Abstract
This work was aimed to determine if 1,5-bis(4-allyldimethylammoniumphenyl)pentan-3-one dibromide (BW284c51), the most selective acetylcholinesterase inhibitor (AchEI), affects the nicotinic acetylcholine (Ach) receptor (AchR) function.
Purified Torpedo nicotinic AchRs were injected into Xenopus laevis oocytes and BW284c51 effects on Ach- and carbamylcholine (Cch)-elicited currents were assessed using the voltage-clamp technique.
BW284c51 (up to 1 mM) did not evoke any change in the oocyte membrane conductance. When BW284c51 (10 pM–100 μM) and Ach were coapplied, Ach-evoked currents (IAch) were reversibly inhibited in a concentration-dependent manner (Hill coefficient, 1; IC50, 0.2–0.5 μM for 0.1–1000 μM Ach). Cch-elicited currents showed a similar inhibition by BW284c51.
IAch blockade by BW284c51 showed a strong voltage dependence, being only apparent at hyperpolarising potentials. BW284c51 also enhanced IAch desensitisation.
BW284c51 changed the Ach concentration-dependence curve of Torpedo AchR response from two-site to single-site kinetics, without noticeably affecting the EC50 value.
The BW284c51 blocking effect was highly selective for nicotinic over muscarinic receptors. BW284c51 inhibition potency was stronger than that of tacrine, and similar to that of d-tubocurarine (d-TC). Coapplication of BW284c51 with either tacrine or d-TC revealed synergistic inhibitory effects.
Our results indicate that BW284c51 antagonises nicotinic AchRs in a noncompetitive way by blocking the receptor channel, and possibly by other, yet unknown, mechanisms.
Therefore, besides acting as a selective AchEI, BW284c51 constitutes a powerful and reversible blocker of nicotinic AchRs that might be used as a valuable tool for understanding their function.
Keywords: BW284c51, nicotinic acetylcholine receptors, Xenopus oocytes, cholinesterase inhibitors, acetylcholine, carbamylcholine, Torpedo marmorata
Introduction
Nicotinic acetylcholine receptors (AchRs) are widespread ligand-gated ion channels that mediate fast cholinergic transmission at both the peripheral and central nervous systems. Thus, in addition to their involvement in neuromuscular and autonomic ganglia synaptic transmission, they play an important role in cognitive and addictive processes (Wonnacott, 1997; Lena & Changeux, 1998; Clementi et al., 2000). Furthermore, their dysfunction has been linked to a number of human diseases, including congenital myasthenia, schizophrenia, familial epilepsy and neurodegenerative disorders such as Alzheimer's and Parkinson's diseases (Lena & Changeux, 1998; Hogg et al., 2003). A special attention is currently given to numerous compounds that are able to modulate AchR function through their interaction with allosteric sites in the receptor molecule (Clapham & Neher, 1984; García-Colunga & Miledi, 1996; Pereira et al., 2002). At present, acetylcholinesterase (AchE) inhibitors (AchEIs) constitute, in fact, the preferred strategy for Alzheimer's treatment (Small, 2004), not only because of their reinforcing action on cholinergic transmission through AchE inhibition but also due to their modulatory effects on nicotinic AchRs, including allosteric potentiation (Pereira et al., 2002). Actually, distinct AchEIs can evoke different effects on nicotinic AchRs depending on their chemical nature and on receptor subunit composition (Zwart et al., 2000). Tacrine (Zwart et al., 2000), physostigmine (Van den Beukel et al., 1998; Zwart et al., 2000) neogstigmine (Nagata et al., 1997; Smulders et al., 2003) and pyridostigmine (Bradley et al., 1986) can alternatively block or potentiate the activity of nicotinic AchRs, depending on the concentrations used and the biological sample tested. The actions of quaternary ammonium AchEIs on nicotinic Ach currents (IAch) have also been extensively studied. Clinical concentrations of edrophonium reduced single channel IAch amplitude in BC3H1 cells (Wachtel, 1990) and in mouse muscle receptors expressed in Xenopus oocytes (Yost & Maestrone, 1994). Decamethonium, a bisquaternary compound, acts as a partial agonist of muscle nicotinic AchRs (del Castillo & Katz, 1957; Adams & Sakmann, 1978; Aoshima, 1990; Bertrand et al., 1992; Liu & Dilger, 1993), and it can also antagonise α7 responses (Bertrand et al., 1992) or block open endplate channels (Adams & Sakmann, 1978). Other bisquaternary AchEIs such as hexamethonium and dodecamethonium block both neuronal and muscle nicotinic responses (Bertrand et al., 1992; Lummis et al., 1992).
Over the years, 1,5-bis(4-allyldimethylammoniumphenyl)pentan-3-one dibromide, BW284c51, a bisquaternary ammonium AchEI, whose chemical structure is shown in Figure 1a, has been used to ascertain the tissue localisation of the AchE, despite the significant structural and functional homology found between the different vertebrate cholinesterases (Koelle, 1963; Mikalsen et al., 1986, Radic et al., 1993; Dupree & Bigbee, 1994). Additionally, its high specificity allows it to discriminate between AchEs from different species, since BW284c51 binding depends on the presence of specific amino-acid residues in the catalytic and peripheral sites of the enzyme (Radic et al., 1993; Eichler et al., 1994). Even though no actions have been described for BW284c5s besides those related to AchE inhibition, there are significant structural and functional similarities between this compound and other cholinesterase inhibitors (ChEIs) that behave as nicotinic AchR modulators, especially those with quaternary ammonium groups. Therefore, this work was addressed to determine whether BW284c51 has any action on nicotinic AchR function and, should this be the case, to unravel the mechanisms underlying such interaction. With this purpose, we transplanted Torpedo marmorata nicotinic AchRs, reconstituted in asolectin lipid vesicles, to Xenopus oocytes (Morales et al., 1995). This procedure allows us to study the behaviour of native nicotinic AchRs incorporated into the oocyte membrane, overcoming any post-translational modification that could occur when heterologous nicotinic AchRs are expressed from exogenous mRNA in oocytes (Buller & White, 1990; Sivilotti et al., 1997). The effect of BW284c51 on the function of nicotinic AchR was assessed using the voltage-clamp technique, which provides a detailed functional assay of receptor activity and allows quantitative pharmacological studies.
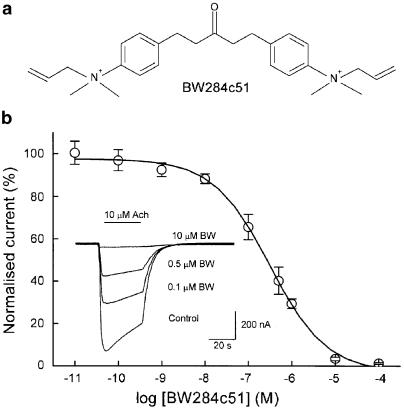
Figure 1.
Effects of BW284c51 on Ach-induced currents (IAch). (a) Chemical structure of BW284c51. (b) Relationship between BW284c51 dose and IAch peak amplitude in Xenopus oocytes injected with purified T. marmorata nicotinic AchRs. Repeated IAch were evoked by 32 s pulses of 10 μM Ach, either alone (control) or coapplied with increasing concentrations of BW284c51 (BW). The interval between applications was of 10 min. Values obtained for each BW284c51 concentration were normalised as the percentage of the maximum IAch response, and data represent the mean±s.e.m. corresponding to five oocytes from three donors. The solid line represents a simple sigmoidal curve that fits to the data with a Hill coefficient close to 1. The inset shows representative, superimposed IAch elicited at a holding potential of −60 mV by Ach alone (control) or coapplied with the indicated BW284c51 concentrations to the same oocyte. In this and following figures, downward deflections denote inward currents and the horizontal bars indicate the time of drug application.
Methods
Purification and reconstitution of nicotinic AchRs
The methodology employed has been described previously (Morales et al., 1995; Ivorra et al., 2002). Briefly, membranes from the electric organ of T. marmorata were solubilised in cholate and nicotinic AchRs isolated by bromoacetylcholine-affinity chromatography carried out in the presence of asolectin lipids. After elution with carbamylcholine (Cch), purified receptors were dialysed and reconstituted in asolectin vesicles at a final protein concentration of 0.3–1.2 mg ml−1. Aliquoted samples were stored in liquid nitrogen until use.
Oocyte preparation and microinjection
Adult Xenopus laevis (purchased from Blades Biological, U.K.) were anaesthetised by immersion in 0.17% MS-222 for 15 min and a piece of ovary was aseptically removed, thereafter allowing the recovery of the toad. Fully grown immature oocytes were isolated from the ovary and their surrounding layers removed either manually or by collagenase treatment, as described previously (Ivorra & Morales, 1997). Cells were kept at 15–16°C in a modified Barth's solution (88 mM NaCl, 1 mM KCl, 2.40 mM NaHCO3, 0.33 mM Ca(NO3)2, 0.41 mM CaCl2, 0.82 mM MgSO4, 10.00 mM HEPES, pH 7.4) supplemented with penicillin (100 U ml−1) and streptomycin (0.1 mg ml−1) until used for electrophysiological recordings. Oocytes were microinjected with 100 nl of an aliquot of reconstituted nicotinic AchRs, after being thawed on ice and rehomogenised before injection (Morales et al., 1995).
Electrophysiological recordings
Membrane current recordings were performed at 21–25°C, 16–72 h after injection, using a high-compliance two-microelectrode voltage-clamp system (TurboTEC-10CD, npi). The recording methodology has been described previously (Morales et al., 1995). Briefly, intracellular electrodes (0.8–3 MΩ) were filled either with 3 M KCl or potassium acetate for voltage recording and current injection, respectively. Oocytes were placed in a 150 μl recording chamber that was continuously superfused with normal frog Ringer's solution (NR, 115 mM NaCl, 2 mM KCl, 1 mM CaCl2, 5 mM HEPES, pH 7.0) supplemented with 0.5 μM atropine sulphate (normal Ringer with atropine, ANR) to block eventual muscarinic responses (Kusano et al., 1982). The membrane potential was held at −60 mV, unless otherwise stated. Ach, Cch and other tested drugs were diluted in ANR solution and applied for 32 s while superfusing the oocyte at a flow rate of 7–15 ml min−1. Membrane currents were low-pass filtered at 30–1000 Hz and recorded on both a chart recorder (Kipp & Zonen BD-112) and a PC computer, after sampling (Digidata 12 bits, 4096 points per record) at five times the filter frequency using the WCP v. 3.2.8 package developed by J. Dempster (Strathclyde Electrophysiology Software, University of Strathclyde, U.K.). Current–voltage relationships (i–v curves) were obtained by giving 800 ms voltage pulses from −120 to +60 mV in 20 mV steps) to the oocyte while superfusing it with Ach alone or coapplied with BW284c51. Concentration–response curves were obtained by exposing injected oocytes to increasing Ach concentrations, alone or together with the indicated drugs. In order to reduce nicotinic AchR desensitisation, the interval between two consecutive Ach applications was of, at least, 7 min.
Data analysis
For receptor activation, dose–response data were fitted to Hill's equation as described by the ratio I/Imax=[1+(EC50/[Ach])n]−1, where I is the peak IAch elicited at a given Ach concentration, Imax the maximum current recorded, EC50 is the concentration of agonist required to obtain one-half of the maximum current and n the Hill coefficient (nH). Inhibition curves were determined by measuring IAch in the presence of different BW284c51 concentrations. Each value given corresponds to the average of, at least, two consecutive measurements obtained under the same conditions. Data were fitted to a single-site inhibition curve using the Origin 6.1 software (OriginLab Corp., Northampton, MA, U.S.A.). For graphical representations, the values of the dose–response curves were normalised as the percentage of the largest induced current for each oocyte. Unless otherwise specified, values given are the mean±s.e.m. When comparing two-group means of normally distributed data, the Student's t-test was used. Otherwise, the Mann–Whitney rank sum test was applied. Among groups differences were determined by the Kruskal–Wallis analysis of variance on ranks, and the comparison of groups by using the Dunn's test. A significance level of P<0.05 was considered for all cases.
Drugs
Ach, atropine sulphate, BW284c51, Cch, MS-222, penicillin, streptomycin, tacrine sulphate and d-tubocurarine (d-TC) chloride were purchased from Sigma (St Louis, MO, U.S.A.). HEPES was obtained from Acros Organics (New Jersey, NJ, U.S.A.). Reagents of general use were purchased from Scharlau Chemie SA (Barcelona, Spain). Unless otherwise stated, all drugs used were dissolved from stock solutions in ANR just before each application. BW284c51-containing solutions were protected from light at all times.
Results
BW284c51 inhibition of nicotinic currents
In both oocytes bearing nicotinic AchRs and uninjected cells, with the membrane potential being held at −60 mV, BW284c51 (0.1 nM–1 mM) superfusion did not appreciably modify the cell membrane conductance. However, when it qwas coapplied with Ach, the amplitude of the elicited IAch was reduced and the current decay pattern became significantly altered. Figure 1b shows the blocking effect of different BW284c51 concentrations on the IAch elicited by 10 μM Ach. The normalised dose-inhibition curve obtained could be fitted to a sigmoidal function with an estimated IC50 of 0.5 μM (pIC50 6.3±0.1) and an nH value of 0.7, suggesting that BW284c51 exerts its inhibitory action through its binding to the nicotinic AchR in a molecular ratio of 1 : 1.
The effect of BW284c51 on IAch desensitisation was determined by measuring, for each oocyte, the IAch amplitude elicited by 10 or 100 μM Ach either alone or coapplied with 0.5 μM BW284c51, at different times after the current peak. As previously reported (Morales et al., 1995), under control conditions (Ach alone), IAch displayed a slow desensitisation at low agonist concentrations (10 μM), whereas at higher Ach doses (100 μM) IAch showed a fast peak followed by a plateau. The presence of BW284c51 markedly increased IAch desensitisation, especially at the higher Ach concentrations (Table 1), and decreased the time between the beginning of the response and the current peak (Table 1). Interestingly, the fast desensitisation phase of the IAch was more pronouncedly affected by BW284c51 than the slow one (Table 1; compare the desensitisation values at 2 and 20 s for currents elicited by Ach alone or coapplied with BW284c51), although both components were significantly speeded up for 100 μM Ach.
Table 1.
BW284c51 effects on IAch time to peak and desensitisation
| Test | Time to peak (s) | Desensitisation (%) | ||
|---|---|---|---|---|
| 2 s | 10 s | 20s | ||
| 10 μM Ach (n=34, 7) | 7.1±0.5 | 3±1 | 14±3 | 25±3 |
| 10 μM Ach+0.5 μM BW284c51 (n=34, 7) | 5.2±0.5** | 5±1** | 17±2* | 23±2 |
| 100 μM Ach (n=67, 13) | 3.4±0.2 | 14±2 | 49±2 | 67±2 |
| 100 μM Ach+0.5 μM BW284c51 (n=67, 13) | 2.0±0.1** | 44±2** | 75±1** | 83±1** |
| 100 μM Cch (n=21, 6) | 9.3±1.1 | 2±1 | 12±2 | 30±4 |
| 100 μM Cch+0.5 μM BW284c51 (n=21, 6) | 9.4±1.2 | 4±1* | 11±2 | 26±5 |
| 1000 μM Cch (n=13, 2) | 3.4±0.2 | 16±2 | 55±4 | 72±3 |
| 1000 μM Cch+0.5 μM BW284c51 (n=13, 2) | 2.6±0.2** | 33±5** | 60±5 | 71±6 |
Data show desensitisation values for either Ach (10 and 100 μM) or Cch (100 and 1000 μM) currents, elicited by each agonist alone or coapplied with 0.5 μM BW284c51. The time to IAch peak data is the time between the current onset and its maximal value. Desensitisation values were obtained using the equation: Dti=100−((Iti/Ipeak) * 100), where Dti is the desensitisation value at the specified time, Ipeak the peak current amplitude, and Iti the current amplitudes remaining at 2, 10 and 20 s after the peak. Note that desensitisation increased with concentration for both Ach- and Cch-elicited responses, and that BW284c51 mainly enhanced the fast desensitisation component (measured as the 2 s desensitisation), although the slow one (measured as the 20 s desensitisation) was also increased for currents elicited by 100 μM ACh. The numbers of cells tested and donors used for each group are given within parentheses. Values from control and the corresponding BW284c51 groups were compared (*P<0.05; **P<0.005).
Although the presence of 0.5 μM atropine in the bathing solution, to block any muscarinic response, has no effect on muscle type nicotinic AchRs (Miledi & Sumikawa, 1982), it might contribute to the observed effects of BW284c51 on IAch. To rule out this possibility, we recorded the IAch elicited by 100 μM Ach (in a Ringer without atropine added) either alone or coapplied with 0.5 μM BW284c51 in five oocytes of a donor lacking muscarinic receptors in their membrane. In these experiments, BW284c51 showed similar blocking effects on AchRs than those observed in the presence of atropine, indicating that BW284c51, by itself, was responsible for IAch blockade.
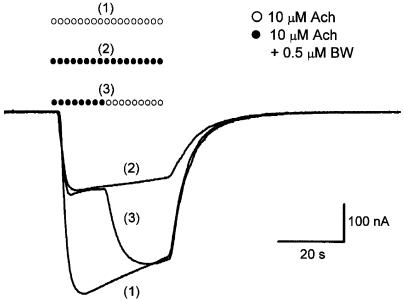
The BW284c51 inhibitory effect on IAch was immediately and completely reversed after BW284c51 removal as shown in Figure 2. Furthermore, the IAch elicited by Ach after its coapplication with BW284c51 (up to 10 μM) showed the same amplitude and time-course kinetics as the initial control response (Figure 2) for any of the Ach concentrations tested, thus indicating the lack of any residual effect of BW284c51 on nicotinic AchRs.
Figure 2.
Fast and reversible IAch blockade by BW284c51. IAch records elicited by 10 μM Ach alone (1) or coapplied with 0.5 μM BW284c51 (2) in the same oocyte. Mixed symbols (3) indicate substitution of the Ringer's solution containing Ach plus BW284c51 by another with Ach alone. Note that once the washout had started, the IAch quickly reached the control amplitude.
Noncompetitive antagonism of BW284c51
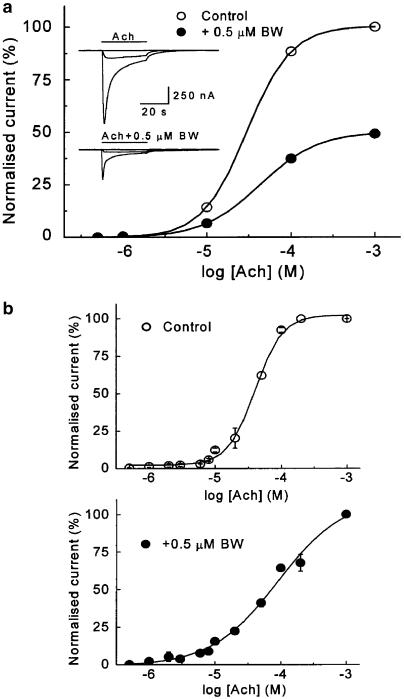
To better characterise BW284c51 effects on IAch, dose–response curves were obtained in the absence and presence of 0.5 μM BW284c51. As shown in Figure 3, bath applications of 0.5–1000 μM Ach to oocytes injected with nicotinic AchRs elicited responses similar to those previously described for these receptors (Morales et al., 1995). The relationship obtained was well fitted to a two-site nonlinear Hill equation with a value of nH of 2.0±0.3 and an EC50 of 41 μM (n=7, four donors).
Figure 3.
Ach dose–IAch relationship for currents evoked by Ach alone or together with BW284c51 in oocytes bearing nicotinic AchRs. (a) The plot shows Ach dose–IAch relationships in an oocyte challenged with increasing Ach concentrations, applied alone or together with 0.5 μM BW284c51. Ach pulses were given for 32 s every 5–30 min, depending on concentrations, to ensure complete recovery between trials. The inset shows superimposed recordings obtained by applying to the same cell, at −60 mV, 0.5, 10 or 1000 μM Ach, either alone (upper records) or together with 0.5 μM BW284c51 (lower records). Note that in the presence of BW284c51, the values are roughly half of those obtained with Ach alone. (b) Graphs were obtained by averaging the IAch from seven oocytes (four donors) covering the total range of Ach concentrations tested. IAch was elicited by either Ach alone (upper graph) or coapplied with BW284c51 (lower graph). The dose–response curve for currents elicited by Ach alone best fitted to a two-site Hill equation, whereas in the presence of BW284c51, the best fit corresponded to a single-site equation.
When the same Ach concentrations were coapplied with BW284c51 at a 0.5 μM dose (approximately its IC50), IAch was reduced by about 50%, as compared to values obtained in the absence of the inhibitor, independent of the agonist concentration used (48±1%, 34 cells from seven donors and 49±1, 67 cells of 13 donors, for 10 and 100 μM Ach, respectively; P>0.457, t-test; Figure 3a), thus indicating a noncompetitive action of BW283c51 in the tested range of Ach concentrations. The concentration–response curve in the presence of BW284c51 was fitted to a simple sigmoidal curve with a slope corresponding to an nH value of 1.0±0.2 and an EC50 for Ach of 55 μM (n=9, six donors), this suggesting that the affinity of the nicotinic AchRs towards its agonist was barely affected by the presence of BW284c51.
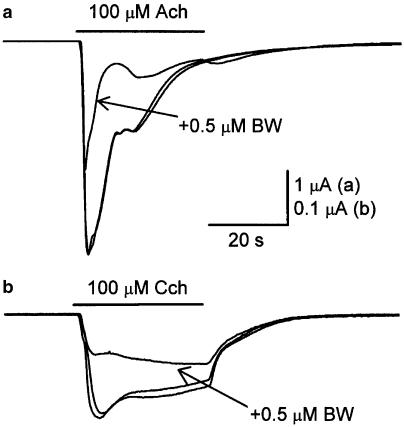
Since Xenopus oocytes bear intrinsic AchE activity (Soreq et al., 1982; Gundersen & Miledi, 1983), we set to address whether the BW284c51 effects on nicotinic currents were somehow associated to its activity as AchEI. With this purpose, we used Cch instead of Ach as the agonist of nicotinic AchRs, since Cch is not hydrolysable by AchE (Sung et al., 1998). This approach also allowed us to test whether a possible interaction between BW284c51 and Ach occurred when both compounds were coapplied. As shown in Figure 4b, superfusion of an injected oocyte with Cch (100 μM) evoked nicotinic currents of smaller amplitude and slower desensitisation than those elicited, in the same cell, by an identical Ach concentration (Figure 4a; Table 1). However, BW284c51 effects on Cch currents were comparable in their potency to those evoked on IAch, since 0.5 μM BW284c51 when coapplied with 100 or 1000 μM Cch evoked currents that were reduced to 49±2% (n=21, six donors) and 46±1% (n=13, two donors), respectively, of the values obtained at the same concentrations of Cch alone (Figure 4b). These values were neither significantly different between them (P>0.05, Mann–Whitney rank sum test) nor from those obtained when Ach was used as the agonist (P>0.05, Kruskal–Wallis analysis of variance on ranks). As shown in Table 1, BW284c51 also increased the rate of desensitisation of Cch currents, but in contrast to Ach currents, only the fast desensitisation component (2 s desensitisation) was significantly affected.
Figure 4.
BW284c51 effects on Ach- and Cch-elicited currents. Recordings show nicotinic currents elicited in the same oocyte by 100 μM Ach either alone (pre- and post-control responses) or together with 0.5 μM BW284c51 (a), and by 100 μM Cch either alone (pre- and post-control responses) or coapplied with 0.5 μM BW284c51 (b). Note that as for IAch, BW284c51 blocked about half of the Cch-induced current. In both cases, the blockade was fully reversible upon removal of BW284c51.
Comparison of the blocking effects of BW284c51 with those of other antagonists
BW284c51 actions on nicotinic AchR receptors were compared to those mediated by other compounds with well-known inhibitory actions. Tested drugs were tacrine, a powerful ChEI of clinical use (Cantí et al., 1998), and d-TC (Jenkinson, 1960; O'Leary et al., 1994), both potent antagonists of muscle and Torpedo nicotinic IAch. For comparative purposes, all antagonists were used at the same concentration, together with 100 μM Ach. Similar results were obtained when applying the selected drugs in different oocytes and sequentially in the same cell, although the latter approach was preferred to decrease deviations due to differences between oocytes, even from the same donor. However, repeated applications of agonist for long periods usually caused a slow, but progressive, decrease in the current amplitude, likely due to receptor desensitisation. To minimise the possible errors derived from this unwanted effect in the quantitation of the inhibitory potency of the drugs on the IAch, the degree of the inhibition elicited by each tested compound was expressed as the percentage of the response obtained in presence of the antagonist with respect to that evoked by Ach alone in the previous and subsequent trials.
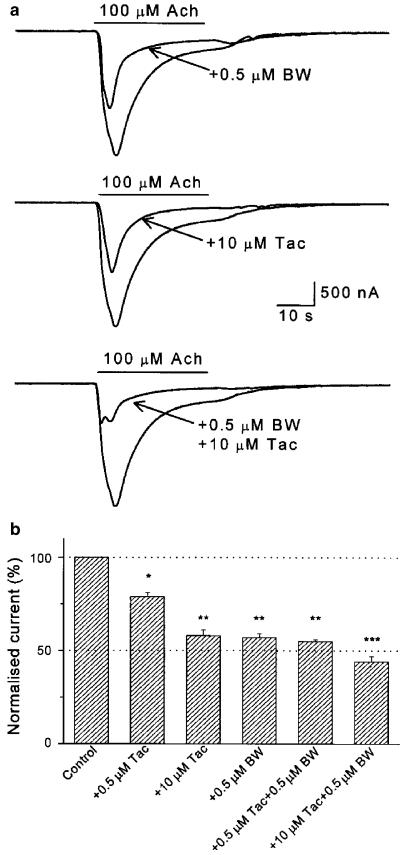
Tacrine at 0.5 μM had a weak blocking effect on IAch, whose amplitude fell to 82±5% (n=7, from three donors; P<0.005) of the control values (see histogram of Figure 5b). The IAch inhibition promoted by 0.5 μM tacrine was much smaller than that obtained in the same cells with BW284c51 at the same concentration (Figure 5b). When both drugs were coapplied at 0.5 μM, the IAch inhibition was similar to that found with BW284c51 alone (Figure 5b). The IC50 for tacrine was close to 10 μM (see Figure 5a middle panel, and Figure 5b). When tacrine and BW284c51 were coapplied at concentrations close to their IC50 values (10 and 0.5 μM, respectively), the IAch was significantly smaller than that obtained with any of these blockers alone (P<0.005; Figure 5a and b). Although these results indicate that tacrine and BW284c51 exerted an additive blocking effect on IAch, the resulting inhibition was smaller than the sum of their individual blocking effects.
Figure 5.
Additive blockade of IAch by BW284c51 and tacrine. (a) Representative currents evoked in an oocyte upon superfusion either with 100 μM Ach alone or together with 0.5 μM BW285c51 (upper records), 10 μM tacrine (Tac; middle records) or both inhibitors simultaneously (lower panel). As shown, the blockade caused by individual application of these drugs was comparable, but their coapplication resulted in an increased inhibition. (b) Column graph showing the inhibitory effects of BW284c51 and/or tacrine on IAch. The height of the bars indicates the fraction of the control IAch remaining after the application of Ach with the indicated drugs. Data are the mean±s.e.m. (five oocytes from two donors). Different number of asterisks atop the bars indicate significant differences between two groups (P<0.05, as determined by the analysis of variance on ranks).
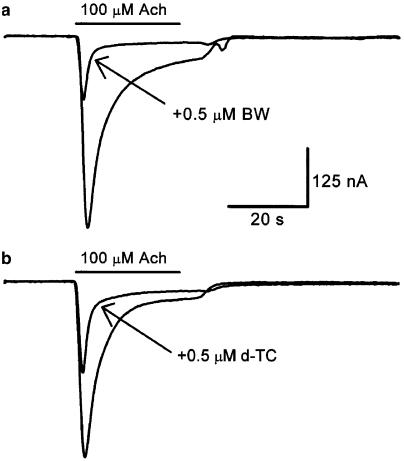
The inhibitory effect of 0.5 μM BW284c51 on IAch was also compared to that of d-TC, used at the same concentration. As shown in Figure 6, the IAch blockade elicited by BW284c51 (to 49±1% of the control IAch value; n=67, 15 donors) was comparable to that evoked by d-TC (to 53±5% of the control IAch, n=10, four donors; P=0.083), when both drugs were separately coapplied with 100 μM Ach. As above, experiments were carried out in oocytes from the same donor injected with a common sample of reconstituted receptors. Both drugs potentiated each other's inhibitory effects on IAch and their synergistic effects were stronger than those obtained by BW284c51 and tacrine (not shown). Interestingly, although BW284c51 and d-TC exerted a fast antagonistic action on IAch, the BW284c51 blockade was quickly and completely removed after this drug was washed out (Figure 2), whereas the recovery from the d-TC block in the same cells (n=10, four donors) was much slower (not shown).
Figure 6.
BW284c51 and d-TC exhibit similar antagonist potencies on IAch. Records obtained in the same oocyte by superfusing the cell with 100 μM Ach, either alone or together with 0.5 μM BW284c51 (a) or 0.5 μM d-TC (b). Note that both drugs had similar inhibiting effects on IAch.
Selectivity of BW284c51 effects on nicotinic over muscarinic AchRs
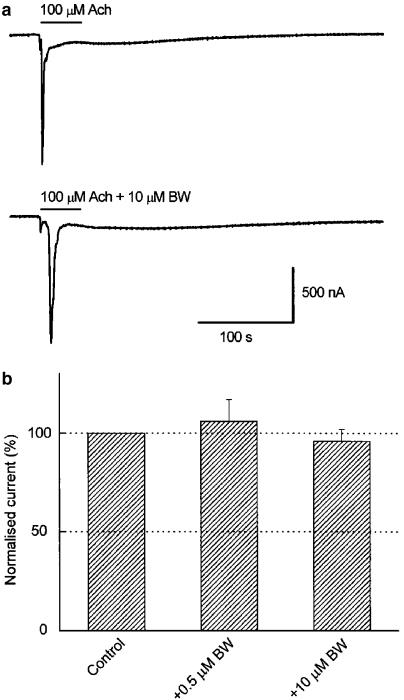
Muscarinic currents can be elicited by Ach in some noninjected oocytes, since M1 and M3 receptors are functionally expressed in the membrane of about 40% of X. laevis oocytes (Lupu-Meiri et al., 1990; Davidson et al., 1991). There is experimental evidence that compounds representative of the main ChEI classes interact with muscarinic Ach receptors (Bakry et al., 1988; Zhang et al., 1997; Lockhart et al., 2001). Since it has been shown that physostigmine, tacrine, edrophonium and galantamine are able to displace the binding of the muscarinic AchR agonist [3H]oxotremorine-M from its receptor in rat cortex and brain stem homogenates (Lockhart et al., 2001), we studied the effects of 0.5–10 μM BW284c51 on muscarinic responses. Figure 7a shows a muscarinic IAch elicited by 100 μM Ach, recorded in NR solution in an uninjected oocyte. This slow current shows some oscillations and is mainly carried by Cl−, as a result of the activation of the inositol trisphosphate signalling cascade (Oron et al., 1985; Parker & Miledi, 1986), which raises intracellular Ca2+ concentration and, subsequently, activates endogenous Ca2+-dependent Cl− channels present in the oocyte membrane. When the Ach pulse was repeated 15 min later in the presence of BW284c51 (0.5–10 μM), there were not significant differences neither in the current amplitude nor in the time course of the response (Figure 7a). Similar results were obtained in other cells isolated from different donors (Figure 7b; P=0.128).
Figure 7.
Lack of effect of BW284c51 on muscarinic AchRs. (a) Muscarinic currents elicited in a noninjected oocyte, with the membrane potential held at −60 mV, by superfusing it with an NR solution containing 100 μM Ach, either alone (upper record) or together with 10 μM BW284c51 (lower record). (b) Column graph showing the effect of Ach coapplication with either 0.5 or 10 μM BW284c51 on the muscarinic current amplitude. Data were normalised as the percentage of the control responses (obtained in the same cell in the absence of the drug) and expressed as the mean±s.e.m. of five cells from three donors. There were not significant differences between any of these three groups.
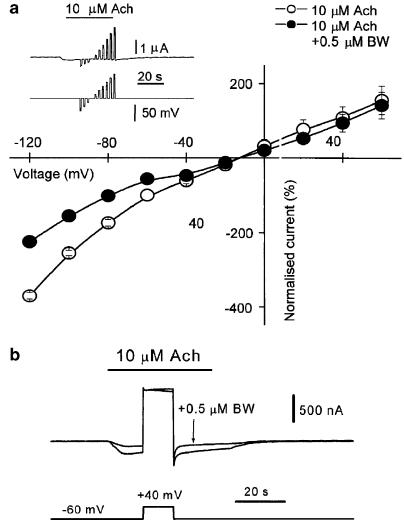
Voltage dependence of the BW284c51 blockade
To determine whether the IAch blocking effect of BW284c51 exhibited any voltage dependence, an i–v curve was obtained by jumping the membrane potential to different voltages during the plateau of the IAch elicited by Ach (10 μM) either alone or coapplied with BW284c51 (0.5 μM). The control IAch, i–v relationship showed an inward rectification at hyperpolarising potentials and a reversal potential of −13±2 mV (n=5, two donors), which was in good agreement with our previous data (Morales et al., 1995). In the presence of BW284c51, IAch was markedly reduced at potentials below −40 mV, but no blocking effect was found at positive potentials (Figure 8a). The voltage dependence of the BW284c51 blockade of IAch could be plainly observed by giving a long-lasting pulse from −60 to +40 mV at the IAch plateau elicited by 10 μM ACh either alone or coapplied with BW284c51 (0.5 μM). As Figure 8b illustrates, the IAch blockade elicited by BW284c51, evident at −60 mV, was fully reversed by the depolarising pulse. After repolarisation, IAch was again quickly blocked, showing a similar degree of inhibition to that found before membrane depolarisation. The ion selectivity of the Ach-gated channel was not affected by BW284c51, since the IAch reversal potential remained unchanged (−12±1 mV, n=5, two donors; P=0.547).
Figure 8.
Voltage dependence of BW284c51 effects on IAch. (a) The i–v relationship for the IAch, elicited by 10 μM Ach either alone or coapplied with 0.5 μM BW284c51, was obtained by giving voltage pulses from −120 up to +60 mV, in 20 mV steps, during the current plateau induced by Ach. Values were normalised, for each oocyte, as the percentage of the IAch obtained at −60 mV while superfusing it with Ach alone. Every point is the mean±s.e.m. of five oocytes from two donors. Note that the blocking effect of BW284c51 vanished at positive potentials. The inset shows the IAch plateau elicited by 10 μM Ach and the time at which voltage pulses were given. (b) Effect of a 10 s depolarising pulse (from the holding potential, −60 mV, up to +40 mV) on the IAch elicited by 10 μM Ach either alone or coapplied with 0.5 μM BW284c51. Note that IAch blockade by BW284c51 was completely abolished at +40 mV and that once the depolarising pulse had finished, the blocking action of BW284c51 was immediately, and completely, recovered.
Discussion
Our results show that BW284c51 blocks Torpedo nicotinic AchRs in a noncompetitive and voltage-dependent manner, and also increases IAch desensitisation. This blocking effect was selective for nicotinic over M1 and M3 muscarinic AchRs and was not related to its ability as ChEI, since a similar inhibition was obtained using Cch as the agonist, a compound which is not hydrolysed by AchE (Sung et al., 1998).
Noncompetitive inhibitors are thought to inhibit receptor function by different mechanisms, including blockade of the open channel and promotion of desensitisation (for a review see Popot & Changeux, 1984). The strong voltage dependence of the IAch inhibition by BW284c51, only present at negative potentials, and the faster IAch peak observed when Ach was coapplied with BW284c51, are consistent with an open-channel blockade by this compound exerted at the vestibule or within the channel itself. On the other hand, though BW284c51 induced a faster IAch decay, it cannot account for the observed blocking effect, since changes in desensitisation were more evident at high Ach doses, whereas the degree of inhibition was dose-independent.
It is well known that some AchEIs exert an inhibitory effect on IAch, including tacrine (Cantí et al., 1998; Prince et al., 2002), its derivatives CI-1002, CI-1017 (Ros et al., 2000) and bis(7)-tacrine (Ros et al., 2001a), and other inhibitors such as physostigmine (Cantí et al., 1998), huprines (Ros et al., 2001b), edrophonium, neostigmine and pyridostigmine (Yost & Maestrone, 1994). Interestingly, most of these compounds, which have quite different molecular structures, share the property of mediating a noncompetitive antagonism with remarkable voltage dependence, suggesting that they operate through an open-channel blocking mechanism. However, the inhibitory potency of different AchEIs on nicotinic IAch was highly variable. Thus, whereas (±)-huprine Y, bis(7)-tacrine and BW284c51 bear IC50 values in the submicromolar range (Ros et al., 2001a, 2001b; our present data), the IC50 values for the rest of the above AchEIs are at least one order of magnitude higher. So, IAch inhibition by BW284c51 was stronger than that mediated by tacrine (Cantí et al., 1998; Prince et al., 2002), and was of a similar potency to that found for d-TC (O'Leary et al., 1994; our present data). Interestingly, the blocking effect of BW284c51 and tacrine or curare were additive, suggesting that these drugs differ, at least partially, in their mechanisms of IAch inhibition. However, since both BW284c51 and tacrine show a noncompetitive antagonism on nicotinic currents, their additive effect might be achieved through interactions with different sites on the receptor. In this sense, it is worth noting to mention that multiple binding sites have been proposed for the tacrine action on human muscle nicotinic AchRs (Prince et al., 2002), and that BW284c51 in addition to plugging the channel it is able to modify the nH for Ach. Nevertheless, our data support a unique binding site for BW284c51, which might be located close to the pore of the channel. Moreover, the additive blocking effects of BW284c51 and d-TC, a competitive inhibitor, and the specificity on nicotinic over muscarinic receptors, agree well with an action of the BW284c51 exerted mainly on, or close to, the channel pore. The change observed in the nH value for AchRs when Ach was coapplied with BW284c51 remains to be explained.
BW284c51 inhibitory effects on Torpedo nicotinic AchRs strongly resembled those elicited by edrophonium, an AchEI with only one quaternary ammonium group, on mouse muscle nicotinic AchRs expressed in Xenopus oocytes. Thus, edrophonium enhanced AchR desensitisation and caused a voltage-dependent blockade, which was more potent at hyperpolarising membrane potentials, this also suggesting a channel blockade within the ion-conducting pore (Yost & Maestrone, 1994). The main difference between these two compounds on their action on nicotinic AchRs is at their IC50 value, which is about two orders of magnitude higher for edrophonium than for BW284c51 (Yost & Maestrone, 1994; S. Olivera-Bravo, I. Ivorra & A. Morales, unpublished results); nevertheless, its effects were also completely reversible upon washout of the inhibitor.
Significant differences were found, however, between the BW284c51 effects on nicotinic AchRs and those exerted by other bisquaternary ammonium drugs, such as hexamethonium and decamethonium. Thus, whereas decamethonium behaves as a partial agonist of nicotinic AchRs (Adams & Sakmann, 1978; Aoshima, 1990; Bertrand et al., 1992; Liu & Dilger, 1993), BW284c51 did not elicit any agonistic effect on these receptors, even at concentrations as high as 1 mM. Unlike BW284c51, hexamethonium and decamethonium inhibitory effects on AchRs could not be immediately reverted upon their washing out; in fact, their effects only disappeared either by depolarising the membrane or by extensive, long-lasting washes (Bertrand et al., 1990). This fact suggests that these two bisquaternary compounds interact with the nicotinic AchR in a site located deeper within the channel than the site for BW284c51 binding. Since BW284c51 effects are more alike to those evoked by edrophonium, a molecule with a single quaternary ammonium, than those elicited by hexamethonium or decamethonium, which are bisquaternary ammonium compounds, it follows that BW284c51 blockade seemingly depends mainly on the presence of this functional group rather than on its number.
To conclude, this is the first report showing a set of BW284c51 actions on AchRs not directly related to its activity as an AchEI (Mikalsen et al., 1986, Radic et al., 1993; Dupree & Bigbee, 1994). It should be pointed out that the morphogenic effects of BW284c51, suppressing neurite outgrowth in several model systems, have been attributed to its interaction with the peripheral site of AchE (Eichler et al., 1994; Sharma & Bigbee, 1998; Brimijoin & Koenigsberger, 1999). Putatively, this interaction might distort the enzyme shape, and/or surface charge, which in turn could disturb critical protein–protein interactions, including those involved in neurites outgrowth (Bigbee et al., 1999). Although BW284c51 constitutes an excellent pharmacological tool for inhibiting AchE activity with high selectivity, our findings must be taken into account in view of its powerful effect on nicotinic receptors. Moreover, this compound should be useful as a tool for the study of nicotinic AchR function given its high blocking potency and immediate recovery of receptor activity after its removal, two properties in which BW284c51 has proven to be similar, or even better, than the classical inhibitor d-TC.
Acknowledgments
We thank Dr J.M. González-Ros for kindly providing the samples of reconstituted nicotinic AchRs, Drs P. Lax and R.A. Jiménez-Moreno for their help with some experiments, Drs J.M. Henley and J. Martín-Nieto for helpful comments on the manuscript and Mr S. Moya for expert technical assistance. This work was supported by a grant from the Spanish MCyT (BFI2001-0756). Silvia Olivera-Bravo held a Fundación Carolina postdoctoral fellowship.
Abbreviations
- Ach
acetylcholine
- AchE
acetylcholinesterase
- AchEI
acetylcholinesterase inhibitor
- AchR
acetylcholine receptor
- ANR
normal Ringer with atropine
- BW284c51
1,5-bis(4-allyldimethylammoniumphenyl)pentan-3-one dibromide
- Cch
carbamylcholine
- ChEI
cholinesterase inhibitor
- d-TC
d-tubocurarine
- IAch
acetylcholine current
References
- ADAMS P.R., SAKMANN B. Decamethonium both opens and blocks endplate channels. Proc. Natl. Acad. Sci. U.S.A. 1978;75:2994–2998. doi: 10.1073/pnas.75.6.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOSHIMA H. Acetylcholine receptor-mediated membrane current in oocytes injected with Electrophorus electricus mRNA: analyses of nicotine, succinylcholine, and decamethonium responses on the basis of the minimal model. J. Biochem. 1990;108:947–953. doi: 10.1093/oxfordjournals.jbchem.a123319. [DOI] [PubMed] [Google Scholar]
- BAKRY N.M., EL-RASHID A.H., ELDEFRAWI A.T., ELDEFRAWI M.E. Direct action of organophosphate anticholinesterases on nicotinic and muscarinic acetylcholine receptors. J. Biochem. Toxicol. 1988;3:235–259. doi: 10.1002/jbt.2570030404. [DOI] [PubMed] [Google Scholar]
- BERTRAND D., BALLIVET M., RUNGGER D. Activation and blocking of neuronal nicotinic acetylcholine receptor reconstituted in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 1990;87:1993–1997. doi: 10.1073/pnas.87.5.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTRAND D., BERTRAND S., BALLIVET M. Pharmacological properties of the homomeric α7 receptor. Neurosci. Lett. 1992;146:87–90. doi: 10.1016/0304-3940(92)90179-b. [DOI] [PubMed] [Google Scholar]
- BIGBEE J.W., SHARMA K.V., GUPTA J.J., DUPREE J.L. Morphogenic role for acetylcholinesterase in axonal outgrowth during neural development. Environ. Health Perspect. 1999;107:81–87. doi: 10.1289/ehp.99107s181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY R.J., STERZ R., PEPER K. Agonist and inhibitory effects of pyridostigmine at the neuromuscular junction. Brain Res. 1986;376:199–203. doi: 10.1016/0006-8993(86)90918-2. [DOI] [PubMed] [Google Scholar]
- BRIMIJOIN S., KOENIGSBERGER C. Cholinesterases in neural development: new findings and toxicologic implications. Environ. Health Perspect. 1999;107:59–64. doi: 10.1289/ehp.99107s159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULLER A.L., WHITE M. Altered patterns of N-linked glycosylation of the Torpedo acetylcholine receptor expressed in Xenopus oocytes. J. Membr. Biol. 1990;115:179–189. doi: 10.1007/BF01869456. [DOI] [PubMed] [Google Scholar]
- CANTÍ C., BODAS E., MARSAL J., SOLSONA C. Tacrine and physostigmine block nicotinic receptors in Xenopus oocytes injected with Torpedo electroplaque membranes. Eur. J. Pharmacol. 1998;363:197–202. doi: 10.1016/s0014-2999(98)00793-6. [DOI] [PubMed] [Google Scholar]
- CLAPHAM D.E, NEHER E. Substance P reduces acetylcholine-induced currents in isolated bovine chromaffin cells. J. Physiol. 1984;347:255–277. doi: 10.1113/jphysiol.1984.sp015065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLEMENTI F., FORNASARI D., GOTTI C. Neuronal nicotinic receptors, important new players in brain function. Eur. J. Pharmacol. 2000;393:3–10. doi: 10.1016/s0014-2999(00)00066-2. [DOI] [PubMed] [Google Scholar]
- DAVIDSON A., MENGOD G., MATUS-LEIBOVITH N., ORON Y. Native Xenopus oocytes express two types of muscarinic responses. FEBS Lett. 1991;284:252–256. doi: 10.1016/0014-5793(91)80697-2. [DOI] [PubMed] [Google Scholar]
- DEL CASTILLO J., KATZ B. Interaction at end-plate receptors between different choline derivatives. Proc. Roy. Soc. Lond. 1957;146:369–381. doi: 10.1098/rspb.1957.0018. [DOI] [PubMed] [Google Scholar]
- DUPREE J.L., BIGBEE J.W. Retardation of neuritic outgrowth and cytoskeletal changes accompany acetylcholinesterase inhibitor treatment in cultured rat dorsal root ganglion neurons. J. Neurosci. Res. 1994;39:567–575. doi: 10.1002/jnr.490390508. [DOI] [PubMed] [Google Scholar]
- EICHLER J., ANSELMENT A., SUSSMAN J.L., MASSOULIE J., SILMAN I. Differential effects of ‘peripheral' site ligands on Torpedo and chicken acetylcholinesterase. Mol. Pharmacol. 1994;45:335–340. [PubMed] [Google Scholar]
- GARCÍA-COLUNGA J., MILEDI R. Serotoninergic modulation of muscle acetylcholine receptors of different subunit composition. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3990–3994. doi: 10.1073/pnas.93.9.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDERSEN C.B., MILEDI R. Acetylcholinesterase activity of Xenopus laevis oocytes. Neuroscience. 1983;10:1487–1495. doi: 10.1016/0306-4522(83)90129-x. [DOI] [PubMed] [Google Scholar]
- HOGG R.C., RAGGENBASS M., BERTRAND D. Nicotinic acetylcholine receptors: from structure to brain function. Rev. Physiol. Biochem. Pharmacol. 2003;147:1–46. doi: 10.1007/s10254-003-0005-1. [DOI] [PubMed] [Google Scholar]
- IVORRA I., FERNANDEZ A., GAL B., ALEU J., GONZALEZ-ROS J.M., FERRAGUT J.A., MORALES A. Protein orientation affects the efficiency of functional protein transplantation into the Xenopus oocyte membrane. J. Membr. Biol. 2002;185:117–127. doi: 10.1007/s00232-001-0118-x. [DOI] [PubMed] [Google Scholar]
- IVORRA I., MORALES A. Membrane currents in immature oocytes of the Rana perezi frog. Pflügers Arch. 1997;434:413–421. doi: 10.1007/s004240050415. [DOI] [PubMed] [Google Scholar]
- JENKINSON D.H. The antagonism between tubocurarine and substances which depolarise the motor end-plate. J. Physiol. (London) 1960;152:309–324. doi: 10.1113/jphysiol.1960.sp006489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOELLE G.B.Cholinesterase and anticholinesterase agents Handbuch der Experimentellen Pharmacologie 1963Berlin: Springer-Verlag; 883–920.ed. Eichler, O. & Farah, A. pp [Google Scholar]
- KUSANO K., MILEDI R., STINNAKRE J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J. Physiol. (London) 1982;328:143–170. doi: 10.1113/jphysiol.1982.sp014257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENA C., CHANGEUX J.P. Allosteric nicotinic receptors, human pathologies. J. Physiol. (Paris) 1998;92:63–74. doi: 10.1016/S0928-4257(98)80140-X. [DOI] [PubMed] [Google Scholar]
- LIU Y., DILGER J.P. Decamethonium is a partial agonist at the nicotinic acetylcholine receptor. Synapse. 1993;13:57–62. doi: 10.1002/syn.890130108. [DOI] [PubMed] [Google Scholar]
- LOCKHART B., CLOSIER M.M., HOWARD K., STEWARD C., LESTAGE P. Differential inhibition of [3H]-oxotremorine-M and [3H]-quinuclinidyl benzilate binding to muscarinic receptors in rat brain membranes with acetylcholinesterase inhibitors. Naunyn Schmiedebergs Arch. Pharmacol. 2001;363:429–438. doi: 10.1007/s002100000382. [DOI] [PubMed] [Google Scholar]
- LUMMIS S.C.R., BUCKINGHAM S.D., BALK M.L., HOLYOKE JR C.W., SATTELLE D.B. Actions of a series of bisquaternary compounds on nicotinic acetylcholine receptors in insects: ligand binding and electrophysiological studies. Neuropharmacology. 1992;31:379–382. doi: 10.1016/0028-3908(92)90070-6. [DOI] [PubMed] [Google Scholar]
- LUPU-MEIRI M., SHAPIRA H., MATUS-LEIBOVITCH N., OROM Y. Two types of intrinsic muscarinic responses in Xenopus oocytes. Pflügers Arch. 1990;417:391–397. doi: 10.1007/BF00370658. [DOI] [PubMed] [Google Scholar]
- MIKALSEN A., ANDERSEN R.F., ALEXANDER J. Use of ethopropazine and BW284c51 as selective inhibitors for cholinesterases from various species. Comp. Biochem. Physiol. 1986;83:447–449. doi: 10.1016/0742-8413(86)90152-0. [DOI] [PubMed] [Google Scholar]
- MILEDI R., SUMIKAWA K. Synthesis of cat muscle acetylcholine receptors by Xenopus oocytes. Biomed. Res. 1982;3:390–399. [Google Scholar]
- MORALES A., ALEU J., IVORRA I., FERRAGUT J.A., GONZALEZ-ROS J.M., MILEDI R. Incorporation of reconstituted acetylcholine receptors from Torpedo into the Xenopus oocyte membrane. Proc. Natl. Acad. Sci. U.S.A. 1995;92:8468–8472. doi: 10.1073/pnas.92.18.8468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAGATA K., HUANG C.S., SONG J.H., NARAHASHI T. Direct actions of anticholinesterases on the neuronal nicotinic acetylcholine receptor channels. Brain Res. 1997;769:211–218. doi: 10.1016/s0006-8993(97)00707-5. [DOI] [PubMed] [Google Scholar]
- O'LEARY M.E., FILATOV G.N., WHITE M.M. Characterization of d-tubocurarine binding site of Torpedo acetylcholine receptor. Am. J. Physiol. 1994;266:C648–C653. doi: 10.1152/ajpcell.1994.266.3.C648. [DOI] [PubMed] [Google Scholar]
- ORON Y., DASCAL N., NADLER E., LUPU M. Inositol 1,4,5,-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985;313:141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- PARKER I., MILEDI R. Changes in intracellular calcium and in membrane currents evoked by injection of inositol trisphosphate into Xenopus oocytes. Proc. Roy. Soc. Lond. Ser. B. 1986;228:307–315. doi: 10.1098/rspb.1986.0057. [DOI] [PubMed] [Google Scholar]
- PEREIRA E.F., HILMAS C., SANTOS M.D., ALKONDON M., MAELICKE A., ALBUQUERQUE E.X. Unconventional ligands and modulators of nicotinic receptors. J. Neurobiol. 2002;53:479–500. doi: 10.1002/neu.10146. [DOI] [PubMed] [Google Scholar]
- POPOT J.L., CHANGEUX J.P. Nicotinic receptor of acetylcholine: structure of an oligomeric integral membrane protein. Physiol. Rev. 1984;64:1162–1239. doi: 10.1152/physrev.1984.64.4.1162. [DOI] [PubMed] [Google Scholar]
- PRINCE R.J., PENNINGTON R.A., SINE S.M. Mechanism of tacrine block at adult human muscle nicotinic acetylcholine receptors. J. Gen. Physiol. 2002;120:369–393. doi: 10.1085/jgp.20028583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RADIC Z., PICKERING N.A., VELLOM D.C., CAMP S., TAYLOR P. Three distinct domains in the cholinesterase molecule confer selectivity for acetyl- and butyrylcholinesterase inhibitors. Biochemistry. 1993;32:12074–12084. doi: 10.1021/bi00096a018. [DOI] [PubMed] [Google Scholar]
- ROS E., ALEU J., GOMEZ DE ARANDA I., CANTÍ C., PANG Y.P., MARSAL J., SOLSONA C. Effects of bis(7)-tacrine on spontaneous synaptic activity and on the nicotinic Ach receptor of Torpedo electric organ. J. Neurophysiol. 2001a;86:183–189. doi: 10.1152/jn.2001.86.1.183. [DOI] [PubMed] [Google Scholar]
- ROS E., ALEU J., GOMEZ DE ARANDA I., MUÑOZ-TORRERO D., CAMPS P., BADIA A., MARSAL J., SOLSONA C. The pharmacology of novel acetylcholinesterase inhibitors, (±)-huprines Y and X, on the Torpedo electric organ. Eur. J. Pharmacol. 2001b;421:77–84. doi: 10.1016/s0014-2999(01)01028-7. [DOI] [PubMed] [Google Scholar]
- ROS E., ALEU J., MARSAL J., SOLSONA C. Effects of CI-1002 and CI-1017 on spontaneous synaptic activity and on the nicotinic acetylcholine receptor of Torpedo electric organ. Eur. J. Pharmacol. 2000;390:7–13. doi: 10.1016/s0014-2999(99)00911-5. [DOI] [PubMed] [Google Scholar]
- SHARMA K.V., BIGBEE J.W. Acetylcholinesterase antibody treatment results in neurite detachment and reduced outgrowth from cultured neurons: further evidence for a cell adhesive role for neuronal acetylcholinesterase. Neurosci. Res. 1998;53:454–464. doi: 10.1002/(SICI)1097-4547(19980815)53:4<454::AID-JNR7>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- SIVILOTTI L.G., MCNEIL D.K., LEWIS T.M., NASSAR M.A., SCHOEPFER R., COLQUHOUN D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. J. Physiol. (London) 1997;500:123–138. doi: 10.1113/jphysiol.1997.sp022004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMALL D.H. Do acetylcholinesterase inhibitors boost synaptic scaling in Alzheimer's disease. Trends Neurosci. 2004;27:245–249. doi: 10.1016/j.tins.2004.03.006. [DOI] [PubMed] [Google Scholar]
- SMULDERS C.J.G.M., BUETERS T.J.H., VAN KLEEF R.G.D.M., VIJVERBERG H.P.M. Selective effects of carbamate pesticides on rat neuronal nicotinic acetylcholine receptors and rat brain acetylcholinesterase. Toxicol. Appl. Pharmacol. 2003;193:139–146. doi: 10.1016/j.taap.2003.07.011. [DOI] [PubMed] [Google Scholar]
- SOREQ H., PARVARI R., SILMAN I. Biosynthesis and secretion of catalytically active acetylcholinesterase in Xenopus oocytes microinjected with mRNA from rat brain and Torpedo electric organ. Proc. Natl. Acad. Sci. U.S.A. 1982;79:830–834. doi: 10.1073/pnas.79.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUNG J.J., KIM S.J., LEE H.B., CHUNG J.M., CHOI Y.M., CHA C.I., SUH Y.H., LEE K.W. Anticholinesterase induces nicotinic modulation. Muscle Nerve. 1998;21:1135–1144. doi: 10.1002/(sici)1097-4598(199809)21:9<1135::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- VAN DEN BEUKEL I., VAN KLEEF R.G., ZWART R., OORTGIESEN M. Physostigmine and acetylcholine differentially activate nicotinic receptor subpopulations in Locusta migratoria neurons. Brain Res. 1998;789:263–273. doi: 10.1016/s0006-8993(98)00007-9. [DOI] [PubMed] [Google Scholar]
- WACHTEL R.E. Physostigmine block of ion channels activated by acetylcholine in BC3HS cells. Mol. Pharmacol. 1990;44:1051–1055. [PubMed] [Google Scholar]
- WONNACOTT S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- YOST C.S., MAESTRONE E. Clinical concentrations of edrophonium enhance desensitisation of the nicotinic acetylcholine receptor. Anaesth. Analg. 1994;78:520–526. doi: 10.1213/00000539-199403000-00018. [DOI] [PubMed] [Google Scholar]
- ZHANG Y., CARLEN P.L., ZHANG L. Kinetics of muscarinic reduction of IsAHP in hippocampal neurons: effects of acetylcholinesterase inhibitors. J. Neurophysiol. 1997;78:2999–3007. doi: 10.1152/jn.1997.78.6.2999. [DOI] [PubMed] [Google Scholar]
- ZWART R., VAN KLEEF R.G., GOTTI C., SMULDERS C.J., VIJVERBERG H.P.M. Competitive potentiation of acetylcholine effects on neuronal nicotinic receptors by acetylcholinesterase-inhibiting drugs. J. Neurochem. 2000;75:2492–2500. doi: 10.1046/j.1471-4159.2000.0752492.x. [DOI] [PubMed] [Google Scholar]