Abstract
SQ109 is a novel [1,2]-diamine-based ethambutol (EMB) analog developed from high-throughput combinatorial screening. The present study aimed at characterizing its pharmacodynamics and pharmacokinetics.
The antimicrobial activity of SQ109 was confirmed in vitro (Mycobacterium tuberculosis-infected murine macrophages) and in vivo (M. tuberculosis-infected C57BL/6 mice) and compared to isoniazid (INH) and EMB. SQ109 showed potency and efficacy in inhibiting intracellular M. tuberculosis that was similar to INH, but superior to EMB. In vivo oral administration of SQ109 (0.1–25 mg kg−1 day−1) to the mice for 28 days resulted in dose-dependent reductions of mycobacterial load in both spleen and lung comparable to that of EMB administered at 100 mg kg−1 day−1, but was less potent than INH at 25 mg kg−1 day−1. Monitoring of SQ109 levels in mouse tissues on days 1, 14 and 28 following 28-day oral administration (10 mg kg−1 day−1) revealed that lungs and spleen contained the highest concentration of SQ109, at least 10 times above its MIC.
Pharmacokinetic profiles of SQ109 in mice following a single administration showed its Cmax as 1038 (intravenous (i.v.)) and 135 ng ml−1 (p.o.), with an oral Tmax of 0.31 h. The elimination t1/2 of SQ109 was 3.5 (i.v.) and 5.2 h (p.o.). The oral bioavailability was 4%. However, SQ109 displayed a large volume of distribution into various tissues. The highest concentration of SQ109 was present in lung (>MIC), which was at least 120-fold (p.o.) and 180-fold (i.v.) higher than that in plasma. The next ranked tissues were spleen and kidney. SQ109 levels in most tissues after a single administration were significantly higher than that in blood. High tissue concentrations of SQ109 persisted for the observation period (10 h).
This study demonstrated that SQ109 displays promising in vitro and in vivo antitubercular activity with favorable targeted tissue distribution properties.
Keywords: Antituberculosis, ethambutol, SQ109, pharmacodynamics, pharmacokinetics
Introduction
Recent years have seen an increased incidence of tuberculosis in both developing and industrialized countries, widespread emergence of drug-resistant tubercular strains and a deadly synergy of tuberculosis with the human immunodeficiency virus (HIV) (Espinal et al., 2001). Increased susceptibility to tuberculosis is associated with early stages of HIV infection, and tuberculosis in turn accelerates the progression of HIV infection to AIDS. The current tuberculosis treatment regimens, although highly effective when administered faithfully for the full dosing period, are far from ideal (Barry III, 1997). Using the optimal combination of available drugs, the duration of treatment required for curing patients cannot be reduced below 6 months. In most low-income countries, an 8-month regimen is used. Furthermore, all four of the most effective oral drugs – isoniazid (INH), rifampin, ethambutol (EMB) and pyrazinamide – must be taken together during the first 2-month treatment (American Thoracic Society Documents, 2003). With an estimated 8.8 million new cases of tuberculosis in 2002, of which 3.9 million were smear-positive (2004 WHO report, www.whoint/tb/publications/global_report/), and an annual mortality of nearly two million (Dye et al., 1999), there is a pressing need for new antitubercular agents acting with greater potency and efficacy than the current existing drugs (O'Brien & Nunn, 2001).
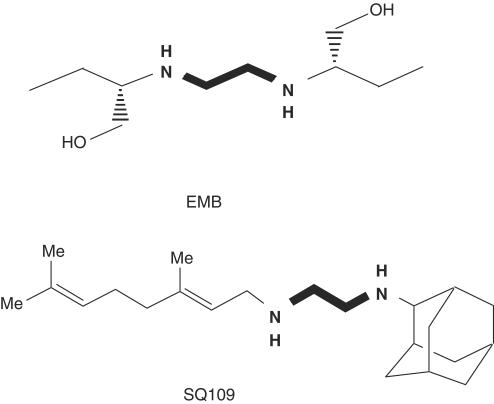
With the completion of the genome sequence and progress on proteomics of Mycobacterium tuberculosis (Cole et al., 1998; Mattow et al., 2001), the promise of a new generation of potent drugs to combat the emerging epidemic of the disease is foreseeable. EMB discovered in 1961 is still a first-line drug for treating all forms of tuberculosis (American Thoracic Society Documents, 2003). Due to its modest efficacy against M. tuberculosis and its chemical simplicity, EMB is amenable to optimization by combinatorial chemistry (Barry III et al., 2000). Identification of diamine analogs with enhanced efficacy over EMB is one approach to improving the existing treatment of tuberculosis, potentially making therapeutic implementation easier and of shorter duration. A diverse library of 63,238 EMB analogs with a 1,2-diamine pharmacophore (Figure 1) was synthesized on solid support using a novel acylation-reduction sequence, and screened to determine minimal inhibitory concentration (MIC) using two techniques employing whole M. tuberculosis, including one where the organism was engineered to express luciferase with disruption of the bacterial cell wall, the mechanism of action of the parent compound EMB (Lee et al., 2003). After the lead optimization and screening, three analogs were found to have an enhanced antitubercular activity compared to EMB. Based on the results obtained from both the efficacious comparison and high-throughput pharmacokinetic screening using cassette dosing combined with liquid chromatography tandem mass spectrometry (LC/MS/MS) (Jia et al., 2003a), we selected N-Geranyl-N′-(2-adamantyl)ethane-1,2-diamine (SQ109; MW 330.2; Figure 1) as a lead compound for advanced drug testing.
Figure 1.
Chemical structures of EMB and SQ109.
The purpose of the present study was to determine, by comparison with EMB and INH, the effectiveness of SQ109 monotherapy on M. tuberculosis-infected murine macrophage cells and examine the therapeutic effects of SQ109 on M. tuberculosis-infected mouse model. In addition, pharmacokinetic characteristics of SQ109 were examined in mice, including its distribution into various tissues after a single administration and its disposition into targeted tissues after a 28-day repeated administration. The goal of these studies was to understand the relationship between SQ109 antitubercular efficacy, its achieved concentration in M. tuberculosis-susceptible tissues, and its pharmacokinetic profile as measured by LC/MS/MS.
Methods
In vitro infection model
The RAW 264.7 (ATCC TIB-71) murine macrophage cell line was seeded overnight at 5 × 105 cells per well in 24-well plates at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM) with essential amino acids and glutamine supplemented with 10% heat-inactivated fetal bovine serum (FBS). Log-phase cultures of recombinant M. tuberculosis H37Rv containing the luciferase reporter construct pSMT1 (hsp60 promoter-driven luciferase) (Snewin et al., 1999) were grown in Middlebrook 7H9 supplemented with bovine serum albumin (BSA), dextrose, catalase and 50 μg ml−1 of hygromycin B at 37°C with 5% CO2 from frozen stocks. Mycobacteria were harvested by centrifugation at 2500 × g for 10 min and washed twice with serum-free DMEM before re-suspension in DMEM supplemented with 5% heat-inactivated FBS at 5 × 106 cells ml−1. Macrophages were infected by incubation with the bacteria at a ratio of 10 : 1 (M. tuberculosis: cell) overnight at 37°C; they were then washed three times in Dulbecco's phosphate-buffered saline (PBS, pH 7.4). The infected cells were treated in triplicate with INH, EMB and SQ109 dissolved in DMEM containing 5% FBS at various MIC for 7 days. Macrophage cells were lysed by the addition of 1 ml per well sterile distilled H2O containing 0.1% Triton X-100 with stirring for 2 min. In all, 100 μl lysate was sampled from each well and an equal amount of 1% N-decyl aldehyde (Sigma) in ethanol was added. Luminescence was immediately quantified using a Luminiskan luminometer (Packard) with a dwell-time of 10 s per well to test the activities of each drug on the infected RAW 264.7 cells. The bioluminescence-based assay employs a reporter strain of M. tuberculosis, which endogenously expresses firefly luciferase that catalyzes the substrate to produce luminescence. Therefore, mycobacterial growth inside the infected cells can be estimated based on luminous intensity (Snewin et al., 1999).
Mycobacterial inoculum and in vivo infection mouse model
An aliquot of a frozen stock of M. tuberculosis H37Rv Pasteur was thawed and added to 5 ml 7H9 broth medium supplemented with 0.2% glycerol, albumin dextrose complex and 0.05% Tween 80, and incubated for 1 week at 37°C. A volume of 1 ml of the mixture was then added into 25 ml fresh medium during week 2. The culture was washed twice with PBS and 0.05% Tween 80, re-suspended in PBS with 0.5% BSA and 0.05% Tween 80, and aliquots were frozen at −80°C. To determine viable colony-forming units (CFU) of the frozen culture, an aliquot was thawed and 10-fold dilutions were plated on agar 7H9 and incubated at 37°C. Mean CFU per treatment was determined 20 days later.
Female C57BL/6 mice (8 weeks old) were purchased from Charles River (Raleigh, NC, U.S.A.). The mice were housed in a BSL-2 facility and allowed to acclimatize for at least 4 days before infection. On the day of inoculation, a frozen sample of stock M. tuberculosis of known viability and CFU count was thawed and diluted to a concentration of approximately 5 × 105 CFU per ml. Mice were infected with the M. tuberculosis H37Rv by lateral tail vein injection with 105 CFU suspended in 0.2 ml of PBS, and final concentration of injected CFU was confirmed by culture of a sample of the inoculum.
Oral treatment of mice with INH (25 mg kg−1), EMB (100 mg kg−1) and SQ109 (0.1, 10 and 25 mg kg−1) was initiated 20 days after inoculation. Control groups of infected but untreated mice were killed at the initiation of therapy (early controls) or at the end of the treatment period. There were six mice per group. Chemotherapy was given daily for 5 days per week until the mice were killed, 4 weeks after initiation of treatment. The spleen and lungs were aseptically removed and weighed. The organs were homogenized in 2 ml of sterile PBS containing 0.05% Tween-80. Homogenate samples from individual tissues were diluted 10-fold in PBS and plated on 7H10 agar dishes. Inoculated dishes were incubated at 37°C in ambient air for 3 weeks prior to calculation of CFU. Viable counts were converted to a logarithmic scale; readings were corrected to represent whole organ totals. The severity of infection and the effectiveness of the treatments were assessed by the survival rate, and the mean number of CFU in mouse organs.
Analytical method for determining SQ109 in biomatrices
Separation of SQ109 from biomatrices was achieved on a Beta Basic C18 analytical column (150 × 2 mm, 5 μm) preceded by a Beta Basic C18 guard column (4 × 2 mm; Keystone Scientific, Bellefonte) at 25°C and a flow rate of 0.6 ml min−1. SQ109 and terfenadine (internal standard) were eluted using a mobile phase composed of buffer A (5 mM CH3COONH4 with 0.1% trifluoroacetic acid, pH 6.8) and buffer B (methanol with 0.1% trifluoroacetic acid) according to the following gradient program: 50% buffer A and 50% buffer B were held for 0.5 min, and then buffer A was linearly decreased to 20% over 3 min and remained constant for 1 min while the analytes were eluted. The column was re-equilibrated to initial conditions via a step gradient for 3 min.
A PE Sciex API 3000 triple quadrupole mass spectrometer equipped with a Turbo Ion spray source and operating at 450°C in the positive ion mode was used for analysis of SQ109. Hydrocarbon-free air was used as both the auxiliary and nebulizer gas, while nitrogen was used as the collision gas. Peak area ratios of SQ109 to terfenadine were used for the construction of calibration curves via weighted (reciprocal of concentration) linear least-square regression of the compound concentrations and the measured area ratios. Data were acquired and analyzed using Analyst 1.4 software program (Biosystems).
Calibration standards were prepared by spiking the corresponding biomatrices (e.g., plasma, urine and tissue homogenates) with known amounts of SQ109 over a concentration range of 1.9–500 ng ml−1. Each standard was also spiked with terfenadine at 500 ng ml−1, except for the blank that was spiked with 10 μl of deionized water. The analytes were then mixed with 2 ml of acetonitrile, vortexed and centrifuged at 1000 × g for 5 min. To erythrocyte, acetonitrile was added after lysis of the cells with an equal volume of deionized water. The resulting organic layer was removed and evaporated to dryness at 50°C with a gentle stream of dry nitrogen. The residue was reconstituted in 200 μl of methanol (0.1% trifluoroacetic acid)/5 mM ammonium acetate (80/20) and centrifuged at approximately 15,000 × g for 5 min. The supernatant was transferred into autosampler vials, and only 10 μl of the supernatant was injected into the LC/MS/MS system for analysis. The lower limit of quantitation of SQ109 in plasma was determined to be 1.95 ng ml−1, with correlation coefficients of greater than 0.99.
Pharmacokinetic studies
Male CD2F1 mice (23–27 g) were administered SQ109 at 3 mg kg−1 (intravenous (i.v.)), or 25 mg kg−1 (p.o.). Four or five mice were anesthetized with isoflurane at the following times after administration of SQ109 to collect blood from the brachial region of each animal: 2, 5, 10, 15 and 30 min and 1, 3, 6, 10 and 24 h after a single i.v. dosing; 5, 15 and 30 min and 1, 2, 4, 6, 10 and 24 h after a single oral dosing. Each blood sample was collected into a tube containing EDTA and centrifuged (2000 × g, 10 min) to separate plasma and red blood cells. To each 200 μl of plasma sample, 10 μl of internal standard solution (10 μg ml−1) was added. SQ109 was then separated and analyzed according to the previously described procedures. Peak area ratios of SQ109 to the internal standard were plotted against theoretical concentrations. Drug concentrations in samples were calculated from the standard calibration curves. Pharmacokinetic parameters were calculated using the computer program WinNonlin (Pharsight Co., Mountain View, CA, U.S.A.), and bioavailability was calculated as (AUCp.o. AUCi.v.−1) × (dosei.v. × dosep.o.−1) × 100%.
SQ109 levels in vital tissues following multiple dosing for 28 days
In order to determine whether tissue levels of SQ109 correlate with its efficacy in the H37Rv-infected mouse model, and whether SQ109 accumulates in the targeted tissues over a long period of multiple dose administration, SQ109 levels in the lung, spleen, liver, kidney and heart were monitored during the period of multiple dose administration. Briefly, C57BL/6 mice were orally given SQ109 by gavage at 10 mg kg−1 day−1 for 28 days. Groups of five mice each were anesthetized at 1 h after oral administration on days 1, 14 and 28 with CO2/O2. Blood and the five vital tissues were collected. Plasma and tissue homogenates were prepared for analysis using the below-mentioned procedures. Standard curves for SQ109 in different tissue matrices were plotted for quantitative purposes.
Tissue distribution and elimination of SQ109 after a single administration
Male CD2F1 mice (25–27 g) were housed in suspended wire metabolism cages in order to collect their urine and feces to determine SQ109 excretion. The mice were fasted overnight before dosing. Water was provided throughout the study. Mice were dosed with SQ109 at either 3 mg kg−1 (i.v.) or 25 mg kg−1 (p.o.). Groups of four mice each were anesthetized with isoflurane at 1, 4 and 10 h after dosing, and blood was collected from the brachial region of each mouse into a tube containing EDTA. Tissues and organs were immediately removed, individually weighed, washed with cold saline and stored at −20°C prior to analysis for levels of SQ109. On the day of analysis, tissues and organs were minced with scissors and homogenized in ice-cold 5 mM ammonium acetate buffer (1 : 5, w : w). Aliquots of the homogenate (200 μl) were extracted as described previously.
For elimination studies with SQ109, groups of four mice resided in metabolism cages, where urine and feces were separated by a cone-shaped device. Pooled urine and feces were cumulatively collected prior to drug administration, and at 4, 8, 24 and 32 h after a single dose (3 mg kg−1, i.v.; 25 mg kg−1, p.o.). Feces were homogenized in 12 volumes by fecal weight of 5 mM ammonium acetate buffer, and 200 μl aliquots were extracted after centrifugation as described previously. Urine samples were diluted 1 : 10 with 5 mM ammonium acetate without further preparation.
Statistical analysis
All results were expressed as mean±s.d. unless otherwise noted. To analyze experimental results, one-way analysis of variance (ANOVA) was applied to compare the means of two or more groups simultaneously and examine the significance of differences, and P<0.05 was considered to be statistically significant.
Results
In vitro activity of SQ109 on M. tuberculosis-infected macrophage cells
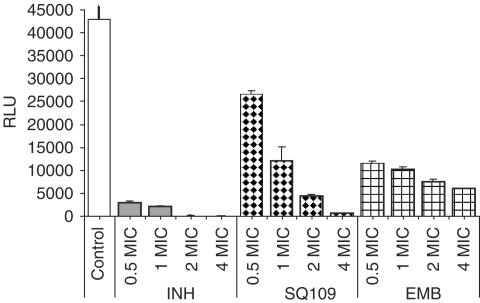
Mycobacterial infection of the murine macrophage RAW264.7 cell line was monitored by measuring relative luminescence units (RLU) of a recombinant M. tuberculosis H37Rv containing the luciferase reporter construct pSMT1. The RLU for untreated (control) macrophages infected with M. tuberculosis remained relatively stable throughout each experiment (42,978±6173, n=9). Drug effects on viability of intracellular M. tuberculosis were evaluated by estimating changes in RLU in drug-treated cultures compared to the control cultures on day 7. In vitro MIC calculated on fresh cultures of M. tuberculosis H37Rv differs for the different antitubercular drugs in this study: the MIC of SQ109 (1.56 μM) is intermediate between that of INH (0.73 μM) and EMB (24.5 μM). SQ109 inhibited RLU of intracellular M. tuberculosis in a dose-dependent fashion (Figure 2), and decreased the RLU to 27.7, 10.4 and 1.2% of the control value at 1, 2 and 4 times its MIC (i.e., 1.56, 3.12 and 6.24 μM), respectively. By comparison, EMB at 1 × MIC, 2 × MIC and 4 × MIC (i.e., 24.5, 49 and 98 μM) decreased the RLU to 23.5, 17.5 and 13.8% of control. It should be noted that the lowest value attained with the EMB, 13.8% at 4 × MIC, was substantially higher than the value obtained with 16-fold less SQ109 (1.2% at 4 × MIC). INH was the most potent inhibitor: INH at 1 × MIC and 2 × MIC reduced the RLU to 5.0 and 0.2% of control. SQ109 potency (the concentration of SQ109 required to produce 90% inhibition, that is, 1.56 μM) and maximal efficacy (a decrease in the RLU by ∼99% of the control value) were close to that of INH and superior to that of EMB, its parent compound.
Figure 2.
Effects of tuberculosis drugs and SQ109 on RAW 264.7 cells infected with M. tuberculosis luciferase reporter strain H37Rv/pSMT1. Cells were treated with drugs for 7 days at various folds of their corresponding MICs: INH (1 MIC=0.73 μM), EMB (1 MIC=24 μM), SQ109 (1 MIC=1.56 μM). Results (mean±s.d., n=3) are expressed as RLU reading on day 7 relative to day 0 for the untreated control.
In vivo activity of SQ109 on M. tuberculosis-infected mice
Table 1 shows the activity of antituberculosis drugs on mice infected with M. tuberculosis H37Rv. Oral INH at 25 mg kg−1 and oral EMB at 100 mg kg−1 were positive controls. INH reduced bacterial concentration in lungs 2.81 logs and 2.34 logs in spleens, compared to bacterial concentration in tissues of untreated controls (P<0.01). The results of INH treatment were consistent with those reported elsewhere (Cynamon et al., 1999; Stover et al., 2000; Lenaerts et al., 2002). EMB treatment of infected mice at 100 mg kg−1 daily for 28 days resulted in a reduction of M. tuberculosis CFU in both spleen and lung that was comparable to that of oral SQ109 at 10 and 25 mg kg−1. SQ109 exhibited inhibition of Mycobacteria growth in both organs in a dose-dependent manner. SQ109 at 0.1 mg kg−1, 1/1000 the effective dose of EMB, still produced a significant decrease in bacteria in spleen (P=0.032). As expected, some of the untreated mice died from TB infection, whereas all of the drug-treated mice survived the duration of the experiment. Of particular interest in these studies, mice treated with 10 mg kg−1 of SQ109 were cleared of M. tuberculosis bacteria to the same degree and in the same time frame as mice treated with the standard 100 mg kg−1 of EMB. INH was demonstrated the most potent drug in reducing mycobacterial burden in both spleen and lung.
Table 1.
CFU counts in organ homogenates after 28-day oral administration of SQ109, EMB and INH to mice inoculated with M. tuberculosis H37Rv
| Log10 CFU/organ (mean±s.d.) | ||
|---|---|---|
| Treatment (daily dose)a | Lung | Spleen |
| Untreated | 7.05±0.13 | 6.58±0.18 |
| INH (25 mg kg−1) | 4.24±0.12* | 4.22±0.05* |
| EMB (100 mg kg−1)** | 5.38±0.19* | 5.13±0.12* |
| SQ109 (0.1 mg kg−1) | 6.69±0.13** | 6.06±0.17*** |
| SQ109 (10 mg kg−1)** | 5.45±0.16* | 5.36±0.20* |
| SQ109 (25 mg kg−1)** | 5.18±0.15* | 5.14±0.14* |
Treatment was started 20 days after the mice (n=8 per group) received inoculation.
Statistically significant difference from the untreated group, P<0.01.
P=0.052, compared to the untreated group.
P=0.032, compared to the untreated group.
SQ109 levels in vital tissues following 28-day multiple dosing
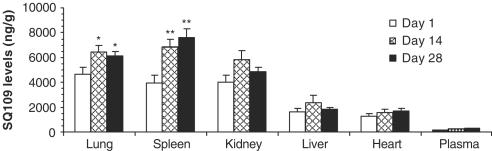
The effects of tissue matrix and homogenization procedures on the extraction recovery of SQ109 were assessed by comparison of chromatographic responses of each tissue extract with those obtained from standards of SQ109 spiked into the mobile phase. The overall recovery of SQ109 ranged from 72 to 127% among various tissues. Figure 3 shows SQ109 levels in mouse vital tissues monitored on days 1, 14 and 28 during a consecutive 28-day oral dosing period with 10 mg kg−1. These results demonstrated that SQ109 was concentrated in lung, spleen and kidney, and concentrations in those tissues were significantly higher than those in liver, heart and plasma (P<0.001). On day 28, SQ109 concentrations in lung (6108±394 ng g−1) and spleen (7596±699 ng g−1) were significantly higher than in kidney (4858±388 ng g−1) (P<0.05), although on days 1 and 14 SQ108 concentrations in the lung and spleen were only slightly higher than in kidney. In general, parallel comparison of organs over time showed that SQ109 accumulated mainly in the lung and spleen. The next ranked tissue was kidney. SQ109 concentrations in the three tissues were at least 45-fold higher than in plasma. During the repeated dosing period, a statistically significant accumulation of SQ109 in the lungs and spleen was found when we compared SQ109 concentrations in various tissues on day 1 with those on days 14 and 28. For example, SQ109 concentration in lungs was 4613±618 ng g−1 on day 1 versus 6508±481 ng g−1 on day 14 (P=0.043) and 6108±394 ng g−1 on day 28 (P=0.048), respectively. In spleen, SQ109 concentration was 3912±656 ng g−1 on day 1 versus 6922±542 ng g−1 on day 14 (P=0.009) and 7596±699 ng g−1 on day 28 (P=0.007), respectively. A slight accumulation of SQ109 in the kidney and liver over the dosing period was seen (P>0.05). These observations suggest that no unusual enzymatic activity was induced following the long period of oral administration of SQ109 to mice, which otherwise could accelerate the normal degradation of SQ109, resulting in no accumulation or even decreases of SQ109 in the tissues.
Figure 3.
SQ109 concentrations (mean±s.d.) in the lung, spleen, kidney, liver, heart and plasma at 1 h after oral administration of the compound to mice (10 mg kg−1 day−1). Groups of 4–5 mice were killed on days 1, 14 and 28 during the 28-day dosing period. SQ109 concentrations in the lung, spleen and kidney were significantly higher than those in liver, heart and plasma (P<0.001). Statistically significant SQ109 accumulation in lungs and spleen was found on days 14 and 28 versus day 1: *P<0.05; **P<0.01.
Pharmacokinetic profiling of SQ109
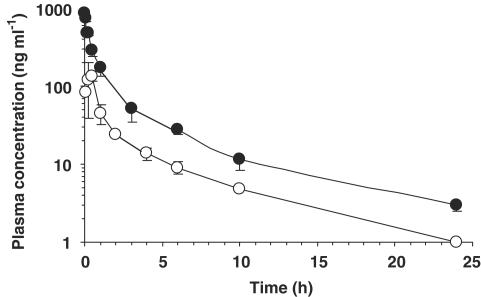
Preferential distribution of SQ109 into the lung and spleen, the target organs of tubercular infection and replication, prompted us to evaluate pharmacokinetic properties of the compound. SQ109 plasma concentration–time course after single oral and i.v. administration to mice is illustrated in Figure 4. The SQ109 plasma concentration–time curve following an i.v. bolus dose of 25 mg kg−1 showed a bi-exponential decline (i.e., the initial and terminal slopes) associated with a two-compartmental model, which suggests a bi-phasic disposition process of the compound in systemic circulation. Table 2 illustrates the pharmacokinetic parameters of SQ109 derived from the compartmental analysis. The Cmax obtained by i.v. dosing was 1.04 μg ml−1, which is higher than the in vitro MIC level (0.5 μg ml−1), while the Cmax obtained by p.o. dosing was about 0.14 μg ml−1. SQ109 exhibited an elimination t1/2 of 3.5 h (i.v.) and 5.2 h (p.o.). Of particular interest was the large volume of distribution of SQ109 (Vss=11,826 ml kg−1), which was significantly higher than those of other compounds we have studied (Jia et al., 1999; 2001; 2002; 2003b). The comparison of oral AUC with i.v. AUC of SQ109 in plasma over time resulted in an apparent oral bioavailability of 4%. Erythrocyte concentration of SQ109 was approximately two-fold lower than the corresponding concentration of SQ109 in plasma through 15 min after p.o. and i.v. administration. At times beyond 15 min post-dose, the concentration of SQ109 in erythrocytes was similar to the corresponding concentration in plasma. The result indicates that erythrocyte uptake accounts for a certain proportion of the total blood concentration of SQ109.
Figure 4.
Plasma concentration–time courses of SQ109 after i.v. (•, 3 mg kg−1) and oral (○, 25 mg kg−1) administration to mice. Each point represents the mean±s.d. of 4–5 mice.
Table 2.
Compartmental analysis of pharmacokinetic parameters (mean±s.e.m.) of SQ109 in mice
| Route | i.v. | p.o. |
|---|---|---|
| Dose (mg kg−1) | 3 | 25 |
| AUC0 → ∞ (ng h−1 ml−1) | 792±369 | 254±184 |
| t1/2α (h)a | 0.07±0.051 | |
| t1/2β (h)b | 0.43±0.35 | |
| t1/2el (h)c | 3.5±6.6 | 5.2±1.1 |
| Cmax (ng ml−1) | 1038±93 | 135±10 |
| Tmax (h) | 0.31±0.06 | |
| CL (ml kg−1 h−1) | 3788±1768 | |
| Vdss (ml kg−1) | 11826±14878 | |
| Bioavailability (%) | 4 |
Half-life of the distribution phase.
Half-life of the initial elimination phase.
Half-life of the terminal elimination phase.
SQ109 tissue distribution and elimination following a single dose
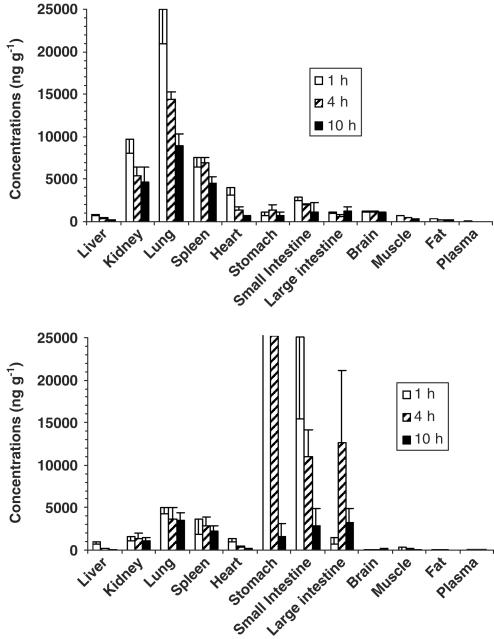
Figure 5 shows SQ109 levels in various tissues at 1, 4 and 10 h after a single dose. In general, SQ109 concentrations in lungs, kidney, spleen and heart were significantly higher than those in plasma measured at the same time, suggesting that SQ109 rapidly penetrated into the extravascular compartment and deposited there. After i.v. administration of SQ109, the highest concentration of SQ109 was in the lung. At 1 h after i.v. dosing, the lung concentration of SQ109 was 25.3±4.0 μg g−1, which was about 25-fold higher than the measured peak plasma concentration of SQ109 at 2 min following the same administration route. Spleen and kidney ranked as the tissues containing the second highest concentrations of SQ109. SQ109 stayed in the lung and spleen at higher levels than observed for other tissues and plasma for at least 10 h. The same trend was observed when SQ109 was absorbed into the systemic circulation after oral administration: by 4 and 10 h, the highest concentration of SQ109 was in the lung, followed by the spleen and kidney (Figure 5), excluding the content of SQ109 in the stomach and small intestine. These organs contained about 52.3 and 25% of the administered dose of SQ109 at 1 and 4 h, respectively, after oral dosing.
Figure 5.
Tissue levels of SQ109 after i.v. (3 mg kg−1, upper panel) and p.o. administration (25 mg kg−1, lower panel) to mice (mean±s.d., n=4–5). For clarity and comparison, the i.v. and p.o. scales were kept the same, resulting in the bars for stomach concentrations in the oral study being off scale. The actual stomach concentrations of SQ109 at 1 and 4 h were 934±300 and 376±183 μg g−1, respectively.
Levels of unchanged SQ109 excreted in the urine and feces by mice were also determined. For mice given SQ109 (3 mg kg−1, i.v.), 0.02–0.04% of the administered dose of intact SQ109 was excreted in urine, and 0.06–1% of the dose was excreted in feces from 0 to 32 h after dosing. For mice given oral SQ109 (25 mg kg−1), the levels of intact SQ109 in urine were low and accounted for less than 0.01% of the dose. The amount of SQ109 in feces from 0 to 32 h after oral administration accounted for only 2% of the dose.
Discussion
The intensity of drug pharmacologic effects of an antimicrobial is a function of the amount of drug in the body and, more specifically, the achievement of effective drug concentration at the site(s) of bacterial infection. In the present study, we evaluated the change in SQ109 concentration over time as assessed by pharmacokinetics and compared these results to the static relationship between the concentration at the infection site and the intensity of observed activity as quantified by pharmacodynamic analysis and efficacy against multiplying M. tuberculosis.
The efficacy of SQ109 against M. tuberculosis was demonstrated through in vitro macrophage model followed by further testing in an in vivo animal model. Both models were infected with the same M. tuberculosis strain H37Rv. SQ109 showed its ability to penetrate into macrophage phagosome, where M. tuberculosis replicates, and result in inhibition of the bacteria that usually behave as an intracellular parasite of macrophages in mammalian hosts. The activity of SQ109 in this regard was comparable to that of INH in the macrophage test system, but superior over that of EMB (Figure 2). With demonstrated in vitro activity, SQ109 also exerted in vivo antitubercular activity in a dose-dependent manner by reducing bacterial load of 1–1.9 log units in liver and spleen homogenates over a period of 28 days by conventional monitoring of CFU levels. INH (25 mg kg−1 day−1) was demonstrated to be more potent than SQ109 (0.1, 10 and 25 mg kg−1 day−1) and EMB (100 mg kg−1 day−1) in reducing mycobacterial burden in both spleen and lung (Table 1). The discrepancies in in vitro and in vivo activities among SQ109, INH and EMB are presently not clear, although it is likely that they reflect the differences of their molecular targets and pharmacokinetic properties.
Once it entered systemic circulation of mice after a single i.v. or p.o. administration, SQ109 rapidly reached peak concentrations in the lung and spleen far above its MIC level for M. tuberculosis, without noticeable side effects such as injection site irritation, inability to move, ruffled fur, ataxia, tremors, convulsions, emesis, diarrhea, labored breathing and acute death. SQ109 concentrations in the respiratory tract remained above the MIC for more than 10 h after oral dosing (Figure 5). This rapid transfer of compound from circulation to vascularized tissue of the lung may be related to the fact that SQ109 has an adamantane tricyclic fragment in the chemical structure (Figure 1). Adamantane-containing antiviral drugs on the market (Rimantadine and Amantadine) are used to combat viral lung pathogens like Influenza A by inhibiting initiation of infection and virus assembly (Hay et al., 1985) and by blocking the ion channels in lipid membranes of virus (Griffin et al., 2003). These drugs distribute specifically to lung and have large Vss (Hoffman et al., 1988). It is very likely that the adamantane structure is also integral for both in vivo and in vitro antitubercular activity: four EMB analogues out of seven of the most potent compounds in vitro and in vivo have an adamantyl moiety in the molecules (unpublished observation).
SQ109 possesses a large Vss, as do the other EMB analogs. Its large Vss might also be attributed to hydrophobic moieties of the compound and diamine structure that results in rapid penetration into extravascular compartments with favorable tissue kinetics, especially in the lung and spleen (Figures 3 and 5). The lung and spleen concentrations of SQ109 oscillated above the MIC over the course of daily administration. This finding suggests that orally delivered SQ109 in animal models and patients may beneficially concentrate in these organs, where replicating Mycobacteria happen during disease. That SQ109 was easily detected in organs of mice administered a 25 mg kg−1 oral dose clearly demonstrated that much of the dose was absorbed into tissues.
However, pharmacokinetic studies indicate that SQ109 has a poorer oral bioavailability than its in vivo efficacy in mycobacterial disease and tissue distribution would indicate (Table 2). The reasons for poor oral bioavailability of any compound include:
Poor oral absorption: The Cmax level of oral SQ109 was about 80-fold less than that of i.v. SQ109 when compared on the basis of same dose, suggesting issues with intestinal absorption. Many candidate drugs fail due to problems with intestinal absorption, since molecules must be able to permeate cell membranes composed of phospholipid bilayers to traverse the intestine and enter circulation. In addition, two findings in the present study argue against profound intestinal permeability issues with SQ109: (i) the drug is able to pass through several cell membranes and achieve effective concentrations in the phagosome of infected macrophages in vitro (Figure 2), and (ii) sufficient drug traversed the intestine and distributed to the lung and spleen to achieve levels far above the in vitro MIC and create an effective antimicrobial effect, even at the lowest dose tested in vivo, 0.1 mg kg−1 (Table 1).
First-pass effect: After i.v. administration, the concentration of SQ109 in liver, the main organ of drug metabolism, was lower than the concentration observed in most tissues, including the brain (Figure 5). Relative to the concentration of SQ109 in other tissues, however, the concentration of SQ109 in liver was higher after oral administration than after i.v. administration to mice (Figures 3 and 5). These data suggest that SQ109 may undergo a first-pass metabolic step in the liver after oral administration. So far we have identified four metabolites of SQ109 after incubation of the compound with mouse and human liver microsomes and recombinant CYP450 isoforms.
High dissociation constants of SQ109 from blood proteins, causing quick disappearance from the systemic circulation by tissue redistribution. The time course of tissue concentrations of SQ109 after oral administration is compatible with an initial hepatic sequestration of this material, with subsequent redistribution to the lung, spleen and kidney (Figures 3 and 5).
There are a number of drugs with apparent bioavailability issues that have good, even excellent efficacy against their target pathogen. For example, Halofuginone is a drug used to prevent coccidiosis in poultry, treat cryptosporidiosis and theileriosis in cattle, and treat various diseases in humans. This drug has no oral bioavailability (Stecklair et al., 2001). Like SQ109, Halofuginone shows concentrations in the targeted tissues 50–2000 times higher than in plasma after oral administration. Most antibiotic drugs exert their effects not within the plasma compartment but in defined targets into which these drugs must be distributed from the central compartment (Muller et al., 2004). Recent studies have further indicated that antibiotic drug levels at the target site may substantially differ from corresponding plasma drug levels, and the concentration profile at the target site is more helpful than concentration in plasma in designing clinical trials, and is an important determinant of clinical outcome (Ryan, 1993; Presant et al., 1994; Joukhadar et al., 2001). In this respect, SQ109 shows favorable targeted tissue distribution properties in relation to its antitubercular activity.
Interestingly, SQ109 appears to preferentially accumulate in the lungs and spleen over a 28-day period of repeated administration. There was statistically significant accumulation of SQ109 in these two tissues and slight increase of SQ109 in other tissues when we compare concentrations of the drug at steady state on days 14 and 28 with that achieved on day 1 after a single administration (Figure 3). When the drug disposition kinetics are in first order, tissue concentrations following multiple doses should be higher at steady state. This suggests that enzymatic involvement in the up- or downregulation of SQ109 in the tissues is not an issue. These results also explain the complex relationship between concentration–time profiles of SQ109 and its observed antitubercular effect (Figures 4 and 5).
In conclusion, SQ109 exhibited both in vitro antimicrobial activity against M. tuberculosis strain H37Rv grown inside the host murine macrophage cells and in vivo antimicrobial activity on the mouse model inoculated with the H37Rv. Oral administration of SQ109 to the mice for 28 consecutive days resulted in significant reductions in mycobacterial burden in both spleen and lungs of the mice. Monitoring SQ109 levels in mouse vital tissues in the course of 28-day oral administration showed that the potential sites of action (e.g., lungs and spleen) contained SQ109 at least 10 times more than its MIC. SQ109 displayed a large volume of distribution to various tissues. Despite its low oral bioavailability, the targeted tissue concentrations of SQ109 were at least 120-fold higher than that in plasma. This study provides important insights into the integration of in vitro efficacy parameters, into the in vivo pharmacokinetic and pharmacodynamic evaluation of SQ109, and should facilitate future clinical trials of the compound.
Acknowledgments
We are deeply grateful to Dr Carol A, Nacy for her valuable comments and suggestions on earlier drafts of this paper. The studies were supported by the Inter-institute Program of National Institutes of Health.
Abbreviations
- BSA
bovine serum albumin
- CFU
colony-forming units
- DMEM
Dulbecco's modified Eagle's medium
- EMB
ethambutol
- FBS
fetal bovine serum
- INH
isoniazid
- LC/MS/MS
liquid chromatography tandem mass spectrometry
- MIC
minimal inhibitory concentration
- PBS
phosphate-buffered saline
- RLU
relative luminescence units
- SQ109
N-Geranyl-N′-(2-adamantyl)ethane-1,2-diamine
References
- American Thoracic Society Documents American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: Treatment of Tuberculosis. Am. J. Respir. Crit. Care Med. 2003;167:603–662. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- BARRY C.E., III New horizons in the treatment of tuberculosis. Biochem. Pharmacol. 1997;54:1165–1172. doi: 10.1016/s0006-2952(97)00163-9. [DOI] [PubMed] [Google Scholar]
- BARRY C.E., III, SLAYDEN R.A., SAMPSON A.E., LEE R.E. Use of genomics and combinatorial chemistry in the development of new antimycobacterial drugs. Biochem. Pharmacol. 2000;59:221–231. doi: 10.1016/s0006-2952(99)00253-1. [DOI] [PubMed] [Google Scholar]
- COLE S.T., BROSCH R., PARKHILL J., GARNIER T., CHURCHER C., HARRIS D., GORDON S.V., EIGLMEIER K., GAS S., BARRY CE., III, TEKAIA F., BADCOCK K., BASHAM D., BROWN D., CHILLINGWORTH T., CONNOR R., DAVIES R., DEVLIN K., FELTWELL T., GENTLES S., HAMLIN N., HOLROYD S., HORNSBY T., JAGELS K., KROGH A., MCLEAN J., MOULE S., MURPHY L., OLIVER K., OSBORNE J., QUAIL M.A., RAJANDREAM M.A., ROGERS J., RUTTER S., SEEGER K., SKELTON J., SQUARES R., SQUARES S., SULSTON J.E., TAYLOR K., WHITEHEAD S., BARRELL B.G. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence (published erratum appears in Nature (1998) 396, 190) Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- CYNAMON MH., KLEMENS S.P., SHARPE C.A., CHASE S. Activities of several novel oxazolidinones against Mycobacterium tuberculosis in a murine model. Antimicrob. Agents Chemother. 1999;43:1189–1191. doi: 10.1128/aac.43.5.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DYE C., SCHEELE S., DOLIN P., PATHANIA V., RAVIGLIONE M.C. Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA. 1999;282:677–686. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- ESPINAL M.A., LASZLO A., SIMONSEN L., BOULAHBAL F., KIM S.J., RENIERO A., HOFFNER S., RIEDER H.L., BINKIN N., DYE C., WILLIAMS R., RAVIGLIONE M. Global trends in resistance to antituberculosis drugs. N. Engl. J. Med. 2001;344:1294–1303. doi: 10.1056/NEJM200104263441706. [DOI] [PubMed] [Google Scholar]
- GRIFFIN S.D.C., BEALES L.P., CLARKE D.S., WORSFOLD O., EVANS S.D., JAEGER J., HARRIS M.P.G., ROWLANDS D.J. The p7 protein of hepatitis C virus forms an ion channel that is blocked by the antiviral drug, Amantadine. FEBS Lett. 2003;535:34–38. doi: 10.1016/s0014-5793(02)03851-6. [DOI] [PubMed] [Google Scholar]
- HAY A.J., WOLSTENHOLME A.J., SKEHEL J.J., SMITH M.H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J. 1985;4:3021–3026. doi: 10.1002/j.1460-2075.1985.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOFFMAN H.E., GAYLORD J.C., BLASECKI J.W., SHALABY L.M., WHITNEY C.W., JR Pharmacokinetics and metabolism of rimantadine hydrochloride in mice and dogs. Antimicrob. Agents Chemother. 1988;32:1699–1704. doi: 10.1128/aac.32.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIA L., LINNIK M.D., JACK R.M., YU L. Biostability and pharmacokinetics of LJP920, an octameric Gal (α1–3) Gal conjugate for the inhibition of xenotransplantation rejection. Pharm. Pharmacol. 2001;53:999–1005. doi: 10.1211/0022357011776243. [DOI] [PubMed] [Google Scholar]
- JIA L., TOMASZEWSKI J.E., NOKER P., GORMAN G., GLAZE E., PROTOPOPOVA M.N. Ethambutol analogs. I. Analytic method and cassette dosing in assessing pharmacokinetics of three leads. Pharm. Sci. 2003a;5:719. [Google Scholar]
- JIA L., WONG H., CERNA C., WEITMAN S.D. Effect of nanonization on absorption of 301029: ex vivo and in vivo pharmacokinetic correlations determined by liquid chromatography/mass spectrometry. Pharm. Res. 2002;19:1090–1095. doi: 10.1023/a:1019829622088. [DOI] [PubMed] [Google Scholar]
- JIA L., WONG H., WANG Y., GARZA M., WEITMAN S.D. Carbendazim: disposition, cellular permeability, metabolite identification and pharmacokinetic comparison with its nanoparticle. J. Pharm. Sci. 2003b;92:161–172. doi: 10.1002/jps.10272. [DOI] [PubMed] [Google Scholar]
- JIA L., YOUNG X., GUO W. Physicochemistry, pharmacokinetics, and pharmacodynamics of S-nitrosocaptopril crystals, a new nitric oxide donor. J. Pharm. Sci. 1999;88:981–986. doi: 10.1021/js990108g. [DOI] [PubMed] [Google Scholar]
- JOUKHADAR C., FROSSARD M., MAYER B.X., BRUNNER M., KLEIN N., SIOSTRZONEK P., EICHLER H.G., MULLER M. Impaired target site penetration of beta-lactams may account for therapeutic failure in patients with septic shock. Crit. Care Med. 2001;29:385–391. doi: 10.1097/00003246-200102000-00030. [DOI] [PubMed] [Google Scholar]
- LEE R., PROTOPOPOVA M., CROOKS E., SLAYDEN R., TERROT M., BARRY C. Combinatorial lead optimization of [1,2]-diamines based on EMB as potential anti-tuberculosis preclinical candidates. J. Combinat. Chem. 2003;5:172–187. doi: 10.1021/cc020071p. [DOI] [PubMed] [Google Scholar]
- LENAERTS A.J.M., GRUPPO V., BROOKS J.V., ORME I.M. Rapid in vivo screening of experimental drugs for tuberculosis using gamma interferon gene-disrupted mice. Antimicrob. Agents Chemother. 2002;47:783–785. doi: 10.1128/AAC.47.2.783-785.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTOW J., JUNGBLUT P.R., MULLER E.C., KAUFMANN S.H.E. Identification of acidic, low molecular mass proteins of Mycobacterium tuberculosis strain H37Rv by matrix-assisted laser desorption/ionization and electrospray ionization mass spectrometry. Proteomics. 2001;1:494–507. doi: 10.1002/1615-9861(200104)1:4<494::AID-PROT494>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- MULLER M., PENA A.D., DERENDORF H. Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue. Antimicrob. Agents Chemother. 2004;48:1441–1453. doi: 10.1128/AAC.48.5.1441-1453.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'BRIEN R.J., NUNN P.P. The need for new drugs against tuberculosis: obstacles, opportunities, and next steps. Am. J. Respir. Crit. Care Med. 2001;162:1055–1058. doi: 10.1164/ajrccm.163.5.2007122. [DOI] [PubMed] [Google Scholar]
- PRESANT C.A., WOLF W., WALUCH V., WISEMAN C., KENNEDY P., BLAYNEY D., BRECHNER R.R. Association of intratumoral pharmacokinetics of fluorouracil with clinical response. Lancet. 1994;343:1184–1187. doi: 10.1016/s0140-6736(94)92399-x. [DOI] [PubMed] [Google Scholar]
- RYAN D.M. Pharmacokinetics of antibiotics in natural and experimental superficial compartments in animals and humans. J. Antimicrob. Chemother. 1993;31 Suppl D:1–6. doi: 10.1093/jac/31.suppl_d.1. [DOI] [PubMed] [Google Scholar]
- SNEWIN V.A., GARES M.P., GAORA P.Ó., HASAN Z., BROWN I.N., YOUNG D.B. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect. Immun. 1999;67:4586–4593. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STECKLAIR K.P., HAMBURGER D.R., EGORIN M.J., PARISE R.A., COVEY J.M., EISEMAN J.L. Pharmacokinetics and tissue distribution of halofuginone (NSC 713205) in CD2F1 mice and Fischer 344 rats. Cancer Chemother. Pharmacol. 2001;48:375–382. doi: 10.1007/s002800100367. [DOI] [PubMed] [Google Scholar]
- STOVER C.K., WARRENER P., VANDEVANTER D.R., SHERMAN D.R., ARAIN T.M., LANGHORNE M.H., ANDERSON S.W., TOWELL J.A., YUAN Y., MCMURRAY D.N., KREISWIRTH B.N., BARRY C.E., BAKER W.R. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]