Abstract
Tyrosine kinase (TK) inhibitors genistein and tyrphostin A23 (A23) inhibited Ca2+ currents in guinea-pig ventricular myocytes investigated under standard whole-cell conditions (K+-free Tyrode's superfusate; EGTA-buffered (pCa–10.5) Cs+ dialysate). However, the inhibitors (100 μM) also induced membrane currents that reversed between −40 and 0 mV, and the objective of the present study was to characterize these currents.
Genistein-induced current behaved like Cl− current, and was unaffected by either the addition of divalent cations (0.5 mM Cd2+; 3 mM Ni2+) that block the Na+–Ca2+ exchanger (NCX), or the removal of external Na+ and Ca2+.
A23-induced current was independent of Cl− driving force, and strongly suppressed by addition of Cd2+ and Ni2+, and by removal of either external Na+ or Ca2+. These and other results suggested that A23 activated an NCX current driven by submembrane Na+ and Ca2+ concentrations higher than those in the bulk cytoplasm.
Improved control of intracellular Na+ and Ca2+ concentrations was obtained by suppressing cation influx (10 μM verapamil) and raising dialysate Na+ to 7 mM and dialysate pCa to 7. Under these conditions, stimulation by A23 was described by the Hill equation with EC50 68±4 μM and coefficient 1.1, tyrphostin A25 was as effective as A23, and TK-inactive tyrphostin A1 was ineffective. Phosphotyrosyl phosphatase inhibitor orthovanadate (1 mM) antagonized the action of 100 μM A23.
The results suggest that activation of cardiac NCX by A23 is due to inhibition of genistein-insensitive TK.
Keywords: Guinea-pig; ventricular myocytes; genistein; tyrphostins A23, A25 and A1; orthovanadate; Cl− current; Na+–Ca2+ exchanger
Introduction
Genistein, tyrphostin A23 (A23), and tyrphostin A25 (A25) are tyrosine kinase (TK) inhibitors (Akiyama et al., 1987; Gazit et al., 1989; Holmes et al., 1996) that are widely used to investigate the involvement of tyrosine phosphorylation in the regulation of ion channels (Cataldi et al., 1996; Wang et al., 1996; Ogura et al., 1999; Albert et al., 2001), pumps (Feraille et al., 1997), and exchangers (Wang et al., 1997; Linck et al., 1998; Kiang et al., 2003). The inhibitors are active on external application, and are typically used at concentrations up to 300 μM, with little effect on the activity of other kinases such as protein kinase A (PKA) and protein kinase C (PKC) (Akiyama et al., 1987; Enright & Booth, 1991; O'Dell et al., 1992). Nevertheless, it is commonly acknowledged that these drugs can have direct effects that are unrelated to their actions on TK (for a review, see Davis et al., 2001). In the case of genistein, the direct effects are believed to include activation of cystic fibrosis transmembrane regulator (CFTR) Cl− channels (French et al., 1997; Weinreich et al., 1997; Zhou et al., 1998) and inhibition of various cation channels (Smirnov & Aaronson, 1995; Paillart et al., 1997; Washizuka et al., 1998; Albert et al., 2001).
In an earlier study on the action of TK inhibitors on L-type Ca2+ current (ICa,L) in guinea-pig ventricular myocytes (Ogura et al., 1999), we observed that both genistein and the structurally and mechanistically different A23 (Levitzki & Gazit, 1995) caused development of outward current at 0 mV. The current appeared to be CFTR Cl− current (ICl(CFTR)), and was not further investigated. The results of the present study indicate that the genistein-induced current behaves like ICl(CFTR), whereas the A23-induced current behaves like Na+–Ca2+ exchanger (NCX) current (INCX).
Methods
All procedures were carried out in accordance with national and university regulations on the care and treatment of laboratory animals.
Ventricular myocytes
Guinea-pigs (250–300 g) were killed by cervical dislocation. Hearts were quickly excised, mounted on a Langendorff column, and perfused through the aorta with Ca2+-free Tyrode's solution (37°C) that contained collagenase (0.08–0.12 mg ml−1; Yakult Pharmaceutical Co., Tokyo, Japan) for 10–15 min. The cells were dispersed and kept in a storage solution (22°C) that contained (mM) KOH 80, KCl 30, KH2PO4 30, MgSO4 3, glutamic acid 50, taurine 20, glucose 20, ethylene glycol-bis(b-aminoethyl)-N,N,N,N-tetraacetic acid (EGTA) 0.5, and N′-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid (HEPES) 10 (pH 7.4 with KOH).
A few drops of the cell suspension were placed in a 0.3-ml perfusion chamber mounted on an inverted microscope stage. After the cells had settled to the bottom, the chamber was perfused (≈3 ml min−1) with Tyrode's solution at 36°C. Whole-cell membrane currents were recorded using an EPC-7 amplifier (List Electronic, Darmstadt, Germany). Recording pipettes were fabricated from thick-walled borosilicate glass capillaries (H15/10/137, Jencons Scientific Ltd, Bedfordshire, U.K.), and had resistances of 1.5–2.5 MΩ when filled with pipette solution. Voltages were corrected by −10 and −5 mV to account for junction potentials between external solution and low- and high-Cl− pipette solutions, respectively. The series resistance ranged between 3 and 7 MΩ, and was compensated by 60–80%. Leakage compensation was not used. The current signals were low-pass filtered at 3 kHz, and digitized with an A/D converter (Digidata 1200A, Axon Instruments, Foster City, CA, U.S.A.) and pCLAMP software (Axon Instruments) at a sampling rate of 8 kHz prior to analysis.
Myocytes were initially superfused with normal Tyrode's solution that contained (mM) NaCl 143, KCl 5.4, CaCl2 1.8, MgCl2 1.2, glucose 10, and HEPES 5 (pH 7.4 with NaOH). Thereafter, the myocytes were superfused with standard K+-free Tyrode's solution (KCl omitted), or with a K+-free Tyrode's solution that was either Na+-free (Na+ replaced by N-methyl-D-glucamine (NMDG+)) or Ca2+-free (CaCl2 replaced by MgSO4).
Myocytes were dialysed with one of the following K+-free Cs+ pipette solutions: standard solution that contained (mM) CsCl 40, CsOH 106, aspartic acid 106, MgATP 5, EGTA 5, and HEPES 5 (pH 7.2 with CsOH) (calculated pCa 10.5); high-Cl− solution (standard solution that contained 140 mM Cl− (aspartate replaced)); pCa 7 solution (standard solution with added CaCl2); and 7 mM Na+-pCa 7 solution (pCa 7 solution that contained 7 mM Na+ (Cs+ replaced) and either 40 or 65 mM Cl− (aspartate replaced)).
Drugs
Genistein (Sigma-Aldrich, Oakville, ON, Canada) and tyrphostins A1, A23 and A25 (Calbiochem, San Diego, CA, U.S.A.) were prepared as 100-mM stock solutions in dimethyl sulphoxide (DMSO). Appropriate amounts of stock solutions were added to external solutions, and mixtures sonicated as required to ensure proper dispersion of drug. Corresponding amounts of DMSO (⩽0.3% v v−1) were also added to the control external solutions. These concentrations of DMSO have little effect on membrane currents in guinea-pig ventricular myocytes (Ogura et al., 1995). Verapamil (Sigma-Aldrich) was dissolved in water and diluted in the bathing solution. Aqueous stock solutions (100 mM) of Na3VO4 (orthovanadate) (Fisher Scientific, Nepeon, ON, Canada) were freshly prepared, and the pH was adjusted to ∼10 (Gordon, 1991; Shuba et al., 1996). Appropriate amounts of stock solution were added to the superfusate just before use, and the pH was readjusted to 7.4 with NaOH.
Statistics
Results are expressed as means±s.e.m.; n indicates the number of experiments. Comparisons were made using Student's t-test, and differences were considered significant when P<0.05.
Results
Differential effect of Cd2+ on membrane currents induced by genistein and tyrphostin A23
Myocytes superfused with standard K+-free Tyrode's solution were dialysed with standard Cs+ solution to suppress K+ currents, and held at –40 mV to inactivate Na+ and T-type Ca2+ currents. Under these conditions, 200-ms depolarizations to 0 mV elicited inward ICa,L that rapidly reached a peak and then inactivated. As previously reported (Ogura et al., 1999), ICa,L was reversibly inhibited by 100 μM genistein and 100 μM A23 (Figure 1a). These drugs also had reversible effects on holding current at –40 mV (Ih) and end-of-pulse current (I200) at 0 mV (Figure 1a). On average (n=10), 100 μM genistein shifted Ih by −0.12±0.06 pA pF−1 (P<0.05) and I200(0 mV) by 0.72±0.2pA pF−1 (P<0.01), whereas 100 μM A23 shifted Ih by –0.21±0.07 pA pF−1 (P<0.01) and I200(0 mV) by 0.55±0.14 pA pF−1 (P<0.01). A plausible explanation for the shifts is that both genistein and A23 induced currents that had a reversal potential (Erev) between –40 and 0 mV.
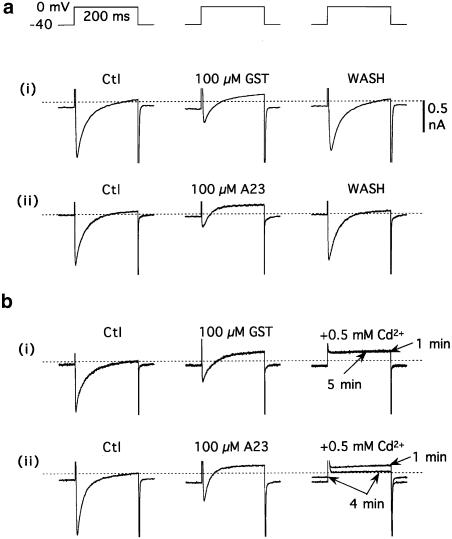
Figure 1.
Differential effect of 0.5 mM Cd2+ on membrane currents induced by 100 μM genistein and 100 μM A23. The myocytes were dialysed with standard Cs+ pipette solution, superfused with standard K+-free Tyrode's solution, and depolarized from –40 to 0 mV for 200 ms at 0.2 Hz. (a) Reversible effects of (i) genistein (GST), and (ii) A23. In addition to inhibiting inward ICa,L at 0 mV, both genistein and A23 induced an inward shift in the holding current (Ih) at –40 mV and an outward shift in the end-of-pulse current (I200) at 0 mV. The records were obtained before (Ctl), 5 min after drug application, and ≈ 5 min after drug removal. (b) Effects of 0.5 mM Cd2+ on the shifts in current induced by (i) 100 μM genistein and (ii) 100 μM A23. Cd2+ rapidly abolished ICa,L in both myocytes, and gradually reversed the current shifts in the A23-treated myocyte. The dashed lines indicate zero-current levels in these and subsequent records.
The first indication that the ionic basis of the genistein-induced current was different from that of the A23-induced current was provided by the responses to Cd2+, a Ca2+-channel blocker that also inhibits NCX activity (Iwamoto & Shigekawa, 1998). When myocytes bathed and dialysed with standard solutions were pretreated with 0.5 mM Cd2+, application of 100 μM genistein alone (n=4) or with 100 μM A23 (n=3) shifted the membrane current as described above (e.g., by 0.81±0.13 pA pF−1 (n=7) (P<0.01) at 0 mV), whereas application of 100–200 μM A23 was without effect (e.g., shift of −0.05±0.08 pA pF−1 (n=6) at 0 mV). To quantify this differential response, 0.5 mM Cd2+ was applied to myocytes that had been treated with 100 μM genistein or A23 for 5 min. Cd2+ had little effect on genistein-induced shifts in Ih and I200(0 mV), but suppressed the A23-induced shifts (Figure 1b). Overall, Cd2+ inhibited 5±4% of the genistein-induced shift in I200(0 mV) (n=4), and 93±6% of the A23-induced shift (n=8). Ni2+ (3 mM) had similar differential effects on drug-induced shifts (n=3 each).
A concern with the outward shifts of I200 (0 mV) caused by genistein and A23 under ICa,L-recording conditions is that they were conceivably due to inhibition of residual inward ICa,L at 200 ms. A minor contribution from this source is not ruled out, but a major contribution can be discounted based on the marked inward shift in I200 (0 mV) caused by Cd2+ in A23 (but not genistein)-treated myocytes (e.g., Figure 1b), and on results obtained in the absence of ICa,L (see below).
Differential effect of dialysate Cl− on currents induced by A23 and genistein
The involvement of Cl− conductance in A23 action was evaluated by determining the effects of altered Cl− distribution on drug-induced current. Current–voltage (I–V) relationships were obtained from myocytes that were bathed with standard superfusate (149 mM Cl−) and dialysed with either standard (40 mM) or high (140 mM) Cl− pipette solution to set calculated ECl at –35 or −2 mV. In the myocytes dialysed with standard solution, the I200 induced by 100 μM A23 (A23 minus control) had an Erev (−22±4 mV, n=7) that was significantly more positive than calculated ECl (Figure 2a). The effects of A23 were unaffected by 140-mM Cl− dialysate; in particular, the Erev of A23-induced I200 was unchanged at −23±2 mV (n=9) (e.g., Figure 2b). In marked contrast, the Erev of genistein-activated current was sensitive to elevated-Cl− pipette solution, shifting from –33±3 mV (n=5) in myocytes dialysed with 40 mM Cl− solution (e.g., Figure 4b) to −21±2 mV (n=6) (Figure 6e), −2±2 mV (Shuba & McDonald, 1997), and −1±3 mV (n=5) (this study) in myocytes dialysed with 65, 130, and 140 mM Cl− solution, respectively.
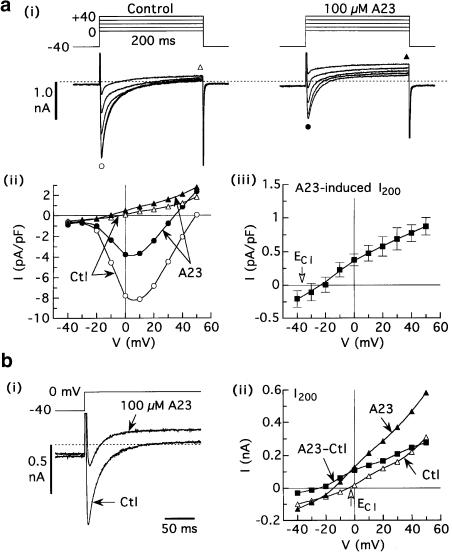
Figure 2.
Lack of effect of dialysate Cl− concentration on the reversal potential of A23-induced current. Myocytes superfused with standard solution were dialysed with Cs+ solution that contained either 40 or 140 mM Cl−, and depolarized with 200-ms pulses from holding potential –40 mV. (a) Data obtained from myocytes dialysed with 40 mM Cl− solution. (i, ii) Current records and I–V relationships of peak inward current (circles) and I200 (triangles) obtained before and 5–6 min after application of 100 μM A23 to a representative myocyte. (iii) Average I–V relationship of A23-induced (A23 minus control) I200. Erev estimated by linear interpolation was –22±4 mV (n=7). (b) Data obtained from a myocyte dialysed with 140 mM Cl− solution. (i) Example records obtained before (Ctl) and 4 min after addition of 100 μM A23. (ii) I–V relationships of I200, illustrating that the Erev of the A23-induced current (−24 mV) was similar to that measured in myocytes dialysed with 40 mM Cl− solution, despite the +33-mV shift in calculated ECl (vertical arrow).
Figure 4.
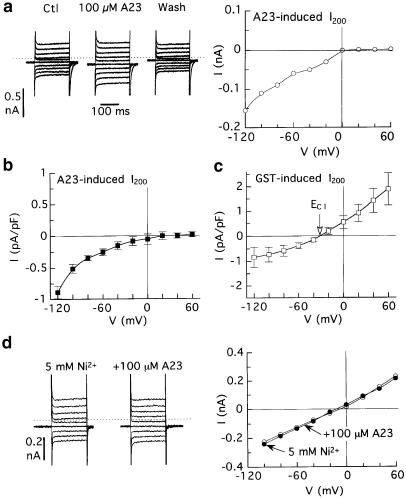
Effects of A23 and genistein on membrane currents in myocytes superfused with Ca2+-free solution and dialysed with standard pCa 10.5 solution. The myocytes were pulsed from –40 mV to other voltages for 200 ms at 0.2 Hz before and 5 min after application of 100 μM A23 or genistein. (a) Lack of effect of A23. Left: original records and difference (A23-induced) current; right: I–V relationship of the A23-induced I200. (b) Activation of outwardly rectifying current by genistein. The current reversed near calculated ECl (−35 mV). The calibration bar indicating 0.5 nA applies to all records.
Figure 6.
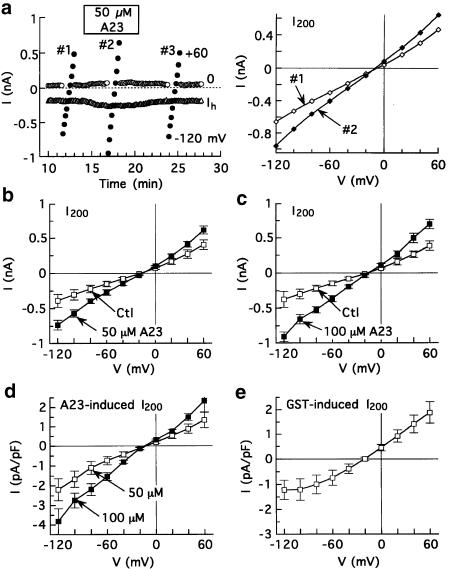
Effects of A23 and genistein on membrane currents in myocytes having near-physiological transmembrane gradients of Na+ and Ca2+. The myocytes were superfused with standard solution that contained 10 μM verapamil, dialysed with pCa 7 solution that contained 7 mM Na+ and 65 mM Cl−, and depolarized from –40 to 0 mV for 200 ms at 0.2 Hz except for determinations of I–V relationships. (a) Effects of 50 μM A23 in a representative experiment. Left: time course of changes in I200 amplitudes. Right: I200–V relationships determined before and after addition of A23. (b, c) I200 measured before and after addition of 50 μM A23 (b, n=5) or 100 μM A23 (c, n=6). (d) A23-induced I200; same myocytes as in (b, c). (e) I200 induced by 100 μM genistein; n=6 myocytes.
Lack of involvement of nonselective cation channels in A23 action
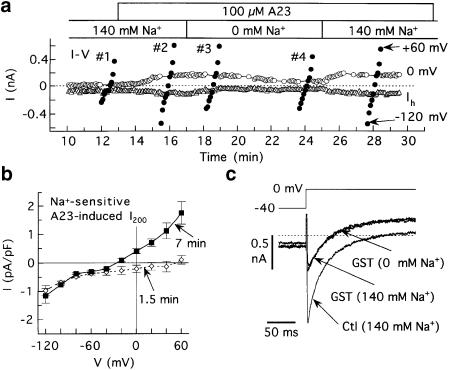
A possible explanation for the effects of A23 on I200 was that the drug activates a Cd2+-sensitive, nonselective cation current with Erev≈ −22 mV under standard conditions. In that case, replacement of standard (Na+) superfusate with Na+-free (NMDG+) superfusate should affect the voltage dependence of the A23-induced current by abolishing the inward component, enhancing the outward component, and shifting Erev to a more negative potential. To test this prediction, myocytes treated with 100 μM A23 were superfused with Na+ solution and then with Na+-free solution. The results from a representative experiment (Figure 3a) indicate that the removal of Na+ had two reversible effects on A23-induced I200; it rapidly suppressed the inward component, and slowly suppressed the outward component. The slow suppression of outward current is inconsistent with involvement of nonselective cation channels in A23 action.
Figure 3.
Differential effects of Na+-free external solution on membrane currents induced by A23 and genistein. Myocytes dialysed with standard Cs+ solution were superfused with standard 140 mM Na+ or 0 mM Na+ solution, and depolarized from –40 to 0 mV for 200 ms at 0.2 Hz, except for determinations of I–V relationships. (a) Reversible early and late effects of Na+-free solution on I200 in a myocyte treated with 100 μM A23. The filled circles indicate the amplitudes of I200 on the I–V runs. Time markers indicate time after patch-breakthrough. (b) Na+-sensitive A23-induced I200 determined at ≈1.5 and 7 min after replacement of standard solution with Na+-free solution (n=5). The current was measured by subtracting I200 (A23, 0 mM Na+) from I200 (A23, 140 mM Na+) (e.g., in (a): #3 from #2 (1.5 min), and #4 from #2 (7 min)). (c) Superimposed records illustrating the lack of effect of an 8-min exposure to Na+-free solution on genistein-modified current.
The I–V relationship of the early Na+-sensitive component (measured after 1.5 min Na+-free exposure) was inwardly rectifying with Erev near +50 mV, and the I–V relationship of the late Na+-sensitive component (measured after ≈7 min exposure) was slightly outwardly rectifying, with an Erev of –26±3 mV (n=5) (Figure 3b). In marked contrast to these findings, removal of Na+ had neither an early nor late effect on genistein-modified current (n=4) (e.g., Figure 3c).
Evidence for involvement of the NCX in A23-induced current
The effects of Cd2+, Ni2+, and Na+ removal pointed to involvement of NCX in A23-induced current. To investigate this possibility in more detail, experiments were conducted on myocytes with modified transmembrane distributions of Ca2+ and Na+.
Experiments on myocytes with modified Ca2+ distribution
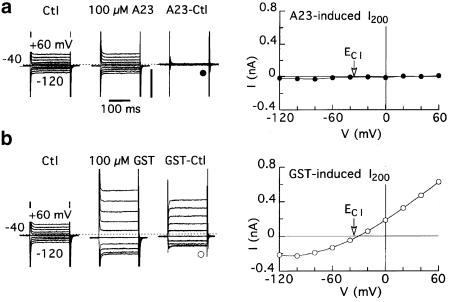
Superfusion with Ca2+-free solution is expected to be permissive for inward but not outward INCX. To determine whether this was the case with A23-induced current, myocytes dialysed with standard solution were superfused with Ca2+-free solution and then treated with A23. The results of these experiments were inconclusive because the drug failed to induce any current at all (Figure 4a); in nine myocytes, 100–250 μM A23 changed the current levels at 0 and −80 mV by negligible 0.03±0.04 and −0.02±0.02 pA pF−1, respectively. By contrast, 100 μM genistein induced an outwardly rectifying current with Erev near ECl (Figure 4b).
The failure of A23 to induce inward current in the foregoing trials was tentatively attributed to the ancillary effects of Ca2+-free superfusion, including suppression of Ca2+ entry and consequent lowering of subsarcolemmal Ca2+ essential for NCX activity. To stabilize intracellular Ca2+ in myocytes superfused with Ca2+-free solution, dialysate Ca2+ was raised from standard pCa 10.5 to pCa 7. Under these conditions, 100 μM A23 caused a reversible increase in current amplitude at negative potentials (Figure 5a). The I–V relationship of the A23-induced current had the properties of a forward-mode INCX (i.e., inwardly rectifying with poorly defined positive Erev) (Figure 5b), whereas the I–V relationship of genistein-induced current had the usual outwardly rectifying form (Figure 5c). In agreement with the view that the A23-induced current was in large measure due to NCX activity, the current was undetectable in myocytes that were superfused with Ca2+-free solution that contained 5 mM Ni2+ (e.g., Figure 5d). In six experiments, the current induced by 100 μM A23 at −100 mV was −0.06±0.05 pA pF−1 (versus −0.51±0.07 pA pF−1 in the absence of Ni2+ (Figure 5b)).
Figure 5.
Effects of A23, genistein, and Ni2+ on membrane currents in myocytes superfused with Ca2+-free solution and dialysed with pCa 7 solution. The myocytes were pulsed from −40 mV to other voltages for 200 ms at 0.2 Hz before, 5 min after application of 100 μM A23 or genistein, and 5–7 min after drug washout. (a) Reversible effects of A23 on membrane current in a representative myocyte. (b) The voltage dependence of A23-induced I200; n=9 myocytes. (c) The voltage dependence of genistein-induced I200; n=4. Erev was near the calculated ECl of −32 mV. (d) Inhibition of A23-induced current by pretreatment with 5 mM Ni2+.
Experiments on myocytes having near-physiological distributions of Ca2+ and Na+
To determine the I–V relationship of A23-induced current under near-physiological ionic conditions, myocytes were dialysed with a 7 mM Na+ pipette solution and superfused with K+-free Tyrode's solution that was supplemented with 10 μM verapamil to block Ca2+ (and Na+) channels with minimal inhibition of NCX (see Van Amsterdam & Zaagsma, 1986). Under these conditions, 50 μM A23 reversibly induced a current that had an Erev near −15 mV (Figure 5a). In five myocytes treated with 50 μM A23, the drug-induced current reversed at –13±5 mV (Figure 5b), a value that is close to the Erev of INCX (−18.7 mV) estimated as 3ENa–2ECa (where ENa is the Na+ equilibrium potential, and ECa is the Ca2+ equilibrium potential). The current induced by 100 μM A23 was larger than that induced by 50 μM, and reversed at −15±3 mV (n=6) (Figure 6c,d). For comparison, the current induced by genistein was outwardly rectifying and reversed at –21±2 mV (n=6) (calculated ECl –22 mV) (Figure 6e).
In myocytes with near-physiological distributions of Na+ and Ca2+, the reversal of the effects of higher (⩾100 μM) A23 (and A25, see below) was not always as satisfactory as it was with lower concentrations or with higher concentrations under standard conditions. Reversal was >85% complete in ≈30% of myocytes treated with high A23, and >60% complete in another 30%; in the remaining myocytes, recovery was interrupted by development of hypercontracture. The data in Figures 7 and 8 (see below) are from all myocytes in which membrane currents were relatively stable before and 5–7 min after addition of a tyrphostin.
Figure 7.
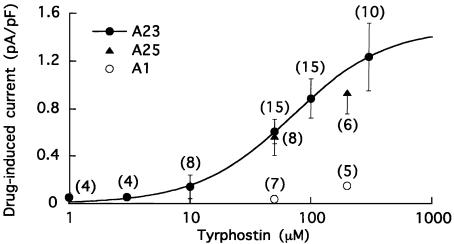
Concentration-dependent effects of A23, A25 and A1. The myocytes were superfused with standard solution that contained 10 μM verapamil, dialysed with pCa 7 solution that contained 7 mM Na+ and 40 mM Cl−, and depolarized from −40 to 0 mV for 200 ms at 0.1 Hz. The amplitude of the voltage-averaged current between –40 and 0 mV (I200(0 mV) minus Ih(−40 mV)) was measured before and 5–7 min after drug application to obtain the drug-induced current. The data obtained with A23 (filled circles) are described by the Hill equation with an EC50 of 68±4 μM and coefficient of 1.1. Also shown on the plot are data obtained from myocytes that were treated with A25 (triangles) and A1 (open circles). Numbers of myocytes in parentheses.
Figure 8.
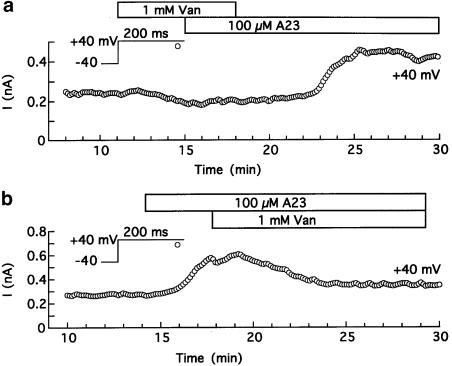
Antagonism of A23 action by orthovanadate. The myocytes were superfused with standard solution that contained 10 μM verapamil, dialysed with 7 mM Na+, pCa 7 solution, and depolarized from −40 to +40 mV for 200 ms at 0.133 Hz. (a) Reversible antagonism of A23 action on I200 (+40 mV) by 1 mM orthovanadate (Van). (b) Inhibition of A23-induced current by 1 mM orthovanadate.
Relative effectiveness of A23 and two other tyrphostins
To determine the concentration dependence of A23 action, myocytes dialysed with 7 mM Na+–pCa 7 solution and superfused with verapamil solution were treated with a concentration of A23 between 1 and 300 μM. The myocytes were pulsed from –40 to 0 mV, and a voltage-averaged current (I200(0 mV) minus Ih(−40 mV)) was measured before and 5 min after application of A23. A plot of A23-induced current versus A23 concentration is shown in Figure 7, and the Hill equation fitting the data has an EC50 of 68±4 μM and a coefficient of 1.1. The plot also shows the results of trials with two other tyrphostins, TK-inhibitor A25 and TK-inactive A1. The current induced by 50 μM A25 (0.57±0.14 pA pF−1; n=8; P<0.001) was as large as the current induced by 50 μM A23 (0.61±0.1 pA pF−1; n=15), and considerably larger (P<0.005) than that induced by 50 μM A1 (0.04±0.03 pA pF−1; n=7). A similar discrepancy between A23 and A1 effectiveness was observed when 200 μM concentrations were applied.
Antagonism of A23 action by orthovanadate
The data in Figure 7 indicate that TK-inhibiting tyrphostins induce a current with properties like INCX. To investigate the possible involvement of tyrosine phosphorylation in the action of the TK inhibitors, we looked for antagonism of A23 action by orthovanadate, an established inhibitor of phosphotyrosyl phosphatase (PTP) (Swarup et al., 1982; Davis et al., 2001). Two types of experiments were performed on myocytes with near-physiological distributions of Na+ and Ca2+. In the first series, myocytes were pretreated with 1 mM orthovanadate and then treated with 100 μM A23; in the second, myocytes were pretreated with 100 μM A23 and then treated with 1 mM orthovanadate.
Figure 8a shows that pretreatment with orthovanadate caused a small reduction in outward current monitored at +40 mV, and prevented the increase in amplitude usually observed after addition of A23. However, subsequent washout of the PTP inhibitor led to a typical A23 response in this and two similar experiments. Representative data from the second series of experiments are shown in Figure 8b. A23 (100 μM) induced an outward current at +40 mV, and addition of 1 mM orthovanadate resulted in a partial inhibition of the current. In six myocytes, the A23-induced current was reduced by 63±8% (P<0.01).
Discussion
NCX plays a major role in the function of cardiac cells. When operating in its forward mode (Ca2+ efflux), the exchanger contributes to diastolic relaxation and counterbalances Ca2+ entry via Ca2+ channels (Carmeliet, 1992; Bers et al., 2003); when operating in its reverse mode, it promotes a Ca2+ influx that contributes to Ca2+ loading of the sarcoplasmic reticulum and/or Ca2+ release from the organelle (Crespo et al., 1990; Leblanc & Hume, 1990; Lipp & Niggli, 1994; Litwin et al., 1998; Meme et al., 2001; Bers et al., 2003). In either mode, INCX affects the configuration of the action potential (Mitchell et al., 1984; Noble et al., 1991; Janvier & Boyett, 1996; Wei et al., 2003).
NCX is upregulated by PKC (Iwamoto et al., 1996) and PKA (Ruknudin & Schulze, 2002; Schulze et al., 2003), but there is little information on a possible role for TK (see below). In the present study, we have investigated the effects of TK inhibitors A23 and genistein on cardiac membrane currents, and found that A23 induced a current that behaved like INCX. We discuss the properties of this current, and consider possible underlying mechanisms.
Properties of the current induced by A23
Under standard ICa,L recording conditions, both genistein and A23 induced reversible outward shifts in late current at 0 mV. The genistein-induced shifts were almost certainly due to activation of outwardly rectifying ICl(CFTR) as previously reported (Shuba et al., 1996; Chiang et al., 1997; Shuba & McDonald, 1997), whereas the A23-induced shifts were unrelated to Cl− pathways because they were unaffected by changes in dialysate Cl− concentration. A further distinction between genistein- and A23-induced current was that A23-induced current was strongly inhibited by Cd2+. The latter result suggested that Cd2+-sensitive nonselective cation conductance and/or Cd2+-sensitive NCX were involved in the A23 response. However, a major contribution by nonselective channels seems unlikely on several grounds, including the finding that removal of external Na+ or Ca2+ inhibited the outward component of A23-induced current. Conversely, the activation of Ni2+-sensitive inwardly rectifying current by A23 in myocytes bathed with Ca2+-free solution and dialysed with pCa 7 pipette solution suggests the involvement of NCX.
The foregoing analysis points to INCX as the identity of A23-induced current. An apparent difficulty with this interpretation concerns the availability of intracellular Na+ to carry the A23-induced outward current recorded under standard experimental conditions (Na+-free pipette solution). However, it seems highly likely that Na+ ions were present in submembrane regions (Carmeliet, 1992) due to technical limitations in controlling intracellular ion concentrations (Pusch & Neher, 1988; Mathias et al., 1990), especially in the face of continuous influx via noninactivated Na+ channels, ‘background' Na+ channels, nonselective cation channels, and forward-mode NCX activity. It has been estimated that this influx can raise global cytoplasmic Na+ by ≈1 mM min−1 in nonpatched myocytes when, as here, Na+ pump sites are inhibited by K+-free external conditions (Eisner et al., 1981; Désilets & Baumgarten, 1986; Bers et al., 2003). Although continuous diffusion of cytoplasmic Na+ into the pipette will have attenuated elevation of global Na+ in our patched myocytes, it is probable that submillimolar concentrations of subsarcolemmal Na+ were only attained after a period of restricted Na+ influx. In this regard, superfusion with Na+-free solution had a biphasic effect on A23-modified current, an early reduction of inward current that we attribute to depletion of external Na+, and a secondary reduction of outward current that we attribute to depletion of subsarcolemmal Na+.
It is also probable that subsarcolemmal Ca2+ concentration was considerably higher than cytoplasmic Ca2+ concentration in myocytes patched with standard (pCa 10.5) pipette solution, especially during pulses that elicited ICa,L (You et al., 1997; Weber et al., 2002). The data in Figure 2a(iii) indicate that the A23-induced current reversed at about –22 mV, an Erev for INCX that would be consistent with respective subsarcolemmal Na+ and Ca2+ concentrations of 3.6 mM and 10 nM (for example). In the absence of additional modulation, a Ca2+ concentration of this magnitude would only secure a mild activation of cardiac NCX via binding of the divalent cation to the allosteric activation site (KD 125–300 nM: (Weber et al., 2001; Reeves & Condrescu, 2003).
In myocytes configured for measurement of ICa,L, 0.5 mM Cd2+ completely suppressed both ICa,L and A23-induced current (Figure 1b). The two events were linked in so far as suppression of ICa,L suppressed Ca2+ influx, lowered submembrane Ca2+ concentration, and inhibited Ca2+-mediated activation of NCX. However, it is probable that Cd2+ also suppressed A23-induced current by competing with Ca2+ for external NCX sites. Cd2+ has been shown to inhibit smooth muscle NCX (Smith et al., 1987), cardiac NCX (Bers et al., 1980; Trosper & Philipson, 1983), and cloned NCX1 (Iwamoto & Shigekawa, 1998) with KD⩽33 μM, and has been used (0.2–1 mM) as a prophylactic measure to block NCX in recent studies on cardiomyocytes (Feraille et al., 1997; Gao et al., 2002). Application of Ni2+ (3 mM), a weaker inhibitor of ICa,L (McDonald et al., 1994) and NCX1 (Iwamoto & Shigekawa, 1998) than is Cd2+, also suppressed A23-induced current.
Earlier findings and possible mechanisms
The effects of 100–200 μM genistein on NCX have been examined in three earlier studies, with one reaching the conclusion that the drug inhibited NCX in cultured neuronal cells (Wang et al., 1997), and the others that it had no effect on the activity of NCX1 expressed in fibroblasts (Condrescu et al., 1996; Linck et al., 1998). In the present study, genistein-induced current was insensitive to application of Cd2+ and modifications of Na+ and Ca2+ concentrations. These results, and the failure of genistein to induce current at any ECl between −35 and −1 mV, lead to the conclusion that (in agreement with the earlier studies) genistein does not stimulate NCX activity.
To our knowledge, there are no previous data on the effects of tyrphostin compounds on the activity of either native or expressed NCX. The present results with A23 are consistent with a stimulatory effect on NCX1 in guinea-pig ventricular myocytes, perhaps by affecting the activatory action of intracellular Ca2+ (see above). A key question is whether the tyrphostin acted by influencing tyrosine phosphorylation. In that regard, it is known that NCX1 has consensus sites for phosphorylation by TK (Quednau et al., 1997), and that tyrosine phosphorylation of NCX regulatory protein can modulate basal NCX activity (Kiang et al., 2003). Evidence in favour of a phosphorylation-related mechanism is that a second TK inhibitor (A25) was as effective as A23, the TK-inactive analogue (A1) was substantially less effective, and PTP inhibitor orthovanadate antagonized the action of A23. On the other hand, the ineffectiveness of TK inhibitor genistein weakens the case for such a mechanism. However, it is important to note that even though both A23 and genistein are classified as broad-spectrum TK inhibitors, they may preferentially interact with one TK family over another (Akiyama & Ogawara, 1991; Ramdas et al., 1994). Since it is well established that specific TK families can have opposing effects on cellular processes (Zhang et al., 2002), it is entirely possible that A23 and genistein have TK-related differential actions on INCX. In that regard, differential action on specific TK families has been held responsible for the divergent effects of 100 μM A23 (ca. 40% inhibition) and 100 μM genistein (120% stimulation) on swelling-activated Cl− current in human atrial myocytes (Du et al., 2004).
Acknowledgments
We thank Ms Gina Dickie for excellent technical assistance. This study was supported by the Canadian Institutes of Health Research.
Abbreviations
- A1
tyrphostin A1
- A23
tyrphostin A23
- A25
tyrphostin A25
- CFTR
cystic fibrosis transmembrane regulator
- DMSO
dimethyl sulphoxide
- ECl
Cl− equilibrium potential
- ENa
Na+ equilibrium potential
- Erev
reversal potential
- EC50
concentration that produces 50% of maximal response
- EGTA
ethylene glycol-bis(b-aminoethyl)-N,N,N,N-tetraacetic acid
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulphonic acid
- I–V
current-voltage
- ICa,L
L-type Ca2+ current
- ICl(CFTR)
cystic fibrosis transmembrane regulator chloride current
- Ih
holding current
- INCX
Na+–Ca2+ exchanger current
- I200
current at end of 200-ms pulse
- KD
dissociation constant
- NCX
Na+–Ca2+ exchanger
- NMDG
N-methyl-D-glucamine
- PKA
protein kinase A
- PKC
protein kinase C
- TK
tyrosine kinase
References
- AKIYAMA T., ISHIDA J., NAKAGAWA S., OGAWARA H., WATANABE S., ITOH N., SHIBUYA M., FUKAMI Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- AKIYAMA T., OGAWARA H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- ALBERT A.P., AROMOLARAN A.S., LARGE W.A. Agents that increase tyrosine phosphorylation activate a non-selective cation current in single rabbit portal vein smooth muscle cells. J. Physiol. 2001;530:207–217. doi: 10.1111/j.1469-7793.2001.0207l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERS D.M., EISNER D.A., VALDIVIA H.H. Sarcoplasmic reticulum Ca2+ and heart failure: roles of diastolic leak and Ca2+ transport. Circ. Res. 2003;93:487–490. doi: 10.1161/01.RES.0000091871.54907.6B. [DOI] [PubMed] [Google Scholar]
- BERS D.M., PHILIPSON K.D., NISHIMOTO A.Y. Sodium–calcium exchange and sidedness of isolated cardiac sarcolemmal vesicles. Biochim. Biophys. Acta. 1980;601:358–371. doi: 10.1016/0005-2736(80)90540-4. [DOI] [PubMed] [Google Scholar]
- CARMELIET E. A fuzzy subsarcolemmal space for intracellular Na+ in cardiac cells. Cardiovasc. Res. 1992;26:433–442. doi: 10.1093/cvr/26.5.433. [DOI] [PubMed] [Google Scholar]
- CATALDI M., TAGLIALATELA M., GUERRIERO S., AMOROSO S., LOMBARDI G., DI RENZO G., ANNUNZIATO L. Protein-tyrosine kinases activate while protein-tyrosine phosphatases inhibit L-type calcium channel activity in pituitary GH3 cells. J. Biol. Chem. 1996;271:9441–9446. doi: 10.1074/jbc.271.16.9441. [DOI] [PubMed] [Google Scholar]
- CHIANG C.E., CHEN S.A., CHANG M.S., LIN C.I., LUK H.N. Genistein directly induces cardiac CFTR chloride current by a tyrosine kinase-independent and protein kinase A-independent pathway in guinea pig ventricular myocytes. Biochem. Biophys. Res. Commun. 1997;235:74–78. doi: 10.1006/bbrc.1997.6739. [DOI] [PubMed] [Google Scholar]
- CONDRESCU M., NICOLL D., PHILIPSON K.D., REEVES J.P. Regulation of the NCX-2 sodium–calcium exchanger by protein kinases in CHO cells. Biophys. J. 1996;70:A206. [Google Scholar]
- CRESPO L.M., GRANTHAM C.J., CANNELL M.B. Kinetics, stoichiometry and role of the Na–Ca exchange mechanism in isolated cardiac myocytes. Nature. 1990;345:618–621. doi: 10.1038/345618a0. [DOI] [PubMed] [Google Scholar]
- DAVIS M.J., WU X., NURKIEWICZ T.R., KAWASAKI J., GUI P., HILL M.A., WILSON E. Regulation of ion channels by protein tyrosine phosphorylation. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H1835–H1862. doi: 10.1152/ajpheart.2001.281.5.H1835. [DOI] [PubMed] [Google Scholar]
- DÉSILETS M., BAUMGARTEN C.M. K+, Na+, and Cl− activities in ventricular myocytes isolated from rabbit heart. Am. J. Physiol. 1986;251:C197–C208. doi: 10.1152/ajpcell.1986.251.2.C197. [DOI] [PubMed] [Google Scholar]
- DU X.L., GAO Z., LAU C.P., CHIU S.W., TSE H.F., BAUMGARTEN C.M., LI G.R. Differential effects of tyrosine kinase inhibitors on volume-sensitive chloride current in human atrial myocytes: evidence for dual regulation by Src and EGFR kinases. J. Gen. Physiol. 2004;123:427–439. doi: 10.1085/jgp.200409013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EISNER D.A., LEDERER W.J., VAUGHAN-JONES R.D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J. Physiol. 1981;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENRIGHT W.J., BOOTH P. Specificity of inhibitors of tyrosine kinases. Focus. 1991;13:79–83. [Google Scholar]
- FERAILLE E., CARRANZA M.L., ROUSSELOT M., FAVRE H. Modulation of Na+,K(+)-ATPase activity by a tyrosine phosphorylation process in rat proximal convoluted tubule. J. Physiol. 1997;498 Pt 1:99–108. doi: 10.1113/jphysiol.1997.sp021844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRENCH P.J., BIJMAN J., BOT A.G., BOOMAARS W.E., SCHOLTE B.J., DE JONGE H.R. Genistein activates CFTR Cl− channels via a tyrosine kinase- and protein phosphatase-independent mechanism. Am. J. Physiol. 1997;273:C747–C753. doi: 10.1152/ajpcell.1997.273.2.C747. [DOI] [PubMed] [Google Scholar]
- GAO J., WYMORE R.S., WANG Y., GAUDETTE G.R., KRUKENKAMP I.B., COHEN I.S., MATHIAS R.T. Isoform-specific stimulation of cardiac Na/K pumps by nanomolar concentrations of glycosides. J. Gen. Physiol. 2002;119:297–312. doi: 10.1085/jgp.20028501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAZIT A., YAISH P., GILON C., LEVITZKI A. Tyrphostins I: synthesis and biological activity of protein tyrosine kinase inhibitors. J. Med. Chem. 1989;32:2344–2352. doi: 10.1021/jm00130a020. [DOI] [PubMed] [Google Scholar]
- GORDON J.A. Use of vanadate as protein-phosphotyrosine phosphatase inhibitor. Methods Enzymol. 1991;201:477–482. doi: 10.1016/0076-6879(91)01043-2. [DOI] [PubMed] [Google Scholar]
- HOLMES T.C., FADOOL D.A., LEVITAN I.B. Tyrosine phosphorylation of the Kv1.3 potassium channel. J. Neurosci. 1996;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IWAMOTO T., PAN Y., WAKABAYASHI S., IMAGAWA T., YAMANAKA H.I., SHIGEKAWA M. Phosphorylation-dependent regulation of cardiac Na+/Ca2+ exchanger via protein kinase C. J. Biol. Chem. 1996;271:13609–13615. doi: 10.1074/jbc.271.23.13609. [DOI] [PubMed] [Google Scholar]
- IWAMOTO T., SHIGEKAWA M. Differential inhibition of Na+/Ca2+ exchanger isoforms by divalent cations and isothiourea derivative. Am. J. Physiol. 1998;275:C423–C430. doi: 10.1152/ajpcell.1998.275.2.C423. [DOI] [PubMed] [Google Scholar]
- JANVIER N.C., BOYETT M.R. The role of Na–Ca exchange current in the cardiac action potential. Cardiovasc. Res. 1996;32:69–84. [PubMed] [Google Scholar]
- KIANG J.G., MCCLAIN D.E., WARKE V.G., KRISHNAN S., TSOKOS G.C. Constitutive NO synthase regulates the Na+/Ca2+ exchanger in human T cells: role of [Ca2+]iand tyrosine phosphorylation. J. Cell. Biochem. 2003;89:1030–1043. doi: 10.1002/jcb.10564. [DOI] [PubMed] [Google Scholar]
- LEBLANC N., HUME J.R. Sodium current-induced release of calcium from cardiac sarcoplasmic reticulum. Science. 1990;248:372–376. doi: 10.1126/science.2158146. [DOI] [PubMed] [Google Scholar]
- LEVITZKI A., GAZIT A. Tyrosine kinase inhibition: an approach to drug development. Science. 1995;267:1782–1788. doi: 10.1126/science.7892601. [DOI] [PubMed] [Google Scholar]
- LINCK B., QIU Z., HE Z., TONG Q., HILGEMANN D.W., PHILIPSON K.D. Functional comparison of the three isoforms of the Na+/Ca2+ exchanger (NCX1, NCX2, NCX3) Am. J. Physiol. 1998;274:C415–C423. doi: 10.1152/ajpcell.1998.274.2.C415. [DOI] [PubMed] [Google Scholar]
- LIPP P., NIGGLI E. Sodium current-induced calcium signals in isolated guinea-pig ventricular myocytes. J. Physiol. 1994;474:439–446. doi: 10.1113/jphysiol.1994.sp020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LITWIN S.E., LI J., BRIDGE J.H. Na–Ca exchange and the trigger for sarcoplasmic reticulum Ca release: studies in adult rabbit ventricular myocytes. Biophys. J. 1998;75:359–371. doi: 10.1016/S0006-3495(98)77520-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATHIAS R.T., COHEN I.S., OLIVA C. Limitations of the whole cell patch clamp technique in the control of intracellular concentrations. Biophys. J. 1990;58:759–770. doi: 10.1016/S0006-3495(90)82418-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCDONALD T.F., PELZER S., TRAUTWEIN W., PELZER D.J. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol. Rev. 1994;74:365–507. doi: 10.1152/physrev.1994.74.2.365. [DOI] [PubMed] [Google Scholar]
- Meme W., O'NEILL S., EISNER D. Low sodium inotropy is accompanied by diastolic Ca2+ gain and systolic loss in isolated guinea-pig ventricular myocytes. J. Physiol. 2001;530:487–495. doi: 10.1111/j.1469-7793.2001.0487k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL M.R., POWELL T., TERRAR D.A., TWIST V.W. The effects of ryanodine, EGTA and low-sodium on action potentials in rat and guinea-pig ventricular myocytes: evidence for two inward currents during the plateau. Br. J. Pharmacol. 1984;81:543–550. doi: 10.1111/j.1476-5381.1984.tb10107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOBLE D., NOBLE S.J., BETT G.C., EARM Y.E., HO W.K., SO I.K. The role of sodium–calcium exchange during the cardiac action potential. Ann. N. Y. Acad. Sci. 1991;639:334–353. doi: 10.1111/j.1749-6632.1991.tb17323.x. [DOI] [PubMed] [Google Scholar]
- O'DELL T.J., GRANT S.G., KARL K., SORIANO P.M., KANDEL E.R. Pharmacological and genetic approaches to the analysis of tyrosine kinase function in long-term potentiation. Cold Spring Harb. Symp. Quant. Biol. 1992;57:517–526. doi: 10.1101/sqb.1992.057.01.057. [DOI] [PubMed] [Google Scholar]
- OGURA T., SHUBA L.M., MCDONALD T.F. Action potentials, ionic currents and cell water in guinea pig ventricular preparations exposed to dimethyl sulfoxide. J. Pharmacol. Exp. Ther. 1995;273:1273–1286. [PubMed] [Google Scholar]
- OGURA T., SHUBA L.M., MCDONALD T.F. L-type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am. J. Physiol. 1999;276:H1724–H1733. doi: 10.1152/ajpheart.1999.276.5.H1724. [DOI] [PubMed] [Google Scholar]
- PAILLART C., CARLIER E., GUEDIN D., DARGENT B., COURAUD F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J. Pharmacol. Exp. Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- PUSCH M., NEHER E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Arch. 1988;411:204–211. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- QUEDNAU B.D., NICOLL D.A., PHILIPSON K.D. Tissue specificity and alternative splicing of the Na+/Ca2+ exchanger isoforms NCX1, NCX2, and NCX3 in rat. Am. J. Physiol. 1997;272:C1250–C1261. doi: 10.1152/ajpcell.1997.272.4.C1250. [DOI] [PubMed] [Google Scholar]
- RAMDAS L., MCMURRAY J.S., BUDDE R.J. The degree of inhibition of protein tyrosine kinase activity by tyrphostin 23 and 25 is related to their instability. Cancer Res. 1994;54:867–869. [PubMed] [Google Scholar]
- REEVES J.P., CONDRESCU M. Allosteric activation of sodium–calcium exchange activity by calcium: persistence at low calcium concentrations. J. Gen. Physiol. 2003;122:621–639. doi: 10.1085/jgp.200308915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUKNUDIN A., SCHULZE D.H. Phosphorylation of the Na+/Ca2+ exchangers by PKA. Ann. N. Y. Acad. Sci. 2002;976:209–213. doi: 10.1111/j.1749-6632.2002.tb04743.x. [DOI] [PubMed] [Google Scholar]
- SCHULZE D.H., MUQHAL M., LEDERER W.J., RUKNUDIN A.M. Sodium/calcium exchanger (NCX1) macromolecular complex. J. Biol. Chem. 2003;278:28849–28855. doi: 10.1074/jbc.M300754200. [DOI] [PubMed] [Google Scholar]
- SHUBA L.M., ASAI T., PELZER S., MCDONALD T.F. Activation of cardiac chloride conductance by the tyrosine kinase inhibitor, genistein. Br. J. Pharmacol. 1996;119:335–345. doi: 10.1111/j.1476-5381.1996.tb15991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHUBA L.M., MCDONALD T.F. Synergistic activation of guinea-pig cardiac cystic fibrosis transmembrane conductance regulator by the tyrosine kinase inhibitor genistein and cAMP. J. Physiol. 1997;505 Pt 1:23–40. doi: 10.1111/j.1469-7793.1997.023bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMIRNOV S.V., AARONSON P.I. Inhibition of vascular smooth muscle cell K+ currents by tyrosine kinase inhibitors genistein and ST 638. Circ. Res. 1995;76:310–316. doi: 10.1161/01.res.76.2.310. [DOI] [PubMed] [Google Scholar]
- SMITH J.B., CRAGOE E.J., Jr, SMITH L. Na+/Ca2+ antiport in cultured arterial smooth muscle cells. Inhibition by magnesium and other divalent cations. J. Biol. Chem. 1987;262:11988–11994. [PubMed] [Google Scholar]
- SWARUP G., COHEN S., GARBERS D.L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem. Biophys. Res. Commun. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- TROSPER T.L., PHILIPSON K.D. Effects of divalent and trivalent cations on Na+–Ca2+ exchange in cardiac sarcolemmal vesicles. Biochim. Biophys. Acta. 1983;731:63–68. doi: 10.1016/0005-2736(83)90398-x. [DOI] [PubMed] [Google Scholar]
- VAN AMSTERDAM F.T., ZAAGSMA J. Modulation of ATP-dependent calcium extrusion and Na+/Ca2+ exchange across rat cardiac sarcolemma by calcium antagonists. Eur. J. Pharmacol. 1986;123:441–449. doi: 10.1016/0014-2999(86)90721-1. [DOI] [PubMed] [Google Scholar]
- WANG C., DAVIS N., COLVIN R.A. Genistein inhibits Na+/Ca2+ exchange activity in primary rat cortical neuron culture. Biochem. Biophys. Res. Commun. 1997;233:86–90. doi: 10.1006/bbrc.1997.6398. [DOI] [PubMed] [Google Scholar]
- WANG Y.T., YU X.M., SALTER M.W. Ca2+-independent reduction of N-methyl-D-aspartate channel activity by protein tyrosine phosphatase. Proc. Natl. Acad. Sci. U.S.A. 1996;93:1721–1725. doi: 10.1073/pnas.93.4.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHIZUKA T., HORIE M., OBAYASHI K., SASAYAMA S. Genistein inhibits slow component delayed-rectifier K currents via a tyrosine kinase-independent pathway. J. Mol. Cell. Cardiol. 1998;30:2577–2590. doi: 10.1006/jmcc.1998.0815. [DOI] [PubMed] [Google Scholar]
- WEBER C.R., GINSBURG K.S., PHILIPSON K.D., SHANNON T.R., BERS D.M. Allosteric regulation of Na/Ca exchange current by cytosolic Ca in intact cardiac myocytes. J. Gen. Physiol. 2001;117:119–131. doi: 10.1085/jgp.117.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBER C.R., PIACENTINO V., III, GINSBURG K.S., HOUSER S.R., BERS D.M. Na+–Ca2+ exchange current and submembrane [Ca2+] during the cardiac action potential. Circ. Res. 2002;90:182–189. doi: 10.1161/hh0202.103940. [DOI] [PubMed] [Google Scholar]
- WEI S.K., RUKNUDIN A., HANLON S.U., MCCURLEY J.M., SCHULZE D.H., HAIGNEY M.C. Protein kinase A hyperphosphorylation increases basal current but decreases beta-adrenergic responsiveness of the sarcolemmal Na+–Ca2+ exchanger in failing pig myocytes. Circ. Res. 2003;92:897–903. doi: 10.1161/01.RES.0000069701.19660.14. [DOI] [PubMed] [Google Scholar]
- WEINREICH F., WOOD P.G., RIORDAN J.R., NAGEL G. Direct action of genistein on CFTR. Pflügers Arch. 1997;434:484–491. doi: 10.1007/s004240050424. [DOI] [PubMed] [Google Scholar]
- YOU Y., PELZER D.J., PELZER S. Modulation of L-type Ca2+ current by fast and slow Ca2+ buffering in guinea pig ventricular cardiomyocytes. Biophys. J. 1997;72:175–187. doi: 10.1016/S0006-3495(97)78656-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG Z.Y., ZHOU B., XIE L. Modulation of protein kinase signaling by protein phosphatases and inhibitors. Pharmacol. Ther. 2002;93:307–317. doi: 10.1016/s0163-7258(02)00199-7. [DOI] [PubMed] [Google Scholar]
- ZHOU S.S., HAZAMA A., OKADA Y. Tyrosine kinase-independent extracellular action of genistein on the CFTR Cl− channel in guinea pig ventricular myocytes and CFTR-transfected mouse fibroblasts. Jpn. J. Physiol. 1998;48:389–396. doi: 10.2170/jjphysiol.48.389. [DOI] [PubMed] [Google Scholar]