Abstract
We examined the effects of an angiotensin-converting enzyme inhibitor (ACEI), captopril, on cerebral arterioles in young and old spontaneously hypertensive rats (SHR).
Animals were anesthetized with sodium pentobarbitone (60 mg kg−1 day−1). We measured cerebral blood flow (CBF, arbitrary units) and cerebral arteriolar internal diameter (ID, μm) prior to and during stepwise hypotension (SH) in 6- (WKY-6) and 15-month-old (WKY-15) Wistar Kyoto rats and in age-matched SHR that were untreated (SHR-6 and SHR-15) or treated for 3 months with captopril (SHR-6C, 105±2 mg kg−1 day−1 and SHR-15C, 94±1 mg kg−1 day−1). ID and cross-sectional area of the vessel wall (CSA) were measured in deactivated (EDTA) cerebral arterioles during a second SH.
Captopril decreased the lower limit of CBF autoregulation (61±6 in SHR-6C and 51±2 in SHR-15C versus 52±6 in WKY-6 and 62±7 in WKY-15 and 83±14 mmHg in SHR-6 and 120±19 mmHg in SHR-15; P<0.05) and CSA (510±21 in SHR-6C and 585±25 in SHR-15C versus 529±12 in WKY-6 and 549±20 in WKY-15 and 644±38 mmHg in SHR-6 and 704±38 mmHg in SHR-15; P<0.05).
Captopril increased cerebral arteriolar external diameter of SHR (105±5 in SHR-6C and 94±4 in SHR-15C vs 125±8 in WKY-6 and 108±3 in WKY-15 and 83±2 mmHg in SHR-6 and 80±2 mmHg in SHR-15 for a pial arteriolar pressure step of 35–39 mmHg; P<0.05). Captopril attenuated increases in cerebral arteriolar distensibility in young SHR.
Thus, ACEIs attenuate eutrophic and hypertrophic inward remodeling of cerebral arterioles in young and old SHR, thus decreasing the lower limit of CBF autoregulation.
Keywords: Hypertension, eutrophic remodeling, hypertrophic remodeling, vascular distensibility, aging, lower limit of cerebral blood flow autoregulation
Introduction
Chronic hypertension in young rat models, such as spontaneously hypertensive rats (SHR) and stroke-prone SHR (SHRSP), induces adaptive remodeling of cerebral arterioles with reorganization of wall material, leading to a reduction in internal diameter (ID) (eutrophic inward remodeling) (Baumbach & Heistad, 1989; Baumbach & Hadju, 1993). This increases cerebrovascular resistance, so maintaining baseline cerebral blood flow (CBF), but at the price of a shift in the lower limit of CBF autoregulation to a higher systemic mean pressure level. As in chronic hypertension both systemic mean pressure and the lower limit of CBF autoregulation increase, the security margin, which indicates the degree to which mean arterial pressure may fall before CBF starts to decrease (Lartaud et al., 1993), remains the same. Thus, the cerebral circulation adapts to a higher input pressure. Problems could arise when hypertension is treated if the treatment normalizes blood pressure but fails to decrease the lower limit of CBF autoregulation at the same time. This would render the brain prone to hypotensive hypoperfusion and ischemia; this may damage the brain, leading to cognitive impairment (Atkinson, 2001). In this respect, angiotensin I-converting enzyme inhibitors (ACEIs) may be the antihypertensive drugs of choice, as they restore the hypertension-induced decrease in cerebral arteriolar diameter (Hadju et al., 1991; Chillon & Baumbach, 1999; 2001) and reverse the increase in the lower limit of CBF autoregulation either after acute (Barry et al., 1984a, 1984b; Paulson et al., 1988) or chronic administration (Muller et al., 1990; Toyoda et al., 1998).
The above hypothesis is based on work in relatively young rats (3–6 months old SHR or SHRSP), which may not be an adequate model for the human situation. Chronic systemic arterial hypertension is a disease of the middle-aged and elderly. However, the impact of chronic hypertension treatment with ACEIs on CBF autoregulation and arteriolar diameter in old animals has, to our knowledge, not been studied. Here, the lower limit of CBF autoregulation was determined following hypotensive hemorrhage in old (15 months) SHR chronically treated or not with the ACEI, captopril. Results were compared to those obtained with captopril treatment of young SHR (6 months). Arteriolar diameter and wall stiffness were also measured. It has previously been reported that one of the main age-related (as opposed to hypertension-related) effects on the arteriolar wall is an increase in stiffness (Hadju et al., 1990; Dupuis et al., 2004).
Methods
Animals and operative procedures
The experiments were conducted on male Wistar Kyoto (WKY) and spontaneously hypertensive (SHR) rats (Iffa-Credo, l'Arbresle, France). SHR were divided into four groups: 6-month-old SHR that were untreated (SHR-6mo, 384±8 g, n=13) or treated from 3 to 6 months of age with captopril (SHR-6moC, 105±2 mg kg−1 day−1 in the drinking water, 371±2 g, n=13) and 15-month-old SHR that were untreated (SHR-15mo, 430±10 g, n=9) or treated from 12 to 15 months of age with captopril (SHR-15moC, 94±1 mg kg−1 day−1 in the drinking water, 423±3 g, n=12). Untreated 6-month-old (WKY-6mo, 399±10 g, n=11) and 15-month-old (WKY-15mo, 459±8 g, n=15) WKY served as normotensive controls. ‘Old', defined as being close to the median lifespan, is 15 months in SHR, as there is substantial mortality in untreated SHR beyond 15 months, such that there are no survivors at 18 months (Giummelly et al., 1999). We used age-matched WKY as normotensive controls, acknowledging that 15-month-old normotensive rats are more ‘mature' than ‘old' animals (Lartaud et al., 1993; 1994; Dupuis et al., 2004).
Animals were housed at 24°C, exposed to 12 h of light (lights on at 06:00 and off at 18:00 h) and allowed free access to food and fluid.
After 3 months' treatment, we evaluated CBF autoregulation and the structure and function of cerebral arterioles. Animals were anesthetized with sodium pentobarbitone (60 mg kg−1, i.p.). We used sodium pentobarbitone anesthesia, which provokes less depression of the cardiovascular and respiratory system than some anesthetic agents (urethane (Fluckiger et al., 1985)), but more than others (chloralose (Fluckiger et al., 1985), ketamine-xylazine (Wixson et al., 1987)). Albeit, although to our knowledge there has been no extensive study on the effects of different anesthetic agents on CBF autoregulation, some reports indicate that barbiturates attenuate less (certain) cardiovascular reflexes, such as the baroreflex (Fluckiger et al., 1985). Furthermore, the blood pressure-lowering effect of captopril is independent of barbiturate anesthesia as it is observed in nonanesthetized rats (Chillon et al., 1992) and in pithed rats (Atkinson et al., 1987). Finally, as the sensitivity to barbiturates increases with age (Stijnen et al., 1992), this may interfere with the effect of age on the lower limit of CBF autoregulation. However, the phenomenon of the shift in the lower limit of CBF autoregulation with age in the normotensive rats is observed using barbiturates (Dupuis et al., 2004) and in nonanesthetized rats (Lartaud et al., 1993). The general conclusion on the use of anesthesia is that there is no reason to believe that this will fundamentally alter our observations and that the choice between the use or not of anesthesia is dictated by the humane principles of experimentation on animals.
Animals were intubated and mechanically ventilated with room air (50 strokes per minute, tidal volume 3.0 ml) to maintain blood gases (pH, pCO2, pO2, blood gas analyzer 238, Ciba Corning, Cergy Pontoise, France) in the physiological range. A silicone catheter (Sigma Medical, Nanterre, France) was introduced into the right femoral vein and connected to a pump (Bioblock Scientific, Paris, France) for continuous infusion of sodium pentobarbitone (0.25 ml h−1; 20 mg kg−1 h−1) to maintain anaesthesia throughout the surgery and the experiment. The depth of anaesthesia was periodically evaluated by testing the corneal reflex and the rate of the pump infusion adjusted individually for each animal to maintain an adequate depth of anesthesia. A polyethylene cannula (Merck Biotrol, Chennevieres, France) was introduced into the right femoral artery; the cannula was connected to a low volume, strain-gauge transducer (Baxter, Bentley Laboratories, Europe) for measurement of blood pressure and heart rate. A second cannula was introduced into the left femoral artery to obtain blood samples for the measurement of arterial blood gases at baseline and for withdrawal of blood to produce hypotension. Rectal temperature was maintained at 37–38°C with a heating pad. Experiments were performed in accordance with the guidelines of the French Ministry of Agriculture, Paris, France (decree 12001-464, 29 May 2001; permits 54-4 and 03575).
Measurement of cerebral arteriolar pressure and diameter and CBF
We measured the pressure and ID of first-order pial arterioles through an open skull preparation as described previously (Dupuis et al., 2004). Briefly, the head was placed in an adjustable head holder and a 1 cm skin incision made to expose the skull. A dam of dental acrylic cement was constructed on the exposed skull and ports were placed for inflow and outflow of artificial cerebrospinal fluid (CSF). Craniotomy was performed over the left parietal cortex within the walls of the dam, and the dura was incised to expose the cerebral vessels. The exposed surface was continuously suffused with artificial CSF, warmed to 37–38°C and equilibrated with a gas mixture of 5% CO2–95% N2. The composition of the CSF was (mmol l−1) KCl, 3.0; MgCl2, 0.6; CaCl2, 1.5; NaCl, 131.9; NaHCO3, 24.6; urea, 6.7; and glucose, 3.7, in order to mimic as closely as possible the composition of naturally produced CSF (Baumbach et al., 1988a). Cerebral arteriolar pressure was continuously measured under sodium pentobarbitone anesthesia, with a micropipette connected to a servo-null pressure-measuring device (model 5A, Vista Electronics Company, Ramona, CA, U.S.A.). Pipettes were sharpened to a beveled tip, 3–5 μm in diameter, filled with 1.5 mmol l−1 sodium chloride, and inserted into the lumen of a cerebral arteriole with a micromanipulator (Dupuis et al., 2004). The presence of the pipette tip in the vessel wall had no discernible effect on the diameter of cerebral arterioles. Arteriolar diameter was monitored through a microscope (Stemi 200-C, Carl Zeiss Jena GMBH, Jena, Germany) connected to a closed-circuit video system with a final magnification of × 600. Images were digitized using a video frame grabber and diameter measured using image analysis software (Saisam®, Microvision Instruments, Evry, France). The precision of this video system is 0.6 μm. CBF (arbitrary units (a.u.)) was measured by laser Doppler flowmetry using a BLF 21 system (Transonic Systems Inc., Ithaca, NY, U.S.A.) equipped with a 1.2 mm diameter needle probe (Fujii et al., 1991).
Experimental protocol
At 30 min after completion of surgery, baseline cerebral arteriolar pressure, diameter and CBF were measured. To determine the lower limit of CBF autoregulation, stepwise hypotension (10 mmHg per step to a systemic mean arterial pressure of 20–30 mmHg) was induced by controlled withdrawal of blood. At 1 min after each stepwise fall in blood pressure, systemic and cerebral arteriolar pressures, arteriolar diameter and CBF were measured. After the final step, blood was re-injected to restore blood pressure.
Wall stiffness was evaluated from arteriolar pressure–diameter relationships obtained in deactivated (EDTA 67 mM (Baumbach et al., 1988a)) cerebral arterioles between mean arteriolar pressures of 40 and 5 mmHg, using hemorrhage to reduce pressure in steps of 5 mmHg. Cerebral arteriolar – not systemic – pressure changes were used in the calculation of distensibility. After the final pressure step, blood was again reinfused to restore pressure to control levels. Arterioles were fixed by suffusion with glutaraldehyde (2.25% v v−1 in 0.10 mol −1 cacodylate buffer) as described previously (Baumbach, 1996; Baumbach et al., 2002; 2003; Dupuis et al., 2004).
Animals were killed with sodium pentobarbital (250 mg kg−1, i.v.) and the arteriolar segment removed. The cross-sectional area (CSA) of the wall was measured in 7 μm sections, using a video image-analyzing system (Dupuis et al., 2004).
Calculations
The lower limit of CBF autoregulation is defined as the value of mean arterial pressure below which CBF significantly falls upon further reduction in pressure. It was determined individually by a best-fit method for two linear regression slopes relating CBF to the systemic mean pressure. The first slope was for the autoregulatory plateau and the second for the passive decrease in CBF with pressure below the lower limit of CBF. The lower limit of CBF was defined as the intersection of the two slopes.
The security margin (%), which indicates the degree to which mean arterial pressure may fall before CBF starts to decrease, was defined as ((baseline mean arterial blood pressure−lower limit of CBF autoregulation)/baseline mean arterial blood pressure) × 100 (Lartaud et al., 1993).
Eutrophic inward remodeling was evaluated by comparison of external diameters (ED) between the different groups of animals. ED was calculated using ID and wall thickness (WT) as: ED=ID+2WT. WT was calculated from CSA and ID: WT=[(4CSA/π+ID2)1/2−ID]/2. WT to lumen ratio (WT/ID) was calculated from WT and ID.
The passive distensibility of cerebral arterioles was evaluated using the stress–strain relationship of deactivated cerebral arterioles. Circumferential stress (σ) at each pressure step was calculated from cerebral mean arteriolar pressure (APm), ID of the cerebral arterioles (ID), and WT: σ=(APm × ID)/(2WT). Mean pressure was converted from millimeters of mercury to newtons per square meter (1 mmHg=1.334 × 102 N m−2). The circumferential strain (ɛ) was calculated as: ɛ=(ID−IDo)/IDo where IDo is the ‘original' ID defined as the diameter at 5–10 mmHg. To obtain tangential elastic modulus, the stress–strain data from each animal were fitted to an exponential curve (y=aebx) using least-squares analysis: σ=σoeβɛ, where σo is the stress at original diameter and β is a constant that is related to the rate of increase of the stress-strain curve. Tangential elastic modulus (ET) was calculated at several different values of stress from the derivative of the exponential curve: ET=dσ/dɛ=βσoeβɛ.
The eutrophic inward remodeling index evaluates the contribution of rearrangement of wall material to the decrease in ID. This index is calculated by evaluating the ID that cerebral arterioles of SHR would have if there had been no hypertrophic inward remodeling, that is, by calculating the ID using the values of ED of SHR and the CSA of WKY: IDcal=(EDSHR2–4CSAWKY/π)1/2. The eutrophic inward remodeling index is thus determined as: 100 × (IDWKY−IDcal)/(IDWKY−IDSHR) (Baumbach & Heistad, 1989).
The hypertrophic inward remodeling index evaluates the contribution of wall hypertrophy to the decrease in ID. This index is calculated by evaluating the ID that cerebral arterioles of SHR would have if there had been no eutrophic inward remodeling, that is, by calculating the ID using the values of ED of WKY and the CSA of SHR: IDcal=(EDWKY2–4CSASHR/π)1/2. The hypertrophic inward remodeling index is thus determined as: 100 × (IDWKY−IDcal)/(IDWKY−IDSHR) (Baumbach & Heistad, 1989).
Eutrophic and hypertrophic inward remodeling indices were calculated versus aged-matched WKY for SHR and versus aged-matched SHR for the captopril-treated rats.
Substances used
Captopril, glutaraldehyde, and cacodylate sodique were purchased from Sigma Chemical Company (St Louis, MO, U.S.A.), nitrogen and carbon dioxide from Air Liquide (Nancy, France), and sodium pentobarbital from Sanofi Santé Animale (Libourne, France).
Statistical analysis
Results are expressed as means±s.e.m. The experimental protocol was designed for the use of a one-way ANOVA with the variable ‘group' (SHR-6mo, SHR-6moC, SHR-15mo, SHR-15moC, WKY-6mo, WKY-15mo). It should be noted that two-way ANOVA (age–hypertension) was not used, as we did not treat WKY with captopril. Chronic treatment of normotensive rats with ACEIs exposes them to chronic hypotension, which may have interesting effects on the structure and function of the cerebral circulation, but these are not within the framework examined here. We have previously reported on the impact of chronic ACEI treatment on the cerebral circulation of the normotensive rats of different ages (Lartaud et al., 1994). Significant differences between means were determined using the Bonferroni test. The probability level chosen was P⩽0.05.
Results
Pressures
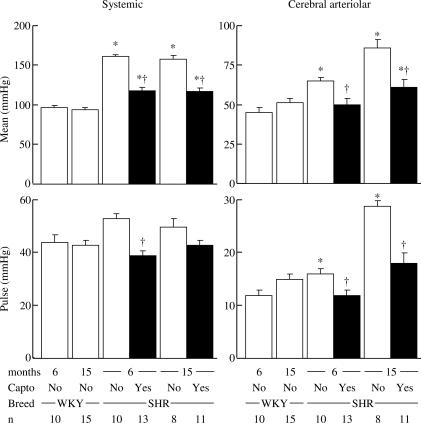
Captopril lowered the systemic mean arterial pressure in a similar fashion in both young and old SHR, to a level that remained significantly higher (+22 and +24%, respectively) than that of WKY (Figure 1). Captopril normalized the cerebral arteriolar mean and pulse pressures in young SHR (Figure 1). In contrast, in old SHR, although captopril normalized cerebral arteriolar pulse pressure, mean pressure remained at a level significantly higher (+20%) than in age-matched WKY (Figure 1).
Figure 1.
Effect of chronic treatment with the ACEI, captopril (100 mg kg−1 day−1, full columns) on systemic (left) and cerebral arteriolar (right) mean (top) and pulse (bottom) pressures in young (6 months) and old (15 months) SHR compared to age-matched normotensive WKY. *P⩽0.05 versus age-matched WKY. †P⩽0.05 versus age-matched SHR.
Arteriolar dilatation and CBF autoregulation
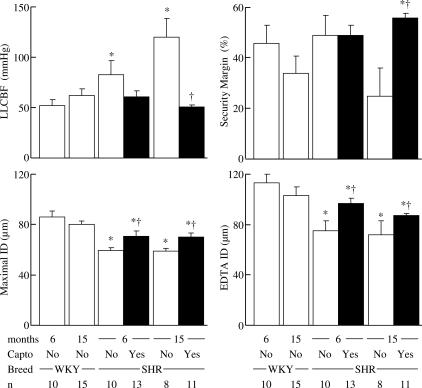
Baseline and maximal active, as well as passive (EDTA) IDs, were significantly reduced in young and old SHR compared to age-matched WKY (Table 1 and Figure 2). Captopril significantly increased the diameters in young and old SHR, but to a level that remained significantly lower than in WKY (Table 1 and Figure 2).
Table 1.
Baseline values for cerebral arterioles after deactivation (EDTA) in WKY and SHR of different ages treated or not with captopril
| Parameters | WKY | SHR | WKY | SHR | SHR | SHR |
|---|---|---|---|---|---|---|
| Age (months) | 6 | 6 | 15 | 15 | 6 | 15 |
| Captopril (mg kg−1 day−1) | No | No | No | No | 105±2 | 94±1 |
| N | 10 | 10 | 15 | 8 | 13 | 11 |
| Baseline ID (μm) | 65±4 | 40±1* | 59±3 | 40±2* | 51±2† | 51±3† |
| EIR Index | NA | 98 | NA | 94 | 95 | 92 |
| HIR Index | NA | 2 | NA | 4 | 4 | 10 |
Eutrophic inward remodeling (EIR) and hypertrophic inward remodeling (HIR) indices were calculated as described previously (Baumbach & Heistad, 1989).
P⩽0.05 versus age-matched WKY.
P⩽0.05 versus age-matched SHR.
Figure 2.
Effect of chronic treatment with the ACEI, captopril (100 mg kg−1 day−1, full columns) on the lower limit of CBF autoregulation (LLCBF), the security margin, maximal hypotension-induced dilatation (maximal ID) and maximal vasodilatation induced by EDTA (EDTA ID) in young (6 months) and old (15 months) SHR compared to age-matched normotensive WKY. Maximal vasodilatation induced by EDTA was measured for pressure steps of 30–35 mmHg. *P⩽0.05 versus age-matched WKY. †P⩽0.05 versus age-matched SHR.
The lower limit of CBF autoregulation was significantly increased in both young and old SHR compared to age-matched WKY (Figure 2). Captopril decreased the lower limit of CBF autoregulation in both young and old SHR, but this decrease was statistically significant in old hypertensive rats only (Figure 2).
The security margin was similar in SHR and age-matched WKY (Figure 2). Captopril had no effect on security margin in young treated SHR, but significantly increased the margin in old SHR, to a value greater than that of old WKY (Figure 2).
Arteriolar structure
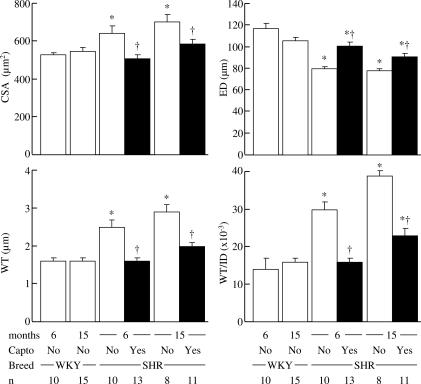
WT and WT/ID increased in hypertensive rats. Captopril lowered WT and WT/ID in young and old SHR. However, captopril was more efficient in young SHR as WT and WT/ID in old treated SHR remained higher than those of age-matched WKY (Figure 3).
Figure 3.
Effect of chronic treatment with the ACEI, captopril (100 mg kg−1 day−1, full columns) on CSA of the vessel wall, external diameter (ED), WT and WT/ID in young (6 months) and old (15 months) SHR compared to age-matched normotensive WKY. External diameter was measured in maximally dilated cerebral arterioles (EDTA) for pressure steps of 30–35 mmHg. WT and WT/ID were calculated from values of CSA and ID measured in maximally dilated cerebral arterioles (EDTA) for pressure steps of 30–35 mmHg. *P⩽0.05 versus age-matched WKY. †P⩽0.05 versus age-matched SHR.
CSA was significantly increased and external diameter, after deactivation with EDTA, was significantly smaller in SHR compared to WKY, regardless of age (Figure 3). Captopril treatment decreased CSA and increased external diameter in SHR regardless of age (Figure 3). The increase in external diameter produced by captopril was smaller in old SHR (Figure 3). Remodeling indices show that the decrease in cerebrovascular resistance produced by captopril is due to its effects on inward eutrophic remodeling (Table 1).
Arteriolar mechanics
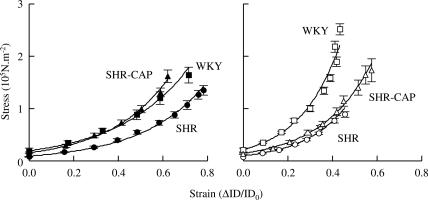
The stress–strain relationship curves in deactivated cerebral arterioles were shifted to the right in SHR compared to age-matched WKY (Figure 4). Treatment with captopril shifted the stress–strain relationship curve to the left in young, but not in old, SHR (Figure 4). Thus, captopril prevented the increase in passive distensibility in young, but not in old, SHR.
Figure 4.
Effect of chronic treatment with the ACEI, captopril (100 mg kg−1 day−1, SHR-CAP), on the stress–strain relationships in young (6 months, left) and old (15 months, right) SHR compared to age-matched normotensive WKY. Values are means±s.e.m. ID, internal diameter of cerebral arterioles for mean cerebral arteriolar pressure steps of 5 mmHg (35–39 to 5–9 mmHg); IDo, ID at the lowest pressure step.
Discussion
One of the main results of this paper is that the lower limit of CBF autoregulation increases with hypertension and that this hypertension-induced shift in the lower limit of CBF autoregulation is amplified by age. Chronic treatment with the ACEI, captopril, lowers the lower limit of CBF autoregulation in young and old SHR, as it partially reverses the hypertension-induced reduction in arteriolar diameter (with and without tonus) and restores arteriolar autoregulatory dilator capacity. This shift in the lower limit of CBF autoregulation to a lower pressure level following chronic ACEI treatment confirms published reports on this subject (Muller et al., 1990; Toyoda et al., 1998). However, it can be argued that what is important in terms of prevention of cerebral ischemia is the relative shift in the lower limit of CBF autoregulation compared to the fall in input blood pressure, that is, the security margin. In young (6 months) SHR, as the increases in the lower limit of CBF autoregulation and in the mean arterial blood pressure are similar, the security margin remains unchanged compared to normotensive controls. In contrast, in old (15 months) SHR, as the increase in the lower limit of CBF autoregulation is greater than that in blood pressure, the security margin is reduced. In old SHR, as the effect of captopril on the lower limit of CBF autoregulation (58% reduction) far exceeds its effect on systemic mean blood pressure (26% decrease), there is a substantial increase in the security margin, which more than doubles.
Several factors may explain the improvement in security margin following ACE inhibition in old SHR. First, the improvement in security margin following chronic ACE inhibition in old SHR could be due to a specific change in arteriolar remodeling in this group. Concerning vascular remodeling, the structural alterations of arterioles during chronic hypertension were first described in the extensive work of Folkow et al. (1958), who showed encroachment of the lumen by the vascular wall. At the time this was attributed to vascular wall hypertrophy, and the latter term is often taken as synonymous of wall remodeling. The term ‘vascular wall remodeling', as introduced in the late 80s by Baumbach & Heistad (1989) to describe the structural alterations observed in cerebral arterioles of hypertensive rats, was defined as a reduction in external diameter of fully dilated cerebral arterioles that could not be attributed to a decrease in distensibility. In other words, the reduction in ID was due to a rearrangement of existing wall material without de novo synthesis of wall material (i.e. hypertrophy). Thus, in hypertensive subjects, a decrease in ID observed in arterioles may be due to either vascular wall hypertrophy or vascular wall remodeling. A consensus letter to the editor (Mulvany et al., 1996) clarified the different terms: ‘hypertrophic inward remodeling' was proposed for vascular wall hypertrophy and ‘eutrophic inward remodeling' for rearrangement of wall material. Results obtained in the present experiment show that cerebral arterioles underwent both hypertrophic inward remodeling (as shown by the increase in CSA) and eutrophic inward remodeling (as shown by the reduction in external diameter) in young and old hypertensive rats. Furthermore, values of eutrophic inward remodeling indices in young and old SHR show that 98 and 94% of the increase in cerebrovascular resistance can be accounted for by inward eutrophic remodeling. We have previously proposed that impaired responses of cerebral arterioles to stimuli such as acute decrease in pressure may be linked to vascular remodeling (Chillon & Baumbach, 2001). Thus, in the present experiment, vascular remodeling may decrease both maximal and autoregulatory dilatation and captopril may improve the security margin in old SHR following the reversal of such changes. However, one argument against this is that changes in ID measured under EDTA are similar at both ages (6 and 15 months), but the effect of captopril on the lower limit of CBF autoregulation is far greater at 15 months (−58 versus −27%). Factors other than a change in the geometry of cerebral arterioles have to be considered to fully explain the effects of captopril on the security margin in old SHR.
Considering firstly wall stiffness, the rightward shift of the stress–strain relationship curves of both young and old SHR indicates that arteriolar wall stiffness decreases in SHR compared to age-matched WKY regardless of age. An increase in passive compliance would be expected to affect arterioles more during dilatation and result in increased diameter when vessels are dilated in response to reduced pressure (Chillon & Baumbach, 2002). The fact that following captopril in old rats distensibility remains high (whereas it is reduced by captopril in young SHR) may be an additional factor in the greater shift in the lower limit of CBF autoregulation produced by captopril in old SHR. We have to remain cautious, however, as we did not observe any differences in the maximal autoregulatory dilatation between young and old SHR.
Finally, the improvement in security margin following ACE inhibition in old SHR could also be due to an effect on arteriolar functional dilator capacity following potentiation of a cerebrovascular bradykinin–NO system (Takada et al., 2001) or blockade of an angiotensin II–AT1 vasoconstrictor pathway (Nishimura et al., 2000; Estrup et al., 2001; Ito et al., 2002) or a decrease in angiotensin II-induced generation of superoxide by NADPH-oxidase (van der Giet et al., 2002). These aspects were not investigated.
Another main result of this paper, as mentioned earlier, is that captopril treatment reverses the hypertension-provoked increase in cerebral arteriolar distensibility in young, but not in old, hypertensive rats. It has been proposed that the increase in distensibility may be consecutive to vascular wall hypertrophy and an increase in the proportion of compliant (smooth muscle cells, elastin, endothelial cells) to stiff (collagen, basement membrane) components of the vessel wall (Baumbach et al., 1988b). The increase in the smooth muscle component may improve vasodilatory capacity. An argument against this hypothesis is that captopril treatment reduced CSA in both young and old SHR, and yet did not normalize passive distensibility in old SHR. This suggests that factors other than the relative composition of the vessel wall, such as modification of the connections between the different components of the wall, may be important in old SHR.
In conclusion, the main result of this paper is that in SHR some, but not all, age-related changes are reversed by chronic captopril treatment. Indeed, chronic ACE inhibition with captopril failed to restore passive distensibility in old SHR. Furthermore, captopril lowers blood pressure and – to a much greater extent – the lower limit of CBF autoregulation, so more than doubling the cerebrovascular security margin in old SHR. Were this to be the case in man, these observations would have marked clinical importance in terms of prevention of iatrogenic cerebral hypoperfusion during antihypertensive treatment of the elderly.
Acknowledgments
This study was funded by grants from the French Ministry of Education, Research and Technology (EA3448, Paris, France), the Lorraine Regional Development Committee (Metz, France), the Greater Nancy Urban Council (Nancy, France) and Henri Poincaré University (Nancy, France), and the Pharmacolor Association, Nancy.
Abbreviations
- ACEI
angiotensin-converting enzyme inhibitor
- CBF
cerebral blood flow
- CSA
cross-sectional area
- CSF
cerebrospinal fluid
- ɛ
circumferential strain
- ID
internal diameter
- SH
stepwise hypotension
- SHR
spontaneously hypertensive rats
- SHRSP
stroke-prone spontaneously hypertensive rats
- σ
circumferential stress
- WKY
Wistar Kyoto rats
- WT
wall thickness
- WT/ID
wall thickness to lumen ratio
References
- ATKINSON J. Cerebrovascular structure and dementia: new drug targets. Trends Pharmacol. Sci. 2001;22:630–635. doi: 10.1016/s0165-6147(00)01866-6. [DOI] [PubMed] [Google Scholar]
- ATKINSON J., SONNAY M., SAUTEL M., FOUDA A.K. Chronic treatment of the spontaneously hypertensive rat with captopril attenuates responses to noradrenaline in vivo but not in vitro. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;335:624–628. doi: 10.1007/BF00166978. [DOI] [PubMed] [Google Scholar]
- BARRY D.I., JARDEN J.O., PAULSON O.B., GRAHAM D.I., STRANDGAARD S. Cerebrovascular aspects of converting-enzyme inhibition I: effects of intravenous captopril in spontaneously hypertensive and normotensive rats. J. Hypertens. 1984a;2:589–597. doi: 10.1097/00004872-198412000-00003. [DOI] [PubMed] [Google Scholar]
- BARRY D.I., PAULSON O.B., JARDEN J.O., JUHLER M., GRAHAM D.I., STRANDGAARD S. Effects of captopril on cerebral blood flow in normotensive and hypertensive rats. Am. J. Med. 1984b;76:79–85. doi: 10.1016/0002-9343(84)90890-8. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., HADJU M.A. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., HEISTAD D.D. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L. Effects of increased pulse pressure on cerebral arterioles. Hypertension. 1996;27:159–167. doi: 10.1161/01.hyp.27.2.159. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., DOBRIN P.B., HART M.N., HEISTAD D.D. Mechanics of cerebral arterioles in hypertensive rats. Circ. Res. 1988a;62:56–64. doi: 10.1161/01.res.62.1.56. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., SIGMUND C.D., FARACI F.M. Cerebral arteriolar structure in mice overexpressing human renin and angiotensinogen. Hypertension. 2003;41:50–55. doi: 10.1161/01.hyp.0000042427.05390.5c. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., SIGMUND C.D., BOTTIGLIERI T., LENTZ S.R. Structure of cerebral arterioles in cystathionine beta-synthase-deficient mice. Circ. Res. 2002;91:931–937. doi: 10.1161/01.res.0000041408.64867.1d. [DOI] [PubMed] [Google Scholar]
- BAUMBACH G.L., WALMSLEY J.G., HART M.N. Composition and mechanics of cerebral arterioles in hypertensive rats. Am. J. Pathol. 1988b;133:464–471. [PMC free article] [PubMed] [Google Scholar]
- CHILLON J.M., BAUMBACH G.L. Effects of an angiotensin-converting enzyme inhibitor and a ß-blocker on cerebral arterioles in rats. Hypertension. 1999;33:856–861. doi: 10.1161/01.hyp.33.3.856. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., BAUMBACH G.L. Effects of an angiotensin-converting enzyme inhibitor and a ß-blocker on cerebral arteriolar dilatation in hypertensive rats. Hypertension. 2001;37:1388–1393. doi: 10.1161/01.hyp.37.6.1388. [DOI] [PubMed] [Google Scholar]
- CHILLON J.M., BAUMBACH G.L.Autoregulation: arterial and intracranial pressure Cerebral Blood Flow and Metabolism 2002New York: Lippincott Williams & Wilkins; 395–412.2nd edn. ed. Edvinsson, L. & Krause, D.N. pp. [Google Scholar]
- CHILLON J.M., CAPDEVILLE-ATKINSON C., LARTAUD I., GUILLOU J., MERTÈS P.M., ATKINSON J. Chronic antihypertensive treatment with captopril plus hydrochlorothiazide improves aortic distensibility in the spontaneously hypertensive rat. Br. J. Pharmacol. 1992;107:710–714. doi: 10.1111/j.1476-5381.1992.tb14511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUPUIS F., RÉGRIGNY O., ATKINSON J., LIMINANA P., DELAGRANGE P., SCALBERT E., CHILLON J.M. Impact of treatment with melatonin on cerebral circulation in old rats. Br. J. Pharmacol. 2004;141:399–406. doi: 10.1038/sj.bjp.0705629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESTRUP T.M., PAULSON O.B., STRANDGAARD S. No effect of angiotensin II AT2-receptor antagonist PD123319 on cerebral blood flow autoregulation. J. Renin Angiotensin Aldosterone Syst. 2001;2:188–192. doi: 10.3317/jraas.2001.026. [DOI] [PubMed] [Google Scholar]
- FLUCKIGER J.P., SONNAY M., BOILLAT N., ATKINSON J. Attenuation of the baroreceptor reflex by general anesthetic agents in the normotensive rat. Eur. J. Pharmacol. 1985;109:105–109. doi: 10.1016/0014-2999(85)90545-x. [DOI] [PubMed] [Google Scholar]
- FOLKOW B., GRIMBY G., THULESIUS O. Adaptative structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol. Scand. 1958;44:255–272. doi: 10.1111/j.1748-1716.1958.tb01626.x. [DOI] [PubMed] [Google Scholar]
- FUJII K., HEISTAD D.D., FARACI F.M. Role of the basilar artery in the regulation of blood flow to the brain stem in rats. Stroke. 1991;22:763–767. doi: 10.1161/01.str.22.6.763. [DOI] [PubMed] [Google Scholar]
- GIUMMELLY P., LARTAUD-IDJOUADIENE I., NIEDERHOFFER N., CHILLON J.M., CAPDEVILLE-ATKINSON C., ATKINSON J. Antihypertensive treatment and aortic internal diameter in SHR. Hypertension. 1999;34:207–211. doi: 10.1161/01.hyp.34.2.207. [DOI] [PubMed] [Google Scholar]
- HADJU M.A., HEISTAD D.D., BAUMBACH G.L. Effects of antihypertensive therapy on mechanics of cerebral arterioles in rats. Hypertension. 1991;17:308–316. doi: 10.1161/01.hyp.17.3.308. [DOI] [PubMed] [Google Scholar]
- HADJU M.A., HEISTAD D.D., SIEMS J.E., BAUMBACH G.L. Effects of aging on mechanics and composition of cerebral arterioles in rats. Circ. Res. 1990;66:1747–1754. doi: 10.1161/01.res.66.6.1747. [DOI] [PubMed] [Google Scholar]
- ITO T., YAMAKAWA H., BREGONZIO C., TERRON J.A., FALCON-NERI A., SAAVEDRA J.M. Protection against ischemia and improvement of cerebral blood flow in genetically hypertensive rats by chronic pretreatment with an angiotensin II AT1 antagonist. Stroke. 2002;33:2297–2303. doi: 10.1161/01.str.0000027274.03779.f3. [DOI] [PubMed] [Google Scholar]
- LARTAUD I., BRAY-DES-BOSCS L., CHILLON J.M., ATKINSON J., CAPDEVILLE-ATKINSON C. In vivo cerebrovascular reactivity in Wistar and Fischer 344 rat strains during aging. Am. J. Physiol. 1993;264:H851–H858. doi: 10.1152/ajpheart.1993.264.3.H851. [DOI] [PubMed] [Google Scholar]
- LARTAUD I., MAKKI T., BRAY-DES BOSCS L., NIEDERHOFFER N., ATKINSON J., CORMAN B., CAPDEVILLE-ATKINSON C. Effect of chronic ANG I-converting enzyme inhibition on aging processes. IV. Cerebral blood flow autoregulation. Am. J. Physiol. 1994;267:R687–R694. doi: 10.1152/ajpregu.1994.267.3.R687. [DOI] [PubMed] [Google Scholar]
- MULLER F., LARTAUD I., BRAY L., ATKINSON J., JANIAN P., BURLET C., CAPDEVILLE C. Chronic treatment with the angiotensin I converting enzyme inhibitor, perindopril, restores the lower limit of autoregulation of cerebral blood flow in the awake renovascular hypertensive rat. J. Hypertens. 1990;8:1037–1042. doi: 10.1097/00004872-199011000-00010. [DOI] [PubMed] [Google Scholar]
- MULVANY M.J., BAUMBACH G.L., AALKJAER C., HEARGERTY A.M., KORSGAARD N., SCHIFFRIN E.L., HEISTAD D.D. Vascular remodeling: letter to the editor. Hypertension. 1996;28:505–506. [PubMed] [Google Scholar]
- NISHIMURA Y., ITO T., SAAVEDRA J.M. Angiotensin II AT1 blockade normalizes cerebrovascular autoregulation and reduces cerebral ischemia in spontaneously hypertensive rats. Stroke. 2000;31:2478–2486. doi: 10.1161/01.str.31.10.2478. [DOI] [PubMed] [Google Scholar]
- PAULSON O.B., WALDEMAR G., ANDERSEN A.R., BARRY D.I., PEDERSEN E.V., SCHMIDT J.F., VORSTRUP S. Role of angiotensin in autoregulation of cerebral blood flow. Circulation. 1988;77:I-55–I-58. [PubMed] [Google Scholar]
- STIJNEN A.M., DANHOF M., VAN BEZOOIJEN C.F. Increased sensitivity to anesthetic effect of phenobarbital in aging BN/BiRij rats. J. Pharmacol. Exp. Ther. 1992;261:81–87. [PubMed] [Google Scholar]
- TAKADA J., IBAYASHI S., NAGAO T., OOBOSHI H., KITAZONO T., FUJISHIMA M. Bradykinin mediates the acute effect of an angiotensin-converting enzyme inhibitor on cerebral autoregulation in rats. Stroke. 2001;32:1216–1219. doi: 10.1161/01.str.32.5.1216. [DOI] [PubMed] [Google Scholar]
- TOYODA K., FUJII K., IBAYASHI S., KITAZONO T., NAGAO T., TAKABA H., FUJISHIMA M. Attenuation and recovery of brain stem autoregulation in spontaneously hypertensive rats. J. Cerebr. Blood Flow Metab. 1998;18:305–310. doi: 10.1097/00004647-199803000-00009. [DOI] [PubMed] [Google Scholar]
- VAN DER GIET M., ERINOLA M., ZIDEK W., TEPEL M. Captopril and quinalapril reduce reactive oxygen species. Eur. J. Clin. Invest. 2002;32:732–737. doi: 10.1046/j.1365-2362.2002.01064.x. [DOI] [PubMed] [Google Scholar]
- WIXSON S.K., WHITE W.G., HUGHES H.C.J., LANG C.M., MARSHALL W.K. The effects of pentobarbital, fentanyl-droperidol, ketamine-xylazine and ketamine-diazepam on arterial blood pH, blood gases, mean arterial blood pressure and heart rate in adult male rats. Lab. Anim. Sci. 1987;37:736–742. [PubMed] [Google Scholar]