Abstract
M Adenosine 5′-triphosphate (ATP) is known to augment cardiac contractile activity and cause an increase in intracellular Ca2+ concentration ([Ca2+]i) in isolated cardiomyocytes. However, no information regarding the ATP-mediated signal transduction in the myocardium in congestive heart failure (CHF) is available.
CHF due to myocardial infarction (MI) in rats was induced by the occlusion of the left coronary artery for 8 weeks. The positive inotropy due to ATP was depressed in failing hearts. Treatment of 3 weeks infarcted animals with imidapril (1 mg kg−1 day−1) for a period of 5 weeks improved the left ventricle function and decreased the attenuation of inotropic response to ATP.
ATP-induced increase in [Ca2+]i was significantly depressed in cardiomyocytes isolated from the failing heart and this change was partially attenuated by imidapril treatment. However, the binding characteristics of 35S-labeled adenosine 5′-(γ-thio) triphosphate in sarcolemma isolated from the failing heart remained unaltered.
ATP-induced increase in [Ca2+]i was depressed by verapamil and cibacron blue in both control and failing heart cardiomyocytes; however, the ATP response in the failing hearts, unlike the control preparations, was not decreased by ryanodine. This insensitivity to ryanodine was attenuated by imidapril treatment.
Treatment of infarcted rats with enalapril and losartan produced effects similar to imidapril.
These findings indicate that the positive inotropic response to ATP and ATP-induced increase in [Ca2+]i in cardiomyocytes are impaired in heart failure. Furthermore, blockade of renin angiotensin system prevented the impairment of the ATP-mediated inotropic and [Ca2+]i responses in the failing heart.
Keywords: ATP-mediated responses, congestive heart failure, isolated cardiomyocytes, intracellular calcium, angiotensin blockade
Introduction
Progressive deterioration of cardiac performance is a major abnormality associated with congestive heart failure (CHF) due to myocardial infarction (MI) (Houser et al., 2000). Several investigators have demonstrated that impairment of cardiomyocyte contractility (Alpert et al., 2002; Hasenfuss & Pieske, 2002) due to abnormal intracellular Ca2+ handling is the principle cause of diminished cardiac performance of the failing heart (Beuckelmann et al., 1992; Holt et al., 1998; Lindner et al., 1998; Zhang et al., 1999). Alterations in the activities of sarcolemmal (SL) Na+– Ca2+ exchanger (Sipido et al., 2002), L-type Ca2+ channels (Dixon et al., 1990; Chen et al., 2002), Na+–K+-ATPase (Schwinger et al., 1999), sarcoplasmic reticulum (SR) Ca2+-pump (Hasenfuss & Pieske, 2002) and Ca2+-release channels or ryanodine receptors (Marks et al., 2002) as well as myofibrillar Ca2+-stimulated ATPase (Wang et al., 2002) have been linked with abnormal intracellular Ca2+ regulation and impaired cardiac performance in heart failure. Although some studies have demonstrated that abnormality of intracellular Ca2+ and contractile dysfunction at the cardiomyocyte level is not a prerequisite for reduced cardiac function in failing hearts (Anand et al., 1997; Gupta et al., 2000; Yoshida et al., 2001), their observations with selected single cardiomyocytes appear to reflect cellular heterogeneity in the failing myocardium and thus their interpretation is of limited nature.
Several lines of experimental and clinical evidence support the view that the sympathetic nervous system is activated in heart failure (Packer et al., 1987; Wang & Dhalla, 2000). Thus an increased sympathetic tone and increased levels of plasma norepinephrine (NE) can be seen to activate β-adrenoceptors to maintain cardiac output in compensated stages of heart failure (Dhalla et al., 1997). However, prolonged activation of sympathetic system has been shown to result in depressing cardiac performance mainly due to the downregulation of β-adrenoceptor-mediated signal transduction (Dhalla et al., 1997). Since adenosine 5′-triphosphate (ATP) is released from the sympathetic nerve terminals as a cotransmitter along with NE (Burnstock, 1995; Vassort, 2001) and is known to exert synergistic effect with NE on cardiac contractility (DeYoung & Scarpa, 1987; Zheng et al., 1992), it is possible that there occurs a loss of purinergic support during the development of heart failure. This view is based on the observations that downregulation of purinergic (P2X1) receptors has been reported in resistance arteries isolated from animals with heart failure due to MI (Malmsjo et al., 1999). Although Hou et al. (1999) have shown that mRNA levels for purinergic P2X1 receptors were upregulated in the hearts undergoing CHF, no information regarding the status of ATP-induced signal transduction mechanisms in heart failure is available in the literature. It should also be pointed out that in addition to sympathetic nerve endings, a substantial amount of ATP is released from different local sources under pathophysiological conditions (Vassort, 2001). Furthermore, ATP has been demonstrated to modify contractile force development by increasing the intracellular concentration of Ca2+ ([Ca2+]i) primarily due to its interaction with purinergic receptors in cardiomyocytes (Wang et al., 1999; Vassort, 2001). Thus it is considered important to examine if the responsiveness of myocardium to ATP is altered in heart failure.
Since rennin–angiotensin system (RAS) is known to play an important role in the pathophysiology of CHF, various pharmacological interventions, such as angiotensin-converting enzyme (ACE) inhibitors and angiotensin II type 1 receptor (AT1R) antagonists, have shown beneficial effects in experimental animals (Sanbe et al., 1995; Wang et al., 2002; 2003; Guo et al., 2003) and patients with heart failure (Konstam, 2003). The protective effects of ACE inhibitors are mainly mediated by reduction of infarct size (Ertl et al., 1982), improvement of cardiac contractility, reduction in cardiac hypertrophy and inhibition of cardiac remodeling (Litwin et al., 1991). In addition, ACE inhibitors have been reported to normalize the β-adrenoceptor signal transduction derangements and subsequent improvement in β-adrenoceptor responses in failing hearts (Makino et al., 2003; Sethi et al., 2004). Likewise, treatment of infarcted animals with AT1R antagonists has been reported to prevent MI-induced changes in cardiac performance and downregulation of β-adrenoceptor mechanisms (Guo et al., 2003; Sethi et al., 2004). However, the effect of ACE inhibitors or AT1R antagonists on ATP receptor-mediated responses in CHF has not been studied. The present study, therefore, was undertaken (a) to evaluate whether the reduced cardiac performance in CHF subsequent to MI is associated with abnormal intracellular Ca2+ handling in cardiomyocytes, (b) to investigate the effect of exogenous ATP on cardiac performance as well as [Ca2+]i in cardiomyocytes from the MI hearts, and (c) to examine if the blockade of RAS by an ACE inhibitor, imidapril (Wang et al., 2002), can modify the purinergic receptor responses to ATP in the failing heart. The selection of imidapril for this study was mainly due to the fact that this long-acting ACE inhibitor has been reported to reduce mortality due to coronary artery disease in mice to a greater extent than other ACE inhibitors (Ogiku et al., 1994). In addition, imidapril has been shown to reduce mortality and prevent subcellular remodeling in the MI rat model of CHF (Ren et al., 1998; Tappia et al., 1999; Wang et al., 2002; 2003). Some experiments were also carried out to test if another ACE inhibitor, enalapril (Fujii et al., 2002), and an AT1R blocker, losartan (Ruzicka et al., 1999), produce effects similar to those seen with imidapril.
Methods
Experimental model
Male Sprague–Dawley rats (175–200 g) were used in the present study. The animals were maintained at 23±1°C with a constant humidity 55±5% and kept at a 12 h day/night cycle. Tap water and rat chow were provided ad libitum. All protocols were approved by the University of Manitoba Animal Care Committee in accordance with standards of the Canadian Council for Animal Care. MI was induced in the rats by occlusion of the left coronary artery as described previously (Afzal & Dhalla, 1992; Tappia et al., 1999). Briefly, the animals were anesthetized with 5% isoflurane in oxygen at a flow rate of 2 l min−1. After shaving the thoracic fur, an incision was made along the left sternal border, the fourth rib was cut proximal to the sternum and retractors were inserted. The pericardial sac was pierced to exteriorize the heart through the intercostal space and the left coronary artery was ligated 2–3 mm from its origin with a suture of 6-0 silk. The heart was repositioned in the chest and the incision was closed with a purse-string suture. Throughout the operative procedure, the rats were maintained on a positive-pressure ventilation system delivering 2.5% isoflurane in oxygen. In this study, we employed 200 animals for coronary artery ligation and 132 animals with MI were employed for further experiments. The mortality of experimental animals operated in this manner was about 30% within first 48 h postsurgery. Sham control animals were operated in the same manner except that the coronary artery was not ligated.
Drug treatments
At 3 weeks after the surgery, the animals were allowed to recover and randomly divided into four groups: sham and MI animals without any treatment (Sham, MI), and sham and MI animals with imidapril treatment (Sham+IMP, MI+IMP). In some of the experiments, animals of MI group were treated with enalapril or losartan (MI+ENL, MI+LOS). All the drugs including imidapril (1 mg kg−1 day−1), enalapril (10 mg kg−1 day−1) and losartan (20 mg kg−1 day−1) were administered orally by gastric tube starting at the end of 3rd week following the surgery to the respective groups; the treatments were continued for 5 weeks. It is pointed out that the scar in the infarcted animals is completely healed at 3 weeks of coronary occlusion whereas the early signs of CHF begin to appear at 4 weeks (Dixon et al., 1990; Afzal & Dhalla, 1992). The doses and routes of administration of all these agents were selected on the basis of previous studies (Wang et al., 2002; Guo et al., 2003; Sethi et al., 2004). For the general measurements, the rats were killed by decapitation, the hearts were surgically removed and ventricles were dissected and weighed. The scar tissue was separated from the left ventricles.
Hemodynamic studies
The animals were anesthetized with an intraperitoneal (i.p.) injection of a mixture of ketamine (60 mg kg−1) and xylazine (10 mg kg−1). The right carotid artery was exposed and cannulated with a microtip pressure transducer (model SPR-249, Millar Instruments, Houston, TX, U.S.A.), which was introduced through a proximal arteriotomy (Afzal & Dhalla, 1992). The systolic pressure and diastolic pressure in aorta were measured and the mean arterial pressure (MAP) was calculated. The catheter was advanced carefully through the lumen of the carotid artery until the tip of the transducer entered the left ventricle (LV); the catheter was secured with a silk ligature around the artery. After 15 min stabilization of the heart function, left ventricular end diastolic pressure (LVEDP), left ventricular systolic pressure (LVSP), maximum rates of pressure development (+dP/dt) and pressure decay (−dP/dt) were recorded by using the computer program AcqKnowledge for Windows 3.03 (MP100, BIOPAC Systems Inc., Goleta, CA, U.S.A.). The left ventricular developed pressure (LVDP) was calculated by subtracting the LVEDP from LVSP. In another set of experiments, ATP (1 μmol kg−1) was injected through the jugular vein. The selection of this dose of ATP was based on our preliminary experiments showing optimal inotropic effect in control animals. The LV function was assessed immediately as ATP is quickly degraded by ectonucleotidases (Vassort, 2001).
Isolation of cardiomyocytes
Ventricular myocytes were isolated by the method as described previously (Xu et al., 1997). Briefly, rats from some experimental groups after 8 weeks of surgery were injected with heparin (10 U g−1 body wt, i.p.) and anesthetized with a mixture of ketamine (60 mg kg−1) and xylazine (10 mg kg−1). The heart was rapidly excised, mounted on Langendorff's apparatus and perfused initially with Ca2+-free buffer (pH 7.4) containing (in mM) NaCl 90, KCl 10, KH2PO4 1.2, MgSO4 5, NaHCO3 15, taurine 30, glucose 20, gassed with 95% O2 and 5% CO2 for 10 min at 37°C followed by perfusion with the same buffer containing 0.04% collagenase, 0.1% bovine serum albumin (BSA) and 50 μM CaCl2. At the end of a 30 min recirculation period, the heart was removed from the cannula and the atria were excised. The viable LV including septum was cut into small pieces and subjected to another 30 min of digestion with a fresh collagenase solution in the presence of 1% BSA gassed with a mixture of 95% O2 and 5% CO2 in a shaking water bath at 37°C. The ventricular fragments were triturated gently (twice per minute) with a plastic pipette. The cells from 3–4 harvests were combined and filtered through a 200 μM nylon mesh. The myocytes were allowed to sediment followed by successive resuspension and sedimentation for 10 min in buffers containing gradually increasing extracellular concentrations of Ca2+ (100, 250, 500 and 750 μM) to a final concentration of 1 mM. While increasing the extracellular concentration of Ca2+, cardiomyocytes were allowed to settle and the supernatant was removed each time to reduce the number of other cells contaminating the cardiomyocyte preparation. The rod-shaped quiescent myocytes comprised more than 85% of the final cell population.
Measurement of [Ca2+]i
Freshly isolated adult cardiomyocytes were incubated with 5 μM Fura-2 acetoxymethylester (Fura-2 AM) for 40 min at 37°C in Krebs–Henseleit buffer (pH 7.4). containing (in mM) NaCl 90, KCl 10, KH2PO4 1.2, MgSO4 5, NaHCO3 15, glucose 20 with 1 mM Ca2+ and then washed twice to remove any extracellular dye. The final cell number in cuvette was adjusted to 0.3 million cells ml−1. The alteration in fluorescence intensity was monitored by an SLM DMX-1100 dual-wavelength spectrofluorometer adjusted at excitation wavelength 340/380 nm, emission wavelength 510 nm, integration time 0.95 s and resolution time 1.0 s at room temperature. The [Ca2+]i levels were calculated according to the Grynkiewicz equation (Grynkiewicz et al., 1985):
Kd is the effective dissociation constant and was taken as 224 for all the [Ca2+]i measurements. The ratio (R) of the fluorescence signals at 340/380 nm was calculated automatically. Rmax and Rmin values were determined by the addition of 20 μl Triton X-100 (10%) and 40 μl EGTA (400 mM), respectively. Both Sf2 and Sb2 are the fluorescence proportionality coefficients obtained at 380 nm (excitation wavelength) under Rmin and Rmax conditions, respectively. Treatment with various pharmacological agents including verapamil, cibacron blue and ryanodine was performed by incubating the Fura-2 AM-loaded cells in the buffer containing the desired concentration of pharmacological agent for 10–20 min at room temperature prior to the measurement of fluorescence. The increase in [Ca2+]i at the peak transient was calculated as the net increase above the basal value in each experiment. This method of [Ca2+]i measurement is similar to that described previously (Xu et al., 1997). It should be pointed out that Fura-2 AM is commonly used for the measurement of [Ca2+]i spectrofluorometrically because the fluorescent ratio obtained at two wavelengths minimizes the artifacts related to changes in [Ca2+]i.
Isolation of SL membranes and [35S]ATPγS binding assay
Cardiac heavy SL membranes, which sediment at low centrifugal forces, were isolated from the hearts by the hypotonic shock–LiBr treatment method (Dhalla et al., 1981). The purified SL membrane pellet was suspended in 25 mM sucrose, 50 mM Tris-HCl (pH 7.4) at a concentration of 3–5 mg ml−1, stored at −80°C, and used within 2–3 weeks without any loss of activity. Protein concentration of the membranes was determined by the method of Lowry et al. (1951). The status of purinergic receptors in the SL membrane was determined by studying the binding characteristics of a slowly hydrolyzable analog of ATP, 35S-labeled adenosine 5′-(γ-thio) triphosphate ([35S]ATPγS) (Eckstein, 1985). Membrane protein (30–50 μg) was incubated in a volume of 0.5 ml medium containing various concentrations of [35S]ATPγS (0.5–10 nM) and 50 mM Tris-HCl (pH 7.5) at 37°C for 30 min as described previously (Zhao & Dhalla, 1990). The reaction was terminated by vacuum filtration over wet Whatman filters (GF/B), using a cell harvester (M-24R, Brandel, Gaithersburg, MD, U.S.A.). The filters were washed three times with 6 ml of ice-cold deionized water and the radioactivity on the filters was counted with a Beckman scintillation counter. The binding was determined in the absence (total) and presence of 4 mM ATP (nonspecific); the specific binding was calculated by subtracting nonspecific binding from the total binding. To avoid possible artifacts, the binding of radioligand GF/B filters was checked in the absence of membrane protein from the assay tubes. The values of dissociation constant (Kd) and maximum receptor density (Bmax) were calculated by Scatchard plot analysis as described earlier (Zhao & Dhalla, 1990).
Statistical analysis
All results were expressed as mean±s.e.m. Statistical analysis was performed by using Microcal Origin Version 6 (Microcal Software Inc.). The differences between two groups were evaluated by Student's t-test. The data from more than two groups were evaluated by one-way analysis of variance (ANOVA) followed by Duncan's multiple comparison tests. P<0.05 was considered statistically significant.
Drugs and chemicals
ATP, verapamil, ryanodine and cibacron blue were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A). Enalapril and losartan were supplied by Merck Research Laboratories (Rahway, NJ, U.S.A.). Fura-2 AM and collagenase (Type II, 295 U mg−1) were purchased from Molecular Probes (Eugene, OR, U.S.A.) and Worthington Biochemical Co. (Freehold, NJ, U.S.A.), respectively. [35S]ATPγS was purchased from NEN Life Sciences Products (Boston, MA, U.S.A.) with specific gravity=65 Ci mmol−1. Imidapril hydrochloride was kindly supplied by Tanabe Seiyaku Co. Ltd, Osaka, Japan. All other reagents were of analytical grade and purchased either from Sigma Chemical Co. (St Louis, MO, U.S.A.) or Fisher Scientific (Fair Lawn, NJ, U.S.A.).
Results
General characteristics and hemodynamic parameters in experimental animals subsequent to MI
Occlusion of the left coronary artery for 8 weeks resulted in scar formation in the LV. Cardiac hypertrophy in infarcted animals was indicated by an increase of viable LV and right ventricle (RV) weights as well as the increased heart to body weight ratio compared to sham control values (Table 1). Treatment of 3 weeks infarcted animals with imidapril (1 mg kg−1 day−1) for 5 weeks caused attenuation of these parameters; however, no change in scar weight in MI animals was observed after imidapril treatment. Additionally, the animals with MI also showed an increase in LVEDP and decrease in contractile function with respect to both +dP/dt and −dP/dt; these changes in LV function were partially normalized by imidapril treatment for 5 weeks (Table 1). On the other hand, heart rate, MAP and LVDP in MI animals were not altered significantly when compared to those in sham control animals. Similarly, no alterations in general characteristics and LV function were observed after imidapril treatment in sham control animals (Table 1).
Table 1.
General and hemodynamic characteristics of animals with CHF with or without imidapril treatment
| Parameter | Sham | Sham+IMP | MI | MI+IMP |
|---|---|---|---|---|
| Body wt (g) | 522±17 | 452±8 | 487±11 | 470±15 |
| RV wt (mg) | 263±10 | 270±20 | 580±12* | 384±10† |
| Scar wt (mg) | ND | ND | 237±28 | 241±20 |
| Viable LV wt (mg) | 902±22 | 900±40 | 1129±30* | 1018±12† |
| Heart wt/body wt (mg g−1) | 2.4±0.3 | 2.5±0.4 | 3.58±0.1* | 2.8±0.3† |
| Heart rate (beats min−1) | 284±13 | 251±17 | 284±23 | 294±10 |
| MAP (mmHg) | 106±5 | 102±4 | 98±5 | 96±7 |
| LVEDP (mmHg) | 3.4±0.4 | 3.2±0.5 | 14.9±0.8* | 6.3±0.4† |
| LVDP (mmHg) | 120±7 | 125±7 | 110±9 | 120±12 |
| +dP/dt (mmHg s−1) | 9500±500 | 7500±800 | 4243±788* | 6665±941† |
| −dP/dt, (mmHg s−1) | 9700±600 | 9200±400 | 4145±478* | 9620±1184† |
Values are mean±s.e.m. of five animals for each group. The viable left ventricle (LV) wt of the experimental animals refers to the weight of the LV plus septum after removal of the scar tissue. Sham, operated but the coronary artery was not ligated; MI, myocardial infarcted with coronary artery ligated; IMP, imidapril was given orally (1 mg kg−1 day−1) starting at the end of 3rd week following the surgery and continued for 5 weeks; RV, right ventricle; MAP, mean arterial pressure; LVEDP, left ventricular end diastolic pressure; LVDP, left ventricular developed pressure; +dP/dt, maximum rate of pressure development; −dP/dt, maximum rate of pressure decay; ND, not detectable;
P<0.05 vs sham control value;
P<0.05 vs MI value.
Inotropic effect of ATP in MI rats
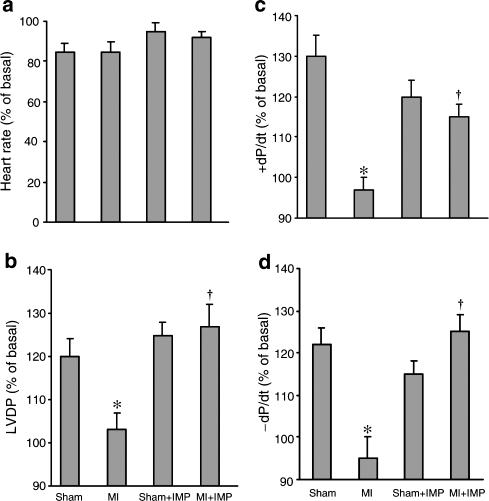
In order to examine if the positive inotropic effect of ATP is attenuated in heart failure, ATP was injected intravenously in the anesthetized animals. ATP was found to increase LVDP, +dP/dt and −dP/dt in both control and MI groups; however, these responses to ATP were significantly depressed (P<0.05) in the MI group in comparison to control (Figure 1). Treatment with imidapril normalized the attenuated inotropic effects of ATP in the failing hearts. The positive inotropic action of ATP in control animals remained unaltered after imidapril treatment (Figure 1). It can also be seen from Figure 1 that no change in the heart rate was observed following ATP administration in any group.
Figure 1.
Positive inotropic and chronotropic responses to ATP (1 μmol kg−1) in animals with CHF with or without imidapril (IMP) treatment (1 mg kg−1 day−1). Values are mean±s.e.m. of six animals in each group. Sham: operated but not ligated; MI: myocardial infarcted with coronary artery ligated; imidapril (IMP) was given orally (1 mg kg−1 day−1). *P<0.05 compared with sham group; †P<0.05 compared with MI.
Effect of ATP on cardiomyocyte [Ca2+]i in MI rats
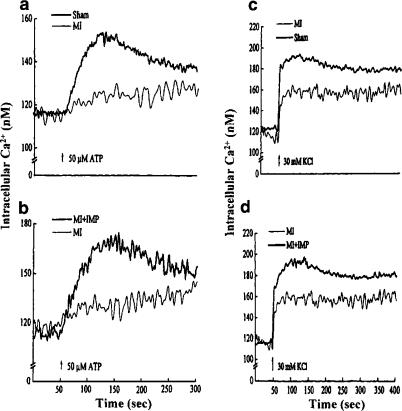
In order to test if the reduced ATP responsiveness in MI occurs at the cardiomyocyte level, [Ca2+]i in cardiomyocytes from failing hearts was measured upon exposure to 50 μM ATP. In agreement with the previous studies in freshly isolated cardiomyocytes (Zheng et al., 1990; Xu et al., 1997), an increase in [Ca2+]i by ATP was observed in cardiomyocytes isolated from sham control animals (Table 2 and Figure 2). A significant depression in ATP-induced increase in [Ca2+]i was observed in MI as shown in Table 2 and Figure 2. Treatment of MI animals with imidapril resulted in complete normalization of ATP-mediated increase in [Ca2+]i in failing cardiomyocytes, whereas no alteration in [Ca2+]i was observed in sham control animals after imidapril treatment (Table 2 and Figure 2). Attenuation of [Ca2+]i response in failing cardiomyocytes was not limited to ATP because the response of KCl (30 mM), a known depolarizing agent (Xu et al., 1997), was also depressed in MI cardiomyocytes as compared to sham control animals (Table 2 and Figure 2). Imidapril treatment caused an improvement in KCl response in failing cardiomyocytes similar to that for ATP (Table 2 and Figure 2). In addition, no alteration in KCl-induced increase in [Ca2+]i was observed in sham control animals after imidapril treatment. Basal [Ca2+]i remained unaltered in cardiomyocytes isolated from all groups in the presence of ATP or KCl (Table 2).
Table 2.
ATP-induced and KCl-induced increase in [Ca2+ ]i in cardiomyocytes from CHF animals with or without imidapril treatment
| ATP | KCl | |||
|---|---|---|---|---|
| Basal [Ca2+]i (nM) | Increase in [Ca2+]i (% of basal value) | Basal [Ca2+]i (nM) | Increase in [Ca2+]i (% of basal value) | |
| Sham | 120±10 | 33±2.3 | 122±6 | 65±3.8 |
| Sham+IMP | 125±12 | 32±4.4 | 125±5 | 69±3.8 |
| MI | 118±6 | 12±2.1* | 116±4 | 29±2.6* |
| MI+IMP | 116±5 | 32±2.8† | 118±5 | 60±2.4† |
Values are mean±s.e.m. of five experiments in each group. The concentration of ATP and KCl were 50 μM and 30 mM, respectively. The increase in [Ca2+ ]i was measured 100 s after the addition of ATP and 75 s after the addition of KCl. Sham, operated but without ligation of coronary artery; MI, myocardial infarcted with coronary artery ligated; imidapril (IMP) was given orally (1 mg kg−1 day−1).
P<0.05 vs sham control value;
P<0.05 vs MI value.
Figure 2.
Representative tracings of [Ca2+]i alteration in cardiomyocytes isolated from left ventricles of animals with CHF after stimulation with exogenous ATP (50 μM) and KCl (30 mM). Sham: operated but not ligated; MI: myocardial infarcted with coronary artery ligated; imidapril (IMP) was given orally (1 mg kg−1 day−1).
Effect of different concentrations of ATP on [Ca2+]i in MI cardiomyocytes
In order to examine if the attenuation of ATP-induced increase in [Ca2+]i was due to a reduced sensitivity of cardiomyocytes to ATP, alterations in [Ca2+]i in both control and experimental preparations were examined at different concentrations of extracellular ATP (5–100 μM). Concentration-dependent increase in [Ca2+]i was observed in cardiomyocytes isolated from sham control animals, whereas a comparable reduction in ATP-mediated increase in [Ca2+]i was observed in cardiomyocytes isolated from MI hearts (Table 3). Treatment with imidapril attenuated the depression of ATP-induced increase in [Ca2+]i in the presence of all concentrations of ATP in cardiomyocytes from the failing heart (Table 3). The concentration of ATP required for half-maximal response in all three groups was about 25 μM. From Table 3, it appears that unlike the MI and MI+IMP groups, the cardiomyocytes in the sham group did not exhibit maximal response at 100 μM ATP. However, the increase in [Ca2+]i in the sham cardiomyocytes by 125 or 150 μM ATP was found not to be different from that with 100 μM ATP (data not shown). Thus 100 μM ATP was observed to produce maximal increase in [Ca2+]i in all three experimental groups (Table 3). It is also pointed out that the concentration of ATP (50 μM) employed for studying changes in [Ca2+]i in cardiomyocytes is much higher than the Kd value (about 12 nM) for ATP binding in SL preparation; this may be due to changes in the characteristics of purinergic receptors or loss of some factors responsible for their sensitivity to ATP during the isolation of cardiomyocytes and/or SL preparations. Although the dose of ATP used in hemodynamic experiments was observed to produce optimal inotropic action, we did not estimate the concentration of ATP in the blood. Thus, some caution should be exercised while seeking a relationship between in vitro and in vivo doses of ATP.
Table 3.
Increase in [Ca2+]i in cardiomyocytes due to different concentrations of ATP in animals with CHF with or without imidapril treatment
| Increase in intracellular concentration of Ca2+ (% of basal) | |||
|---|---|---|---|
| ATP (μM) | Sham | MI | MI+IMP |
| 5 | 6±0.4 | 2.3±0.3* | 5.2±0.2† |
| 10 | 14±0.8 | 5.1±0.7* | 11.1±0.6† |
| 25 | 21±1.7 | 7.5±1.1* | 14.8±0.9† |
| 50 | 36±2.3 | 13.8±0.8* | 28.5±1.4† |
| 100 | 44±3.5 | 15.2±0.6* | 27.6±1.7† |
Values are mean±s.e.m. of four animals in each group. The basal value for [Ca2+]i in cardiomyocytes varied between 108 and 125 nM Ca2+ and there was no difference (P<0.05) among groups. The increase in [Ca2+]i was measured 100 s after the addition of ATP. Sham, operated but not ligated; MI, myocardial infarcted with coronary artery ligated; imidapril (IMP) was given orally (1 mg kg−1 day−1).
P<0.05 vs sham control value;
P<0.05 vs MI value.
Significance and mechanisms of ATP-induced increase in [Ca2+]i in failing cardiomyocytes
In order to determine the functional significance of ATP-induced increase in [Ca2+]i, cardiomyocytes were isolated from infarcted animals at different intervals after occluding the coronary artery. It should be pointed out that studies from our laboratory (Dixon et al., 1990; Afzal & Dhalla, 1992; Wang et al., 2002) have revealed that the infarcted animals at 4, 8 and 16 weeks of coronary occlusion were at early, moderate and severe stages of CHF, whereas those at 2 weeks were at prefailure stage. The data in Table 4 indicate a progressive decrease in ATP-induced increase in [Ca2+]i during 4–16 weeks of inducing MI, whereas no changes in ATP-induced increase in [Ca2+]i was evident at 2 weeks. These alterations were prevented upon treating the infarcted animals with imidapril. No changes in basal [Ca2+]i in cardiomyocytes from untreated and treated animals at different periods following coronary occlusion were observed.
Table 4.
ATP-induced increase in [Ca2+]i in cardiomyocytes at different times after the induction of MI in animals with or without imidapril
| Untreated animals | Treated animals | |||
|---|---|---|---|---|
| Basal [Ca2+]i (nM) | ATP-induced increase in [Ca2+]i (% of basal) | Basal [Ca2+]i (nM) | ATP-induced increase in [Ca2+]i (% of basal) | |
| Sham | 113±5.4 | 35±1.7 | 119±6.6 | 32±0.8 |
| 2 weeks | 119±4.7 | 34±1.5 | 116±3.9 | 30±1.7 |
| 4 weeks | 116±7.5 | 26±1.4* | 121±4.1 | 33±1.5† |
| 8 weeks | 121±4.9 | 11±0.8* | 119±3.9 | 26±2.3† |
| 16 weeks | 116±5.2 | 6±1.2* | 120±5.2 | 24±3.4† |
Values are mean±s.e.m. of six animals in each group. [Ca2+]i was measured 100 s after the addition of ATP (50 μM).
P<0.05 vs sham control values;
P<0.05 vs corresponding values for the untreated group.
To investigate the mechanism of the improved ATP-induced increase in [Ca2+]i in cardiomyocytes isolated from CHF animals treated with imidapril, isolated cardiomyocytes from all the groups were incubated with verapamil, an L-type Ca2+-channel blocker (Afzal et al., 1989), cibacron blue, an ATP-receptor blocker (Musat & Dhalla, 1996), and ryanodine, an agent that prevents the release of Ca2+ from SR stores (Chen & van Breemen, 1993) prior to ATP exposure. Treatment with verapamil and cibacron blue resulted in a significant reduction of ATP-induced increase in [Ca2+]i in both sham control cardiomyocytes and failing cardiomyocytes (Table 5). On the other hand, ryanodine treatment caused a significant depression in ATP-induced increase in [Ca2+]i in cardiomyocytes isolated from control hearts but did not affect the ATP-induced increase in [Ca2+]i in cardiomyocytes from failing hearts (Table 5). However, verapamil, cibacron blue and ryanodine caused a significant reduction in the ATP response in cardiomyocytes isolated from the imidapril-treated group (Table 5).
Table 5.
Effects of Ca2+ antagonist (verapamil), Ca2+-release channel blocker (ryanodine) and ATP receptor blocker (cibacrone blue) on the increase in [Ca2+]i due to ATP in cardiomyocytes isolated from animals with CHF with or without imidapril treatment
| Sham | Sham+IMP | MI | MI+IMP | |
|---|---|---|---|---|
| Without drug | 34±4 | 33±3 | 14±2 | 30±2 |
| Verapamil (10 μM) | 2±2* | 1±1* | 2±3* | 3±3* |
| Ryanodine (10 μM) | 15±4* | 12±3* | 11±3 | 19±4* |
| Cibacron blue (100 μM) | 9±5* | 7±4* | 4±4* | 9±5* |
Values are mean±s.e.m. of five animals and are presented as % increase of the basal value of [Ca2+]i in each group. Sham, operated but not ligated; MI, myocardial infarcted with coronary artery ligated; imidapril (IMP) was given orally (1 mg kg−1 day−1) starting at the end of 3rd week following the surgery and continued for 5 weeks. The cells were preincubated with the drugs for 10 min at room temperature.
P<0.05 vs without drug. The concentration of ATP was 50 μM.
In order to examine if the depressed increase in [Ca2+]i in failing cardiomyocytes was due to a defect at the purinergic receptor level, the specific binding of [35S]ATPγS, a slow-hydrolyzable analog of ATP (Eckstein, 1985), to cardiac SL membranes isolated from sham control and infarcted animals treated with or without imidapril was determined. No differences in the ATP binding characteristics (Kd and Bmax) were observed in all experimental groups (Table 6).
Table 6.
Changes in ATP binding characteristics in sarcolemma from left ventricle of animals with CHF with or without imidapril treatment
| Kd (nM) | Bmax (pmol/mg) | |
|---|---|---|
| Sham | 11.9±0.8 | 10.1±0.5 |
| Sham+IMP | 10.2±1.1 | 9.2±1.0 |
| MI | 10.2±0.7 | 9.3±0.5 |
| MI+IMP | 10.4±0.7 | 11.6±0.8 |
Values are mean±s.e.m. of four experiments in each group. Sham, operated but not ligated; MI, myocardial infarcted with coronary artery ligated; imidapril (IMP) was given orally (1 mg kg−1 day−1) starting at the end of 3rd week following the surgery and continued for 5 weeks. Maximal binding (Bmax) and dissociation constant (Kd) were determined from the Scatchard plot for the specific ATP binding.
Modification of MI-induced changes by enalapril and losartan treatments
In order to determine if the actions of imidapril are simulated by the blockade of RAS, we examined the effects of enalapril, another ACE inhibitor (Fujii et al., 2002), and losartan, an AT1 receptor blocker (Ruzicka et al., 1999). The data presented in Table 7 show that both enalapril and losartan improved the LV function of the failing heart as the depressed contractile activities with respect to both +dP/dt and −dP/dt were increased and the LVEDP was decreased. No significant change in heart rate and MAP was observed in any of the groups. Partial normalization of the ATP-induced increase in [Ca2+]i in failing cardiomyocytes was also observed with both enalapril and losartan treatments (Table 7). On the other hand, LV function and ATP-induced increase in [Ca2+]i were not altered in sham control animals after treatment with enalapril and losartan (data not shown in Table 7). Similarly, basal [Ca2+]i was not changed after treatments with these pharmacological interventions in both control and experimental groups (Table 7).
Table 7.
Cardiac performance and ATP-induced increase in [Ca2+]i in cardiomyocytes from animals with congestive heart failure with or without enalapril and losartan treatments
| Sham | MI | MI+ENL | MI+LOS | |
|---|---|---|---|---|
| Cardiac performance | ||||
| Heart rate (beats min−1) | 291±10 | 282±14 | 278±13 | 284±13 |
| MAP (mmHg) | 103±7 | 101±8 | 96±11 | 104±9 |
| LVEDP (mmHg) | 4.1±0.3 | 16.2±0.7* | 7.1±0.5† | 6.9±0.6† |
| LVDP (mmHg) | 118±5 | 108±6 | 120±7 | 123±6 |
| +dP/dt (mmHg s−1) | 9652±520 | 4243±788* | 6712±879† | 6843±633† |
| −dP/dt (mm Hg s−1) | 9581±645 | 4100±233* | 9675±1256† | 9822±1236† |
| [Ca2+]i in cardiomyocytes | ||||
| Basal [Ca2+]i (nM) | 112±7.5 | 117±5.4 | 120±4.6 | 118±3.7 |
| ATP-induced increase in [Ca2+]i (% of basal) | 34.1±2.8 | 14.2±1.4* | 26.5±1.8† | 27.2±1.4† |
Values are mean±s.e.m. of six animals in each group. [Ca2+]i was measured 100 s after the addition of ATP (50 μM). Sham, operated but not ligated; MI, myocardial infarcted with coronary artery ligated; enalapril (ENL) (10 mg kg−1 day−1) and losartan (LOS) (20 mg kg−1 day−1) were given orally starting at the end of 3rd week following the surgery and continued for 5 weeks; MAP, mean arterial pressure; LVEDP, left ventricular end diastolic pressure; LVDP, left ventricular developed pressure; +dP/dt, maximum rate of pressure development; −dP/dt, maximum rate of pressure decay;
P<0.05 vs sham control value;
P<0.05 vs MI value.
Discussion
In the present study, we have shown that MI in rats was associated with cardiac hypertrophy and heart dysfunction as reflected by elevated LVEDP, and decreased LV +dP/dt and −dP/dt. These changes due to MI are in agreement with our previous observations in the same experimental model of CHF (Shao et al., 1999; Wang et al., 2002). Administration of exogenous ATP, a known purinergic receptor agonist (Vassort, 2001), caused a significant increase in LV function in control hearts, whereas such an increase was attenuated in MI hearts. This observation indicates a depression in the positive inotropic response of the failing heart to ATP. Malmsjo et al. (1999) have also shown the depression in ATP-mediated contractile response in the mesenteric arteries from rats with heart failure. Similarly, the pressor response to an ATP analog, that causes activation of purinergic receptors was markedly attenuated in CHF (Zhao et al., 2000). Since the inotropic effect of ATP is mediated by its action on purinergic receptors on cardiomyocytes and subsequent increase in [Ca2+]i (Danziger et al., 1988; Legssyer et al., 1988), it is possible that the attenuated positive inotropic response to ATP in the failing heart may be due to the changes in the purinergic receptors or associated signal transduction mechanisms. Although extracellular ATP-induced increase in [Ca2+]i was attenuated in cardiomyocytes isolated from failing hearts, no changes in the affinity (1/Kd) or density (Bmax) of ATP receptors were observed in failing hearts due to MI. Furthermore, mRNA levels for purinergic receptors in the failing hearts were increased rather than decreased (Hou et al., 1999). In addition, sensitivity of cardiomyocytes to ATP remained unaltered in failing hearts as the concentrations of ATP required for producing half-maximal increase in [Ca2+]i in cardiomyocytes from control and MI hearts were comparable. Thus it appears that the attenuated positive inotropic responses to ATP in heart failure may not be due to any defect in the ATP receptors. Any defect in the purinergic receptors is further excluded by our own observations that the ATP-induced increase in [Ca2+]i in cardiomyocytes from the failing hearts was markedly decreased by cibacron blue, a well-known ATP receptor antagonist (Musat & Dhalla, 1996). Since the increase in [Ca2+]i due to KCl, a known depolarizing agent (Xu et al., 1997), was also depressed in cardiomyocytes from the failing heart, it is evident that the postreceptor defect in [Ca2+]i handling may be responsible for altered responsiveness to ATP in failing hearts.
ATP-induced increase in [Ca2+]i has been suggested to be due to an influx of Ca2+ via the L-type Ca2+ channel (Christie et al., 1992), which further triggers the mobilization of Ca2+ from SR (Sheu et al., 1986; DeYoung & Scarpa, 1987). Since verapamil, a Ca2+-channel antagonist was observed to decrease markedly the ATP-induced increase in [Ca2+]i in cardiomyocytes from both control and failing hearts, it is likely that the influx of Ca2+ via L-type Ca2+ channel may be preserved in CHF. In this regard, it should be noted that, although a reduction in the L-type Ca2+-channel density in the failing heart has been reported (Dixon et al., 1990), an increase in its phosphorylation has also been demonstrated (Chen et al., 2002), and this may serve as a compensatory mechanism to maintain L-type Ca2+-channel function in heart failure. On the other hand, the ATP-induced increase in [Ca2+]i in failing cardiomyocytes, unlike control preparations, was not depressed by ryanodine, an agent that blocks Ca2+-release channels or depletes SR Ca2+ stores (Chen & van Breemen, 1993). Accordingly, it is suggested that abnormal [Ca2+]i handling at the SR level may be partly responsible for attenuation of the ATP responses in the failing heart. Indeed, defective SR Ca2+ handling due to altered expression of SR proteins has been reported in CHF due to MI (Afzal & Dhalla, 1992; Shao et al., 1999). In addition, protein kinase A-mediated hyperphosphorylation of the ryanodine receptor has also been suggested to contribute to an impaired regulation of ryanodine receptors in the failing heart (Marx et al., 2000). In view of the observations that the ATP-mediated signal transduction mechanism involves the activation of phospholipase C (PLC) (Legssyer et al., 1988) and dramatic changes in PLC isoforms have been identified in the MI-induced heart failure (Tappia et al., 1999), an abnormality associated with PLC-mediated signal transduction can also be seen to explain the attenuation of ATP-induced increase in [Ca2+]i in failing cardiomyocytes. A progressive decrease in the ATP-induced increase in [Ca2+]i during 4–16 weeks of inducing MI seems to suggest that such a change in Ca2+ mobilization in cardiomyocytes may play an important role in the progression of heart failure because early, moderate and severe stages of heart failure have been reported to occur at 4, 8 and 16 weeks of the coronary occlusion (Dixon et al., 1990; Afzal & Dhalla, 1992).
In this study, we have shown that treatment with imidapril, a well-known long-acting ACE inhibitor (Wang et al., 2002), caused a significant improvement of the reduced ATP responses in both failing hearts and failing cardiomyocytes. Although the attenuation of ATP-mediated increase in [Ca2+]i during the progression of CHF was markedly improved after imidapril, this effect of imidapril treatment was not specific for ATP responses because the depression in [Ca2+]i in KCl-depolarized cells was also prevented by imidapril. Since ACE inhibitors have been reported to improve SR Ca2+ transport and cardiac function in CHF (Shao et al., 1999), the beneficial effects of imidapril are most likely related to the recovery of SR function in terms of [Ca2+]i handling abnormalities in cardiomyocytes. However, an improvement of the purinoceptor signal transduction mechanisms cannot be excluded as imidapril has also been shown to correct partially the MI-induced changes in PLC (Tappia et al., 1999), which is linked with ATP-induced Ca2+-induced Ca2+ release from SR (Puceat & Vassort, 1996). It seems likely that the observed effects of imidapril may be due to ACE inhibition per se because enalapril, another ACE inhibitor (Fujii et al., 2002), was also found to exert similar beneficial effects in MI hearts. Normalization of the ATP response with AT1R blocker, losartan (Ruzicka et al., 1999), further supports the contention that the beneficial effects of imidapril are due to blockade of the RAS. Nonetheless, the role of bradykinin, which is known to be accumulated upon treatment with ACE inhibitors and produce protective effect on the heart (Gohlke et al., 1994), cannot be completely ruled out. Furthermore, the direct effects of angiotensin II on ATP-induced increase in [Ca2+]i in cardiomyocytes from control and failing hearts remain to be examined for making a meaningful conclusion.
Although the mechanisms of imidapril-mediated protection in the present study are not fully elucidated, it has been shown that the protective effect of imidapril treatment in MI is partially mediated by prevention of changes in protein kinase C (Wang et al., 2003), which is associated with the regulation of [Ca2+]i in cardiomyocytes (Zheng et al., 1992). In addition, ACE inhibitors have also been shown to improve the [Ca2+]i handling abnormalities and β-adrenoceptor responsiveness in the failing heart (Litwin & Morgan, 1992; Yoshida et al., 2001; Makino et al., 2003). In particular, imidapril has been reported to prevent the attenuation of isoproterenol-induced increase in [Ca2+]i in depolarized cardiomyocytes from the MI hearts (Sethi et al., 2004). Taken together, it is suggested that the beneficial effects of imidapril in improving ATP responsiveness in the failing heart are due to improvement in Ca2+ handing as well as purinoceptor-mediated and β-adrenoceptor-mediated signaling in cardiomyocytes. However, some caution should be taken while interpreting the data presented here because of some apparent limitations of this study. For example, heart rates recorded in this study are low due to the use of ketamine plus xylazine anesthesia in comparison to the unanesthetized rats (Gratton et al., 1995). It can be argued that relatively low LVEDP observed in this study (as a consequence of the anesthetic mixture) may indicate that the MI rats are in the compensated state rather than in heart failure. However, this may not be the case because MI rats with large infarcts have been characterized to be at a moderate stage of CHF (Dixon et al., 1990). Furthermore, examination of the MI unanesthetized rats with echocardiography has revealed that the ejection fraction was about 30% (data not shown) indicating that the experimental animals employed in this study were in heart failure. It is also pointed out that this experimental model of CHF has been employed in our previous studies (Dixon et al., 1990; Afzal & Dhalla, 1992; Shao et al., 1999; Wang et al., 2002; 2003; Guo et al., 2003).
Another limitation of this study relates to the possibility that ATP may modify the effect of NE in the failing hearts because ATP is released along with NE from the sympathetic nerve terminals (Burnstock, 1995). Although NE has been shown to potentiate the ATP-induced increase in [Ca2+]i in control cardiomyocytes (Zheng et al., 1992), we did not carry out such experiments with the failing cardiomyocytes. However, the interpretation of such experiments would be complicated due to the fact that this preparation was found to exhibit depressed responses to isoproterenol in depolarized cardiomyocytes from MI rats (Sethi et al., 2004). In spite of the progressive decline in ATP-induced increase in [Ca2+]i at different intervals of inducing heart failure, extensive experiments are needed to settle the question regarding the cause/effect relationship between the observed changes in responses to ATP and the development of heart failure. Nonetheless, the data in this study suggest that there occurs downregulation of ATP-mediated signal transduction in the failing heart. Such a defect in responsiveness of failing hearts to ATP is different from that in the β-adrenoceptor-mediated signal transduction because, unlike β-adrenoceptors, the density of purinergic receptors was not altered in CHF.
Acknowledgments
This work was supported by a grant from the Canadian Institute of Health Research (CIHR Group in Experimental Cardiology). N.S.D. holds a CIHR/Pharmaceutical Research and Development Chair in Cardiovascular Research supported by Merck Frosst Canada.
Abbreviations
- ACE
angiotensin-converting enzyme
- ANOVA
analysis of variance
- ATP
adenosine 5′-triphosphate
- AT1R
angiotensin II type 1 receptor
- Bmax
maximum receptor density
- BSA
bovine serum albumin
- [Ca2+]i
intracellular calcium concentration
- CHF
congestive heart failure
- +dP/dt
maximum rate of pressure development
- −dP/dt
maximum rate of pressure decay
- ENL
enalapril
- IMP
imidapril
- Kd
dissociation constant
- LOS
losartan
- LV
left ventricle
- LVDP
left ventricular developed pressure
- LVEDP
left ventricular end diastolic pressure
- LVSP
left ventricular systolic pressure
- MAP
mean arterial pressure
- MI
myocardial infarction
- NE
norepinephrine
- PKA
protein kinase A
- PLC
phospholipase C
- [35S]ATPγS
35S-labled adenosine 5′-(γ-thio) triphosphate
- RAS
rennin–angiotensin system
- RV
right ventricle
- SL
sarcolemma
- SR
sarcoplasmic reticulum
References
- AFZAL N., DHALLA N.S. Differential changes in left and right ventricular SR calcium transport in congestive heart failure. Am. J. Physiol. Heart Circ. Physiol. 1992;262:H868–H874. doi: 10.1152/ajpheart.1992.262.3.H868. [DOI] [PubMed] [Google Scholar]
- AFZAL N., PIERCE G.N., ELIMBAN V., BEAMISH R.E., DHALLA N.S. Influence of verapamil on some subcellular defects in diabetic cardiomyopathy. Am. J. Physiol. Endocrinol. Metab. 1989;256:E453–E458. doi: 10.1152/ajpendo.1989.256.4.E453. [DOI] [PubMed] [Google Scholar]
- ALPERT N.R., MULIERI L.A., WARSHAW D. The failing human heart. Cardiovasc. Res. 2002;54:1–10. doi: 10.1016/s0008-6363(02)00248-1. [DOI] [PubMed] [Google Scholar]
- ANAND I.S., LIU D., CHUGH S.S., PRAHASH A.J., GUPTA S., JOHN R., POPESCU F., CHANDRASHEKHAR Y. Isolated myocyte contractile function is normal in postinfarct remodeled rat heart with systolic dysfunction. Circulation. 1997;96:3974–3984. doi: 10.1161/01.cir.96.11.3974. [DOI] [PubMed] [Google Scholar]
- BEUCKELMANN D.J., NABAUER M., ERDMANN E. Intracellular calcium handling in isolated ventricular myocytes from patients with terminal heart failure. Circulation. 1992;85:1046–1055. doi: 10.1161/01.cir.85.3.1046. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Noradrenaline and ATP: cotransmitters and neuromodulators. J. Physiol. Pharmacol. 1995;46:365–384. [PubMed] [Google Scholar]
- CHEN Q., VAN BREEMEN C. The superficial buffer barrier in venous smooth muscle: sarcoplasmic reticulum refilling and unloading. Br. J. Pharmacol. 1993;109:336–343. doi: 10.1111/j.1476-5381.1993.tb13575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN X., PIACENTINO V., III, FURUKAWA S., GOLDMAN B., MARGULIES K.B., HOUSER S.R. L-type Ca2+ channel density and regulation are altered in failing human ventricular myocytes and recover after support with mechanical assist devices. Circ. Res. 2002;91:517–524. doi: 10.1161/01.res.0000033988.13062.7c. [DOI] [PubMed] [Google Scholar]
- CHRISTIE A., SHARMA V.K., SHEU S.S. Mechanism of extracellular ATP-induced increase of cytosolic Ca2+ concentration in isolated rat ventricular myocytes. J. Physiol. 1992;445:369–388. doi: 10.1113/jphysiol.1992.sp018929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANZIGER R.S., RAFFAELI S., MORENO-SANCHEZ R., SAKAI M., CAPOGROSSI M.C., SPURGEON H.A., HANSFORD R.G., LAKATTA E.G. Extracellular ATP has a potent effect to enhance cytosolic calcium and contractility in single ventricular myocytes. Cell Calcium. 1988;9:193–199. doi: 10.1016/0143-4160(88)90023-1. [DOI] [PubMed] [Google Scholar]
- DE YOUNG M.B., SCARPA A. Extracellular ATP induces Ca2+ transients in cardiac myocytes which are potentiated by norepinephrine. FEBS Lett. 1987;223:53–58. doi: 10.1016/0014-5793(87)80508-2. [DOI] [PubMed] [Google Scholar]
- DHALLA N.S., ANAND-SRIVASTAVA M.B., TUANA B.S., KHANDELWAL R.L. Solubilization of a calcium dependent adenosine triphosphatase from rat heart sarcolemma. J. Mol. Cell. Cardiol. 1981;13:413–423. doi: 10.1016/0022-2828(81)90283-2. [DOI] [PubMed] [Google Scholar]
- DHALLA N.S., WANG X., SETHI R., DAS P.K., BEAMISH R.E. β-Adrenergic linked signal transduction mechanisms in failing hearts. Heart Failure Rev. 1997;2:55–65. [Google Scholar]
- DIXON I.M., LEE S.L., DHALLA N.S. Nitrendipine binding in congestive heart failure due to myocardial infarction. Circ. Res. 1990;66:782–788. doi: 10.1161/01.res.66.3.782. [DOI] [PubMed] [Google Scholar]
- ECKSTEIN F. Nucleoside phosphorothioates. Annu. Rev. Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- ERTL G., KLONER R.A., ALEXANDER R.W., BRAUNWALD E. Limitation of experimental infarct size by an angiotensin-converting enzyme inhibitor. Circulation. 1982;65:40–48. doi: 10.1161/01.cir.65.1.40. [DOI] [PubMed] [Google Scholar]
- FUJII M., WADA A., TSUTAMOTO T., OHNISHI M., ISONO T., KINOSHITA M. Bradykinin improves left ventricular diastolic function under long-term angiotensin-converting enzyme inhibition in heart failure. Hypertension. 2002;39:952–957. doi: 10.1161/01.hyp.0000015613.78314.9e. [DOI] [PubMed] [Google Scholar]
- GOHLKE P., LINZ W., SCHOLKENS B.A., KUWER I., BARTENBACH S., SCHNELL A., UNGER T. Angiotensin-converting enzyme inhibition improves cardiac function. Role of bradykinin. Hypertension. 1994;23:411–418. doi: 10.1161/01.hyp.23.4.411. [DOI] [PubMed] [Google Scholar]
- GRATTON J.P., MAURICE M.C., RAE G.A., D'ORLEANS-JUSTE P. Pharmacological properties of endothelins and big endothelines in ketamine/xylazine or urethane anesthetized rats. Am. J. Hypertens. 1995;8:1121–1127. doi: 10.1016/0895-7061(95)00227-G. [DOI] [PubMed] [Google Scholar]
- GRYNKIEWICZ G., POENIE M., TSIEN R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J. Biol. Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- GUO X., CHAPMAN D., DHALLA N.S. Partial prevention of changes in SR gene expression in congestive heart failure due to myocardial infarction by enalapril or losartan. Mol. Cell. Biochem. 2003;254:163–172. doi: 10.1023/a:1027321130997. [DOI] [PubMed] [Google Scholar]
- GUPTA S., PRAHASH A.J., ANAND I.S. Myocyte contractile function is intact in the post-infarct remodeled rat heart despite molecular alterations. Cardiovasc. Res. 2000;48:77–88. doi: 10.1016/s0008-6363(00)00160-7. [DOI] [PubMed] [Google Scholar]
- HASENFUSS G., PIESKE B. Calcium cycling in congestive heart failure. J. Mol. Cell. Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- HOLT E., TONNESSEN T., LUNDE P.K., SEMB S.O., WASSERSTROM J.A., SEJERSTED O.M., CHRISTENSEN G. Mechanisms of cardiomyocyte dysfunction in heart failure following myocardial infarction in rats. J. Mol. Cell. Cardiol. 1998;30:1581–1593. doi: 10.1006/jmcc.1998.0724. [DOI] [PubMed] [Google Scholar]
- HOU M., MALMSJO M., MOLLER S., PANTEV E., BERGDAHL A., ZHAO X.H., SUN X.Y., HEDNER T., EDVINSSON L., ERLINGE D. Increase in cardiac P2X1-and P2Y2-receptor mRNA levels in congestive heart failure. Life Sci. 1999;65:1195–1206. doi: 10.1016/s0024-3205(99)00353-7. [DOI] [PubMed] [Google Scholar]
- HOUSER S.R., PIACENTINO V., III, WEISSER J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J. Mol. Cell. Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- KONSTAM M.A. Improving clinical outcomes with drug treatment in heart failure: what have trials taught. Am. J. Cardiol. 2003;91:9D–14D. doi: 10.1016/s0002-9149(02)03374-x. [DOI] [PubMed] [Google Scholar]
- LEGSSYER A., POGGIOLI J., RENARD D., VASSORT G. ATP and other adenine compounds increase mechanical activity and inositol trisphosphate production in rat heart. J. Physiol. 1988;401:185–199. doi: 10.1113/jphysiol.1988.sp017157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDNER M., ERDMANN E., BEUCKELMANN D.J. Calcium content of the sarcoplasmic reticulum in isolated ventricular myocytes from patients with terminal heart failure. J. Mol. Cell. Cardiol. 1998;30:743–749. doi: 10.1006/jmcc.1997.0626. [DOI] [PubMed] [Google Scholar]
- LITWIN S.E., LITWIN C.M., RAYA T.E., WARNER A.L., GOLDMAN S. Contractility and stiffness of noninfarcted myocardium after coronary ligation in rats. Effects of chronic angiotensin converting enzyme inhibition. Circulation. 1991;83:1028–1037. doi: 10.1161/01.cir.83.3.1028. [DOI] [PubMed] [Google Scholar]
- LITWIN S.E., MORGAN J.P. Captopril enhances intracellular calcium handling and beta-adrenergic responsiveness of myocardium from rats with postinfarction failure. Circ. Res. 1992;71:797–807. doi: 10.1161/01.res.71.4.797. [DOI] [PubMed] [Google Scholar]
- LOWRY O.H., ROSENBROUGH N.J., FARR A.L., RANDALL R.J. Protein measurements with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- MAKINO T., HATTORI Y., MATSUDA N., ONOZUKA H., SAKUMA I., KITABATAKE A. Effects of angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on beta-adrenoceptor signaling in heart failure produced by myocardial infarction in rabbits: reversal of altered expression of beta-adrenoceptor kinase and Gi alpha. J. Pharmacol. Exp. Ther. 2003;304:370–379. doi: 10.1124/jpet.102.040956. [DOI] [PubMed] [Google Scholar]
- MALMSJO M., BERGDAHL A., MOLLER S., ZHAO X.H., SUN X.Y., HEDNER T., EDVINSSON L., ERLINGE D. Congestive heart failure induces downregulation of P2X1-receptors in resistance arteries. Cardiovasc. Res. 1999;43:219–227. doi: 10.1016/s0008-6363(99)00060-7. [DOI] [PubMed] [Google Scholar]
- MARKS A.R., REIKEN S., MARX S.O. Progression of heart failure: is protein kinase A hyperphosphorylation of the ryanodine receptor a contributing factor. Circulation. 2002;105:272–275. [PubMed] [Google Scholar]
- MARX S.O., REIKEN S., HISAMATSU Y., JAYARAMAN T., BURKHOFF D., ROSEMBLIT N. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- MUSAT S., DHALLA N.S. Alteration in cardiac sarcolemmal ATP receptors by oxyradicals. Ann. NY Acad. Sci. 1996;793:1–12. doi: 10.1111/j.1749-6632.1996.tb33500.x. [DOI] [PubMed] [Google Scholar]
- OGIKU N., SUMIKAWA H., NISHIMURA T., NARITA H., ISHIDA R. Reduction of the mortality rate by imidapril in a small coronary artery disease model, (NZW × BXSB) F1 male mice. Jpn. J. Pharmacol. 1994;64:129–133. doi: 10.1254/jjp.64.129. [DOI] [PubMed] [Google Scholar]
- PACKER M., LEE W.H., KESSLER P.D., GOTTLIEB S.S., BERNSTEIN J.L., KUKIN M.L. Role of neurohormonal mechanisms in determining survival in patients with severe chronic heart failure. Circulation. 1987;75:IV80–IV92. [PubMed] [Google Scholar]
- PUCEAT M., VASSORT G. Purinergic stimulation of rat cardiomyocytes induces tyrosine phosphorylation and membrane association of phospholipase C gamma: a major mechanism for InsP3 generation. Biochem. J. 1996;318:723–728. doi: 10.1042/bj3180723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REN B., LUKAS A., SHAO Q., GUO M., TAKEDA N., AITKEN R.M., DHALLA N.S. Electrocardiographic changes and mortality due to myocardial infarction in rats with or without imidapril treatment. J. Cardiovasc. Pharmacol. Ther. 1998;3:11–22. doi: 10.1177/107424849800300102. [DOI] [PubMed] [Google Scholar]
- RUZICKA M., YUAN B., LEENEN F.H. Blockade of AT1 receptors and Na+/H+ exchanger and LV dysfunction after myocardial infarction in rats. Am. J. Physiol. Heart Circ. Physiol. 1999;277:H610–H616. doi: 10.1152/ajpheart.1999.277.2.H610. [DOI] [PubMed] [Google Scholar]
- SANBE A., TSUKADA J., TAKEO S. Effects of trandolapril on cardiac angiotensin I converting enzyme activity in rats with chronic heart failure following myocardial infarction. Jpn. Heart J. 1995;36:451–463. doi: 10.1536/ihj.36.451. [DOI] [PubMed] [Google Scholar]
- SCHWINGER R.H., WANG J., FRANK K., MULLER-EHMSEN J., BRIXIUS K., MCDONOUGH A.A., ERDMANN E. Reduced sodium pump alpha1, alpha3, and beta1-isoform protein levels and Na+,K+-ATPase activity but unchanged Na+− Ca2+ exchanger protein levels in human heart failure. Circulation. 1999;99:2105–2112. doi: 10.1161/01.cir.99.16.2105. [DOI] [PubMed] [Google Scholar]
- SETHI R., SHAO Q., REN B., SAINI H.K., TAKEDA N., DHALLA N.S. Changes in β-adrenoceptors in heart failure due to myocardial infarction are attenuated by blockade of rennin–angiotensin system. Mol. Cell. Biochem. 2004;263:11–20. doi: 10.1023/B:MCBI.0000041844.24424.35. [DOI] [PubMed] [Google Scholar]
- SHAO Q., REN B., ZARAIN-HERZBERG A., GANGULY P.K., DHALLA N.S. Captopril treatment improves the sarcoplasmic reticular Ca2+ transport in heart failure due to myocardial infarction. J. Mol. Cell. Cardiol. 1999;31:1663–1672. doi: 10.1006/jmcc.1999.1000. [DOI] [PubMed] [Google Scholar]
- SHEU S.S., SHARMA V.K., UGLESITY A. Na+–Ca2+ exchange contributes to increase of cytosolic Ca2+ concentration during depolarization in heart muscle. Am. J. Physiol. Cell Physiol. 1986;250:C651–C656. doi: 10.1152/ajpcell.1986.250.4.C651. [DOI] [PubMed] [Google Scholar]
- SIPIDO K.R., VOLDERS P.G., VOS M.A., VERDONCK F. Altered Na+/Ca2+ exchange activity in cardiac hypertrophy and heart failure: a new target for therapy. Cardiovasc. Res. 2002;53:782–805. doi: 10.1016/s0008-6363(01)00470-9. [DOI] [PubMed] [Google Scholar]
- TAPPIA P.S., LIU S.Y., SHATADAL S., TAKEDA N., DHALLA N.S., PANAGIA V. Changes in sarcolemmal PLC isoenzymes in postinfarct congestive heart failure: partial correction by imidapril. Am. J. Physiol. Heart Circ. Physiol. 1999;277:H40–H49. doi: 10.1152/ajpheart.1999.277.1.H40. [DOI] [PubMed] [Google Scholar]
- VASSORT G. Adenosine 5′-triphosphate: a P2-purinergic agonist in the myocardium. Physiol. Rev. 2001;81:767–806. doi: 10.1152/physrev.2001.81.2.767. [DOI] [PubMed] [Google Scholar]
- WANG J., LIU X., REN B., RUPP H., TAKEDA N., DHALLA N.S. Modification of myosin gene expression by imidapril in failing heart due to myocardial infarction. J. Mol. Cell. Cardiol. 2002;34:847–857. doi: 10.1006/jmcc.2002.2023. [DOI] [PubMed] [Google Scholar]
- WANG J., LIU X., SENTEX E., TAKEDA N., DHALLA N.S. Increased expression of protein kinase C isoforms in heart failure due to myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H2277–H2287. doi: 10.1152/ajpheart.00142.2002. [DOI] [PubMed] [Google Scholar]
- WANG X., DAKSHINAMURTI K., MUSAT S., DHALLA N.S. Pyridoxal 5′-phosphate is an ATP-receptor antagonist in freshly isolated rat cardiomyocytes. J. Mol. Cell. Cardiol. 1999;31:1063–1072. doi: 10.1006/jmcc.1999.0936. [DOI] [PubMed] [Google Scholar]
- WANG X., DHALLA N.S. Modification of beta-adrenoceptor signal transduction pathway by genetic manipulation and heart failure. Mol. Cell. Biochem. 2000;214:131–155. doi: 10.1023/a:1007131925048. [DOI] [PubMed] [Google Scholar]
- XU Y.J., SHAO Q., DHALLA N.S. Fura-2 fluorescent technique for the assessment of Ca2+ homeostasis in cardiomyocytes. Mol. Cell. Biochem. 1997;172:149–157. [PubMed] [Google Scholar]
- YOSHIDA H., TANONAKA K., MIYAMOTO Y., ABE T., TAKAHASHI M., ANAND-SRIVASTAVA M.B., TAKEO S. Characterization of cardiac myocyte and tissue beta-adrenergic signal transduction in rats with heart failure. Cardiovasc. Res. 2001;50:34–45. doi: 10.1016/s0008-6363(01)00203-6. [DOI] [PubMed] [Google Scholar]
- ZHANG X.Q., MUSCH T.I., ZELIS R., CHEUNG J.Y. Effects of impaired Ca2+ homeostasis on contraction in postinfarction myocytes. J. Appl. Physiol. 1999;86:943–950. doi: 10.1152/jappl.1999.86.3.943. [DOI] [PubMed] [Google Scholar]
- ZHAO D.Y., DHALLA N.S. [35S]ATP gamma S binding sites in the purified heart sarcolemma membrane. Am. J. Physiol. Cell Physiol. 1990;258:C185–C188. doi: 10.1152/ajpcell.1990.258.1.C185. [DOI] [PubMed] [Google Scholar]
- ZHAO X.H., SUN X.Y., ERLINGE D., EDVINSSON L., HEDNER T. Downregulation of adenosine and P2X receptor-mediated cardiovascular responses in heart failure rats. Blood Pressure. 2000;9:152–161. doi: 10.1080/080370500453500. [DOI] [PubMed] [Google Scholar]
- ZHENG J.S., CHRISTIE A., DE YOUNG M.B., LEVY M.N., SCARPA A. Synergism between cAMP and ATP in signal transduction in cardiac myocytes. Am. J. Physiol. Cell Physiol. 1992;262:C128–C135. doi: 10.1152/ajpcell.1992.262.1.C128. [DOI] [PubMed] [Google Scholar]
- ZHENG J.S., DE YOUNG M.B., WIENER E., LEVY M.N., SCARPA A. Extracellular ATP-induced Ca2+ transients in cardiomyocytes are potentiated by an increase in cellular cAMP. Ann. NY Acad. Sci. 1990;603:448–451. [Google Scholar]