Abstract
Macrolides have long been used as anti-bacterial agents; however, there is some evidence that may exert anti-inflammatory activity. Therefore, erythromycin was used to characterize the mechanisms involved in their in vivo anti-inflammatory activity.
Erythromycin pretreatment (30 mg kg−1 day−1 for 1 week) reduced the lipopolysaccharide (LPS; intratracheal, 0.4 mg kg−1)-induced increase in neutrophil count and elastase activity in the bronchoalveolar lavage fluid (BALF) and lung tissue myeloperoxidase activity, but failed to decrease tumor necrosis factor-α and macrophage-inflammatory protein-2 augmented levels in BALF. Erythromycin pretreatment also prevented lung P-selectin, E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) mRNA upregulation in response to airway challenge with LPS.
Mesentery superfusion with LPS (1 μg ml−1) induced a significant increase in leukocyte–endothelial cell interactions at 60 min. Erythromycin pretreatment abolished the increases in these parameters.
LPS exposure of the mesentery for 4 h caused a significant increase in leukocyte rolling flux, adhesion and emigration, which were inhibited by erythromycin by 100, 93 and 95%, respectively.
Immunohistochemical analysis showed that LPS exposure of the mesentery for 4 h caused a significant enhancement in P-selectin, E-selectin, ICAM-1 and VCAM-1 expression that was downregulated by erythromycin pretreatment.
Flow cytometry analysis indicated that erythromycin pretreatment inhibited LPS-induced CD11b augmented expression in rat neutrophils.
In conclusion, erythromycin inhibits leukocyte recruitment in the lung and this effect appears mediated through downregulation of CAM expression. Therefore, macrolides may be useful in the control of neutrophilic pulmonary diseases.
Keywords: Erythromycin, cell adhesion molecules, lipopolysaccharide, leukocyte, endothelium, intravital microscopy
Introduction
A common feature in septic patients and in animal models of sepsis is that, regardless of the organ in which the sepsis originates, the lungs are generally the first to fail (Welbourn & Young, 1992). The sequestration of neutrophils in the pulmonary microcirculation and their activation appears to be a key event in the development of the acute lung injury. Neutrophils, when activated, are a source of proteases, reactive oxygen species and inflammatory mediators, which can contribute to endothelial cell and alveolar epithelial cell damage (Worthen & Downey, 1996). Indeed, depletion of neutrophils in animal models preserves the lung during endotoxemia (Sheridan et al., 1997).
A major factor contributing to the neutrophil infiltration into the lungs is the shedding of the lipopolysaccharide (LPS) from Gram-negative bacteria into the circulation. This proinflammatory molecule interacts with a variety of cell types including neutrophils, monocytes and endothelial cells. A number of studies have demonstrated that LPS increases microvascular permeability, neutrophil chemotaxis and its accumulation into the airway wall, cell adhesion molecule (CAM) expression and, hence, neutrophilic airway inflammation (Libby et al., 1986; Pober et al., 1986; Schleimer & Rutledge, 1986; Osborn et al., 1989; Johnston et al., 1997; Ridger et al., 2001). The LPS model is widely used to assess the effects of drugs on acute lung injury (Miotla et al., 1998).
Leukocyte recruitment has generally been described as a multistep cascade involving endothelial selectins (E- and P-selectin) and leukocyte selectins (L-selectin), which permit the initial phase of leukocyte recruitment, that is, its transient attachment to the endothelial surface followed by leukocyte rolling along the vessel wall. The second phase of leukocyte recruitment involves the activation of integrins that mediate firm adhesion, which precedes the subsequent transmigration through the vascular endothelium (Butcher, 1991; Springer, 1994).
Macrolide antibiotics have long been used as anti-microbial agents in bacterial exacerbations of chronic bronchitis and community-acquired pneumonia. These antibiotics are supposed to have various biological effects apart from their anti-bacterial activity. In this context, Kudoh et al. (1998) observed that administration of erythromycin to patients with diffuse panbronchiolitis improved their survival and indicated that the efficacy might be derived from their anti-inflammatory and immunomodulatory activities. Indeed, it has been suggested that macrolides exert their anti-inflammatory activity by acting at different levels, including inhibition of inflammatory cell chemotaxis, cytokine synthesis, adhesion molecule expression and reactive oxygen species production (Wales & Woodhead, 1999; Culic et al., 2001; Tamaoki, 2004).
Despite these findings, most of the studies carried out with regard to the mechanism of the anti-inflammatory activity displayed by macrolides have been performed using in vitro models, particularly in isolated neutrophils, with variable results. Therefore, the present study was undertaken to evaluate the possible inhibition by erythromycin pretreatment of neutrophil accumulation in the bronchoalveolar lavage fluid (BALF) after intratracheal (i.t.) administration of LPS and the inhibitory mechanisms involved in this response. The effects on elastase and myeloperoxidase (MPO) activities, and two proinflammatory cytokines released in BALF, tumor necrosis factor-α (TNF-α) and macrophage-inflammatory protein-2 (MIP-2), as well as on lung CAMs expression were also determined. In addition, intravital microscopy within the rat mesenteric microcirculation was used to examine the effect of erythromycin pretreatment on CAMs mediating leukocyte–endothelial cell interactions during acute (1 h) and subacute (4 h) inflammation using an LPS-induced model. Finally, immunohistochemical studies of the mesenteric vascular bed and flow cytometry analysis in neutrophils were carried out to characterize the effect of erythromycin on constitutive, preformed and inducible CAMs expression.
Methods
LPS-induced lung inflammation
Pathogen-free male Sprague–Dawley rats weighing 225–250 g were used for these experiments. The experimental protocol complies with regulations established for use of laboratory animals by European Community and by the Spanish and Regional Governments and has been approved by local Ethics Committee. Animals received water and commercial chow ad libitum. Rats were treated with an oral dose of erythromycin (30 mg kg−1) once a day for 1 week, with the last dose administered 1 h before LPS exposure. This schedule of treatment and dose level were chosen from previous reports (Kohno et al., 1989; Tamaoki et al., 1995; Miyajima et al., 1999; Ianaro et al., 2000), and also based on the negative results obtained in pilot experiments with the oral administration of a single dose of erythromycin (30 mg kg−1, 1 h before LPS challenge), which failed to decrease neutrophil counts in BALF (data not shown). The plasma and lung tissue levels obtained in the rat from this dose of oral erythromycin are around 3 μg ml−1 (Kohno et al., 1989), which is close to the therapeutic range in the clinical setting (Anderson et al., 1984). Control rats received drug vehicle (0.5% carboxymethylcellulose and 1% Tween 80). Freshly prepared erythromycin was administered in a volume of 10 ml kg−1 (∼2.5 ml rat−1) by oral gavage with a 21-gauge feeding tube fitted to a 5.0 ml syringe. Rats received endotracheally, by the transoral route, a single dose of 0.4 mg kg−1 of LPS administered as 250 μl of a solution of LPS of 0.4 mg ml−1 in saline, that is, ∼100 μg rat−1. Tracheal instillation was carried out under halothane anesthesia. The animals were killed by an overdose of urethane at the indicated time intervals after endotracheal LPS or saline administration. A group of control animals was also studied. These naïve rats received i.t. saline instead of LPS and were not treated with drugs. An additional group of animals were pretreated with erythromycin and challenged with saline.
Lungs from animals were lavaged with four aliquots of 5 ml each of saline with heparin 10 IU ml−1. Cell suspensions were concentrated by low speed centrifugation, and the cell pellet suspended. Total cell counts were made in a hemocytometer. Differential counts were obtained from cytospin preparations stained with May–Grünwald–Giemsa. Neutrophil counts in BALF were determined at 10 h post-LPS challenge, since previous experiments showed that peak values were observed around this time point (Spond et al., 2001). The elastase activity in BALF, used as an indicator of neutrophil activation, was measured by means of a spectrofluorometric method as previously described (Cortijo et al., 1999). The elastase activity was determined at 10 h post-LPS, since peak changes are observed at this time point (Pauwels et al., 1990). In separate experimental groups, LPS-challenged rats were killed at 4 h and TNF-α and MIP-2 concentrations in BALF were measured with an enzyme-linked immunoabsorbent assay (ELISA) kit as indicated by the manufacturer. The lung tissue MPO activity was measured spectrophotometrically using 3,3′,5,5′-tetramethylbenzidine as substrate as reported previously (Serrano-Mollar et al., 2003). The MPO activity was determined at 4 h and used as an indicator of neutrophil infiltration in the lung tissue. The time point for determination of TNF-α, MIP-2 and MPO was selected from previous reports (Schmal et al., 1996; Yi et al., 1996; Blackwell et al., 1999; Spond et al., 2001). Protein quantitation in lung tissue homogenates (4 h post-LPS) and in BALF supernatants (10 h post-LPS) was performed using the Bradford assay (1976). Protein concentration in BALF was used as a marker of microvascular leakage in the lung (Ortiz et al., 1996).
Lung P-selectin, E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1) gene expression (RT–PCR)
The P-selectin, E-selectin, ICAM-1 and VCAM-1 mRNA transcripts were measured by real-time quantitative reverse transcriptase–polymerase chain reaction (RT–PCR). The method used for obtaining quantitative data of relative gene expression was the comparative Ct method (ΔΔCt method) as described by the manufacturer (PE-ABI PRISM 7700 Sequence Detection System; Perkin-Elmer Applied Biosystems; Foster City, CA, U.S.A.) and as reported previously (Blesa et al., 2003). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was chosen as the endogenous control gene. The lung tissue samples were obtained at 4 h postchallenge. This time point for measurements of CAM's mRNA was selected from previous reports (Sanders et al., 1992; Fries et al., 1993; Beck-Schimmer et al., 1997). Total RNA was extracted from lung tissue homogenates by using TriPure Isolation Reagent (Roche Applied Science, Indianapolis, IN, U.S.A.). Reverse transcription of RNA to generate cDNA was carried out by using TaqMan Reverse Transcription Reagents (Applied Biosystems), and PCR was performed by using TaqMan Universal PCR Master Mix (Applied Biosystems) as indicated by the manufacturer. TaqMan primer-probe sets for the following genes were obtained from Applied Biosystems (TaqMan Assay-on-Demand™ Gene Expression Products): P-selectin (Rn00565416_m1), E-selectin (Rn00568021_m1), ICAM-1 (Rn00564227_m1) and VCAM-1 (Rn00563627_m1). The PCR primer for rat GAPDH was designed using the Primer Express software (PE Biosystems, Morrisville, NC, U.S.A.) according to the published rat GAPDH cDNA sequence (GenBank NM17008) as reported previously (Blesa et al., 2003).
Intravital microscopy
Male Sprague–Dawley rats (200–250 g) were fasted for 20–24 h prior to experiments with free access to water. The animals were anesthetized with sodium pentobarbital (65 mg kg−1, i.p.). A tracheotomy was performed to facilitate breathing and the right jugular vein was cannulated for intravenous administration of additional anesthetic as required. The right carotid artery was cannulated to monitor systemic arterial blood pressure through a pressure transducer (Spectramed Stathan P-23XL) connected to a recorder (GRASS RPS7C8B, Quincy, MA, U.S.A.).
A midline abdominal incision was made and a segment of the mid-jejunal mesentery exteriorized and carefully placed on an optically clear viewing pedestal to allow transillumination of a 3 cm−2 segment of the mesenteric microvasculature. The temperature of the pedestal was maintained at 37°C. The exposed intestine was continuously superfused with a bicarbonate buffer saline (BBS, pH 7.4, 2 ml min−1, 37°C) and covered with a BBS-soaked gauze to prevent evaporation. Mesenteric microcirculation was observed through an orthostatic microscope (Nikon Optiphot-2, SMZ1, Badhoevedorp, The Netherlands) with a × 20 objective lens (Nikon SLDW) and a × 10 eyepiece as described previously (Sanz et al., 2002). A video camera (Sony SSC-C350P, Koeln, Germany) mounted on the microscope projected the image onto a color monitor (Sony Trinitron PVM-14N2E) and the images were captured on videotape (Sony SVT-S3000P) with superimposed time and date for subsequent playback analysis. The final magnification of the image on the monitor was × 1300.
Single unbranched mesenteric venules with diameters ranging between 25 and 40 μm were studied. Venular diameter (Dv) was measured on-line using a video caliper (Microcirculation Research Institute, Texas A&M University, College Station, TX, U.S.A.). Centerline red blood cell velocity (Vrbc) was also measured on-line with an optical Doppler velocimeter (Microcirculation Research Institute, Texas A&M University). Venular blood flow was calculated from the product of mean red blood cell velocity (Vmean=Vrbc 1.6−1) and microvascular cross-sectional area, assuming cylindrical geometry. Venular wall shear rate (γ) was calculated based on the Newtonian definition: γ=8 × (Vmean/Dv) s−1, in which Dv is venular diameter (House & Lipowsky, 1987).
The number of rolling, adherent and emigrated leukocytes was determined off-line during playback analysis of videotaped images. Rolling leukocyte flux was determined by counting the number of leukocytes rolling passing a fixed reference point in the microvessel per min. The same reference point was used throughout the experiment as leukocytes may roll for only a section of the vessel before rejoining the blood flow or becoming firmly adherent. Leukocyte rolling velocity (Vwbc) was determined by measuring the time required for a leukocyte to traverse a distance of 100 μm along the length of the venule and was expressed as μm s−1. A leukocyte was considered to be adherent to venular endothelium if it remained stationary for a period equal to or exceeding 30 s. Adherent cells were expressed as the number per 100 μm length of venule. Leukocyte emigration was expressed as the number of white blood cells per microscopic field.
Experimental protocol
In these experiments, to determine the effect of erythromycin on leukocyte infiltration elicited by LPS, erythromycin was given at an oral dose of 30 mg kg−1 once a day for 1 week, with the last dose administered 1 h before LPS exposure. In the control groups, rats received the same volume of vehicle for the same period of time. After a 30 min stabilization period, baseline measurements (time 0) of mean arterial blood pressure (MABP), Vrbc, vessel diameter, shear rate, leukocyte rolling flux and velocity and leukocyte adhesion and emigration were made. The superfusion buffer was then supplemented with LPS (1 μg ml−1) and recordings were performed for 5 min at 15 min intervals over a 60 min period and the aforementioned leukocyte and hemodynamic parameters measured. In a separate group of experiments, the effect of buffer superfusion on leukocyte responses was evaluated for the same time period after vehicle or erythromycin pretreatment.
In another set of experiments, animals were similarly pretreated with erythromycin or vehicle and 1 h later 5 ml of LPS (0.2 μg ml−1) was i.p. injected. Leukocyte and hemodynamic parameters were evaluated 4 h after LPS administration. Similarly, a group of rats were pretreated with vehicle or erythromycin and then i.p. injected with saline and responses determined 4 h later.
Immunohistochemistry
Immunohistochemistry was used to examine the expression of P-selectin, E-selectin, ICAM-1 and VCAM-1. Once the experiment using intravital microscopy was completed, the portion superfused with buffer and LPS for 60 min or that exposed to saline and LPS for 4 h, with or without erythromycin treatment, was then isolated and further fixed in 4% paraformaldehyde for 90 min at 4°C as described previously (Sanz et al., 2002). After fixation, the tissue was dehydrated using graded acetone washes at 4°C, embedded in paraffin wax and 4-μm-thick sections were cut.
Immunohistochemical localization of P-selectin, E-selectin, ICAM-1 and VCAM-1 was accomplished using a modified avidin and biotin immunoperoxidase technique as described previously (Sanz et al., 2002). Tissue sections were incubated with anti-rat-P-selectin monoclonal antibody (mAb) (RMP-1), anti-rat-E-selectin mAb (RME-1), anti-rat-ICAM-1mAb (1A29) or anti-rat-VCAM-1mAb (5F10) for 24 h at 200 μg ml−1. Control preparations consisted in the incubation with the isotype-matched murine antibody MOPC 21 (IgG1) or UPC 10 (IgG2a) as primary antibodies for the same period of time at 200 μg ml−1. Positive staining was defined as a venule displaying brown reaction product.
Determination of surface expression of CD11b/CD18 integrins by flow cytometry
The expression of CD11b/CD18 (αMβ2) integrins was determined in leukocytes from peripheral blood from rats. As described previously, erythromycin was given at an oral dose of 30 mg kg−1 once a day for 1 week, with the last dose administered 1 h before blood sample collection. In the control groups, rats received the same volume of vehicle for the same period of time. After 1 h of vehicle or erythromycin administration, animals were sedated with ether and blood samples were obtained by cardiac puncture. Duplicate samples (100 μl) of citrated whole blood were then incubated with saturating amounts (10 μl) of the conjugated mAb anti-rat-CD11b-FITC for 20 min on ice in the dark to determine the effect of erythromycin pretreatment on basal expression of CD11b/CD18 integrins. In another set of experiments, 100 μl samples of citrated whole blood were incubated for 4 h at 37°C with either vehicle (control sample) or LPS (1 ng ml−1) before labeling with conjugated antibodies. For the removal of red blood cells and for fixing leukocytes, an automated lysing procedure was performed with an EPICS Q-PREP system (Coulter Electronics, Hialeah, FL, U.S.A.).
Flow cytometric analyses were performed with an EPICS XL-MCL Flow Cytometer (Beckman-Coulter, Hialeah, FL, U.S.A.) with a 15 mW argon laser tuned at 488 nm. The instrument was set up to measure forward-angle light scatter (FS), side-angle light scatter (SS) and FITC-fluorescence (FL1). FL1 was collected through a 488 nm blocking filter, a 550 nm long-pass dichroic plus a 525 nm band pass. Measurements were amplified linearly (FS and SS) or logarithmically (FL1). The expression of surface antigens (FL1) was analyzed separately in granulocytes by their specific features of size (FS) and granularity (SS) in the flow cytometer.
Statistical analysis
Data are presented as mean±s.e.m. of n experiments. Statistical analysis of data was carried out by analysis of variance followed by Bonferroni test or by Student's t-test as appropriate (GraphPad Software Inc., San Diego, CA, U.S.A.). Significance was accepted when P<0.05.
Materials
Erythromycin, pentobarbital, LPS (Escherichia coli serotype 0127:B8), MOPC 21, UPC 10 were purchased from Sigma Chemical Co., St Louis, MO, U.S.A. Antibodies anti-rat-P-selectin (RMP-1), anti-rat-E-selectin (RME-1) and anti-rat-VCAM-1 (5F10) were acquired as stated previously (Sanz et al., 1997; Walter et al., 1997a, 1997b). Anti-rat-VCAM-1 (5F10) was kindly donated by Biogen Inc., Cambridge, MA, U.S.A. Anti-rat-ICAM-1 (1A29) was supplied by LabClinics S.A., Barcelona, Spain. Anti-rat-CD11b-FITC and stuf-Mark antigen unmasking fluid were from Serotec, Spain. Rat TNF-α and MIP-2 ELISA kits were from Chemicon International (Temecula, CA, U.S.A.) and CytoscreenTM Biosource Int. (Camarillo, CA, U.S.A.).
Results
LPS-induced inflammation in rat lung
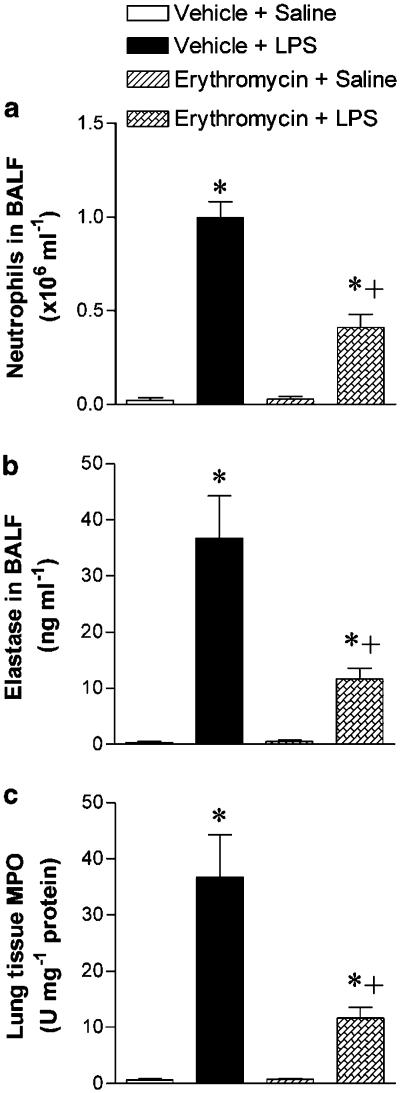
Compared to naïve untreated rats, animals instilled with LPS (0.4 mg kg−1, i.t.) showed, at 10 h postexposure, an increased number of total cells (from 0.36±0.13 in controls to 1.62±0.09 × 106 cells ml−1 BALF; n=10 in control and 14 in LPS group; P<0.05). This augmentation in total cells was mainly due to the increase in neutrophils (from 8.6±1.9 to 60.9±3.0% in control and LPS groups, respectively; P<0.05). The neutrophil counts in BALF are shown in Figure 1a. Pretreatment with erythromycin did not alter cell counts in saline-challenged rats but reduced total cell and neutrophil numbers at 10 h post-LPS challenge by 47 and 59%, respectively (Figure 1a). No relevant changes were observed in other cells types in BALF (eosinophils and mononuclear cells) nor did erythromycin have any significant effect on these cells types.
Figure 1.
Effect of erythromycin on LPS-induced leukocyte recruitment in rat lung. Neutrophil counts (a) and elastase activity (b) in the BALF and lung tissue MPO activity (c) in the following experimental groups: untreated rats challenged with saline (negative control); untreated rats exposed to LPS (0.4 mg kg−1, i.t.; ∼100 μg rat−1) (positive control); and saline- or LPS-exposed rats pretreated with erythromycin (30 mg kg−1 day−1 for 1 week before challenge). BALF for counting of neutrophils and determination of elastase activity was obtained at 10 h postexposure to saline or LPS. The lung tissue samples for determination of MPO activity were obtained at 4 h postsaline or LPS exposure. Data are mean±s.e.m. of 10 (negative control, that is, vehicle+saline), 14 (positive control, that is, vehicle+LPS), 5 (erythromycin+saline) and 15 (erythromycin+LPS) animals in each group; *P<0.05 compared to negative control; +P<0.05 compared to positive control.
Naïve rats have almost undetectable levels of elastase activity in BALF, but this activity was notably increased at 10 h after LPS challenge. Erythromycin significantly reduced elastase activity in this fluid by 68% (Figure 1b). The LPS-induced augmentation of lung MPO activity measured at 4 h postexposure indicates tissue infiltration with neutrophils preceding their pass into BALF. Consistent with the findings in BALF, the augmented lung tissue MPO was also significantly reduced in erythromycin-treated rats (Figure 1c). Erythromycin pretreatment did not affect the elastase and MPO activities in saline-challenged rats.
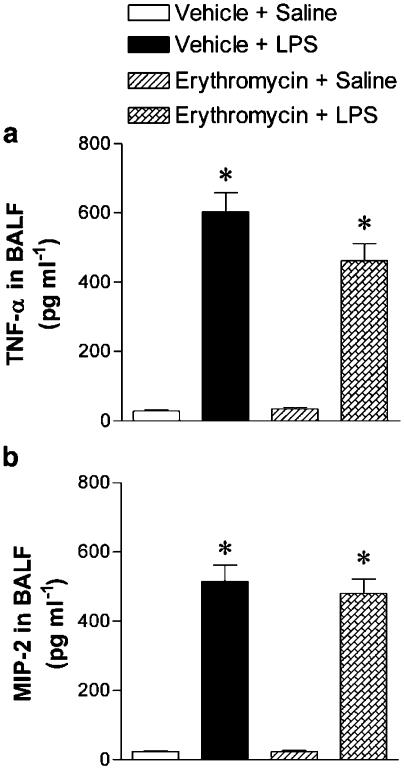
In order to investigate whether the reduction in LPS-induced neutrophil accumulation by erythromycin is due to inhibition of the release of proinflammatory cytokines, TNF-α and MIP-2 levels were measured in the BALF fluid of untreated and treated animals after 4 h exposure to LPS. The BALF concentration of TNF-α and MIP-2 was augmented at 4 h post-LPS challenge compared to levels in naïve rats, but erythromycin failed to decrease the LPS-induced augmentation of TNF-α and MIP-2 content in BALF (Figure 2). Erythromycin pretreatment did not affect TNF-α and MIP-2 levels in saline-challenged rats.
Figure 2.
Effect of erythromycin on LPS-induced release of TNF-α (a) and MIP-2 (b) in BALF. TNF-α and MIP-2 levels in BALF of rats in the following experimental groups: untreated rats challenged with saline (negative control); untreated rats exposed to LPS (0.4 mg kg−1, i.t.; ∼100 μg rat−1) (positive control); and saline- or LPS-exposed rats pretreated with erythromycin (30 mg kg−1 day−1 for 1 week before challenge). BALF was obtained at 4 h postexposure to saline or LPS. Data are mean±s.e.m. of 10 (negative control, that is, vehicle+saline), 14 (positive control, that is, vehicle+LPS), 5 (erythromycin+saline) and 15 (erythromycin) rats per group; *P<0.05 compared to negative control.
Protein levels in BALF were not significantly increased at 10 h post-LPS, and erythromycin had no effect on BALF proteins (protein concentrations in BALF supernatants were 0.22±0.02, 0.30±0.03 and 0.23±0.02 mg ml−1 in naïve, untreated LPS and erythromycin-treated rats exposed to LPS for n=10, 14 and 15 rats in each group, respectively). These results indicate that the dose level of LPS used in these experiments is not sufficient to elicit the microvascular leakage of proteins in the rat airways.
Lung P-selectin, E-selectin, ICAM-1 and VCAM-1 gene expression (RT–PCR)
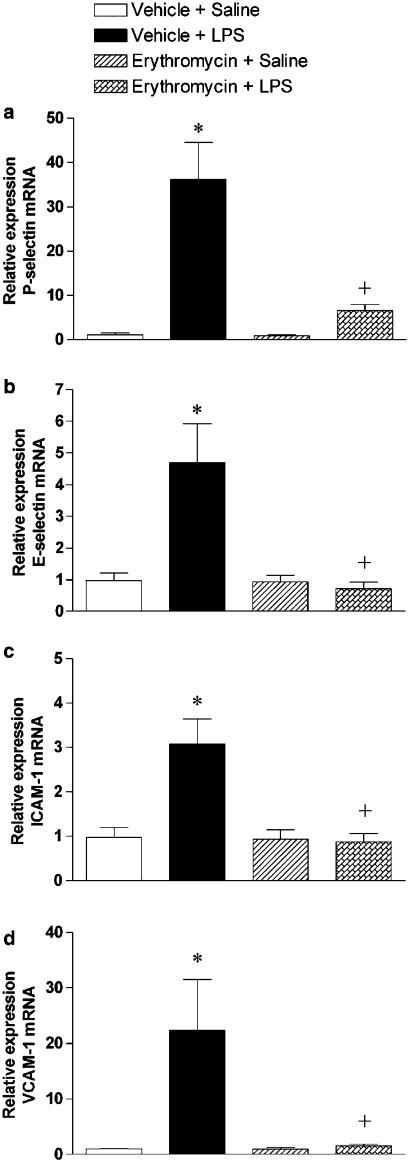
Since erythromycin did not affect the release of MIP-2 and TNF-α, it is likely that this macrolide exert its anti-inflammatory activity by modulating the expression of both leukocyte and endothelial CAMs. To investigate such possibility, RT–PCR was used to determine the expression of P-selectin, E-selectin, ICAM-1 and VCAM-1 mRNA in the lung. LPS challenge significantly upregulated the expression of these CAMs, and this augmentation in their transcripts was blocked in rats pretreated with erythromycin (Figure 3). Erythromycin pretreatment did not affect the CAMs mRNA levels in saline-challenged rats.
Figure 3.
Effect of erythromycin on LPS-induced upregulation of cell adhesion molecule expression in the lung tissue. Relative quantification of the mRNA levels of P-selectin (a), E-selectin (b), ICAM-1 (c), VCAM-1 (d) and GAPDH by real-time quantitative RT–PCR using the comparative Ct method (ΔΔCt method). Data from five independent experiments are shown for the following experimental groups: vehicle+saline, vehicle+LPS, erythromycin+saline and erythromycin+LPS. Measurements are made at 4 h after challenge with LPS or saline. The Ct values for GAPDH were similar in the different samples, thus confirming the value of this housekeeping gene as endogenous control. Columns show the fold-increase in the expression of the cell adhesion molecules relative to control GAPDH values as mean±s.e.m. of five experiments in each group; *P<0.05 compared to vehicle+saline; +P<0.05 from vehicle+LPS.
Intravital microscopy in rat mesentery
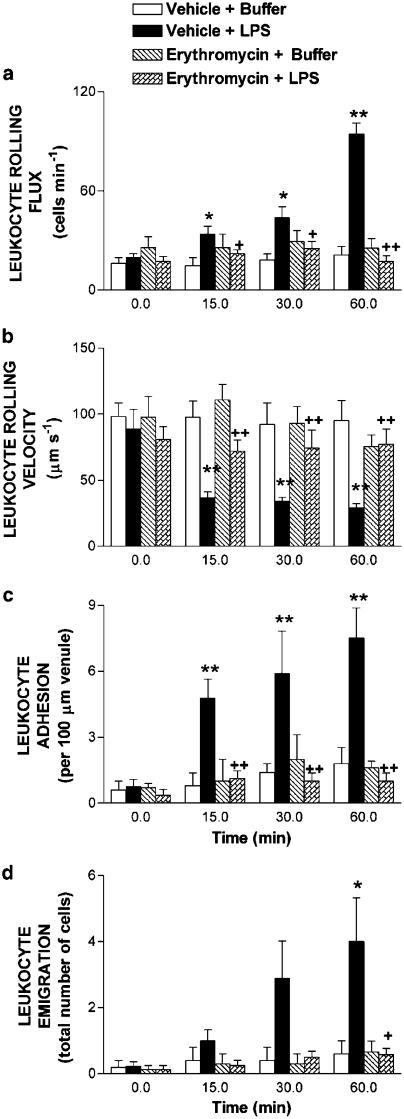
To investigate further the influence of erythromycin pretreatment on the expression of CAMs, intravital microscopy was used to examine leukocyte trafficking in the mesentery as leukocyte–endothelial cell interactions would be expected to precede the tissue accumulation of leukocytes. Figure 4 illustrates acute LPS-induced leukocyte responses. Leukocyte rolling flux and adhesion were significantly increased within 15 min of 1 μg ml−1 LPS superfusion. After 60 min superfusion with LPS, increases in leukocyte rolling flux and concomitant significant decreases in the leukocyte rolling velocity were observed vs buffer (Figures 4a and b). Similarly, at the same time point, LPS induced increases in leukocyte adhesion and emigration as shown in Figures 4c and d. Pretreatment with erythromycin abolished LPS-induced increase in leukocyte rolling flux, adhesion and emigration (Figure 4). In addition, the decrease in leukocyte rolling velocity induced by LPS at 60 min was reversed by the administration of this macrolide (Figure 4). Erythromycin pretreatment did not affect these parameters in rats not exposed to LPS. LPS superfusion for 60 min neither affected MABP nor venular shear rate (Table 1). Similarly, erythromycin pretreatment had no effect in these responses.
Figure 4.
Effect of erythromycin on acute LPS-induced leukocyte rolling flux (a), leukocyte rolling velocity (b), leukocyte adhesion (c) and leukocyte emigration (d) in rat mesenteric postcapillary venules. Parameters were measured 0, 15, 30 and 60 min after superfusion with buffer or with LPS (1 μg ml−1) in the following experimental groups: untreated rats exposed to buffer (negative control); untreated rats exposed to LPS (positive control); and saline- or LPS-exposed rats pretreated with erythromycin (30 mg kg−1 day−1 for 1 week before superfusion). Data are mean±s.e.m. of 5 (negative control, that is, vehicle+buffer), 9 (positive control, that is, vehicle+LPS), 5 (erythromycin+buffer) and 8 (erythromycin+LPS) rats per group; *P<0.05 or **P<0.01 compared to negative control; +P<0.05 or ++P<0.01 compared to positive control.
Table 1.
Hemodynamic parameters in vehicle and erythromycin-treated animals before (0 min) and after (60 min) LPS (1 μg ml−1) superfusion
| MABP (mm Hg) | Shear rate (s−1) | |||
|---|---|---|---|---|
| Treatment (min) | 0 | 60 | 0 | 60 |
| Buffer (vehicle) | 95.1±8.3 | 103.2±9.8 | 577.1±68.4 | 588.1±74.1 |
| Buffer (erythromycin) | 103.0±11.6 | 109.8±7.4 | 502.8±160.7 | 565.7±135.4 |
| LPS (vehicle) | 105.1±7.3 | 99.8±4.5 | 538.7±113.7 | 537.7±107.5 |
| LPS (erythromycin) | 94.1±10.1 | 98.8±11.2 | 590.1±74.0 | 624.9±84.4 |
Values are mean±s.e.m. No significant changes among the different groups were observed.
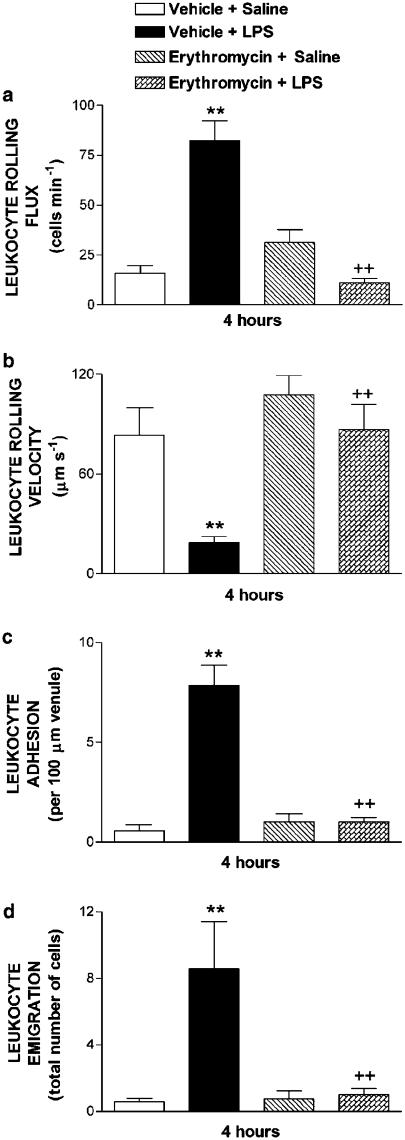
Figure 5 shows the effect of erythromycin on subacute LPS-induced leukocyte–endothelial cell interactions. After 4 h i.p. injection of 5 ml of 0.2 μg ml−1 LPS, significant increases in leukocyte rolling flux, adhesion and emigration, and significant decreases in the leukocyte rolling velocity were detected compared to values obtained in the saline-treated group. Erythromycin pretreatment significantly reduced LPS-induced leukocyte rolling flux, adhesion and emigration by 100, 93 and 95%, respectively, after 4 h exposure to LPS (Figure 5), and significantly increased the reduction in the leukocyte rolling velocity elicited by LPS. Erythromycin pretreatment did not affect these parameters in rats not exposed to LPS. None of these treatments had significant effects on circulating leukocyte counts, MABP and shear rate (Table 2).
Figure 5.
Effect of erythromycin on subacute LPS-induced leukocyte rolling flux (a), leukocyte rolling velocity (b), leukocyte adhesion (c) and leukocyte emigration (d) in rat mesenteric postcapillary venules. Parameters were measured 4 h after i.p. injection of 5 ml of saline or 5 ml of LPS (0.2 μg ml−1) in the following experimental groups: untreated rats exposed to buffer (negative control); untreated rats exposed to LPS (positive control); and saline- or LPS-exposed rats pretreated with erythromycin (30 mg kg−1 day−1 for 1 week before LPS injection). Data are mean±s.e.m. of 7 (negative control, that is, vehicle+saline), 7 (positive control, that is, vehicle+LPS), 5 (erythromycin+saline) and 9 (erythromycin+LPS) rats per group; **P<0.01 compared to negative control; ++P<0.01 compared to positive control.
Table 2.
Hemodynamic parameters in vehicle and erythromycin-treated animals after 4 h buffer and 5 ml of LPS (0.2 μg ml−1) exposure
| Leukocyte counts (cells μl−1) | MABP (mmHg) | Shear rate (s−1) | |
|---|---|---|---|
| Treatment (h) | 4 | 4 | 4 |
| Saline | 3544.2±443.3 | 104.2±8.9 | 677.2±70.8 |
| Saline (erythromycin) | 4100.0±655.7 | 115.7±11.6 | 577.9±116.1 |
| LPS (vehicle) | 5275.5±605.1 | 109.5±9.7 | 500.8±36.9 |
| LPS (erythromycin) | 4788.9±893.2 | 133.2±25.7 | 631.2.±82.4 |
Values are mean±s.e.m. No significant changes among the different groups were observed.
Immunohistochemical and flow cytometry analysis of CAM expression
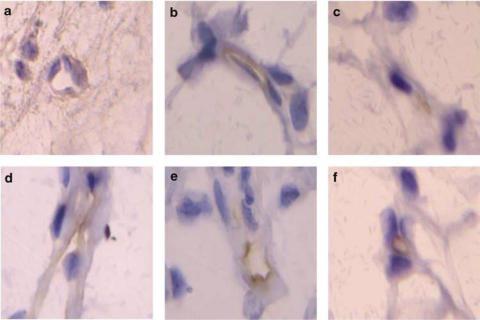
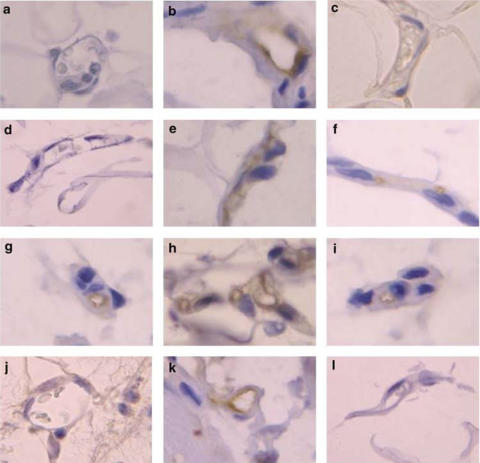
Immunohistochemical experiments revealed that when the mesenteric tissue was subjected to 60 min buffer superfusion or 4 h saline exposure, only the ICAM-1 constitutive expression was detected (Figures 6 and 7). Interestingly, while erythromycin decreased P-selectin expression in animals suffused with LPS for 60 min, constitutive ICAM-1 expression was unaffected by this treatment (Figure 6). Furthermore, after 4 h LPS exposure significant increases in P-selectin, E-selectin, ICAM-1 and VCAM-1 expression were detected (Figure 7). Erythromycin pretreatment resulted in the downregulation of all of these endothelial CAMs (Figure 7).
Figure 6.
Representative photomicrographs of rat mesenteric venules showing immunolocalization of P-selectin and ICAM-1 expression in animals untreated and pretreated with erythromycin after acute LPS superfusion. P-selectin expression after buffer (a) and LPS 60 min superfusion in the untreated group (b) and erythromycin-pretreated group (c). ICAM-1 expression after buffer (d) and LPS 60 min superfusion in the untreated group (e) and erythromycin-pretreated group (f). Brown reaction product indicates positive immunoperoxidase localization for both CAMs on the vascular endothelium. All six panels are lightly counterstained with hematoxylin and have the same magnification ( × 400). Results are representative of n=5–6 experiments with each treatment.
Figure 7.
Representative photomicrographs of rat mesenteric venules showing immunolocalization of P-selectin, E-selectin, ICAM-1 and VCAM-1 expression in animals untreated and pretreated with erythromycin after subacute LPS exposure. P-selectin expression after 4 h saline (a) or LPS exposure in the untreated group (b) and erythromycin-pretreated group (c). E-selectin expression after 4 h saline (d) or LPS exposure in the untreated group (e) and erythromycin-pretreated group (f). ICAM-1 expression after 4 h saline (g) or LPS exposure in the untreated group (h) and erythromycin-pretreated group (i). VCAM-1 expression after 4 h saline (j) or LPS exposure in the untreated group (k) and erythromycin-pretreated group (l). Brown reaction product indicates positive immunoperoxidase localization for all CAMs on the vascular endothelium. All panels are lightly counterstained with hematoxylin and have the same magnification ( × 400). Results are representative of n=6–7 experiments with each treatment.
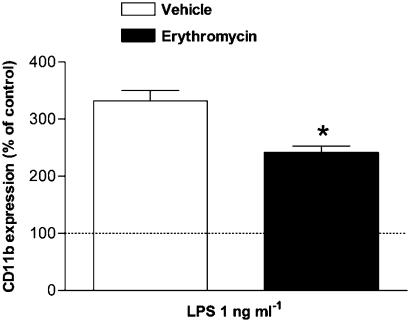
Finally, since there is some evidence that erythromycin treatment might affect CD11b/CD18-integrin expression on human neutrophils (Lin et al., 2000), we next investigated the expression of this CAM in rat peripheral blood granulocytes after 1 h of the last dose of vehicle or erythromycin. Erythromycin treatment did not affect the basal expression of CD11b/CD18 integrins. As expected, when samples were stimulated with LPS for 4 h, a significant increase in CD11b/CD18-integrin expression was observed (Figure 8). Erythromycin pretreatment significantly reduced LPS-induced CD11b/CD18-integrin upregulation (Figure 8).
Figure 8.
Effect of erythromycin on surface expression of αMβ2-integrins (CD11b/CD18) on peripheral rat neutrophils. Rats were untreated or pretreated with erythromycin (30 mg kg−1 day−1 for 1 week) and 1 h later blood samples were collected. Samples were incubated for 4 h with either vehicle (saline) or 1 ng ml−1 LPS, and then stained for 20 min with conjugated mAb and analyzed by flow cytometry. FITC fluorescence values are expressed as the percentage of mean fluorescence intensity of basal values (dotted line). Data are mean±s.e.m. of n=4 experiments. *P<0.05 compared to the mean fluorescence intensity in the vehicle-treated group.
Discussion
In the first part of the present study, we have used an LPS model of lung inflammation to evaluate the anti-inflammatory activity displayed by erythromycin. In this model, increases of neutrophil numbers in BALF were accompanied by augmentation of the BALF elastase activity and lung MPO, as well as TNF-α and MIP-2 levels in BALF, but without significant extravasation as measured by BALF protein levels. The dose of LPS (∼100 μg rat−1) used in the present study was within the range of those used by other researchers in rats to investigate the anti-inflammatory effects of different compounds in this model of acute lung injury (O'Leary et al., 1996; Yi et al., 1996; O'Leary & Zuckerman, 1997; Spond et al., 2001).
As expected, neutrophil infiltration in the lung tissue, assessed by the lung MPO, and in the BALF was significantly diminished by pretreatment of the animals with erythromycin. This finding is in agreement with the beneficial effect reported by Tamaoki et al. (1995) for erythromycin (10 mg kg−1 per day p.o. for 1 week). In addition, elastase activity in BALF, which is considered as a marker of neutrophil activation, since this enzyme is released almost exclusively from these cells, was also increased at 10 h post-LPS exposure, and this augmentation was significantly decreased in erythromycin-treated rats. In agreement with our results, a reduction in neutrophil-derived elastolytic activity was reported in BALF and sputum from erythromycin-treated patients with chronic airway disease (Mikami 1991; Ichikawa et al., 1992; Oishi et al., 1994). The observed decrease of elastase activity in BALF supernatants may just reflect the reduced entry of neutrophils in the lung tissue and BALF, but the contribution of a direct effect of erythromycin on neutrophils inhibiting degranulation cannot be excluded by these experiments. In this regard, we reported that erythromycin (25–100 μg ml−1) inhibited elastase release from human isolated neutrophils up to ∼30% (Villagrasa et al., 1997).
Erythromycin did not show activity on TNF-α and MIP-2 levels in BALF. The levels of MIP-2 and TNF-α found in this study are similar to values published by others (O'Leary et al., 1996; Schmal et al., 1996; Yi et al., 1996). We are not aware of previous references in the literature for the effects of macrolides on TNF-α and MIP-2 levels in the LPS model, but Miyajima et al. (1999) reported that erythromycin failed to modify TNF-α, MIP-2 and other chemotactic activities in a neutrophilic model of immunologically mediated pulmonary inflammation in rats. Erythromycin (100 μg ml−1) also failed to inhibit the production of leukotriene B4 by human neutrophils activated with the chemotactic peptide N-formylmethionyl-leucyl-phenylalanine (Villagrasa et al., 1997). In addition, we did not observe any significant increase of protein levels in BALF after LPS (∼100 μg rat−1) challenge. This finding is not contradicting the reports of others since higher doses of LPS are required to elicit airway microvascular leakage (Tamaoki et al., 1995).
Further research is therefore required to ascertain the mechanisms underlying the effect of erythromycin on LPS-induced pulmonary inflammation. A possibility is the action of macrolides on the sequential expression of CAMs, which are decisive for modulating the entry of neutrophils into the airways, since it is expected that they precede leukocyte accumulation in the lung and BALF. Certainly, another mechanism by which LPS promotes acute lung inflammation is by upregulating the expression of CAMs. Thus, the transcript levels of P-selectin, E-selectin, ICAM-1 and VCAM-1 are augmented in the lung, with peaks observed at 3–6 h after exposure to LPS (Sanders et al., 1992; Fries et al., 1993; Beck-Schimmer et al., 1997; Panes et al., 1999). In keeping with these studies, we found that i.t. LPS augmented the P-selectin, E-selectin, ICAM-1 and VCAM-1 mRNA in the lung from rats i.t. challenged with LPS. Erythromycin pretreatment abrogated the expression of these CAMs.
To investigate further the influence of erythromycin pretreatment on the expression of CAMs, intravital microscopy was used to examine leukocyte–endothelial cell interactions in rat mesentery vasculature. Under acute stimulation of the mesentery with LPS, pretreatment with erythromycin resulted in the total abolition of these interactions. Concordant with these results, immunohistochemical studies revealed that there was scarce P-selectin expression in the endothelium of the rats pretreated with the macrolide. To our knowledge, this is the first report that demonstrates that macrolides inhibit endothelial P-selectin expression. Additionally, it has been suggested that macrolides can also inhibit syalil-Lewisx, a well-known selectin ligand (Azuma et al., 1998). Therefore, the dramatic effect elicited by erythromycin on LPS-induced increase on leukocyte rolling could be due to P-selectin downregulation and blockade of syalil-Lewisx function.
In order to evaluate the effect of erythromycin pretreatment on the expression of inducible CAMs, a subacute model (4 h) of LPS-induced leukocyte recruitment in mesentery was used. After 4 h LPS exposure, a significant increase in leukocyte–endothelial cell interaction in postcapillary venules was observed. Immunohistochemistry showed a clear enhancement of P-selectin, E-selectin, ICAM-1 and VCAM-1 endothelial expression. Erythromycin downregulated the expression of all the endothelial CAMs investigated. These results are in agreement with previous findings in which the anti-inflammatory effects exerted by macrolides are primarily mediated through nuclear transcriptional regulation (Aoki & Kao, 1999). Nuclear factor-κB (NF-κB) is a protein that is essential for the transcription of genes that encode a number of proinflammatory molecules such as E-selectin, ICAM-1 and VCAM-1 (Collins et al., 1995). In addition, in rodents, P-selectin upregulation requires NF-κB activation (Manning et al., 1995). Thus, the P-selectin downregulation elicited by erythromycin pretreatment may be due to inhibition of NF-κB activation. Although this effect was not described previously, there are some evidences that indirectly associate macrolides with E-selectin downregulation. In this context, treatment with azithromycin improved the endothelial function in patients with coronary artery disease, resulting in a significant decrease of E-selectin plasma levels (Parchure et al., 2002), and, in vitro, incubation with roxithromycin was found to inhibit TNF-α-induced E-selectin expression in endothelial cells from the dermal microvasculature (Akamatsu et al., 2001).
In the sequential adhesive cascade that mediates the trafficking of leukocytes from blood to sites of inflammation, leukocytes need to adhere firmly to the endothelium before transmigrating. Activation of leukocytes precedes firm adhesion and is mediated by their previous interaction with selectins and chemoattractants (Butcher 1991; Springer 1994). Two types of integrins are involved in this process β2- and α4-integrins, which interact with their counter-receptors on endothelial cells. The main endothelial ligand for β2-integrins is ICAM-1. In this study, we have found that, while the basal expression of αMβ2-integrin and ICAM-1 was not affected by erythromycin pretreatment, LPS-induced ICAM-1 upregulation after 4 h exposure to the stimulus was clearly diminished. In agreement with our observations, there are several reports indicating that macrolide antibiotics reduce ICAM-1 expression. In vitro, Kawasaki et al. (1998) demonstrated that roxithromycin reduced interferon-γ (IFN-γ)-induced ICAM-1 expression albeit in a human bronchial epithelial cell line, and Akamatsu et al. (2001) demonstrated the same effect but in endothelial cells from the dermal microvasculature stimulated with TNF-α. In vivo, erythromycin has been shown to decrease the expression of ICAM-1 in different animal models of lung inflammation (Miyajima et al., 1999; Li et al., 2002). Furthermore, as found by Lin et al. (2000) in isolated human neutrophils, we have encountered a small but significant decrease in LPS-induced CD11b-integrin expression in rat neutrophils pretreated with erythromycin.
Another interaction that contributes to leukocyte adhesion is the α4-integrins/VCAM-1 pathway. We have found a clear decrease in LPS-induced VCAM-1 upregulation by erythromycin. Despite neutrophils are the primary cells recruited in this model, it is well established that, as opposed to humans, rat neutrophils do express functional α4- and β1-integrins (Davenpeck et al., 1998). In this regard, in a model of bleomycin challenged mice, macrolides were found to inhibit the induction of VCAM-1 mRNA in the lung tissue (Azuma et al., 2001; Li et al., 2002). Finally, the inhibition of leukocyte transmigration displayed by treatment with the macrolide would be the result of the sequential inhibition of the CAMs investigated.
In conclusion, in the present study, we have provided evidence that erythromycin is an in vivo inhibitor of neutrophil recruitment in the lung. This effect is not mediated through inhibition of TNF-α and MIP-2 release. Conversely, it has a powerful effect modulating endothelial CAM expression. In this context, the effects observed are likely mediated through P- and E-selectin, ICAM-1 and VCAM-1 downregulation. Thus, macrolide antibiotics exert anti-inflammatory activity in vivo and may be useful in the control of neutrophilic pulmonary diseases acting on different adhesive pathways.
Acknowledgments
The present study was supported by Grants SAF 2002-01482 and SAF-2003-07206-C02-01 from Spanish Ministry of Science and Technology, Research Groups Grant 03/166 from Generalitat Valenciana, and Grant MT-7684 from the Canadian Institutes of Health Research (A.C.I.). Y.N.A.N. was supported by a grant from Spanish Ministry of Education, Culture and Sports.
Abbreviations
- CAM
cell adhesion molecule
- Dv
venular diameter
- ICAM-1
intercellular adhesion molecule-1
- i.t.
intratracheal
- LPS
lipopolysaccharide
- mAb
monoclonal antibody
- MABP
mean arterial blood pressure
- MIP-2
macrophage-inflammatory protein-2
- MPO
myeloperoxidase
- TNF-α
tumor necrosis factor-α
- VCAM-1
vascular cell adhesion molecule-1
- Vmean
mean red blood cell velocity
- Vrbc
centerline red blood cell velocity
- Vwbc
leukocyte rolling velocity
References
- AKAMATSU H., YAMAWAKI M., HORIO T. Effects of roxithromycin on adhesion molecules expressed on endothelial cells of the dermal microvasculature. J. Int. Med. Res. 2001;29:523–527. doi: 10.1177/147323000102900609. [DOI] [PubMed] [Google Scholar]
- ANDERSON R., FERNANDES A.C., EFTYCHIS H.E. Studies on the effects of ingestion of a single 500 mg oral dose of erythromycin stearate on leukocyte motility and transformation and on release in vitro of prostaglandin E2 by stimulated leukocytes. J. Antimicrob. Chemother. 1984;14:41–50. doi: 10.1093/jac/14.1.41. [DOI] [PubMed] [Google Scholar]
- AOKI Y., KAO P.N. Erythromycin inhibits transcriptional activation of NF-kappaB, but not NFAT, through calcineurin-independent signaling in T cells. Antimicrob. Agents Chemother. 1999;43:2678–2684. doi: 10.1128/aac.43.11.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZUMA A., FURUTA T., ENOMOTO T., HASHIMOTO Y., UEMATSY K., NUKARIYA N., MURATA A., KUDOH S. Preventive effect of erythormycin on experimental bleomycin-induced acute lung injury in rats. Thorax. 1998;53:186–189. doi: 10.1136/thx.53.3.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZUMA A., LI Y., USUKI J., AOYAMA A., ENOMOTO T., KUDOH S. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule-1 messenger RNA induction preventing neutrophil-induced lung injury and fibrosis in bleomycin-challenged mice. Chest. 2001;120 Suppl:20S–22S. doi: 10.1378/chest.120.1_suppl.s20-a. [DOI] [PubMed] [Google Scholar]
- BECK-SCHIMMER B., SHIMMER R.C., WARNER R.L., SCHMAL H., NORDBLOM G., FLORY C.M., LESCH M.E., FRIEDL H.P., SCHRIER D.J., WARD P.A. Expression of lung vascular and airway ICAM-1 after exposure to bacterial lipopolysaccharide. Am. J. Respir. Cell. Mol. Biol. 1997;17:344–352. doi: 10.1165/ajrcmb.17.3.2861. [DOI] [PubMed] [Google Scholar]
- BLACKWELL T.S., LANCASTER L.H., BLACKWELL T.R., VENKATAKRISHNAN A., CHRISTMAN J.W. Chemotactic gradients predict neutrophilic alveolitis in endotoxin-treated rats. Am. J. Respir. Crit. Care Med. 1999;159:1644–1652. doi: 10.1164/ajrccm.159.5.9806166. [DOI] [PubMed] [Google Scholar]
- BLESA S., CORTIJO J., MATA M., SERRANO A., CLOSA D., SANTANGELO F., ESTRELA J.M., SUCHANKOVA J., MORCILLO E.J. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur. Respir. J. 2003;21:394–400. doi: 10.1183/09031936.03.00039602. [DOI] [PubMed] [Google Scholar]
- BRADFORD M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- BUTCHER E.C. Leukocyte–endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- COLLINS T., READ M.A., NEISH A.S., WHITLEY M.Z., THANOS D., MANIATIS T. Transcriptional regulation of endothelial cell adhesion molecules: NF-kappa B and cytokine-inducible enhancers. FASEB J. 1995;9:899–909. [PubMed] [Google Scholar]
- CORTIJO J., VILLAGRASA V., PONS R., BERTO L., MARTÍ-CABRERA M., MARTINEZ-LOSA M., DOMENECH T., BELETA J., MORCILLO E.J. Bronchodilator and anti-inflammatory activities of glaucine: in vitro studies in human airway smooth muscle and polymorphonuclear leukocytes. Br. J. Pharmacol. 1999;127:1641–1651. doi: 10.1038/sj.bjp.0702702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CULIC O., ERAKOVIC V., PARNHAM M.J. Anti-inflammatory effects of macrolide antibiotics. Eur. J. Pharmacol. 2001;429:209–229. doi: 10.1016/s0014-2999(01)01321-8. [DOI] [PubMed] [Google Scholar]
- DAVENPECK K.L., STERBINSKY S.A., BOCHNER B.S. Rat neutrophils express alpha4 and beta1 integrins and bind to vascular cell adhesion molecule-1 (VCAM-1) and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) Blood. 1998;91:2341–2346. [PubMed] [Google Scholar]
- FRIES J.W., WILLIAMS A.J., ATKINS R.C., NEWMAN W., LIPSCOMB M.F., COLLINS T. Expression of VCAM-1 and E-selectin in an in vivo model of endothelial activation. Am. J. Pathol. 1993;143:725–737. [PMC free article] [PubMed] [Google Scholar]
- HOUSE S.D., LIPOWSKY H.H. Leukocyte–endothelium adhesion: microhemodynamics in mesentery of the cat. Microvasc. Res. 1987;34:363–379. doi: 10.1016/0026-2862(87)90068-9. [DOI] [PubMed] [Google Scholar]
- IANARO A., IALENTI A., MAFFIA P., SAUTEBIN L., ROMBOLÀ L., CARNUCCIO R., IUVONE T., D'ACQUISTO F., DI ROSA M. Anti-inflammatory activity of macrolide antibiotics. J. Pharmacol. Exp. Ther. 2000;292:156–163. [PubMed] [Google Scholar]
- ICHIKAWA Y., NINOMIYA H., KOGA H., TANAKA M., KINOSHITA M., TOKUNAGA N., YANO T., OIZUMI K. Erythromycin reduces neutrophils and neutrophil-derived elastolytic-like activity in the lower respiratory tract of bronchiolitis patients. Am. Rev. Respir. Dis. 1992;146:196–203. doi: 10.1164/ajrccm/146.1.196. [DOI] [PubMed] [Google Scholar]
- JOHNSTON B., WALTER U.M., ISSEKUTZ A.C., ISSEKUTZ T.B., ANDERSON D.C., KUBES P. Differential roles of selectins and the α4-integrin in acute, subacute, and chronic leukocyte recruitment in vivo. J. Immunol. 1997;159:4514–4523. [PubMed] [Google Scholar]
- KAWASAKI S., TAKIZAWA H., OHTOSHI T., TAKEUCHI N., KOHYAMA T., NAKAMURA H., KASAMA T., KOBAYASHI K., NAKAHARA K., MORITA Y., YAMAMOTO K. Roxithromycin inhibits cytokine production by and neutrophil attachment to human bronchial epithelial cells in vitro. Antimicrob. Agents Chemother. 1998;42:1499–1502. doi: 10.1128/aac.42.6.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOHNO Y., YOSHIDA H., SUWA T., SUGA T. Comparative pharmacokinetics of clarithromycin (TE-031), a new macrolide antibiotic, and erythromycin in rats. Antimicrob. Agents Chemother. 1989;33:751–756. doi: 10.1128/aac.33.5.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUDOH S., AZUMA A., YAMAMOTO M., IZUMI T., ANDO M. Improvement of survival in patients with diffuse panbronchiolitis treated with low-dose erythromycin. Am. J. Respir. Crit. Care Med. 1998;157:1829–1832. doi: 10.1164/ajrccm.157.6.9710075. [DOI] [PubMed] [Google Scholar]
- LI Y., AZUMA A., TAKAHASHI S., USUKI J., MATSUDA K., AOYAMA A., KUDOH S. Fourteen-membered ring macrolides inhibit vascular cell adhesion molecule 1 messenger RNA induction and leukocyte migration: role in preventing lung injury and fibrosis in bleomycin-challenged mice. Chest. 2002;122:2137–2145. doi: 10.1378/chest.122.6.2137. [DOI] [PubMed] [Google Scholar]
- LIBBY P., ORDOVAS J.M., AUGER K.R., ROBBINS A.H., BIRINYI L.K., DINARELLO C.A. Endotoxin and tumor necrosis factor induce interleukin-1 gene expression in adult human vascular endothelial cells. Am. J. Pathol. 1986;124:179–185. [PMC free article] [PubMed] [Google Scholar]
- LIN H.C., WANG C.H., LIU C.Y., YU C.T., KUO H.P. Erythromycin inhibits beta2-integrins (CD11b/CD18) expression, interleukin-8 release and intracellular oxidative metabolism in neutrophils. Respir. Med. 2000;94:654–660. doi: 10.1053/rmed.1999.0781. [DOI] [PubMed] [Google Scholar]
- MANNING A.M., BELL F.P., ROSENBLOOM C.L., CHOSAY J.G., SIMMONS C.A., NORTHRUP J.L., SHEBUSKI R.J., DUNN C.J., ANDERSON D.C. NF-kappa B is activated during acute inflammation in vivo in association with elevated endothelial cell adhesion molecule gene expression and leukocyte recruitment. J. Inflamm. 1995;45:283–296. [PubMed] [Google Scholar]
- MIKAMI M. Clinical and pathophysiological significance of neutrophil elastase in sputum and the effect of erythromycin in chronic respiratory disease. Nihon Kyobu Shikkan Gakkai Zasshi. 1991;29:72–83. [PubMed] [Google Scholar]
- MIOTLA J.M., TEIXEIRA M.M., HELLEWELL P.G. Suppression of acute lung injury in mice by an inhibitor of phosphodiesterase type 4. Am. J. Respir. Cell. Mol. Biol. 1998;18:411–420. doi: 10.1165/ajrcmb.18.3.2913. [DOI] [PubMed] [Google Scholar]
- MIYAJIMA M., SUGA M., NAKAGAWA K., ITO K., ANDO M. Effects of erythromycin on experimental extrinsic allergic alveolitis. Clin. Exp. Allergy. 1999;29:253–261. doi: 10.1046/j.1365-2222.1999.00430.x. [DOI] [PubMed] [Google Scholar]
- O'LEARY E.C., MARDER P., ZUCKERMAN S.H. Glucocorticoid effects in an endotoxin-induced rat pulmonary inflammation model: Differential effects on neutrophil influx, integrin expression, and inflammatory mediators. Am. J. Respir. Cell. Mol. Biol. 1996;15:97–106. doi: 10.1165/ajrcmb.15.1.8679228. [DOI] [PubMed] [Google Scholar]
- O'LEARY E.C., ZUCKERMAN S.H. Glucocorticoid-mediated inhibition of neutrophil emigration in an endotoxin-induced rat pulmonary inflammation model occurs without an effect on airways MIP-2 levels. Am. J. Respir. Cell. Mol. Biol. 1997;16:267–274. doi: 10.1165/ajrcmb.16.3.9070611. [DOI] [PubMed] [Google Scholar]
- OISHI K., SONODA F., KOBAYASHI S., IWAGAKI A., NAGATAKE T., MATSUSHIMA K., MATSUMOTO K. Role of interleukin-8 (IL-8) and an inhibitory effect of erythromycin on IL-8 release in the airways of patients with chronic airway diseases. Infect. Immun. 1994;62:4145–4152. doi: 10.1128/iai.62.10.4145-4152.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORTIZ J.L., VALLÉS J.M., MARTÍ-CABRERA M., CORTIJO J., MORCILLO E.J. Effects of selective phosphodiesterase inhibitors on platelet-activating factor- and antigen-induced airway hyperreactivity, eosinophil accumulation, and microvascular leakage in guinea pigs. Naunyn-Schmiedeberg's Arch. Pharmacol. 1996;353:200–206. doi: 10.1007/BF00168758. [DOI] [PubMed] [Google Scholar]
- OSBORN L., HESSION C., TIZARD R., VASSALLO C., LUHOWSKYJ S., CHI-ROSSO G., LOBB R. Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 1989;59:1203–1211. doi: 10.1016/0092-8674(89)90775-7. [DOI] [PubMed] [Google Scholar]
- PANES J., PERRY M.A., GRANGER D.N. Leukocyte–endothelial cell adhesion: avenues for therapeutic interventions. Br. J. Pharmacol. 1999;126:537–550. doi: 10.1038/sj.bjp.0702328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARCHURE N., ZOURIDAKIS E.G., KASKI J.C. Effect of azithromycin treatment on endothelial function in patients with coronary artery disease and evidence of Chlamydia pneumoniae infection. Circulation. 2002;105:1298–1303. doi: 10.1161/hc1102.105649. [DOI] [PubMed] [Google Scholar]
- PAUWELS R.A., KIPS J.C., PELEMAN R.A., VAN DER STRAETEN M.E. The effect of endotoxin on airway responsiveness and cellular influx in rats. Am. Rev. Respir. Dis. 1990;141:540–545. doi: 10.1164/ajrccm/141.3.540. [DOI] [PubMed] [Google Scholar]
- POBER J.S., GIMBRONE M.A., Jr, LAPIERRE L.A., MENDRICK D.L., FIERS W., ROTHLEIN R., SPRINGER T.A. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J. Immunol. 1986;137:1893–1896. [PubMed] [Google Scholar]
- RIDGER V.C., WAGNER B.E., WALLACE W.A., HELLEWELL P.G. Differential effects of CD18, CD29, and CD49 integrin subunit inhibition on neutrophil migration in pulmonary inflammation. J. Immunol. 2001;166:3484–3490. doi: 10.4049/jimmunol.166.5.3484. [DOI] [PubMed] [Google Scholar]
- SANDERS W.E., WILSON R.W., BALLANTYNE C.M., BEAUDET A.L. Molecular cloning and analysis of in vivo expression of murine P-selectin. Blood. 1992;80:795–800. [PubMed] [Google Scholar]
- SANZ M.J., ALVAREZ A., PIQUERAS L., CERDA M., ISSEKUTZ A.C., LOBB R.R., CORTIJO J., MORCILLO E.J. Rolipram inhibits leukocyte–endothelial cell interactions in vivo through P- and E-selectin downregulation. Br. J. Pharmacol. 2002;135:1872–1881. doi: 10.1038/sj.bjp.0704644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANZ M.J., HARTNELL A., CHISHOLM P., WILLIAMS C., DAVIES D., WEG V.B., FELDMAN M., BOLANOWSKI M.A., LOBB R.R., NOURSHARGH S. Tumor necrosis factor-α-induced eosinophil accumulation in rat skin is dependent on α4 integrin/vascular cell adhesion molecule-1 adhesion pathways. Blood. 1997;90:4144–4152. [PubMed] [Google Scholar]
- SCHLEIMER R.P., RUTLEDGE B.K. Cultured human vascular endothelial cells acquire adhesiveness for neutrophils after stimulation with interleukin 1, endotoxin, and tumor-promoting phorbol diesters. J. Immunol. 1986;136:649–654. [PubMed] [Google Scholar]
- SCHMAL H., SHANLEY T., JONES M.L., FRIEDL H.P., WARD P.A. Role for macrophage inflammatory protein-2 in lipopolysaccharide-induced lung injury in rats. J. Immunol. 1996;156:1963–1972. [PubMed] [Google Scholar]
- SERRANO-MOLLAR A., CLOSA D., PRATS N., BLESA S., MARTINEZ-LOSA M., CORTIJO J., ESTRELA J.M., MORCILLO E.J., BULBENA O. In vivo antioxidant treatment protects against bleomycin-induced lung damage in rats. Br. J. Pharmacol. 2003;138:1037–1048. doi: 10.1038/sj.bjp.0705138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERIDAN B.C., MCINTYRE R.C., JR, MOORE E.E., MELDRUM D.R., AGRAFOJO J., FULLERTON D.A. Neutrophils mediate pulmonary vasomotor dysfunction in endotoxin-induced acute lung injury. J. Trauma. 1997;42:391–396. doi: 10.1097/00005373-199703000-00005. [DOI] [PubMed] [Google Scholar]
- SPOND J., CHAPMAN R., FINE J., JONES H., KREUTNER W., KUNG T.T., MINNICOZZI M. Comparison of PDE 4 inhibitors, rolipram and SB 207499 (ArifloTM), in a rat model of pulmonary fibrosis. Pulm. Pharmacol. Ther. 2001;14:157–164. doi: 10.1006/pupt.2001.0291. [DOI] [PubMed] [Google Scholar]
- SPRINGER T.A. Traffic signals for lymphocyte recirculation and leukocyte emigration: The multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- TAMAOKI J. The effects of macrolides on inflammatory cells. Chest. 2004;125:41S–51S. doi: 10.1378/chest.125.2_suppl.41s. [DOI] [PubMed] [Google Scholar]
- TAMAOKI J., TAGAYA E., YAMAWAKI I., SAKAI N., NAGAI A., KONNO K. Effect of erythromycin on endotoxin-induced microvascular leakage in the rat trachea and lungs. Am. J. Respir. Crit. Care Med. 1995;151:1582–1588. doi: 10.1164/ajrccm.151.5.7735618. [DOI] [PubMed] [Google Scholar]
- VILLAGRASA V., BERTO L., CORTIJO J., PERPINA M., SANZ C., MORCILLO E.J. Effects of erythromycin on chemoattractant-activated human polymorphonuclear leukocytes. Gen. Pharmacol. 1997;29:605–609. doi: 10.1016/s0306-3623(96)00566-6. [DOI] [PubMed] [Google Scholar]
- WALES D., WOODHEAD M. The anti-inflammatory effects of macrolides. Thorax. 1999;54 Suppl 2:S58–S62. doi: 10.1136/thx.54.2008.s58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALTER U.M., AYER L.M., MANNING A.M., FRENETTE P.S., WAGNER D.D., HYNES R.O., WOLITZKY B.A., ISSEKUTZ A.C. Generation and characterization of a novel adhesion function blocking monoclonal antibody recognizing both rat and mouse E-selectin. Hybridoma. 1997a;16:355–361. doi: 10.1089/hyb.1997.16.355. [DOI] [PubMed] [Google Scholar]
- WALTER U.M., AYER L.M., WOLITZKY B.A., WAGNER D.D., HYNES R.O., MANNING A.M., ISSEKUTZ A.C. Characterization of a novel adhesion function blocking monoclonal antibody to rat/mouse P-selectin generated in P-selectin-deficient mouse. Hybridoma. 1997b;16:249–257. doi: 10.1089/hyb.1997.16.249. [DOI] [PubMed] [Google Scholar]
- WELBOURN C.R., YOUNG Y. Endotoxin, septic shock and acute lung injury: neutrophils, macrophages and inflammatory mediators. Br. J. Surg. 1992;79:998–1003. doi: 10.1002/bjs.1800791006. [DOI] [PubMed] [Google Scholar]
- WORTHEN G.S., DOWNEY G.P.Mechanisms of neutrophil mediated injury ARDS Acute Respiratory Distress in Adults 1996London: Chapman & Hall; 99–114.ed. Evans, T.W. & Haslett, C [Google Scholar]
- YI E.S., REMICK D.G., LIM Y., TANG W., NADZIENKO C.E., BEDOYA A., YIN S., ULICH T.R. The intratracheal administration of endotoxin: X. Dexamethasone downregulates neutrophil emigration and cytokine expression in vivo. Inflammation. 1996;20:165–175. doi: 10.1007/BF01487403. [DOI] [PubMed] [Google Scholar]