Abstract
Minocycline has anti-inflammatory and antiapoptotic effects on cartilage, neurons and periodontal tissues, and both properties are central to the pharmaceutical treatment of liver diseases. We investigated the effects of minocycline on fulminant hepatitis in C57BL/6J mice induced by lethal challenge of the activating anti-Fas antibody, Jo2.
Intraperitoneal injection of Jo2 (0.6 μg g−1) to mice resulted in fulminant hepatitis, as evidenced by increase of serum alanine/aspartate transaminase activities and histopathological alterations in liver sections, as well as animal death. Nevertheless, mice pretreated with three doses of minocycline (5 mg kg−1) resisted this lethal effect significantly. Minocycline treatment improved the survival kinetics, although to a lesser extent, when mice were challenged simultaneously with Jo2 or even treated 30 min after the lethal challenge.
Jo2-induced activation of caspase-3 or -9 in liver tissues was inhibited by minocycline pretreatment, and yet the direct addition of minocycline to liver extracts from Jo2-challenged mice failed to block caspase activation in vitro. Moreover, minocycline efficiently suppressed the release of cytochrome c from mitochondria of the liver tissues from Jo2-challenged mice. In contrast, caspase-8 activation and Bid truncation triggered by Jo2 were not diminished by minocycline pretreatment in mouse livers.
Our results suggest that easing of Fas-triggered fulminant hepatitis by minocycline may involve a mitochondrial apoptotic pathway, probably through preventing cytochrome c release and thereby blocking downstream caspase activation.
Keywords: Fulminant hepatitis, minocycline, Fas, caspase, cytochrome c, mitochondria
Introduction
Acute or fulminant liver failure (ALF or FLF), a syndrome caused by diverse aetiologies, including viral hepatitis, immunological insults and hepatotoxins, debilitates many patients worldwide (Atillasoy & Berk, 1995). In their severe forms, ALF and FLF are often devastating and life threatening. However, the current remedy for these depends mainly on supportive treatment and liver transplantation. Further understanding of the molecular pathogenesis appears to be essential for the development of effective therapies.
Fas (CD95/APO-1), a 43-kDa cell surface glycoprotein, belongs to the tumour necrosis factor receptor superfamily, and mediates apoptosis upon binding with its cognate ligand, or artificially with specific agonistic antibodies (Ogasawara et al., 1993). The Fas/Fas-ligand (Fas/FasL) system plays a pivotal role in diverse types of liver diseases (Galle et al., 1995; Strand et al., 1998). When injected with the anti-Fas monoclonal antibody Jo2, mice die within hours, mimicking certain forms of ALF in humans (Ogasawara et al., 1993). During Fas activation, caspase-8 is activated upon recruitment to the death-induced signalling complex (DISC) through the Fas-associated death domain (FADD) (Ashkenazi & Dixit, 1998; Peter & Krammer, 1998). Activated caspase-8 may either directly proteocleave and activate the effector caspase-3, or indirectly activate the mitochondrial apoptotic pathway through processing the Bcl-2 family member BH3-interacting domain death agonist (Bid) (Scaffidi et al., 1998). The Bid-modified mitochondria, in turn, release cytochrome c, which triggers apoptosome assembly in the presence of caspase-9, apoptotic protease activating factor-1 (Apaf-1) and dATP, thereby leading to caspase-3 activation (Li et al., 1997). Mice deficient in Bid (Yin et al., 1999) or overexpressing a liver-specific Bcl-2 (Lacronique et al., 1996; Rodriguez et al., 1996a) readily resist fulminant hepatitis triggered by anti-Fas antibody, suggesting a central role of mitochondria-dependent pathways in ALF or FLF. In addition, caspases are also the critical effectors in the Fas-mediated apoptotic processes, as demonstrated by genetic studies using caspase knockout mice (Woo et al., 1999) and by peptidic caspase inhibitors (Rodriguez et al., 1996b; Bajt et al., 2000). Caspase-3 in hepatocytes may activate additional caspase-8, thereby creating a positive amplification loop between two cascades of caspase-8 and -9. Conceivably, blocking caspase or mitochondrial activation in the Fas signalling pathway might have therapeutic potential for treating ALF.
Minocycline, a tetracycline derivative, is commonly prescribed for a variety of infections, including atypical pneumonia, infectious diarrhoea and acne (Klein & Cunha, 1995). Independent of its antimicrobial activity, minocycline exerts pleiotrophic anti-inflammatory effects on the brain (Yrjanheikki et al., 1998; 1999; Chen et al., 2000; Du et al., 2001; Sanchez Mejia et al., 2001; Zhu et al., 2002), and on connective (Sadowski & Steinmeyer, 2001) and periodontal tissues (Golub et al., 1985). Minocycline might confer protection in models of amyotrophic lateral sclerosis (Zhu et al., 2002), cerebral ischaemia (Yrjanheikki et al., 1998; 1999), traumatic brain injury (Sanchez Mejia et al., 2001), Huntington's (Chen et al., 2000) and Parkinson's diseases (Du et al., 2001), as well as in osteoarthritis (Amin et al., 1996) and rheumatoid arthritis (Tilley et al., 1995). Plausible mechanisms for such protection involve the inhibition of caspase-1 (Yrjanheikki et al., 1998; 1999; Chen et al., 2000; Du et al., 2001; Sanchez Mejia et al., 2001) and caspase-3 (Chen et al., 2000; Zhu et al., 2002) and suppression of inducible nitric oxide synthase (iNOS) (Amin et al., 1996; Yrjanheikki et al., 1998; Chen et al., 2000; Du et al., 2001; Sadowski & Steinmeyer, 2001). In addition, interference with cytochrome c release from mitochondria has recently been implicated in minocycline-mediated neuroprotection (Zhu et al., 2002). In this study, we investigated whether minocycline could be beneficial for the treatment of ALF in a mouse model. We found that minocycline is indeed capable of easing the symptoms of fulminant hepatitis triggered via a Fas-mediated pathway.
Methods
Animals and materials
Male C57BL/6J mice (8- to 10-week-old; National Laboratory Animal Center, Taipei, Taiwan) were maintained for at least 1 week at 22°C before being used in all experiments. Animals were kept under specific pathogen-free conditions in a 12 h light : dark rhythm with free access to food and water, and received humane care in compliance with Institutional Guidelines. All chemicals were purchased from Sigma Chemical Co. (St Louis, MO, U.S.A.) unless otherwise stated.
Experimental procedures
Mice were pretreated intraperitoneally (i.p.) with three doses of 5 mg kg−1 of minocycline, or phosphate-buffered saline (PBS: controls), at 24 h, 12 h and immediately before the i.p. administration of an agonistic anti-Fas Jo2 antibody (PharMingen, San Diego, CA, U.S.A.) at 0.6 μg g−1, a dose that could kill approximately 90% of mice tested within 20 h. After this lethal challenge, mice were monitored for viability for 7 days. Serum activities of aspartate transaminase (AST) and alanine transaminase (ALT) were determined using the test kits OSR6107 and OSR6109, respectively (Olympus, Clare, Ireland). Immediately after taking the blood samples retroorbitally, mice were killed by cervical dislocation. The ratios of liver to body weight were measured, and the excised liver mass was sectioned, fixed overnight at 4°C in 10% formalin solution, dehydrated, paraffin-embedded, cut at 4-μm thickness and stained with haematoxylin and eosin for histological examination.
In another set of experiments, mice were treated with 0.6 μg g−1 of anti-Fas antibody. A total of 15 mice per group were simultaneously or sequentially treated with single injection of 5 mg kg−1 minocycline, or not treated (controls). Animal survival was monitored for 24 h.
Caspase activities
Caspase-3 and -9 activities were measured in liver extracts after Jo2 antibody challenge from mice pretreated with minocycline or not. After euthanizing the animals, the freshly excised liver was homogenized in 25 mM HEPES buffer (pH 7.3) containing 5 mM EDTA, 5 mM DTT, 0.1% CHAPS plus a protease inhibitor cocktail (Roche, Mannheim, Germany). After centrifugation at 20,000 × g for 20 min at 4°C, the resulting supernatant was assayed for caspase-3 and -9 activities using specific fluorogenic substrates of 50 μM DEVD-AFC and 250 μM LEHD-AMC, respectively, according to the manufacturer's instructions (BD Biosciences Clontech, Palo Alto, CA, U.S.A.). Briefly, enzymatic activities were monitored using a fluorescence microplate reader (Tecan, Durham, NC, U.S.A.) at excitation wavelengths of 430 and 360 nm and emission wavelengths of 535 and 465 nm for caspase-3 and -9, respectively. The fluorescence intensity was calibrated using standard concentrations of AFC and AMC; the caspase activity was calculated from the slope of the recorder trace and expressed in pmol h−1 mg protein−1. Reaction mixtures lacking fluorogenic substrates or after adding specific inhibitors (DEVD-CHO for caspase-3 or LEHD-CHO for caspase-9) were used as controls. For evaluation of the direct effect of minocycline on caspase activity, liver extracts from anti-Fas antibody-challenged mice were assayed with various concentrations of minocycline or specific inhibitors for caspase-3 and -9 as controls. Protein concentrations in the liver extracts were determined by spectrophotometry using a protein assay kit from Bio-Rad Laboratories (Hercules, CA, U.S.A.).
Western blotting of cytochrome c, caspase-8 and Bid
Western blot analysis was used as previously described (Bajt et al., 2000) with minor modifications to detect the amounts of cytosolic cytochrome c, caspase-8 and Bid in liver extracts. After Jo2 lethal challenge, liver tissues from mice pretreated with minocycline or PBS were homogenized in 25 mM HEPES (pH 7.3) containing 5 mM EDTA, 5 mM DTT, 0.1% CHAPS and a cocktail of protease inhibitors (Roche). The homogenate was first centrifuged at 1000 × g for 10 min at 4°C, and the supernatant was further centrifuged at 20,000 × g for 20 min. A 40 μg portion of the resulting supernatant, used as the cytosolic component of liver tissues, was electrophoresed on 15% sodium dodecyl sulphate–polyacrylamide gels. By using primary antibodies against cytochrome c, Bid (PharMingen, San Diego, CA, U.S.A.) and caspase-8 (Santa Cruz Biotechnology, California, U.S.A.), Western blots were performed with enhanced chemiluminescence reagents (Amersham Biosciences, Piscataway, NJ, U.S.A.). Protein expression levels were analysed by densitometry with normalization to β-actin in each sample.
Statistical analyses
Data are given as mean±s.e.m. of n observations. Differences between groups were evaluated using ANOVA or Mann–Whitney's nonparametric U test. The survival curve obtained from the Kaplan–Meier procedure was analysed using a log-rank test, and P<0.05 was considered statistically significant.
Results
Effect of minocycline on anti-Fas-induced animal death and fulminant hepatitis
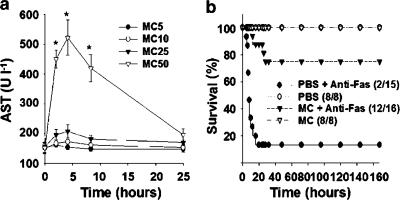
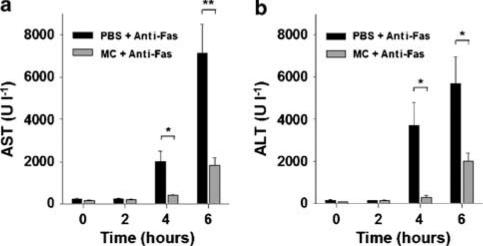
Because of previous reports concerned with the adverse potential of minocycline to the liver (Bocker et al., 1991; Gough et al., 1996; Malcolm et al., 1996; Lawrenson et al., 2000), we first examined its hepatotoxicity in mice measured from serum AST activities. As shown in Figure 1a, only minocycline administered at 50 mg kg−1, but not at 5, 10 or 25 mg kg−1, caused transient hepatic injury, consistent with a previous observation in mice (Bocker et al., 1991). We therefore used 5 mg kg−1 of minocycline (one-tenth of the hepatotoxic dose) throughout the following experiments. Minocycline pretreatment has been extensively used to study several degenerative diseases of the central nerve system in mice (Yrjanheikki et al., 1998; 1999; Chen et al., 2000; Du et al., 2001; Sanchez Mejia et al., 2001; Zhu et al., 2002). To investigate its effects on fulminant hepatitis, the minocycline-pretreated mice were subjected to a lethal challenge with 0.6 μg g−1 of anti-Fas Jo2 antibody. Groups of 8–16 mice were untreated or pretreated with three consecutive doses of 5 mg kg−1 of minocycline at 24 and 12 h and immediately before the administration of Jo2, and the survivors were observed for 1 week. The results (Figure 1b) show that after Jo2 challenge, 75% (12/16) of the minocycline-pretreated mice survived compared with 13% (2/15) of the PBS-pretreated controls (P<0.001). In contrast, the mice from the untreated group began to die at 5 h after Jo2 injection and 67% (10/15) of mice were killed at 10 h; by the time at 20 h after lethal challenge, more than 85% (13/15) of mice had been killed (Figure 1b). The hepatic protection of minocycline was further analysed from the serum activities of liver enzymes and the liver-to-body weight ratios, which represent the extents of pathological enlargement in response to hepatotoxic insult. Minocycline pretreatment significantly decreased the elevated activities of AST and ALT (Figure 2) and the enlarged liver-to-body weight ratios caused by Jo2 (data not shown).
Figure 1.
Effect of minocycline on mouse survival compromised by anti-Fas antibody. (a) Evaluation of minocycline hepatotoxicity. Doses of minocycline (MC) at 5, 10, 25 or 50 mg kg−1 were injected i.p. into groups of 5–9 C57BL/6J mice and at the indicated times the mice were killed for the determination of serum AST activities. *P<0.05 compared with AST activity (170±7 U l−1) of the control groups (PBS alone). (b) Effect of minocycline on Jo2-treated mice. Mice were pretreated with three doses of 5 mg kg−1 of minocycline, or PBS as controls, at 24 h, 12 h and immediately before a challenge with 0.6 μg g−1 of Jo2. Survival was monitored daily for 7 days. Numbers in parentheses indicate surviving mice vs totals.
Figure 2.
Effect of minocycline pretreatment on anti-Fas antibody-induced elevation of serum AST and ALT activities. Groups of 4–8 mice were pretreated with three doses of either 5 mg kg−1 of minocycline or PBS and then lethally challenged with 0.6 μg g−1 of Jo2. The resulting mice were killed for determination of their individual AST (a) and ALT (b) activities at the indicated times. Bars represent mean±s.e. (*P<0.05; **P<0.005).
Histopathological study of minocycline protection against fulminant hepatitis provoked by anti-Fas antibody
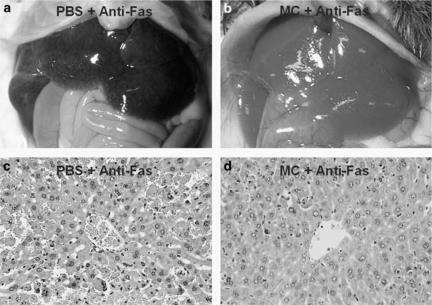
To further characterize the hepatoprotective effects of minocycline, macroscopic and microscopic examinations of mouse livers were performed after the lethal Jo2 challenge. When examined at 6 h, the untreated livers grossly showed extensive haemorrhages (Figure 3a), and minocycline pretreatment appeared to effectively minimize this damage (Figure 3b). Moreover, histological sections of liver tissues from Jo2-challenged mice revealed a typical pattern of diffuse haemorrhage, pyknotic nuclei, rupture of the sinusoid lining and deranged parenchyma (Figure 3c). In contrast, minocycline pretreatment drastically diminished this injury and preserved the normal hepatic architecture (Figure 3d). Thus, minocycline pretreatment in this study protected mice against anti-Fas antibody-induced fulminant hepatitis, thereby rescuing most animals from death.
Figure 3.
Histopathological study of minocycline protection against fulminant hepatitis triggered by anti-Fas antibody. Mice were triggered to manifest fulminant hepatitis by 0.6 μg g−1 of Jo2 in conjunction with three doses of 5 mg kg−1 of minocycline pretreatment (b, d) or with PBS alone (a, c). Photographs of gross liver features were taken at 6 h postchallenge (a, b), and micrographs of the given liver tissue sections stained with haematoxylin–eosin are shown in (c, d) (× 400).
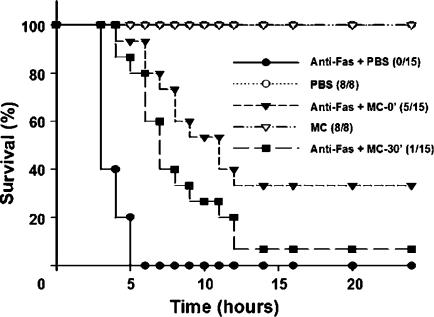
Therapeutic potential of minocycline in fulminant hepatitis
Because the pretreatment described above appeared to suppress hepatitis, we evaluated the therapeutic potential of minocycline on ALF. During a 24-h period (Figure 4), we found that, although to a lesser extent than the results shown in Figure 1b, a simultaneous or a 30-min postchallenge treatment with minocycline also delayed mortality kinetics and rescued Jo2-challenged mice from death by 33% (5/15; P<0.001 as compared with untreated controls) and 7% (1/15; P<0.05), respectively.
Figure 4.
Therapeutic effect of minocycline against anti-Fas-induced fulminant hepatitis. Groups of 8–15 mice were treated simultaneously with 0.6 μg g−1 of Jo2 and 5 mg kg−1 of minocycline, Jo2 and PBS, minocycline alone or PBS. One group of 15 mice was treated with minocycline 30 min after Jo2 challenge. Survival rates were monitored for 24 h. Numbers in parentheses indicate surviving mice vs totals.
Effect of minocycline on caspase activation in fulminant hepatitis
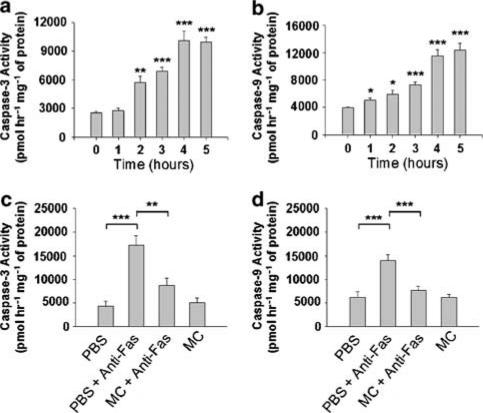
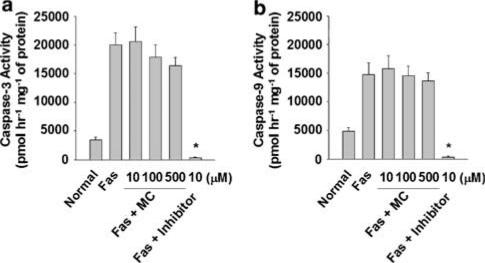
We observed that prior inhibition of caspase activation by i.p. injection of the pan-caspase inhibitor z-VAD could effectively prevent Fas-mediated ALF in mice (data not shown), consistent with previously published results (Rodriguez et al., 1996b). Additionally, caspase-3 and -9 were shown to be activated in mouse livers after Jo2 challenge (Figure 5a and b). These caspase activities increased significantly at 1–2 h and reached their plateau at 4 h after Jo2 injection. When comparing the kinetic patterns between caspase activation (Figure 5a and b) and serum activities of AST and ALT (Figure 2), we observed that caspases were activated earlier than the elevation of serum AST and ALT activities, consistent with the results from a previous report in that apoptotic process emerged before necrotic cell death in a Fas-induced hepatitis model (Bajt et al., 2000). We next investigated whether minocycline pretreatment could influence the activation of caspase-3 or -9 in fulminant hepatitis elicited by Jo2. As compared with PBS-treated controls shown in Figure 5c and d, we found that minocycline could inhibit Jo2-activated caspase-3 and -9 activities. We further tested whether minocycline had a direct inhibitory effect on the indicated caspases. Liver extracts were prepared from the mice lethally challenged by Jo2. As shown in Figure 6, Jo2 could trigger the activation of caspase-3 and -9, and the addition of minocycline, even at the final concentrations up to 500 μM, failed to attenuate the caspase-3 and -9 activities in vitro; as controls, both of the caspase activities were readily abolished by their specific inhibitors. These results indicate that minocycline suppresses Fas-mediated caspase activation through an indirect inhibitory mechanism that only acts adequately in vivo.
Figure 5.
Effect of minocycline on anti-Fas antibody-induced elevation of caspase-3 and -9 activities in vivo. (a) Time-course study of hepatic caspase activities after anti-Fas antibody injection. Activities of hepatic caspase-3 and -9 were measured at the indicated times after lethal Jo2 (0.6 μg g−1) challenge, and as a control caspase activities were also determined before the Jo2 injection (t=0). Data represent mean+s.e. of four animals per group. Significantly different from controls: *P<0.05; **P<0.005; ***P<0.001. (b) Effect of minocycline pretreatment on hepatic caspase-3 and -9 activities in Jo2-challenged mice. Groups of 8–10 mice were pretreated with three doses of either 5 mg kg−1 of minocycline or PBS (controls) and then lethally challenged by Jo2. At 4 h after the Jo2 injection, the mice were killed and their livers were removed for the preparation of protein extracts to measure caspase activities by fluorometry using synthetic substrates DEVD-AFC (for caspase-3) and LEHD-AMC (for caspase-9). (Bars represent mean±s.e.; *P<0.005; **P<0.001.)
Figure 6.
Effects of minocycline on Fas-activated caspases in mouse liver extracts. Mice were lethally challenged with 0.6 μg g−1 of Jo2, and 4 h after treatment their livers were removed for protein extraction. As described previously (Okamoto et al., 2003) with slight modification, 100 μg of each extract, without or with either the indicated concentrations of minocycline or a specific caspase inhibitor, was used to measure the activities of caspase-3 or -9 by fluorometry. Data of all groups were obtained from four independent experiments. (Bars represent mean±s.e.; Normal, nontreated; Inhibitor, DEVD-CHO for caspase-3 or LEHD-CHO for caspase-9; *P<0.001 vs Fas control.)
Effect of minocycline on cytochrome c release, caspase-8 activation and Bid truncation after anti-Fas antibody administration
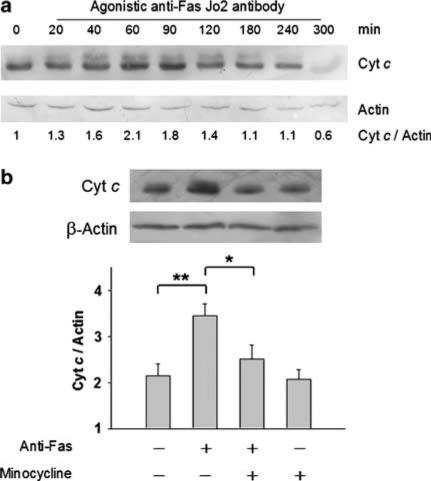
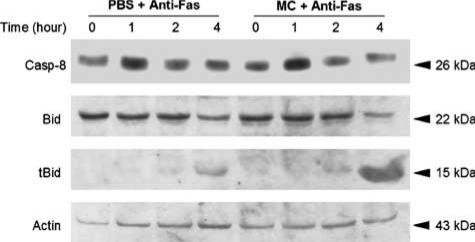
Cytoplasmic cytochrome c is a potent stimulus for sequential activation of caspase-9 and -3 leading to apoptosis (Green & Reed, 1998). We observed that lethal Jo2 injection to the mice could readily trigger cytochrome c release from hepatic mitochondria, as the amounts of cytosolic cytochrome c peaked approximately at 1–1.5 h after challenge (Figure 7a). Since minocycline did not inhibit directly caspase-9 and -3 (Figure 6), we examined its effect on cytochrome c release in the liver extracts of Jo2-challenged mice. Our results shown in Figure 7b reveal that, as compared with the untreated group, minocycline significantly inhibited the release of cytochrome c in Jo2-challenged mice. Caspase-8 could directly activate effector caspase-3 without mitochondrion participation (Scaffidi et al., 1998), or alternatively caspase-8 could trigger caspase-9 through cleavage and insertion of Bid to mitochondria (Scaffidi et al., 1999). To investigate the effect of minocycline on caspase-8 activation, Western blot of hepatic caspase-8 was performed during 0–4 h after Jo2 challenge. As shown in Figure 8, Jo2 appeared to trigger hepatic caspase-8 activation at 1 h after injection, whereas minocycline pretreatment failed to influence this alteration. In addition, Bid and its truncated form have been used as indicators of caspase-8 activity after anti-Fas antibody challenge (Li et al., 1998; Luo et al., 1998). By the same approach, we found that, similar to the untreated group (Figure 8), Bid from minocycline-pretreated mice still underwent proteocleavage within 2–4 h after Jo2 challenge, indicating that minocycline treatment did not alter caspase-8-mediated Bid truncation. These observations suggest that hepatoprotection by minocycline against Fas-mediated fulminant hepatitis involves mitochondrial pathways, probably by blocking cytochrome c release and by this means abolishes downstream caspase activation.
Figure 7.
Effect of minocycline on cytochrome c release in anti-Fas-induced hepatitis. (a) Time-course study of Jo2-induced cytochrome c release in mouse livers after lethal Jo2 challenge. The livers from mice lethally challenged by Jo2 were removed at the indicated times for Western determination of cytosolic cytochrome c content. Each lane represents a protein sample derived from an individual mouse, and the representative data are shown from two independent experiments. (b) Effect of minocycline on hepatic cytochrome c release in Jo2-treated mice. Groups of 6–9 mice were pretreated with three doses of either 5 mg kg−1 of minocycline or with PBS (control) and then lethally challenged with 0.6 μg g−1 of Jo2. At 1-h postchallenge, the cytosolic fraction prepared from the indicated liver tissues was evaluated for cytochrome c release using Western blot analysis plus densitometric quantification (bars represent mean±s.e.; *P<0.05; **P<0.005). Each lane represents a sample from a single mouse, and the representative data are shown from three independent experiments.
Figure 8.
Effects of minocycline on hepatic caspase-8 or Bid activation in anti-Fas-induced fulminant hepatitis. Mice pretreated with three doses of either 5 mg kg−1 of minocycline or PBS were lethally challenged with 0.6 μg g−1 of Jo2. At the indicated times, the mice were killed to remove their livers for the preparation of Western blotting. The cytosolic fractions were used to detect the active form of caspase-8 (casp-8), full length of Bid and its truncated form (tBid). Each lane represents a sample from a single mouse, and the representative data are shown from three independent experiments.
Discussion
We explored the potential of minocycline to treat experimental ALF induced by an anti-Fas antibody, Jo2, in a mouse model. Minocycline pretreatment appeared to alleviate ongoing disease symptoms (Figures 2 and 3) and to rescue mice from lethal Jo2 challenge (Figure 1). The Fas system plays a major role in the pathogenesis of many liver diseases, such as viral hepatitis, alcoholic hepatitis and Wilson's disease (Galle et al., 1995; Strand et al., 1998). It has recently been demonstrated that, using antisense (Zhang et al., 2000) or RNA-interference (Song et al., 2003) techniques, the specific inhibition of Fas expression protects mice from lethal Jo2 challenge, consistent with a previous result (Ogasawara et al., 1993) in that the Fas-deficient mice were generally resistant to Jo2-induced ALF. In addition, targeting caspase-8 by small interfering RNA (Zender et al., 2003) or a specific inhibitor IETD-CHO (Bajt et al., 2000), or by overexpression of the FADD dominant-negative mutant in liver (Seino et al., 2001), prevents Fas-triggered ALF in mice. These observations establish an indispensable role of the death receptor-mediated apoptotic pathway in ALF using this mouse model. On the other hand, overexpression of Bcl-2 in the mouse liver (Lacronique et al., 1996; Rodriguez et al., 1996a), suppression of Bid by antisense oligonucleotides (Zhang et al., 2003) and genetic deficiency of bid (Yin et al., 1999) can all protect mice from Jo2-induced ALF. In this study, we showed that Jo2 challenge not only triggered cytochrome c release (Figure 7a) but also activated caspase-9 (Figure 5b) in mouse liver. Together, these results clearly indicate that Fas-mediated fulminant hepatitis also involves the mitochondria-dependent pathway. Signalling blockage of the Fas-mediated death pathway may thus have a therapeutic potential for the treatment of ALF.
Minocycline has been shown to suppress caspase activation in several neural diseases using mouse models, including caspase-1 in brain ischaemia (Yrjanheikki et al., 1998; 1999), Parkinson's disease (Du et al., 2001) and traumatic brain injury (Sanchez Mejia et al., 2001), caspase-3 in amyotrophic lateral sclerosis (Zhu et al., 2002), as well as caspase-1 and -3 in Huntington's disease (Chen et al., 2000). In this study, we also demonstrated that minocycline inhibited the activation of caspase-3 and -9 in mouse fulminant hepatitis triggered by Jo2 challenge (Figure 5). However, in our system, minocycline did not appear to block directly caspase activities in vitro (Figure 6), similar to a previous observation in that activities of caspase-1 and -3 were not suppressed by direct addition of minocycline to protein extracts of HeLa cells (Chen et al., 2000). In fact, it has been shown in the mice challenged by Jo2 that only through i.p. injection into animals, but not by direct addition to the liver extracts, osthole (Okamoto et al., 2003), aminoguanidine (Okamoto & Okabe, 2000) or glycyrrhizin (Okamoto, 2000) inhibits caspase-3 activation thereby alleviating the symptoms of hepatitis. These observations suggest that minocycline along with other drugs mentioned above should target the component(s) upstream of caspases in the liver, thus blocking Fas-mediated hepatitis.
Cytochrome c and/or other apoptotic factors released from mitochondria play a central role in the activation of caspase-9 and the downstream effector caspase-3 (Green & Reed, 1998). The release of cytochrome c triggered by calcium or Bid from purified liver mitochondria is inhibited by minocycline (Zhu et al., 2002). The results shown in Figure 7b demonstrate that the release of cytochrome c from hepatic mitochondria triggered by Jo2 challenge could also be effectively suppressed by minocycline pretreatment. Yet, such a minocycline pretreatment failed to interfere with the caspase-8 activation and its downstream Bid truncation in response to Jo2 challenge from the same liver extracts (Figure 8). These results thus suggest that mitochondria may serve as one of the major pharmaceutical targets for minocycline to relieve disease symptoms resulting from Fas-mediated hepatitis. Indeed, through the interference with mitochondrial permeability transition (MPT), genipin, a metabolite of herbal Inchin-ko-to (Yamamoto et al., 2000), and cyclosporin A, an MPT inhibitor (Okamoto et al., 1999; Feldmann et al., 2000), have been shown to alleviate acute liver injury and subsequent lethality in Jo2-challenged mice (Yamamoto et al., 2000). Moreover, the superoxide dismutase mimic MnTBAP has been shown to abrogate Jo2-induced hepatic mitochondrial alterations, thereby nullifying subsequent ALF in mice (Malassagne et al., 2001). This indicates that disturbed intracellular oxidative homeostasis is involved in Fas-mediated fulminant hepatitis. Conceivably, the antioxidative capability of minocycline (Miyachi et al., 1986; Lin et al., 2003) may also cooperate in the mouse liver by preserving mitochondrial function sufficiently to cope with an anti-Fas antibody attack. Jo2-induced hepatic damage can be markedly reduced using a specific iNOS inhibitor (Okamoto & Okabe, 2000) or iNOS-knockout mice (Chang et al., 2003), implicating a role of iNOS in Fas-mediated hepatotoxicity. Because neuroprotection by minocycline has been in part attributed to its inhibitory effect on iNOS (Yrjanheikki et al., 1998; Chen et al., 2000; Du et al., 2001), we speculate that minocycline may suppress the hepatotoxicity of Jo2 by blocking hepatic iNOS activity.
Overall, our results demonstrate that, in addition to its well-studied role in neuroprotection, minocycline is also hepatoprotective against anti-Fas-induced ALF in mice, probably through interference with the signalling of the mitochondria-dependent death pathway. Further study on the effects of minocycline on liver organ function as a whole is thus justified. Minocycline, together with its derivatives, may enable us to understand the molecular basis of ALF pathogenesis, and thereby offer better therapies for this devastating disease.
Acknowledgments
We thank Ming-Shiou Chang and Chia-Yi Yu for technical assistance. This study was supported in parts by National Science Council (NSC 89-2320-B-016-020, 91-2320-B-016-054, 91-2314-B-016-109), National Health Research Institute (NHRI-CN-CL8902P-3), Department of Defense, Republic of China (DOD90-43, DOD91-36, DOD93-23) and C.Y. Chai Foundation for Advancement of Education, Sciences and Medicine.
Abbreviations
- ALF
acute liver failure
- ALT
alanine transaminase
- Apaf-1
apoptotic protease activating factor-1
- AST
aspartate transaminase
- Bid
BH3-interacting domain death agonist
- DISC
death-induced signalling complex
- FADD
Fas-associated death domain
- FLF
fulminant liver failure
- iNOS
inducible nitric oxide synthase
- Jo2
anti-Fas antibody
- MPT
mitochondrial permeability transition
- PBS
phosphate-buffered saline
References
- AMIN A.R., ATTUR M.G., THAKKER G.D., PATEL P.D., VYAS P.R., PATEL R.N., PATEL I.R., ABRAMSON S.B. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc. Natl. Acad. Sci. U.S.A. 1996;93:14014–14019. doi: 10.1073/pnas.93.24.14014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ASHKENAZI A., DIXIT V.M. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- ATILLASOY E., BERK P.D. Fulminant hepatic failure: pathophysiology, treatment, and survival. Annu. Rev. Med. 1995;46:181–191. doi: 10.1146/annurev.med.46.1.181. [DOI] [PubMed] [Google Scholar]
- BAJT M.L., LAWSON J.A., VONDERFECHT S.L., GUJRAL J.S., JAESCHKE H. Protection against Fas receptor-mediated apoptosis in hepatocytes and nonparenchymal cells by a caspase-8 inhibitor in vivo: evidence for a postmitochondrial processing of caspase-8. Toxicol. Sci. 2000;58:109–117. doi: 10.1093/toxsci/58.1.109. [DOI] [PubMed] [Google Scholar]
- BOCKER R., ESTLER C.J., LUDEWIG-SANDIG D. Evaluation of the hepatotoxic potential of minocycline. Antimicrob. Agents Chemother. 1991;35:1434–1436. doi: 10.1128/aac.35.7.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG B., NISHIKAWA M., SATO E., INOUE M. Mice lacking inducible nitric oxide synthase show strong resistance to anti-Fas antibody-induced fulminant hepatitis. Arch. Biochem. Biophys. 2003;411:63–72. doi: 10.1016/s0003-9861(02)00723-3. [DOI] [PubMed] [Google Scholar]
- CHEN M., ONA V.O., LI M., FERRANTE R.J., FINK K.B., ZHU S., BIAN J., GUO L., FARRELL L.A., HERSCH S.M., HOBBS W., VONSATTEL J.P., CHA J.H., FRIEDLANDER R.M. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 2000;6:797–801. doi: 10.1038/77528. [DOI] [PubMed] [Google Scholar]
- DU Y., MA Z., LIN S., DODEL R.C., GAO F., BALES K.R., TRIARHOU L.C., CHERNET E., PERRY K.W., NELSON D.L., LUECKE S., PHEBUS L.A., BYMASTER F.P., PAUL S.M. Minocycline prevents nigrostriatal dopaminergic neurodegeneration in the MPTP model of Parkinson's disease. Proc. Natl. Acad. Sci. U.S.A. 2001;98:14669–14674. doi: 10.1073/pnas.251341998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FELDMANN G., HAOUZI D., MOREAU A., DURAND-SCHNEIDER A.M., BRINGUIER A., BERSON A., MANSOURI A., FAU D., PESSAYRE D. Opening of the mitochondrial permeability transition pore causes matrix expansion and outer membrane rupture in Fas-mediated hepatic apoptosis in mice. Hepatology. 2000;31:674–683. doi: 10.1002/hep.510310318. [DOI] [PubMed] [Google Scholar]
- GALLE P.R., HOFMANN W.J., WALCZAK H., SCHALLER H., OTTO G., STREMMEL W., KRAMMER P.H., RUNKEL L. Involvement of the CD95 (APO-1/Fas) receptor and ligand in liver damage. J. Exp. Med. 1995;182:1223–1230. doi: 10.1084/jem.182.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLUB L.M., GOODSON J.M., LEE H.M., VIDAL A.M., MCNAMARA T.F., RAMAMURTHY N.S. Tetracyclines inhibit tissue collagenases. Effects of ingested low-dose and local delivery systems. J. Periodontol. 1985;56:93–97. doi: 10.1902/jop.1985.56.11s.93. [DOI] [PubMed] [Google Scholar]
- GOUGH A., CHAPMAN S., WAGSTAFF K., EMERY P., ELIAS E. Minocycline induced autoimmune hepatitis and systemic lupus erythematosus-like syndrome. BMJ. 1996;312:169–172. doi: 10.1136/bmj.312.7024.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN D.R., REED J.C. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- KLEIN N.C., CUNHA B.A. Tetracyclines. Med. Clin. N. Am. 1995;79:789–801. doi: 10.1016/s0025-7125(16)30039-6. [DOI] [PubMed] [Google Scholar]
- LACRONIQUE V., MIGNON A., FABRE M., VIOLLET B., ROUQUET N., MOLINA T., PORTEU A., HENRION A., BOUSCARY D., VARLET P., JOULIN V., KAHN A. Bcl-2 protects from lethal hepatic apoptosis induced by an anti-Fas antibody in mice. Nat. Med. 1996;2:80–86. doi: 10.1038/nm0196-80. [DOI] [PubMed] [Google Scholar]
- LAWRENSON R.A., SEAMAN H.E., SUNDSTROM A., WILLIAMS T.J., FARMER R.D. Liver damage associated with minocycline use in acne: a systematic review of the published literature and pharmacovigilance data. Drug Saf. 2000;23:333–349. doi: 10.2165/00002018-200023040-00006. [DOI] [PubMed] [Google Scholar]
- LI H., ZHU H., XU C.J., YUAN J. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell. 1998;94:491–501. doi: 10.1016/s0092-8674(00)81590-1. [DOI] [PubMed] [Google Scholar]
- LIN S., WEI X., XU Y., YAN C., DODEL R., ZHANG Y., LIU J., KLAUNIG J.E., FARLOW M., DU Y. Minocycline blocks 6-hydroxydopamine-induced neurotoxicity and free radical production in rat cerebellar granule neurons. Life Sci. 2003;72:1635–1641. doi: 10.1016/s0024-3205(02)02442-6. [DOI] [PubMed] [Google Scholar]
- LI P., NIJHAWAN D., BUDIHARDJO I., SRINIVASULA S.M., AHMAD M., ALNEMRI E.S., WANG X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- LUO X., BUDIHARDJO I., ZOU H., SLAUGHTER C., WANG X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- MALASSAGNE B., FERRET P.J., HAMMOUD R., TULLIEZ M., BEDDA S., TREBEDEN H., JAFFRAY P., CALMUS Y., WEILL B., BATTEUX F. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology. 2001;121:1451–1459. doi: 10.1053/gast.2001.29590. [DOI] [PubMed] [Google Scholar]
- MALCOLM A., HEAP T.R., ECKSTEIN R.P., LUNZER M.R. Minocycline-induced liver injury. Am. J. Gastroenterol. 1996;91:1641–1643. [PubMed] [Google Scholar]
- MIYACHI Y., YOSHIOKA A., IMAMURA S., NIWA Y. Effect of antibiotics on the generation of reactive oxygen species. J. Invest. Dermatol. 1986;86:449–453. doi: 10.1111/1523-1747.ep12285793. [DOI] [PubMed] [Google Scholar]
- OGASAWARA J., WATANABE-FUKUNAGA R., ADACHI M., MATSUZAWA A., KASUGAI T., KITAMURA Y., ITOH N., SUDA T., NAGATA S. Lethal effect of the anti-Fas antibody in mice. Nature. 1993;364:806–809. doi: 10.1038/364806a0. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T. The protective effect of glycyrrhizin on anti-Fas antibody-induced hepatitis in mice. Eur. J. Pharmacol. 2000;387:229–232. doi: 10.1016/s0014-2999(99)00807-9. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., HITOMI Y., HARA A. The protective effect of cyclosporine A on anti-Fas antibody-induced hepatitis in mice. Jpn. J. Pharmacol. 1999;79:485–488. doi: 10.1254/jjp.79.485. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., KAWASAKI T., HINO O. Osthole prevents anti-Fas antibody-induced hepatitis in mice by affecting the caspase-3-mediated apoptotic pathway. Biochem. Pharmacol. 2003;65:677–681. doi: 10.1016/s0006-2952(02)01606-4. [DOI] [PubMed] [Google Scholar]
- OKAMOTO T., OKABE S. Inhibition of anti-Fas antibody-induced hepatitis by aminoguanidine in mice. Eur. J. Pharmacol. 2000;403:277–280. doi: 10.1016/s0014-2999(00)00479-9. [DOI] [PubMed] [Google Scholar]
- PETER M.E., KRAMMER P.H. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr. Opin. Immunol. 1998;10:545–551. doi: 10.1016/s0952-7915(98)80222-7. [DOI] [PubMed] [Google Scholar]
- RODRIGUEZ I., MATSUURA K., KHATIB K., REED J.C., NAGATA S., VASSALLI P. A bcl-2 transgene expressed in hepatocytes protects mice from fulminant liver destruction but not from rapid death induced by anti-Fas antibody injection. J. Exp. Med. 1996a;183:1031–1036. doi: 10.1084/jem.183.3.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODRIGUEZ I., MATSUURA K., ODY C., NAGATA S., VASSALLI P. Systemic injection of a tripeptide inhibits the intracellular activation of CPP32-like proteases in vivo and fully protects mice against Fas-mediated fulminant liver destruction and death. J. Exp. Med. 1996b;184:2067–2072. doi: 10.1084/jem.184.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SADOWSKI T., STEINMEYER J. Minocycline inhibits the production of inducible nitric oxide synthase in articular chondrocytes. J. Rheumatol. 2001;28:336–340. [PubMed] [Google Scholar]
- SANCHEZ MEJIA R.O., ONA V.O., LI M., FRIEDLANDER R.M.Minocycline reduces traumatic brain injury-mediated caspase-1 activation, tissue damage, and neurological dysfunction Neurosurgery 2001481393–1399.discussion 1399–1401 [DOI] [PubMed] [Google Scholar]
- SCAFFIDI C., FULDA S., SRINIVASAN A., FRIESEN C., LI F., TOMASELLI K.J., DEBATIN K.M., KRAMMER P.H., PETER M.E. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCAFFIDI C., SCHMITZ I., ZHA J., KORSMEYER S.J., KRAMMER P.H., PETER M.E. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J. Biol. Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- SEINO K., SETOGUCHI Y., OGINO T., KAYAGAKI N., AKIBA H., NAKANO H., TANIGUCHI H., TAKADA Y., YUZAWA K., TODOROKI T., FUKUCHI Y., YAGITA H., OKUMURA K., FUKAO K. Protection against Fas-mediated and tumor necrosis factor receptor 1-mediated liver injury by blockade of FADD without loss of nuclear factor-kappaB activation. Ann. Surg. 2001;234:681–688. doi: 10.1097/00000658-200111000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SONG E., LEE S.K., WANG J., INCE N., OUYANG N., MIN J., CHEN J., SHANKAR P., LIEBERMAN J. RNA interference targeting Fas protects mice from fulminant hepatitis. Nat. Med. 2003;9:347–351. doi: 10.1038/nm828. [DOI] [PubMed] [Google Scholar]
- STRAND S., HOFMANN W.J., GRAMBIHLER A., HUG H., VOLKMANN M., OTTO G., WESCH H., MARIANI S.M., HACK V., STREMMEL W., KRAMMER P.H., GALLE P.R. Hepatic failure and liver cell damage in acute Wilson's disease involve CD95 (APO-1/Fas) mediated apoptosis. Nat. Med. 1998;4:588–593. doi: 10.1038/nm0598-588. [DOI] [PubMed] [Google Scholar]
- TILLEY B.C., ALARCON G.S., HEYSE S.P., TRENTHAM D.E., NEUNER R., KAPLAN D.A., CLEGG D.O., LEISEN J.C., BUCKLEY L., COOPER S.M., DUNCAN H., PILLEMER S.R., TUTTLEMAN M., FOWLER S.E. Minocycline in rheumatoid arthritis. A 48-week, double-blind, placebo-controlled trial. MIRA Trial Group. Ann. Intern. Med. 1995;122:81–89. doi: 10.7326/0003-4819-122-2-199501150-00001. [DOI] [PubMed] [Google Scholar]
- WOO M., HAKEM A., ELIA A.J., HAKEM R., DUNCAN G.S., PATTERSON B.J., MAK T.W. In vivo evidence that caspase-3 is required for Fas-mediated apoptosis of hepatocytes. J. Immunol. 1999;163:4909–4916. [PubMed] [Google Scholar]
- YAMAMOTO M., MIURA N., OHTAKE N., AMAGAYA S., ISHIGE A., SASAKI H., KOMATSU Y., FUKUDA K., ITO T., TERASAWA K. Genipin, a metabolite derived from the herbal medicine Inchin-ko-to, and suppression of Fas-induced lethal liver apoptosis in mice. Gastroenterology. 2000;118:380–389. doi: 10.1016/s0016-5085(00)70220-4. [DOI] [PubMed] [Google Scholar]
- YIN X.M., WANG K., GROSS A., ZHAO Y., ZINKEL S., KLOCKE B., ROTH K.A., KORSMEYER S.J. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- YRJANHEIKKI J., KEINANEN R., PELLIKKA M., HOKFELT T., KOISTINAHO J. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc. Natl. Acad. Sci. U.S.A. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YRJANHEIKKI J., TIKKA T., KEINANEN R., GOLDSTEINS G., CHAN P.H., KOISTINAHO J. A tetracycline derivative, minocycline, reduces inflammation and protects against focal cerebral ischemia with a wide therapeutic window. Proc. Natl. Acad. Sci. U.S.A. 1999;96:13496–13500. doi: 10.1073/pnas.96.23.13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZENDER L., HUTKER S., LIEDTKE C., TILLMANN H.L., ZENDER S., MUNDT B., WALTEMATHE M., GOSLING T., FLEMMING P., MALEK N.P., TRAUTWEIN C., MANNS M.P., KUHNEL F., KUBICKA S. Caspase 8 small interfering RNA prevents acute liver failure in mice. Proc. Natl. Acad. Sci. U.S.A. 2003;100:7797–7802. doi: 10.1073/pnas.1330920100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZHANG H., COOK J., NICKEL J., YU R., STECKER K., MYERS K., DEAN N.M. Reduction of liver Fas expression by an antisense oligonucleotide protects mice from fulminant hepatitis. Nat. Biotechnol. 2000;18:862–867. doi: 10.1038/78475. [DOI] [PubMed] [Google Scholar]
- ZHANG H., TAYLOR J., LUTHER D., JOHNSTON J., MURRAY S., WYATT J.R., WATT A.T., KOO S., YORK-DEFALCO C., STECKER K., DEAN N.M. Antisense oligonucleotide inhibition of Bcl-xL and Bid expression in liver regulates responses in a mouse model of Fas-induced fulminant hepatitis. J. Pharmacol. Exp. Ther. 2003;31:31. doi: 10.1124/jpet.103.050435. [DOI] [PubMed] [Google Scholar]
- ZHU S., STAVROVSKAYA I.G., DROZDA M., KIM B.Y., ONA V., LI M., SARANG S., LIU A.S., HARTLEY D.M., WU DU C., GULLANS S., FERRANTE R.J., PRZEDBORSKI S., KRISTAL B.S., FRIEDLANDER R.M. Minocycline inhibits cytochrome c release and delays progression of amyotrophic lateral sclerosis in mice. Nature. 2002;417:74–78. doi: 10.1038/417074a. [DOI] [PubMed] [Google Scholar]