Abstract
The present study investigates the effects of gastrin-17 on human colon cancer HT-29 cells to examine whether gastrin receptor (CCK-2), cyclooxygenase (COX-1, COX-2) isoforms and prostaglandin receptor pathways interact to control cell growth.
Reverse transcription (RT)–polymerase chain reaction (PCR) analysis demonstrated that HT-29 cells are endowed with the naïve expression of CCK-2 receptor (short splice variant), COX-1, COX-2 and prostaglandin EP4 receptor, but not gastrin.
Gastrin-17 significantly promoted cell growth and DNA synthesis. Both these stimulating effects were abolished by L-365,260 or GV150013 (CCK-2 receptor antagonists), but were unaffected by SC-560 (COX-1 inhibitor). L-745,337 (COX-2 inhibitor) or AH-23848B (EP4 receptor antagonist) partly reversed gastrin-17-induced cell growth, while they fully antagonized the enhancing action on DNA synthesis.
HT-29 cells responded to gastrin-17 with a significant increase in prostaglandin E2 release. This enhancing effect was completely counteracted by L-365,260, GV150013 or L-745,337, while it was insensitive to cell incubation with SC-560.
Exposure of HT-29 cells to gastrin-17 was followed by an increased phosphorylation of both extracellular regulated kinases (ERK-1/ERK-2) and Akt. Moreover, gastrin-17 enhanced the transcriptional activity of COX-2 gene promoter and stimulated COX-2 expression. These latter effects were antagonized by L-365,260 or GV150013, and could be blocked also by PD98059 (inhibitor of ERK-1/ERK-2 phosphorylation) or wortmannin (inhibitor of phosphatidylinositol 3-kinase). Analogously, gastrin-17-induced prostaglandin E2 release was prevented by PD98059 or wortmannin.
The present results suggest that (a) in human colon cancer cells endowed with CCK-2 receptors, gastrin-17 is able to enhance the transcriptional activity of COX-2 gene through the activation of ERK-1/ERK-2- and phosphatidylinositol 3-kinase/Akt-dependent pathways; (b) these stimulant actions lead to downstream increments of COX-2 expression, followed by prostaglandin E2 production and EP4 receptor activation; (c) the recruitment of COX-2/prostaglandin pathways contributes to the growth-promoting actions exerted by gastrin-17.
Keywords: Cyclooxygenase-2, gastrin, CCK-2 receptor, prostaglandin EP receptor, colon cancer, MAP kinase, PI3 kinase, Akt
Introduction
Colorectal cancer accounts for about 15% of human malignancies and represents one of the most frequent cause of cancer death in western countries. The optimal therapeutic option for this neoplasia remains surgical resection, whereas pharmacological treatments are far from being satisfactory. In the search for novel therapies, the roles played by gut hormones and cyclooxygenase (COX) pathways in the control of colorectal cancer growth and progression are under active investigation (Smith & Watson, 2000; Subbaramaiah & Dannenberg, 2003).
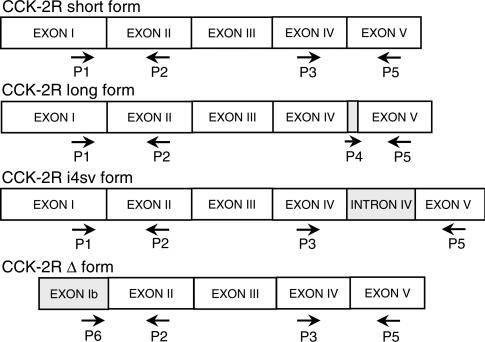
Gastrin is a peptide hormone synthesized and released mainly from G cells in the gastric antrum (Dockray et al., 2001). The biological effects of this gut hormone are mediated by membrane receptors, named CCK-2, which can be selectively blocked by nonpeptidic antagonists, such as L-365,260 and GV150013 (Noble et al., 1997). Cloning of CCK-2 receptor gene has revealed the existence of distinct molecular variants, designated as short, long, truncated (Δ) and i4sv forms (Song et al., 1993; Miyake, 1995; Hellmich et al., 2000).
Gastrin is mainly deputed to the regulation of gastric acid secretion and gastrointestinal motility (Dockray et al., 2001). Furthermore, it exerts trophic actions on digestive cells (Smith & Watson, 2000; Rozengurt & Walsh, 2001). Clinical studies have indicated that hypergastrinemia can be associated with an increased risk of colorectal cancer, particularly in patients with Helicobacter pylori (H. pylori) infection (Hartwich et al., 2001). At preclinical level, gastrin stimulates both in vitro and xenografted human colon cancer cells (Smith & Watson, 2000). Moreover, in animal models of colonic carcinogenesis, hypergastrinemia increases the incidence and growth rate of epithelial neoplasms (Watson & Smith, 2001). CCK-2 receptors have been detected in primary colorectal tumours (Schmitz et al., 2001), but their role in the control of cancer cell growth remains controversial and deserve further investigation.
Epidemiological studies have demonstrated a consistent decrease in mortality for colorectal cancer in subjects treated with nonsteroidal anti-inflammatory drugs. The reduced risk has been ascribed to the pharmacological blockade of COX, the dominant enzyme in the metabolic pathway responsible for conversion of arachidonic acid into prostanoids (Du Bois et al., 1998). Following the identification of two COX isoforms (COX-1, COX-2), evidence was obtained that COX-2 can be overexpressed in colonic neoplasms, where it plays pivotal roles in promoting tumorigenesis, growth and progression of cancer cells (Subbaramaiah & Dannenberg, 2003).
Clinical findings indicate that most of patients with colorectal cancer display increased expression of CCK-2 receptors and COX-2 in their neoplastic tissues (Hartwich et al., 2001). Accordingly, gastrin might upregulate COX-2 expression, and this enzyme might mediate the proliferative actions of gastrin on colonic cancer cells. Previous studies on murine cell lines have lent some support to this hypothesis (Guo et al., 2002; Yao et al., 2002).
We have screened the expression of CCK-2 receptor and COX isoforms in different colon cancer cell lines, and found that HT-29 cells express CCK-2 receptors as well as COX-1 and COX-2. An experimental study was then designed in order (1) to examine the role of CCK-2 receptors in the control of colon cancer cell proliferation, and assess the relative contribution of COX pathways in this process; (2) to characterize the mechanisms and cellular signals mediating the interaction between CCK-2 receptors and COX pathways.
Methods
Cell culture and assays for growth and DNA synthesis
The human colon adenocarcinoma cell line HT-29 was obtained from American Type Culture Collection (Manassas, VA, U.S.A.) and cultured in McCoy's 5A medium (Life Technologies Inc., Rockville, MD, U.S.A.) containing 10% fetal bovine serum and supplemented with penicillin and streptomycin (both at 2%). Cells were maintained in a humidified incubator at 37°C, in the presence of 5% CO2. To perform cell growth studies, HT-29 cells at 70% confluence were collected by trypsinization, seeded in 96-well plates (5 × 104 cells per well), and allowed to adhere in the presence of standard culture medium. After 24 h, cells were washed with phosphate-buffered saline (PBS) and serum-free medium was added. The day after, test drugs were added to culture medium for different time intervals, ranging from 24 h to 8 days. In experiments extending over 24 h, serum-free medium containing test drugs was replaced every 24 h. At the end of experiments, cell growth was determined by means of a kit for colorimetric assay, based on the use of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-4-sulphophenyl)-2H-tetrazolium (CellTiter 96 AQueous One Solution Proliferation Assay, Promega, Madison, WI, U.S.A.), in accordance with the manufacturer's instructions. In control experiments, cells were collected by trypsinization and subjected also to direct count.
DNA synthesis was determined by measuring incorporation of 5-bromo-2′-deoxyuridine (BrdU) into DNA using a cell proliferation kit (Roche Diagnostics, Mannheim, Germany). For this purpose, BrdU 10 μM was added to culture medium after treatment with test drugs. After 6 h, cells were fixed and BrdU incorporation was determined by enzyme-linked immunosorbent assay (ELISA) using kit reagents in accordance with the manufacturer's instructions. In all cases, experiments were performed in triplicate and the results are reported as the mean of four to seven independent experiments.
Assay of prostaglandin E2
Levels of prostaglandin E2 (PGE2) released by HT-29 cells into the culture medium, either under basal conditions or following their exposure to test drugs, were determined by means of a kit for competitive enzyme-linked immunoassay (Cayman, Ann Arbor, MI, U.S.A.). Experiments were performed as reported above for proliferation studies. At the end of incubation, either in the absence or in the presence of test drugs, aliquots of culture medium (0.5 ml) were collected into ice-cold vials containing indomethacin 1 mM (final concentration) and stored at −80°C until the subsequent analysis. Cells were then collected by trypsinization and subjected to direct count. At the time of immunoenzyme analysis, which was carried out in accordance with the manufacturer's instructions, each sample was diluted 1 : 10 (vv−1) and assayed in triplicate. PGE2 levels were then expressed as pg 105 cells−1.
RNA extraction and reverse transcription (RT)–polymerase chain reaction (PCR)
The expression of mRNAs encoding different target proteins was assessed by RT followed by PCR. For this purpose, total RNA was isolated from HT-29 cells by means of Trizol® (Life Technologies, Carlsbad, CA, U.S.A.) and chloroform. Total RNA (1 μg) served as template for single-strand cDNA synthesis in a reaction using 2 μl random hexamer oligonucleotide primers (0.5 μg μl−1) with 200 U of MMLV-reverse transcriptase in manufacturer's buffer containing 500 μM deoxynucleotide triphosphate mixture (dNTP) and 10 mM dithiothreitol. cDNA samples were subjected to PCR in the presence of specific oligonucleotide primers based on the nucleotide sequence of CCK-2 receptor splice variants (Figure 1 and Table 1) as well as gastrin (McWilliams et al., 1998), COX isoforms (Hla & Neilson, 1992), and EP receptor subtypes (Belley & Chadee, 1999) (Table 1). PCR, consisting of 5 μl of RT products, Taq polymerase 2.5 U, dNTP 100 μM and oligonucleotide primers 0.5 μM, was carried out by a PCR-Express thermocycler (Hybaid, Ashford, Middlesex, U.K.). Unless otherwise stated, the following standard conditions were used: 3 min of initial denaturation at 94°C, followed by 35–40 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C for 1 min, with a final extension period of 7 min at 72°C. Aliquots of RNA not subjected to RT were included in PCR reactions to verify the absence of genomic DNA. The efficiency of RNA extraction, RT and PCR was evaluated by specific sets of oligonucleotide primers for the constitutively expressed human β-actin gene (Table 1). The amplified cDNA products were separated by 1.5% agarose gel electrophoresis, in a Tris buffer 40 mM containing 2 mM EDTA, 20 mM acetic acid (pH 8), and stained with ethidium bromide. cDNA bands were then visualized by UV light and quantitated by densitometric analysis with NIH Image computer program (Scion Corporation, Frederick, MD, U.S.A.). The relative expression of various mRNAs was normalized to that of β-actin.
Figure 1.
Schematic representation of oligonucleotide primers designed for RT–PCR analysis of mRNA encoding the splice variants of human CCK-2 receptor (CCK-2R). With respect to the short form, the long form includes a 15-bp nucleotide cassette of intron IV (Song et al., 1993), whereas the i4sv form retains the whole sequence of intron IV (Hellmich et al., 2000). In the Δ variant, exon I is replaced with a novel exon named exon Ib (Miyake, 1995). Different primer combinations and size of PCR products allowed to detect the expression of specific splice variants in accordance with the following criteria: short form, P1xP2 (307 bp) and P3xP5 (562 bp); long form, P1xP2 (307 bp) and P4xP5 (473 bp); i4sv form, P1xP2 (307 bp), P3xP5 (769 bp) and P4xP5 (668); Δ form, P6xP2 (280 bp) and P3xP5 (562 bp). Nucleotide sequences of the above primers are displayed in Table 1.
Table 1.
Specifications of oligonucleotide primer pairs used for RT–PCR assays
| Gene | Nucleotide sequence | Product size (bp) |
|---|---|---|
| CCK-2 receptor | P1, S: 5′-
CGGGGGCCATGGAGCTGTCAAAGCTGAACCGGAGC-3′ P2, A: 5′- TCGCTGACTGCCAGTGAGAG-3′ P3, S: 5′- GTGGCCTACGGGCTTATCTCTCGCGAGCTCTACTTA-3′ P4, S: 5′-CAGGTGGGGCTGGAC-3′ P5, A: 5′- CATCGGGAAGAGCCCTGGGGCGAGCTCGTGGAGGCC-3′ P6, S: 5′- CAGGAGAAGAACTGAACTGTCC-3′ |
see Figure 1 |
| Gastrin | S: 5′-
GCCCAGCCTCTCATCATC-3′ A: 5′- CACTCCAGCCCTGTCCCC-3′ |
215 |
| COX-1 | S: 5′-
TGCCCAGCTCCTGGCCCGCCGCTT-3′ A: 5′- GTGCATCAACACAGGCGCCTCTTC-3′ |
301 |
| COX-2 | S: 5′-
TTCAAATGAGATTGTGGGAAAATTGCT-3′ A: 5′- AGATCATCTCTGCCTGAGTATCTT-3′ |
305 |
| EP1 receptor | S: 5′-
CGCGCTGCCCATCTTCTCCAT-3′ A: 5′- CCCAGGCCGATGAAGCACCAC-3′ |
471 |
| EP2 receptor | S: 5′-
CAACCTCATCCGCATGCAC-3′ A: 5′- CTCAAAGGTCAGCCTG-3′ |
419 |
| EP3 receptor | S: 5′-
CCATCCCCTCCTCACCTC-3′ A: 5′- CGATGTGCTCCCAACGCT-3′ |
397 |
| EP4 receptor | S: 5′-
TGGTATGTGGGCTGGCTG-3′ A: 5′- TGCGGAGCAGCGCGCCGC-3′ |
468 |
| β-Actin | S: 5′-
TCATGAAGTGTGACGTTACATCCGT-3′ A: 5′- CTTAGAAGCATTTGCGGTGCACGATG-3′ |
286 |
After 3 min of initial denaturation at 94°C, PCR assays were performed by 35–40 cycles of denaturation at 94°C for 1 min, annealing at 55°C for 1 min, extension at 72°C for 1 min, final extension at 72°C for 7 min, with the exception of gastrin (denaturation at 95°C for 45 s, annealing at 60°C for 1.5 min and extension at 72°C for 1.5 min), and EP1 receptor (denaturation at 98°C for 1 min). S, sense; A, antisense.
Western blot assay
Western blot analysis was used to examine the expression of COX isoforms as well as the phosphorylation status of regulatory proteins belonging to the mitogen-activated protein (MAP) kinase and phosphatidylinositol 3 (PI3)-kinase pathways. Cells were lysed for 30 min on ice in radioimmune precipitation assay buffer containing 50 mM Tris–HCl (pH 7.4), 1% Nonidet P-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EGTA, 1 mM phenylmethylsulphonyl fluoride, 10 μg ml−1 aprotinin, 1 mM sodium orthovanadate, 0.1% sodium dodecylsulphate (SDS). Lysates were then centrifuged at 15,000 × g for 10 min at 4°C. The supernatants were separated from pellets and stored at −80°C until subsequent procedures. Protein concentration was determined by the Bradford method (Bio-Rad protein assay reagent, Hercules, CA, U.S.A.). Equivalent amounts of protein lysates (30 μg) were denatured by heating for 5 min, separated by electrophoresis on a SDS–polyacrylamide gel (10%) and transferred onto a nitrocellulose membrane. The blots were blocked overnight with 5% nonfat dried-milk in a buffer containing 140 mM NaCl, 20 mM Tris–HCl at pH 7.5, and 0.1% Tween-20 (TBS-T), and incubated with an anti-COX-1 or anti-COX-2 antibody. Phosphorylation of extracellular regulated kinases (ERK-1/ERK-2) and Akt was evaluated by incubation of blots with anti-phospho-p42/44 MAP-kinase (Thr202/Tyr204) and anti-phospho-Akt (Ser473) antibodies. After repeated washings with TBS-T buffer, blots were incubated with a peroxidase-conjugated secondary antibody (SantaCruz Biotechnology, Santa Cruz, CA, U.S.A.) for 1 h at room temperature. Following repeated washings with TBS-T buffer, the immunoreactive bands were visualized by enhanced chemiluminescence (ECL, Amersham Biosciences Europe, Cologno Monzese, Italy) and autoradiography by means of X-ray films. Blots were then stripped and reprobed with anti-β-actin antibody. Immunoreactive bands were quantitated by densitometric analysis with NIH Image computer program (Scion Corporation, Frederick, MD, U.S.A.), and their relative intensity was normalized to that of β-actin.
Luciferase assay
To evaluate the influence of test drugs on transcriptional activity of the promoter of COX-2 gene, HT-29 cells were transiently transfected with 1.8-kb COX-2 promoter cloned into pGL3-Luc-Basic plasmid (Promega Corporation, Madison, WI, U.S.A.), or with control plasmid pGL3-Luc, by means of LipofectamineTM (Life Technologies Inc., Rockville, MD, U.S.A.). A β-galactosidase-expressing plasmid was included in each transfection to control for transfection efficiency. For this purpose, cells seeded in six-well plates were exposed to plasmids for 24 h in the presence of complete medium. Transfected cells were then exposed to serum-free medium containing test drugs. After 24-h incubation, transfected cells were washed twice with PBS, lysed in Reporter Lysis Buffer and assayed for luciferase activity using the Luciferase Assay System (both from Promega Corporation, Madison, WI, U.S.A.). β-Galactosidase activity in 50 μl of cell lysate was estimated after incubation at 37°C with 2 mM chlorophenol red β-galactopyranoside in 20 mM MgCl2, 0.1 mM MnCl2, 45 mM 2-mercaptoethanol and 100 mM NaHPO4, pH 8.0. The reaction was stopped by addition of 0.5 M EDTA, pH 8.0, and the absorbance was measured at 570 nm by means of a spectrophotometer. Luciferase activity was assayed in triplicate for each experiment and normalized to β-galactosidase activity. The results are reported as the mean of five to six independent experiments.
Drugs, antibodies and reagents
The following drugs, antibodies and reagents were used: human gastrin-17, SC-560 (5-(4-chlorophenyl)-1-(4-methoxyphenyl)-3-trifluoromethylpyrazole), indomethacin, AH-23848B ((1α[z],2β5α)-(±)-7-(5-[{(1,1′-biphenyl)-4-yl}methoxy]- 2-[4-morpholinyl]-3-oxocyclopentyl)-4-heptonoic acid calcium salt), ethylenediamine tetracetic acid, sodium dodecylsulphate, polyacrylamide, Tween-20, phenylmethylsulphonyl fluoride, aprotinin, sodium orthovanadate (Sigma Chemicals Co., St Louis, MO, U.S.A.); human gastrin-17-glycine (Neosystem, Strasbourgh, France); L-365,260 ((R)N-(2,3-dihydro-1-methyl-2-oxo-5-phenyl-1H-1,4-benzo-diazepin-3-yl)-N(3-methylphenyl)-urea) and L-745,337 (5-methanesulphonamido-6-(2,4-difluorothiophenyl)-1-indanone) (kindly provided by Merck Frosst Canada Inc., Dorval, Canada); GV150013 (N-(+)-[1-adamant-1-ylmethyl)-2,4-dioxo-5-phenyl-2,3,4,5-tetrahydro -1H-1,5-benzodiazepin-3-yl]-N-phenylurea) (kindly provided by Glaxo-Wellcome, Verona, Italy); PD98059 (2-(2-amino-3-methoxyphenyl)-4H-1-benzopyran-4-one), wortmannin (Tocris Cookson Ltd, Avonmouth, U.K.); anti-COX-1, anti-COX-2 and anti-β-actin antibodies (Santa-Cruz Biotechnology Inc., Santa Cruz, CA, U.S.A.); anti-phospho-p42/44 MAP-kinase and anti-phospho-Akt antibodies (Cell Signaling Technology, Beverly, MA, U.S.A.); random hexamers, dithiothreitol, MMLV-reverse transcriptase, Taq polymerase, deoxynucleotidetriphosphate mixture, ethidium bromide (Promega, Madison, WI, U.S.A.); chlorophenol red β-galactopyranoside (Boehringer Mannheim, Mannheim, Germany). Other reagents were of analytical grade.
Statistical analysis
Results are given as mean±standard error of mean (s.e.m.). The significance of differences was evaluated by one-way analysis of variance (ANOVA) for unpaired data followed by post hoc analysis by Dunnett or Bonferroni test, as appropriate. P-values lower than 0.05 were considered significant; ‘n' indicates the number of experiments.
Results
Expression of CCK-2 receptor variants, gastrin, COX isoforms and EP receptor subtypes in HT-29 cells
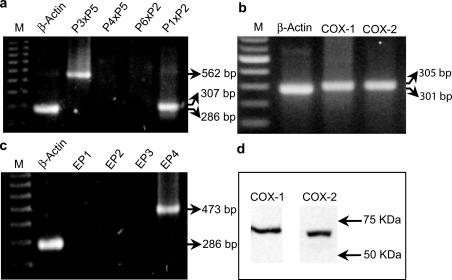
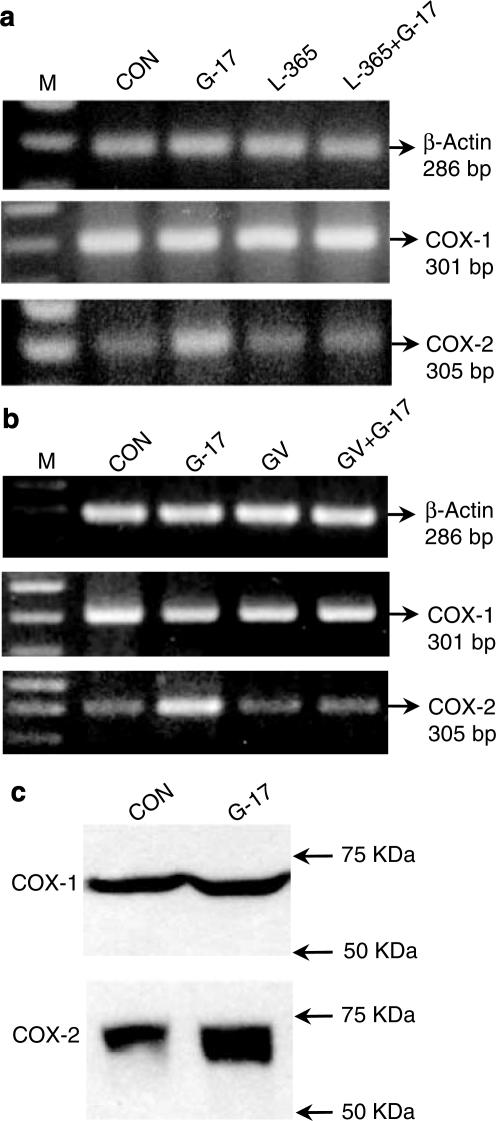
RT–PCR analysis, carried out to detect the presence of CCK-2 receptor mRNA, displayed cDNA fragments of 307 and 562 bp for primer combinations P1xP2 and P3xP5, respectively, while no positive cDNA bands were detected with primers P4xP5 or P6xP2, indicating that HT-29 cells express the short form of CCK-2 receptor, but not long, Δ or i4sv splice variants (Figure 2a). There was also no evidence for the presence of gastrin gene transcripts in HT-29 cells (not shown). Primer combinations used to detect mRNA of COX isoforms allowed the amplification of cDNA bands specific for COX-1 and COX-2 (Figure 2b). Western blot analysis confirmed that both COX-1 and COX-2 proteins are basally expressed in HT-29 cells (Figure 2d). In addition, RT–PCR revealed the expression of EP4, but not EP1, EP2 or EP3 receptor subtypes (Figure 2c).
Figure 2.
RT–PCR analysis of mRNA encoding CCK-2 receptor splice variants (a), cyclooxygenase isoforms (COX-1, COX-2) (b) and EP receptor subtypes (EP1, EP2, EP3, EP4) (c) in HT-29 cells. In panel (a), different combinations of primers P1, P2, P3, P4, P5, P6 and respective size of PCR products allowed to detect the expression of specific splice variants of CCK-2 receptor mRNA, in accordance with criteria illustrated in Figure 1. M=size markers. (d) Western blot analysis of protein expression of cyclooxygenase isoforms in HT-29 cells.
Effects of gastrin-17, glycine extended gastrin-17, CCK-2 receptor antagonists, COX inhibitors and EP4 receptor antagonist on cell growth
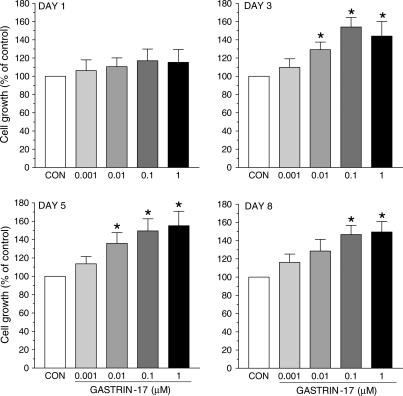
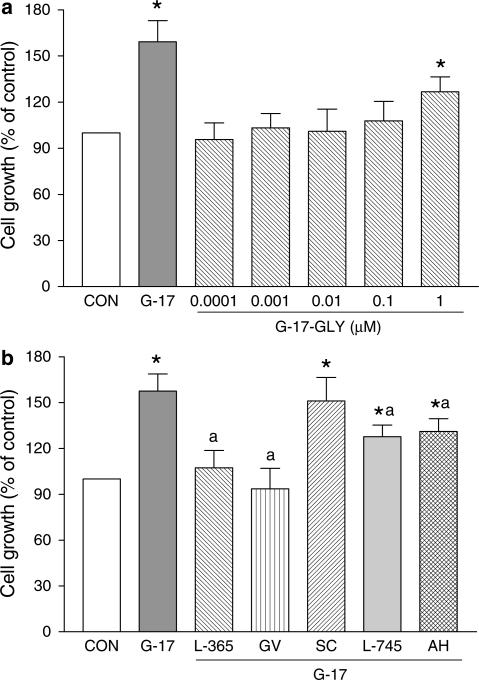
Gastrin-17 (0.001–1 μM) enhanced cell growth in a concentration-dependent manner. Its stimulant action reached a level of statistical significance on day 3, and the maximal effect occurred with the concentration of 0.1 μM at all time points (Figure 3). Under control conditions, cell count at day 1, 3, 5 and 8 accounted for 2.2±0.3, 5.9±0.7, 13.1±1.6 and 21.4±1.9 105 cells, respectively (n=4 for each time point). Since in previous studies on HT-29 cells gastrin-17 induced maximal and CCK-2-dependent stimulations of intracellular signals and proliferation when assayed at 0.1 μM (Ishizuka et al., 1994; Smith et al., 1996), subsequent experiments on cell growth were performed upon incubation of HT-29 cells with test drugs for 3 days, and gastrin-17 was used at the concentration of 0.1 μM. Under these conditions, glycine extended gastrin-17 (G-17-GLY, 0.0001–1 μM) moderately increased cell growth only when assayed at 1 μM (Figure 4a).
Figure 3.
Growth of HT-29 cells following their exposure to increasing concentrations of gastrin-17 (0.001–1 μM) for 1, 3, 5 or 8 days. Each column represents the mean of five to six experiments±s.e.m. (vertical bars). Significant difference from control values (CON): *P<0.05.
Figure 4.
Growth of HT-29 cells. (a) Effects of gastrin-17 (G-17, 0.1 μM) or increasing concentrations of glycine extended gastrin-17 (G-17-GLY, 0.0001–1 μM). (b) Effects of G-17 (0.1 μM), either alone or in combination with L-365,260 (L-365, 1 μM), GV150013 (GV, 0.1 μM), SC-560 (SC, 0.1 μM), L-745,337 (L-745, 0.1 μM) or AH-23848B (AH, 1 μM). Each column represents the mean of five to seven experiments±s.e.m. (vertical bars). Significant difference from the respective control values (CON): *P<0.05; significant difference from the respective values obtained in the presence of G-17 alone: aP<0.05.
When tested alone, L-365,260 (0.001–1 μM) and GV150013 (0.001–1 μM) did not modify cell growth (not shown). However, both L-365,260 (1 μM) and GV150013 (0.1 μM) prevented the growth stimulant effect of gastrin-17 (Figure 4b). SC-560 (0.1 μM), a selective COX-1 blocker, neither affected cell growth per se (not shown), nor influenced the stimulant action of gastrin-17 (Figure 4b). Under the same conditions, cell growth was reduced by the selective COX-2 blocker L-745,337 (0.01–100 μM), with a significant effect at 100 μM (−34.3%). Since L-745,337 did not exert significant antiproliferative actions at concentrations equal or lower than 10 μM (−15.6% at 10 μM), the subinhibitory concentration of 0.1 μM was selected for subsequent interaction assays with gastrin-17. In this setting, L-745,337 partly prevented the effect of gastrin-17 on cell growth (Figure 4b). The EP4 receptor antagonist AH-23848B (1 μM) was also able to partly counteract gastrin-17-induced cell growth (Figure 4b). No significant changes in cell growth occurred with AH-23848B per se (not shown).
Effects of gastrin-17, CCK-2 receptor antagonists, COX inhibitors and EP4 receptor antagonist on cell DNA synthesis
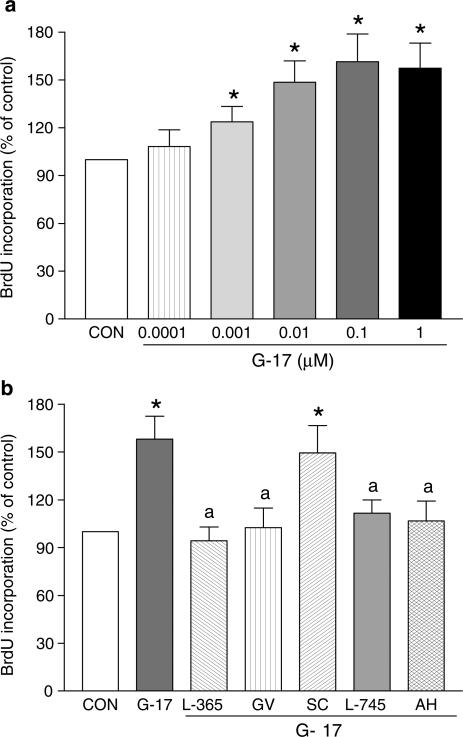
Gastrin-17 (0.0001–1 μM) concentration-dependently stimulated DNA synthesis in HT-29 cells, as indicated by BrdU incorporation, with a maximal effect at the concentration of 0.1 μM (Figure 5a). L-365,260 (1 μM), GV150013 (0.1 μM), SC-560 (0.1 μM), L-745,337 (0.1 μM) or AH-23848B (1 μM) did not affect DNA synthesis per se (not shown), whereas these inhibitors, with exception of SC-560, prevented the stimulant effect of gastrin-17 0.1 μM (Figure 5b).
Figure 5.
Evaluation of 5-bromo-2′-deoxyuridine (BrdU) incorporation in HT-29 cells. (a) Effects of increasing concentrations of gastrin-17 (G-17, 0.0001–1 μM). (b) Effects of G-17 (0.1 μM), either alone or in combination with L-365,260 (L-365, 1 μM), GV150013 (GV, 0.1 μM), SC-560 (SC, 0.1 μM), L-745,337 (L-745, 0.1 μM) or AH-23848B (AH, 1 μM). Each column represents the mean of four to five experiments±s.e.m. (vertical bars). Significant difference from the respective control values (CON): *P<0.05; significant difference from the respective values obtained in the presence of G-17 alone: aP<0.05.
Effects of gastrin-17, CCK-2 receptor antagonists and COX inhibitors on PGE2 production
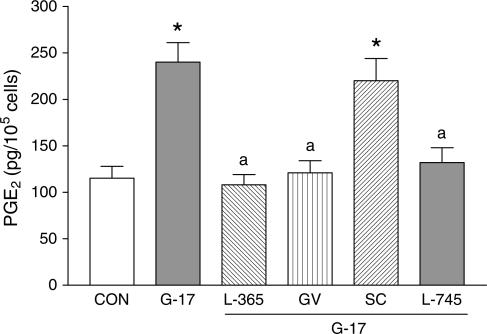
As for the experiments on cell growth, PGE2 assay was carried out following incubation of HT-29 cells with different test drugs. Under control conditions, basal PGE2 secretion was not modified by L-365,260 (1 μM), GV150013 (0.1 μM) or L-745,337 (0.1 μM), but was abolished by SC-560 (0.1 μM; not shown). Gastrin-17 (0.1 μM) significantly increased PGE2 release into culture medium. Such an effect was prevented by L-365,260 (1 μM), GV150013 (0.1 μM), or L-745,337 (0.1 μM), whereas SC-560 (0.1 μM) was without effect (Figure 6).
Figure 6.
PGE2 release from HT-29 cells following their incubation with gastrin-17 (G-17, 0.1 μM), either alone or in combination with L-365,260 (L-365, 1 μM), GV150013 (GV, 0.1 μM), SC-560 (SC, 0.1 μM) or L-745,337 (L-745, 0.1 μM). Each column represents the mean of six experiments±s.e.m. (vertical bars). Significant difference from the respective control values (CON): *P<0.05; significant difference from the respective values obtained in the presence of G-17 alone: aP<0.05.
Effects of gastrin-17 and CCK-2 receptor antagonists on COX-2 gene expression and promoter activity
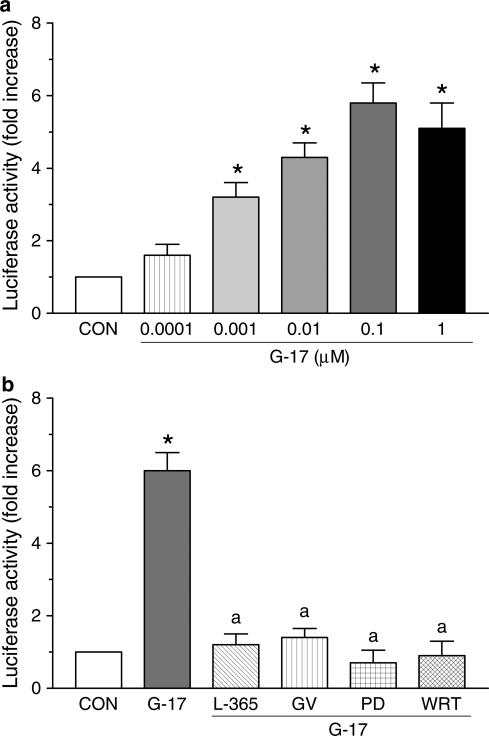
Following exposure of HT-29 cells to gastrin-17, both RT–PCR and Western blot analysis showed an enhancement of basal COX-2 expression, without concomitant changes in COX-1 expression (Figure 7). L-365,260 (1 μM) or GV150013 (0.1 μM) did not modify basal COX-1 or COX-2 expression, while preventing the effects of gastrin-17 on COX-2 induction (Figure 7a, b). The influence of gastrin-17 (0.0001–1 μM) on the transcriptional activity of COX-2 promoter was then examined in cells transiently transfected with COX-2-promoter-Luc plasmid. The peptide increased luciferase activity in a concentration-dependent manner, with a maximal effect at 0.1 μM (Figure 8a). L-365,260 (1 μM) or GV150013 (0.1 μM) did not affect the transcriptional activity of COX-2 promoter when tested alone (not shown). However, both CCK-2 receptor antagonists counteracted the enhancing action of gastrin-17 (Figure 8b).
Figure 7.
(a, b) RT–PCR analysis of mRNA expression for cyclooxygenase isoforms (COX-1, COX-2) in HT-29 cells. (a) Effects of gastrin-17 (G-17, 0.1 μM), L-365,260 (L-365, 1 μM) or G-17 plus L-365,260. (b) Effects of G-17 (0.1 μM), GV150013 (GV, 0.1 μM) or G-17 plus GV150013. M=size markers. (c) Western blot analysis showing the effects of G-17 (0.1 μM) on protein expression of cyclooxygenase isoforms. CON=control.
Figure 8.
Transcriptional activity of COX-2 promoter in HT-29 cells transiently transfected with a COX-2-promoter-Luc plasmid. (a) Effects of increasing concentrations of gastrin-17 (G-17, 0.0001-1 μM) on luciferase activity. (b) Effects of G-17 (0.1 μM), either alone or in combination with L-365,260 (L-365, 1 μM), GV150013 (GV, 0.1 μM), PD98059 (PD, 50 μM) or wortmannin (WRT, 0.1 μM), on luciferase activity. Each column represents the mean of five to six experiments±s.e.m. (vertical bars). Significant difference from the respective control values (CON): *P<0.05; significant difference from the respective value obtained in the presence of G-17 alone: aP<0.05.
Role of MAP-kinase and PI3-kinase pathways in the regulatory actions of gastrin-17 on COX-2 gene expression, promoter activity and PGE2 production
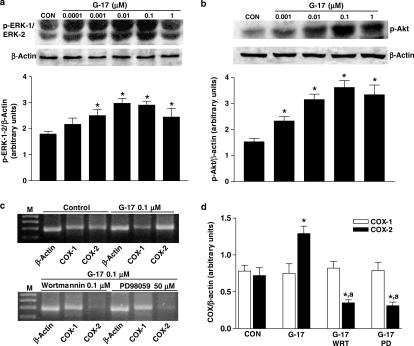
Western blot analysis showed that gastrin-17 (0.0001–1 μM) concentration-dependently enhanced the phosphorylation of kinases ERK-1/ERK-2 and Akt, belonging to the MAP-kinase and PI3-kinase signalling pathways, respectively (Figure 9a, b). RT–PCR indicated that gastrin-17-induced COX-2 expression was blocked by incubation of HT-29 cells with PD98059 (10–50 μM), a selective inhibitor of MEK, the kinase responsible for the phosphorylation of ERK-1/ERK-2. Analogously, gastrin-17-induced COX-2 expression was prevented by wortmannin (0.01–1 μM), a blocker of PI3-kinase (Figure 9c, d). In these experiments, both PD98059 and wortmannin reduced COX-2 mRNA below control levels, suggesting an involvement of ERK-1/ERK-2 and PI3-kinase in the control of basal COX-2 expression, as previously reported for other colonic and extradigestive cell lines (Shao et al., 2000; St-Germain et al., 2004). In cells transfected with COX-2-promoter-Luc plasmid, PD98059 or wortmannin inhibited the increase in luciferase activity elicited by gastrin-17 (Figure 8b). Furthermore, the increase in PGE2 release elicited by gastrin-17 0.1 μM (235.3±27.2 pg 105 cells−1; n=5) was also significantly counteracted by PD98059 50 μM (99.7±13.3 pg 105 cells−1; n=5; P<0.05) or wortmannin 0.1 μM (114.5± 17.8 pg 105 cells−1; n=5; P<0.05).
Figure 9.
(a, b) Western blot analysis showing the effects of increasing concentrations of gastrin-17 (G-17, from 0.0001 or 0.001 to 1 μM) on phosphorylation of ERK-1/ERK-2 (p-ERK-1/ERK-2) or Akt (p-Akt) in HT-29 cells. Column graphs refer to the densitometric analysis of p-ERK-1/ERK-2 or p-Akt bands normalized to the expression of β-actin. Each column represents the mean of four experiments±s.e.m. (vertical bars). Significant difference from the respective control values (CON): *P<0.05. (c, d) RT–PCR analysis showing the effects of G-17 (0.1 μM), either alone or in combination with wortmannin (WRT, 0.1 μM) or PD98059 (PD, 50 μM), on mRNA expression of cyclooxygenase isoforms (COX-1, COX-2) in HT-29 cells. M=size markers. Column graphs refer to the densitometric analysis of COX-1 or COX-2 cDNA bands normalized to the expression of β-actin. Each column represents the mean of four experiments±s.e.m. (vertical bars). Significant difference from the respective control values (CON): *P<0.05; significant difference from the respective values obtained in the presence of G-17 alone: aP<0.05.
Discussion
In the present study, evidence was obtained that in human colon cancer cells, characterized by naïve coexpression of CCK-2 receptors and COX-2 enzyme, the growth-stimulating actions of gastrin-17 can be partly ascribed to the induction of COX-2, which then increases cellular proliferative activity through generation of PGE2 and downstream recruitment of EP4 receptors.
The role played by fully processed gastrin and CCK-2 receptors in the control of colon cancer growth is currently the subject of a considerable debate, due to different reasons: (1) colon cancer cells may express the gastrin gene and produce unprocessed forms of gastrin peptide (i.e., progastrin and glycine extended gastrins), which are increasingly regarded as most relevant in the pathophysiology of colon cancer growth (Dockray et al., 2001; Singh et al., 2003); (2) gastrin-17 can exert inhibitory effects on different target cells, including colon cancer, and it has been proposed that CCK-2 receptors may mediate direct inhibitory or growth factor-dependent stimulant actions on cell growth (Varro et al., 2002); (3) some models of intestinal epithelial cells do not express CCK-2 receptors and need to be transfected to examine the effects mediated by gastrin-17 (Guo et al., 2002; Singh et al., 2003); (4) while the presence of CCK-2 receptors in primary colorectal tumours has been described (Watson et al., 2002), doubts have been cast on the functionality of these receptors (Rozengurt & Walsh, 2001). Uncertainties are further fostered by the fact that CCK-2 receptor gene encodes different splice variants and, although the presence of these molecular forms has been detected in colonic tumours, their respective relevance in the control of colon cancer growth remains unclear (Hellmich et al., 2000; Smith & Watson, 2000). However, while these arguments question the importance of gastrin-17, other authors suggest that mature gastrin peptides, acting on CCK-2 receptors via systemic or paracrine/autocrine mechanisms, are implicated in the pathophysiology of colorectal adenoma–carcinoma sequence and may contribute to regulate cell growth (Smith & Watson, 2000; Watson et al., 2002). Moreover, as in vitro cultured cell models are concerned, while some human colon cancer cell lines may lack detectable amounts of CCK-2 receptor, other cell lines are endowed with functioning CCK-2 receptors (Ishizuka et al., 1994; Smith et al., 1996).
Considering the above issues, prior to address the interactions between gastrin-17 and COX pathways, in the present study care was taken to examine the characteristics of the cellular model, in terms of CCK-2 receptor expression, constitutive induction of gastrin gene and sensitivity to different gastrin peptides. The present observation, showing that HT-29 cells are equipped with short-form CCK-2 receptors mediating proliferative actions of gastrin-17, was not unexpected since several investigations had previously demonstrated the expression of CCK-2 receptors (binding and RT–PCR) and the occurrence of CCK-2-mediated growth responses upon exposure of HT-29 cells to gastrin-17 (Ishizuka et al., 1994; Mauss et al., 1994; Smith et al., 1996; Watson et al., 2002). In line with data by Mauss et al. (1994), who did not detect gastrin by radioimmunoassay in culture medium from HT-29 cells, we did not observe gastrin mRNA expression by RT–PCR, and two distinct CCK-2 antagonists (L-365,260, GV150013) failed to affect the basal growth of these cells, suggesting that in our model the short variant of CCK-2 receptor is not activated by endogenous gastrin to stimulate cell proliferation via paracrine/autocrine loops. In our settings, HT-29 cells increased their growth rate when exposed to micromolar concentrations of G-17-GLY, a peptide known to bind specific receptor sites in the nanomolar range (Dockray et al., 2001). However, the majority of previous studies demonstrated CCK-2-mediated growth actions of gastrin-17 in HT-29 cells (Ishizuka et al., 1994; Mauss et al., 1994; Smith et al., 1996). In addition, even if our results with micromolar G-17-GLY might reflect its competition with endogenous unprocessed gastrins, we did not detect gastrin mRNA in HT-29 cells and, consistently with our findings, Stepan et al. (1999) could measure only negligible amounts of G-17-GLY in the same cell line. Overall, the gastrin gene does not appear to be transcribed in HT-29 cells, and the present growth effect of micromolar G-17-GLY is likely to result from nonspecific interaction of this peptide with CCK-2 receptors.
When considering COX pathways, PGE2 has been implicated in the pathophysiology of colon cancer (Sheng et al., 2001) and, consistently with our observations, the constitutive expression of COX-1 and COX-2 in HT-29 cells has been previously described (Yang & Frucht, 2000; Sharma et al., 2001). Therefore, in the present study, PGE2 concentration was assayed in cell culture medium. The results indicate that HT-29 cells exhibit a very low basal rate of PGE2 production and that COX-1 is likely to account for such biosynthetic activity, since PGE2 release was suppressed by SC-560, while being insensitive to L-745,337. At the same time, growth of colonic cells was not sensitive to COX-1 blockade, and L-745,337 exerted significant inhibitory effects at higher concentrations than those required to block COX-2 (Santini et al., 1999), suggesting that neither COX-1 nor COX-2 are implicated in the maintenance of basal HT-29-cell growth. In keeping with this proposal, selective COX-2 inhibitors can depress basal proliferation of colon cancer cell lines through mechanisms unrelated to COX-2 blockade (for a review see, Tegeder et al., 2001). It was also previously shown that, even if constitutively expressed, COX-2 did not produce PGE2 in HT-29 cells (Shao et al., 2000), and L-745,337 significantly decreased growth of this cell line at concentrations higher than those required for COX-2 blockade (Santini et al., 1999).
In the present study, when exposed to increasing concentrations of gastrin-17, HT-29 cells responded with significant increments of their growth and DNA synthesis, and such events were associated with a marked increase in the release of PGE2 into culture medium. Subsequent experiments suggested that such stimulant actions are related to the activation of CCK-2 receptors and induction of COX-2 activity. Four lines of evidence support this view: (1) the growth actions of gastrin-17 were antagonized by L-365,260 or GV150013, reversed by L-745,337 and unaffected by SC-560; (2) the increase in PGE2 release no longer occurred after CCK-2 receptor or COX-2 blockade, whereas it was insensitive to COX-1 inhibition; (3) incubation of HT-29 cells with gastrin-17 resulted in an enhancement of COX-2, but not COX-1, expression which was suppressed by two distinct CCK-2 antagonists (L-365,260, GV150013); (4) when HT-29 cells were transiently transfected with COX-2 promoter coupled with a luciferase reporter gene, gastrin-17 significantly enhanced the transcriptional activity of COX-2 promoter and such effect was abolished by CCK-2 receptor blockade. Overall, it can be proposed that gastrin-17 targets the promoter of COX-2 gene via naïve CCK-2 receptors in HT-29 cells and induce COX-2 activity, which contributes to the growth actions of gastrin-17 through the biosynthesis of PGE2. Although colon cancer cell growth appears to be mostly controlled by unprocessed gastrin peptides (Dockray et al., 2001), our findings support the new concept that mature gastrin can significantly contribute to the proliferation of colonic tumours endowed with CCK-2 receptor and COX-2 coexpression. In keeping with this view, hypergastrinemia has been associated with an increased risk of colorectal carcinoma, and colonic cancer tissues dissected from patients with hypergastrinemia and H. pylori infection display an upregulation of both CCK-2 receptor and COX-2 expression, with respect to normal surrounding mucosa (Thorburn et al., 1998; Hartwich et al., 2001). Of note, the concept that COX-2 pathway can be recruited by G-protein-coupled receptors to regulate relevant functions in cells of gastrointestinal tract has been highlighted in previous studies. For instance, the activation of muscarinic receptors by carbachol caused a concomitant enhancement of COX-2 expression and stimulation of PGE2 biosynthesis in HT-29 cells (Yang & Frucht, 2000). Furthermore, in a rat model of gastric injury, gastrin enhanced the mucosal integrity via COX-2 upregulation and increased PGE2 production (Komori et al., 2002).
The molecular mechanisms responsible for the growth-promoting effects of gastrin-17 have been the subject of extensive investigation. It is recognized that this peptide promotes the activation of both MAP-kinase and PI3-kinase/Akt pathways (Rozengurt & Walsh, 2001). In the present study, the incubation of HT-29 cells with gastrin-17 resulted in the activation of both ERK-1/ERK-2 and Akt. Furthermore, the blockade of ERK-1/ERK-2 phosphorylation and PI3-kinase activity reversed the stimulant actions exerted by gastrin-17 on the transcriptional function of COX-2 promoter, the expression of COX-2 and the production of PGE2. Therefore, in colon cancer cells equipped with naïve CCK-2 receptors, gastrin-17 can generate ERK- and PI3-kinase-mediated signals, which then operate on COX-2 promoter to upregulate the expression of COX-2 gene, leading to a subsequent increase in PGE2 release.
With regard to the role played by ERK-1/ERK-2, the present observations are consistent with the view that the MAP-kinase pathway is involved in the positive control of COX-2 gene transcription in various cellular systems, including colon cancer cells (Shao et al., 2000; Subbaramaiah & Dannenberg, 2003). Moreover, gastrin-17 increased COX-2 expression in rat intestinal epithelial cells stably transfected with human CCK-2 receptor (Guo et al., 2002). Considering the PI3-kinase/Akt pathway, the role of this cascade in the control of COX-2 expression is controversial. In most cases, the PI3-kinase/Akt system mediates stimulant actions on COX-2 expression (Di Popolo et al., 2000). However, in some reports, PI3-kinase activation did not influence or even downregulated the expression of this COX isoform (Weaver et al., 2001). The present study provides novel evidence that PI3-kinase/Akt can be coactivated with ERK pathway to promote the induction of COX-2 in colon cancer cells. In keeping with this view, the existence of multiple crosstalks between MAP-kinase and PI3-kinase/Akt pathways has been demonstrated, and the simultaneous activation of these transduction mechanisms in different cellular systems is described (Yart et al., 2002).
The exact contribution of COX-2-dependent prostaglandin production to cancer progression is still debated, but it is accepted that PGE2 generated within colorectal neoplasms may enhance cell growth/survival (Sheng et al., 2001). Since in the present study gastrin-17-induced COX-2 expression was coupled with an increase in PGE2 production and this event could be causally related to the stimulant action of gastrin-17 on cell growth, we attempted to identify the EP receptor mediating the effects of PGE2. RT–PCR assay showed that only the EP4 subtype is expressed in HT-29 cells, and targeting of this receptor by a specific antagonist reduced the proliferative effects of gastrin-17. These findings suggest that signalling via EP4 receptor is responsible for the COX-2-dependent effect of gastrin-17 in HT-29 cells, thus further supporting previous data on the involvement of this receptor pathway in the control of colon cancer growth (Sheng et al., 2001; Mutoh et al., 2002).
In conclusion, the present study provides evidence that, in human colon cancer cells with naïve expression of CCK-2 receptors and COX-2 isoforms, gastrin can stimulate the transcriptional activity of COX-2 gene, through ERK- and PI3-kinase/Akt-dependent transduction mechanisms. These effects then lead to downstream increments of COX-2 expression, followed by PGE2 production and EP4 receptor activation, which contribute to the growth-enhancing action exerted by gastrin-17.
Abbreviations
- ANOVA
analysis of variance
- BrdU, 5-bromo-2′-deoxyuridine, COX
cyclooxygenase
- dNTP
deoxynucleotide triphosphate mixture
- EDTA
ethylenediamine tetraacetic acid
- EGTA
ethyleneglycol-bis-(2-aminoethyl)-tetraacetic acid
- ERK-1/ERK-2
extracellular regulated kinases
- G-17-GLY
glycine extended gastrin-17
- MAP-kinase
mitogen-activated protein
- PBS
phosphate-buffered saline
- PCR
polymerase chain reaction
- PGE2
prostaglandin E2
- PI3-kinase
phosphatidylinositol-3 kinase
- RT
reverse transcription
- SDS
sodium dodecylsulphate
- s.e.m.
standard error of mean
- TBS–T, NaCl, Tris–HCl
Tween-20 buffer
References
- BELLEY A., CHADEE K. Prostaglandin E2 stimulates rat and human colonic mucin exocytosis via the EP4 receptor. Gastroenterology. 1999;117:1352–1362. doi: 10.1016/s0016-5085(99)70285-4. [DOI] [PubMed] [Google Scholar]
- DI POPOLO A., MEMOLI A., APICELLA A., TUCCILLO C., DI PALMA A., RICCHI P., ACQUAVIVA A.M., ZARRILLI R. IGF-II/IGF-I receptor pathway up-regulates COX-2 mRNA expression and PGE2 synthesis in Caco-2 human colon carcinoma cells. Oncogene. 2000;19:5517–5524. doi: 10.1038/sj.onc.1203952. [DOI] [PubMed] [Google Scholar]
- DOCKRAY G.J., VARRO A., DIMALINE R., WANG T. The gastrins: their production and biological activities. Annu. Rev. Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- DU BOIS R.N., ABRAMSON S.B., CROFFORD L., GUPTA R.A., SIMON L.S., VAN DE PUTTE L.B., LIPSKY P.E. Cyclooxygenase in biology and disease. FASEB J. 1998;12:1063–1073. [PubMed] [Google Scholar]
- GUO Y.-S., CHENG J.-Z., JIN G.-F., GUTKIND J.S., HELLMICH M.R., TOWNSEND M.T. Gastrin stimulates cyclooxygenase-2 expression in intestinal epithelial cells through multiple signaling pathways. J. Biol. Chem. 2002;277:48755–48763. doi: 10.1074/jbc.M209016200. [DOI] [PubMed] [Google Scholar]
- HARTWICH A., KONTUREK S.J., PIERZCHALSKI P., ZUCHOWICZ M., LABZA H., KONTUREK P.C., KARCZEWSKA E., BIELANSKI W., MARLICZ K., STARZYNSKA T., LAWNICZAK M., HAHN E.G. Helicobacter pylori infection, gastrin, cyclooxygenase-2, and apoptosis in colorectal cancer. Int. J. Colorectal. Dis. 2001;16:202–210. doi: 10.1007/s003840100288. [DOI] [PubMed] [Google Scholar]
- HELLMICH M.R., RUI X.-L., HELLMICH H.L., FLEMING R.Y.D., EVERS B.M., TOWNSEND C.M. Human colorectal cancers express a constitutively active cholecystokinin-B/gastrin receptor that stimulates cell growth. J. Biol. Chem. 2000;275:32122–32128. doi: 10.1074/jbc.M005754200. [DOI] [PubMed] [Google Scholar]
- HLA T., NEILSON K. Human cyclooxygenase-2 cDNA. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHIZUKA J., TOWNSEND C.M., BOLD R.J., MARTINEZ J., RODRIGUEZ M., THOMPSON J.C. Effects of gastrin on 3′,5′-cyclic adenosine monophosphate, intracellular calcium, and phosphatidylinositol hydrolysis in human colon cancer cells. Cancer Res. 1994;54:2129–2135. [PubMed] [Google Scholar]
- KOMORI M., TSUJI S., SUN W.H., TSUJII M., KAWAI N., YASUMARU M., KAKIUCHI Y., KIMURA A., SASAKI Y., HIGASHIYAMA S., KAWANO S., HORI M. Gastrin enhances gastric mucosal integrity through cyclooxygenase-2 upregulation in rats. Am. J. Physiol. 2002;283:G1368–G1378. doi: 10.1152/ajpgi.00006.2002. [DOI] [PubMed] [Google Scholar]
- MAUSS S., NIEDERAU C., HENGELS K.J. Effects of gastrin, proglumide, loxiglumide and L-365,260 on growth of human colon carcinoma cells. Anticancer Res. 1994;14:215–220. [PubMed] [Google Scholar]
- MCWILLIAMS D.F., WATSON S.A., CROSBEE D.M., MICHAELI D., SETH R. Coexpression of gastrin and gastrin receptors (CCK-B and delta CCK-B) in gastrointestinal tumour cell lines. Gut. 1998;42:795–798. doi: 10.1136/gut.42.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIYAKE A. A truncated isoform of human CCK-B/gastrin receptor generated by alternative usage of a novel exon. Biochem. Biophys. Res. Commun. 1995;208:230–237. doi: 10.1006/bbrc.1995.1328. [DOI] [PubMed] [Google Scholar]
- MUTOH M., WATANABE K., KITAMURA T., SHOJI Y., TAKAHASHI M., KAWAMORI T., TANI K., KOBAYASHI M., MARUYAMA T., KOBAYASHI K., OHUCHIDA S., SUGIMOTO Y., NARUMIYA S., SUGIMURA T., WAKABAYASHI K. Involvement of prostaglandin E receptor subtype EP4 in colon carcinogenesis. Cancer Res. 2002;62:28–32. [PubMed] [Google Scholar]
- NOBLE F., WANK S.A., CRAWLEY J.N., BRADWEJN J., SEROOGY K.B., HAMON M., ROQUES B.P. International Union of Pharmacology. XXI. Structure, distribution and functions of cholecystokinin receptors. Pharmacol. Rev. 1997;51:745–781. [PubMed] [Google Scholar]
- ROZENGURT E., WALSH J.H. Gastrin, CCK, signaling, and cancer. Annu. Rev. Physiol. 2001;63:49–76. doi: 10.1146/annurev.physiol.63.1.49. [DOI] [PubMed] [Google Scholar]
- SANTINI G., SCIULLI M.G., MARINACCI R., FUSCO O., SPOLETINI L., PACE A., RICCIARDULLI A., NATOLI C., PROCOPIO A., MACLOUF J., PATRIGNANI P. Cyclooxygenase-independent induction of p21WAF−1/cip1, apoptosis and differentiation by L-745,337, a selective PGH synthase-2 inhibitor, and salicylate in HT-29 cells. Apoptosis. 1999;4:151–162. doi: 10.1023/a:1009631204581. [DOI] [PubMed] [Google Scholar]
- SCHMITZ F., OTTE J.M., STECHELE H.U., REIMANN B., BANASIEWICZ T., FOLSCH U.R., SCHMIDT W.E., HERZIG K.H. CCK-B/gastrin receptors in human colorectal cancer. Eur. J. Clin. Invest. 2001;31:812–820. doi: 10.1046/j.1365-2362.2001.00870.x. [DOI] [PubMed] [Google Scholar]
- SHAO J., SHENG H., INOUE H., MORROW J.D., DU BOIS R.N. Regulation of constitutive cyclooxygenase-2 expression in colon carcinoma cells. J. Biol. Chem. 2000;43:33951–33956. doi: 10.1074/jbc.M002324200. [DOI] [PubMed] [Google Scholar]
- SHARMA R.A., GESCHER A., PLASTARAS J.P., LEURATTI C., SINGH R., GALLACHER-HORLEY B., OFFORD E., MARNETT L.J., STEWARD W.P., PLUMMER S.M. Cyclooxygenase-2, malondialdehyde and pyrimidopurinone adducts of deoxyguanosine in human colon cells. Carcinogenesis. 2001;22:1557–1560. doi: 10.1093/carcin/22.9.1557. [DOI] [PubMed] [Google Scholar]
- SHENG H., SHAO J., WASHINGTON M.K., DU BOIS R.N. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J. Biol. Chem. 2001;276:18075–18081. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- SINGH P., LU X., COBB S., MILLER B.T., TARASOVA N., VARRO A., OWLIA A. Progastrin1–80 stimulates growth of intestinal epithelial cells in vitrovia high-affinity binding sites. Am. J. Physiol. 2003;284:G328–G339. doi: 10.1152/ajpgi.00351.2002. [DOI] [PubMed] [Google Scholar]
- SMITH A.M., WATSON S.A. Review article: gastrin and colorectal cancer. Aliment. Pharmacol. Ther. 2000;14:1231–1247. doi: 10.1046/j.1365-2036.2000.00842.x. [DOI] [PubMed] [Google Scholar]
- SMITH J.P., STOCK E.A., WOTRING M.G., MCLAUGHLIN P.J., ZAGON I.S. Characterization of the CCK-B/gastrin-like receptor in human colon cancer. Am. J. Physiol. 1996;271:R797–R805. doi: 10.1152/ajpregu.1996.271.3.R797. [DOI] [PubMed] [Google Scholar]
- SONG I., BROWN D.R., WILTSHIRE R.N., GANTZ I., TRENT J.M., YAMADA T. The human gastrin/cholecystokinin type B receptor gene: alternative splice donor site in exon 4 generates two variant mRNAs. Proc. Natl. Acad. Sci. U.S.A. 1993;90:9085–9089. doi: 10.1073/pnas.90.19.9085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEPAN V.M., SAWADA M., TODISCO A., DICKINSON C.J. Glycine-extended gastrin exerts growth-promoting effects on human colon cancer cells. Mol. Med. 1999;5:147–159. [PMC free article] [PubMed] [Google Scholar]
- ST-GERMAIN M.E., GAGNON V., PARENT S., ASSELIN E. Regulation of COX-2 protein expression by Akt in endometrial cancer cells is mediated through NF-kappaB/IkappaB pathway. Mol. Cancer. 2004;3:7–11. doi: 10.1186/1476-4598-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUBBARAMAIAH K., DANNENBERG A.J. Cyclooxygenase 2: a molecular target for cancer prevention and treatment. Trends Pharmacol. Sci. 2003;24:96–102. doi: 10.1016/S0165-6147(02)00043-3. [DOI] [PubMed] [Google Scholar]
- TEGEDER I., PFEILSCHIFTER J., GEISSLINGER G. Cyclooxygenase-independent actions of cyclooxygenase inhibitors. FASEB J. 2001;15:2057–2072. doi: 10.1096/fj.01-0390rev. [DOI] [PubMed] [Google Scholar]
- THORBURN C.M., FRIEDMAN G.D., DICKINSON C.J., VOGELMAN J.H., ORENTRICH N., PARSONNET J. Gastrin and colorectal cancer: a prospective study. Gastroenterology. 1998;115:275–280. doi: 10.1016/s0016-5085(98)70193-3. [DOI] [PubMed] [Google Scholar]
- VARRO A., NOBLE P.J., WROBLEWSKI L.E., BISHOP L., DOCKRAY G.J. Gastrin-cholecystokininB receptor expression in AGS cells is associated with direct inhibition and indirect stimulation of cell proliferation via paracrine activation of the epidermal growth factor receptor. Gut. 2002;50:827–833. doi: 10.1136/gut.50.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON S.A., MORRIS T.M., MCWILLIAMS D.F., HARRIS J., EVANS S., SMITH A., CLARKE P.A. Potential role of endocrine gastrin in the colonic adenoma carcinoma sequence. Br. J. Cancer. 2002;87:567–573. doi: 10.1038/sj.bjc.6600509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON S.A., SMITH A.M. Hypergastrinemia promotes adenoma progression in the APC(Min-/+) mouse model of familial adenomatous polyposis. Cancer Res. 2001;6:625–631. [PubMed] [Google Scholar]
- WEAVER S.A., RUSSO M.P., WRIGHT K.L., KOLIOS G., JOBIN C., ROBERTSON D.A.F., WARD S.G. Regulatory role of phosphatidylinositol 3-kinase on TNF-α-induced cyclooxygenase 2 expression in colonic epithelial cells. Gastroenterology. 2001;120:1117–1127. doi: 10.1053/gast.2001.23257. [DOI] [PubMed] [Google Scholar]
- YANG W.L., FRUCHT H. Cholinergic receptor up-regulates COX-2 expression and prostaglandin E2 production in colon cancer cells. Carcinogenesis. 2000;21:1789–1793. doi: 10.1093/carcin/21.10.1789. [DOI] [PubMed] [Google Scholar]
- YAO M., SONG D.H., RANA B., WOLFE M.M. COX-2 selective inhibition reverses the trophic properties of gastrin in colorectal cancer. Br. J. Cancer. 2002;87:574–579. doi: 10.1038/sj.bjc.6600495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YART A., CHAP H., RAYNAL P. Phosphoinositide 3-kinases in lysophosphatidic acid signalling: regulation and cross-talk with the Ras/mitogen-activated protein kinase pathway. Biochim. Biophys. Acta. 2002;1582:107–111. doi: 10.1016/s1388-1981(02)00144-0. [DOI] [PubMed] [Google Scholar]