Abstract
The aim of the present study was to investigate the involvement of adenosine 3′,5′-cyclic monophosphate (cAMP) cascade in the acute impairment of contraction by 17β-estradiol in porcine coronary arteries, and to elucidate the signaling pathway leading to the activation of this cascade by the hormone.
Isometric tension was recorded in isolated rings of porcine coronary arteries.
The contraction to U46619 was reduced significantly following 30 min incubation with 1 nM 17β-estradiol or 1 nM isoproterenol. There was no additive effect when 17β-estradiol and isoproterenol were administered together. The effect of 17β-estradiol was mimicked by both the cyclic AMP analogue 8-Br-cAMP and the guanosine 3′,5′-cyclic monophosphate (cyclic GMP) analogue 8-Br-cGMP.
In rings with and without endothelium, the modulatory effect of 17β-estradiol was abolished by the adenylyl cyclase inhibitor, SQ 22536, but was unaffected by the guanylyl cyclase inhibitor, ODQ.
Both the cAMP antagonist Rp-8-Br-cAMPS and the cGMP antagonist inhibitor Rp-8-Br-cGMPS inhibited the effect of 17β-estradiol.
The effect of 17β-estradiol was unaffected by the protein kinase A inhibitor, KT5720, but was abolished by the protein kinase G (PKG) inhibitor, KT5823, which also abolished the effect of isoproterenol.
These data support our earlier findings that 17β-estradiol (1 nM) acutely impairs contractile responses of porcine coronary arteries in vitro. This acute effect of 17β-estradiol involves cAMP in vascular smooth muscles and the activation of PKG.
Keywords: 17β-estradiol; cAMP; cGMP; cAMP-dependent protein kinase; cGMP-dependent protein kinase, porcine coronary artery; isoproterenol; vascular smooth muscle
Introduction
Epidemiological studies have shown that women rarely suffer from cardiovascular diseases during their premenopausal years. However, the incidence of cardiovascular diseases increases as they reach menopause (Barrett-Connor, 1997). This increase has been attributed to the lack of the female sex hormone, estrogen (Stampfer et al., 1991; Ettinger et al., 1996). Indeed, there is evidence in both clinical and animal studies that the lack of estrogen can aggravate hypertension. Estrogen supplement has been found to decrease blood pressure in women (Stonier et al., 1992; Seely et al., 1999). In spontaneously hypertensive rats, estradiol replacement also reduces blood pressure (Williams et al., 1990).
The exact mechanisms behind the cardioprotective effects of estrogen are still unknown despite the use of hormone replacement therapy. The benefits of estrogen may involve a favorable modulation of lipoprotein metabolism, with a decrease in low-density and an increase in high-density lipoproteins (Bush et al., 1987; Whitcroft et al., 1994). Antioxidant effects have also been implicated (Keaney et al., 1994; Huang et al., 1999). Besides acting indirectly on the cardiovascular system, estrogen can also exert its action directly on the vasculature. Estrogen can enhance the expression of endothelial nitric oxide synthase (eNOS) (Weiner et al., 1994), which increases the level of nitric oxide, leading to an increase in endothelium-dependent relaxation in vitro or an increase in blood flow in vivo (Gisclard et al., 1988; Williams et al., 1990).
The effects of estrogen were previously thought to involve solely genomic mechanisms. However, in recent years, short-term effects of estrogen, which do not involve the transcription and translation of genes, have been demonstrated in a vast number of cell types such as pancreatic cells (Ropero et al., 1999), uterine cells (Doolan et al., 2000), colon cells (Doolan & Harvey, 2003) and neural cells (Kelly et al., 1999). In the vascular system, nongenomic effects have been demonstrated both in vivo (Gilligan et al., 1994) and in vitro (Chen et al., 1999). Estrogen can acutely inhibit vasoconstriction by inhibiting calcium influx in vascular smooth muscles (Kitazawa et al., 1997). These effects, however, are observed with the use of high concentrations (micromolar) of estrogen, while the highest normal physiological concentration of the hormone in plasma is only at the nanomolar range (Christ & Wehling, 1998). In vascular endothelial cells, nanomolar concentrations of estrogen can acutely activate eNOS, leading to an increase in nitric oxide production (Stefano et al., 2000), which is mediated via the PI3-kinase-AKt pathway (Haynes et al., 2000). This effect is suggested to involve a novel truncated estrogen receptor α (Figtree et al., 2003), which is expressed in the plasma membrane of endothelial cells (Ihionkhan et al., 2002; Figtree et al., 2003). However, few studies demonstrated the nongenomic effect of estrogen in vascular smooth muscles at the physiological range.
Previous results from our laboratory demonstrated that even low concentrations of estrogens (1–10 nM), as opposed to the high concentrations commonly used in most studies, caused endothelium-independent effects in the vasculature (Teoh et al., 1999; Teoh & Man, 2000). This concentration of 17β-estradiol is close to the circulating concentration of 17β-estradiol in females. Nanomolar concentrations of the hormone enhanced endothelium-independent relaxation by a adenosine 3′,5′-cyclic monophosphate (cAMP)-dependent mechanism, while having no direct relaxing effect (Teoh & Man, 2000). 17β-Estradiol also impairs agonist-induced contractions in porcine coronary arteries, and this effect is independent of the presence of functional endothelium (Teoh & Man, 2000). The enhancement of relaxation together with the impairment of contraction may prove significant in the modulation of vascular tone. The present study was designed to further investigate the involvement of the cAMP cascade in the acute impairment of contraction by estrogens in porcine coronary arteries, and to elucidate the signaling pathway involved. For comparison, the effects of the β-adrenergic agonist, isoproterenol, were also studied.
Methods
Tissue preparation
Pigs were killed according to the regulation laid down by the Food and Environmental Hygiene Department of the Hong Kong Special Administrative Region. Hearts from pigs of 6 months old and of either sex were collected from the local slaughter house and rinsed in cold, oxygenated (95% O2; 5% CO2) Krebs–Henseleit solution (KHS; composition in mM: NaCl 120, KCl 4.76, NaHCO3 25, NaH2PO4 H2O 1.18, CaCl2 1.25, MgSO47H2O 1.18, glucose 5.5) before the left anterior descending and right coronary arteries were isolated. The hearts collected for experiments were from pigs that were not sexually mature. The coronary arteries were prepared for experiments as previously described (Teoh et al., 1999). After removal of the surrounding connective tissues, arteries were cut into 3 mm wide rings and suspended between stainless-steel hooks and stationary support rods positioned in 5 ml jacketed organ baths filled with oxygenated KHS maintained at 37°C. In experiments where arterial rings without functional endothelium were used, arteries were perfused with 0.25% Triton X-100 (diluted with KHS) for 30 s at a rate of 1 ml min−1 before 3 mm rings were cut. The rings were then placed under 2 × g tension for 120 min during which bath KHS was changed periodically. Isometric tension was measured by force transducers (FT03, Grass Instrument Co., Quincy, U.S.A.) coupled to an amplifier and a personal computer for data collection (PICO Data Logger, Pico Technology Ltd, Cambridge, U.K.).
Functional studies
The viability of each porcine coronary arterial ring was determined by contracting twice with 30 mM KCl before relaxing with 1 μM bradykinin. Rings that failed to produce an average contraction of greater than or equal to 4.0 g when challenged with KCl and greater than or equal to 40% relaxation to bradykinin were excluded from the study (about 10–20%). In endothelium-denuded preparations, rings with greater than or equal to 5% relaxation were discarded. Bradykinin and KCl were then removed by repeated changes of bath KHS. After baseline tensions were re-established, rings were incubated again with various drugs or vehicle. Where necessary, antagonists were introduced into the baths 20 min before addition of vehicle solvent or the studied agonist. 17β-Estradiol, cAMP and guanosine 3′,5′-cyclic monophosphate (cGMP) analogues were added 30 min prior to testing. Except where noted, all drugs remained present throughout the experiment. Contractions were produced by a stepwise addition of U46619 (9,11-dideoxy-9α-methanoepoxy prostaglandin F2; 0.1 nM to 1 μM). Each tissue was exposed to only one contracting agent. Data are expressed as percent KCl-induced contraction obtained during the viability test.
Statistical analysis
Data are reported as means±standard error of the mean (s.e. m.) with n indicating the number of porcine hearts from which arterial rings were obtained. Maximal contractions and pD2 values were determined with the aid of a curve-fitting program (SigmaPlot, SPSS Inc., Chicago, IL, U.S.A.). Statistical tests were performed using a computer statistical package (SPSS, SPSS Inc., Chicago, IL, U.S.A.). Analysis of variance (one-way ANOVA), followed by post hoc Dunnett's tests or LSD tests were applied to determine individual differences between multiple groups of data. A P-value of less than 0.05 was considered to indicate statistically significant differences.
Drugs and chemicals
U46619 was obtained from Biomol (PA, U.S.A.), and 8-Br-cAMP, 8-Br-cGMP, Rp-8-Br-cGMPS and Rp-8-Br-cAMPS were purchased from BioLog Life Science Institute (Bremen, Germany). KT5720 and KT5823 were obtained from Calbiochem Novabiochem Corporation (La Jolla, CA, U.S.A.), ODQ, PKG inhibitor, 17β-estradiol and the remaining chemicals were obtained from Sigma (St Louis, MO, U.S.A.). Stocks of 17β-estradiol, U46619 and rolipram were made up in ethanol. The final concentration of ethanol in each bath did not exceed 0.2% of the total bath volume. KT5720 and KT5823 were first dissolved in dimethyl sulfoxide. The final concentration of dimethyl sulfoxide in each bath did not exceed 0.1% of the total bath volume. All other drugs were dissolved in deionized water and all working solutions were obtained by dilution in KHS.
Results
Effect of 17β-estradiol, isoproterenol and cyclic nucleotides
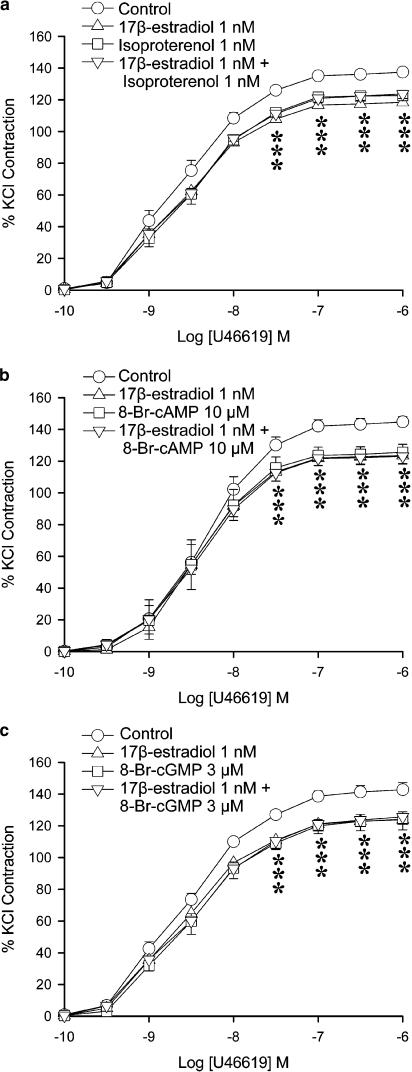
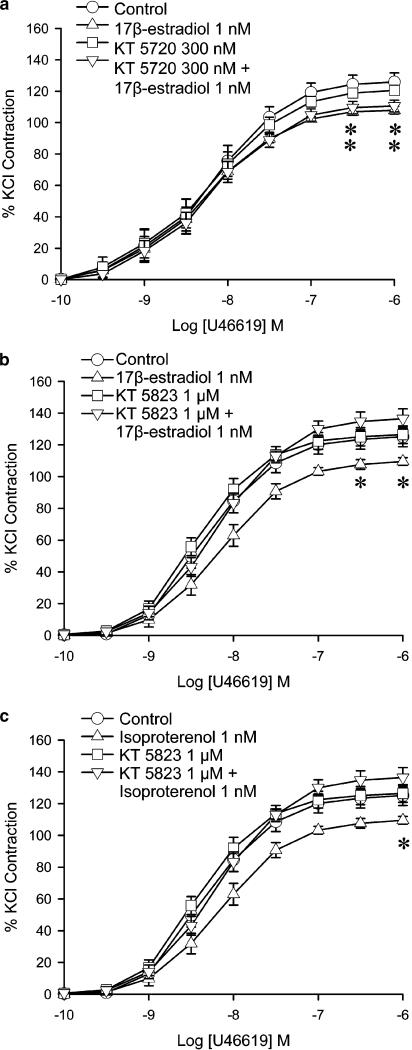
In order to study the relationship between 17β-estradiol and the cyclic nucleotide cascades, the effects of 17β-estradiol, isoproterenol and cyclic nucleotide analogues were compared. Under control conditions (vehicle control), U46619 (0.1 nM–1 μM) elicited concentration-dependent contractions, with a maximal contraction averaging 145±3.6% of the response that 30 mM KCl induced (Table 1). 17β-Estradiol (1 nM) significantly reduced the contraction at 0.1 to 1 μM of U46619 (Figure 1a). Maximal contractions were also reduced significantly (Table 1) but the curves were not shifted. Isoproterenol (1 nM–10 μM) caused a concentration-dependent relaxation in porcine coronary arteries contracted with U46619 (data not shown). At 1 nM, the percent relaxation elicited by isoproterenol was negligible. This dose (1 nM) was chosen for further studies to ensure that the β-adrenergic agonist caused no direct relaxation. At 1 nM, isoproterenol reduced the contraction to U46619 (Figure 1a), with a maximal contraction significantly different from control. The effect was similar to that of 17β-estradiol at 1 nM. When given in combination, the depression caused by 17β-estradiol (1 nM) plus isoproterenol (1 nM) was not significantly different from the effect of either agent alone. The levels of maximal contractions obtained with U46619 were also not significantly different (Figure 1a).
Table 1.
Effects of different pharmacological agents on the acute effect of 1 nM 17β-estradiol in porcine coronary arteries
| Treatment | Maximum contraction (% KCl) | |
|---|---|---|
| Control | 138±1.9 | |
| 17β-estradiol 1 nM | 119±1.4 | * |
| Isoproterenol 1 nM | 124±2.7 | * |
| 17β-estradiol 1 nM+isoproterenol 1 nM | 123±2.9 | * |
| Control | 145±3.6 | |
| 17β-estradiol 1 nM | 124±4.8 | * |
| 8-Br-cAMP 10 μM | 125±5.1 | * |
| 17β-estradiol 1 nM+8-Br-cAMP 10 μM | 123±4.8 | * |
| Control | 141±4.0 | |
| 17β-estradiol 1 nM | 122±7.1 | * |
| 8-Br-cGMP 3 μM | 122±4.1 | * |
| 17β-estradiol 1 nM+8-Br-cGMP 3 μM | 126±5.1 |
Data represent mean±s.e.m. n=6–7 in each treatment group.
P<0.05 vs corresponding controls (ANOVA–Dunnett's).
Figure 1.
Effects of 17β-estradiol and (a) isoproterenol, (b) 8-Br-cAMP and (c) 8-Br-cGMP on U46619-induced contraction. Porcine coronary arterial rings were incubated with 17β-estradiol (1 nM) and/or isoproterenol (1 nM), and/or 8-Br-cAMP (10 μM) or and/or 8-Br-cGMP (3 μM) for 30 min before cumulative addition of U46619. For each treatment group, n=6–7. *P<0.05 vs control group (ANOVA followed by post hoc Dunnett's test).
8-Br-cAMP (10 μM), a cAMP analogue, caused a similar reduction of the contraction to U46619 with a significant reduction of the maximal response (Figure 1b). Combination of 17β-estradiol (1 nM) and 8-Br-cAMP (10 μM) also caused a reduction of contraction to U46619. However, the effect was not significantly different from that obtained with either 17β-estradiol or 8-Br-cAMP alone.
8-Br-cGMP (3 μM), a cGMP analogue, caused a similar reduction of the contraction to U46619 as obtained with 17β-estradiol. The inhibitory effect of the combination of 17β-estradiol (1 nM) plus 8-Br-cGMP (3 μM) was not significantly different from that obtained with either drug alone (Figure 1c and Table 1).
Inhibitors of adenylyl cyclase and guanylyl cyclase
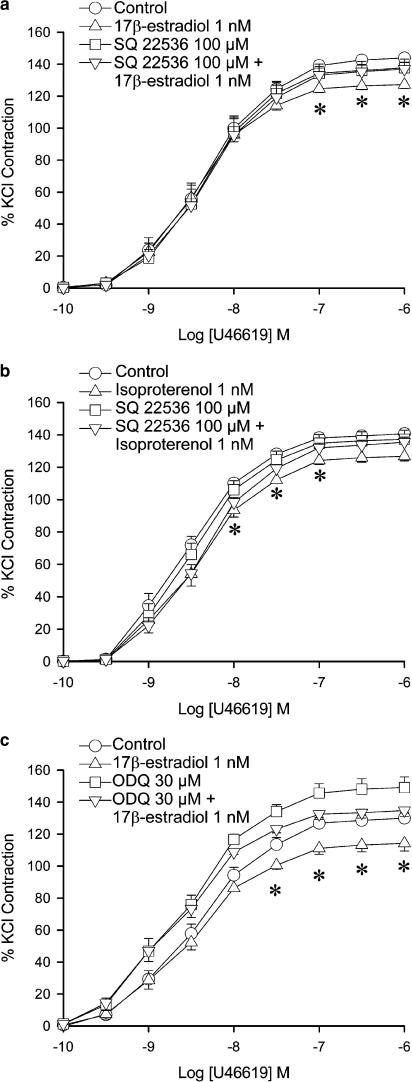
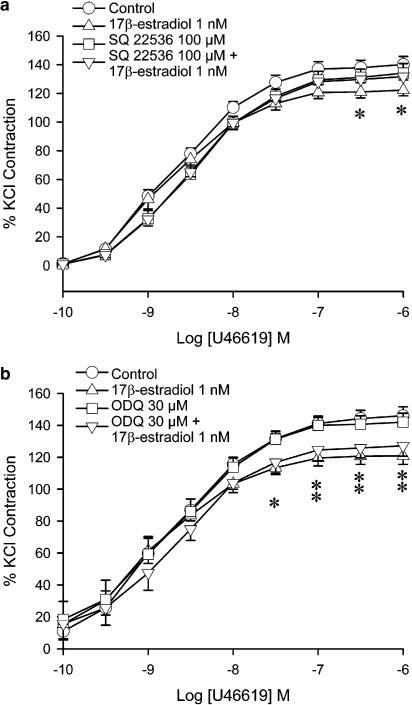
Adenylyl cyclase inhibitors and guanylyl cyclase inhibitors were used in experiments to investigate the involvement of adenylyl cyclase and guanylyl cyclase in the effect of 17β-estradiol. SQ 22536 (100 μM), an adenylyl cyclase inhibitor, exerted no significant effect on the contraction to U46619. When administered together with 17β-estradiol (1 nM), SQ 22536 abolished the reduction of contraction by the hormone (Figure 2a). The maximal contraction was no longer statistically different from that obtained in control arteries. SQ 22536 also abolished the reduction of contraction caused by isoproterenol (Figure 2b). To confirm the role of adenylyl cyclase, another inhibitor of adenylyl cyclase, 2′,5′-dideoxyadenosine was used. 2′,5′-dideoxyadenosine, which itself did not exert any effect on the contraction to U46619, abolished the effect of 17β-estradiol when the two drugs were administered together (data not shown).
Figure 2.
Effects of 17β-estradiol, adenylyl cyclase inhibitor and guanylyl cyclase inhibitor on U46619-induced contraction. Porcine coronary arterial rings were incubated with 17β-estradiol (1 nM) for 30 min. (a) SQ 22536 (100 μM) was added 20 min prior to administration of 17β-estradiol. (b) SQ 22536 (100 μM) was added 20 min prior to administration of isoproterenol. (c) ODQ (30 μM) was added 20 min prior to administration of 17β-estradiol. U46619 was then added cumulatively. Responses were calculated as a percent of the average of two KCl-induced contractions. Data are expressed as mean±s.e.m. with n=7–8. *P<0.05 vs control group (ANOVA followed by post hoc Dunnett's test).
The NO-sensitive guanylyl cyclase inhibitor, ODQ (30 μM), caused a significant increase in the maximal contraction to U46619 in rings with endothelium (Figure 2c). Administration of ODQ plus 17β-estradiol caused a reduction in the maximal contraction, compared to arteries treated with ODQ alone. The contraction was however not significantly different from that under the control condition.
Rp-diastereomers of cyclic nucleotides
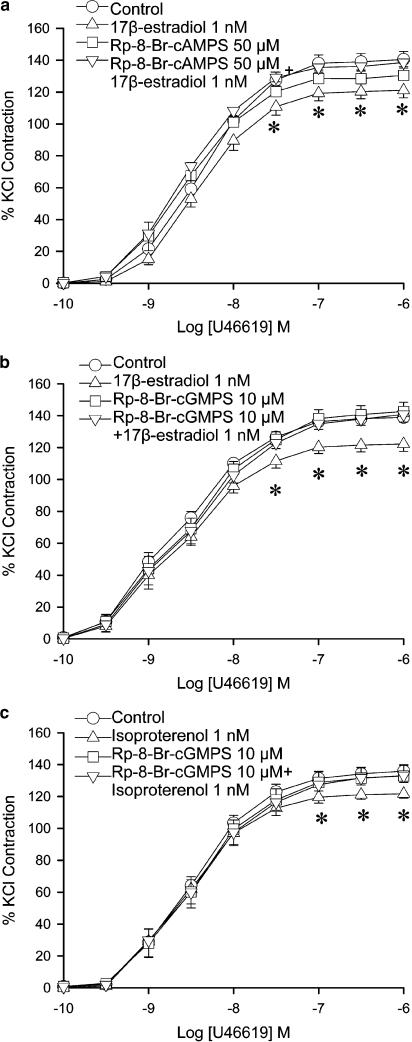
To study the role of the cAMP and cGMP in the effect of 17β-estradiol, competitive antagonists of cAMP and cGMP were used. Rp-8-Br-cAMPS (50 μM), a competitive cAMP antagonist, did not alter the contraction to U46619 in arteries with endothelium. Combined incubation with Rp-8-Br-cAMPS plus 17β-estradiol abolished the reduction in contraction caused by 17β-estradiol alone (Figure 3a). The maximal contraction to U46619 was no longer statistically different from control.
Figure 3.
Effects of 17β-estradiol and Rp-diastereomers on U46619-induced contraction. Porcine coronary arterial rings were incubated with (a) Rp-8-Br-cAMPS (50 μM) or (b) Rp-8-Br-cGMPS (10 μM) 20 min prior to administration of 17β-estradiol. (c) Rp-8-Br-cGMPS (10 μM) were added 20 min prior to administration of isoproterenol. U46619 was then added cumulatively. For each treatment group, n=5–7. *P<0.05 vs control group (ANOVA followed by post hoc Dunnett's test).
Rp-8-Br-cGMPS (10 μM) did not alter the contraction to U46619 in arteries with endothelium. The combination of Rp-8-Br-cGMPS and 17β-estradiol abolished the reduction in contraction caused by 17β-estradiol alone (Figure 3b). The maximal contraction to U46619 was comparable to control.
Contraction to U46619 in isoproterenol (1 nM)-treated arteries was significantly lower than control in arteries (at 0.1 to 1 μM, Figure 3c). The effect was blocked by the combined administration of arteries with isoproterenol plus Rp-8-Br-cGMPs (Figure 3c).
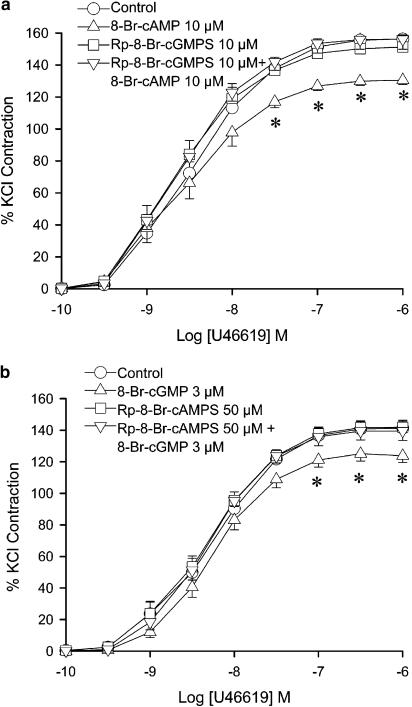
Administration of 8-Br-cAMP (10 μM) caused a decrease in contraction at U46619 concentrations of 30 nM to 1 μM, which was abolished by in the presence of Rp-8-Br-cGMPS (Figure 4a).
Figure 4.
Inhibitory effects Rp-diastereomers of cyclic nucleotides on cyclic nucleotides. Porcine coronary arterial rings were incubated with (a) 8-Br-cAMP (10 μM) and (b) 8-Br-cGMP (3 μM) for 30 min. (a) Rp-8-Br-cGMPS (10 μM) and (b) Rp-8-Br-cAMPS (50 mM) were added 20 min prior to administration of 8-Br-cAMP or 8-Br-cGMP. U46619 was then added cumulatively. Responses were calculated as a percent of the average of two KCl-induced contractions. Data are expressed as mean±s.e.m. with n=6–7. *P<0.05 vs control group (ANOVA followed by post hoc Dunnett's test).
Incubation with 8-Br-cGMP (3 μM) caused a reduction of contraction in the arteries. However, administration of Rp-8-Br-cAMPS reversed the effect of 8-Br-cGMP (Figure 4b).
Protein kinase inhibitors
Protein kinase A (PKA) inhibitors and protein kinase G (PKG) inhibitors were used in experiments to determine whether PKA or PKG were activated when 17β-estradiol was administered. KT5720 (300 nM), a PKA antagonist, did not alter the contraction to U46619 in rings with endothelium. The combination of KT5720 plus 17β-estradiol did not significantly differ from the effect of 17β-estradiol alone. The maximal contraction to U46619 was reduced to a comparable extent to that of arteries treated with 17β-estradiol alone (Figure 5a).
Figure 5.
Effects of 17β-estradiol and PKA inhibitor and PKG inhibitor on U46619-induced contraction. Porcine coronary arterial rings were incubated with 17β-estradiol (1 nM) or isoproterenol (1 nM) for 30 min. (a) The PKA inhibitor KT5720 (300 nM) was added 20 min prior to administration of 17β-estradiol. Control denotes vehicle control of 0.03% DMSO diluted in KHS. (b) The PKG inhibitor KT5823 (1 μM) was added 20 min prior to administration of 17β-estradiol. Control denotes vehicle control of 0.1% DMSO diluted in KHS. (c) KT5823 (1 μM) was added 20 min prior to administration of isoproterenol. Control denotes vehicle control of 0.1% DMSO diluted in KHS. U46619 was then added cumulatively. Responses were calculated as a percent of the average of two KCl-induced contractions. Data are expressed as mean±s.e.m. with n=6–8. *P<0.05 vs control group (ANOVA followed by post hoc Dunnett's test).
KT5823 (300 nM), a PKG antagonist, did not alter the contraction to U46619 in rings with endothelium. The presence of KT5823 abolished the effect of 17β-estradiol given alone (Figure 5b). KT5823 also abolished the effect of isoproterenol (1 nM) on the contraction (Figure 5c). Another PKG antagonist, PKG Inhibitor, also abolished the effect of 17β-estradiol (data not shown).
Involvement of endothelium on the acute impairing effects of 1 nM 17β-estradiol
The effects of SQ 22536 (100 μM) and ODQ (30 μM) on the acute impairing effect of 1 nM 17β-estradiol obtained in rings without functional endothelium were not different from those observed in rings with endothelium (Figure 6). SQ 22536 caused a reversal of effect of 17β-estradiol in endothelium-disrupted arterial rings (Figure 6a). ODQ did not affect the impairment of contraction by 17β-estradiol (Figure 6b). Rp-8-Br-cAMPS and Rp-8-Br-cGMPS abolished the effect of 17β-estradiol on contraction to agonists in arteries with disrupted endothelium (data not shown).
Figure 6.
Effects of 17β-estradiol, adenylyl cyclase inhibitor and guanylyl cyclase inhibitor on U46619-induced contraction in endothelium-disrupted arterial rings. Endothelium-disrupted porcine coronary arterial rings were incubated with 17β-estradiol (1 nM) for 30 min. (a) SQ 22536 (100 μM) was added 20 min prior to administration of 17β-estradiol. (b) ODQ (30 μM) was added 20 min prior to administration of 17β-estradiol. U46619 was then added cumulatively. Responses were calculated as a percent of the average of two KCl-induced contractions. Data are expressed as mean±s.e.m. with n=6–8. *P<0.05 vs control group (ANOVA followed by post hoc Dunnett's test).
Discussion
Our earlier studies showed that agonist-induced contractions in porcine coronary arteries are attenuated by 17β-estradiol (1 nM) after 30 min exposure (Teoh et al., 1999). This concentration of 17β-estradiol has no direct relaxing effect on the arteries. The modulatory effects with physiologically relevant concentrations of 17β-estradiol did not appear to involve the classical cytosolic steroid receptors or result from nuclear transcription and translation. The present study, using the same model, demonstrates pharmacologically that the acute impairment of U46619 contractions of porcine coronary arteries by 17β-estradiol is cAMP dependent. Similar results were observed with 5-hydroxytryptamine (data not shown). Indeed, one important feature of the present study is the use of physiologically relevant concentrations of 17β-estradiol to elicit a functionally measurable decrease in arterial contraction, and to demonstrate the involvement of cAMP in this phenomenon. While it has been shown previously in vascular smooth muscle cells (Farhat et al., 1996; Christ et al., 1999) that estrogen can increase the production of cAMP, this was only observed using pharmacological concentrations of the hormone. Indeed, using the porcine coronary artery model, we also found an increase in cAMP level in the arterial rings after exposure to 17β-estradiol at a high (100 μM) concentration (data not shown). However, at 1 nM, we could not demonstrate an appreciable change in the level of cAMP. To our knowledge, only the present study and our previous study (Teoh & Man, 2000) showed that the effect of physiological concentration of estrogen in blood vessels involves cAMP. Although the magnitude of impairment demonstrated in this study is relatively small, the combined effects of impairing contraction (this study) and enhancing relaxation (Teoh & Man, 2000) could contribute significantly to the modulation of vascular tone, preventing blood pressure variability, organ damage and vascular remodeling.
In previous works, we demonstrated that 8-Br-cAMP caused an enhancement of endothelium-independent relaxation (Teoh & Man, 2000). Here, we showed that the same concentration of 8-Br-cAMP caused a decrease in maximal contraction to U46619. This shift was similar in terms of both magnitude and latency to that produced by 1 nM 17β-estradiol. The β-adrenoceptor agonist, isoproterenol, also caused a similar shift in the maximal contraction. When 17β-estradiol was administered with either 8-Br-cAMP or isoproterenol, there was no significant difference between the results obtained with each of the drugs alone. This suggests that 17β-estradiol and isoproterenol act through the same intracellular pathway, namely, by elevating cAMP levels.
The adenylyl cyclase inhibitors, SQ 22536 and 2′,5′-dideoxyadenosine, abolished the effect of 17β-estradiol. This suggests that the acute effect of 17β-estradiol is dependent on the activation of adenylyl cyclase, as is the case with isoproterenol (Bhalla & Sharma, 1982). The finding that the effect of 17β-estradiol involves the activation of adenylyl cyclase is in accord with studies in other tissues, including neurons (Kelly et al., 1999), uterine smooth muscles (Doolan et al., 2000) and kidney cells (Stock et al., 1992). In hypothalamic neurons, the activation of adenylyl cyclase by estrogen has been found to be G-protein coupled (Kelly et al., 1999; Qiu et al., 2003). The role of G-protein in the observed increase in cAMP in this study needs to be further investigated.
Administration of ODQ, an NO-sensitive guanylyl cyclase inhibitor, failed to attenuate the impairment of contraction by 17β-estradiol. The effect of 17β-estradiol was also not affected by the removal of the endothelium. This eliminates the contribution of nitric oxide to the observed effect. It has been demonstrated in rat aorta that short-term treatment with 17β-estradiol at micromolar concentration reduced maximal contraction to vasoconstricting agents via an endothelium-independent mechanism (Andersen et al., 1999). In the human coronary artery, the effect of 17β-estradiol on relaxation is also endothelium independent (Mügge et al., 1993). The inability to demonstrate an increase in NO by 17β-estradiol could be due to the fact that 17β-estradiol only causes a transient activation of eNOS with a duration of not more than 20 min (Chen et al., 1999; Stefano et al., 2000).
Treatment of porcine coronary arteries with Rp-8-Br-cAMPS inhibited the impairment of contraction by 17β-estradiol and 8-Br-cGMP. However, treatment of porcine coronary arteries with the PKA inhibitor, KT5720, did not significantly block the effect of 17β-estradiol. Rp-8-Br-cAMPS competes for the cAMPS binding domain on PKA. However, binding of Rp-8-Br-cAMPS results in the ‘locking' of the regulatory subunit to the catalytic subunit (Jackson, 1996). KT5720 is a staurosporine-like compound that binds to the ATP binding of the catalytic subunit of PKA (Spicuzza et al., 2001), inhibiting the catalysis of its phosphorylation of target proteins. The use of KT5720 as an effective PKA inhibitor has been demonstrated in isolated tissues (Dhankoti et al., 2000) as well as in cultured vascular smooth muscle cells (Purdy & Arendshorst, 2001). The lack of inhibition of KT5720 on the effect of 17β-estradiol in our study suggests that the effect is not mediated by PKA. The major mechanism for relaxation of the cAMP-elevating agent isoproterenol in vascular smooth muscles is PKA independent and may involve the activation of BKCa channels (White et al., 2001).
The effect of 17β-estradiol was abolished by the competitive antagonist of cGMP, Rp-8-Br-cGMPS, which binds to the cyclic nucleotide-binding sites of PKG. Rp-8-Br-cGMPS also inhibits the impairment of contraction by 8-Br-cAMP. Indeed, it has been demonstrated that competitive antagonists of cGMP block the effect elicited by cAMP in the vascular smooth muscle (White et al., 2000). The findings that the specific PKG antagonists KT5823 and PKGI, which inhibit the ATP-binding site of PKG, inhibited the effect of 17β-estradiol strongly suggest that activation of PKG by cAMP is the major pathway mediating the effect of 17β-estradiol in reducing contraction of vascular smooth muscles. In porcine coronary arteries, cAMP analogues can open BKCa channels. The effect can only be blocked by the PKG inhibitor KT5823 but not PKA inhibitors (White et al., 2000). The finding that the impairment of contraction elicited by isoproterenol (1 nM) was also inhibited by KT5823 and PKGI suggests that the cross-activation of PKG by cAMP is not unique to 17β-estradiol. Indeed, cross-activation of PKG by cAMP elevating agents has been reported. It has been demonstrated in porcine coronary arteries that dopamine increases the level of cAMP, which cross-activates PKG to open BKCa channels (Han et al., 1999). Similar effects are also obtained with forskolin (White et al., 2000).
One of the possible limitations of this study is that the coronary arteries of pig hearts were obtained from a local slaughter house and we were not able to determine the sex of the animals from which the arteries were obtained. In our study, these pigs were around 6 months old and about 60 kg in size. Male pigs were castrated soon after birth while female pigs were not ovariectomized. Pigs normally reach sexual maturity at around 1 year of age. Breeding does not start until they reach 1.5 years of age. Thus, we can assume that the animals used in our study were not sexually mature and had a low circulating level of sex hormones (both testosterone and estrogen). It is therefore reasonable to find no differential effects between data obtained from the two sexes, and the combination of data from both sexes should not affect our conclusion. However, further work is needed to address the issue of the effect of 17β-estradiol in coronary arteries of hearts from sexually mature male and female pigs.
In conclusion, we report that 17β-estradiol (1 nM) acutely impairs the contractile responses in porcine coronary arteries. This effect is dependent on the activation of the adenylyl cyclase and involves an increase in cAMP levels in vascular smooth muscles, which cross-activates PKG. The mechanism of action may be similar to the mechanism of other cAMP elevating agents that affect vascular tone. This acute impairment of contraction may partially explain the beneficial effects of estrogen on the vasculature.
External data objects
Acknowledgments
We would like to thank the Sheung Shui Slaughter House for supplying the porcine hearts. We also thank Godfrey S.K. Man for his indispensable technical assistance. W.K. was a recipient of postgraduate studentship from the University of Hong Kong. The study was supported by a grant from the Seed Funding Programme for Basic Research of the University of Hong Kong.
Abbreviations
- cAMP
adenosine 3′,5′-cyclic monophosphate
- cGMP
guanosine 3′,5′-cyclic monophosphate
- eNOS
endothelial nitric oxide synthase
- KCl
potassium chloride
- KHS
Krebs–Henseleit solution
- PKA
protein kinase A
- PKG
protein kinase G
- U46619
9,11-dideoxy-9α-methanoepoxyprostaglandin F2
Supplementary information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- ANDERSEN H., WEIS J.U., FJALLAND B., KORSGAARD N. Effect of acute and long-term treatment with 17-β-estradiol on the vasomotor responses in the rat aorta. Br. J. Pharmacol. 1999;126:159–168. doi: 10.1038/sj.bjp.0702289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARRETT-CONNOR E. Sex differences in coronary heart disease. Why are women so superior? The 1995 Ancel Keys Lecture. Circulation. 1997;95:252–264. doi: 10.1161/01.cir.95.1.252. [DOI] [PubMed] [Google Scholar]
- BHALLA R.C., SHARMA R.V. Characteristics of hormone-stimulated adenylate cyclase in vascular smooth muscle: altered activity in spontaneously hypertensive rat. Blood Vessels. 1982;19:109–116. [PubMed] [Google Scholar]
- BUSH T.L., BARRETT-CONNOR E., COWAN L.D., CRIQUI M.H., KIND B.M. Cardiovascular mortality and noncontraceptive use of estrogen in women: results from the Lipid Research Clinics Program Follow-up Study. Circulation. 1987;75:1102–1109. doi: 10.1161/01.cir.75.6.1102. [DOI] [PubMed] [Google Scholar]
- CHEN Z., YUHANNA I.S., GALCHEVA-GARGOVA Z., RICHARD H.K., MENDELSOHN M.E., SHAUL P.W. Estrogen receptor α mediates the nongenomic activation of endothelial nitric oxide synthase by estrogen. J. Clin. Invest. 1999;103:401–406. doi: 10.1172/JCI5347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRIST M., GUNTHER A., HECK M., SCHMIDT B.M.W., FALKENSTEIN E., WEHLING M. Aldosterone, not estradiol, is the physiological agonist for rapid increases in cAMP in vascular smooth muscle cells. Circulation. 1999;99:1485–1491. doi: 10.1161/01.cir.99.11.1485. [DOI] [PubMed] [Google Scholar]
- CHRIST M., WEHLING M. Cardiovascular steroid actions: swift swallows or sluggish snails. Cardiovasc. Res. 1998;40:34–44. doi: 10.1016/s0008-6363(98)00147-3. [DOI] [PubMed] [Google Scholar]
- DHANKOTI S.N., GAO Y., NGUYEN M.Q., RAJ J.U. Involvement of cGMP-dependent protein kinase in the relaxation of ovine pulmonary arteries to cGMP and cAMP. J. Appl. Physiol. 2000;88:1637–1642. doi: 10.1152/jappl.2000.88.5.1637. [DOI] [PubMed] [Google Scholar]
- DOOLAN C.M., CONDLIFFE S.B., HARVEY B.J. Rapid non-genomic activation of cytosolic cAMP-dependent protein kinase activity and [Ca2+]I by 17β-estrardiol in female rat distal colon. Br. J. Pharmacol. 2000;129:1375–1386. doi: 10.1038/sj.bjp.0703193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOOLAN C.M., HARVEY B.J. A Gαs protein-coupled membrane receptor, distinct from the classical oestrogen receptor, tranduces rapid effects of oestradiol on [Ca2+]I in female rat distal colon. Mol. Cell. Endocrinol. 2003;199:87–103. doi: 10.1016/s0303-7207(02)00303-9. [DOI] [PubMed] [Google Scholar]
- ETTINGER N., FRIEDMAN G.D., BUSH T., QUESENBERRY C.P., JR Reduced mortality associated with long-term postmenopausal estrogen therapy. Obstet. Gynecol. 1996;87:6–12. doi: 10.1016/0029-7844(95)00358-4. [DOI] [PubMed] [Google Scholar]
- FARHAT M.Y., ABI-YOUNES S., DINGAAN B., VARGAS R., RAMWELL P.W. Estradiol increases cyclic adenosine monophosphate in rat pulmonary vascular smooth muscle cells by a nongenomic mechanism. J. Pharmacol. Exp. Ther. 1996;276:652–657. [PubMed] [Google Scholar]
- FIGTREE G.A., MCDONALD D., WATKINS H., CHANNON K.M. Truncated estrogen receptor α 46-kDa isoform in human endothelial cells: relationship to acute activation of nitric oxide synthase. Circulation. 2003;107:120–126. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- GILLIGAN D.M., BADAR D.M., PANZA J.A., QUYYUMI A.A., CANNON R.O., III Acute vascular effects of estrogen in postmenopausal women. Circulation. 1994;90:786–791. doi: 10.1161/01.cir.90.2.786. [DOI] [PubMed] [Google Scholar]
- GISCLARD V., MILLER V.M., VANHOUTTE P.M. Effect of 17β-estradiol on endothelium-dependent responses in the rabbit. J. Pharmacol. Exp. Ther. 1988;244:19–22. [PubMed] [Google Scholar]
- HAN G., KRYMAN J.P., MCMILLIN P.J., WHITE R.E., CARRIER G.O. A novel transduction mechanism mediating dopamine-induced vascular relaxation: opening of BKCa channels by cyclic AMP-induced stimulation of the cyclic GMP-dependent protein kinase. J. Cardiovasc. Pharmacol. 1999;34:619–627. doi: 10.1097/00005344-199911000-00001. [DOI] [PubMed] [Google Scholar]
- HAYNES M.P., SINHA D., RUSSELL K.S., COLLINGE M., FULTON D., MORALES-RUIZ M., SESSA W.C., BENDER J.R. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- HUANG M., LI J., TEOH H., MAN R.Y.K. Low concentrations of 17beta-estradiol reduce oxidative modification of low-density lipoproteins in the presence of vitamin C and vitamin E. Free Radic. Biol. Med. 1999;27:438–441. doi: 10.1016/s0891-5849(99)00086-6. [DOI] [PubMed] [Google Scholar]
- IHIONKHAN C.E., CHAMBLISS K.L., GIBSON L.L., HAHNER L.D., MENDELSOHN M.E., SHAUL P.W. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ. Res. 2002;91:814–820. doi: 10.1161/01.res.0000038304.62046.4c. [DOI] [PubMed] [Google Scholar]
- JACKSON W.F. Rp diastereomeric analogs of cAMP inhibit both cAMP- and cGMP-induced dilation of hamster mesenteric small arteries. Pharmacology. 1996;52:226–234. doi: 10.1159/000139387. [DOI] [PubMed] [Google Scholar]
- KEANEY J.F., SHWAERY G.T., XU A.-M. 17β-Estradiol preserves endothelial vasodilator function and limits low-density lipoprotein oxidation in hypercholesterolemic swine. Circulation. 1994;89:2251–2259. doi: 10.1161/01.cir.89.5.2251. [DOI] [PubMed] [Google Scholar]
- KELLY M.J., LAGRANGE A.H., WAGNER E.J., RONNEKLEIV O.K. Rapid effects of estrogen to modulate G protein-coupled receptors via activation of protein kinase A and protein kinase C pathways. Steroid. 1999;64:64–75. doi: 10.1016/s0039-128x(98)00095-6. [DOI] [PubMed] [Google Scholar]
- KITAZAWA T., HAMADA E., KITAZAWA K., GAZNABI A.K.M. Non-genomic mechanism of 17β-oestradiol-induced inhibition of contraction in mammalian vascular smooth muscle. J. Physiol. (London) 1997;499:497–511. doi: 10.1113/jphysiol.1997.sp021944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MÜGGE A., RIEDEL M., BARTON M., KUHN M., LICHTLEN P.R. Endothelium independent relaxation of human coronary arteries by 17β-estradiol in vitro. Cardiovasc. Res. 1993;27:1939–1942. doi: 10.1093/cvr/27.11.1939. [DOI] [PubMed] [Google Scholar]
- PURDY K.E., ARENDSHORST W.J. Iloprost inhibits inositol-1,4,5-trisphosphate-mediated calcium mobilization stimulated by angiotensin II in cultured preglomerular vascular smooth muscle cells. J. Am. Soc. Nephrol. 2001;12:19–28. doi: 10.1681/ASN.V12119. [DOI] [PubMed] [Google Scholar]
- QIU J., BOSCH M.A., TOBIAS S.C., GRADY D.K., SCALAN T.S., RONNEKLEIV O.K., KELLY M.J. Rapid signaling of estrogen in hypothalamic neurons involves a noel G-protein-coupled estrogen receptor that activates protein kinase C. J. Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROPERO A.B., FUENTES E., ROVIRA J.M., RIPOLL C., SORIA B., NADAL A. Non-genomic actions of 17beta-oestradiol in mouse pancreatic beta-cells are mediated by a cGMP-dependent protein kinase. J. Physiol. 1999;521:397–407. doi: 10.1111/j.1469-7793.1999.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEELY E.W., WALSH B.W., GERHARD M.D., WILLIAMS G.W. Estradiol with or without progesterone and ambulatory blood pressure in postmenopausal women. Hypertension. 1999;33:1190–1194. doi: 10.1161/01.hyp.33.5.1190. [DOI] [PubMed] [Google Scholar]
- SPICUZZA L., BELVISI M.G., BIRRELL M.A., BARNES P.J., HELE D.J., GIEMBYCZ M.A. Evidence that the anti-spasmogenic effect of the β-adrenoceptor agonist, isoprenaline, on guinea-pig trachealis is not mediated by cAMP-dependent protein kinase. Br. J. Pharmacol. 2001;133:1201–1212. doi: 10.1038/sj.bjp.0704213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STAMPFER J.M., COLDITZ F.A., WILLETT W.C., MANSON J.E., ROSNER B., SPEIZER F.E., HENNEKENS C.H. Postmenopausal estrogen therapy and cardiovascular disease. N. Engl. J. Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- STEFANO G.B., PREVOT V., BEAUVILLAIN J.-C., CADET P., FIMIANI C., WELTERS I., FRICCHIONE G.L., BRETON C., LASSALLE P., SALZET M., BILFINGER T.V. Cell-surface estrogen receptors mediate calcium-dependent nitric oxide release in human endothelia. Circulation. 2000;101:1594–1597. doi: 10.1161/01.cir.101.13.1594. [DOI] [PubMed] [Google Scholar]
- STOCK J.L., CODERRE J.A., BURKE E.M., DANNER D.B., CHIPMAN S.D., SHAPIRO J.R. Identification of estrogen receptor mRNA and the estrogen modulation of parathyroid hormone-stimulated cAMP accumulation in opossum kidney cells. J. Cell. Physiol. 1992;150:517–525. doi: 10.1002/jcp.1041500312. [DOI] [PubMed] [Google Scholar]
- STONIER C., BENNETT J., MESSENGER E.A., ABER G.M. Oestradiol-induced hypotension in spontaneously hypertensive rats: putative role for intracellular cations, sodium-potassium flux and prostanoids. Clin. Sci. 1992;82:389–395. doi: 10.1042/cs0820389. [DOI] [PubMed] [Google Scholar]
- TEOH H., LEUNG S.W.S., MAN R.Y.K. Short-term exposure to physiological levels of 17 beta-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc. Res. 1999;42:224–231. doi: 10.1016/s0008-6363(98)00265-x. [DOI] [PubMed] [Google Scholar]
- TEOH H., MAN R.Y.K. Enhanced relaxation of porcine coronary arteries after acute exposure to a physiological level of 17β-estradiol involves non-genomic mechanisms and the cyclic AMP cascade. Br. J. Pharmacol. 2000;198:1739–1747. doi: 10.1038/sj.bjp.0703252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THROCKMORON D.C., PACKER C.S., BROPHY C.M. Protein kinase C activation during Ca2+-independent vascular smooth muscle contraction. J. Surg. Res. 1997;78:48–53. doi: 10.1006/jsre.1998.5368. [DOI] [PubMed] [Google Scholar]
- WEINER C.P., LIZASOAIN I., BAYLIS S.A., KNOWLES R.G., CHARLES I.G., MONCADA S. Induction of calcium-dependent nitric oxide synthases by sex hormones. Proc. Natl. Acad. Sci. U.S.A. 1994;91:5212–5216. doi: 10.1073/pnas.91.11.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITCROFT S.I., CROOK D., MARSH M.S., ELLERINGTON M.C., WHITEHEAD M.I., STEVENSON J.C. Long-term effects of oral and transdermal hormone replacement therapies on serum lipid and lipoprotein concentrations. Obstet. Gynecol. 1994;84:222–226. [PubMed] [Google Scholar]
- WHITE R., BOTTRILL F., SIAU D., HILEY C.R. Protein kinase A-dependent and -independent effects of isoproterenol in rat isolated mesenteric artery: interactions with levcromakalim. J. Pharmacol. Exp. Ther. 2001;298:917–924. [PubMed] [Google Scholar]
- WHITE R., KRYMAN J.P., EL-MOWAFY A.M., HAN G., CARRIER G.O. cAMP-dependent vasodilators cross-activate the cGMP-dependent protein kinase to stimulate BKCa channel activity in coronary artery smooth muscle cells. Circ. Res. 2000;86:897–905. doi: 10.1161/01.res.86.8.897. [DOI] [PubMed] [Google Scholar]
- WILLIAMS J.K., ADAMS M.R., KLOPFENSTEIN H.S. Estrogen modulates responses of atherosclerotic coronary arteries. Circulation. 1990;81:1680–1687. doi: 10.1161/01.cir.81.5.1680. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.