Abstract
α3IA (6-(4-pyridyl)-5-(4-methoxyphenyl)-3-carbomethoxy-1-methyl-1H-pyridin-2-one) is a pyridone with higher binding and functional affinity and greater inverse agonist efficacy for GABAA receptors containing an α3 rather than an α1, α2 or α5 subunit. If doses are selected that minimise the occupancy at these latter subtypes, then the in vivo effects of α3IA are most probably mediated by the α3 subtype.
α3IA has good CNS penetration in rats and mice as measured using a [3H]Ro 15-1788 in vivo binding assay.
At doses in rats that produce relatively low levels of occupancy (12%) in the cerebellum (i.e. α1-containing receptors), α3IA (30 mg kg−1 i.p.), like the nonselective partial inverse agonist N-methyl-β-carboline-3-carboxamide (FG 7142), not only caused behavioural disruption in an operant, chain-pulling assay but was also anxiogenic in the elevated plus maze, an anxiogenic-like effect that could be blocked with the benzodiazepine antagonist Ro 15-1788 (flumazenil).
Neurochemically, α3IA (30 mg kg−1 i.p.) as well as FG 7142 (15 mg kg−1 i.p.) increased the concentration of the dopamine metabolite 3,4-dihydroxyphenylacetic acid in rat medial prefrontal cortex by 74 and 68%, respectively, relative to vehicle-treated animals, a response that mimicked that seen following immobilisation stress.
Taken together, these data demonstrate that an inverse agonist selective for GABAA receptors containing an α3 subunit is anxiogenic, and suggest that since α3-containing GABAA receptors play a role in anxiety, then agonists selective for this subtype should be anxiolytic.
Keywords: GABAA receptor; anxiety; inverse agonist; benzodiazepine; anxiogenic; 3,4-dihydroxyphenylacetic acid
Introduction
The GABAA receptor is generally considered to be a pentamer comprising subunits of members of the GABAA receptor family (α1–6, β1–3, γ1–3, δ, ɛ, θ and π), with the majority of native receptors containing two α, two β and a single γ subunit (Sieghart & Sperk, 2002). In addition to the agonist (GABA) recognition site, the GABAA receptor also contains binding sites for a number of pharmacologically relevant substances such as neurosteroids, barbiturates, ethanol, convulsants, anaesthetics and benzodiazepines (Korpi et al., 2002). In light of the clinical use of benzodiazepines based upon their anxiolytic, sedative, myorelaxant, cognition impairing and anticonvulsant properties, the binding site for these compounds has been the focus of considerable attention.
Detailed analyses of recombinant GABAA receptors have established that the benzodiazepine binding site occurs at the interface of the α and γ subunits (Sieghart & Sperk, 2002). Since the predominant γ subunit occurring in native GABAA receptors is the γ2, then the benzodiazepine site pharmacology of GABAA receptors in the brain is dictated primarily by the α subunit present (McKernan & Whiting, 1996). The influence of the α subunit on benzodiazepine binding site pharmacology is best illustrated by the fact that GABAA receptors containing either an α4 or α6 subunit have essentially no affinity for classical benzodiazepines such as diazepam or lorazepam, a difference that can be solely attributed to the presence of an arginine residue in α4 and α6 subunits, which in α1, α2, α3 and α5 subunits is histidine (Wieland et al., 1992). Thus, the benzodiazepine binding site is associated with GABAA receptors containing a β and γ2 subunit in conjunction with either an α1, α2, α3 or α5 subunit, a receptor population accounting for roughly three-quarters of the total brain GABAA receptor population (McKernan & Whiting, 1996; Sieghart & Sperk, 2002).
The clinically used ‘classical' benzodiazepines (e.g. diazepam, lorazepam, flunitrazepam, alprazolam) are nonselective full agonists in that they potentiate the effects of GABA at α1-, α2-, α3- and α5-containing GABAA receptors as a result of increasing the number of channel opening events when GABA is bound (Sieghart & Sperk, 2002). This causes an increased chloride ion flux into the cell, resulting in a hyperpolarisation of the resting membrane potential, the behavioural manifestations of which are anxiolysis, sedation, myorelaxation, cognitive impairment and anticonvulsant activity (Korpi et al., 2002). On the other hand, nonselective inverse agonists, such as N-methyl-β-carboline-3-carboxamide (FG 7142) or methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate (DMCM), have the opposite effects in that they decrease the number of GABA-induced channel opening events, resulting in depolarisation and increased neuronal excitability. The opposite effects of benzodiazepine site agonists and inverse agonists are reflected at the behavioural level, since inverse agonists are anxiogenic, increase vigilance and are either convulsant in their own right or enhance the efficacy of a convulsant compound (i.e. are proconvulsant) (Haefely et al., 1993). Between the extremes of full agonism or full inverse agonism (e.g. DMCM) lie a spectrum of efficacies, which include partial agonists such as bretazenil or imidazenil, partial inverse agonists such as FG 7142 and antagonists, the prototypic example of which is Ro 15-1788 (flumazenil) (Haefely et al., 1993). With respect to Ro 15-1788, it is important to note that this compound has no effect on GABA-induced channel opening effects and therefore does not affect the resting membrane potential nor does it have marked effects in vivo (Haefely, 1988).
Recent molecular genetic approaches have begun to define which of the specific pharmacological features of benzodiazepines are associated with particular (i.e. α1-, α2-, α3- or α5- containing) subtypes of GABAA receptor (Rudolph & Möhler, 2004). For example, mice in which the α1-containing GABAA receptors are rendered insensitive to diazepam are less sensitive to the sedative effects of diazepam, establishing the role of α1-containing GABAA receptors in mediating the sedative properties of nonselective benzodiazepines (Rudolph et al., 1999; Crestani et al., 2000a; McKernan et al., 2000). Pharmacological confirmation that α1-containing GABAA receptors play a key role in mediating the sedative properties of nonselective benzodiazepines comes from observations that the α1 binding selective imidazopyridine zolpidem is hypnotic (Crestani et al., 2000a), whereas a compound lacking α1 efficacy, L-838417, has a much reduced sedation liability (McKernan et al., 2000).
While GABAA receptors containing an α1 subunit are associated with sedation and anticonvulsant activity and those containing α5 are associated with certain cognitive processes (Rudolph et al., 1999; Collinson et al., 2002; Crestani et al., 2002), the role of α3-containing GABAA receptors is less well defined. Thus, the α3 subtype does not appear to be associated with diazepam-induced changes in either sleep architecture (Kopp et al., 2003), motor performance, sedation or anticonvulsant activity (Löw et al., 2000). Moreover, although a comparison of transgenic mice containing diazepam-insensitive α2 or α3 GABAA populations suggests that the α3 subtype does not mediate the anxiolytic effects of diazepam, whereas α2 does (Löw et al., 2000), the interpretation of these behavioural data is confounded by methodological issues (Crestani et al., 2000b; Reynolds et al., 2001).
Clearly, it would be useful to resolve the relative anxiolytic contributions of the α2 and α3 subtypes pharmacologically, but as yet there are no compounds reported to have subtype selective agonism for α2- versus α3- or α3- versus α2-containing GABAA receptors (Cooke & Hamilton, 2002). However, in the present study, we describe the properties of 6-(4-pyridyl)-5-(4-methoxyphenyl)-3-carbomethoxy-1-methyl-1H-pyridin-2-one (α3IA), which has α3 subtype selective inverse agonism. Thus, α3IA possesses a degree of binding and inverse agonist efficacy selectivity for α3-containing receptors such that its in vivo effects are presumably mediated primarily through this GABAA receptor subtype. In rats, this compound not only disrupted behaviour in the chain-pulling assay but was also anxiogenic in the elevated plus maze and produced changes in the dopamine metabolite 3,4-dihydroxyphenylacetic acid (DOPAC) in the medial prefrontal cortex consistent with a stress response. These data implicate α3-containing GABAA receptors in mediating at least part of the anxiogenic effects of nonselective inverse agonists and suggest that a compound with agonist efficacy at the α3 subtype may be anxiolytic.
Methods
All animal procedures were performed in accordance with the U.K. Animals (Scientific Procedures) Act, 1986.
Drugs
Ro 15-1788 (flumazenil) and bretazenil were gifts from Hoffman La Roche (Basel, Switzerland), whereas FG-7142, DMCM, chlordiazepoxide and flunitrazepam were all obtained from Sigma-Aldrich (Gillingham, U.K.). [3H]Ro 15-1788 (70–87 Ci mmol−1) and [3H]Ro 15-4513 (20–40 Ci mmol−1) were purchased from NEN (Perkin-Elmer Life Sciences, Boston, MA, U.S.A.). α3IA was synthesised in-house as described in detail elsewhere (Collins et al., 2002).
In vitro binding
The affinity of α3IA for various human recombinant GABAA receptors (β3, γ2 plus either α1, α2, α3, α4, α5 or α6 subunits) was measured in mouse fibroblast L(tk−) cells as described in more detail elsewhere (Hadingham et al., 1993; 1996; Wafford et al., 1996). In summary, membrane preparations from cells expressing α1-, α2-, α3- or α5-containing GABAA receptors were incubated with 1.8 nM [3H]Ro 15-1788, whereas the radioligand used for α4- or α6-containing receptors was 8.0 nM [3H]Ro 15-4513. Nonspecific binding (NSB) was defined using 10 μM (final concentration) flunitrazepam for the α1, α2, α3 and α5 subtypes and 10 μM Ro 15-4513 for the α4 and α6 subtypes. IC50 values were calculated using XLfit (IDBS, Guildford, U.K.) and converted to KI values using the Cheng–Prussof equation (Cheng & Prussof, 1973) assuming respective affinities (KD values) for [3H]Ro 15-1788 of 0.92, 1.05, 0.58 and 0.45 nM at α1-, α2-, α3- or α5-containing receptors and 5.0 and 6.5 nM for [3H]Ro 15-4513 at α4- and α6-containing receptors.
In vitro efficacy measurements
Efficacy was measured versus human GABAA receptors stably expressed in mouse fibroblast L(tk−) cells using whole-cell patch clamping essentially as described in more detail elsewhere (Brown et al., 2002). Briefly, L(tk−) cells of the same type used for in vitro affinity measurements (Hadingham et al., 1993; 1996; Wafford et al., 1996) were grown as a monolayer on glass microscope coverslips and continually perfused with artificial CSF on the stage of a Nikon Diaphot inverted microscope. Cell membrane patches with resistances of around 5–10 MΩ were formed using patch pipettes with tips of around 1.5–2.5 μm and cells were voltage clamped at −20 mV using an Axopatch 200B amplifier (Axon Instr., Foster City, CA, U.S.A.) with a triple-barrelled pipette system being used to apply drugs or wash solutions. The efficacy of α3IA (made up as a 10 mM stock in DMSO with a DMSO concentration in the assay of <0.1%) was measured by preapplying increasing concentrations of the drug for 30 s, and then adding an approximate EC20 concentration of GABA for 5 s. Data were analysed in GraphPad Prism (GraphPad Software Inc., San Diego, CA, U.S.A.) with curve fitting being performed using a nonlinear least-squares method for each individual cell. From these data, maximum efficacy and EC50 were determined for each cell (Brown et al., 2002).
In vivo binding
Receptor occupancy of α3IA was measured in male Swiss Webster mice (25–30 g; B & K International, Hull, U.K.) or male Sprague–Dawley rats (250–275 g; B & K International) using in vivo binding of [3H]Ro 15-1788 as described earlier (Atack et al., 1999). Briefly, for doses of 10 and 30 mg kg−1, α3IA was suspended in vehicle (0.5% aqueous carboxymethylcellulose) at concentrations of 1 and 3 mg ml−1 for mice or 10 and 30 mg ml−1 for rats (dose volume=10 and 1 ml kg−1 for mice and rats, respectively; n=5–6/group for mice and 7–8 for rats). In order to define the levels of NSB, a separate group of animals received a dose of bretazenil (5 mg kg−1 i.p. in 100% PEG 300) sufficient to occupy all benzodiazepine binding sites. After 12 min, [3H]Ro 15-1788 (diluted 1:150 with saline) was injected via a tail vein (5 μl g−1 for mice, 1 μl g−1 for rats), and 3 min later, animals were killed and the brain removed and the cerebellum was dissected free. The spinal column was removed and the spinal cord was blown out of the column using compressed air. The cerebellum and spinal cord (representing populations of primarily α1- and α2- plus α3-containing receptors, respectively; Atack et al., 1999) were weighed and homogenised in 10 volumes of ice-cold buffer (10 mM phosphate buffer/100 mM KCl, pH 7.4) and 200 μl aliquots were filtered and washed over Whatman GF/B glass fibre filters. Washed filters were then placed into scintillation vials and 10 ml scintillation fluid added. Radioactivity retained on the filters (=membrane-bound radioactivity) was counted and % occupancy of drug-treated samples was calculated as follows:
where c.p.m.vehicle and c.p.m.NSB=average counts in vehicle- and bretazenil-treated animals.
Elevated plus maze
Male Sprague–Dawley rats (250–300 g; B & K International) were divided equally into four groups (n=18/group), which received i.p. either vehicle (0.5% carboxymethylcellulose, 1 ml kg−1; pretreatment time=15 min), 10 or 30 mg kg−1 α3IA (15 min pretreatment) or FG 7142 (30 mg kg−1, 30 min pretreatment). The shorter pretreatment time for α3IA (15 min) compared to FG 7142 (30 min) was chosen based on the rapid metabolism of α3IA (Collins et al., 2002). Separate studies showed that there is no difference in plus maze performance for control rats dosed with vehicle either 15 or 30 min prior to the assay (data not shown).
After the appropriate pretreatment interval, rats were placed on the elevated plus maze for a 5 min trial (Dawson & Tricklebank, 1995). During this time, the behaviour of the rats was monitored by closed circuit TV and analysed using Flexible Maze Software (HVS Image, Hampton, Middlesex, U.K.), which calculated the time the rats spent in various parts of the maze.
In order to establish whether the effects observed with α3IA were mediated via the benzodiazepine binding site, a second experiment was performed in which rats were divided into four groups (n=12/group) and received either vehicle alone (0.5% carboxymethylcellulose; pretreatment time=15 min), vehicle followed by an additional injection of the benzodiazepine antagonist Ro 15-1788 (flumazenil; 10 mg kg−1; pretreatment time=15 min), 30 mg kg−1 α3IA (15 min pretreatment) or 30 mg kg−1 α3IA plus Ro 15-1788 (both 15 min pretreatments).
Rat response sensitivity test
The response sensitivity test is a rodent model of sedation and/or behavioural disruption in which food-deprived rats are trained to pull a chain for access to food pellets according to a random interval 30 s schedule (Bayley et al., 1996). In this test, standard operant conditioning chambers were equipped with a chain suspended from the centre of the ceiling and connected to a micro switch. For this task, rats are maintained at 85% of their free feeding weight by postsessional feeding. Following simple initial training where one pellet is dispensed into a food magazine positioned at floor level after a single chain pull, rats are exposed to a progressively increasing, regulated probability interval schedule until the final average pellet–pellet interval was 60 s.
Male PVG rats (300–350 g; B & K International) were assigned to one of four groups, which received i.p. (dose volume=1 ml kg−1) either vehicle (0.5% carboxymethylcellulose), 10 or 30 mg kg−1 α3IA or 30 mg kg−1 FG 7142 (n=11–12/group). Immediately after injection, rats were placed individually in operant boxes and the rate of chain pulling was recorded in 4 min intervals over a total test period of 32 min. Although PVG rats were used in this particular experiment, this assay works equally well in Sprague–Dawley rats.
Neurochemical analyses
Male Sprague–Dawley rats (200–250 g; B&K International) were assigned to one of four groups. Thus, rats received injections (1 ml kg−1 i.p.) of either vehicle (0.5% methyl cellulose, 15 min pretreatment), 30 mg kg−1 α3IA (15 min pretreatment) or 15 mg kg−1 FG 7142 (30 min pretreatment), the latter being a dose above the minimum effective anxiolytic dose of 10 mg kg−1 FG 7142 (Cole et al., 1995) and previously shown to have an effect on DA turnover (Deutch & Roth, 1990). In the fourth group of animals, stress was induced by taping animals to a wire grid for 30 min. Rats were killed, brains removed and the medial prefrontal cortex dissected on ice and rapidly frozen on dry ice and stored at −70°C. Subsequently, samples of the medial prefrontal cortex were analysed for the dopamine metabolite DOPAC using high-performance liquid chromatography (HPLC) with electrochemical detection as described in more detail elsewhere (Hutson et al., 1991; 2004). Samples of the medial prefrontal cortex were homogenised in 10 volumes 0.4 M perchloric acid containing 0.1% cysteine, 0.01% sodium ethylene diaminetetraacetic acid (NaEDTA) and 0.01% sodium metabisulphite. Homogenates were centrifuged at 3000 × g for 10 min and the supernatants were injected onto a 3 μm Techsphere ODS column (4.6 mm × 7.5 cm; HPLC Technology Co. Ltd, Welwyn Garden City, U.K.) using a mobile phase of 0.07 M K2HPO4, 0.0035% NaEDTA, 0.023% octyl sodium sulphate and 12.5% methanol (pH 2.75), with a flow rate of 1 ml min−1. An Antec electrochemical detector (Presearch, Hitchin, U.K.) was used to detect DOPAC by using a working electrode potential of +0.65 V relative to the silver/silver chloride reference electrode.
Results
In vitro properties of α3IA
Table 1 shows the affinity of α3IA (the structure of which is presented in Figure 1) compared to FG 7142 for the benzodiazepine binding site of human recombinant GABAA receptors containing different α subunits. This compound has essentially no affinity for GABAA receptors containing either an α4 or α6 subunit but has modest affinity for subtypes containing either an α1, α2, α3 or α5 subunit. Of these latter subtypes, α3IA had modest binding selectivity for α3-containing receptors compared to α2 or α5 (four- to five-fold selectivity) and α1 (12-fold)-containing receptors. In comparison, and like other β-carbolines, FG 7142 has a modest binding selectivity for the α1 subtype, possessing between 3- and 20-fold higher affinity for the α1 compared to the α2, α3 and α5 subtypes.
Table 1.
Affinity of FG 7142 and α3IA for human recombinant GABAA receptors containing different α subunits stably expressed in mouse fibroblast L(tk−) cells
| Ki (nM), at GABAA receptors containing β3, γ2 plus | ||||||
|---|---|---|---|---|---|---|
| Compound | α1 | α2 | α3 | α4 | α5 | α6 |
| FG 7142 | 91±22 | 330±62 | 492±105 | N/A | 2150±725 | N/A |
| α3IA | 1029±118* | 323±22* | 82±12* | >10,000 | 410±134 | >10,000 |
N/A, not assayed. Values shown are mean±s.e.m. (n=3–4 separate determinations).
Data from Collins et al. (2002).
Figure 1.
Structure of α3IA.
In addition to a modest α3 binding selectivity, α3IA also possesses greater inverse agonist efficacy for the α3 compared to α1, α2 and α5 subtypes (Figure 2). Hence, when measured in recombinant human GABAA receptors expressed in L(tk−) cells, the modulation of the GABA EC20-induced currents at the α3 subtype (−45%) was consistent with appreciable inverse agonism at this subtype (Table 2). By comparison, the full inverse agonist DMCM produces an inverse agonist efficacy of −62% in this particular efficacy assay (Chambers et al., 2003), whereas the partial inverse agonist FG 7142 has an efficacy of −37% (Table 2). On the other hand, α3IA modulation at the α1, α2 and α5 subtypes (−31, −24 and −4%, respectively) was lower than seen with FG 7142 (−43, −34 and −30%), and is consistent with α3IA being either a lower efficacy inverse agonist or an antagonist (Figure 2 and Table 2). Not only does α3IA have greater inverse agonism at the α3 subtype compared to α1, α2 or α5 but additionally the functional affinity for α3 (EC50=70 nM; Table 2) is greater than for the α1 or α2 subtypes (EC50 values=1300 and 185 nM, respectively). Similarly, the moderate α1 binding selectivity of FG 7142 (Table 1) is reflected in a higher functional affinity at the α1 subtype (EC50=137 nM) relative to the α2, α3 and α5 subtypes (EC50 values ranging from 507 to 1439 nM; Table 2).
Figure 2.
Efficacy of α3IA measured by whole-cell patch clamping in human GABAA receptors containing β3 and γ2 subunits plus either an α1, α2, α3 or α5 subunit stably transfected in L(tk−) mouse fibroblast cells. Efficacy was measured as the ability of the compound to modulate the current produced by a concentration of GABA corresponding to 20% of the maximal response (EC20), with negative values indicating a reduction in the GABA-induced current. Values shown are mean±s.e.m. of recordings from four to seven individual cells (n=6, 5, 7 and 4 for α1, α2, α3 and α5 cells, respectively). The shaded areas represent the range of inverse agonist efficacies across subtypes for the nonselective partial inverse agonist FG 7142 (range of efficacies=−30 to −43%) or the nonselective full inverse agonist DMCM (range of efficacies=−53 to −71%; Chambers et al., 2003).
Table 2.
Efficacy of FG 7142 and α3IA for human recombinant GABAA receptors containing different α subunits stably expressed in mouse fibroblast L(tk−) cells
| Human recombinant GABAA receptors containing β3, γ2 plus | ||||
|---|---|---|---|---|
| Compound | α1 | α2 | α3 | α5 |
| FG 7142 | ||||
| Max. % modulationa | −43±2 (5) | −34±5 (4) | −37±3 (6) | −30±2 (6) |
| EC50 (nM)b | 137 | 507 | 1021 | 1439 |
| α3IA | ||||
| Max. % modulationa | −31±4 (6) | −24±4 (5) | −45±5 (7) | −4±4 (4) |
| EC50 (nM)b | 1300 | 185 | 70 | N/D |
N/D, not determined.
Mean±s.e.m. of modulation observed in each individual cell (figures within parentheses=n).
Calculated from the curve fitted through the mean data.
Occupancy of benzodiazepine binding sites by α3IA
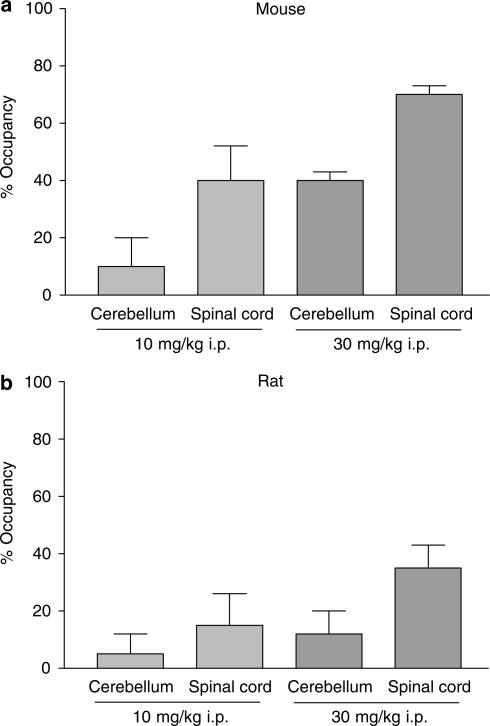
The ability of α3IA to occupy mouse and rat cerebellum and spinal cord benzodiazepine binding sites in vivo is illustrated in Figure 3. In mice, at both 10 and 30 mg kg−1 i.p., occupancy was not only dose-dependent but was also greater in the spinal cord (40±12 and 70±3% at 10 and 30 mg kg−1, respectively) relative to the cerebellum (10±10 and 40±3%) (Figure 3a). Based on these data, the dose of α3IA required to inhibit in vivo [3H]Ro 15-1788 by 50% (ID50) was 14 mg kg−1 in the spinal cord and the corresponding extrapolated value for the cerebellum was 52 mg kg−1. In rat, occupancy in both the cerebellum and spinal cord was lower dose-for-dose than that observed in mice, suggesting that the pharmacokinetics of α3IA differs between these two species. Nevertheless, there was greater occupancy in rat spinal cord (15±11 and 35±8% at 10 and 30 mg kg−1, respectively) relative to rat cerebellum (5±7 and 12±8%) (Figure 3b).
Figure 3.
Occupancy of benzodiazepine binding sites by 10 or 30 mg kg−1 α3IA (i.p. in 0.5% methyl cellulose suspension, pretreatment time=15 min) in the cerebellum and spinal cord, tissues enriched in GABAA receptors containing α1 or α2/α3 subunits, respectively, of (a) mice and (b) rats. In both species, both doses of α3IA gave greater occupancy in the spinal cord relative to the cerebellum, indicating that the in vitro binding selectivity (affinity at α2 and α3>α1) is reflected in vivo. Values shown are mean±s.e.m. (n=5–6 and 7–8/group for mice and rats, respectively).
α3IA is anxiogenic in the rat elevated plus maze
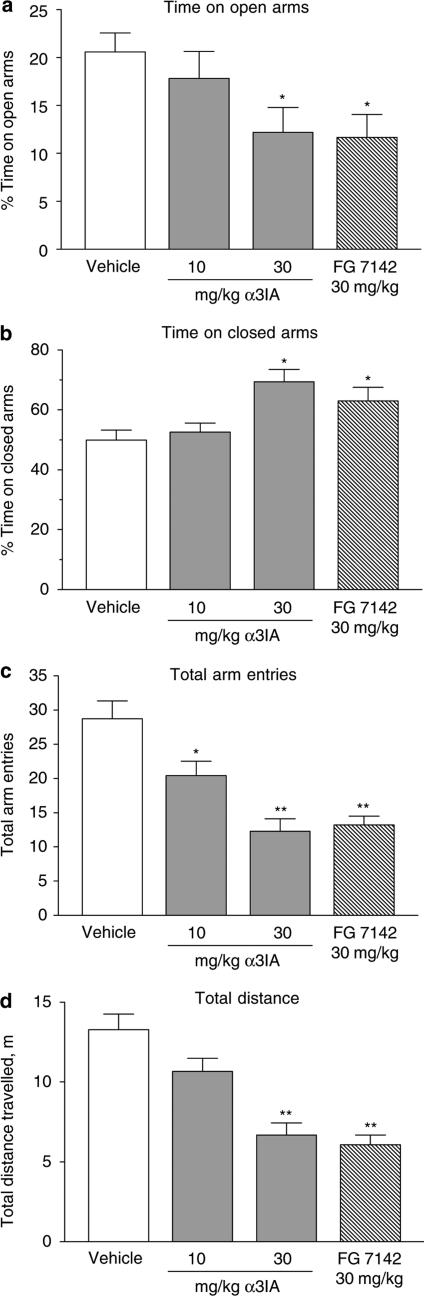
Figure 4 shows the effect of α3IA on the performance of rats in the elevated plus maze. In this task, a number of parameters are recorded during a 5 min total trial spent with the general principle being that in the novel, threatening (i.e. illuminated and elevated) environment, the less time spent on the open arms (and therefore more time spent on the closed arms) the greater the anxiety level of the animal. Using this paradigm, the nonselective inverse agonist FG 7142 produced a robust anxiogenic response, with the percent time on the open arms falling from 21±2 to 12±2%. Similarly, 30 mg kg−1 α3IA produced a significant anxiogenic response, the extent of which (percent time on open arms=12±3%) was comparable to that seen with FG 7142. The decrease in time spent on the open arms corresponded to an increase in time spent on the closed arms (Figure 4b) as well as a decrease in total arm entries (Figure 4c). In addition, both α3IA and FG 7142 produced significant decrements in the total distance traveled during the 5 min plus maze trial (Figure 4d).
Figure 4.
Effects of 10 or 30 mg kg−1 α3IA or 30 mg kg−1 FG 7142 relative to rats treated with vehicle (0.5% carboxymethylcellulose) on various parameters measured during a 5 min trial on the elevated plus maze. Parameters included: (a) percent time on the open arms; (b) percent time on the closed arms; (c) total arm entries; and (d) total distance traveled. Compared to vehicle-treated rats, both 30 mg kg−1 α3IA and FG 7142 produced significant effects on each of these parameters. Values shown are mean±s.e.m. (n=18/group). *P<0.05 and **P<0.01 relative to vehicle group using an analysis of variance followed by Dunnett's post hoc t-tests.
Verification that the anxiogenic effects of α3IA were mediated by the benzodiazepine binding site of the GABAA receptor was demonstrated in a second experiment in which the anxiogenesis produced by α3IA could be blocked by pretreatment of rats with the antagonist Ro 15-1788 (data only shown for % time on the open arms; Figure 5). That the effect of Ro 15-1788 was due to blockade of α3IA was confirmed by the observation that Ro 15-1788 alone did not affect behavioural performance, consistent with Ro 15-1788 being an antagonist in vivo.
Figure 5.
Significant anxiogenic response of α3IA (30 mg kg−1), shown here as a decrease in the time spent on the open arms, was blocked by the prototypic benzodiazepine antagonist Ro 15-1788 (flumazenil) (10 mg kg−1 i.p.), the latter of which by itself had no effect on plus maze performance. Values shown are mean±s.e.m. (n=12/group). *P<0.05 relative to vehicle/vehicle group using an analysis of variance followed by Dunnett's post hoc t-tests.
α3IA impairs performance in rat response sensitivity test
In Figure 6, the rate of responding (chain-pulling) in the response sensitivity test is shown for α3IA in comparison with vehicle and FG 7142. In vehicle-treated animals, the rate of responding was 82±8% of baseline (Figure 6a), primarily because during the 32 min total trial period performance drops off, presumably due to fatigue (Figure 6b). FG 7142 (30 mg kg−1 i.p.) significantly impaired performance in this task, as did 30 mg kg−1, but not 10 mg kg−1, α3IA. The effects of FG 7142 and 30 mg kg−1 α3IA were most noticeable at earlier points during the trial, with the effects of both compounds having essentially gone by the end of the trial.
Figure 6.
(a) Average rate of chain-pulling during a 32 min response sensitivity trial expressed as a percentage of baseline responding prior to i.p. administration of either vehicle (0.5% methyl cellulose), 10 or 30 mg kg−1 α3IA or 30 mg kg−1 FG 7142 immediately prior to commencing the trial. Values shown are mean±s.e.m. (n=11–12/group). (b) The same data presented in panel (a) broken down into 4 min time bins. Each data point represents the mean (error bars omitted for clarity; n=11–12/group). *Significantly different from vehicle using an analysis of variance followed by Dunnett's post hoc t-tests.
α3IA increases rat medial prefrontal cortex DOPAC concentrations
Following immobilisation stress, levels of the dopamine metabolite DOPAC were significantly elevated to 210±19% of control values in the rat medial prefrontal cortex (Figure 7). Administration of either α3IA (30 mg kg−1) or FG 7142 (15 mg kg−1) significantly increased DOPAC concentrations to 174±14 and 168±25% of control values, respectively.
Figure 7.
Comparison of DOPAC concentrations in medial prefrontal cortex of rats receiving immobilisation stress or i.p. injections of vehicle (0.5% carboxymethylcellulose), 30 mg kg−1 α3IA (15 min pretreatment) or 15 mg kg−1 FG 7142 (30 min pretreatment). Values shown are mean±s.e.m. (n=9–11/group). *Statistically different from vehicle using analysis of variance followed by post hoc Dunnett's t-test.
Discussion
In vivo effects of α3IA are due to inverse agonism at α3-containing GABAA receptors
α3IA has a degree of α3 versus α1, α2 and α5 binding selectivity (respective Ki values=82, 1029, 323 and 410 nM), which is reflected by the higher higher functional affinity (EC50) for the α3 subtype (70 nM at α3 versus 1300 and 185 nM at α1 and α2, respectively). In addition to this binding selectivity, α3IA has greater inverse agonist efficacy at α3- (−45% at a GABA EC20) compared to α1-, α2- or α5-containing receptors (−31, −24 and −4%, respectively). It should be noted that in a previous study, α3IA was reported to have antagonist or very weak agonist efficacy at human α1-, α2- and α5-containing GABAA receptors transiently expressed human GABAA receptors in Xenopus oocytes (Collins et al., 2002), whereas in the present study, it behaves as a weak inverse agonist at the same receptor subtypes stably expressed in a mouse fibroblast cell line. Although these differences are probably in part related to the different expression systems used, data obtained from the stably expressed receptors are derived from concentration–response curves and are presumably therefore more reliable than the efficacies measured using a single drug concentration (Collins et al., 2002). It should be further emphasised that while differences in efficacy measured in Xenopus oocytes and L(tk−) cells do occur (Chambers et al., 2002; Mitchinson et al., 2004; Szekeres et al., 2004), these differences tend to be quantitative rather than qualitative insofar as it is the magnitude of the potentiation that differs rather than the type of modulation. (i.e. robust inverse agonists in one system do not become agonists in the other and vice versa). For example, DMCM has an α5 inverse agonist efficacy of −34% in Xenopus oocytes and −57% in L(tk−) cells (Chambers et al., 2002; Szekeres et al., 2004), and comparable differences in efficacy also occur for compounds with agonist efficacy (Mitchinson et al., 2004).
α3IA was CNS penetrant in mice and rats giving dose-dependent occupancy in both species and greater occupancy in spinal cord relative to the cerebellum, reflecting the preferential localisation of α2/α3-containing receptors in the spinal cord and α1-containing receptors in the cerebellum (Atack et al., 1999), and indicating that the in vitro binding selectivity is reflected in vivo.
The extent of receptor occupancy is crucial to the interpretation of the in vivo effects. For example, despite the preponderance of α1-containing receptors in the rat brain (McKernan & Whiting, 1996; Sieghart & Sperk, 2002), the assumption is that at relatively low levels of occupancy (less than 20%) and with a low inverse agonist efficacy, the in vivo effects of α3IA would not be mediated via the α1 subtype but that the much greater occupancy and efficacy of the less populous α3 receptors would be responsible for the in vivo effects. On the other hand, at doses that produce appreciable α1 occupancy (i.e. >50%), the relatively modest inverse agonist efficacy at a predominant receptor population might be expected to outweigh the greater inverse agonist efficacy at the less abundant α3-containing receptor population. Accordingly, in the present study, in vivo effects were examined at doses (10 and 30 mg kg−1 i.p.) that gave comparatively low levels of receptor occupancy (Figure 3b).
α3IA and the nonselective inverse agonist FG 7142 have similar anxiogenic effects
FG 7142 has previously been reported to be anxiogenic not only in animals (Thiébot et al., 1988) but also in man (Dorow et al., 1983). More specifically, in the elevated plus maze, which is a widely used model of anxiety (Dawson & Tricklebank, 1995), the decreased percent time spent on the open arms and increased time spent on the closed arms observed in the present study is in agreement with previous reports (Pellow & File, 1986; Cole et al., 1995; Dawson et al., 1995). α3IA was also anxiogenic in the elevated plus maze, an effect attributable to the benzodiazepine site-mediated modulation of presumably α3-containing GABAA receptors since the benzodiazepine antagonist Ro 15-1788 (flumazenil) blocked this effect.
In the rat chain-pulling assay, both α3IA and FG 7142 caused an appreciable impairment in performance, which was time-dependent in that the greatest effects were seen 5–15 min after injection and by 30 min there was no longer a significant impairment, in agreement with pharmacokinetic data showing both compounds to have a high rate of clearance in the rat (data not shown). Although a reduced responding rate in this paradigm has been used to assess the level of sedation produced by benzodiazepine site agonists (Bayley et al., 1996), the impairments produced by α3IA and FG 7142 are unlikely to be due to sedation since FG 7142 increases, rather than decreases, attention (Sarter et al., 2001). Rather, the decreased performance presumably serves as a marker of a lack of well-being (Dawson et al., 1994). Consequently, the effects of α3IA and FG 7142 on reducing chain-pulling performance may well reflect an anxiogenic state that could manifest itself as a decrease in responding rate (Dawson et al., 1995).
GABAA receptors are implicated in the stress-induced increase in mesocortical dopamine efflux or dopamine metabolite levels, since these effects can be attenuated by benzodiazepine agonists (Fadda et al., 1978; Feenstra et al., 1995; Hutson & Barton, 1997). Moreover, FG 7142 and DMCM alone can mimic the stress-induced increase in medial prefrontal cortex dopamine turnover (Tam & Roth, 1985; Imperato et al., 1991; Hutson & Barton, 1997). In the present study, we chose DOPAC as a marker of dopamine turnover since it has been shown to be a reliable marker of stress-induced activation of the dopamine system in the prefrontal cortex (Deutch & Roth, 1990). The fact that α3IA produces an increase in medial prefrontal cortex DOPAC concentrations comparable to FG 7142 implicates α3-containing GABAA receptor subtype in the activation of medial prefrontal cortex dopamine systems, although the anatomical basis for this interaction is unclear (Hutson & Barton, 1997).
Role of α3-containing GABAA receptors in anxiety
Taken together, the data described above suggest that α3IA is anxiogenic using either behavioural (elevated plus maze or chain-pulling) or neurochemical (medial prefrontal cortex DOPAC concentration) measures, implicating α3-containing GABAA receptors in anxiety. Clearly, α3-containing GABAA receptors are not solely responsible for mediating the anxiogenic properties of benzodiazepines since evidence from transgenic mice indicate that the α2 subtype is also involved in anxiety (Löw et al., 2000). This discrepancy may, in part, be methodological (Crestani et al., 2000b; Reynolds et al., 2001) or could reflect the difference between a pharmacological versus molecular genetic approach. Further clarification of this issue would come from the use of α3IA in conjunction with α2 and α3 point mutated mice.
It is not possible to relate the anatomical distribution of α3-containing GABAA receptors to anxiety since, although the amygdala plays a key role in the association between explicit cues and conditioned fear (LeDoux, 2000; Davis & Whalen, 2001), the anatomical substrates of the presumably more diffuse cues associated with anxiety are less well understood. Nevertheless, the bed nucleus of the stria terminalis, which constitutes part of the extended amygdala, has been implicated in mediating the sustained responses to threat that are analogous to anxiety rather than fear (Walker et al., 2003). However, although the bed nucleus of the stria terminalis contains α3-containing GABAA receptors (Pirker et al., 2000), the anatomical association between this receptor subtype and anxiety is tenuous since additional GABAA receptor subtypes are also expressed in this region (Pirker et al., 2000). Similarly, while the cortex undoubtedly plays a role in the processing of sensory information that forms the basis of anxiety and α3-containing GABAA receptors have a preferential cortical localisation (Pirker et al., 2000), other GABAA receptor subtypes are also highly expressed in the cortex (Pirker et al., 2000).
As regards the activation of dopaminergic transmission in the medial prefrontal cortex, FG 7142 also elevates DOPAC concentrations in the ventral tegmental area (VTA) but not the substantia nigra (Deutch & Roth, 1990), suggesting that activation of the VTA by FG 7142 and, by analogy, α3IA may precede activation within the medial prefrontal cortex (Kaneyuki et al., 1991). However, it is not clear whether this is due to direct activation of α3-containing GABAA receptors within the VTA since, although the α3 subunit is expressed in the VTA, expression of this, and, indeed, other α subunits are relatively low (Pirker et al., 2000).
Recently, the α5 binding selective compound L-655708 has been reported to be anxiogenic on the rat elevated plus maze, implicating this GABAA receptor subtype in anxiety (Navarro et al., 2002). While this compound is an inverse agonist at the α5 subtype, it also possesses equivalent inverse agonism at the α1 subtype, and presumably, therefore, also the α2 and α3 subtypes (Casula et al., 2001). Accordingly, in the absence of receptor occupancy data, it is not clear to what extent the anxiogenic behaviour induced by L-655708 can be attributed to the α5 subtype or the more abundant α1, α2 and α3 subtypes. The possibility that the anxiogenic behaviour induced by L-655708 may not be due to the α5 subtype is consistent with observations that there is no alteration in the anxiety levels of α5 knockout mice (Collinson et al., 2002), and that a compound with inverse agonist efficacy selective for the α5 subtype is not anxiogenic (Sternfeld et al., 2004).
As a corollary to the present study, it would be expected that an α3 selective agonist would be anxiolytic. Currently, compounds have been described with selective inverse agonist efficacy for single GABAA receptor subtypes such as α3IA for the α3 subtype or compounds which are selective for the α5 subtype (Chambers et al., 2002; 2003; Sternfeld et al., 2004; Szekeres et al., 2004). As regards subtype selective agonists, selective agonist efficacy for more than one GABAA receptor subtype can be achieved with compounds such as L-838417, which is an antagonist at α1- and a partial agonist at α2-, α3- and α5-containing receptors (McKernan et al., 2000), but compounds with selective agonist efficacy for single subtypes have not been reported. More recently, a subtype-selective dihydroquinoline, ‘Compound 4', has been described, which potentiates the effects of GABA at α2- but not α1-containing GABAA receptors via a novel biding site distinct from the benzodiazepine, barbiturate, neurosteroid or loreclezole binding sites (Johnstone et al., 2004). Consistent with L-838417, the lack of α1 efficacy of this compound resulted in no sedation. On the other hand, Compound 4 was anxiolytic and while it would be tempting to ascribe this anxiolytic activity to the α2 subtype, it should be noted that efficacy of this compound at the α3 and other subtypes was not measured, and therefore the subtype selectivity of this compound remains uncertain (Johnstone et al., 2004).
Clearly, the use of genetic and pharmacological approaches act as complementary strategies for defining the role of particular GABAA receptors in mediating the specific pharmacological effects of nonselective benzodiazepine sites ligands such as diazepam. This information should then form the basis for developing compounds that selectively target individual populations of GABAA receptors and that would be expected to have novel pharmacological profiles.
Abbreviations
- α3IA
6-(4-pyridyl)-5-(4-methoxyphenyl)-3-carbomethoxy-1-methyl-1H-pyridin-2-one
- FG 7142
N-methyl-β-carboline-3-carboxamide
- DMCM
methyl-6,7-dimethoxy-4-ethyl-β-carboline-3-carboxylate
- DOPAC
3,4-dihydroxyphenylacetic acid
References
- ATACK J.R., SMITH A.J., EMMS F., MCKERNAN R.M. Regional differences in the inhibition of mouse in vivo [3H]Ro 15-1788 binding reflect selectivity for α1 versusα2 and α3 subunit-containing GABAA receptors. Neuropharmacology. 1999;20:255–262. doi: 10.1016/S0893-133X(98)00052-9. [DOI] [PubMed] [Google Scholar]
- BAYLEY P.J., BENTLEY G.D., JACKSON A., WILLIAMSON D., DAWSON G.R. Comparison of benzodiazepine (BZ) receptor agonists in two rodent activity tests. J. Psychopharmacol. 1996;10:206–213. doi: 10.1177/026988119601000305. [DOI] [PubMed] [Google Scholar]
- BROWN N., KERBY J., BONNERT T.P., WHITING P.J., WAFFORD K.A. Pharmacological characterization of a novel cell line expressing human α4β3δ GABAA receptors. Br. J. Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASULA M.A., BROMIDGE F.A., PILLAI G.V., WINGROVE P.B., MARTIN K., MAUBACH K., SEABROOK G.R., WHITING P.J., HADINGHAM K.L. Identification of amino acid residues responsible for the α5 subunit binding selectivity of L-655,708, a benzodiazepine binding site ligand at the GABAA receptor. J. Neurochem. 2001;77:445–451. doi: 10.1046/j.1471-4159.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- CHAMBERS M.S., ATACK J.R., BROMIDGE F.A., BROUGHTON H.B., COOK S., DAWSON G.R., HOBBS S.C., MAUBACH K.A., REEVE A.J., SEABROOK G.R., WAFFORD K., MACLEOD A.M. 6,7-Dihydro-2-benzothiophen-4(5H)-ones: a novel class of GABA-A α5 receptor inverse agonists. J. Med. Chem. 2002;45:1176–1179. doi: 10.1021/jm010471b. [DOI] [PubMed] [Google Scholar]
- CHAMBERS M.S., ATACK J.R., BROUGHTON H.B., COLLINSON N., COOK S., DAWSON G.R., HOBBS S.C., MARSHALL G., MAUBACH K.A., PILLAI G.V., REEVE A.J., MACLEOD A.M. Identification of a novel, selective GABAAα5 receptor inverse agonist which enhances cognition. J. Med. Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- CHENG Y.C., PRUSSOF W.H. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem. Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- COLE B.J., HILLMAN M., SEIDELMANN D., KLEWER M., JONES G.H. Effects of benzodiazepine receptor partial inverse agonists in the elevated plus maze test of anxiety in the rat. Psychopharmacology. 1995;121:118–126. doi: 10.1007/BF02245598. [DOI] [PubMed] [Google Scholar]
- COLLINS I., MOYES C., DAVEY W.B., ROWLEY M., BROMIDGE F.A., QUIRK K., ATACK J.R., MCKERNAN R.M., THOMPSON S.-A., WAFFORD K., DAWSON G.R., PIKE A., SOHAL B., TSOU N.N., BALL R.G., CASTRO J.L. 3-Heteroaryl-2-pyridones: benzodiazepine site ligands with functional selectivity for α2/α3-subtypes of human GABAA receptor-ion channels. J. Med. Chem. 2002;45:1887–1900. doi: 10.1021/jm0110789. [DOI] [PubMed] [Google Scholar]
- COLLINSON N., KUENZI F.M., JAROLIMEK W., MAUBACH K.A., COTHLIFF R., SUR C., SMITH A., OTU F.M., HOWELL O., ATACK J.R., MCKERNAN R.M., SEABROOK G.R., DAWSON G.R., WHITING P.J., ROSAHL T.W. Enhanced learning and memory and altered GABAergic synaptic transmission in mice lacking the α5 subunit of the GABAA receptor. J. Neurosci. 2002;22:5572–5580. doi: 10.1523/JNEUROSCI.22-13-05572.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOKE A.J., HAMILTON N.M. α-Subunit selective modulators of GABAA receptor function as CNS therapeutics. Expert Opin. Ther. Patents. 2002;12:1491–1501. [Google Scholar]
- CRESTANI F., KEIST R., FRITSCHY J.-M., BENKE D., VOGT K., PRUT L., BLÜTHMANN H., MÖHLER H., RUDOLPH U. Trace fear conditioning involves hippocampal α5 GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTANI F., MARTIN J.R., MÖHLER H., RUDOLPH U. Mechanism of action of the hypnotic zolpidem in vivo. Br. J. Pharmacol. 2000a;131:1251–1254. doi: 10.1038/sj.bjp.0703717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTANI F., MARTIN J.R., MÖHLER H., RUDOLPH U. Resolving differences in GABAA receptor mutant mouse studies. Nat. Neurosci. 2000b;3:1059. doi: 10.1038/80553. [DOI] [PubMed] [Google Scholar]
- DAVIS M., WHALEN P.J. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- DAWSON G.R., BAYLEY P., CHANNELL S., IVERSEN S.D. A comparison of the effects of the novel muscarinic receptor agonists L-689,660 and AF102B in tests of reference and working memory. Psychopharmacology. 1994;113:361–368. doi: 10.1007/BF02245210. [DOI] [PubMed] [Google Scholar]
- DAWSON G.R., CRAWFORD S.P., COLLINSON N., IVERSEN S.D., TRICKLEBANK M.D. Evidence that the anxiolytic-like effects of chlordiazepoxide on the elevated plus maze are confounded by increases in locomotor activity. Psychopharmacology. 1995;118:316–323. doi: 10.1007/BF02245961. [DOI] [PubMed] [Google Scholar]
- DAWSON G.R., TRICKLEBANK M.D. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol. Sci. 1995;16:33–36. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- DEUTCH A.Y., ROTH R.H. The determinants of stress-induced activation of the prefrontal cortical dopamine system. Prog. Brain Res. 1990;85:367–402. doi: 10.1016/s0079-6123(08)62691-6. [DOI] [PubMed] [Google Scholar]
- DOROW R., HOROWSKI R., PASCHELKE G., AMIN M. Severe anxiety induced by FG 7142, a β-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- FADDA F., ARGIOLAS A., MELIS M.R., TISSARI A.H., ONALI P.L., GESSA G.L. Stress-induced increase in 3,4-dihydroxyphenylacetic acid (DOPAC) levels in the cerebral cortex and in N. accumbens: Reversal by diazepam. Life Sci. 1978;23:2219–2224. doi: 10.1016/0024-3205(78)90207-2. [DOI] [PubMed] [Google Scholar]
- FEENSTRA M.G.P., BOTTERBLOM M.H.A., VAN UUM J.F.M. Novelty-induced increase in dopamine release in the rat prefrontal cortex in vivo: inhibition by diazepam. Neurosci. Lett. 1995;189:81–84. doi: 10.1016/0304-3940(95)11456-7. [DOI] [PubMed] [Google Scholar]
- HADINGHAM K.L., GARRETT E.M., WAFFORD K.A., BAIN C., HEAVENS R.P., SIRINATHSINGHJI D.J.S., WHITING P.J. Cloning of cDNAs encoding the human γ-aminobutyric acid type A receptor α6 subunit and characterization of the pharmacology of α6-containing receptors. Mol. Pharmacol. 1996;49:253–259. [PubMed] [Google Scholar]
- HADINGHAM K.L., WINGROVE P., LE BOURDELLES B., PALMER K.J., RAGAN C.I., WHITING P.J. Cloning of cDNA sequences encoding human α2 and α3 γ-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant α1-, α2-, α3, and α5-containing human γ-aminobutyric acidA receptors. Mol. Pharmacol. 1993;43:970–975. [PubMed] [Google Scholar]
- HAEFELY W. The preclinical pharmacology of flumazenil. Eur. J. Anaesthesiol. (Suppl.) 1988;2:25–36. [PubMed] [Google Scholar]
- HAEFELY W.E., MARTIN J.R., RICHARDS J.G., SCHOCH P. The multiplicity of actions of benzodiazepine receptor ligands. Can. J. Psychiatry. 1993;38:S102–S108. [PubMed] [Google Scholar]
- HUTSON P.H., BARTON C.L. L-701,324, a glycine/NMDA receptor antagonist, blocks the increase of cortical dopamine metabolism by stress and DMCM. Eur. J. Pharmacol. 1997;326:127–132. doi: 10.1016/s0014-2999(97)85406-4. [DOI] [PubMed] [Google Scholar]
- HUTSON P.H., BRISTOW L.J., THORN L., TRICKLEBANK M.D. R-(+)-HA-966, a glycine/NMDA receptor antagonist selectively blocks the activation of the mesolimbic dopamine system by amphetamine. Br. J. Pharmacol. 1991;103:2037–2044. doi: 10.1111/j.1476-5381.1991.tb12372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTSON P.H., PATEL S., JAY M.T., BARTON C.L. Stress-induced increase of cortical dopamine metabolism: attenuation by a tachykinin NK1 receptor antagonist. Eur. J. Pharmacol. 2004;484:57–64. doi: 10.1016/j.ejphar.2003.10.057. [DOI] [PubMed] [Google Scholar]
- IMPERATO A., STEFANO P.-A., CASOLINI P., ANGELUCCI L. Changes in brain dopamine and acetylcholine release during and following stress are independent of the pituitary–adrenocortical axis. Brain Res. 1991;538:111–117. doi: 10.1016/0006-8993(91)90384-8. [DOI] [PubMed] [Google Scholar]
- JOHNSTONE T.B.C., HOGENKAMP D.J., COYNE L., SU J., HALLIWELL R.F., TRAN M.B., YOSHIMURA R.F., LI W.Y., WANG J., GEE K.W. Modifying quinolone antibiotics yields new anxiolytics. Nat. Med. 2004;10:31–32. doi: 10.1038/nm967. [DOI] [PubMed] [Google Scholar]
- KANEYUKI H., YOKOO H., TSUDA A., YOSHIDA M., MIZUKI Y., YAMADA M., TANAKA M. Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain Res. 1991;557:154–161. doi: 10.1016/0006-8993(91)90129-j. [DOI] [PubMed] [Google Scholar]
- KOPP C., RUDOLPH U., KEIST R., TOBLER I. Diazepam-induced changes on sleep and the EEG spectrum in mice: role of the α3-GABAA receptor subtype. Eur. J. Neurosci. 2003;17:2226–2230. doi: 10.1046/j.1460-9568.2003.02651.x. [DOI] [PubMed] [Google Scholar]
- KORPI E.S., GRÜNDER G., LÜDDENS H. Drug interactions at GABAA receptors. Prog. Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- LEDOUX J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LÖW K., CRESTANI F., KEIST R., BENKE D., BRÜNIG I., BENSON J.A., FRITSCHY J.-M., RÜLICKE T., BLUETHMANN H., MÖHLER H., RUDOLPH U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- MCKERNAN R.M., ROSAHL T.W., REYNOLDS D.S., SUR C., WAFFORD K.A., ATACK J.R., FARRAR S., MYERS J., COOK G., FERRIS P., GARRET L., BRISTOW L., MARSHALL G., MACAULAY A., BROWN N., HOWELL O., MOORE K.W., CARLING R.W., STREET L.J., CASTRO J.L., RAGAN C.I., DAWSON G.R., WHITING P.J. Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABAA receptor α1 subtype. Nat. Neurosci. 2000;3:587–592. doi: 10.1038/75761. [DOI] [PubMed] [Google Scholar]
- MCKERNAN R.M., WHITING P.J. Which GABAA-receptor subtypes really occur in the brain. Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- MITCHINSON A., ATACK J.R., BLURTON P., CARLING R.W., CASTRO J.L., CURLEY K.S., RUSSELL M.G.N., MARSHALL G., MCKERNAN R.M., MOORE K.W., NARQUIZIAN R., SMITH A., STREET L.J., THOMPSON S.A., WAFFORD K. 2,5-Dihydropyrazolo[4,3-c]pyridin-3-ones: functionally selective benzodiazepine binding site ligands on the GABAA receptor. Bioorg. Med. Chem. Lett. 2004;14:3441–3444. doi: 10.1016/j.bmcl.2004.04.085. [DOI] [PubMed] [Google Scholar]
- NAVARRO J.F., BURON E., MARTIN-LOPEZ M. Anxiogenic-like activity of L-655,708, a selective ligand for the benzodiazepine site of GABAA receptors which contain the alpha-5 subunit, in the elevated plus-maze test. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2002;26:1389–1392. doi: 10.1016/s0278-5846(02)00305-6. [DOI] [PubMed] [Google Scholar]
- PELLOW S., FILE S.E. Anxiolytic and angiogenic drug effects on exploratory activity in the elevated plus maze: a novel test of anxiety in the rat. Pharmacol. Biochem. Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- PIRKER S., SCHWARZER C., WIESELTHALER A., SIEGHART W., SPERK G. GABAA receptors: immunocytochemical distribution of 13 subunits in the adult brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- REYNOLDS D.S., MCKERNAN R.M., DAWSON G.R. Anxiolytic-like action of diazepam: which GABAA receptor subtype is involved. Trends Pharmacol. Sci. 2001;22:402–403. doi: 10.1016/s0165-6147(00)01773-9. [DOI] [PubMed] [Google Scholar]
- RUDOLPH U., CRESTANI F., BENKE D., BRÜNIG I., BENSON J.A., FRITSCHY J.-M., MARTIN J.R., BLUETHMANN H., MÖHLER H. Benzodiazepine actions mediated by specific γ-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- RUDOLPH U., MÖHLER H. Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 2004;44:475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429. [DOI] [PubMed] [Google Scholar]
- SARTER M., BRUNO J.P., BERNSTON G.G. Psychotogenic properties of benzodiazepine receptor inverse agonists. Psychopharmacology. 2001;156:1–13. doi: 10.1007/s002130100756. [DOI] [PubMed] [Google Scholar]
- SIEGHART W., SPERK G. Subunit composition, distribution and function of GABAA receptor subtypes. Curr. Top. Med. Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- STERNFELD F., CARLING R.W., JELLEY R.A., LADDUWAHETTY T., MERCHANT K.J., MOORE K.W., REEVE A.J., STREET L.J., O'CONNOR D., SOHAL B., ATACK J.R., COOK S., SEABROOK G., WAFFORD K., TATTERSALL F.D., COLLINSON N., DAWSON G.R., CASTRO J.L., MACLEOD A.M. Selective, orally active γ-aminobutyric acidAα5 receptor inverse agonists as cognition enhancers. J. Med. Chem. 2004;47:2176–2179. doi: 10.1021/jm031076j. [DOI] [PubMed] [Google Scholar]
- SZEKERES H.J., ATACK J.R., CHAMBERS M.S., COOK S.M., MACAULAY A.J., PILLAI G.V., MACLEOD A.M. 3,4-Dihydronaphthalen-1(2H)-ones: novel ligands for the benzodiazepine site of α5-containing GABAA receptors. Bioorg. Med. Chem. Lett. 2004;14:2871–2875. doi: 10.1016/j.bmcl.2004.03.054. [DOI] [PubMed] [Google Scholar]
- TAM S.Y., ROTH R.H. Selective increase in dopamine metabolism in the prefrontal cortex by the anxiogenic beta carboline FG 7142. Biochem. Behav. Pharmacol. 1985;34:1594–1598. doi: 10.1016/0006-2952(85)90708-7. [DOI] [PubMed] [Google Scholar]
- THIÉBOT M.-H., SOUBRIÉ P., SANGER D. Anxiogenic properties of beta-CCE and FG 7142: a review of promises and pitfalls. Psychopharmacology. 1988;94:465–463. doi: 10.1007/BF00212837. [DOI] [PubMed] [Google Scholar]
- WAFFORD K., THOMPSON S., THOMAS D., SIKELA J., WILCOX A., WHITING P.J. Functional characterization of human gamma-aminobutyric acidA receptors containing the α4 subunit. Mol. Pharmacol. 1996;50:670–678. [PubMed] [Google Scholar]
- WALKER D.L., TOUFEXIS D.J., DAVIS M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- WIELAND H.A., LÜDDENS H., SEEBURG P.H. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J. Biol. Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]