Abstract
In 6- and 10-week-old obesity-prone (fa/fa) Zucker diabetic fatty (ZDF) rats, effects of prevention and intervention therapies, respectively, were compared between PPARα/γ agonist, ragaglitazar (RAGA) and separate PPARγ and α agonists, pioglitazone (PIO) and bezafibrate (BF).
In a separate study, lean (+/+) ZDF rats fed highly palatable chow to induce dietary obesity and insulin resistance were treated similarly. To test insulin-secretory capacity, all animals underwent a hyperglycaemic clamp.
Insulin sensitivity was improved equally by RAGA and PIO in fa/fa rats subjected to both prevention and intervention treatments (e.g., prevention HOMA-IR: −71 and −72%, respectively), as was hyperglycaemia (both −68%). BF had no effect on either parameter in any study. Plasma lipids were markedly reduced (by 48–77%) by RAGA in all studies, equivalent to PIO, but to a greater extent than BF.
RAGA improved β-cell function (HOMA-β) more than three-fold with prevention and intervention therapies, whereas PIO showed improvement only in intervention therapy. Consistent with improved insulin sensitivity, glucose infusion rate during the clamp was 60% higher in RAGA-treated animals subjected to prevention therapy, but there was little additional insulin-secretory response, suggesting that insulin secretion was already maximal.
Thus, RAGA and PIO equally improve metabolic profile in ZDF rats, particularly when administered early in the course of diabetes. They also improve β-cell function, although this is better demonstrated through indices incorporating fasting insulin and glucose concentrations than through the hyperglycaemic clamp technique in this model.
Keywords: Dietary obesity, hyperglycaemic clamp, insulin secretion, insulin resistance, PPARα/γ agonist, pioglitazone, ragaglitazar, Zucker diabetic fatty rat
Introduction
Drugs which treat type II diabetes should preferably improve insulin resistance, correct dyslipidaemia and preserve pancreatic β-cell function, the main factors characterizing this progressive syndrome (Reaven, 1988; Unger, 1995). Agonists of the γ isoform of the peroxisome proliferator-activated receptor (PPAR) family, such as the thiazolidinediones (TZDs), stimulate genes which favour storage of triglycerides (TGs), thus lowering circulating free fatty acid (FFA) concentrations (Moore et al., 2001). This shift from FFAs to glucose as a fuel substrate is one mechanism whereby these drugs improve insulin sensitivity in peripheral tissues (Spiegelman, 1998). Furthermore, TZDs have been shown to improve β-cell survival and function in vitro and prevent TG accumulation in islets (Shimabukuro et al., 1998; Murakami et al., 1999; Finegood et al., 2001; Brand et al., 2003; Pickavance et al., 2003; Yajima et al., 2003).
PPAR isoforms exhibit distinct tissue distributions, with PPARγ being expressed predominantly in adipose tissue, and PPARα, the target for the fibrate group of lipid-lowering drugs, being most abundant in liver (Braissant et al., 1996; Fajas et al., 1997). Drugs with activity at both PPARα and γ are able to combine insulin-sensitising and lipid-lowering effects (Murakami et al., 1998; 1999; Etgen et al., 2002; Ljung et al., 2002; Brand et al., 2003; Ye et al., 2003), but whether this is associated with better in vivo preservation of β-cell function than obtained with γ agonists alone is unknown.
Both ZDF and Zucker obese rats have an identical homozygote mutation (fa/fa) in the gene encoding for the leptin receptor that leads to obesity and insulin resistance. In addition, the ZDF strain has a genetic defect in β-cell gene expression (Griffen et al., 2001) that leads to the development of diabetes in this animal model. The ZDF rat is prone to β-cell failure in response to weight gain and is a useful model of type II diabetes (Unger, 1997). Therefore, we used this ZDF rat model to test the effects of treatments with ragaglitazar (RAGA), a dual PPARα/γ agonist, pioglitazone (PIO), a PPARγ agonist, and bezafibrate (BF), a PPARα agonist, early and late in the development of diabetes. A third group of lean (+/+) ZDF rats, genetically equivalent to their obese counterparts, albeit without the leptin receptor mutation (Peterson, 1994), were treated with the above-mentioned compounds after making them obese by feeding them a highly palatable diet (HPD). This had the secondary aim of investigating whether obesity induced by such a diet might reveal the genetic susceptibility to diabetes in the ZDF rat.
Methods
Animals and treatment
The prevention and intervention studies were initiated in 6-week-old pre-diabetic and in 10-week-old diabetic (fa/fa) ZDF rats, respectively. Rats were singly housed, with free access to pelletted chow (CRM (P), Biosure, Cambridge, U.K.) and water. They were randomly assigned to treatment groups (n=8 per group) and gavaged once daily for 28 days with either vehicle (CON, 1% carboxymethylcellulose, 1 ml kg−1), RAGA (Novo Nordisk A/S, Denmark; 1.5 mg kg−1), PIO (Actos®, Takeda, Japan; 30 mg kg−1) or BF (Sigma-Aldrich Co., Ltd, Poole, Dorset, U.K.; 100 mg kg−1). Dosages were selected on the basis of previous findings on efficacy (Haubenwallner et al., 1995; Matsui et al., 1997; Arakawa et al., 1998; Kawamori et al., 1998; Cabrero et al., 1999; Lohray et al., 2001; Sauerberg et al., 2002; Brand et al., 2003; Chakrabarti et al., 2003; Skrumsager et al., 2003; Ye et al., 2003; Saad et al., 2004). Age-matched vehicle-treated lean (+/+) rats (LEAN) served as nondiabetic controls. Importantly, all lean ZDF rats used in these experiments were genotyped and found to be homozygous for the wild-type fa allele and thus did not have a defect in the leptin receptor.
In the diet-induced obesity (DIO) study, 40 6–7-week-old lean (+/+) male ZDF rats (148±2 g; Genetic Models, Inc., Indianapolis, IN, U.S.A.) were housed in groups of four. Eight controls (CHOW) were fed pelletted chow and 32 rats were fed HPD for 10 weeks. The HPD consisted of 33% powdered chow, 33% condensed milk (Nestlé U.K. Ltd, York, U.K.) and 7% sucrose (Tate & Lyle, London, U.K.) by weight, with remainder water, as described previously (Wilding et al., 1992). After 6 weeks on the HPD, rats underwent intervention treatment for 4 weeks with either vehicle (CON), RAGA, BF or PIO at the doses mentioned above.
Animals followed a 12-h light–dark cycle. Body weight and food intake were measured daily. U.K. Home Office regulations (Animal Scientific Procedures Act, 1986) were adhered to throughout.
Hyperglycaemic clamp
After the 28-day dosing period, and after a 12-h fast, all rats were subjected to a hyperglycaemic clamp. Rats were deeply anaesthetised using a combination of Hypnorm™ and Valium® (fentanyl/fluanisone (Janssen Pharmaceutical Ltd, Oxford, U.K.) and diazepam (Roche, Welwyn Garden City, Herts, U.K.); both 0.8 ml kg−1, i.m.), followed by additional doses of Hypnorm™ (∼0.1 ml kg−1, i.m.) every 30–40 min. Following catheterisation of the right jugular vein, a 500-μl sample of blood was taken for later measurement of pre-clamp insulin concentrations by RIA (Pharmacia/Upjohn Diagnostics U.K., Lewes, Sussex, U.K.) and glucose by diagnostic kit (Roche Diagnostics, Milton Keynes, U.K.). Baseline tail-vein blood glucose concentration was measured with an electrochemical meter and electrodes (Precision Q.I.D.™ Sensor and Precision Plus electrodes, Medisense®, Abingdon, Oxon, U.K.).
A priming infusion of 20% D-glucose in water (w v−1) was then begun at a rate of 100 mg kg min−1, and was gradually reduced to 70, 57, 46 and 35 mg kg min−1 every 2 min for the first 11 min of infusion, based on the method of Vettor et al. (1998). The glucose infusion rate (GIR) was then adjusted according to blood glucose readings taken every 10 min during a further 70-min maintenance period to clamp the glucose concentration at 24 mM. Tail-vein blood (∼100 μl) was collected at these time points for later measurement of plasma insulin levels. These values (μU ml−1) were plotted against time (min) and the area under the curve (AUC) calculated for the early- (t=0–11 min) and late-phase (t=20–90 min) insulin-secretory responses, as described previously (Giaccari et al., 1995). Pre-clamp indices of insulin resistance and insulin secretion were calculated using homeostasis model assessment (HOMA) formulae (Matthews et al., 1985): HOMA-IR=fasting insulin (μU ml−1) × fasting glucose (mM)/22.5; HOMA-β=20 × fasting insulin (μU ml−1)/(fasting glucose (mM)-3.5).
Metabolic data and fat mass
At the end of the clamp, rats were killed by cardiac exsanguination. Post-clamp plasma concentrations of FFAs and TGs (Boehringer Mannheim, Milton Keynes, Bucks and Sigma-Aldrich Co., Ltd, Poole, Dorset, U.K.) were measured by diagnostic kits, and those of insulin and leptin by RIA (Biogenesis, Poole, Dorset, U.K.).
Body fat content was estimated by weighing dissected subcutaneous (SC, from below the sternum), perirenal and epididymal fat pads and expressing these as a percentage of final body weight.
Pancreatic insulin concentration
The pancreas was removed and snap-frozen in liquid nitrogen for later measurement of insulin content. Proteins were extracted from ∼50-mg pieces of frozen pancreas by boiling in 0.5 ml of 0.1 M acetic acid for 5 min, cooling to room temperature and then sonicating for 30 s until well-homogenised. Protein was then pelletted by centrifugation at 14,000 r.p.m. for 5 min. A volume of 250 μl of the supernatant was then neutralised with 215 μl of 1 M NaOH, and insulin concentration then measured by RIA in the same way as for plasma concentration. The concentration of pancreatic insulin was then expressed per gram of tissue. As an estimate of insulin-secretory capacity, the ratio of pancreatic insulin content (nmol g−1) to fasting plasma glucose (mM) was calculated.
Statistical analyses
Data are expressed as mean±s.e.m. Differences were considered statistically significant at P<0.05 unless otherwise stated. Parameters were compared between diabetic (ZDF-CON) and LEAN controls (prevention and intervention studies) or between DIO-CON and CHOW (DIO study) by unpaired (two-sample) t-tests or Mann–Whitney test for non-normally distributed data. Significance of trend across obese groups was tested using Cuzick's test. The drug effect on each of the parameters was measured by comparing values in each of the treated ZDF or DIO groups with those in their obese control groups by one-way ANOVA followed by Dunnett's test for multiple comparisons with a control. Using the trapezoidal rule, insulin secretion (μU ml min−1) was calculated as area under the curve (AUC) of plasma insulin concentration (μU ml−1) during the 90-min hyperglycaemic clamp, and the incremental change from baseline was calculated. Steady-state (S-S) GIR was taken as the mean rate over the final 30 min of the clamp. All statistical analyses were carried out using StatsDirect statistical software (v. 2.3.8, © 1990–2001 StatsDirect Ltd).
Results
Prevention study in ZDF rats: effects on energy intake, body weight and body composition
After the prevention treatment period, CON rats were heavier than their LEAN counterparts (+19%; 344±6 vs 289±3 g; P<0.0001), with increased fat mass. RAGA and PIO, but not BF, increased food intake, doubled food efficiency and enhanced weight gain in these animals (Table 1).
Table 1.
Effects of PPAR agonists on cumulative energy intake, body weight and body composition in obese and lean ZDF rats
| Treatment | Energy intake (kJ) | Food efficiency (g MJ−1) | Body weight gain (g) | % epididymal fat | % perirenal fat | % SC fat |
|---|---|---|---|---|---|---|
| Prevention study in ZDF | ||||||
| CON | 14,579±421 | 127±9 | 8.0±0.8 | 0.79±0.03 | 0.98±0.05 | 0.093±0.008 |
| BF | 15,037±655 | 122±10 | 7.7±1.2 | 0.75±0.02 | 0.93±0.05 | 0.111±0.014 |
| PIO | 17,583±262*** | 245±4*** | 13.4±0.4*** | 1.08±0.03*** | 1.30±0.08** | 0.106±0.020 |
| RAGA | 17,863±250*** | 247±5*** | 13.2±0.4*** | 1.04±0.02*** | 1.26±0.06** | 0.118±0.017 |
| LEAN | 9604±111** | 113±6 | 10.9±0.7* | 0.29±0.01*** | 0.27±0.02** | 0.048±0.006** |
| Intervention study in ZDF | ||||||
| CON | 18,356±418 | 17±4 | 0.24±0.22 | 0.80±0.03 | 1.07±0.05 | 0.101±0.009 |
| BF | 19,232±286 | 22±4 | 0.05±0.23 | 0.80±0.03 | 1.05±0.03 | 0.096±0.010 |
| PIO | 20,071±457** | 130±13*** | 6.19±0.86*** | 1.13±0.04*** | 1.34±0.02*** | 0.126±0.014 |
| RAGA | 19,777±738 | 132±22*** | 6.20±1.32*** | 1.10±0.02*** | 1.40±0.04*** | 0.142±0.015* |
| LEAN | 10,127±179** | 64±3*** | 5.38±0.17*** | 0.29±0.01*** | 0.34±0.03*** | 0.054±0.003** |
| Intervention study in DIO | ||||||
| CON | 9760±80 | 2.3±0.3 | 23±3 | 0.58±0.02 | 0.70±0.03 | 0.086±0.009 |
| BF | 10,573±230** | 2.1±0.2 | 22±2 | 0.59±0.03 | 0.69±0.04 | 0.076±0.016 |
| PIO | 11,171±163*** | 4.4±0.2*** | 50±2*** | 0.78±0.04*** | 0.74±0.04 | 0.081±0.017 |
| RAGA | 11,146±232*** | 4.2±0.2*** | 46±2*** | 0.78±0.01*** | 0.86±0.02** | 0.105±0.023 |
| CHOW | 8859±120*** | 1.5±0.3 | 14±3* | 0.35±0.01*** | 0.39±0.02*** | 0.058±0.004* |
Animals were treated for 28 days with ragaglitazar (RAGA, 1.5 mg kg−1), pioglitazone (PIO, 30 mg kg−1) and bezafibrate (BF, 100 mg kg−1). Values are expressed as mean±s.e.m. (n=8);
P<0.05;
P<0.01;
P⩽0.0001 as compared to obese control (CON). Food efficiency=Δ body weight/Σ energy intake. Fat pad mass expressed as weight of a single pad as % of terminal body weight.
Prevention effects on metabolic parameters
CON rats were insulin-resistant (HOMA-IR: +763%; P<0.05), had reduced β-cell function (HOMA-β: −68%; P<0.01), and about half the pancreatic insulin content of LEAN rats, although this latter value was not significant. RAGA and PIO significantly improved insulin sensitivity (both by 72% vs CON; P<0.01), and there were trends toward improved β-cell function and increased pancreatic insulin content, both absolute and relative to plasma glucose, across treatments (P=0.0001, 0.059 and 0.03, respectively). Indeed, RAGA increased HOMA-β several-fold (+607%; P<0.01).
These rats also showed increased FFA and TG levels, which were reduced by all treatments (TGs only in the case of BF, although reduction in FFAs approached significance (P=0.08)). Circulating leptin concentrations were approximately doubled by RAGA and PIO treatments (Table 2).
Table 2.
Effects of PPAR agonists on metabolic parameters in obese and lean ZDF rats
| Pre-clamp | Post-clamp | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment | Insulin (μU ml−1) | Glucose (mM) | HOMA-IR | HOMA-β (%) | Pancreatic insulin (nmol g−1) | Pancreatic insulin/plasma glucose | FFA (mM) | TG (mM) | Leptin (ng ml−1) |
| Prevention study in ZDF | |||||||||
| CON | 16.2±1.8 | 22.8±1.5 | 16.4±2.3 | 17.8±2.9 | 2.8±0.8 | 0.10±0.03 | 1.23±0.10 | 3.97±0.77 | 6.5±0.6 |
| BF | 15.0±4.4 | 23.0±1.6 | 16.2±5.0 | 15.0±4.2 | 3.9±0.9 | 0.13±0.04 | 0.96±0.12 | 1.63±0.47** | 6.6±0.6 |
| PIO | 13.3±0.9 | 7.4±0.7*** | 4.5±0.6** | 71.6±12.2 | 5.8±2.1 | 0.60±0.21** | 0.57±0.04*** | 0.91±0.10*** | 12.2±1.1*** |
| RAGA | 15.9±2.7 | 7.2±0.6*** | 4.7±0.7** | 125.9±40.2** | 5.2±0.9 | 0.56±0.15** | 0.60±0.06*** | 1.11±0.09*** | 12.5±1.2*** |
| LEAN | 7.3±0.9** | 6.3±0.5** | 1.9±0.2* | 56.1±12.1** | 4.5±0.8 | 0.77±0.17** | 0.70±0.09** | 0.80±0.13** | 2.7±0.3*** |
| Intervention study in ZDF | |||||||||
| CON | 15.3±2.3 | 23.9±1.6 | 16.6±3.3 | 13.0±1.3 | 1.2±0.3 | 0.06±0.02 | 1.17±0.10 | 3.38±0.45 | 5.8±0.2 |
| BF | 14.8±1.9 | 19.9±1.8 | 13.3±2.6 | 19.8±3.3 | 1.1±0.5 | 0.04±0.01 | 1.01±0.10 | 2.90±0.69 | 5.2±0.2 |
| PIO | 18.3±2.9 | 11.0±1.2*** | 9.2±2.2 | 55.9±10.2** | 2.2±0.4 | 0.21±0.05* | 0.61±0.07*** | 1.15±0.15** | 8.0±0.4* |
| RAGA | 15.4±1.4 | 10.4±1.5*** | 6.8±0.8* | 60.4±12.9** | 2.9±1.0 | 0.21±0.06 | 0.62±0.03*** | 1.59±0.23* | 9.7±1.1** |
| LEAN | 15.2±0.4 | 6.7±0.5** | 4.6±0.4** | 105.6±15.7** | 2.4±0.4 | 0.36±0.08** | 0.51±0.10** | 0.92±0.19** | 3.1±0.4*** |
| Intervention study in DIO | |||||||||
| CON | 9.8±1.5 | 6.3±0.3 | 2.8±0.5 | 74.7±12.5 | 5.8±2.4 | 0.55±0.12 | 1.97±0.10 | 1.90±0.15 | 6.8±0.7 |
| BF | 6.4±2.0 | 7.8±1.1 | 2.5±1.2 | 36.9±7.6 | 7.3±2.2 | 0.93±0.30 | 1.21±0.12*** | 1.09±0.14*** | 5.2±1.0 |
| PIO | 6.8±1.1 | 4.7±0.9 | 1.3±0.3 | 37.8±4.7 | 5.9±1.2 | 1.69±0.55 | 0.70±0.04*** | 0.78±0.04*** | 7.4±0.6 |
| RAGA | 8.4±1.2 | 5.4±1.1 | 2.0±0.4 | 104.5±39.6 | 4.6±1.0 | 0.79±0.26 | 0.90±0.07*** | 0.93±0.09*** | 7.2±0.6 |
| CHOW | 5.1±0.3** | 6.4±0.5 | 1.4±0.1* | 43.6±9.7 | 1.8±0.4* | 0.28±0.09 | 1.22±0.04** | 1.00±0.011** | 3.2±0.4** |
Animals were treated for 28 days with ragaglitazar (RAGA, 1.5 mg kg−1), pioglitazone (PIO, 30 mg kg−1) and bezafibrate (BF, 100 mg kg−1). Values are expressed as mean±s.e.m. (n=8);
P<0.05;
P<0.01;
P⩽0.0001 as compared to obese control (CON). HOMA-IR=insulin resistance index=glucose (mM) × insulin (μU ml−1)/22.5; HOMA-β=insulin secretion index=20 × insulin (μU ml−1)/(glucose (mM)-3.5). Pancreatic insulin/plasma glucose ratio=(nmol g−1)/mM (fasting plasma glucose).
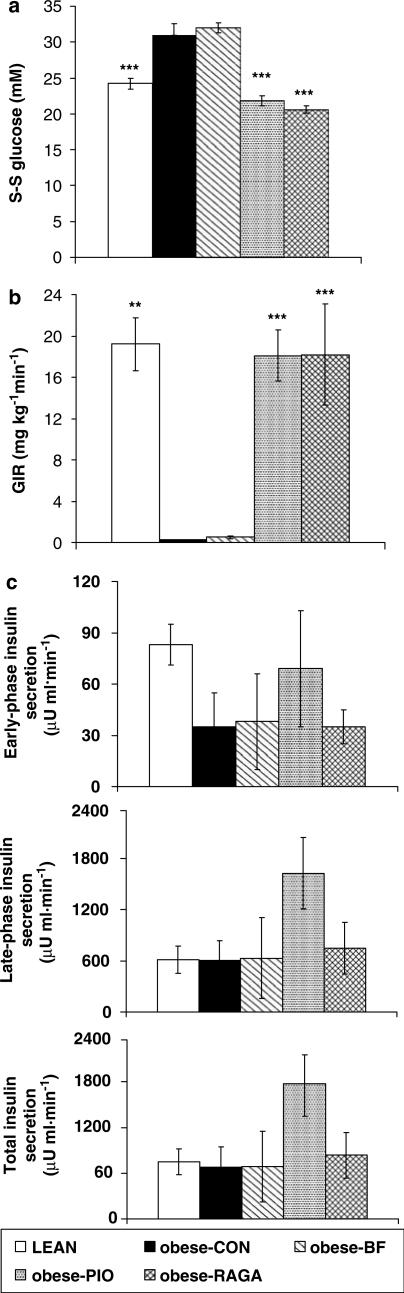
Desired S-S blood glucose concentration during the clamp could not be attained in the CON- and BF-treated groups because these animals were already hyperglycaemic beyond 24 mM (Figure 1a). Consequently, GIR was negligible. GIR in PIO- and RAGA-treated groups was normalised (Figure 1b), although there was no change in insulin-secretory response. The PIO-treated group showed enhanced insulin release throughout the clamp, but this was not significant (Figure 1c).
Figure 1.
Effects of PPAR agonist prevention therapy in ZDF (fa/fa) rats. (a) S-S blood glucose concentration, (b) GIR and (c) incremental change from baseline in insulin secretion during the hyperglycaemic clamp. Animals were treated once daily for 28 days with RAGA (1.5 mg kg−1), PIO (30 mg kg−1) and BF (100 mg kg−1), then clamped at 24 mM glucose, as described in Methods. Vehicle-treated obese (CON) and lean (LEAN) rats were used as controls. Values are expressed as mean±s.e.m. (n=8); **P<0.01; ***P<0.0001 as compared to CON (ANOVA).
Intervention study in ZDF rats: effects on energy intake, body weight and body composition
Despite almost identical body weight to lean controls (−0.02%; 345±6 vs 348±3 g; P>0.05), CON rats had greater fat pad masses. Interestingly, whereas only PIO enhanced food intake, both PIO and RAGA induced weight gain to match that of LEAN rats. This is consistent with increased food efficiency of more than six-fold (both P<0.0001; Table 1).
Intervention effects on metabolic parameters
CON rats showed marked insulin resistance (HOMA-IR: +261%; P<0.01) and reduced β-cell function (HOMA-β: −88%; P<0.01), with a corresponding (nonsignificant) decrease in pancreatic insulin content compared to LEAN. RAGA and PIO improved insulin sensitivity (HOMA-IR: −59% (RAGA) and −44% (PIO); P<0.05 and P=0.09, respectively) and β-cell function, as shown by HOMA-β (>3-fold; both P<0.01) and pancreatic insulin/plasma glucose ratio (both +250%; P=0.052 and 0.045, respectively), and there was a significant trend toward improvement in pancreatic insulin content (+142% (RAGA) and +83% (PIO); P=0.03). Plasma FFA and TG levels were also elevated in CON rats but significantly reduced by RAGA and PIO. BF had no effect on lipids. Both RAGA and PIO increased circulating leptin (Table 2).
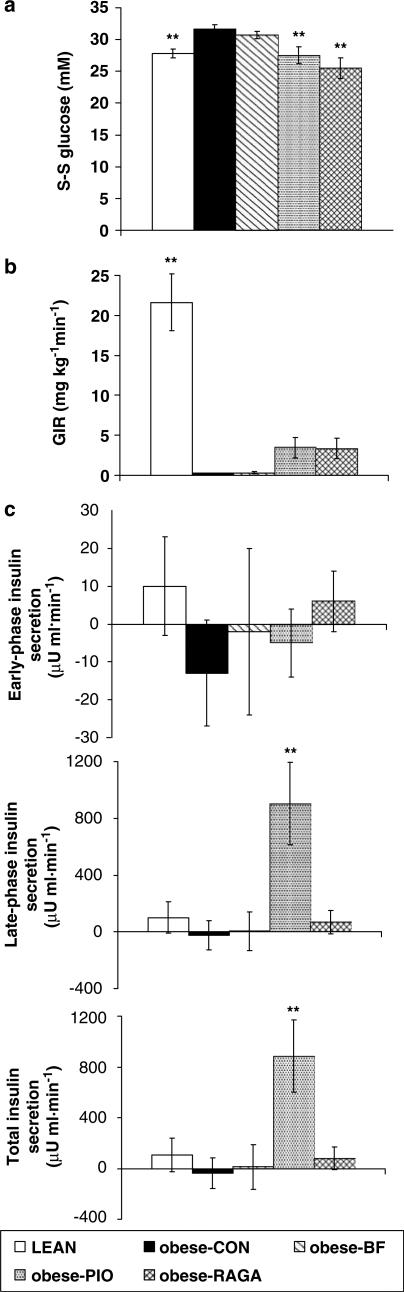
As in the prevention study, because of pre-existing hyperglycaemia, S-S blood glucose concentration could not be attained, and glucose was not infused in CON- and BF-treated groups (Figure 2a, b). S-S GIR in RAGA- and PIO-treated groups, although greater than that required in CON- and BF-treated groups, was not significantly so (Figure 2b), but nevertheless was sufficient in PIO-treated animals to signficantly increase late-phase and total insulin secretion (Figure 2c).
Figure 2.
Effects of PPAR agonist intervention therapy in ZDF (fa/fa) rats. (a) S-S blood glucose concentration, (b) GIR and (c) incremental change from baseline in insulin secretion during the hyperglycaemic clamp. Animals were treated once daily for 28 days with RAGA (1.5 mg kg−1), PIO (30 mg kg−1) and BF (100 mg kg−1), then clamped at 24 mM glucose, as described in Methods. Vehicle-treated obese (CON) and lean (LEAN) rats were used as controls. Values are expressed as mean±s.e.m. (n=8); **P<0.01 as compared to CON (ANOVA).
Intervention study in DIO rats: effects on energy intake, body weight and body composition
After 10 weeks on the HPD, CON rats were 12% heavier than CHOW rats (410±4 vs 365±6 g; P<0.0001). During the 4-week treatment period, energy intake had remained higher in these animals than in CON rats (+10%; P<0.0001), and this was associated with greater body weight gain (final body weight: 426±6 vs 380±8 g; P<0.01). PIO-, BF- and RAGA-treated animals showed a further increase in food intake (+11–14%; all P<0.01) that was associated with greater weight gain in PIO and RAGA groups. Consistent with this, food efficiency nearly doubled and percentage body fat content, greater in CON than LEAN rats, was further increased by PIO and RAGA (Table 1).
Intervention effects on metabolic parameters in DIO rats
CON rats were more insulin-resistant than CHOW rats (HOMA-IR: +100%; P<0.05). This was not significantly affected by any treatment, although HOMA-IR was numerically lower in all treated groups and similar to that in CHOW rats (one-way ANOVA: P>0.05). β-Cell function was not significantly different between the groups (HOMA-β or pancreatic insulin/plasma glucose ratio), although pancreatic insulin content was three-fold higher in CON than CHOW rats (+222%; P<0.05). This was not altered by treatment, however. All treatments reduced FFA and TG concentrations, with PIO and RAGA having a greater effect than BF. Plasma leptin concentration was higher in CON than CHOW rats, but was not altered by any treatment (Table 2).
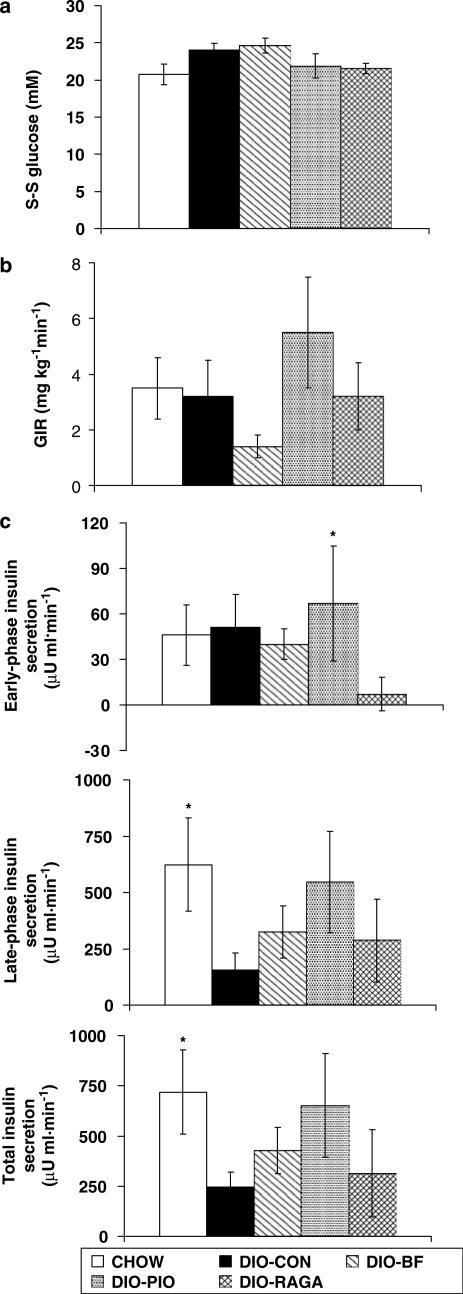
Blood glucose concentration of approximately 22 mM was attained in all groups over the final 30 min of the clamp (all P>0.05; Figure 3a). There were no significant differences between S-S GIRs required to maintain hyperglycaemia in each group (all P>0.05; Figure 3b). However, as observed in the studies of diabetic ZDF rats, insulin secretion in the PIO group was enhanced throughout the clamp, but only significantly in the early phase (P<0.05 vs CON), consistent with the observed increase, albeit nonsignificant, in the GIR of these animals. CHOW rats, however, showed significant increases above baseline compared to CON rats in both the late phase and total insulin secretion (Figure 3c).
Figure 3.
Effects of PPAR agonist intervention therapy in ZDF (+/+) DIO rats. (a) S-S blood glucose concentration, (b) GIR and (c) incremental change from baseline in insulin secretion during the hyperglycaemic clamp. Animals were treated once daily for 28 days with RAGA (1.5 mg kg−1), PIO (30 mg kg−1) and BF (100 mg kg−1), then clamped at 24 mM glucose, as described in Methods. Vehicle-treated DIO (CON) and lean (CHOW) rats were used as controls. Values are expressed as mean±s.e.m. (n=8); *P<0.05 as compared to CON (ANOVA).
Discussion
In these studies, we have shown that the PPARγ agonist, PIO and the PPARα/γ agonist, RAGA, are effective at improving insulin action in the obese, diabetic ZDF rat. These benefits were greater earlier in the course of diabetes, but only nonsignificant trends were seen in the insulin-resistant DIO model. Indices showing improved β-cell function (HOMA-β and pancreatic insulin content/plasma glucose ratio) were generally consistent, agreeing with in vitro observations suggesting that PPARγ and dual PPARα/γ agonists, including RAGA, prevent β-cell death and alter expression of markers of β-cell survival such as insulin and GLUT2 in diabetic rodent models (Murakami et al., 1999; Finegood et al., 2001; Sturis et al., 2002; Brand et al., 2003; Pickavance et al., 2003; Yajima et al., 2003).
Paradoxically, we have not been able to demonstrate improved insulin secretion with RAGA under hyperglycaemic clamp conditions, and only inconsistent improvement with PIO. This may be because insulin secretion is already maximal in these insulin-resistant animals. The apparent increase in insulin secretion seen with PIO but not RAGA intervention therapy, in the face of similar levels of hyperglycaemia and GIRs, could represent a superior effect of RAGA on insulin sensitivity, despite similar HOMA-IR values, or on non-insulin-mediated glucose disposal after a glucose load; that is, an effect which is independent of any change in insulin sensitivity. However, further studies would be required to investigate this phenomenon.
Despite their obesity and insulin resistance, DIO ZDF lean rats did not develop diabetes. Thus, a leptin receptor mutation appears to be necessary for the development of diabetes in this model. Diabetes developed at a younger age and lower body weight in the obese mutant than in the DIO model, suggesting that the islet defect is related directly to the leptin receptor mutation, rather than to the degree of obesity. This is in agreement with findings that overexpression of the normal leptin receptor in the islets of pre-diabetic ZDF rats reverses the diabetic phenotype (Zhou et al., 1998). Our findings seem at odds with those of Griffen et al. (2001), which show that a β-cell defect in insulin gene transcription is present in lean ZDF rats and leads to the development of diabetes in the face of insulin resistance. However, it is possible that the modest insulin resistance observed in our DIO rats was not severe or prolonged enough to allow expression of this phenotype.
Consistent with recent human data showing comparable glycaemic control and lipid profile between RAGA and PIO (Saad et al., 2004), but in contrast to some other reported animal data (Brand et al., 2003; Chakrabarti et al., 2003; Larsen et al., 2003), we have not found any greater effect of RAGA compared to PIO on circulating FFAs and TGs, despite an equivalent effect on insulin resistance and glucose-lowering. Moreover, because both PIO and RAGA caused weight gain, increasing fat mass and leptin concentrations, we can deduce that there was no neutralisation of weight gain by the α component of RAGA, which appears to contradict previous reports (Larsen et al., 2003; Yajima et al., 2003; Ye et al., 2003). However, trends were present for weight gain in these studies, and the lack of an effect may simply represent shorter treatment duration or lack of statistical power. The weak retention by PIO of α activity (Sakamoto et al., 2000) could also explain the lack of difference between PIO and RAGA. The rodent model (diet and strain) and fat depot examined may also explain why our results conflict with those of others. Whereas other workers have tested RAGA in wild-type Sprague–Dawley or Wistar rats fed a high-fat diet (Larsen et al., 2003; Ye et al., 2003), we have used a monogenic obesity-prone model. Certainly, in conjunction with findings that expression of PPARα target genes in liver is altered by RAGA within these other paradigms (Brand et al., 2003; Larsen et al., 2003; Ye et al., 2003), the above rationalisation suggests that it would be imprudent to conclude that RAGA has no PPARα effect in the ZDF rat.
ZDF rats may be poor responders to PPARα agonism in general due to reduced islet PPARα expression (Zhou et al., 1998). If this were also true of expression in the liver, the other tissue to which PPARα is localised, it might explain why BF had no significant effect on insulin resistance, whereas others have shown α agonists to improve insulin sensitivity in rodent models of obesity such as Zucker (fa/fa) rats and high-fat-fed mice and rats (Guerre-Millo et al., 2000; Ye et al., 2001). BF may not have had significant effects on most parameters because it is only a moderately weak PPARα agonist compared to fenofibrate in rodents, although potency in humans is approximately equivalent (Willson et al., 2000). We know that the batch of drug used was effective insofar as weight gain was attenuated and FFA and TG levels, at least in DIO and younger diabetic animals, were reduced. Although others have effectively reduced circulating insulin, FFA and TG concentrations and increased pancreatic insulin content with BF treatment in older, overtly diabetic ZDF rats, this may be due to their use of a different dosage and longer duration of treatment (Harmon et al., 2001).
Conclusion
In summary, treatment with PPARγ and α/γ agonists is more effective when applied early in the natural development of diabetes in the ZDF rat. At the doses used here, RAGA improves insulin sensitivity, β-cell function and metabolic profile equally with PIO and better than BF alone, although enhanced insulin secretion could not be demonstrated dynamically, perhaps because of limitations of hyperglycaemic clamp methodology in this model. Unchanged insulin-secretory response in younger ZDF rats treated with RAGA, despite increased GIR, suggests that the main effect of this compound is through improved insulin sensitivity. Lack of effect on energy accumulation and other outcome variables normally suppressed by BF treatment further emphasise the point that in future the ZDF rat should be employed with caution as a model for studies on PPARα compounds. In addition, we have shown that although the lean (+/+) ZDF rat develops obesity and insulin resistance on a HPD, it is not prone to diabetes like its obese (fa/fa) mutant counterpart.
Acknowledgments
We are very grateful to the Biomedical Services Unit staff at the University of Liverpool, in particular Miss Juliet McAdams, Miss Joanne Sanders and Miss Catherine Edwards, for their excellent care of the animals.
Abbreviations
- BF
bezafibrate
- DIO
dietary obese
- DIO-CON
dietary obese control
- FFA
free fatty acid
- GIR
glucose infusion rate
- HOMA
homeostasis model assessment
- HPD
highly palatable diet
- PIO
pioglitazone
- PPAR
peroxisome proliferator-activated receptor
- RAGA
ragaglitazar
- SC
subcutaneous
- S-S
steady state
- TG
triglyceride
- TZD
thiazolidinedione
References
- ARAKAWA K., ISHIHARA T., AOTO M., INAMASU M., SAITO A., IKEZAWA K. Actions of novel antidiabetic thiazolidinedione, T-174, in animal models of non-insulin-dependent diabetes mellitus (NIDDM) and in cultured muscle cells. Br. J. Pharmacol. 1998;125:429–436. doi: 10.1038/sj.bjp.0702066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAISSANT O., FOUFELLE F., SCOTTO C., DAUCA M., WAHLI W. Differential expression of peroxisome proliferator-activated receptors (PPARs): tissue distribution of PPAR-alpha, -beta, and -gamma in the adult rat. Endocrinology. 1996;137:354–366. doi: 10.1210/endo.137.1.8536636. [DOI] [PubMed] [Google Scholar]
- BRAND C.L., STURIS J., GOTFREDSEN C.F., FLECKNER J., FLEDELIUS C., HANSEN B.F., ANDERSEN B., YE J.-M., SAUERBERG P., WASSERMAN K. Dual PPARα/γ activation provides enhanced improvement of insulin sensitivity and glycemic control in ZDF rats. Am. J. Physiol. 2003;284:E841–E854. doi: 10.1152/ajpendo.00348.2002. [DOI] [PubMed] [Google Scholar]
- CABRERO A., LLAVERIAS G., ROGLANS N., ALEGRET M., SANCHEZ R., ADZET T., LAGUNA J.C., VASQUEZ M. Uncoupling protein-3 mRNA levels are increased in white adipose tissue and skeletal muscle of bezafibrate-treated rats. Biochem. Biophys. Res. Commun. 1999;260:547–556. doi: 10.1006/bbrc.1999.0926. [DOI] [PubMed] [Google Scholar]
- CHAKRABARTI R., VIKRAMADITHYAN R.K., MISRA P., HIRIYAN J., RAICHUR S., DAMARLA R.K., GERSHOME C., SURESH J., RAJAGOPALAN R. Ragaglitazar: a novel PPARα & PPARγ agonist with potent lipid-lowering and insulin-sensitizing efficacy in animal models. Br. J. Pharmacol. 2003;140:527–537. doi: 10.1038/sj.bjp.0705463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETGEN G.J., OLDHAM B.A., JOHNSON W.T., BRODERICK C.L., MONTROSE C.R., BROZINICK J.T., MISENER E.A., BEAN J.S., BENSCH W.R., BROOKS D.A., SHUKER A.J., RITO C.J., MCCARTHY J.R., ARDECKY R.J., TYHONAS J.S., DANA S.L., BILAKOVICS J.M., PATERNITI J.R., JR, OGILVIE K.M., LIU S., KAUFFMAN R.F. A tailored therapy for the metabolic syndrome: the dual peroxisome proliferator-activated receptor-alpha/gamma agonist LY465608 ameliorates insulin resistance and diabetic hyperglycemia while improving cardiovascular risk factors in preclinical models. Diabetes. 2002;51:1083–1087. doi: 10.2337/diabetes.51.4.1083. [DOI] [PubMed] [Google Scholar]
- FAJAS L., AUBOEUF D., RASPÉ E., SCHOONJANS K., LEFEBVRE A.M., SALADIN R., NAJIB J., LAVILLE M., FRUCHART J.C., DEEB S., VIDAL-PUIG A., FLIER J., BRIGGS M.R., STAELS B., VIDAL H., AUWERX J. The organization, promoter analysis, and expression of the human PPARγ gene. J. Biol. Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- FINEGOOD D.T., MCARTHUR M.D., KOJWANG D., THOMAS M.J., TOPP B.G., LEONARD T., BUCKINGHAM R.E. β-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. [DOI] [PubMed] [Google Scholar]
- GIACCARI A., MORVIDUCCI L., ZORRETTA D., SBRACCIA P., LEONETTI F., CAIOLA S., BUONGIORNO A., BONADONNA R.C., TAMBURRANO G. In vivo effects of glucosamine on insulin secretion and insulin sensitivity in the rat: possible relevance to the maladaptive responses to chronic hyperglycaemia. Diabetologia. 1995;38:518–524. doi: 10.1007/BF00400719. [DOI] [PubMed] [Google Scholar]
- GRIFFEN S.C., WANG J., GERMAN M.S. A genetic defect in β-cell gene expression segregrates independently from the fa locus in the ZDF rat. Diabetes. 2001;50:63–68. doi: 10.2337/diabetes.50.1.63. [DOI] [PubMed] [Google Scholar]
- GUERRE-MILLO M., GERVOIS P., RASPE E., MADSEN L., POULAIN P., DERUDAS B., HERBERT J.M., WINEGAR D.A., WILLSON T.M., FRUCHART J.C., BERGE R.K., STAELS B. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J. Biol. Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- HARMON J.S., GLEASON C.E., TANAKA Y., POITOUT V., ROBERTSON R.P. Antecedent hyperglycemia, not hyperlipidemia, is associated with increased islet triacylglycerol content and decreased insulin gene mRNA level in Zucker diabetic fatty rats. Diabetes. 2001;50:2481–2486. doi: 10.2337/diabetes.50.11.2481. [DOI] [PubMed] [Google Scholar]
- HAUBENWALLNER S., ESSENBURG A.D., BARNETT B.C., PAPE M.E., DEMATTOS R.B., KRAUSSE B.R., MINTON L.L., AUERBACH B.J., NEWTON R.S., LEFF T., BISGAIERL C.L. Hypolipidemic activity of select fibrates correlates to changes in hepatic lipolipoprotein C-III expression: a potential physiologic basis for their mode of action. J. Lipid Res. 1995;36:2541–2551. [PubMed] [Google Scholar]
- KAWAMORI R., MATSUHISA M., KINOSHITA J., MOCHIZUTI K., NIWA M., ARISAKA T., IKEDA M., KUBOTA M., WADA M., KANDA T., IKEBUCHI M., TOHDO R., YAMASAKI Y. Pioglitazone enhances splanchnic glucose uptake as well as peripheral glucose uptake in non-insulin-dependent diabetes mellitus. AD-4833 Clamp OGL Study Group. Diabetes Res. Clin. Pract. 1998;41:35–43. doi: 10.1016/s0168-8227(98)00056-4. [DOI] [PubMed] [Google Scholar]
- LARSEN P.J., JENSEN P.B., SØRENSEN R.V., LARSEN L.K., VRANG N., WULFF E.M., WASSERMANN K. Differential influences of peroxisome proliferator-activated receptor γ and -α on food intake and energy homeostasis. Diabetes. 2003;52:2249–2259. doi: 10.2337/diabetes.52.9.2249. [DOI] [PubMed] [Google Scholar]
- LJUNG B., BAMBERG K., DAHLLÖF B., KJELLSTEDT A., OAKES N.D., OSTLING J., SVENSSON L., CAMEJO G. AZ 242, a novel PPARα/γ agonist with beneficial effects on insulin resistance and carbohydrate and lipid metabolism in ob/ob mice and obese Zucker rats. J. Lipid Res. 2002;43:1855–1863. doi: 10.1194/jlr.m200127-jlr200. [DOI] [PubMed] [Google Scholar]
- LOHRAY B.B., LOHRAY V.B., BAJJI A.C., KALCHAR S., POONDRA R.R., PADAKANTI S., CHAKRABARTI R., VIKRAMADITHYAN R.K., MISRA P., JULURI S., MAMIDI N.V., RAJAGOPALAN R. (−)3-(4-(2-(Phenoxasin-10-yl)ethoxy)phenyl)-2-ethoxypropanoic acid ((−)DRF 2725): a dual PPAR agonist with potent antihyperglycemic and lipid modulating activity. J. Med. Chem. 2001;44:2675–2678. doi: 10.1021/jm010143b. [DOI] [PubMed] [Google Scholar]
- MATSUI H., OKUMURA K., KAWAKAMI K., HIBINO M., TOKI Y., ITO T. Improved insulin sensitivity by bezafibrate in rats: relationship to fatty acid composition of skeletal-muscle triglycerides. Diabetes. 1997;46:348–353. doi: 10.2337/diab.46.3.348. [DOI] [PubMed] [Google Scholar]
- MATTHEWS D.R., HOSKER J.P., RUDENSKI A.S., NAYLOR B.A., TREACHER D.F., TURNER R.C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- MOORE G.B., CHAPMAN H., HOLDER J.C., LISTER C.A., PIERCY V., SMITH S.A., CLAPHAM J.C. Differential regulation of adipocytokine mRNAs by rosiglitazone in db/db mice. Biochem. Biophys. Res. Commun. 2001;286:735–741. doi: 10.1006/bbrc.2001.5460. [DOI] [PubMed] [Google Scholar]
- MURAKAMI K., TOBE K., IDE T., MOCHIZUKI T., OHASHI M., AKANUMA Y., YAZAKI Y., KADOWAKI T. A novel insulin sensitizer acts as a coligand for peroxisome proliferator-activated receptor-alpha (PPAR-alpha) and PPAR-gamma: effect of PPAR-alpha activation on abnormal lipid metabolism in liver of Zucker fatty rats. Diabetes. 1998;47:1841–1847. doi: 10.2337/diabetes.47.12.1841. [DOI] [PubMed] [Google Scholar]
- MURAKAMI K., TSUNODA M., IDE T., OHASHI M., MOCHIZUKI T. Amelioration by KRP-297, a new thiazolidinedione, of impaired glucose uptake in skeletal muscle from obese insulin-resistant animals. Metabolism. 1999;48:1450–1454. doi: 10.1016/s0026-0495(99)90158-0. [DOI] [PubMed] [Google Scholar]
- PETERSON R.G.The Zucker diabetic fatty (ZDF) rat Lessons from Animal Diabetes 1994London: Smith-Gordon; 225–230.ed. Shafrir, E. pp. [Google Scholar]
- PICKAVANCE L., WIDDOWSON P.S., FOSTER J.R., WILLIAMS G., WILDING J.P. Chronic treatment with the thiazolidinedione, MCC-555, is associated with reductions in nitric oxide synthase activity and β-cell apoptosis in the pancreas of the Zucker diabetic fatty rat. Int. J. Exp. Path. 2003;84:83–89. doi: 10.1046/j.1365-2613.2003.00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REAVEN G.M. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- SAAD M.F., GRECO S., OSEI K., LEWIN A.J., EDWARDS C., NUNEZ M., REINHARDT R.R. Ragaglitazar improves glycemic control and lipid profile in type 2 diabetic subjects: a 12-week, double-blind, placebo-controlled dose-ranging study with an open pioglitazone arm. Diabetes Care. 2004;27:1324–1329. doi: 10.2337/diacare.27.6.1324. [DOI] [PubMed] [Google Scholar]
- SAKAMOTO J., KIMURA H., MORIYAMA S., ODAKA H., MOMOSE Y., SUGIYAMA Y., SAWADA H. Activation of human peroxisome proliferator-activated receptor (PPAR) subtypes by pioglitazone. Biochem. Res. Commun. 2000;278:704–711. doi: 10.1006/bbrc.2000.3868. [DOI] [PubMed] [Google Scholar]
- SAUERBERG P., PETTERSSON I., JEPPESEN L., BURY P.S., MOGENSEN J.P., WASSERMANN K., BRAND C.L., STURIS J., WOLDIKE H.F., FLECKNER J., ANDERSEN A.S., MORTENSEN S.B., SVENSSON L.A., RASMUSSEN H.B., LEHMANN S.V., POLIVKA Z., SINDELAR K., PANAJOTOVA V., YNDDAL L., WULFF E.M. Novel tricyclic-α-alkyloxyphenylpropionic acids: dual PPARα/γ agonists with hypolipidemic and antidiabetic activity. J. Med. Chem. 2002;45:789–804. doi: 10.1021/jm010964g. [DOI] [PubMed] [Google Scholar]
- SHIMABUKURO M., ZHOU Y.-T., LEE Y., UNGER R.H. Troglitazone lowers islet fat and restores beta cell function of Zucker diabetic fatty rats. J. Biol. Chem. 1998;273:3547–3550. doi: 10.1074/jbc.273.6.3547. [DOI] [PubMed] [Google Scholar]
- SKRUMSAGER B.K., NIELSEN K.K., MULLER M., PABST G., DRAKE P.G., EDSBERG B. Ragaglitazar: the pharmacokinetics, pharmacodynamics, and tolerability of a novel dual PPAR alpha and gamma agonist in healthy subjects and patients with type 2 diabetes. J. Clin. Pharmacol. 2003;43:1244–1256. doi: 10.1177/0091270003257230. [DOI] [PubMed] [Google Scholar]
- SPIEGELMAN B.M. PPAR-gamma: adipogenic regulator and thiazolidinedione receptor. Diabetes. 1998;47:507–514. doi: 10.2337/diabetes.47.4.507. [DOI] [PubMed] [Google Scholar]
- STURIS J., GOTFREDSEN C.F., RØMER J., TORNEHAVE D., GALSGAARD E.D., VØLUND A., WASSERMANN K., KNUDSEN L.B., BRAND C.L. Synergistic effects of the dual-acting PPARα and γ agonist ragaglitazar and the long-acting GLP-1 derivative NN2211 on glycemic control in diabetic ZDF rats. Diabetes. 2002;51 Suppl. 2:A34. [Google Scholar]
- UNGER R.H. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM: Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- UNGER R.H. How obesity causes diabetes in Zucker diabetic fatty rats. Trends Endocrinol. Metab. 1997;7:276–282. doi: 10.1016/s1043-2760(97)00094-5. [DOI] [PubMed] [Google Scholar]
- VETTOR R., PAGANO C., GRANZOTTO M., ENGLARO P., ANGELI P., BLUM W.F., FEDERSPIL G., ROHNER-JEANRENAUD F., JEANRENAUD B. Effects of intravenous neuropeptide Y on insulin secretion and insulin sensitivity in skeletal muscle in normal rats. Diabetologia. 1998;41:1361–1367. doi: 10.1007/s001250051077. [DOI] [PubMed] [Google Scholar]
- WILDING J.P., GILBEY S.G., MANNAN M., ASLAM N., GHATEI M.A., BLOOM S.R. Increased neuropeptide Y content in individual hypothalamic nuclei, but not neuropeptide Y mRNA, in diet-induced obesity in rats. J. Endocrinol. 1992;132:299–304. doi: 10.1677/joe.0.1320299. [DOI] [PubMed] [Google Scholar]
- WILLSON T.M., BROWN P.J., STERNBACH D.D., HENKE B.R. The PPARs: from orphan receptors to drug discovery. J. Med. Chem. 2000;43:527–550. doi: 10.1021/jm990554g. [DOI] [PubMed] [Google Scholar]
- YAJIMA K., HIROSE H., FUJITA H., SETO Y., FUJITA H., UKEDA K., MIYASHITA K., KAWAI T., YAMAMOTO Y., OGAWA T., YAMADA T., SARUTA T. Combination therapy with PPARγ and PPARα agonists increases glucose-stimulated insulin secretion in db/db mice. Am. J. Physiol. Endocrinol. Metab. 2003;284:E966–E971. doi: 10.1152/ajpendo.00149.2002. [DOI] [PubMed] [Google Scholar]
- YE J.-M., DOYLE P.J., IGLESIAS M.A., WATSON D.G., COONEY G.J., KRAEGEN E.W. Peroxisome proliferator-activated receptor (PPAR)-α activation lowers muscle lipids and improves insulin sensitivity in high fat-fed rats. Comparison with PPAR-γ activation. Diabetes. 2001;50:411–417. doi: 10.2337/diabetes.50.2.411. [DOI] [PubMed] [Google Scholar]
- YE J.-M., IGLESIAS M.A., WATSON D.G., ELLIS B., WOOD L., JENSEN P.B., SØRENSEN R.V., LARSEN P.J., COONEY G.J., WASSERMANN K., KRAEGEN E.W. PPARα/γ ragaglitazar eliminates fatty liver and enhances insulin action in fat-fed rats in the absence of hepatomegaly. Am. J. Physiol. Endocrinol. Metab. 2003;284:E531–E540. doi: 10.1152/ajpendo.00299.2002. [DOI] [PubMed] [Google Scholar]
- ZHOU Y.T., SHIMABUKURO M., WANG M.Y., LEE Y., HIGA M., MILBURN J.L., NEWGARD C.B., UNGER R.H. Role of peroxisome proliferator-activated receptor α in disease of pancreatic β cells. Proc. Natl. Acad. Sci. U.S.A. 1998;95:8898–8903. doi: 10.1073/pnas.95.15.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]