Abstract
The present study examined the ability of the selective imidazoline I2-site ligands 2-(-2-benzofuranyl)-2-imidazoline (2-BFI) and 2-[4,5-dihydroimidaz-2-yl]-quinoline (BU224) and selected monoamine oxidase (MAO) inhibitors to evoke locomotor activity in rats bearing a lesion of the nigrostriatal pathway.
Male Sprague–Dawley rats were injected with 12.5 μg 6-hydroxydopamine (6-OHDA) into the right median forebrain bundle to induce a unilateral lesion of the nigrostriatal tract. After 6 weeks, test drugs were administered either alone or in combination with L-DOPA (L-3,4-dihydroxyphenylamine) and the circling behaviour of animals was monitored as an index of anti-Parkinsonian activity.
Intraperitoneal (i.p.) administration of the irreversible MAO-B inhibitor deprenyl (20 mg kg−1) or the imidazoline I2-site ligands BU224 (14 mg kg−1) and 2-BFI (7 and 14 mg kg−1) produced significant increases in ipsiversive rotations compared to vehicle controls totaling, at the highest respective doses tested, 521±120, 131±37 and 92.5±16.3 net contraversive rotations in 30 (deprenyl) or 60 (BU224 and 2-BFI) min. In contrast, the reversible MAO-A inhibitor moclobemide (2.5–10 mg kg−1) and the reversible MAO-B inhibitor lazabemide (2.5–10 mg kg−1) failed to instigate significant rotational behaviour compared to vehicle.
Coadministration of lazabemide (10 mg kg−1), moclobemide (10 mg kg−1) or 2-BFI (14 mg kg−1) with L-DOPA (20 mg kg−1) significantly increased either the duration or total number of contraversive rotations emitted over the testing period in comparison to L-DOPA alone.
These data suggest that I2-specific ligands have dual effects in the 6-OHDA-lesioned rat model of Parkinson's disease; a first effect associated with an increase in activity in the intact hemisphere, probably via an increase in striatal dopamine content, and a secondary action which, through the previously documented inhibition of MAO-A and/or MAO-B, increases the availability of dopamine produced by L-DOPA.
Keywords: 2-BFI, deprenyl, imidazoline I2 site, rat, in vivo, lazabemide, moclobemide, monoamine oxidase, Parkinson's disease
Introduction
Imidazoline-binding sites (I sites) constitute a unique component of the binding profile of many imidazolines, guanidiniums and structurally related derivatives (Eglen et al., 1998). Such sites have been separated into at least three entities, imidazoline-1 (I1), imidazoline-2 (I2), imidazoline-3 (I3), based on their respective preference for the α2-adrenoceptor ligands, clonidine, idazoxan and methoxy-idazoxan (Michel & Ernsberger, 1992; Chan et al., 1995). Recently, ligands have been developed with high selectivity for imidazoline I2 sites, notably 2-BFI (2-(2-benzofuranyl)-2-imidazoline) and its quinoline and isoquinoline analogues, BU216 (3-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride), BU224 (2-[4,5-dihydroimidaz-2-yl]-quinoline) and BU226 (2-[4,5-dihydroimidaz-2-yl]-isoquinoline hydrochloride; Lione et al., 1998).
Autoradiographic studies with [3H]idazoxan, [3H]2-BFI and [3H]BU224 indicate that I2 sites exhibit a differential distribution in rat brain (MacInnes & Handley, 2001), with low levels of binding found throughout the basal ganglia motor loop (Lione et al., 1998; Robinson et al., 2002). The functional significance of the sites in the basal ganglia is unknown, since neither their molecular structure nor their second-messenger systems have been elucidated. However, there is extensive evidence that imidazoline I2-binding sites exist on monoamine oxidase (MAO), at a location distinct from the catalytic site (Alemany et al., 1995; Raddatz et al., 1997; Remaury et al., 2000). In vitro, many imidazolines, including 2-BFI and BU224, reversibly inhibit MAO-A with a similar potency to that of the reversible MAO-A inhibitor moclobemide (IC50: 2-BFI, 16.5±2.7 μM; BU224, 4.8±0.2 μM; moclobemide, 36±3.6 μM; Lalies et al., 1999). These imidazolines also similarly inhibit MAO-B, although with less potency than the selective reversible MAO-B inhibitor, lazabemide (IC50: 2-BFI, 27.9±2.2 μM; BU224, 44.8±6.6 μM (Lalies et al., 1999); lazabemide, 0.03 μM (Da Prada et al., 1987)) and in vivo studies indicate that moclobemide, lazabemide and deprenyl show substitution for 2-BFI in an two-lever drug-discrimination paradigm (MacInnes & Handley, 2002). However, there appears to be little correlation between these agents' affinity for I2 sites and their inhibition of MAO. Thus, despite their above-mentioned similar potencies against MAO-A, moclobemide displays negligible affinity for the I2-binding site (Ki>100 μM) compared to the high affinity displayed by both 2-BFI (Ki 1.7 nM) and BU224 (Ki 2.1 nM) (Lione et al, 1998). This discrepancy suggests that 2-BFI and BU224 may bind to MAOs at sites distinct from that of established MAO inhibitors.
In Parkinson's disease (PD), degeneration of the nigrostriatal pathway results in reduced striatal dopamine levels and the single most effective treatment for this is L-DOPA (L-3,4-dihydroxyphenylamine). The therapeutic benefit of L-DOPA is ascribed to the central action of dopamine that is synthesised in the brain by decarboxylation of L-DOPA (Barbeau, 1981). Coadministration of a peripheral decarboxylase inhibitor helps to reduce peripheral side effects of L-DOPA but long-term treatment is still plagued by debilitating centrally mediated side effects, such as L-DOPA-induced dyskinesia (Nutt, 1990). For this reason, alternative treatments or refinements to existing ones are being investigated (Stocchi et al., 1997). Much attention has focused on the use of MAO-B inhibitors as adjuncts to L-DOPA treatment (Le Witt & Nyholm, 2004). Deprenyl, for example, has been shown to have therapeutic benefit when given alone in younger patients to defer the use of L-DOPA or as an adjunct to L-DOPA in the later stages to ameliorate L-DOPA-induced motor fluctuations (Parkinson Study Group 1994; 1996; Jankovic, 2000). Indeed a new generation of MAO inhibitors are currently being investigated both preclinically (e.g. Aubin et al., 2004) and in phase three clinical trials (rasagiline; Parkinson Study Group, 2004).
Previous radioligand-binding studies have indicated that I2 sites, as defined by [3H]2-BFI but not [3H]idazoxan, are increased in the putamen of PD sufferers (Reynolds et al., 1996; Gargalidis-Moudanos et al., 1997). Given that many I2-site ligands also inhibit MAO, this elevated density of I2 sites may offer an additional treatment target. The aims of the present study were, therefore, to examine the ability of I2-site ligands and specific MAO-A and MAO-B inhibitors to produce locomotor activity when administered alone or to enhance the effects of coadministered L-DOPA, in rats bearing a unilateral 6-OHDA lesion of the nigrostriatal tract.
Methods
Production of 6-OHDA lesions of the nigrostriatal pathway
A total of 16 male, Sprague–Dawley rats (Tucks, U.K.; 200–220 g) were housed in pairs in temperature- and humidity-controlled environment, on a 12 h light/dark cycle with free access to food and water. All procedures conformed to the U.K. Animals (Scientific Procedures) Act, 1986 and all efforts were made to minimise animals' suffering and the number of animals used. Unilateral 6-OHDA lesions of the nigrostriatal tract were produced as described previously (Chadha et al., 2000). At 30min prior to surgery, rats were injected with pargyline (5 mg kg−1; i.p., intraperitoneally) and desipramine (25 mg kg−1; i.p.) to elevate 6-OHDA availability and specificity for dopaminergic neurones. Under general anaesthesia (2.5% isoflurane in 95% O2, 5% CO2), animals were placed in a Kopf small animal stereotaxic frame, the mouthpiece set at 3.3 mm below the ear bars. A single injection of 6-OHDA (12.5 μg in 2.5 μl sterile water containing 0.02% ascorbic acid, 1 μl min−1) was made into the right median forebrain bundle (coordinates; 2.8 mm anterior, 2 mm lateral and 9 mm ventral to bregma) according to the rat brain atlas of Paxinos and Watson (1998). At 2 weeks after lesioning, animals were injected with amphetamine (5 mg kg−1) and placed in automated rotometers (Med. Associates) and rotations recorded for 60 min. Based on previous studies (Hefti et al., 1980; Murray et al., 2002), rats that exhibited >50 full ipsiversive rotations in the 10 min time-bin between 40 and 50 min postinjection were deemed suitably lesioned. These rats (n=14) were randomly allocated into two groups of seven for inclusion in the subsequent studies. Administration of test compounds commenced 4 weeks after amphetamine challenge.
Administration of test compounds
Animals were placed in automated rotometers (Med. Associates) and exposed to a 30 min adjustment period. The apparatus consisted of stainless steel bowls inside which each rat was placed in a jacket that was linked to an infrared sensor directly above the animal. The sensor detected the number of partial (45°) rotations ipsiversive and contraversive to the lesion and these data were recorded with ROTORAT software. When examining the effects of drug alone (deprenyl, 2-BFI, BU224, moclobemide or lazabemide), animals were administered the test compound and recording continued for up to 60 min. For the L-DOPA combination experiments, animals were administered either 2-BFI (14 mg kg−1), moclobemide (10 mg kg−1), lazabemide (10 mg kg−1) or vehicle combined with the peripheral decarboxylase inhibitor benserazide (15 mg kg−1), and then, 30 min later, L-DOPA (10 mg kg−1) was administered. Recording continued for a further 240 min. Drug treatments were distributed between groups as follows: Group 1; 2-BFI, moclobemide+L-DOPA, lazabemide+L-DOPA. Group 2; BU224, moclobemide, lazabemide, 2-BFI+L-DOPA, deprenyl. Based on our previous studies (MacInnes & Handley, 2002), single-drug experiments were conducted every other day, while L-DOPA combination studies were conducted on Mondays and Thursdays. Different doses, including vehicle control, were distributed across sessions and rats in pseudorandom order except for deprenyl, which, because it is an irreversible inhibitor, was given as the last dose to group 2. All drugs were dissolved in 0.9% physiological saline, except for moclobemide which was made up in deionised water, and administered i.p in a dose volume of 2 ml kg−1. Doses of I2-specific ligands and reversible MAO inhibitors were based on those that were effective in previous drug discrimination studies (MacInnes & Handley, 2002) and that retained full solubility in saline. The dose of deprenyl (20 mg kg−1) was chosen on the basis of previous preclinical studies (Heikkila et al., 1981; Prat et al., 2000) and was towards the higher end of the effective dose range to ensure maximum chance of obtaining an effect with only a single dose of this irreversible inhibitor. The dose of L-DOPA (10 mg kg−1) was chosen on the basis of the previous L-DOPA potentiation studies of (Heeringa et al., 1997).
Verification of lesion by tyrosine hydroxylase (TH) immunohistochemistry
At 24 h after administration of the final test drug, rats were deeply anaesthetised (pentobarbitone 60 mg kg−1, i.p.) and perfused via the left ventricle with 100 ml ice-cold phosphate buffered saline (PBS) (0.1 M; pH 7.4) followed by 100 ml ice-cold PBS containing 4% paraformaldehyde. Brains were immediately removed and postfixed for a further 48 h in 0.1 M PBS containing 4% paraformaldehyde at 4°C. Brains were cryoprotected in 30% sucrose for up to 96 h or until brains sank and then 30 μm coronal sections of the substania nigra were cut using a freezing microtome. Sections were stored in 0.1 M PBS containing 0.05% sodium azide until assay. Sections were labelled with TH -specific antibody according to the protocol of Iravani et al. (2002). The extent of nigral lesion was derived from comparison of the mean number of TH immunoreactive cell bodies detected under light microscopy between the lesion and nonlesion hemispheres (3–4 sections per rat).
Statistical analysis
For single-injections studies, rotational behaviour was compared between doses of a given drug or vehicle using a repeated measures one-way analysis of variance with Student–Newman–Keuls post hoc test (GraphPAD Prism version 3) or, for deprenyl alone, using paired t-tests after confirming that there were no deviations from Gaussian distribution. For L-DOPA combination studies, the total number and duration of rotations produced by L-DOPA+drug were compared to those of L-DOPA+vehicle using repeated measures two-way analysis of variance with a Dunnett's post hoc test. Unless otherwise indicated, data represent mean±standard error of the mean (s.e.m.)
Drugs
BU224 (2-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride) was donated by Alan Hudson, Bristol University, U.K; moclobemide (p-chloro-N-(2-morpholinoethyl) benzamide) and lazabemide (N-(2-aminoethyl)-5-chloro-2-pyridinecarboxamide hydrochloride) were donated by Hoffman La Roche, Switzerland. 2-BFI (2-(-2-benzofuranyl)-2-imidazoline) was purchased from Tocris, U.K. Deprenyl, 6-OHDA (6-hydroxydopamine, pargyline, desipramine, L-DOPA (L-3,4-dihydroxyphenylamine), benserazide, amphetamine and all other reagents were purchased from Sigma, U.K. All drugs were dosed as HCl salts, except for 6-OHDA which was dosed as the HBr salt.
Results
Lesion verification
All rats that had undergone surgery to produce a 6-OHDA lesion were screened prior to inclusion in the following studies by administration of 5 mg kg−1 amphetamine. Out of the 16 rats screened, 14 exhibited robust ipsiversive rotational behaviour (4111±729 partial rotations/86±12 full rotations in 10 min; n=14) in response to amphetamine challenge. After experimentation was completed, assessment of TH immunoreactivity confirmed that these animals had over 93% loss of dopamine cell bodies in the SNc (substantia nigra pars compacta) between the lesion (6.7±0.9, cells per SNc; n=14,) and nonlesion (104.6±5.9, cells per SNc; n=14) hemispheres.
Single-injection studies
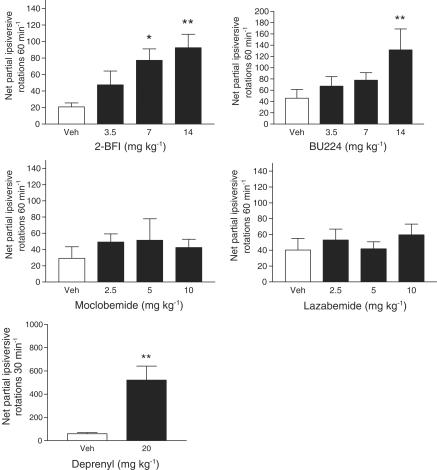
As shown in Figure 1, significant increases in ipsiversive rotational behaviour compared to vehicle were produced following the administration of 2-BFI (1-way ANOVA; F(3,18)=4.79, P<0.05) and BU224 (1-way ANOVA; F(3,18)= 4.07, P<0.05). The Student–Newman–Keuls post hoc analysis revealed that the responses to 7 and 14 mg kg−1 2-BFI and 14 mg kg−1 BU224 reached significance with maximum net partial ipsiversive rotations of 92.5±16.3 in 60 min and 131.7±37.2 in 60 min being achieved, respectively. Deprenyl (20 mg kg−1) also produced a significant increase in net partial ipsiversive rotations compared to vehicle (T(6)=3.51; P<0.05), achieving 520.7±120.5 rotations in 30 min. In contrast, the reversible MAO-A inhibitor moclobemide (F(3,18)=0.30, P=0.82) and the reversible MAO-B inhibitor lazabemide (F(3,18)=0.49, P=0.68) failed to elicit significant increases in ipsiversive rotations in comparison to vehicle.
Figure 1.
Ability of i.p. administration of I2-site ligands (2-BFI and BU224) and MAO inhibitors (moclobemide, lazabemide and deprenyl) to elicit ipsiversive rotations in rats bearing a unilateral 6-OHDA lesion. Rotational behaviour was measured for 30 or 60 min post drug or vehicle administration. Data are mean±s.e.m. (n=7). *P<0.05 and **P<0.01 indicate a statistically significant difference from vehicle using either the Student–Newman–Keuls test after a significant one-way ANOVA or a paired t-test (deprenyl alone).
L-DOPA combination studies
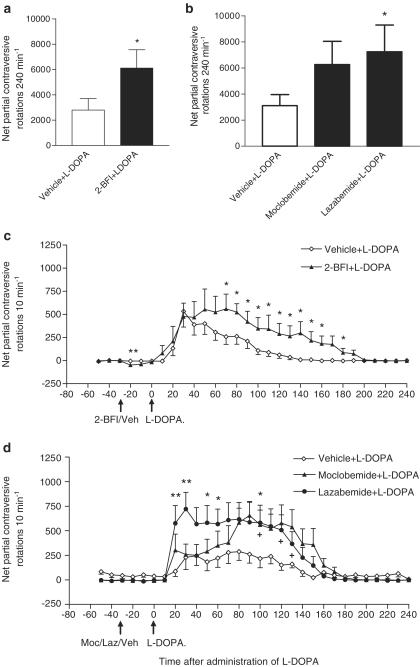
In comparison to L-DOPA (10 mg kg−1) alone, the I2-specific ligand 2-BFI (14 mg kg−1)+L-DOPA (10 mg kg−1) yielded a significant main effect for treatment (F(1,180)=34.78, P<0.0001) and time (F(29,180)=6.87, P<0.0001), and Dunnett's post hoc test indicated that 2-BFI significantly increased the total number of partial contraversive rotations (Figure 2a) and the duration of this rotational behaviour (Figure 2c). Consistent with the single-drug studies reported above, the administration of 2-BFI significantly increased the number of ipsiversive rotations that occurred in the two 10-min time bins directly after its administration, as reflected by the negative dip in net contraversive rotations (Figure 2c).
Figure 2.
Ability of the I2-site ligand 2-BFI (14 mg kg−1 i.p.) or the MAO inhibitors, moclobemide (10 mg kg−1 i.p.) and lazabemide (10 mg kg−1 i.p.) to potentiate L-DOPA (10 mg kg−1 i.p.)-induced contraversive rotations in rats bearing a unilateral 6-OHDA lesion. (a, b) Total number of rotations over 240 min are shown. *P<0.05 indicates a statistically significant difference between drug+L-DOPA versus vehicle+L-DOPA using either a paired t-test (a) or Dunnett's test after a significant two-way ANOVA (b). (c, d) Animals were administered test drugs in conjunction with benserazide (15 mg kg−1) at time T-30 min, with 10 mg kg−1 L-DOPA being given 30 min later at time T0. In (c), *P<0.05 indicates a statistically significant difference between 2-BFI+L-DOPA versus vehicle+L-DOPA (paired t-test; after a significant 2-way ANOVA). In (d), *P<0.05, and **P<0.01 indicate a statistically significant difference between lazabemide+L-DOPA versus vehicle+L-DOPA; +P<0.05 indicates a statistically significant difference between moclobemide+L-DOPA versus vehicle+L-DOPA (Dunnett's test after a significant two-way ANOVA). Data are mean±s.e.m. (n=7). Abbreviations: Laz, lazabemide. Moc, moclobemide. Veh, vehicle.
Administration of L-DOPA (10 mg kg−1) either alone or in combination with 10 mg kg−1 moclobemide or lazabemide gave rise to a significant main effect for treatment (F(2,360)=27.02, P<0.0001) and time (F(29,360)=6.56, P<0.0001). Dunnett's post hoc test indicated that the lazabemide+L-DOPA combination produced significantly more partial contraversive rotations over the 240 min recording period than L-DOPA alone. In contrast, the potentiating effect of moclobemide over this whole period just failed to reach significance (Figure 2b). However, both moclobemide and lazabemide significantly increased the duration of L-DOPA-induced rotational behaviour (Figure 2d) compared to that seen with L-DOPA alone.
Discussion
The data presented here show, for the first time, that administration of the I2-specific ligands, 2-BFI and BU224, produce ipsiversive rotational behaviour in rats bearing a full 6-OHDA lesion of the nigrostriatal tract. The full extent of the 6-OHDA lesion was evidenced in two ways: firstly, by the production of marked ipsiversive rotations with 5 mg kg−1 amphetamine, which, in animals bearing a sham lesion, would produce no ipsiversive rotations (Murray et al., 2002) and secondly, by the loss of >93% TH-positive cells in the SNc of the lesioned hemisphere in contrast to a sham lesion where no significant loss of TH-positive cell numbers is seen from a similar baseline of ∼100 cells per SNc (O'Neill et al., 2004). The degree of rotations produced by both I2-specific ligands, although small, was significantly elevated compared to that produced by vehicle alone. Additionally, over the dose range studied, a plateau response and hence true maximum effect was not reached, thus it is possible that much larger rotational responses may be produced with increased doses of 2-BFI and BU224. That vehicle alone elicits a low level of spontaneous ipsiversive rotational behaviour in these animals is consistent with other studies of this type and is thought to reflect basal levels of dopamine release in the intact hemisphere induced by the injection procedure per se (Chopin et al., 1999).
Triggering release of dopamine from nigrostriatal neurones in the intact hemisphere is a well-established and robust means of producing ipsiversive rotations in unilateral lesioned rats (e.g. Ungerstedt, 1971; Pycock, 1980). This phenomenon is further evidenced in the present study by the above-mentioned marked degree of ipsiversive rotations produced by the dopamine-releasing agent amphetamine. It follows that one possible explanation for the ipsiversive rotational behaviour seen here with 2-BFI and BU224 is that these I2-site ligands may also act to release dopamine in the striatum of the intact hemisphere. That I2-site ligands may act as ‘dopamine releasers' has been suggested previously (Sastre-Coll et al., 2001) and is backed up by the in vivo microdialysis studies of Hudson et al. (1999), which showed that acute administration of similar doses of 2-BFI and BU224 as used here (20 mg kg−1) increased extrasynaptic levels of dopamine in the striatum by 0.5- and 2.5-fold above baseline, respectively. That this increase in striatal dopamine levels is small compared to the 10-fold increase above baseline that would be achieved by 5 mg kg−1 amphetamine (Lamensdorf et al., 1999) is consistent with the level of rotations achieved with 2-BFI and BU224 being small compared to that produced by amphetamine. Moreover, that BU224 (20 mg kg−1) produced a greater elevation of extracellular striatal dopamine than an identical dose of 2-BFI (Hudson et al., 1999) is also consistent with the presently observed number of ipsiversive rotations (∼131) produced by a similar dose of BU224 (14 mg mg kg−1) being greater than that produced by an identical dose of 2-BFI (∼92). Taken together, these data support a correlation between these two events, elevation of extracellular striatal dopamine and ipsiversive rotational behaviour.
An increase in extracellular striatal dopamine levels could, of course, reflect many things in addition to increasing dopamine release, such as reduced reuptake of dopamine or reduced metabolism via catechol-O-methyl transferase (COMT) or MAO, especially considering that this previously documented rise in striatal dopamine levels was accompanied by a concomitant decrease in the levels of dopamine metabolites, homonovanillic acid and 3,4-dihydroxyphenylacetic acid (Hudson et al., 1999). Since no study has yet shown that the I2-specific ligands can either bind to or inhibit COMT, a contribution from this source is unlikely, although remains possible. In contrast, it is well established, as outlined earlier, that both 2-BFI and BU224 can inhibit MAO-A and MAO-B and the profile of increased dopamine levels and reduced dopamine turnover is indeed similar to that previously reported for inhibitors of MAO (Kato et al., 1986; Burkward et al., 1989; Butcher et al., 1990). However, since neither of the reversible MAO-A or MAO-B inhibitors used in the present study (moclobemide or lazabemide) instigated ipsiversive rotational behaviour in the 6-OHDA lesioned rat when given alone, such enzyme inhibition seems unlikely to underpin the rotational behaviour seen with 2-BFI or BU224 given alone. In further support of this, the MAO inhibitor deprenyl seen here and at this same dose in previous studies to elicit ipsiversive rotations when administered alone in 6-OHDA-lesioned rats (Heikkila et al., 1981), is believed to do so through its ability to enhance dopamine release and/or inhibit dopamine reuptake, as demonstrated in vitro in striatal slices (e.g. Heikkila et al., 1981; Fang & Yu, 1994; Neusch et al., 1997), rather then via MAO inhibition (Finberg & Youdim, 1994). Since the ability of I2-site ligands to interfere with dopamine uptake mechanisms has not yet been investigated, such an action cannot be discounted as potentially contributing to the proposed elevation in striatal extracellular dopamine levels.
Ipsiversive rotations may also be elicited via blockade of presynaptic α2-adrenoceptors on nigrostriatal terminals, leading to facilitation of dopaminergic transmission in the intact hemisphere. Such a mechanism underlies the rotational response to the α2-adrenoceptor antagonist efaroxan, which itself also contains the imidazoline moiety (Chopin et al., 1999). However, such an action is again unlikely to underlie the ipsiversive rotational response of the I2-site ligands since, at least for 2-BFI, the doses tested here have negligible affinity for α2-adrenoceptors in vitro (Nutt et al., 1995) and fail to inhibit α2-adrenoceptor-mediated responses in vivo (Jordan et al., 1996). Thus, while enhanced extracellular striatal dopamine levels remain the most likely mediator of the ipsiversive rotational response of I2-site ligands in the 6-OHDA lesioned rat, the exact cellular mechanisms underlying this enhancement remain to be fully established.
The present study also demonstrated for the first time that coadministration of the specific I2-site ligand 2-BFI with L-DOPA lead to a potentiation of L-DOPA-induced contraversive rotational behaviour in rats bearing a full 6-OHDA lesion of the nigrostriatal tract. L-DOPA instigates contraversive rotational behaviour in the unilateral 6-OHDA-lesioned rat via a well-established mechanism. Thus, while peripheral administration of L-DOPA increases dopamine on both sides of the brain, its action at the supersensitive dopamine receptors within the denervated striatum leads to an exaggerated response in the lesioned hemisphere that culminates in contraversive rotational behaviour. Coadministration of an MAO inhibitor such as deprenyl potentiates the actions of L-DOPA by preventing dopamine breakdown (Heikkila et al., 1981; Prat et al., 2000). The present study confirmed these findings with the reversible MAO-A inhibitor, moclobemide and the reversible MAO-B inhibitor, lazabemide, both of which significantly increased the duration of L-DOPA-induced contraversive rotations, while lazabemide also significantly increased the total number of rotations produced by L-DOPA. While previous studies have shown that another reversible MAO-A inhibitor, Ro41-1049 (N-(2-aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide hydrochloride), also increases the duration of L-DOPA-induced contraversive rotations (Heeringa et al., 1997), these same authors failed to demonstrate a significant effect of lazabemide to alter either the duration or number of L-DOPA-induced rotations (Heeringa et al., 1997). This discrepancy in lazabemide's efficacy may lie with the timing of administration of the peripheral aromatic amino-acid decarboxylase inhibitor, benserazide. In the present study, benserazide was administered 30 minutes before L-DOPA, thereby maximising the quantity of L-DOPA that reaches the brain. In contrast, Heeringa et al. (1997) administered benserazide at the same time as L-DOPA, potentially resulting in more L-DOPA being converted to dopamine in the periphery, thereby providing less chance for L-DOPA conversion to dopamine in the brain.
As previously described, it is well established that I2-binding sites exist on MAO (Alemany et al., 1995; Raddatz et al., 1997; Remaury et al., 2000) and that I2-site ligands including 2-BFI reversibly inhibit MAO-A and MAO-B activity in vitro (Ozaita et al., 1997; Lalies et al., 1999). Therefore, the ability of 2-BFI to inhibit MAO most likely underlies its ability to potentiate both the duration and number of L-DOPA-induced contraversive rotations in the 6-OHDA-lesioned rat. Although it will be important to replicate these findings with other I2 ligands such as BU224, to strengthen this supposition, given the similar potencies of BU224 and 2-BFI to inhibit MAO and their similar profiles of action when given alone to 6-OHDA lesioned rats, it seems reasonable, at this stage, to predict that BU224 might also potentiate the actions of L-DOPA in these animals.
Conclusions
The present study confirms the findings of others that administration of MAO-A and MAO-B inhibitors potentiates L-DOPA-induced contraversive rotational behaviour in the 6-OHDA-lesioned rat and extends these finding to include the I2-specific ligand, 2-BFI. In addition, the study also demonstrates that the I2-site-specific ligands, 2-BFI and BU224, are able to induce ipsiversive rotations when administered alone in the 6-OHDA-lesioned rat. These data suggest that I2-specific ligands may have dual effects in the 6-OHDA-lesioned rat model of PD; an immediate effect associated with an increase in activity in the intact hemisphere, probably via an increase in striatal dopamine levels, and a secondary action which, through the previously documented inhibition of MAO-A and/or MAO-B, increases the availability of dopamine produced by L-DOPA. This pharmacological profile suggests that I2-specific ligands may be worthy of further investigation as alternative adjuncts to L-DOPA in the treatment of PD.
Acknowledgments
We would like to thank Dr Mahmood Iravani for his help with the immunohistochemistry. The gift of selected compounds from Alan Hudson, University of Bristol and Hoffman La Roche, Switzerland is gratefully acknowledged. NM is in receipt of a Merck, Sharp and Dohme fellowship.
Abbreviations
- 2-BFI
2-(-2-benzofuranyl)-2-imidazoline
- BU216
3-[4,5-dihydroimidaz-2-yl]-quinoline hydrochloride
- BU224
2-[4,5-dihydroimidaz-2-yl]-quinoline
- BU226
2-[4,5-dihydroimidaz-2-yl]-isoquinoline hydrochloride
- COMT
catechol-O-methyl transferase
- L-DOPA
L-3,4-dihydroxyphenylamine
- MAO
monoamine oxidase
- 6-OHDA
6-hydroxydopamine
- PBS
phosphate-buffered saline
- PD
Parkinson's disease
- Ro41-1049
N-(2-aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide hydrochloride
- SNc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
References
- ALEMANY R., OLMOS G., GARCIA-SEVILLA J.A. The effects of phenelzine and other monoamine oxidase inhibitor antidepressants on brain and liver I2 imidazoline-preferring receptors. Br. J. Pharmacol. 1995;114:837–845. doi: 10.1111/j.1476-5381.1995.tb13280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AUBIN N., BARNEOUD P., CARTER C., CAILLE D., SONTAG N., MARC C., LOLIVIER J., GARDES A., PERRON C., LE KIM A., CHARIERAS T., BURNIER P., PUECH F., JEGHAM S., GEORGE P., SCATTON B., CURET O. New developments SL25.1131, a new, reversible and mixed inhibitor of MAO-A and MAO-B: biochemical and behavioural profile. J. Pharmacol. Exp. Ther. 2004;310:1171–1182. doi: 10.1124/jpet.103.064782. [DOI] [PubMed] [Google Scholar]
- BARBEAU A. The use of L-DOPA in Parkinson's disease: a 20 year follow up. Trends Pharmacol. Sci. 1981;2:297–301. [Google Scholar]
- BURKWARD W.P., BONETTI E.P., DA PRADA M., MARTIN J.R., POLC P., SCHAFFNER R., SCHERSCHLICT R., HEFTI F., MULLER R.K.M., WYSS P., HAEFELY W.E. Pharmacological profile of moclobemide, a short-acting and reversible inhibitor of monoamine oxidase type A. J. Pharmacol. Exp. Ther. 1989;248:391–399. [PubMed] [Google Scholar]
- BUTCHER S.P., FAIRBROTHER I.S., KELLY J.S., ARBUTHNOTT G.W. Effects of selective monoamine oxidase inhibitors on the in vivo release and metabolism of dopamine in the rat striatum. J. Neurochem. 1990;55:981–988. doi: 10.1111/j.1471-4159.1990.tb04587.x. [DOI] [PubMed] [Google Scholar]
- CHADHA A., DAWSON L.G., JENNER P.G., DUTY S. Effect of unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway on GABA(A) receptor subunit gene expression in the rodent basal ganglia and thalamus. Neuroscience. 2000;95:119–126. doi: 10.1016/s0306-4522(99)00413-3. [DOI] [PubMed] [Google Scholar]
- CHAN S.L.F., BROWN C.A., SCARPELLO K.E., MORGAN N.G. Pancreatic β-cells express an imidazoline binding site that is distinct from I1 and I2 sites. Ann. N. Y. Acad. Sci. 1995;763:153–156. doi: 10.1111/j.1749-6632.1995.tb32400.x. [DOI] [PubMed] [Google Scholar]
- CHOPIN P., COLPAERT F.C., MARIEN M. Effects of alpha-2 adrenoceptor agonists and antagonists on circling behavior in rats with unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway. J. Pharmacol. Exp. Ther. 1999;288:798–804. [PubMed] [Google Scholar]
- DA PRADA M., KETTLER R., KELLER H.H., KYBURZ E., HAEFELY W.E. Ro 19-6327, a novel highly selective and reversible MAO-B inhbitor. Pharmacol. Toxicol. 1987;60 Suppl.1:10. [Google Scholar]
- EGLEN R.M., HUDSON A.L., KENDALL D.A., NUTT D.J., MORGAN N.G., WILSON V.G., DILLON M.P. ‘Seeing through a glass darkly': casting a light on imidazoline ‘I' sites. Trends Pharmacol. Sci. 1998;19:381–390. doi: 10.1016/s0165-6147(98)01244-9. [DOI] [PubMed] [Google Scholar]
- FANG J., YU P.H. Effects of l-deprenyl, its analogues and some monoamine oxidase inhibitors on dopamine uptake. Neuropharmacology. 1994;33:763–768. doi: 10.1016/0028-3908(94)90116-3. [DOI] [PubMed] [Google Scholar]
- FINBERG J.P., YOUDIM M.B. Pharmacological actions of l-deprenyl (selegiline) and other selective monoamine oxidase B inhibitors. Clin. Pharmacol Ther. 1994;56:725–733. doi: 10.1038/clpt.1994.202. [DOI] [PubMed] [Google Scholar]
- GARGALIDIS-MOUDANOS C., PIZZINAT N., JAVOY-AGID F., REMAURY A., PARINI A. I2-imidazoline binding sites and monoamine oxidase activity in human postmortem brain from patients with Parkinson's disease. Neurochem Int. 1997;30:31–36. doi: 10.1016/s0197-0186(96)00035-6. [DOI] [PubMed] [Google Scholar]
- HEERINGA M.J., D'AGOSTINI D., DEBOER P., DAPRADA M., DAMSMA G. Effect of monoamine oxidase A and B and of catechol-O-methyltransferase inhibition on L-DOPA-induced circling behaviour. J. Neural Transm. 1997;104:593–603. doi: 10.1007/BF01291878. [DOI] [PubMed] [Google Scholar]
- HEFTI F., MELAMED E., SAHAKIAN B.J., WURTMAN R.J. Circling behaviour in rats with partial, unilateral nigrostriatal lesions: effect of amphetamine, apomorphine and DOPA. Pharmacol. Biochem. Behav. 1980;12:185–188. doi: 10.1016/0091-3057(80)90353-6. [DOI] [PubMed] [Google Scholar]
- HEIKKILA R.E., CABBAT F.S., MANZINO L., DUVOISIN R.C. Potentiation by deprenil of 1-dopa induced circling in nigral-lesioned rats. Pharmacol. Biochem. Behav. 1981;15:75–79. doi: 10.1016/0091-3057(81)90342-7. [DOI] [PubMed] [Google Scholar]
- HUDSON A.L., GOUGH R.E., TYACKE R.J., LIONE L., LALIES M., LEWIS J., HUSBANDS S., KNIGHT P., MURRAY F., HUTSON P., NUTT D.J. Novel selective compounds for the investigation of imidazoline receptors. Ann N. Y. Acad. Sci. 1999;881:81–91. doi: 10.1111/j.1749-6632.1999.tb09344.x. [DOI] [PubMed] [Google Scholar]
- IRAVANI M.M., KASHEFI K., MANDER P., ROSE S., JENNER P. Involvement of inducible nitric oxide synthase in inflammation-induced dopaminergic neurodegeneration. Neuroscience. 2002;110:49–58. doi: 10.1016/s0306-4522(01)00562-0. [DOI] [PubMed] [Google Scholar]
- JANKOVIC J. Parkinson's disease therapy: tailoring choices for early and late disease, young and old patients. Clin. Neuropharmacol. 2000;23:252–261. doi: 10.1097/00002826-200009000-00003. [DOI] [PubMed] [Google Scholar]
- JORDAN S., JACKSON H.C., NUTT D.J., HANDLEY S.L. Discriminative stimulus produced by the imidazoline I2 site ligand, 2-BFI. J. Psychopharmacol. 1996;10:273–278. doi: 10.1177/026988119601000403. [DOI] [PubMed] [Google Scholar]
- KATO T., DONG B., ISHII K., KINENUCHI H. Brain dialysis in vivo metabolism of dopamine and serotonin by monoamine oxidase A but not B in the striatum of unrestrained rats. J. Neurochem. 1986;46:1277–1282. doi: 10.1111/j.1471-4159.1986.tb00650.x. [DOI] [PubMed] [Google Scholar]
- LALIES M.D., HIBELL A., HUDSON AL NUTT D.J. Inhibition of central monoamine oxidase by imidazoline2 site selective ligands. Ann. N. Y. Acad. Sci. 1999;881:114–117. doi: 10.1111/j.1749-6632.1999.tb09350.x. [DOI] [PubMed] [Google Scholar]
- LAMENSDORF I., PORAT S., SIMANTOV R., FINBERG J.P.M. Effect of low-dose treatment with selegiline on dopamine transporter (DAT) expression and amphetamine-induced dopamine release in vivo. Br. J. Pharmacol. 1999;126:997–1002. doi: 10.1038/sj.bjp.0702389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LE WITT P.A., NYHOLM D. New developments in levodopa therapy. Neurology. 2004;62:S9–S16. doi: 10.1212/wnl.62.1_suppl_1.s9. [DOI] [PubMed] [Google Scholar]
- LIONE L.A., NUTT D.J., HUDSON A.L. Characterisation and localisation of [3H]2-(2-benzofuranyl)-2-imidazoline binding in the rat brain: a selective ligand for imidazoline I2 receptors. Eur. J. Pharmacol. 1998;353:123–135. doi: 10.1016/s0014-2999(98)00389-6. [DOI] [PubMed] [Google Scholar]
- MACINNES N., HANDLEY S.L. Region-dependent effects of acute and chronic tranylcypromine in vivo on [3H]2-BFI binding to brain imidazoline I2 sites. Eur. J. Pharmacol. 2001;428:221–225. doi: 10.1016/s0014-2999(01)01259-6. [DOI] [PubMed] [Google Scholar]
- MACINNES N., HANDLEY S.L. Characterisation of the discriminable stimulus produced by 2-BFI: effects of imidazoline I2-site ligands, MAOIs, β-carbolines, agmatine and ibogaine. Br. J. Pharmacol. 2002;135:1227–1234. doi: 10.1038/sj.bjp.0704579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICHEL M.C., ERNSBERGER P. Keeping an eye on the I site: Imidazoline-preferring receptors. Trends Pharmacol. Sci. 1992;13:270–369. [PubMed] [Google Scholar]
- MURRAY T.K., MESSENGER M.J., WARD M.A., WOODHOUSE S., OSBORNE D.J., DUTY S., O'NEILL M.J. Evaluation of the mGluR2/3 agonist LY379268 in rodent models of Parkinson's disease. Pharmacol. Biochem. Behav. 2002;73:455–466. doi: 10.1016/s0091-3057(02)00842-0. [DOI] [PubMed] [Google Scholar]
- NEUSCH C., SCHNIERLE S., MOSER A. Selegiline induces dopamine release through ATP-sensitive potassium channels in the rat caudate-putamen in vitro. Neurochem. Int. 1997;31:307–311. doi: 10.1016/s0197-0186(96)00132-5. [DOI] [PubMed] [Google Scholar]
- NUTT D.J., FRENCH N., HANDLEY S.L., HUDSON A., HUSBANDS S., JACKSON H., JORDAN S., LALIES M.D., LEWIS J., LIONE L.A., MALLARD N., PRATT J. Functional studies of specific imidazoline-2 receptor ligands. Ann. N. Y. Acad. Sci. 1995;763:125–139. doi: 10.1111/j.1749-6632.1995.tb32397.x. [DOI] [PubMed] [Google Scholar]
- NUTT J.G. Levodopa-induced dyskinesia: review, observations and speculation. Neurology. 1990;40:340–345. doi: 10.1212/wnl.40.2.340. [DOI] [PubMed] [Google Scholar]
- O'NEILL M.J., MURRAY T.K., WHALLEY K., WARD M.A., HICKS C.A., OSBORNE D.J., SKOLNICK P. Neurotrophic actions of the novel AMPA receptor potentiator, LY404187, in rodent models of Parkinson's disease. Eur. J. Pharmacol. 2004;486:163–174. doi: 10.1016/j.ejphar.2003.12.023. [DOI] [PubMed] [Google Scholar]
- OZAITA A., OLMOS G., BORONAT M.A., LIZCANO J.M., UNZETA M., GARCIA-SEVILLA J.A. Inhibition of monoamine oxidase A and B activities by imidazol(ine)/guanidine drugs, nature of the interaction and distinction from I2-imidazoline receptors in rat liver. Br. J. Pharmacol. 1997;121:901–912. doi: 10.1038/sj.bjp.0701214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study Group A controlled trial of lazabemide (Ro 19-6327) in levodopa-treated Parkinson's disease. Arch. Neurol. 1994;51:342–347. doi: 10.1001/archneur.1994.00540160036006. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group Effect of lazabemide on the progression of disability in early Parkinson's disease. Ann. Neurol. 1996;40:99–107. doi: 10.1002/ana.410400116. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group A controlled, randomized, delayed-start study of rasagiline in early Parkinson disease. Arch Neurol. 2004;61:561–566. doi: 10.1001/archneur.61.4.561. [DOI] [PubMed] [Google Scholar]
- PAXINOS G., WATSON C. The Rat Brain in Stereotaxic Co-ordinates. UK: Academic Press; 1998. [Google Scholar]
- PRAT G., PEREZ V., RUBI A., CASAS M., UNZETA M. The novel type B MAO inhibitor PF9601N enhances the duration of L-DOPA-induced contralateral turning in 6-hydroxydopamine lesioned rats. J. Neural Transm. 2000;107:409–417. doi: 10.1007/s007020070083. [DOI] [PubMed] [Google Scholar]
- PYCOCK C.J. Turning behaviour in animals. Neuroscience. 1980;5:461–514. doi: 10.1016/0306-4522(80)90048-2. [DOI] [PubMed] [Google Scholar]
- RADDATZ R., PARINI A., LANIER S.M. Localization of the imidazoline binding domain on monoamine oxidase B. Mol. Pharmacol. 1997;52:549–553. doi: 10.1124/mol.52.4.549. [DOI] [PubMed] [Google Scholar]
- REMAURY A., RADDATZ R., ORDENER C., SAVIC S., SHIH J.C., CHE K., SEIF I., DE MAEYER E., LANIER S.M., PARINI A. Analysis of the pharmacological and molecular heterogeneity of I2-imidazoline-binding proteins using monoamine oxidase-deficient mouse models. Mol. Pharmacol. 2000;58:1085–1090. doi: 10.1124/mol.58.5.1085. [DOI] [PubMed] [Google Scholar]
- REYNOLDS G.P., BOULTON R.M., PEARSON S.J., HUDSON A.L., NUTT D.J. Imidazoline binding sites in Huntington's and Parkinson's disease putamen. Eur J Pharmacol. 1996;301:R19–R21. doi: 10.1016/0014-2999(96)00196-3. [DOI] [PubMed] [Google Scholar]
- ROBINSON E.S., TYACKE R.J., NUTT D.J., HUDSON A.L. Distribution of [(3)H]BU224, a selective imidazoline I(2) binding site ligand, in rat brain. Eur. J. Pharmacol. 2002;450:55–60. doi: 10.1016/s0014-2999(02)02076-9. [DOI] [PubMed] [Google Scholar]
- SASTRE-COLL A., ESTEBAN S., MIRALLES A., ZANETTI R., GARCIA-SEVILLA J.A. The imidazoline receptor ligand 2-(2-benzofuranyl)-2-imidazoline is a dopamine releasing agent in the rat striatum in vivo. Neurosci. Lett. 2001;301:29–32. doi: 10.1016/s0304-3940(01)01599-3. [DOI] [PubMed] [Google Scholar]
- STOCCHI F., NORDERA G., MARSDEN C.D. Strategies for treating patients with advanced Parkinson's disease with disastrous fluctuations and dyskinesias. Clin. Neuropharmacol. 1997;20:95–115. doi: 10.1097/00002826-199704000-00001. [DOI] [PubMed] [Google Scholar]
- UNGERSTEDT U. Postsynaptic supersensitivity after 6-hydroxy-dopamine induced degeneration of the nigro-striatal dopamine system. Acta. Physiol. Scand., Suppl. 1971;367:69–93. doi: 10.1111/j.1365-201x.1971.tb11000.x. [DOI] [PubMed] [Google Scholar]