 1974;190:395–400. [PubMed] [Google Scholar]
- TURCHAN J., LASON W., BUDZISZEWSKA B., PRZEWLOCKA B. Effects of single and repeated morphine administration on the prodynorphin, proenkephalin and dopamine D2 receptor gene expression in the mouse brain. Neuropeptides. 1997;31:24–28. doi: 10.1016/s0143-4179(97)90015-9. [DOI] [PubMed] [Google Scholar]
- UNTERWALD E.M., RUBENFELD J.M., IMAI Y., WANG J.B., UHL G.R., KREEK M.J. Chronic opioid antagonist administration upregulates mu opioid receptor binging without altering mu opioid receptor mRNA levels. Mol. Brain Res. 1995;33:351–355. doi: 10.1016/0169-328x(95)00143-g. [DOI] [PubMed] [Google Scholar]
- WAY E.L., LOH H.H., SHEN F. Morphine tolerance, physical dependence, and synthesis of brain 5-hydroxytryptamine. Science. 1968;162:1290–1292. doi: 10.1126/science.162.3859.1290. [DOI] [PubMed] [Google Scholar]
- WAY E.L., LOH H.H., SHEN F.-H. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J. Pharmacol. Exp. Ther. 1969;167:1–8. [PubMed] [Google Scholar]
- ZHAO G.M., WU D.L., SOONG Y., SHIMOYAMA M., BEREZOWAKA I., SCHILLER P.W., SZETO H.H. Profound spinal tolerance after repeated exposure to a highly selective mu-opioid peptide agonist: role of delta-opioid receptors. J. Pharmacol. Exp. Ther. 2002;302:188–196. doi: 10.1124/jpet.302.1.188. [DOI] [PubMed] [Google Scholar]