Abstract
The present studies were designed to test the hypothesis that neuronal-specific protein kinase Cγ (PKCγ) plays a critical role in acute ethanol withdrawal hyper-responsiveness in spinal cord.
Patch-clamp studies were carried out in motor neurons in neonatal rat spinal cord slices. Postsynaptic currents were evoked by brief pulses of 2 mM N-methyl-D-aspartic acid (NMDA) in the presence of bicuculline methiodide 10 μM; strychnine 5 μM and tetrodotoxin 0.5 μM.
Both ethanol depression and withdrawal hyper-responsiveness of NMDA-evoked currents are dependent on increases in intracellular Ca2+. Blocking intracellular increase in Ca2+ by 30 mM 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA) not only decreased the ethanol-induced depression of NMDA-evoked currents (33±5% in control vs 20±3% in BAPTA, P<0.05) but also eliminated acute ethanol withdrawal hyper-responsiveness.
Immunohistochemistry studies revealed that neonatal spinal cord motor neurons contain an abundance of nuclear PKCγ.
Exposure to ethanol (100 mM) induced PKCγ translocation from the nucleus to cytoplasm in motor neurons. Pretreatment with the γ-isozyme-specific peptide PKC inhibitor, γV5-3, blocked ethanol-induced translocation and also blocked withdrawal hyper-responsiveness.
The results show that PKCγ mediates ethanol withdrawal hyper-responsiveness in spinal motor neurons; the results may be relevant to some symptoms of ethanol withdrawal in vivo.
Keywords: Ethanol withdrawal, NMDA, calcium dependence, hyper-responsiveness, protein kinase C, long-term potentiation
Introduction
Ethanol withdrawal symptoms present a serious clinical problem. Symptoms include tremors, convulsions and heightened responses to sensory stimuli (Goldstein & Pal, 1971). In previous studies, this laboratory has investigated the manifestations of ethanol withdrawal in isolated spinal cord preparations acutely exposed to ethanol, which may be related to the motor symptoms of ethanol withdrawal in vivo. In intact neonatal rat spinal cord, ethanol depresses a glutamate-mediated motor neuron-evoked potential (Wong et al., 1997), which recovers to a level above control following washout (Wong et al., 1998). We have also investigated the role of motor neurons in withdrawal hyper-responsiveness, since these, the final common path to motor output, may themselves play a part. In motor neurons in spinal cord slice, we have shown that withdrawal hyper-responsiveness is due to rebound of N-methyl-D-aspartic acid (NMDA)-evoked currents to levels above control following ethanol treatment and wash, and documented a role for kinases in mediating the phenomenon; protein kinase C (PKC) or tyrosine kinase inhibitors but not protein kinase A (PKA) inhibitors prevented withdrawal hyper-responsiveness (Li & Kendig, 2003a). Similarly, in intact isolated neonatal rat spinal cord, the broad-spectrum PKC inhibitor GF-109203X suppressed ethanol withdrawal hyper-responsiveness (Wong et al., 2004). PKC is involved in many of the actions of ethanol (Stubbs & Slater, 1999). Studies with PKCγ null mutant mice showed that PKCγ influences both ethanol consumption and behavioral impulsivity (Bowers & Wehner, 2001). In receptors expressed in oocytes, some ethanol actions are mediated by PKC (Dildy-Mayfield & Harris, 1995). Furthermore, in mice lacking PKCγ, behavioral actions of ethanol are altered as is the function of GABAA receptors (Harris et al., 1995). These data suggest a role for PKC in mediating some actions of ethanol, including the NMDA receptor-mediated withdrawal hyper-responsiveness that follows ethanol exposure.
Expression of PKCγ in the brain and spinal cord is developmentally regulated, being very low at birth and increasing progressively up to 2–3 weeks of age in the rat (Hashimoto et al., 1988). Although the neuronal functions of PKCγ in spinal cord dorsal horn have been widely studied (Mori et al., 1990; Mao et al., 1995; Malmberg et al., 1997; Polgar et al., 1999; Martin et al., 2001), little information is available on PKCγ in spinal cord ventral horn, except one immunohistochemistry study by Miki (1995). Using light and electron microscopic immunohistochemistry, Miki reported that during the embryonic and early postnatal days, the PKCγ isozyme was expressed in both pre- and postsynaptic regions. Immunoreactivity for PKCγ in the postsynaptic regions became stronger with postnatal age, whereas it weakened or disappeared in most of the presynaptic regions (Miki, 1995, 1996). In addition to playing roles in neuronal differentiation and synaptogenesis, PKCγ may be involved in other postsynaptic functions, such as regulation of ion channel activities and transmitter receptors. However, possible postsynaptic neuronal roles of PKCγ in motor neurons have not yet been studied. Using an isozyme-specific peptide inhibitor of PKCγ, γV5-3, the present study was designed to test the hypothesis that PKCγ regulates NMDA-dependent ethanol withdrawal in motor neurons in spinal cord slice.
Methods
Spinal cord motor neurons were studied using patch-clamp techniques as we have described previously (Li & Kendig, 2003a; Li et al., 2003b). Experiments were carried out according to protocols approved by the Stanford Institutional Animal Care and Use Committee. Sprague–Dawley rats aged P7–10 (P0=date of birth) were anesthetized with halothane and decapitated, and spinal cords quickly removed and placed in a cold (under 4°C) oxygenated artificial cerebrospinal fluid (ACSF), which contained (in mM): NaCl, 123; KCl, 4; NaH2PO4, 1.2; MgSO4, 1.3; NaHCO3, 26; dextrose, 10; and CaCl2, 2, pH 7.4. Slices (350 μM thick) were sectioned from the lumbar region on a vibratome (Technical Products International, St Louis, MO, U.S.A.) and transferred to oxygenated ACSF at room temperature for 1-h incubation. Individual slices were transferred to a perfusion chamber for recordings. All the experiments were carried out at room temperature.
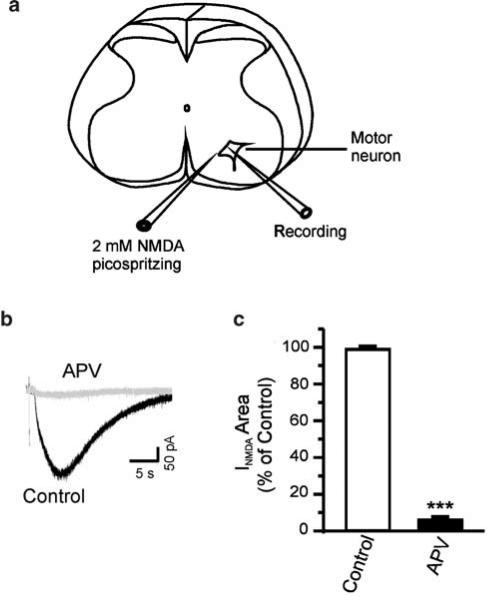
Patch pipettes were pulled on a Flaming-Brown pipette puller (Sutter Instruments, Novato, CA, U.S.A.) and had an impedance of 2–5 MΩ when filled with intracellular solution containing (mM): NaCl, 15; K-gluconate, 110; N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), 10; MgCl2, 2; ethylene glycolbis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid (EGTA), 11; CaCl2·2 H2O, 1; ATP-Na, 2; and GTP, 0.4, pH 7.3, adjusted with KOH. To probe the role of intracellular Ca2+ in withdrawal, in some experiments the fast Ca2+ chelator 1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid (BAPTA, 30 mM) was substituted for EGTA to decrease the rise in internal Ca2+ following NMDA receptor activation; in that case, the K-gluconate was reduced to maintain the osmolarity constant. The osmolarity of the pipette solution was adjusted to 285–295 mosM. According to the criteria of identifying motor neurons reported by Wang et al. (1999) and Wang & Kendig (2000), whole-cell voltage-clamp recordings were made from visually identified motor neurons using infrared video microscopy and a × 40 water-immersion lens (Zeiss Axioskop) and a MultiClamp 700A amplifier (Axon Instruments). A diagram of the recording setup is shown in Figure 1a. Cells were held at a holding potential of −60 mV in perfusate containing bicuculline methiodide (BMI) 10 μM; strychnine 5 μM; and tetrodotoxin (TTX) 0.5 μM. An estimated liquid junction potential of 9 mV was not subtracted from the recorded membrane potentials. Postsynaptic currents were evoked by direct pressure application of 2 mM NMDA (10 psi, 8–12 ms) from a pipette positioned near the recorded cell (Picospritzer, General Valve Division of Parker Hannefin, Fairfield, NJ, U.S.A.) at 1–2 min intervals (Figure 1a). Responses to repeated NMDA application were stable, with no evidence of receptor desensitization. Ethanol (95% pure, Gold Shield Chemical Company, Hayward, CA, U.S.A.) was diluted to 100 mM in ACSF. Concentrations of ethanol in the bath were verified by gas chromatography of the vapor phase in equilibrium with the solution in the chamber. Following a 10 min control period, slices were exposed to 100 mM ethanol for 15–20 min, followed by a 20 min wash period in ethanol-free ACSF. All drugs, except the peptide inhibitor γV5-3 and the Tat carrier, were from Sigma (St Louis, MO, U.S.A.); they are TTX, BMI, strychnine hydrochloride, 2-amino-5-phosphonovaleric acid (APV) and BAPTA. The peptide γV5-3 (γPKC antagonist, amino acids 659–664 [CRLVLAS]) was synthesized in one of the authors' laboratory (DM-R) and conjugated to Tat transmembrane carrier peptide (amino acids 47–57 [YGRKKRRQRRR]) via a cysteine–cysteine bond at its N-terminus (Chen et al., 2001). The peptide competes with activated PKCγ for binding to the isozyme-specific docking proteins, receptors for activated C kinase. This strategy prevents PKC isozyme translocation and functioning in an isozyme-specific manner (Johnson et al., 1996; Schechtman & Mochly-Rosen, 2002).
Figure 1.
(a) Schematic diagram of a lumbar spinal cord slice showing the placement of a recording electrode and a pipette containing 2 mM NMDA. (b) Individual traces elicited by 2 mM NMDA (10 psi, 10 ms) from the same motor neuron before and after application of 50 μM APV. (c) Graph showing a near complete block of NMDA-evoked currents by APV. ***, two-tailed Student's t-test, P<0.0001.
The area of evoked currents during and following ethanol application was measured and normalized to the average baseline current area during the 10-min preceding ethanol. The series resistance was monitored throughout the experiment, and if it changed by more than 15%, the data were discarded. Data are expressed as mean±s.e.m. Statistical significance was determined by one-way ANOVA followed by Dunn's or Tukey's multiple comparison test with significance set at P<0.05. A single neuron was studied in each slice.
Fluorescence immunocytochemical studies were performed on spinal cord sections (30 μm) from control slices (perfused with ACSF for 70 min) and ethanol-treated slices (perfused with ACSF for 30 min, followed by 20 min washing and 20 min washout of 100 mM ethanol). The sections were incubated with a rabbit anti-PKCγ polyclonal antibody (1 : 500) (Santa Cruz Biotechnology, Inc., CA, U.S.A.) overnight at 4°C. A mouse anti-neuronal nuclei monoclonal antibody (NeuN, 1 : 750) (Chemicon International Inc., CA, U.S.A.) was added to identify neurons. After several washes, the sections were labeled for 2 h at room temperature with fluorescein-labeled secondary antibodies: Alexa Fluor® 568 goat anti-mouse IgG and Alexa Fluor® 488 goat anti-rabbit IgG (Molecular Probes, Eugene, OR, U.S.A.). Double immunofluorescence was assessed with a laser confocal microscope (Molecular Dynamics, Sunnyvale, CA, U.S.A.). Quantitative counts of the numbers of ventral horn cells containing PKCγ were made on an average of three fields per section; two to three sections were obtained per animal. Significance of differences was assessed by one-way ANOVA.
Results
Depressant effects of ethanol on NMDA-evoked currents in spinal cord motor neurons are concentration dependent
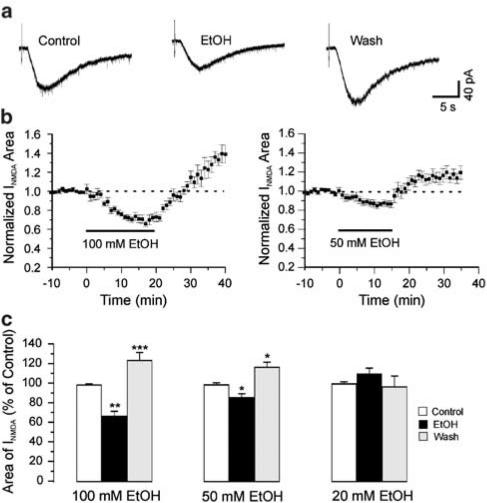
Under the conditions of the present experiment, the current evoked by NMDA was completely blocked by the NMDA receptor antagonist APV (Figure 1b and c) and thus was due entirely to the activation of NMDA receptors. A measure of 100 mM ethanol, about half the anesthetic concentration for rats of this age (Fang et al., 1997), depressed the NMDA-evoked currents to 67±5% of control (n=11, P<0.01) measured at 15 min after application of ethanol. During washout of ethanol, NMDA-evoked currents increased to 124±8% of control at 18 min of wash with ethanol-free ACSF (n=11, P<0.001, Figure 2). Lower concentrations of ethanol had less effect on the area of NMDA-evoked currents; 50 mM ethanol depressed the NMDA-evoked currents to 85±4% of control (n=6, P<0.05). After 18 min of wash with ethanol-free ACSF, NMDA-evoked currents increased to 117±6% of control (n=6, P<0.05). Ethanol (20 mM) induced neither depression (109±5%, n=5, P>0.05) nor withdrawal hyper-responsiveness (93±6%, P>0.05) of the NMDA-evoked currents. Figure 2c summarizes the depressant and withdrawal effects of different concentrations of ethanol on NMDA-evoked currents.
Figure 2.
Ethanol depression and withdrawal effects on NMDA-evoked currents are concentration dependent. (a) Individual traces from a motor neuron showing withdrawal hyper-responsiveness as an increase in NMDA-evoked current above control. The concentration of ethanol is 100 mM. (b) Time course of the mean effects of EtOH (n=11) on the area of NMDA-evoked currents. (c) Histogram showing EtOH depression and withdrawal hyper-responsiveness is concentration dependent. EtOH and wash were measured at 15 min after 100 mM EtOH application and 18 min washout, respectively. One-way ANOVA test, *P<0.05; **P<0.01; ***P<0.001 compared to control. Error bars are s.e.m.
NMDA withdrawal hyper-responsiveness is calcium dependent
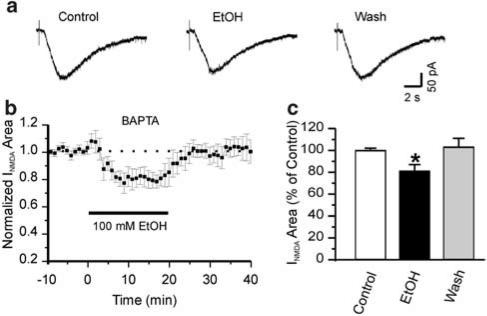
As ethanol withdrawal effects are blocked by NMDA and calcium channel antagonists (Morrisett et al., 1990; Ripley et al., 1996), we hypothesized that withdrawal in motor neurons is dependent on calcium entry mediated at least in part via the NMDA receptor. We tested whether withdrawal hyper-responsiveness is calcium dependent by adding the fast calcium chelator BAPTA to the pipette solution to block the increase in postsynaptic calcium due to influx. In the presence of 30 mM BAPTA, 100 mM ethanol depressed NMDA-evoked currents to 80±3% of control (n=8, P<0.05). Ethanol depression of NMDA-evoked currents was significantly less in the presence of BAPTA than EGTA (P<0.05) measured 18 min after application of ethanol. Furthermore, ethanol-induced withdrawal hyper-responsiveness was blocked by BAPTA (104±7%, n=8, P>0.05 compared to control), suggesting that both ethanol depression of NMDA-evoked currents and withdrawal hyper-responsiveness are calcium-dependent phenomena (Figure 3).
Figure 3.
EtOH depression and withdrawal hyper-responsiveness are calcium dependent. (a) Individual traces from a motor neuron showing no withdrawal hyper-responsiveness when the recording pipette contains 30 mM BAPTA instead of 11 mM EGTA. (b) Time course of the mean effects of ethanol (n=8) on the area of NMDA-evoked currents. (c) Histogram showing less ethanol depression and no withdrawal hyper-responsiveness. Ethanol and wash were measured at 18 min after 100 mM ethanol application and washout, respectively. One-way ANOVA test, *P<0.05 compared to control.
Ethanol induces translocation of PKCγ from the nucleus to cytoplasm
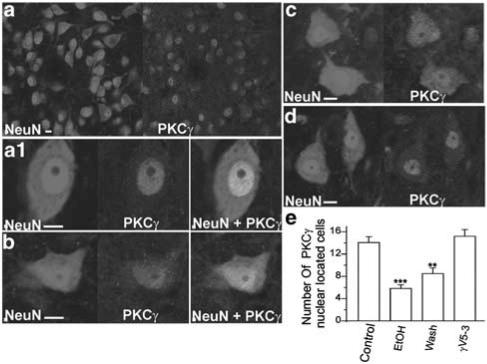
Owing to conflicts in the literature concerning the presence of PKCγ in neonatal rat spinal cord (Hashimoto et al., 1988; Mori et al., 1990; Miki, 1995, 1996), we first examined whether neonatal rat motor neurons contain PKCγ by performing immunohistochemistry. In spinal cord sections, we observed abundant PKCγ isozyme in motor neurons (Figure 4a). In control motor neurons, the PKCγ isozyme was located primarily in the nuclei, but not in the nucleoli. The cytoplasm had very limited staining (Figure 4a1). After treatment with 100 mM ethanol for 20 min, the PKCγ isozyme translocated from the nucleus to cytoplasm and the staining became weaker compared to control (Figure 4b). Following 20 min wash after ethanol, the majority of PKCγ remained in the cytoplasm (Figure 4c). Treatment of the slice with the PKCγ-specific peptide inhibitor γV5-3 30 min before application of 100 mM ethanol inhibited PKCγ translocation (Figure 4d). The number of neurons with PKCγ dense staining in the nucleus was significantly less in both ethanol-treated and washout sections compared to control and γV5-3-treated sections (Figure 4e). Although the number of neurons with PKCγ in the nucleus appears to be greater in withdrawal sections than in ethanol-treated sections, there was no statistically significant difference between these two groups.
Figure 4.
Ethanol induces PKCγ translocation from the nucleus to cytoplasm. (a) Immunostaining image for spinal cord ventral horn neurons (NeuN, 1 : 750) and PKCγ isozyme (PKCγ, 1 : 500). (a1) In control conditions, the PKCγ isozyme is located in the nucleus. (b) After treatment with 100 mM ethanol for 20 min, the PKCγ translocated from the nucleus to cytoplasm. (c) After treatment with 100 mM ethanol for 20 min and wash 20 min, the majority of PKCγ staining remains in the cytoplasm. (d) Pretreatment with the PKCγ selective inhibitor, γV5-3, prevented the ethanol-induced PKCγ translocation. (e) Histogram showing ethanol-treated spinal cord ventral horn contains significantly fewer neurons with PKCγ located solely in the nucleus as compared with control (control: n=5; ethanol treated: n=5; wash: n=5; ethanol plus γV5-3 treated: n=4. n is the number of animals used; the numbers of neurons are averages of three fields per section. Two to three sections were taken randomly per animal. A total of 800–1100 neurons were analyzed for each condition.) **P<0.01; ***P<0.001. Bar=10 μm.
PKCγ mediates withdrawal hyper-responsiveness of NMDA-evoked EPSCs
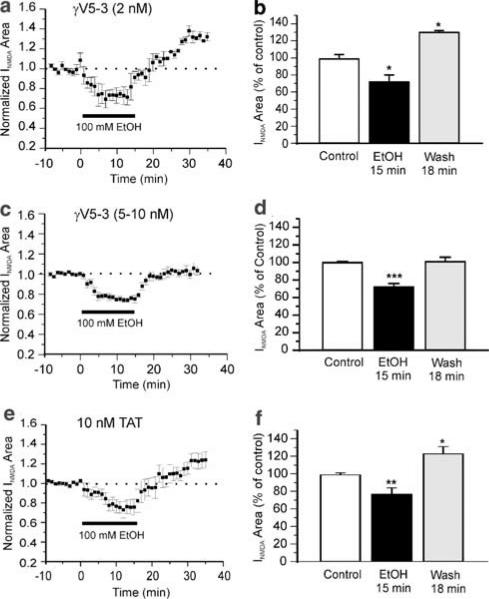
To examine the effect of PKCγ on ethanol-induced withdrawal hyper-responsiveness, we applied the PKCγ inhibitor, γV5-3, 30 min before ethanol to inhibit the translocation of PKCγ. γV5-3 (5 or 10 nM) transiently increased the NMDA-evoked currents to 112±3% (n=8, P<0.05) for 10 min, followed by a return to near control levels (108±5%). The Tat carrier by itself had no effect on NMDA-evoked currents at the corresponding concentration. We found γV5-3 blocked withdrawal hyper-responsiveness in a dose-dependent manner. At a low concentration (2 nM), γV5-3 failed to block withdrawal (Figure 5a). At 18 min of wash, the average area of NMDA-evoked EPSCs was 133±4% (n=3, P<0.05, Figure 5b). When the concentration was increased to 5–10 nM, the PKCγ inhibitor significantly depressed withdrawal hyper-responsiveness (Figure 5c); the average area of NMDA-evoked EPSCs was 101±5% (n=11, P>0.05, Figure 5d) at 18 min of wash. Although ethanol appeared to exert a smaller depressant effect on the NMDA-evoked currents in the presence of the PKCγ inhibitor (75.6±2% of control), this difference did not reach statistical significance compared to the ethanol depression effect without γV5-3 (67±5% of control). The Tat carrier peptide alone at the same 10 nM concentration had no significant effect on withdrawal (Figure 5e). At 18 min of wash, the average area of NMDA-evoked currents was 123±8 (n=7, P<0.05, Figure 5f).
Figure 5.
Elimination of ethanol withdrawal hyper-responsiveness by PKCγ isozyme inhibitor peptide is dose dependent. (a) Time course of the mean effect of ethanol (100 mM) on NMDA-evoked EPSCs (n=3) in the presence of 2 nM PKCγ isozyme inhibitor peptide γV5-3. (b) Histogram showing withdrawal hyper-responsiveness. *P<0.05 compared to control. (c) Time course of the mean effect of ethanol (100 mM) on NMDA-evoked EPSCs (n=11) in the presence of 5–10 nM γV5-3. (d) Histogram showing no withdrawal hyper-responsiveness. ***P<0.001 compared to control. (e) Time course of the mean effect of ethanol (100 mM) on NMDA-evoked EPSCs (n=7) in the presence of 10 nM Tat, the cell-permeable carrier of PKCγ isozyme inhibitor peptide. (f) Histogram showing withdrawal hyper-responsiveness. *P<0.05; **P<0.01.
Discussion
We examined the effect of acute ethanol exposure on NMDA-evoked currents in neonatal rat spinal cord slices as well as the role of the PKCγ isozyme in acute ethanol-induced withdrawal hyper-responsiveness. Our major findings are (1) acute ethanol exposure caused depression of NMDA-evoked currents and withdrawal hyper-responsiveness in a concentration-dependent manner; (2) an increase in intracellular calcium plays an important role in both ethanol depression and withdrawal hyper-responsiveness; (3) although PKCγ translocation is observed during as well as following ethanol exposure, PKCγ activation is not required for ethanol depression but is essential for withdrawal hyper-responsiveness.
Dose-dependent ethanol depression and withdrawal hyper-responsiveness: postsynaptic contributions
Our results demonstrated that ethanol depresses NMDA-evoked currents in a concentration-dependent manner. The extent of ethanol depression in this spinal cord preparation is comparable to that found in cultured neurons (see review by (Allgaier, 2002)), brain stem slices (Lai et al., 2004) and brain slices (Siggins et al., 2003). The NMDA receptors in the brain and spinal cord are developmentally regulated (Subramaniam & Mcgonigle, 1994; Kim et al., 1995; Li and Kendig, unpublished observation). It has been shown that sensitivity to ethanol is developmentally regulated such that adult animals are more sensitive than neonates (Fang et al., 1997). A report by Ziskind-Conhaim et al. (2003) demonstrated a lack of effect of 70 mM ethanol on motor neuron postsynaptic NMDA receptor properties recorded from p1 to 4 rat spinal cord slices. Our study using motor neurons in p7–10 rat spinal cord slices showed a greater sensitivity to ethanol. As PKCγ also increases after birth up to 2–3 weeks of age (Hashimoto et al., 1988), the withdrawal response to ethanol is likely to develop postnatally and may reflect increased expression of PKCγ in these neurons (Miki, 1995, 1996).
NMDA applied as in this study may activate both synaptic and extrasynaptic NMDA receptors. Although we could not rule out the contributions of extrasynaptic NMDA receptors to ethanol withdrawal hyper-responsiveness in the present experiments, our previous results obtained from intact isolated spinal cord preparations showing that dorsal root elicited EPSPs display ethanol withdrawal hyper-responsiveness (Wong et al., 1998), suggests that at least some of the withdrawal phenomenon is due to actions on synaptic receptors.
Calcium-dependent ethanol depression and withdrawal hyper-responsiveness
The role of NMDA receptors and calcium in ethanol withdrawal hyper-responsiveness has been widely studied in vivo and in vitro (Morrisett et al., 1990; Perez-Velazquez et al., 1994; Ripley et al., 1996; Thomas et al., 1998). A significant increase in high-voltage-activated calcium currents during the ethanol withdrawal period is reported in alcohol withdrawal seizure prone mice (Perez-Velazquez et al., 1994). Administration of a calcium channel antagonist and NMDA receptor antagonist during ethanol treatment significantly decreased the withdrawal syndrome, both in vivo and in isolated mouse hippocampal slices (Morrisett et al., 1990; Ripley et al., 1996), suggesting both NMDA receptors and calcium are necessary participants in ethanol withdrawal hyper-responsiveness. However, it has not been previously determined whether presynaptic or postsynaptic mechanisms mediate ethanol withdrawal. We have attempted to localize these aspects of ethanol withdrawal hyper-responsiveness to postsynaptic structures and to determine their molecular basis in neonatal rat spinal cord. Our earlier study showed that acute ethanol withdrawal hyper-responsiveness is at least partially a postsynaptic phenomenon, and is dependent on NMDA receptor activation (Li & Kendig, 2003a). Previous studies in oocytes demonstrated that ethanol sensitivity of certain NMDA receptors is modulated by an intracellular, calcium-dependent process that requires the C0 domain of the NR1 subunit (Mirshahi et al., 1998). The results of the present study suggest that in spinal cord motor neurons, a postsynaptic increase in intracellular calcium is involved in both acute ethanol depression and withdrawal hyper-responsiveness of NMDA receptor-mediated currents. The use of TTX to block sodium channel-related presynaptic transmitter release does not rule out a presynaptic action of NMDA to induce calcium entry and transmitter release; however, the complete inhibition of withdrawal hyper-responsiveness by BAPTA in the postsynaptic motor neurons suggests that a presynaptic action does not play a major role. Our previous findings together with the results of the present study suggest that NMDA receptor activation produces calcium entry into the postsynaptic cell; the increase in intracellular calcium not only regulates the extent of ethanol inhibition but also is critical in induction of ethanol withdrawal hyper-responsiveness.
PKCγ isozyme mediates neuronal plasticity in response to ethanol
Immunohistochemistry showed that neonatal rat motor neurons express abundant PKCγ. The findings are in agreement with Miki's results (Miki, 1995, 1996) that nucleus and dendrites of motor neurons exhibit extensive immunoreactivity for the PKCγ isozyme. The postsynaptic location of PKC has been reported in several brain structures (Miki, 1995, 1996; Saito & Shirai, 2002), such as visual cortex (Wolf et al., 1986; Jia et al., 1990), hippocampus (Hashimoto et al., 1988; Kose et al., 1990) and midbrain (Wolf et al., 1986). In the CA1 region of rat hippocampus, PKCγ is localized in the soma including the nucleus and in dendrites including dendritic spines, axon and synaptic terminals (Kose et al., 1990). In embryonic chick brain, ethanol caused a decreased expression of PKCγ on days 7 to 10 (McIntyre et al., 1999), suggesting that this PKC isozyme is regulated by ethanol. PKCγ has been implicated in synaptic plasticity (Saito & Shirai, 2002). Inhibition of postsynaptic PKC or CaMKII by intracellular injection of PKC(19–31), a selective PKC inhibitor, or CaMKII (273–302), a selective inhibitor of multifunctional Ca2+-calmodulin-dependent protein kinase has been reported to block induction of long-term potentiation (LTP) in the hippocampus (Malinow et al., 1989). LTP is greatly diminished in PKCγ mutant mice (Abeliovich et al., 1993). The present study shows that ethanol-induced synaptic plasticity in motor neurons is also dependent on PKCγ activation and translocation in the postsynaptic cell. In this, withdrawal hyper-responsiveness resembles other forms of synaptic plasticity such as LTP, opioid withdrawal excitation and inflammatory and nerve injury-induced pain (Mao et al., 1995). We have previously partially characterized some of these forms of plasticity in intact isolated neonatal rat spinal cord (Feng & Kendig, 1995a, 1995b; Lozier & Kendig, 1995). However, ethanol withdrawal hyper-responsiveness differs from the others in being localized at least in part to motor neurons rather than interneurons in dorsal horn (Wong et al., 1998; Li & Kendig, 2003a).
In a previous study, we showed that ethanol withdrawal hyper-responsiveness could be blocked either by a tyrosine kinase inhibitor or a broad-spectrum PKC inhibitor, but not by an antagonist to PKA (Li & Kendig, 2003a). Several lines of studies have provided evidence that modulation of NMDA receptor function could be through activation of a PKC-dependent tyrosine kinase-signaling cascade (Grosshans & Browning, 2001). In hippocampus, Lu et al. (1999) reported that activation of PKC by 4β-PMA enhanced NMDA-evoked currents. This PKC-dependent enhancement of NMDA current was blocked by inhibitors of tyrosine kinase (Src family). Intracellular perfusion of c-Src also enhanced NMDA-activated current (Lu et al., 1999). Inhibition of PKC did not alter c-Src caused enhancement of NMDA-evoked currents; however, in neurons from mice lacking c-Src, PKC-dependent upregulation of NMDA current was absent, demonstrating that activation of Src is downstream of PKC. Thus, potentiation of NMDA receptor function by PKC may be via activation of nonreceptor tyrosine kinase, and consequent phosphorylation of NMDA receptors (Grosshans & Browning, 2001). The present study documents that calcium-dependent PKCγ is the isozyme largely responsible for ethanol withdrawal hyper-responsiveness in motor neurons, possibly via tyrosine phosphorylation of sites on NMDA receptors (Harris et al., 1986; Linden et al., 1988).
Clinical significance
Withdrawal from ethanol entails a severe set of clinical symptoms, which include hypersensitivity to sensory stimuli and exaggerated responses including seizures. In parallel behavioral studies in rats, we observed withdrawal hyperalgesia following a single dose of ethanol, with similar PKCγ dependence to the present in vitro results (Shumilla et al., 2004, unpublished data). The heightened responsiveness of NMDA currents in motor neurons could contribute to this behavioral response. The results of the present study suggest that the PKC second messenger system, in particular PKCγ, is an important mediator of this heightened responsiveness and therefore should be considered as a target for developing therapeutic strategies to prevent or ameliorate symptoms of ethanol withdrawal.
Acknowledgments
This work was supported by NIH Grants NS13108 and 47818 to J.J.K., and AA11147 to D.M.-R.
Disclosures: J.J.K. and D.M.-R. have filed for patent protection for PKCγ for the treatment of ethanol withdrawal, and the patents have been licensed to Kai Pharmaceuticals, a company in which D.M.-R. and J.J.K. have equity positions.
Abbreviations
- BAPTA
1,2-bis(2-aminophenoxy)-ethane-N,N,N′,N′-tetraacetic acid
- EGTA
ethylene glycolbis (β-aminoethyl ether)-N,N,N′,N′-tetraacetic acid
- NMDA
N-methyl-D-aspartic acid
- PKCγ
γ-isoform of protein kinase C
References
- ABELIOVICH A., CHEN C., GODA Y., SILVA A.J., STEVENS C.F., TONEGAWA S. Modified hippocampal long-term potentiation in PKC gamma-mutant mice. Cell. 1993;75:1253–1262. doi: 10.1016/0092-8674(93)90613-u. [DOI] [PubMed] [Google Scholar]
- ALLGAIER C. Ethanol sensitivity of NMDA receptors. Neurochem. Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- BOWERS B.J., WEHNER J.M. Ethanol consumption and behavioral impulsivity are increased in protein kinase Cgamma null mutant mice. J. Neurosci. 2001;21:RC180. doi: 10.1523/JNEUROSCI.21-21-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN L., WRIGHT L.R., CHEN C.H., OLIVER S.F., WENDER P.A., MOCHLY-ROSEN D. Molecular transporters for peptides: delivery of a cardioprotective epsilonPKC agonist peptide into cells and intact ischemic heart using a transport system, R(7) Chem. Biol. 2001;8:1123–1129. doi: 10.1016/s1074-5521(01)00076-x. [DOI] [PubMed] [Google Scholar]
- DILDY-MAYFIELD J.E., HARRIS R.A. Ethanol inhibits kainate responses of glutamate receptors expressed in Xenopus oocytes: role of calcium and protein kinase C. J. Neurosci. 1995;15:3162–3171. doi: 10.1523/JNEUROSCI.15-04-03162.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FANG Z., GONG D., IONESCU P., LASTER M.J., EGER E.I., II, KENDIG J. Maturation decreases ethanol minimum alveolar anesthetic concentration (MAC) more than desflurane MAC in rats. Anesth. Analg. 1997;84:852–858. doi: 10.1097/00000539-199704000-00028. [DOI] [PubMed] [Google Scholar]
- FENG J., KENDIG J.J. N-methyl-D-aspartate receptors are implicated in hyperresponsiveness following naloxone reversal of alfentanil in isolated rat spinal cord. Neurosci. Lett. 1995a;189:128–130. doi: 10.1016/0304-3940(95)11465-9. [DOI] [PubMed] [Google Scholar]
- FENG J., KENDIG J.J. Selective effects of alfentanil on nociceptive-related neurotransmission in neonatal rat spinal cord. Br. J. Anaesth. 1995b;74:691–696. doi: 10.1093/bja/74.6.691. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN D.B., PAL N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- GROSSHANS D.R., BROWNING M.D. Protein kinase C activation induces tyrosine phosphorylation of the NR2A and NR2B subunits of the NMDA receptor. J. Neurochem. 2001;76:737–744. doi: 10.1046/j.1471-4159.2001.00034.x. [DOI] [PubMed] [Google Scholar]
- HARRIS K.M., KONGSAMUT S., MILLER R.J. Protein kinase C mediated regulation of calcium channels in PC-12 pheochromocytoma cells. Biochem. Biophys. Res. Commun. 1986;134:1298–1305. doi: 10.1016/0006-291x(86)90391-8. [DOI] [PubMed] [Google Scholar]
- HARRIS R.A., MCQUILKIN S.J., PAYLOR R., ABELIOVICH A., TONEGAWA S., WEHNER J.M. Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc. Natl. Acad. Sci. U.S.A. 1995;92:3658–3662. doi: 10.1073/pnas.92.9.3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASHIMOTO T., ASE K., SAWAMURA S., KIKKAWA U., SAITO N., TANAKA C., NISHIZUKA Y. Postnatal development of a brain-specific subspecies of protein kinase C in rat. J. Neurosci. 1988;8:1678–1683. doi: 10.1523/JNEUROSCI.08-05-01678.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIA W.G., BEAULIEU C., HUANG F.L., CYNADER M.S. Cellular and subcellular localization of protein kinase C in cat visual cortex. Brain Res. Mol. Brain Res. 1990;8:311–317. doi: 10.1016/0169-328x(90)90044-e. [DOI] [PubMed] [Google Scholar]
- JOHNSON J.A., GRAY M.O., CHEN C.H., MOCHLY-ROSEN D. A protein kinase C translocation inhibitor as an isozyme-selective antagonist of cardiac function. J. Biol. Chem. 1996;271:24962–24966. doi: 10.1074/jbc.271.40.24962. [DOI] [PubMed] [Google Scholar]
- KIM H.G., FOX K., CONNORS B.W. Properties of excitatory synaptic events in neurons of primary somatosensory cortex of neonatal rats. Cereb. Cortex. 1995;5:148–157. doi: 10.1093/cercor/5.2.148. [DOI] [PubMed] [Google Scholar]
- KOSE A., ITO A., SAITO N., TANAKA C. Electron microscopic localization of gamma- and beta II-subspecies of protein kinase C in rat hippocampus. Brain Res. 1990;518:209–217. doi: 10.1016/0006-8993(90)90974-g. [DOI] [PubMed] [Google Scholar]
- LAI C.C., CHANG M.C., LIN H.H. Acute tolerance to ethanol inhibition of NMDA-induced responses in rat rostral ventrolateral medulla neurons. J. Biomed. Sci. 2004;11:482–492. doi: 10.1007/BF02256097. [DOI] [PubMed] [Google Scholar]
- LI H.F., KENDIG J.J. Ethanol withdrawal hyper-responsiveness mediated by NMDA receptors in spinal cord motor neurons. Br. J. Pharmacol. 2003a;139:73–80. doi: 10.1038/sj.bjp.0705198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI H.F., WANG M.Y., KNAPE J., KENDIG J.J. Ethanol tachyphylaxis in spinal cord motorneurons: role of metabotropic glutamate receptors. Br. J. Pharmacol. 2003b;138:1417–1424. doi: 10.1038/sj.bjp.0705175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEN D.J., WONG K.L., SHEU F.S., ROUTTENBERG A. NMDA receptor blockade prevents the increase in protein kinase C substrate (protein F1) phosphorylation produced by long-term potentiation. Brain Res. 1988;458:142–146. doi: 10.1016/0006-8993(88)90506-9. [DOI] [PubMed] [Google Scholar]
- LOZIER A.P., KENDIG J.J. Long-term potentiation in an isolated peripheral nerve-spinal cord preparation. J. Neurophysiol. 1995;74:1001–1009. doi: 10.1152/jn.1995.74.3.1001. [DOI] [PubMed] [Google Scholar]
- LU W.Y., XIONG Z.G., LEI S., ORSER B.A., DUDEK E., BROWNING M.D., MACDONALD J.F. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat. Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- MALINOW R., SCHULMAN H., TSIEN R.W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989;245:862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- MALMBERG A.B., CHEN C., TONEGAWA S., BASBAUM A.I. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- MAO J., PRICE D.D., PHILLIPS L.L., LU J., MAYER D.J. Increases in protein kinase C gamma immunoreactivity in the spinal cord dorsal horn of rats with painful mononeuropathy. Neurosci. Lett. 1995;198:75–78. doi: 10.1016/0304-3940(95)11975-3. [DOI] [PubMed] [Google Scholar]
- MARTIN W.J., MALMBERG A.B., BASBAUM A.I. PKCgamma contributes to a subset of the NMDA-dependent spinal circuits that underlie injury-induced persistent pain. J. Neurosci. 2001;21:5321–5327. doi: 10.1523/JNEUROSCI.21-14-05321.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCINTYRE T.A., SOUDER M.G., HARTL M.W., SHIBLEY I.A. Ethanol-induced decrease of developmental PKC isoform expression in the embryonic chick brain. Brain Res. Dev. Brain Res. 1999;117:191–197. doi: 10.1016/s0165-3806(99)00122-4. [DOI] [PubMed] [Google Scholar]
- MIKI A. Developmental changes in the expression of alpha-, beta- and gamma-subspecies of protein kinase C at synapses in the ventral horn of the embryonic and postnatal rat spinal cord. Brain Res. Dev. Brain Res. 1995;87:46–54. doi: 10.1016/0165-3806(95)00051-e. [DOI] [PubMed] [Google Scholar]
- MIKI A. Expression of alpha-, beta-, and gamma-subspecies of protein kinase C in the motor neurons in the embryonic and postnatal rat spinal cord. Neuroscience. 1996;72:805–814. doi: 10.1016/0306-4522(95)00576-5. [DOI] [PubMed] [Google Scholar]
- MIRSHAHI T., ANDERS D.L., RONALD K.M., WOODWARD J.J. Intracellular calcium enhances the ethanol sensitivity of NMDA receptors through an interaction with the C0 domain of the NR1 subunit. J. Neurochem. 1998;71:1095–1107. doi: 10.1046/j.1471-4159.1998.71031095.x. [DOI] [PubMed] [Google Scholar]
- MORI M., KOSE A., TSUJINO T., TANAKA C. Immunocytochemical localization of protein kinase C subspecies in the rat spinal cord: light and electron microscopic study. J. Comp. Neurol. 1990;299:167–177. doi: 10.1002/cne.902990204. [DOI] [PubMed] [Google Scholar]
- MORRISETT R.A., REZVANI A.H., OVERSTREET D., JANOWSKY D.S., WILSON W.A., SWARTZWELDER H.S. MK-801 potently inhibits alcohol withdrawal seizures in rats. Eur. J. Pharmacol. 1990;176:103–105. doi: 10.1016/0014-2999(90)90138-v. [DOI] [PubMed] [Google Scholar]
- PEREZ-VELAZQUEZ J.L., VALIANTE T.A., CARLEN P.L. Changes in calcium currents during ethanol withdrawal in a genetic mouse model. Brain Res. 1994;649:305–309. doi: 10.1016/0006-8993(94)91077-4. [DOI] [PubMed] [Google Scholar]
- POLGAR E., FOWLER J.H., MCGILL M.M., TODD A.J. The types of neuron which contain protein kinase C gamma in rat spinal cord. Brain Res. 1999;833:71–80. doi: 10.1016/s0006-8993(99)01500-0. [DOI] [PubMed] [Google Scholar]
- RIPLEY T.L., WHITTINGTON M.A., BUTTERWORTH A.R., LITTLE H.J. Ethanol withdrawal hyperexcitability in vivo and in isolated mouse hippocampal slices. Alcohol Alcohol. 1996;31:347–357. doi: 10.1093/oxfordjournals.alcalc.a008161. [DOI] [PubMed] [Google Scholar]
- SAITO N., SHIRAI Y. Protein kinase Cgamma (PKCgamma): function of neuron specific isotype. J. Biochem. (Tokyo) 2002;132:683–687. doi: 10.1093/oxfordjournals.jbchem.a003274. [DOI] [PubMed] [Google Scholar]
- SCHECHTMAN D., MOCHLY-ROSEN D. Isozyme-specific inhibitors and activators of protein kinase C. Methods Enzymol. 2002;345:470–489. doi: 10.1016/s0076-6879(02)45039-2. [DOI] [PubMed] [Google Scholar]
- SIGGINS G.R., MARTIN G., ROBERTO M., NIE Z., MADAMBA S., DE LECEA L. Glutamatergic transmission in opiate and alcohol dependence. Ann. NY Acad. Sci. 2003;1003:196–211. doi: 10.1196/annals.1300.012. [DOI] [PubMed] [Google Scholar]
- STUBBS C.D., SLATER S.J. Ethanol and protein kinase C. Alcohol. Clin. Exp. Res. 1999;23:1552–1560. [PubMed] [Google Scholar]
- SUBRAMANIAM S., MCGONIGLE P. Regional profile of developmental changes in the sensitivity of the N-methyl-D-aspartate receptor to polyamines. J. Neurochem. 1994;62:1408–1415. doi: 10.1046/j.1471-4159.1994.62041408.x. [DOI] [PubMed] [Google Scholar]
- THOMAS M.P., MONAGHAN D.T., MORRISETT R.A. Evidence for a causative role of N-methyl-D-aspartate receptors in an in vitro model of alcohol withdrawal hyperexcitability. J. Pharmacol. Exp. Ther. 1998;287:87–97. [PubMed] [Google Scholar]
- WANG M.Y., KENDIG J.J. Patch clamp studies of motor neurons in spinal cord slices: a tool for high-resolution analysis of drug actions. Acta Pharmacol. Sin. 2000;21:507–515. [PubMed] [Google Scholar]
- WANG M.Y., RAMPIL I.J., KENDIG J.J. Ethanol directly depresses AMPA and NMDA glutamate currents in spinal cord motor neurons independent of actions on GABAA or glycine receptors. J. Pharmacol. Exp. Ther. 1999;290:362–367. [PubMed] [Google Scholar]
- WOLF M., BURGESS S., MISRA U.K., SAHYOUN N. Postsynaptic densities contain a subtype of protein kinase C. Biochem. Biophys. Res. Commun. 1986;140:691–698. doi: 10.1016/0006-291x(86)90787-4. [DOI] [PubMed] [Google Scholar]
- WONG S.M., FONG E., TAUCK D.L., KENDIG J.J. Ethanol as a general anesthetic: actions in spinal cord. Eur. J. Pharmacol. 1997;329:121–127. [PubMed] [Google Scholar]
- WONG S.M., TAUCK D.L., FONG E.G., KENDIG J.J. Glutamate receptor-mediated hyperexcitability after ethanol exposure in isolated neonatal rat spinal cord. J. Pharmacol. Exp. Ther. 1998;285:201–207. [PubMed] [Google Scholar]
- WONG S.M.E., SWEITZER S.M, PETERS M.C., KENDIG J.J.Hyperresponsiveness on washout of volatile anesthetics from isolated spinal cord compared to withdrawal from ethanol Anethesia and Analgesia 2004. in press [DOI] [PubMed]
- ZISKIND-CONHAIM L., GAO B.X., HINCKLEY C. Ethanol dual modulatory actions on spontaneous postsynaptic currents in spinal motoneurons. J. Neurophysiol. 2003;89:806–813. doi: 10.1152/jn.00614.2002. [DOI] [PubMed] [Google Scholar]