Abstract
We investigated the role of deoxycholic acid in pacemaker currents using whole-cell patch-clamp techniques at 30°C in cultured interstitial cells of Cajal (ICC) from murine small intestine.
The treatment of ICC with deoxycholic acid resulted in a decrease in the frequency and amplitude of pacemaker currents and increases in resting outward currents. Also, under current clamping, deoxycholic acid produced the hyperpolarization of membrane potential and decreased the amplitude of the pacemaker potentials.
These observed effects of deoxycholic acid on pacemaker currents and pacemaker potentials were completely suppressed by glibenclamide, an ATP-sensitive K+ channel blocker.
NS-398, a specific cyclooxygenase-2 (COX-2) inhibitor, significantly inhibited the deoxycholic acid-induced effects. The treatment with prostaglandin E2 (PGE2) led to a decrease in the amplitude and frequency of pacemaker currents and to an increase in resting outward currents, and these observed effects of PGE2 were blocked by glibenclamide.
We next examined the role of deoxycholic acid in the production of PGE2 in ICC, and found that deoxycholic acid increased PGE2 production through the induction of COX-2 enzyme activity and its gene expression.
The results suggest that deoxycholic acid inhibits the pacemaker currents of ICC by activating ATP-sensitive K+ channels through the production of PGE2.
Keywords: ATP-sensitive K+ channels, deoxycholic acid, interstitial cells of Cajal, pacemaker currents, prostaglandin E2
Introduction
Bile acids are synthesized from cholesterol and are conjugated to either taurine or glycine. These conjugated bile acids are secreted by the liver, stored in the gallbladder, and then secreted into the duodenum. The primary bile acids are dehydroxylated by intestinal bacteria, which give rise to secondary bile acids, such as deoxycholic acid. Most of them are then absorbed from the terminal ileum and returned to the liver by the enterohepatic circulation. Bile acids are essential for the solubilization of lipids and the absorption of fat from the gastrointestinal tract (Bomzon & Ljubuncic, 1995). However, in certain pathological conditions, such as, irritable bowel syndrome and ileal surgical resection, bile acid reabsorption is compromised. Moreover, bile acids mal-absorbed by the small intestine are transported to the colon, where they affect colonic motility, and may produce, for example, diarrhea (Hoffman & Poley, 1969; Balistreri et al., 1983). In addition, it has been reported that biliary deoxycholic acid concentrations are increased in subjects with cholesterol gallstones and a delayed small bowel transit time, and that they altered gallbladder motor functions (Azzaroli et al., 1999). A jejunal or ileal infusion of bile acids in human subjects prolonged jejunocaecal transit time, decreased intestinal motility and delayed intestinal transit time in the jejunum and ileum (Penagini et al., 1988; 1989). Similarly, a gastric infusion of bile acids in the rat delayed gastric emptying and small bowel transit time (Feldman & Gibaldi, 1968). In addition, bile acids inhibited ileal motility in the isolated perfused rabbit terminal ileum (Armstrong et al., 1993). Also, acute intraduodenal bile salt depletion led to strong gallbladder contraction, and altered antroduodenal motility in humans (Portincasa et al., 2000). Moreover, bile acids inhibited gallbladder smooth muscle contractility in muscle strips of guinea-pig gallbladder smooth muscle (Xu et al., 1997), and inhibited spontaneous mechanical contractions in canine colonic smooth muscle (Lee & Lee, 2000). These results suggest that bile acids can affect gastrointestinal motility. In nonmuscle cells, bile acids induce effects through a variety of signal mechanisms, which include activating protein kinase C, cyclooxygenase, or generating reactive oxygen species (ROS) (Sokol et al., 1995; Zhang et al., 1998; Zuh et al., 2002).
The gastrointestinal tract shows spontaneous mechanical contractions, mediated by the periodic generation of electrical pacemaker potentials, which are basic determinant of gastrointestinal smooth muscle activity (Szurszewski, 1987). The interstitial cells of Cajal (ICC) are the pacemaker cells in the gastrointestinal tract (Huizinga et al., 1995). They generate pacemaker potentials, and also mediate signals from the enteric nerves to the smooth muscles (Sanders, 1996). This slow wave generation is due to spontaneous inward currents (pacemaker currents) (Koh et al., 1998; Thomsen et al., 1998). It has been previously reported that bile acids inhibit spontaneous mechanical contractions in canine colon tissue. However, bile acids were found to activate nonselective cation channels in isolated single myocytes, which in turn led to the activation of smooth muscle. The different effect of bile acids in tissue and in isolated myocytes suggests that bile acids can modulate cells other than smooth muscle cells (Lee & Lee, 2000). Therefore, in the present study, we chose to directly address the question as to whether deoxycholic acids affect small bowel motility by modulating pacemaker currents and their downstream pathway in ICC of the murine small intestine. Our results show that deoxycholic acid inhibits pacemaker currents in ICC by enhancing prostaglandin E2 (PGE2) synthesis.
Methods
Reagents, inhibitors, oligonucleotides, and antibodies
Glibenclamide, deoxycholic acid, PGE2, and Tri Reagent were purchased from Sigma (St Louis, MO, U.S.A.). Pinacidil, NS-398 and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ) were purchased from RBI (Natick, NA, U.S.A.), enzyme immunoassay reagents for PGE2 assays were from Cayman Co. (Ann Arbor, MI, U.S.A.), goat polyclonal anti-mouse COX-2 was from Santa Cruz Biotechnology, Inc. (Santa Cruz Biotechnology, CA, U.S.A.), and anti-[rabbit IgG]-peroxidase was from Roche Molecular Biochemicals (Mannheim, Germany).
Preparation of cells and tissue
Balb/C mice (8–13 days old) of either sex were anesthetized with ether and killed by cervical dislocation. The small intestine from 1 cm below the pyloric ring to the cecum was removed and opened along the mesenteric border. The luminal contents were washed away with Krebs–Ringer bicarbonate solution. The opened intestine was then pinned to the base of a Sylgard dish and the mucosa was removed by sharp dissection. Small strips of the intestinal muscle (consisting of both circular and longitudinal muscles) were equilibrated in Ca2+-free Hank's solution (containing in mM; KCl 5.36, NaCl 125, NaOH 0.34, Na2HCO3 0.44, glucose 10, sucrose 2.9 and HEPES 11, adjusted to pH 7.4 with tris) for 30 min, and the cells were dispersed with an enzyme solution containing collagenase (Worthington Biochemical Co., Lakewood, U.S.A.), 1.3 mg ml−1, bovine serum albumin (Sigma), 2 mg ml−1, trypsin inhibitor (Sigma), 2 mg ml−1, and ATP, 0.27 mg ml−1. Cells were then plated onto sterile glass coverslips coated with murine collagen (2.5 μg ml−1, Falcon/BD) in a 35 mm culture dish. The cells were then cultured at 37°C in a 95% O2–5% CO2 incubator in smooth muscle growth medium (SMGM, Clonetics Corp., San Diego, CA, U.S.A.) supplemented with 2% antibiotics/antimycotics (Gibco, Grand Island, NY, U.S.A.) and murine stem cell factor (SCF, 5 ng ml−1, Sigma).
Labeling of cultured ICC by c-kit immunofluorescence
Cultured ICC were fixed in acetone (4°C/5 min). Following fixation, preparations were washed for 60 min in phosphate-buffered saline (PBS; 0.01 M, pH 7.4). Cultured ICC were then incubated in 10% goat serum containing 1% bovine serum albumin for 1 h at RT to reduce nonspecific antibody binding. To examine the ICC, cultured ICC were incubated overnight at 4°C with a rat monoclonal antibody raised against Kit protein (ACK2; 5 μg ml−1 in PBS; Gibco-BRL, Gaithersburg, MD, U.S.A.). Immunoreactivity was detected using fluorescein isothiocyanate (FITC)-conjugated secondary antibody (FITC-anti-rat; Vector Laboratories, 1 : 100 in PBS, 30 min, room temperature). Control cultured ICC were prepared in a similar manner, but omitting ACK2 from the incubation medium. Cells were examined under a confocal laser scanning microscope (FV300, Olympus, Japan) at an excitation wavelength appropriate for FITC (488 nm).
Patch-clamp experiments
The whole-cell configuration of the patch-clamp technique was used to record membrane currents (voltage clamp) and membrane potentials (current clamp) from cultured ICC. Axopatch 1-D (Axon Instruments, Foster, CA, U.S.A.) amplified membrane currents or potentials. The command pulse was applied with an IBM-compatible personal computer and pClamp software (version 6.1; Axon Instruments). The data were filtered at 5 kHz and displayed on an oscilloscope, a computer monitor, and a pen recorder (Gould 2200, Gould, Valley view, OH, U.S.A.). Results were analyzed using pClamp and Graph Pad Prizem (version 2.01) software. The cells were bathed in a solution containing (mM): KCl 5, NaCl 135, CaCl2 2, glucose 10, MgCl2 1.2, and HEPES 10, adjusted to pH 7.4 with tris. The pipette solution contained (mM): KCl 140, MgCl2 5, K2ATP 2.7, Na2GTP 0.1, creatine phophate disodium 2.5, HEPES 5, EGTA 0.1, adjusted to pH 7.2 with tris.
PGE2 production
ICC were cultured in six-well plates (1 × 106 cell per well) and stimulated with deoxycholic acid for 24 h. The medium was then removed, replaced with serum-free medium containing 5 μM of sodium arachidonate, and after 30 min, the PGE2 in this medium was determined by enzyme-linked immunosorbent assay (Cayman Chemical), as described previously (Subbaramaiah et al., 1996).
Western blotting
ICC were cultured with deoxycholic acid for 24 h and cell lysates were prepared by treating the cells with lysis buffer (150 mM NaCl, 100 mM Tris (pH 8.0), 1% Tween-20, 50 mM diethyldithiocarbamate, 1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, 10 μg ml−1 aprotinin, and 10 μg ml−1 leupeptin). The lysates obtained were sonicated for 20 s on ice, centrifuged at 10,000 × g for 10 min to sediment the particulate material, and the protein concentration was determined using the Bio-Rad dye-binding microassay (Bio-Rad, Hercules, CA, U.S.A.), and 20 μg protein per lane was electrophoresed on 12% SDS polyacrylamide gels after boiling for 5 min in Laemmli sample buffer. Proteins were blotted onto Hybond ECL membranes (Amersham-Pharmacia Biotech), and the membrane was incubated with primary antisera. Secondary antibody to IgG conjugated to horseradish peroxidase was used. The blots were probed with the enhanced chemiluminescence detect system (iNtRON, Biotech, Seoul, Korea).
Semiquantitative reverse transcriptase–polymerase chain reaction (RT–PCR)
ICC were cultured with deoxycholic acid for 3 h and total cellular RNA was extracted from the cells using Tri Reagent, according to the manufacturer's instructions. cDNA was reverse-transcribed from 1 μg of total cellular RNA using random hexamer primers and murine leukemia virus reverse transcriptase. cDNA (1 μg) was amplified for 23 cycles using the following mouse gene-specific primers: COX2: 5′-ACTTGCCTCACTTTGTTGAGTCATTC-3′ (sense) and 5′-TTTGATTAGTACTGTAGGGTTAATG-3′ (antisense), and cyclooxygenase (COX-1): 5′-ACTTGCCTCACTTTGTTGAGTCATTC-3′ (sense) and 5′-TTTGATTAGTACTGTAGGGTTAATG-3′ (antisense). The cycling parameters were: 30 s at 94°C for denaturation, 30 s at 60°C for primer annealing, and 1 min at 72°C for polymerization. The same amount of cDNA was amplified for 20 cycles using the specific GAPDH primers: 5′-GAGACCTTCAACACCCC-3′ (sense) and 5′-GTGGTGGTGAAGCTGTAGCC-3′ (antisense). RT–PCR products were visualized and analyzed after electrophoresis in a 2% agarose gel containing ethidium bromide. Gel images were captured on a Gel Doc Image Analysis System (Kodak). The yield of PCR products was normalized with respect to GAPDH after quantitation using NIH Image software (Bethesda, MD, U.S.A.). Relative expression levels were arbitrarily set at 1.0 in the control group.
Statistical analysis
Data are expressed as means±standard errors (s.e.). Differences between the data were evaluated by Student's t-test. A P-value less than 0.05 was taken to indicate a statistically significant difference. The n values reported in the text refer to the number of cells used in the patch-clamp experiments.
Results
Deoxycholic acid inhibits the pacemaker currents in cultured ICC
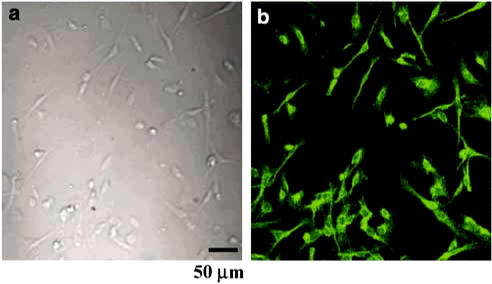
The patch-clamp technique was tested from ICC that showed the network-like structures in cultures (2–4 days) and was identified by immunostaining for Kit protein (Figure 1). At this time, spontaneous rhythmicity was routinely recorded from cultured ICC under current and voltage-clamp conditions and ICC within networks had a more robust electrical rhythmicity, and tissue-like spontaneous slow waves were recorded from these cells (Koh et al., 1998).
Figure 1.
Cultured ICC from the murine small intestine. The tunica muscularis of the small bowel was digested with collagenase, and the dispersed cells were cultured for 2 days. (a) Light microscope image of a small ICC network and (b) confocal microscope image of Kit-immunopositive ICC network in culture (a and b are of same field image).
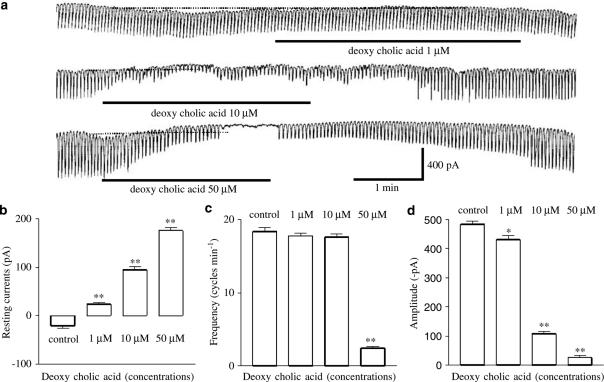
To understand the relationship between deoxycholic acid and the modulation of pacemaker currents in ICC, we examined the effects of deoxycholic acid on pacemaker currents. Under a voltage clamp at a holding potential of −70 mV, ICC generated spontaneous inward currents, which have been called ‘pacemaker currents'. The frequency of the pacemaker currents was 18±0.3 cycles min−1, and the resting current level and amplitude were –43±21 pA and –452±47 pA, respectively (n=8). The addition of deoxycholic acid (1–50 μM) decreased both the frequency and the amplitude of the pacemaker currents, and increased the resting currents in the outward direction (outward currents) in a concentration-dependent manner (Figure 2a). Under control conditions at a holding potential of −70 mV, the resting current was −43±21 pA, and the frequency and amplitudes of the pacemaker currents were 18±0.5 cycles min−1 and −483±11 pA, respectively. In the presence of deoxycholic acid, the resting currents were 36±33 pA at 1 μM, 95±8 pA at 10 μM, and 176±7 pA at 50 μM (Figure 2b, n=8) and the corresponding frequencies and amplitudes were 17±0.3 cycles min−1, 14±1.1 cycles min−1 and 2.4±0.3 cycles min−1, and −430±14 pA, −108±10 pA and −27±12 pA (Figure 2c and d). These results suggest that deoxycholic acid inhibits pacemaker currents in a dose-dependent manner in ICC.
Figure 2.
Effects of deoxycholic acid on pacemaker currents in cultured ICC of the murine small intestine. (a) Pacemaker currents from ICC exposed to deoxycholic acid (1–50 μM) at a holding potential of −70 mV. Deoxycholic acid caused a concentration-dependent decrease in the frequency, and amplitude of pacemaker currents, and increased basal outward currents. The responses to deoxycholic acid are summarized in (b), (c), and (d). Bars represent mean values±s.e. *(P<0.05), **(P<0.01) Significantly different from the untreated control. The dot lines indicate the zero current levels.
Deoxycholic acid activates ATP-sensitive K+ (KATP) channels in cultured ICC
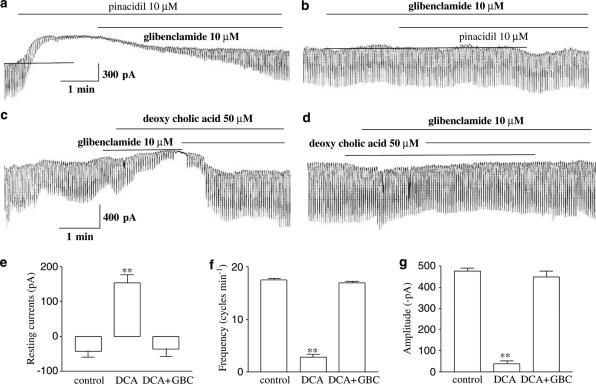
To determine whether deoxycholic acid affects KATP channels in ICC, we used the KATP channels opener (pinacidil) and blocker (glibenclamide). As shown in Figure 3a, pinacidil decreased the frequency and amplitude of the pacemaker currents, and also increased the resting currents in the outward direction. The pinacidil-induced effects were returned to the base-line level by treating with glibenclamide (10 μM). Furthermore, when glibenclamide was pretreated, pinacidil did not inhibit the pacemaker currents (Figure 3b), and glibenclamide when treated alone did not affect the pacemaker currents. These observations suggest that the activation of KATP channels may modulate pacemaker currents of ICC.
Figure 3.
Effects of pinacidil and deoxycholic acid on pacemaker currents in cultured ICC of the murine small intestine. (a) Pacemaker currents of ICC exposed to pinacidil (10 μM) at a holding potential of −70 mV. Pinacidil decreased the frequency and amplitude of the pacemaker currents, and increased the basal outward currents, and these effects were reversed by adding glibenclamide (10 μM). (b) The effects of pinacidil (10 μM) on pacemaker currents after pretreatment with glibenclamide. GBC: glibenclamide (10 μM). (c) Pacemaker currents exposed to deoxycholic acid (50 μM) at a holding potential of −70 mV. The effects of deoxycholic acid were qualitatively the same as the effects of pinacidil on pacemaker currents. Also, these effects were reversed adding glibenclamide (10 μM). (d) The effect of deoxycholic acid (10 μM) on pacemaker currents after pretreating cells with glibenclamide (10 μM). (e, f, and g) Bar graphic representation of the blocking response to glibenclamide on effects of deoxycholic acid. Bars represent mean values±s.e. **(P<0.01) Significantly different from the untreated control. The dot lines indicate the zero current levels. DCA: deoxycholic acid, GBC: glibenclamide.
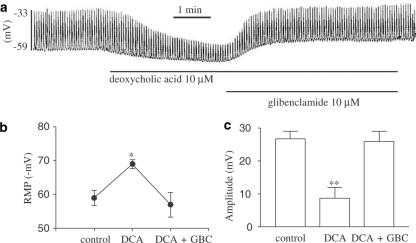
In voltage-clamp mode at a holding potential −70 mV, we checked the effects of deoxycholic acid on pacemaker current in ICC. When deoxycholic acid (50 μM) was applied in ICCs, both the frequency and the amplitude of pacemaker currents were decreased, and the resting currents were increased in the outward direction under voltage-clamp conditions (Figure 3c). The decreased frequency (i.e. from a control of 17.6±0.3 cycles min−1 to 2.8±0.5 cycles min−1) and amplitude (i.e. from a control −476±13 to −38±14 pA) of the pacemaker currents by deoxycholic acid (50 μM) were returned to the base line (i.e., 17±0.3 cycles min−1 and −450±25 pA) by adding glibenclamide (n=5, Figure 3f and g). The activated resting currents (i.e. from a control of −43±17 to 154±23 pA) also returned to the control level (i.e. −37±21 pA) (Figure 3e). Glibenclamide pretreatment (10 μM) prevented deoxycholic acid inhibiting the pacemaker currents (n=3, Figure 3d). The effects of deoxycholic acid were qualitatively the same as the effects of pinacidil on pacemaker currents. Also, in current clamp mode, we examined the effect of deoxycholic acid on membrane potentials and pacemaker potentials of ICC. Deoxycholic acid produced membrane hyperpolarization and decreased the amplitude of the pacemaker potentials (n=4, Figure 4a). Under control conditions at I=0, the resting membrane potential was −59±2.3 mV, and the amplitude of the pacemaker potentials was 26.7±2.3 mV. In the presence of deoxycholic acid (10 μM), the membrane was hyperpolarized to −69±2 mV (Figure 4b) and the amplitude of the pacemaker potentials decreased to 8.6±3.4 mV (Figure 4c). This deoxycholic acid-induced membrane hyperpolarization was blocked by the glibenclamide (10 μM) (Figure 4a), as were the deoxycholic acid-induced effects on pacemaker currents (Figure 3c). Taken together, these results suggest that deoxycholic acid may activate KATP channels in ICC.
Figure 4.
Effects of deoxycholic acid on pacemaker potentials in cultured ICC of murine small intestine. (a) Pacemaker potentials of ICC exposed to deoxycholic acid (10 μM) in the current clamping mode (I=0). The deoxycholic acid induced membrane hyperpolarization and the decreased amplitude of pacemaker potentials was reversed by glibenclamide. Responses to deoxycholic acid are summarized in (b) and (c). Bars represent mean values±s.e. *(P<0.05), **(P<0.01) Significantly different from the untreated control. DCA: deoxycholic acid, GBC: glibenclamide.
PGE2 involved in deoxycholic acid-induced pacemaker current inhibition
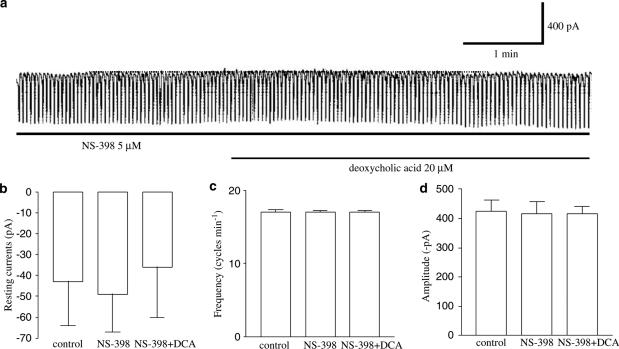
To examine whether the production of PGE2 is involved in the pacemaker current inhibition induced by deoxycholic acid, we examined the effects of NS-398, a specific cyclooxygenase-2 (COX-2) inhibitor (Futaki et al., 1994) and found that the deoxycholic acid-induced inhibition of pacemaker currents was significantly blocked by NS-398 pretreatment (5 μM) (Figure 5a). Under control conditions, at a holding potential of –70 mV, the resting current was −43±21 pA, and the frequency and the amplitude of the pacemaker currents were 17±0.4 cycles min−1 and −425±37 pA, respectively (n=7), and in the presence of NS-398, these values became −36±25 pA (Figure 5b), 17±0.4 cycles min−1 and −415±26 pA, respectively (Figure 5c and d), which were not significantly different from those obtained under control conditions. These results suggest that PGE2 may be involved in the pacemaker current inhibition induced by deoxycholic acid.
Figure 5.
Effect of NS-398 upon deoxycholic acid-induced responses on pacemaker currents in cultured murine small intestine ICC. (a) NS-398 (5 μM) blocked the effect of deoxycholic acid (20 μM) on pacemaker currents. The blocking effect of NS-398 is summarized in (b), (c) ,and (d). Bars represent mean values±s.e. DCA: deoxycholic acid. The dot lines indicate the zero current levels.
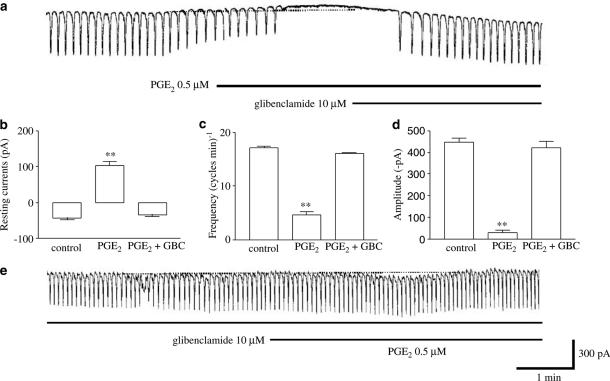
To investigate this possibility, we next examined whether PGE2 mimics the effect of deoxycholic acid on pacemaker currents, and we found that PGE2 affected the pacemaker currents in the same manner as deoxycholic acid. PGE2 (at 0.5 μM) inhibited the pacemaker current and activated outward current (Figure 6a). Under control conditions, at a holding potential −70 mV, the resting current was −42±5 pA, and the frequency and the amplitude of the pacemaker current were 17±0.3 cycles min−1 and −448±19 pA, respectively (n=4). In the presence of PGE2, the resting current was 103±10 pA (Figure 6b), and the frequency and the amplitude of the pacemaker current were 4.7±0.6 cycles min−1 and −28±14 pA, respectively (Figure 6c and d). The effect of PGE2 on the pacemaker current was reversed by treatment with glibenclamide (10 μM) (Figure 6a). In addition, PGE2 had not shown these actions on the pacemaker currents by pretreatment of glibenclamide (10 μM) (Figure 6e) (n=4). These results suggest that PGE2 may be involved in the effects of deoxycholic acid on pacemaker currents.
Figure 6.
Effects of PGE2 on pacemaker currents in cultured murine small intestine ICC. (a) Pacemaker currents exposed to PGE2 (0.5 μM) at a holding potential of −70 mV. PGE2 decreased the frequency and amplitude of the pacemaker currents, and increased the basal outward currents. These effects were reversed by adding glibenclamide (10 μM). The responses to PGE2 are summarized in (b), (c), and (d). Bars represent mean values±s.e. **(P<0.01) Significantly different from the untreated control. (e) PGE2 effects on pacemaker currents in the pretreatment of glibenclamide. The dot lines indicate the zero current levels. GBC: glibenclamide.
Deoxycholic acid increased COX-2 activity and its gene expression in ICC
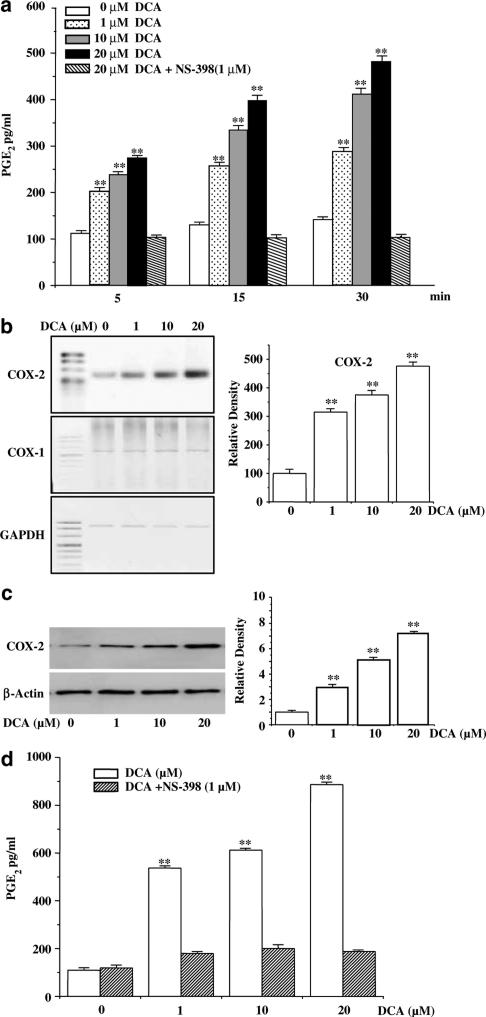
Next, in order to find how deoxycholic acid could affect PGE2 production in ICC, we assayed the PGE2 production, expression of COX-2 mRNA and of its protein, and their responsiveness to deoxycholic acid. As shown in Figure 7a, PGE2 levels in the ICC cells treated with deoxycholic acid increased in a time- and dose-dependent manner, compared with untreated control cultures. The exposure of ICC to deoxycholic acid (1–20 μM) led to the increase in PGE2 production within 5 min. To investigate which isoforms of COX are responsible for deoxycholic acid-related PGE2 production, we performed the experiment in the presence of NS-398, and found that a dose of 1 μM of NS-398 resulted in the complete inhibition of deoxycholic acid-induced PGE2 production. These results suggest that deoxycholic acid may directly affect COX-2 activity.
Figure 7.
(a) Deoxycholic acid increased PGE2 in a time-dependent manner. ICC were incubated with indicated doses of deoxycholic acid for 5, 15, and 30 min. The results are means±s.e. **(P<0.01). DCA: deoxycholic acid. (b) Deoxycholic acid upregulated COX-2 mRNA expression. ICC were treated with different quantities of deoxycholic acid for 3 h. Total RNA was isolated and subjected to RT–PCR analysis with COX-1 and COX-2 primers. The result of a quantitative analysis of COX-2 mRNA expression from three independent experiments is shown in the right panel. The results are expressed as ratios of COX-2 to GAPDH mRNA, and bars represent mean values±s.e. **(P<0.01) Significantly different from the untreated control. (c) Deoxycholic acid upregulated COX-2 protein expression. ICC were treated with 1–20 μM deoxycholic acid for 24 h. Protein was extracted and subjected to Western blot analysis with a polyclonal anti-COX-2 antibody. The levels of COX-2 expression, which were quantified by densitometry from three independent experiments, are shown in the lower panel. The results are means±s.e. **(P<0.01) Significantly different from the untreated control. (d) ICC were incubated with indicated doses of deoxycholic acid for 24 h in the presence or absence 1 μM NS-398. The results are means±s.e. **(P<0.01) Significantly different from the untreated control.
Several previous studies have shown that deoxycholic acid could induce the COX-2 gene expression (Zhang et al., 1998; Glinghammar et al., 2002). Thus, we also checked whether deoxycholic acid was capable of COX-2 gene expression in ICC. As shown in Figure 7b, the exposure of ICC to various concentrations of deoxycholic (1–20 μM) for 3 h led to dose-dependent increases in COX-2 mRNA expression. At the highest concentration of deoxycholic acid examined (20 μM), the COX-2 mRNA content was five times higher than the control value. In contrast, COX-1 gene expression was not induced by deoxycholic acid. To determine whether deoxycholic acid-induced COX-2 mRNA in ICC influences its protein expression, we used Western blot. Figure 7c shows that deoxycholic acid induced an increase in COX-2 protein in a dose-dependent manner. A concentration of 20 μM of deoxycholic acid led to an eight-fold increase in COX-2 protein content. To examine whether increased COX-2 expression is associated with elevated PGE2 synthesis, we measured the levels of PGE2 released from ICC, and found that PGE2 levels in the ICC cells treated with deoxycholic acid increased and this increased PGE2 was completely inhibited by treatment of NS-398 (Figure 7d). Taken all together, these results indicate that treatment of ICC with deoxycholic acid leads to increase in the PGE2 production in ICC through the enhancement of COX-2 activity and its gene expression.
Discussion
The major findings of this study are that pacemaker currents in ICC are inhibited by deoxycholic acid treatment, that this inhibition of pacemaker currents is reversed by glibenclamide treatment, and that pretreatment with NS-398, a specific COX-2 inhibitor, completely suppresses deoxycholic acid-mediated pacemaker current inhibition. In addition, we observed that PGE2 treatment decreased pacemaker currents, and that these observed effect of PGE2 was blocked by glibenclamide. Furthermore, we also found that deoxycholic acid increased COX-2 enzyme activity and its expression in ICC. These results strongly support the idea that deoxycholic acid may inhibit the pacemaker currents of ICC by activating KATP channels through the production of PGE2. To our knowledge, the findings of this study represent the first evidence that PGE2 serves as a deoxycholic acid mediator to inhibit pacemaker currents of ICC.
Deoxycholic acid affects gastrointestinal motility, which exhibits spontaneous mechanical contractions that depend on pacemaker potentials. Since ICC generate spontaneous inward pacemaker currents that are responsible for these pacemaker potentials in gastrointestinal smooth muscles (Huizinga et al., 1995; Koh et al., 1998; Thomsen et al., 1998), deoxycholic acids may affect small bowel motility by modulating pacemaker currents in ICC. To examine this possibility, we investigated the effect of deoxycholic acid on pacemaker currents, and found that deoxycholic acid suppresses pacemaker currents in ICC (Figure 2).
KATP channels play an important role in regulating the resting membrane potential and membrane excitability of a variety of tissues. The activation of these channels causes membrane hyperpolarization and decreases cell excitability. KATP channels are activated by K+ channel openers, such as pinacidil, lemakalim, and diazoxide, and inhibited by glibenclamide (Edwards & Weston, 1993; Quayle et al., 1997). In gastrointestinal smooth muscle tissue, K+ channel openers induced membrane hyperpolarization and reduced the frequency and amplitude of pacemaker potentials. Moreover, these effects were blocked by glibenclamide, suggesting that KATP channel activity modulates pacemaker potentials (Farraway & Huizinga, 1991; Huang et al., 1999). Thus, we asked whether the suppression of pacemaker currents in ICC by deoxycholic acid is mediated KATP channel modulation. In this study, we have demonstrated that pinacidil inhibits pacemaker currents, and activates outward currents, which are antagonized by glibenclamide (Figure 3), suggesting that KATP channels exist in ICC, and that the activity of KATP channels in ICC may be involved in intestinal motility. We also observed that deoxycholic acid treatment of ICC produced the same effects on pacemaker currents as pinacidil, and this observed effect of deoxycholic acid on pacemaker currents was completely inhibited by glibenclamide (Figures 3 and 4). These results suggest that deoxycholic acid suppresses intestinal motility by the activating KATP channels in the ICC of the murine small intestine.
KATP channels in gastrointestinal smooth muscles are regulated by dual signal pathways. One involves acetylcholine, which inhibits the activity of KATP channels by protein kinase C activation (Hatakeyama et al., 1995; Jun et al., 2001). The other involves calcitonin gene-related peptide (CGRP), which activates KATP channels by a cyclic AMP-dependent mechanism by adenylate cyclase activation (Zhang et al., 1994). The stimulatory effect of bile acids upon cyclic AMP has been shown in nonmuscular preparations. Using the adult rabbit distal colon in vitro, Potter et al. (1991) showed that deoxycholic acid significantly increases cyclic AMP, which leads to enhanced chloride secretion. Thus, bile acids may exert their relaxant action on ICC by activating the cyclic AMP second messenger pathway. However, we previously reported that cyclic AMP has no effect on pacemaker currents of ICC, but that ODQ, a guanylate cyclase inhibitor, can block the SNAP (S-nitroso-N-acetylpenicillamine; NO donor)-mediated suppression of pacemaker currents in ICC, and 8-bromo-cyclic GMP mimics the effects of SNAP (Jun et al., 2004). These results suggest that the cyclic GMP system may be an important mediator of pacemaker currents in ICC. Thus, we investigated whether cyclic GMP is involved in the generation of deoxycholic acid-mediated pacemaker currents. However, we found that ODQ could not block the deoxycholic acid-induced inhibition of pacemaker currents (data not shown).
The important question remaining is how does deoxycholic acid activate KATP channels in ICC, and what are the identities of the downstream effectors involved in mediating the effects of deoxycholic acid? Several papers have shown that the PGs may be a downstream effector of bile acid. For example, bile acids were found to induce COX-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line (Yoon et al., 2002) and in an esophageal cancer cell line (Zhang et al., 1998). In addition, bile acids could alter the cellular proliferation in Barrette esophagus by inducing the release of PGE2 through COX-2 enzyme (Kaur & Triadafilopoulos, 2002). Moreover, it is known that deoxycholic acid stimulates PGE2 synthesis in human colonic fibroblasts (Zuh et al., 2002), rabbit gastric cells (Hata et al., 1994), and the gallbladder smooth muscle cells of guinea-pig (Xiao et al., 2002), suggesting that PGE2 may serve as a downstream effector of deoxycholic acid. PGs are biologically active substances, which are synthesized by the gastrointestinal musculature, and are known to be extremely potent regulators of the electrical and mechanical activities of gastrointestinal smooth muscle (Sanders & Northrup, 1983; Sanders, 1984). PGs are generated through the activities of the bifunctional COX-1 and COX-2 enzymes. COX-1 is constitutively expressed in a wide range of tissue, at a constant level of expression. In contrast, COX-2 may be highly induced by cytokines and other participants in inflammatory and neoplastic processes (Herschman, 1994; Seibert et al., 1997). Recent research has found that prostanoids induced by COX-2 regulate the mechanical activities of murine gastric muscles. Since ICC have been shown to be involved in the mediation of enteric motor neurotransmission in GI muscles, ICC might be important sites for the production of prostanoid within the muscle layers (Porcher et al., 2002). In the present study, we found that the inhibitory action of deoxycholic acid on pacemaker currents was completely blocked by NS-398, a specific COX-2 inhibitor (Figure 5). In addition, PGE2 mimicked the effects of deoxycholic acid, and furthermore, PGE2 inhibited pacemaker currents and its action was blocked by glibenclamide (Figure 6). Taken together, these results strongly support the idea that PGE2 is a candidate substance for the deoxycholic acid-mediated species for the direct inhibition of pacemaker currents in ICC. Therefore, we next directly addressed the question as to whether deoxycholic acid contributes to the production of PGE2 in ICC, and found that deoxycholic acid is capable of stimulating the COX-2 enzyme activity and its expression in ICC (Figure 7). These results allowed us to delineate the following relationship: deoxycholic acid → activation of COX-2 activity and enhancement of COX-2 expression (increase the PGE2 production → activation of KATP channels → inhibition of pacemaker currents).
In conclusion, this work demonstrates that deoxycholic acid inhibits pacemaker currents by activating KATP channels in ICC, and that this suppression of pacemaker currents is mediated, at least in part, through PGE2.
Acknowledgments
This work was supported by the Ministry of Science and Technology, Korea and the KOSEF through the Research Center for Proteineous Materials, and the Korean Ministry of Science of Information and Communication (IMT-2000-C3-C5).
Abbreviations
- COX-1
cyclooxygenase-1
- COX-2
cyclooxygenase-2
- ICC
interstitial cells of Cajal
- KATP channels
ATP-sensitive K+ channels
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxaline-1-one
- PGE2
prostaglandin E2
- PGs
prostaglandins
References
- ARMSTRONG D.N., KRENZ H.K., MODLIN I.M., BALLANTYNE G.H. Bile salt inhibition of motility in the isolated perfused rabbit terminal ileum. Gut. 1993;34:483–488. doi: 10.1136/gut.34.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AZZAROLI F., MAZZELLA G.F., MAZZEO C., SIMONI P., FESTI D., COLECCHIA A., MONTAGNANI M., MARTINO C., VILLANOVA N., RODA A., RODA E. Sluggish small bowel motility is involved in determining increased biliary deoxycholic acid in cholesterol gallstone patients. Am. J. gastroenterol. 1999;94:2453–2459. doi: 10.1111/j.1572-0241.1999.01375.x. [DOI] [PubMed] [Google Scholar]
- BALISTRERI W.F., HEUBI J.E., SUCHY F.J. Bile acid metabolism: relationship of bile acid malabsorption and diarrhea. J. Pediatr. Gastroenterol. Nutr. 1983;2:105–121. [PubMed] [Google Scholar]
- BOMZON A., LJUBUNCIC P. Bile acids as endogenous vasodilators. Bioche. Pharmacol. 1995;49:581–589. doi: 10.1016/0006-2952(94)00428-o. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., WESTON A.H. The pharmacology of ATP-sensitive potassium channels. Annu. Rev. Pharmacol. Toxicol. 1993;33:597–637. doi: 10.1146/annurev.pa.33.040193.003121. [DOI] [PubMed] [Google Scholar]
- FARRAWAY L., HUIZINGA J.D. Potassium channel activation by cromakalim affects slow wave type action potential of colonic smooth muscle. J. Pharmacol. Exp. Ther. 1991;257:35–41. [PubMed] [Google Scholar]
- FELDMAN S., GIBALDI M. Effect of bile salts on gastric emptying and intestinal transit in the rat. Gastroenterology. 1968;54:918–921. [PubMed] [Google Scholar]
- FUTAKI N., TAKAHASHI S., YOKOYAMA M., ARAI I., HIGUCHI S., OTOMO S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994;47:55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- GLINGHAMMAR B., INOUE H., RAFTER J.J. Deoxycholic acid causes DNA damage in colonic cells with subsequent induction of caspases, COX-2 promoter activity and the transcription factors NF-kB and AP-1. Carcinogenesis. 2002;23:839–845. doi: 10.1093/carcin/23.5.839. [DOI] [PubMed] [Google Scholar]
- HATA Y., OTA S., TERANO A., KOHMOTO O., YOSHIURA K., OKANO K., IVWEY K.J., SUGIMOTO T. Stimulation of prostaglandin E2 release from cultured rabbit gastric cells by sodium deoxycholate. Prostaglandins. 1994;47:423–436. doi: 10.1016/0090-6980(94)90043-4. [DOI] [PubMed] [Google Scholar]
- HATAKEYAMA N., WANG Q., GOYAL R.K., AKBARALL H.I. Muscarinic suppression of ATP-sensitive K+ channel in rabbit esophageal smooth muscle. Am. J. Physiol. 1995;268:C877–C885. doi: 10.1152/ajpcell.1995.268.4.C877. [DOI] [PubMed] [Google Scholar]
- HERSCHMAN H.R. Regulation of prostaglandin synthase-1 and prostaglandin synthase-2. Cancer Metast. Rev. 1994;13:241–256. doi: 10.1007/BF00666095. [DOI] [PubMed] [Google Scholar]
- HOFFMAN A.F., POLEY J.R. Cholestyramine treatment of diarrhea associated with ileal resection. N. Eng. J. Med. 1969;281:397–402. doi: 10.1056/NEJM196908212810801. [DOI] [PubMed] [Google Scholar]
- HUANG S.M., NAKAYAMA S., IINO S., TOMITA T. Voltage sensitivity of slow wave frequency in isolated circular muscle strips from guinea-pig gastric antrum. Am. J. Physiol. 1999;276:G518–G528. doi: 10.1152/ajpgi.1999.276.2.G518. [DOI] [PubMed] [Google Scholar]
- HUIZINGA J.D., THUNEBERG L., KLUPPEL M., MALYSZ J., MILLELSEN H.B., BERNSTEIN A. W/kit gene required for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- JUN J.Y., CHOI S., YEUM C.H., CHANG I.Y., PARK C.K., KIM M.Y., KONG I.D., SO I., KIM K.W., YOU H.J. Noradrenaline inhibits pacemaker currents through stimulation of β1-adrenoceptors in cultured interstitial cells of Cajal from murine small intestine. Br. J. Pharmacol. 2004;141:670–677. doi: 10.1038/sj.bjp.0705665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUN J.Y., KONG I.D., KOH S.D., WANG S.Y., PERINO B.A., WARD S.M., SANDERS K.M. Regulation of ATP-sensitive K+ channels by protein kinase C in murine colonic myocytes. Am. J. Physiol. 2001;281:C857–C864. doi: 10.1152/ajpcell.2001.281.3.C857. [DOI] [PubMed] [Google Scholar]
- KAUR B.S., TRIADAFILOPOULOS G. Acid and bile induce prostaglandin E2 release and proliferation in Barret's esophagus through COX-2 and PKCe-dependent mechanisms. Am. J. Physiol. 2002;283:G309–G318. doi: 10.1152/ajpgi.00543.2001. [DOI] [PubMed] [Google Scholar]
- KOH S.D., SANDERS K.M., WARD S.M. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J. Physiol. 1998;513:203–213. doi: 10.1111/j.1469-7793.1998.203by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEE H.K., LEE K.H. Bile acid modulation of gastrointestinal smooth muscle contraction and ionic currents. Kor. J. Physiol. Pharmacol. 2000;4:333–338. [Google Scholar]
- PENAGINI R., MISIEWICZ J.J., FROST P.G. Effect of jejunal infusion of bile acids on small bowel transit and fasting jejunal motility in man. Gut. 1988;29:789–794. doi: 10.1136/gut.29.6.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PENAGINNI R., SPILLER R.C., MISIEWICZ J.J., FROST P.G. Effect of ileal infusion of glycochenodeoxycholic acid on segmental transit, motility, and flow in the human jejunum and ileum. Gut. 1989;30:609–617. doi: 10.1136/gut.30.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORCHER C., HOROWITZ B., BAYGUINOV O., WARD S.M., SANDERS K.M. Constitutive expression and function of cyclooxygenase-2 in murine gastric muscles. Gastroenterolgy. 2002;122:1442–1454. doi: 10.1053/gast.2002.33065. [DOI] [PubMed] [Google Scholar]
- PORTINCASA P., PEETERS T.L., VAN BERGE-HENEGOUWEN G.P., VAN SOLINGE W.W., PALASCIANO G., VAN ERPECUM K.J. Acute intraduodenal bile salt depletion leads to strong gallbladder contraction, altered antroduodenal motility and high plasma motilin levels in humans. Neurogastroenterol. Mot. 2000;12:421–430. doi: 10.1046/j.1365-2982.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- POTTER G.D., SELLIN J.H., BURLINGAME S.M. Bile acid stimulation of cyclic AMP and ion transport in developing rabbit colon. J. Pediatr. Gastroenterol. Nutr. 1991;13:335–341. doi: 10.1097/00005176-199111000-00001. [DOI] [PubMed] [Google Scholar]
- QUAYLE J.M., NELSON M.T., STANDEN N.B. ATP-sensitive and inwardly rectifying potassium channels in smooth muscle. Physiol. Rev. 1997;77:1165–1232. doi: 10.1152/physrev.1997.77.4.1165. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M. Role of prostaglandins in regulating gastric motility. Am. J. Physiol. 1984;247:G117–G126. doi: 10.1152/ajpgi.1984.247.2.G117. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M. A case of interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- SANDERS K.M., NORTHRUP T.E. Prostaglandin synthesis by microsomes of circular and longitudinal gastrointestinal muscles. Am. J. Physiol. 1983;244:G442–G448. doi: 10.1152/ajpgi.1983.244.4.G442. [DOI] [PubMed] [Google Scholar]
- SEIBERT K., ZHANG Y., LEAHY K., HAUSER S., MASFERRER J., ISAKSON P. Distribution of COX-1 and COX-2 in normal and inflamed tissue. Adv. Exp. Med. Biol. 1997;400A:167–170. doi: 10.1007/978-1-4615-5325-0_24. [DOI] [PubMed] [Google Scholar]
- SOKOL R.J., WINKLHOFER-ROOB B.M., DEVEREAUX M.W., McKIM J.M. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:249–256. doi: 10.1016/0016-5085(95)90585-5. [DOI] [PubMed] [Google Scholar]
- SUBBARAMAIAH K., TELANG N., RAMONETTI J.T., ARAKI R., DEVITO B., WEKSLER B.B., DANNENBERG A.J. Transcription of cyclooxygenase-2 is enhanced in transformed mammary epithelial cells. Cancer Res. 1996;56:4424–4429. [PubMed] [Google Scholar]
- SZURSZEWSKI J.H.Electrical basis for gastrointestinal motility Physiology of the Gastrointestinal Tract 1987New York: Raven Press; 383–422.2nd edn., ed. Johnson L.R. pp [Google Scholar]
- THOMSEN L., ROBINSON T.L., LEE J.C., FARRAWAY M.J., ANDREWA D.W., HUIZINGA J.D. Interstitial cells of Cajal generate a rhythmic pacemaker current. Nat. Med. 1998;4:848–851. doi: 10.1038/nm0798-848. [DOI] [PubMed] [Google Scholar]
- XIAO Z.U., RHO A.K., BIANCANI P., BEHAR J. Effects of bile acids on the muscle functions of guinea pig gallbladder. Am. J. Physiol. 2002;283:G87–G94. doi: 10.1152/ajpgi.00536.2001. [DOI] [PubMed] [Google Scholar]
- XU Q.W., FREEDMAN S.M., SHAFFER E.A. Inhibitory effect of bile salts on gallbladder smooth muscle contractility in the guinea-pig in vitro. Gastroenterology. 1997;112:1699–1706. doi: 10.1016/s0016-5085(97)70053-2. [DOI] [PubMed] [Google Scholar]
- YOON J.H., HIGUCHI H., WERNBURG N.M., KAUFMANN S.H., GORES G.J. Bile acids induce cyclooxygenase-2 expression via the epidermal growth factor receptor in a human cholangiocarcinoma cell line. Gastroenterology. 2002;122:985–993. doi: 10.1053/gast.2002.32410. [DOI] [PubMed] [Google Scholar]
- ZHANG F., SUBBARAMAIAH K., ALTORKI N., DANNENBERG A.J. Dihydroxy bile acids activate the transcription of cyclooxygenase-2. J. Biol. Chem. 1998;273:2424–2428. doi: 10.1074/jbc.273.4.2424. [DOI] [PubMed] [Google Scholar]
- ZHANG L., BONEV A.D., NELSON M.T., MAWE G. Activation of ATP-sensitive potassium currents in guinea-pig gall-bladder smooth muscle by the neuropeptide CGRP. J. Physiol. 1994;478:483–491. doi: 10.1113/jphysiol.1994.sp020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZUH Y., HUA P., RAFIG S., WAFFNER E.J., DUFFEY M.E., LANCE P. Ca2+-and PKC-dependent stimulation of PGE2 synthesis by deoxycholic acid in human colonic fibroblasts. Am. J. Physiol. 2002;283:G503–G510. doi: 10.1152/ajpgi.00525.2001. [DOI] [PubMed] [Google Scholar]