Abstract
The effects of extracellular and intracellular polyamines (PAs), spermine and putrescine, on the cation current (mICAT) evoked either by activating muscarinic receptors with carbachol or by intracellularly applied GTPγS (in the absence of carbachol) were studied using patch-clamp recording techniques in single guinea-pig ileal myocytes.
Extracellular spermine and putrescine rapidly and reversibly inhibited mICAT in a concentration- and voltage-dependent manner with the IC50 values at −40 mV of about 1 and 5 mM, respectively. Membrane depolarization relieved the blocking action of PAs although cation conductance activation curve remained N-shaped. The inhibition was similar for both carbachol- and GTPγS-evoked currents, suggesting that the cation channel rather than the muscarinic receptor was the primary site of the PA action. In outside-out membrane patches, both cation channel unitary conductance and open probability were reduced.
In perforated-patch experiments used to retain cytoplasmic PAs sustained 100 μM carbachol-induced mICAT was significantly smaller (478±76 pA, n=7) compared to that recorded using conventional whole-cell configuration with nominally PA-free pipette solution (1314±76 pA, n=12), but comparable in size to mICAT with 0.3 mM spermine in the pipette solution (509±41 pA, n=19). Intracellular putrescine inhibited mICAT less potently compared to spermine.
In conclusion, these results show a novel role of intestinal PAs in mICAT inhibition, which can contribute to their well-known suppressing effect on the gastrointestinal smooth muscle excitability and contractility.
Keywords: Gastrointestinal smooth muscle, muscarinic receptor, carbachol, GTPγS, cation current, polyamines, putrescine, spermine
Introduction
The polyamines (PAs) putrescine, spermidine and spermine are a ubiquitous class of aliphatic polycations, which play an important role in protein synthesis, cell division and cell growth. PAs are positively charged at physiological pH; therefore, they are suggested to participate in many cellular processes through their binding to various negatively charged molecules such as DNA, RNA, phospholipids, etc., which can explain the diversity of their cellular effects. Consistent with the idea of electrostatic interaction as the principal mechanism of PA action in many cases, spermine (four positive charges) was found to be more potent compared to spermidine or putrescine (three and two charges, respectively). However, the complexity of the PA-dependent regulation of many cellular functions argues that some additional effects, not related to direct charge interaction, may also be involved (recently reviewed by Wallace et al., 2003).
Being charged molecules, PAs do not readily cross the cell membrane. Thus, intracellular PA concentration is largely dependent on their synthesis, but transport of PA also makes a significant contribution to their homeostasis, particularly under conditions when PA synthesis is impaired (Wallace et al., 2003; Jänne et al., 2004). The PA transporter is carrier-mediated, energy- and temperature-dependent, although endocytosis has been recently suggested as an alternative route of PA internalization. A relatively minor component of PA transport is also dependent on the sodium gradient. Therefore, intracellular concentration of PA is tightly regulated by a complex system of the biosynthesis, transport and degradation.
Generally, tissues with high rates of proliferation and protein synthesis, such as gastrointestinal smooth muscles and liver, have higher PA levels. Intracellular concentration of putrescine, spermidine and spermine in the guinea-pig ileum was estimated at 18, 206 and 385 μM, respectively (Swärd et al., 1994). However, their concentration in the intestinal lumen can be much higher, for example, in the mM range (Osborne & Seidel, 1990).
Extracellular PAs have been shown to relax smooth muscles, to inhibit electrical activity and to decrease the intracellular Ca2+ concentration (Onodera et al., 1968; Nilsson & Hellstrand, 1993; Gomez & Hellstrand, 1995). These effects appear to be mediated by the calcium channel inhibition (Gomez & Hellstrand, 1995; 1999; Nilsson et al., 2002). Acetylcholine, the major excitatory neurotransmitter in visceral smooth muscles, causes membrane depolarization via monovalent cation selective channel opening and thus induces Ca2+ influx through voltage-gated Ca2+ channels. Therefore, cation channels, providing they were targeted by PAs, could also play a role in these processes. However, despite increasing recognition of PAs as specific and important regulators of various ion channels (for a review, see Williams, 1997; Lu, 2004), the effects of PAs on the muscarinic cation current (mICAT) have not yet been investigated.
The effects of PAs on ion channel properties have been reported at nano- to millimolar concentration range acting intra- and/or extracellularly. One well-studied example of physiologically important PA ion channel modulation is strong inward rectification of certain types of KIR channels. PAs produce such rectification by virtue of their ability to block KIR channels from the cytoplasmic side in a steeply voltage-dependent fashion (Lopatin et al., 1994). In contrast, in case of cyclic nucleotide-gated cation channels extra- and intracellular PAs act, depending on voltage, either as permeant or nonpermeant blockers, resulting in complex conductance curves with two or more humps (Guo & Lu, 2000). In smooth muscle myocytes, muscarinic cation conductance curve also shows complex N-shape voltage dependence and its origin is not yet completely known. Thus, as membrane voltage is increased, cation conductance increases in two phases: at potentials positive to about −20 mV, a prominent decline of the conductance occurs due to flickery channel behaviour (Zholos et al., 2003; 2004b). In the present work, we found that in gastrointestinal myocytes both intra- and extracellular PAs cause significant mICat inhibition at physiologically relevant concentrations, but at the same time PAs do not seem to be involved, at least primarily, in mICAT rectification properties.
Methods
Cell preparation and current recording
Adult male guinea-pigs (300–400 g) were killed by dislocation of the neck, followed by immediate exsanguinations according to humane animal care, as required by the Ukrainian law. Experiments were performed at room temperature on single ileal smooth muscle myocytes isolated from the longitudinal muscle layer after tissue treatment with collagenase (1 mg ml −1) at 36°C for 25 min.
Whole-cell membrane current was recorded using low-resistance borosilicate patch pipettes (1–3 MΩ) and an Axopatch 200B (Axon Instruments Inc., Foster City, CA, U.S.A.) voltage-clamp amplifier. For single-channel recordings patch pipettes (5–10 MΩ) coated with elastomer R6101 were used. Single-channel records were filtered at 1 kHz and sampled at 10 kHz.
Solutions
Pipettes were filled with the following solution (in mM): CsCl 80, adenosine 5′-triphosphate magnesium salt (ATP) 1, creatine 5, guanosine 5′-triphosphate lithium salt (GTP) 1, D-glucose 5, N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10, 1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid (BAPTA) 10, CaCl2 4.6 (e.g. [Ca2+]i=100 nM), pH adjusted to 7.4 with CsOH (total Cs+ 124 mM). The presence of 1 mM GTP in this solution reduced desensitization to a minimum (Zholos & Bolton, 1996). In experiments designed to activate cationic channels directly, without the activation of muscarinic receptors, GTP in the pipette solution was replaced with 200 μM guanosine 5′-O-(3-thiotriphosphate (GTPγS). For [Ca2+]i measurements, pipettes were filled with the following solution (in mM): CsCl 120, ATP 1, creatine 5, GTP 1, D-glucose 5, HEPES 10, bis-Fura-2 0.1, pH adjusted to 7.4 with CsOH (total Cs+ 124 mM). For perforated patch recordings, amphotericin B was added to the standard pipette solution at 100 μg ml−1. It was prepared daily as a stock solution (3 mg dissolved in 50 μl of DMSO).
During seal formation, PSS of normal composition was used. After whole cell configuration had been established and about 3 min for equilibration had elapsed, a Ca2+- and Mg2+-free solution was applied of the following composition (in mM): CsCl 120, D-glucose 12, HEPES 10, pH adjusted to 7.4 with CsOH (total Cs+ 124 mM). The cells prior to experiment were kept in the following solution (PSS) (mM): NaCl 120, KCl 6, CaCl2 2.5, MgCl2 1.2, D-glucose 12, HEPES 10, pH adjusted to 7.4 with NaOH. Complete exchange of the external solution was achieved within about 1 s as described previously (Zholos & Bolton, 1995).
Measurement and data analysis
mICAT was continuously monitored at the holding potential of −40 mV. Its steady-state current–voltage (I–V) relationship was obtained by applying a slow 6 s duration voltage ramp from 80 to −120 mV before and after carbachol application with an off-line correction for the background current.
Concentration–effect curves were constructed by plotting mICAT amplitude against the PA concentration on a semi-logarithmic scale. They were fitted by the Hill equation in the following form:
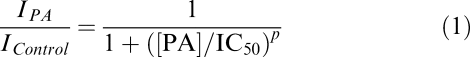 |
where IControl and IPA are, respectively, the amplitudes of the current before and in the presence of various PA concentrations, [PA]; IC50 is the PA concentration at which the current amplitude is reduced by 50% and p is the slope factor of the inhibition curve. The data were analysed and plotted using MicroCal Origin software (MicroCal Software, Inc., Northampton, MA, U.S.A.). Values are given as the means±s.e.m; n represents the number of cells tested. To determine the statistical significance of differences between the means, a t-test was used. Differences were judged to be significant when the two-tailed P-value was less than 0.05.
Calcium measurements
Changes of [Ca2+]i were monitored by using digital imaging microscopy. Bis-Fura-2 introduced into the cell from the patch pipette was excited at 340 and 380 nm using the DeltaRAM-V monochromator (Photon Technology International Inc., Ford, West Sussex, U.K.). The fluorescence light was collected using CFI S.Fluor × 40 objective and an inverted microscope Eclipse TE200 (Nikon UK Ltd, Kingston upon Thames, U.K.), and was detected using an intensified charge-coupled device (CCD) camera IC-200 (Photon Technology International Inc., Ford, West Sussex, U.K.) after passing through a band-pass interference filter centred at 510 nm.
[Ca2+]i was calculated according to the formula (Grynkiewicz et al., 1985)
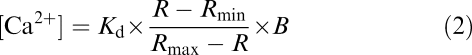 |
where R is the ratio of fluorescence intensities obtained with the excitation at λ1=340 and λ2=380 nm; Rmax and Rmin are the same ratios of Ca2+-saturated and Ca2+-free dye, respectively; B is the ratio of fluorescence intensities at 380 nm in zero calcium to that at saturating calcium; and Kd is the effective dissociation constant of bis-Fura-2 for calcium (525 nm in the presence of 1 mM Mg2+). Rmin was obtained by dialysing the cells with 10 mM EGTA-containing solution (Rmin=0.354±0.007, n=5). Rmax was obtained by clamping the membrane potential to −200 mV, which caused membrane breakage and induced a large increase of R in the presence of 2.5 mM external Ca2+ (Rmax=2.98±0.16, n=5). Thus, the mean value of B was 3.64±0.23 (n=5).
Chemicals used
1,4-Diaminobutane (putrescine), collagenase (type 1A), ATP (magnesium or sodium salt), GTP (sodium salt), GTPγS (tetralithium salt), creatine, HEPES, BAPTA, and carbamylcholine chloride (carbachol) were obtained from Sigma Chemical Co. (Poole, Dorset, U.K.). Spermine tetrahydrochloride was from Calbiochem-Novabiochem Ltd, Beeston, Nottingham, U.K. Bis-Fura 2 was obtained from Molecular Probes Europe BV, Leiden, The Netherlands. All other chemicals were from BDH Laboratory Supplies (AnalaR grade), Pool, U.K.
Results
Effects of extracellular spermine and putrescine on carbachol- and GTPγS-induced cation currents
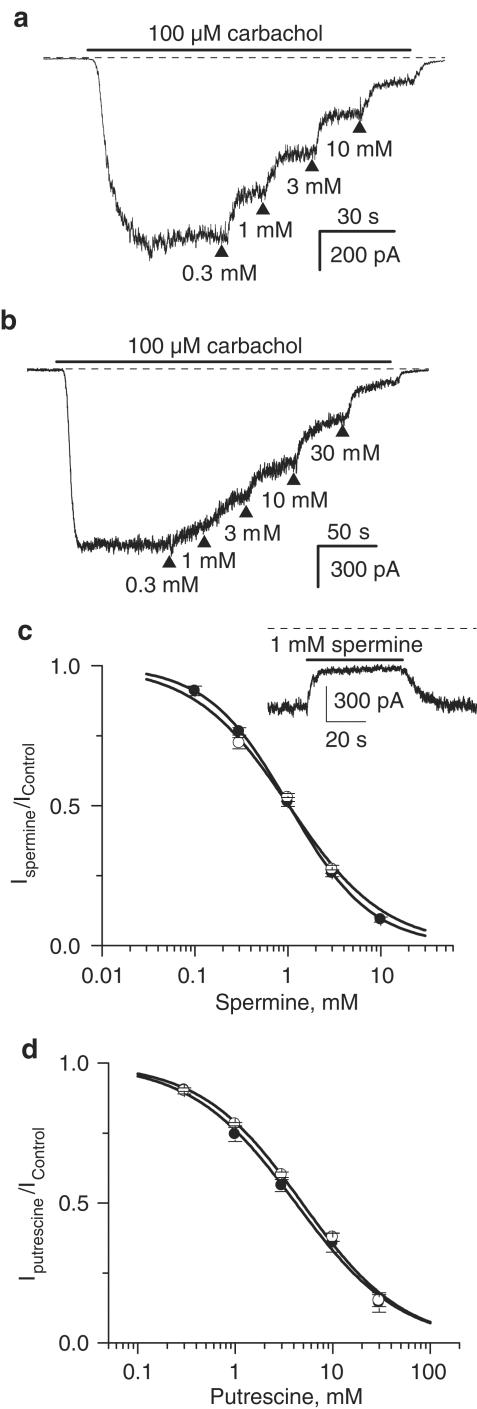
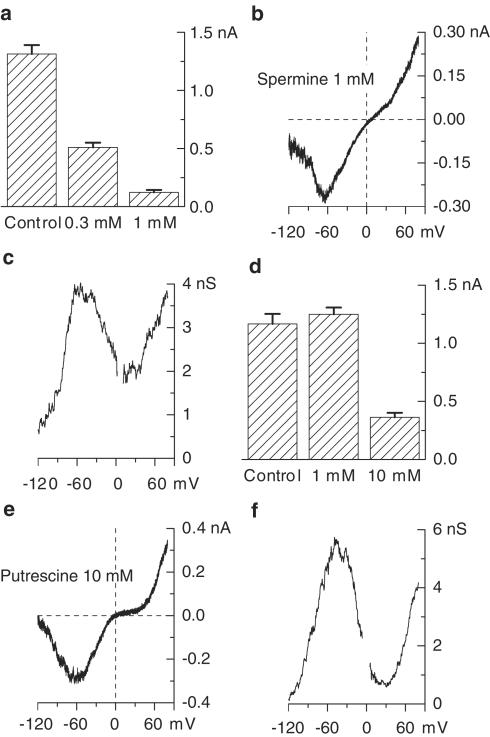
In these experiments, carbachol was applied at 100 μM, a concentration which produces a nearly maximal mICAT in these cells (Bolton & Zholos, 1997). Spermine added to the external solution cumulatively at ascending concentrations produced significant mICAT inhibition (Figure 1a). The inhibition was fast and readily reversible upon wash-out (Figure 1c, inset). Thus, this effect could be attributed mostly to the extracellular action of spermine since PA uptake takes much longer even at 37°C (e.g., 20 min as shown using confocal imaging of fluoresceinated PAs, Aziz et al., 1998). The inhibition concentration–effect curve had the following mean parameters: IC50=1.03±0.01 mM; P=0.98±0.01 (n=16) (Figure 1c, closed circles).
Figure 1.
Inhibitory effect of external spermine (a) and putrescine (b) applied at ascending concentrations on 100 μM carbachol-activated cationic current measured at −40 mV. Relative current amplitude plotted against spermine (c) and putrescine (d) concentration on semilogarithmic scale and fitted according to equation (1) (solid circles for carbachol-activated current, open circles for GTPγS-activated cationic current). The inset shows rapid current inhibition and recovery during spermine application and wash-out.
It was possible that spermine inhibited mICAT by binding to muscarinic receptors. To test this possibility in the next series of experiments, GTPγS was applied intracellularly in order to activate G-proteins directly, bypassing the muscarinic receptors. We have recently shown that in ileal myocytes carbachol and GTPγS activated the same main 57 pS cation channel with virtually identical single-channel conductance and gating kinetics (Zholos et al., 2004b). Following break-through with patch pipettes containing 200 μM GTPγS (no carbachol in the bath), cationic current measured at −40 mV developed slowly, presumably due to the accumulation of activated G proteins, to reach a steady-state level within approximately 3–5 min and then remained sustained for many tens of minutes (cf. Zholos & Bolton, 1996; Zholos et al., 2003). This GTPγS-induced current was inhibited by spermine in a similar manner (IC50=1.02±0.10 mM; P=0.84±0.10, n=5) (Figure 1c, open circles).
Qualitatively similar results were obtained for the putrescine action on mICAT, although putrescine was about four-fold less potent compared to spermine. Such difference could be explained by the lower number of positive charges in case of putrescine, assuming that the effect of both PAs was due to electrostatic interaction with the charged groups on or near the cation channel. A representative example of putrescine-mediated inhibition of carbachol-induced mICAT is shown in Figure 1b, while Figure 1d summarizes the mean results for carbachol- and GTPγS-induced currents as shown by closed and open circles, respectively. The following mean inhibition parameters were obtained: IC50=4.17±0.30 mM, P=0.81±0.05 (carbachol, n=10) and IC50=4.85±0.32 mM, P=0.83±0.05) (GTPγS, n=4). In the latter case, the IC50 value was somewhat larger but did not differ significantly (P=0.92; unpaired t-test) from that obtained for the putrescine action on carbachol-induced current.
Since spermine and putrescine inhibited both carbachol- and GTPγS-induced currents in a closely similar manner, we suggest that cation channel rather than the muscarinic receptor was the primarily site of PA action.
Effects of external PAs on mICAT biophysical properties
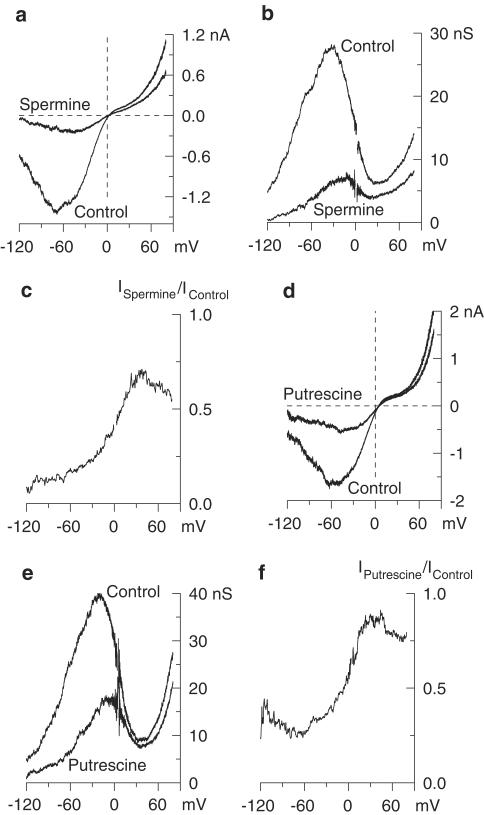
To evaluate the effects of PAs on mICAT biophysical properties and their possible voltage-dependent effects, we measured steady-state mICAT I–V relationships by applying slow voltage ramps in control and, in the same cell, following spermine or putrescine application (Figure 2a, d). mICAT activation curves could then be obtained from the steady-state I–V relationships by dividing current amplitude at each potential by the driving force at that potential (membrane potential–reversal potential; the latter was close to 0 mV since symmetrical Cs+ solutions were used). The I–V curves were U-shaped at negative potentials and showed double rectification around 0 mV – it should be noted that the latter was neither due to a reduced driving force close to the reversal potential nor due to intracellular Mg2+ (Zholos et al., 2003). Cationic conductance activation curve during the PA action did not change its characteristic N-shape (Figure 2b, e). However, the ratio of currents measured in the presence of PAs and in control revealed that membrane depolarization significantly relieved the inhibitory effect of the PAs (Figure 2c, f). The same results were obtained for GTPγS-induced current (data not shown). Notably, the effects of external PAs on mICAT properties were closely similar to those produced by external divalent cations; the latter were attributed to the charge screening effect altering the surface potential (Zholos & Bolton, 1995).
Figure 2.
Effect of external spermine (a–c) and putrescine (d–f) on mICAT I–V relationship. (a, d) Steady-state I–V relationships in control and, in the same myocyte, in the presence of either spermine (3 mM) or putrescine (10 mM). (b, e) Corresponding activation curves. (c, f) Voltage dependence of the inhibitory effect was evaluated by plotting the ratio of currents recorded in the presence of spermine (3 mM) or putrescine (10 mM) to those in control.
Effects of spermine and putrescine on single muscarinic cation channel properties
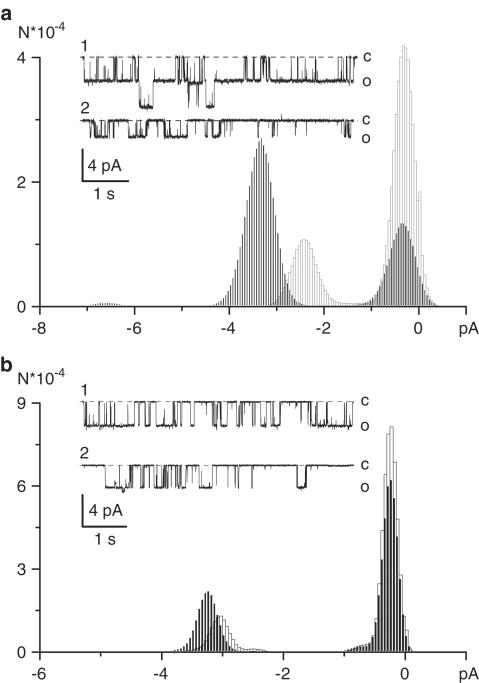
We have previously identified three types of cationic channels (10, 57 and 130 pS) opened by muscarinic receptor activation in ileal myocytes (Zholos et al., 2004b). The 10 and 50 pS channels mediate the voltage-dependent part of the muscarinic conductance as their P0 strongly increases with membrane depolarization. However, the relative contribution of the 10 pS channel was small, estimated at less than 5% at negative potentials. The 130 pS channel mediates a voltage-independent, likely Ca2+-dependent component of the muscarinic cationic current. Thus, the 57 pS channel activity is the major determinant of mICAT, at least at 100 nM [Ca2+]i as used in the present experiments. Therefore, we have studied the action of external PAs on this channel type using outside-out patches. The patches were formed after whole-cell GTPγS-induced current had been fully activated. Only those membrane patches which showed no or only minimal 10 and 130 pS activity were selected in order to investigate the effect of PAs on the 57 pS channel gating.
External application of spermine (3 mM) significantly reduced single-channel conductance (Figure 3a). Thus, at −50 mV unitary current decreased from 3.07±0.19 in control to 1.72±0.27 in the presence of spermine (P<0.001; paired t-test). Channel open probability (e.g., NP0) was also reduced 2.18±0.25 fold (n=4). These effects were concentration-dependent. Spermine at 1 mM weakly reduced single-channel current (from 3.09±0.29 to 2.57±0.22 pA; P<0.05), but noticeably decreased NP0 (1.80-fold).
Figure 3.
Effects of external spermine (a) or putrescine (b) on the 57 pS channel activity recorded in outside-out patches. Both PAs were applied at 3 mM at the holding potential of −50 mV. Solid and open columns show all-point amplitude histograms in control and after PA application, respectively. Representative current traces recorded in control (1) and in the presence of respective PA (2) are shown in the insets.
Putrescine added to the external solution at 3 mM produced similar but less significant effects on the 57 pS channel activity (Figure 3b). Thus, at −50 mV unitary current amplitude modestly decreased from 3.21±0.18 in control to 2.94±0.34 in the presence of putrescine (P=0.035; paired t-test), but the effect on NP0 was more prominent (1.79±0.19-fold reduction, n=5). Notably, for both PAs, the block did not increase open-channel noise (as evident from the unchanged peak width of all-point amplitude histograms shown in Figure 3a, b), indicating that external PAs are unlikely to interact with the channel pore. Also, consistent with the above-described lack of PA action on the N-shaped whole-cell conductance curve, there was no additional channel flicker evident after spermine or putrescine application.
Effect of intracellular PAs on mICAT
One indication that an endogenous cation channel blocker may be present in intact ileal myocytes came from our recent studies of cation single-channel gating (Zholos et al., 2004b). We noted that channel activity was remarkably stable (e.g. within tens of minutes) in isolated outside-out patches or in cell-attached patches formed on previously dialysed cells (or, alternatively, using double-patch configuration), but it was almost impossible to record single-channel activity using conventional cell-attached configuration. Since PAs are highly soluble in water, they can be expected to diffuse readily from the cell cytoplasm into the pipette solution during cell perfusion when low-resistance patch pipettes are used.
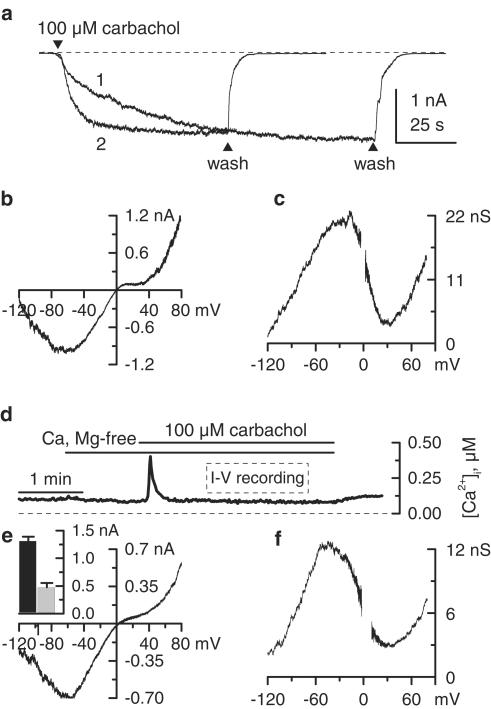
Another indication that a putative channel blocker may be removed by cell perfusion is illustrated in Figure 4a. This figure compares mICAT on-rates in two different experiments. In one case (trace 1), external PSS was replaced by our standard high Cs+ solution before membrane break-through and carbachol was applied shortly after establishing whole-cell configuration (e.g. with a minimal delay of 10–20 s as needed to compensate series resistance). In the other case (trace 2), the current was activated after 3 min of cell perfusion, as usual (compare to Figure 1a, b). In the first case, the current had a noticeably slower kinetics of activation, achieving its nearly maximal 95% amplitude 40–60 s later compared to the current evoked in 3 min-dialysed cells (n=9). We also noted that the time lag in mICAT activation was more prominent in cells that had larger membrane capacitance (e.g., >50 pF). These observations imply that freely diffusible cation channel-blocking molecules, possibly PAs, are present in the cytoplasm. One can estimate that putrescine and spermine are removed with the time constants proportional to the cubic root of molecular weight – 28 and 63 s, respectively (Pusch & Neher, 1988). These values are compatible with the slow current activation in Figure 4a, trace 1 (e.g., τ=36 s).
Figure 4.
Possible mICAT inhibition by a diffusible intracellular factor. (a) Delayed current activation when carbachol was applied immediately after break-through (trace 1; note that PSS has been already replaced by high-Cs+ external solution before establishing the whole-cell configuration) compared to standard 3 min equilibration experiment (trace 2). (b, c) Lack of intracellular ATP (20 mM) effect on the 100 μM carbachol-induced mICAT I–V relationship and corresponding cation conductance activation curve. Cell dialysis with PA-free pipette solution containing 1 mM ATP appears to be sufficient to remove PAs from the cytoplasm. (d) Changes of [Ca2+]i in single ileal smooth muscle cell voltage-clamped at −40 mV during the application of 100 μM carbachol in Ca2+-free high-Cs+ external solution. I–V relationships in perforated patch experiments were measured when [Ca2+]i was stabilized at about 100 nM, as indicated. (e, f) Carbachol (100 μM) induced mICAT I–V relationship and corresponding cation conductance activation curve measured using perforated patch configuration. Mean current amplitudes using this (grey column, n=7) and conventional whole-cell configuration (black column, n=12) are shown in the inset.
Since PAs can strongly bind to some intracellular macromolecules, we were also interested to see whether residual PAs in dialysed cells can account for the above-described double rectification of mICAT around 0 mV. ATP can bind PAs with a high affinity, for example, in bovine lymphocytes the moles of spermine bound to DNA, RNA, phospholipids and ATP were 0.79, 3.7, 0.23 and 4.3 per 100 mol of phosphate of macromolecules or ATP, respectively (Watanabe et al., 1991). Thus, we used a standard pipette solution in which 1 mM MgATP was replaced by 20 mM Na2ATP, an efficient PA scavenger. Using our standard protocol, mICAT in response to 100 μM carbachol was evoked. However, no noticeable change of the mICAT I–V relationship (Figure 4b) or conductance curve shape (Figure 4c) was observed in these experiments (n=5; compare to corresponding control curves in Figure 2).
In contrast, in perforated patch experiments which allow retention of intracellular macromolecules, significantly reduced sustained mICAT was observed (at −40 mV 478±76 pA, n=6 compared to 1314±76 pA, n=12 using conventional whole-cell configuration, P<0.0001). Ca2+-dependent modulation of mICAT was unavoidable in perforated patch experiments, since [Ca2+]i could not be controlled and its variations caused concurrent fluctuations of mICAT. Thus, when [Ca2+]i was not buffered mICAT consisted of two phases; an initial large transient current and a subsequent smaller sustained component. The initial peak current was caused by Ca2+ release and was accompanied by the intracellular calcium rise up to 1–2 μM. [Ca2+]i during the sustained phase usually did not exceed a few hundred nM in the presence of external Ca2+, but declined to about 100 nM when Ca2+-free external solution was used (Figure 4d). Therefore, to account for the differences in the experimental conditions in perforated patch experiments, we measured current amplitude (as well as I–V relationships, see below) about 60 s after carbachol application, that is during the sustained phase when [Ca2+]i had declined to about 100 nM. Although the current was much reduced, its I–V relationship (Figure 4e) and conductance curve shape (Figure 4f) were similar to those obtained using conventional patch-clamp recording techniques with intracellular Ca2+ ‘clamped' at 100 nM (n=7).
Cytoplasmic concentration of spermine in native ileal smooth muscle cells is about 0.4 mM. To prevent its loss when conventional whole-cell configuration was used, 0.3 mM spermine was added to the pipette solution. The cells responded to 100 μM carbachol application by generating a smaller inward current compared to control. To statistically characterize the difference, we patched cells alternately using either spermine-containing or spermine-free pipette solution on the same day. The mean amplitudes of currents induced by carbachol 3 min after the whole-cell configuration had been established were 509±41 pA (n=19) and 1314±76 pA (n=12), respectively (P<0.0001 by unpaired t-test) (Figure 5a). At 1 mM spermine strongly suppressed the current (122±20 pA, n=12).
Figure 5.
Inhibitory effects of intracellular spermine (a–c) and putrescine (d–f) on cation current activated by 100 μM carbachol. (a, d) Mean current amplitude measured at −40 mV in control and when either spermine (a) or putrescine (d) was added to the pipette solution at the indicated concentration. (b, e) Steady-state I–V relationships measured with 1 mM spermine (b) or 10 mM putrescine (e) in the pipette solution. (c, f) Corresponding activation curves.
The effects of intracellular putrescine were studied similarly by alternating cells which were voltage-clamped using putrescine-free (n=14), 1 mM putrescine-containing (n=14) or 10 mM putrescine-containing (n=18), pipette solution. The mean current amplitudes are plotted in Figure 5d. The inhibition was detectable only with 10 mM putrescine (P<0.0001).
Thus, both intracellular spermine and putrescine clearly inhibited mICAT, but there was no noticeable change of the current voltage-dependent behaviour in either case (Figure 5b, c, e, f).
Discussion
Specific interaction of PAs with different ion channels has been reported (reviewed by Williams, 1997; Igarashi & Kashiwagi, 2000). These interactions include the block of inwardly rectifying K+ channels (Lopatin et al., 1994), cyclic nucleotide-gated channels (Lu & Ding, 1999; Guo & Lu, 2000), glutamate-activated receptor channels (Araneda et al., 1999), nicotinic acetylcholine receptor channels (Haghighi & Cooper, 1998), sodium channels (Huang & Moczydlowski, 2001) and voltage-gated calcium channels (Gomez & Hellstrand, 1995; 1999). Many types of monovalent cation selective channels are also blocked by extracellular polycations like spermine in the μM range (Nilius, 2003; Zakharov et al., 2003). The molecular nature of channels carrying mICAT is not yet completely defined, but they appear to be homo- or heteromultimers of transient receptor potential proteins (Lee et al., 2003). At least one member of this novel family of ion channels, TRPM4b, has been recently reported to be potently blocked by internal spermine at all potentials (Nilius et al., 2004).
mICAT is rather insensitive to the inhibitory action of external PAs compared to some other channels. Spermine, which was a more potent blocker compared to putrescine, revealed a noticeable effect at concentrations in the range of hundreds μM. The observed blocking action was most similar to the blocking action of PAs on voltage-gated calcium channels in ileal myocytes (Gomez & Hellstrand, 1995; 1999). Thus, PAs were able to affect calcium channels both from the inside and the outside of the cell membrane; spermine was a more potent inhibitor than spermidine, while putrescine was inactive. Spermine (1 mM) blocked calcium current by half from the outside and almost completely from the inside of the cell membrane, as is the case for mICAT inhibition.
Efficacy of the PA action typically correlates with the number of positive charges on the PA molecule. Thus, spermine, which has twice as many positive charges as putrescine, was four times more potent.
It is unlikely that spermine crosses the membrane and then interacts with the channels from the inside. Blocking action of spermine and putrescine was fast and rapidly reversible (Figure 1c, inset). Also, under the experimental conditions used in our experiments (e.g., room temperature, symmetrical Cs+ solutions), PA transport is expected to be inhibited (compare to Aziz et al., 1998). In ileal myocytes, single-channel calcium currents in the cell-attached recording were unaffected by the addition of spermine to the bath (Gomez & Hellstrand, 1999); thus spermine could not reach the inner part of the membrane patch isolated from the external solution by the pipette seal via its transmembrane transport.
The block of mICAT by intracellular spermine seems to arise due to a more specific channel interaction with the spermine molecule. The possibility exists that this interaction is not direct. For example, PAs are well known to bind with phospholipids. In rat GH3 cells, millimolar spermine has been shown to inhibit polyphosphoinositide break-down and InsP3 production in response to stimulation by GTPγS (Wojcikiewicz & Fain, 1988). A similar effect on ileal myocytes has not been reported, but spermine (1 mM) was shown to decrease the contractile response to carbachol in Ca2+-free medium to 14% of control in cells permeabilized with β-escin, whereas no effect was seen in intact muscle (Swärd et al., 1994). As the responses to InsP3 and caffeine were potentiated, spermine does not seem to inhibit Ca2+ release directly. It is thus possible that spermine can interfere with G-protein-mediated Ca2+ release at a point distal to receptor activation. The mechanism of this action is therefore likely to involve decreased formation of InsP3 from phosphatidylinositol 4,5-bisphosphate catalysed by phospholipase C.
Recent studies of mICAT modulation have shown that phospholipase C inhibition greatly decreases this current and positively shifts the activation curve (Zholos et al., 2004a). Thus, this mechanism can be partially involved in the inhibition of mICAT by spermine, at least when spermine concentration in the pipette solution was high. On the other hand, at lower spermine concentration, phospholipase C inhibition is less likely to be involved since large initial transient mICAT was observed in perforated patch experiments when the concentration of endogenous spermine was presumably about 0.3 mM. This current is thought to be due to InsP3-induced Ca2+ release (see Zholos et al., 2003 for a review); thus, production of InsP3 remains sufficiently high to cause substantial Ca2+ release.
Intracellular concentration of spermine in the guinea-pig ileum is close to that which we have used in the present experiments. The blocking action of intracellular spermine at 0.3 mM on the current may thus indicate that this PA plays a role in the functional regulation of the muscarinic cation current.
In conclusion, our present results provide new insights into the mechanisms of PA-dependent regulation of smooth muscle excitation and contraction. mICAT tonic inhibition by PAs can be functionally relevant, since PA metabolism changes under both physiological and pathophysiological conditions. However, the inhibitory action of PAs on smooth muscle excitation (inhibition of mICAT as shown in the present study and voltage-gated Ca2+ channels as shown earlier by Gomez & Hellstrand, 1995; 1999) could be at least partially compensated by the potentiating effect on muscle contractility, since PAs can also increase sensitivity of the contractile proteins to Ca2+ through inhibition of myosin phosphatase (Swärd et al., 1994; Nilsson et al., 2002).
Acknowledgments
This work was supported by The Wellcome Trust Grant number 062926.
Abbreviations
- ATP
adenosine 5′ triphosphate magnesium or sodium salt
- BAPTA, 1,2-bis(2-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid; GTP
guanosine 5′-triphosphate lithium salt
- GTPγS
guanosine 5′-O-(3-thiotriphosphate)
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- I–V relationship
current–voltage relationship
- mICAT
muscarinic receptor cationic current
- PAs
polyamines
References
- ARANEDA R.C., LAN J.Y., ZHENG X., ZUKIN R.S., BENNETT M.V. Spermine and arcaine block and permeate N-methyl-D-aspartate receptor channels. Biophys. J. 1999;76:2899–2911. doi: 10.1016/S0006-3495(99)77445-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZIZ S.M., YATIN M., WORTHEN D.R., LIPKE D.W., CROOKS P.A. A novel technique for visualizing the intracellular localization and distribution of transported polyamines in cultured pulmonary artery smooth muscle cells. J. Pharm. Biomed. Anal. 1998;17:307–320. doi: 10.1016/s0731-7085(98)00016-8. [DOI] [PubMed] [Google Scholar]
- BOLTON T.B., ZHOLOS A.V. Activation of M2 muscarinic receptors in guinea-pig ileum opens cationic channels modulated by M3 muscarinic receptors. Life Sci. 1997;60:1121–1128. doi: 10.1016/s0024-3205(97)00056-8. [DOI] [PubMed] [Google Scholar]
- GOMEZ M., HELLSTRAND P. Effects of polyamines on voltage-activated calcium channels in guinea-pig intestinal smooth muscle. Pflugers Arch. 1995;430:501–507. doi: 10.1007/BF00373886. [DOI] [PubMed] [Google Scholar]
- GOMEZ M., HELLSTRAND P. Endogenous polyamines modulate Ca2+ channel activity in guinea-pig intestinal smooth muscle. Pflugers Arch. 1999;438:445–451. doi: 10.1007/s004249900068. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GUO D., LU Z. Mechanism of cGMP-gated channel block by intracellular polyamines. J. Gen. Physiol. 2000;115:783–797. doi: 10.1085/jgp.115.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGHIGHI A.P., COOPER E. Neuronal nicotinic acetylcholine receptors are blocked by intracellular spermine in a voltage-dependent manner. J. Neurosci. 1998;18:4050–4062. doi: 10.1523/JNEUROSCI.18-11-04050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUANG C.J., MOCZYDLOWSKI E. Cytoplasmic polyamines as permeant blockers and modulators of the voltage-gated sodium channel. Biophys. J. 2001;80:1262–1279. doi: 10.1016/S0006-3495(01)76102-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IGARASHI K., KASHIWAGI K. Polyamines: mysterious modulators of cellular functions. Biochem. Biophys. Res. Commun. 2000;271:559–564. doi: 10.1006/bbrc.2000.2601. [DOI] [PubMed] [Google Scholar]
- JÄNNE J., ALHONEN L., PIETILÄ M., KEINÄNEN T.A. Genetic approaches to the cellular functions of polyamines in mammals. Eur. J. Biochem. 2004;271:877–894. doi: 10.1111/j.1432-1033.2004.04009.x. [DOI] [PubMed] [Google Scholar]
- LEE Y.M., KIM B.J., KIM H.J., YANG D.K., ZHU M.H., LEE K.P., SO I., KIM K.W. TRPC5 as a candidate for the nonselective cation channel activated by muscarinic stimulation in murine stomach. Am. J. Physiol. 2003;284:G604–G616. doi: 10.1152/ajpgi.00069.2002. [DOI] [PubMed] [Google Scholar]
- LOPATIN A.N., MAKHINA E.N., NICHOLS C.G. Potassium channel block by cytoplasmic polyamines as the mechanism of intrinsic rectification. Nature. 1994;372:366–369. doi: 10.1038/372366a0. [DOI] [PubMed] [Google Scholar]
- LU Z. Mechanism of rectification in inward-rectifier K+ channels. Annu. Rev. Physiol. 2004;66:103–129. doi: 10.1146/annurev.physiol.66.032102.150822. [DOI] [PubMed] [Google Scholar]
- LU Z., DING L. Blockade of a retinal cGMP-gated channel by polyamines. J. Gen. Physiol. 1999;113:35–43. doi: 10.1085/jgp.113.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B. Calcium-impermeable monovalent cation channels: a TRP connection. Br. J. Pharmacol. 2003;138:5–7. doi: 10.1038/sj.bjp.0705073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., PRENEN J., VOETS T., DROOGMANS G. Intracellular nucleotides and polyamines inhibit the Ca2+-activated cation channel TRPM4b. Pflugers Arch. 2004;448:70–75. doi: 10.1007/s00424-003-1221-x. [DOI] [PubMed] [Google Scholar]
- NILSSON B.O., GOMEZ M.F., SWÄRD K., HELLSTRAND P. Regulation of Ca2+ channel and phosphatase activities by polyamines in intestinal and vascular smooth muscle – implications for cellular growth and contractility. Acta Physiol. Scand. 2002;176:33–41. doi: 10.1046/j.1365-201X.2002.01013.x. [DOI] [PubMed] [Google Scholar]
- NILSSON B.O., HELLSTRAND P. Effects of polyamines on intracellular calcium and mechanical activity in smooth muscle of guinea-pig taenia coli. Acta Physiol. Scand. 1993;148:37–43. doi: 10.1111/j.1748-1716.1993.tb09529.x. [DOI] [PubMed] [Google Scholar]
- ONODERA K., UNEMOTO T., MIYAKI K., HAYASHI M. Pharmacological studies on polyamines. I. Relaxing effect of spermine and spermidine on smooth muscle of guinea pig ileum contracted by 5-hydroxytryptamine and nicotine. Arch. Int. Pharmacodyn. Ther. 1968;174:491–494. [PubMed] [Google Scholar]
- OSBORNE D.L., SEIDEL E.R. Gastrointestinal luminal polyamines: cellular accumulation and enterohepatic circulation. Am. J. Physiol. 1990;258:G576–G584. doi: 10.1152/ajpgi.1990.258.4.G576. [DOI] [PubMed] [Google Scholar]
- PUSCH M., NEHER E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflugers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- SWÄRD K., NILSSON B.O., HELLSTRAND P. Polyamines increase Ca2+ sensitivity in permeabilized smooth muscle of guinea pig ileum. Am. J. Physiol. 1994;266:C1754–C1763. doi: 10.1152/ajpcell.1994.266.6.C1754. [DOI] [PubMed] [Google Scholar]
- WALLACE H.M., FRASER A.V., HUGHES A. A perspective of polyamine metabolism. Biochem. J. 2003;376:1–14. doi: 10.1042/BJ20031327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE S., KUSAMA E., KOBAYASHI H., IGARASHI K. Estimation of polyamine binding to macromolecules and ATP in bovine lymphocytes and rat liver. J. Biol. Chem. 1991;266:20803–20809. [PubMed] [Google Scholar]
- WILLIAMS K. Interactions of polyamines with ion channels. Biochem. J. 1997;325:289–297. doi: 10.1042/bj3250289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOJCIKIEWICZ R.J., FAIN J.N. Polyamines inhibit phospholipase C-catalysed polyphosphoinositide hydrolysis. Studies with permeabilized GH3 cells. Biochem. J. 1988;255:1015–1021. doi: 10.1042/bj2551015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZAKHAROV S.I., SMANI T., LENO E., MACIANSKIENE R., MUBAGWA K., BOLOTINA V.M. Monovalent cation (MC) current in cardiac and smooth muscle cells: regulation by intracellular Mg2+ and inhibition by polycations. Br. J. Pharmacol. 2003;138:234–244. doi: 10.1038/sj.bjp.0705074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. Effects of divalent cations on muscarinic receptor cationic current in smooth muscle from guinea-pig small intestine. J. Physiol. 1995;486:67–82. doi: 10.1113/jphysiol.1995.sp020791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., BOLTON T.B. A novel GTP-dependent mechanism of ileal muscarinic metabotropic channel desensitization. Br. J. Pharmacol. 1996;119:997–1012. doi: 10.1111/j.1476-5381.1996.tb15770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., TSVILOVSKYY V.V., BOLTON T.B. Muscarinic cholinergic excitation of smooth muscle: signal transduction and single cationic channel properties. Neurophysiology. 2003;35:283–301. [Google Scholar]
- ZHOLOS A.V., TSYTSYURA YA.D., GORDIENKO D.V., TSVILOVSKYY V.V., BOLTON T.B. Phospholipase C, but not InsP3 or DAG, dependent activation of the muscarinic receptor-operated cation current in guinea-pig ileal smooth muscle cells. Br. J. Pharmacol. 2004a;141:23–36. doi: 10.1038/sj.bjp.0705584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHOLOS A.V., ZHOLOS A.A., BOLTON T.B. G-protein-gated TRP-like cationic channel activated by muscarinic receptors: effect of potential on single-channel gating. J. Gen. Physiol. 2004b;123:581–598. doi: 10.1085/jgp.200309002. [DOI] [PMC free article] [PubMed] [Google Scholar]