Abstract
Somatostatin is a potent inhibitor of gastric acid secretion. Its effects are mediated through five specific receptor subtypes (sst1–5), of which sst2 is dominant on the enterochromaffin-like (ECL) cell and the parietal cell. To study the paracrine mechanisms of somatostatin, the sst2-specific antagonist PRL-2903 was used.
Effects of PRL-2903 on acid secretion and release of histamine were studied in the totally isolated, vascularly perfused rat stomach. Further, the release of histamine and gastrin after bombesin, alone and in combination with PRL-2903, were studied. Results are presented as mean±standard error of the mean (s.e.m.).
PRL-2903 concentration-dependently increased the venous histamine concentration from basal 55.6±7.5 to 367±114 nM at 50 μM PRL-2903. With 10 μM PRL-2903, venous histamine output increased from baseline 6.2±0.5 to 20.9±4.9 nmol h−1; P=0.008. The combination of 520 pM gastrin and 10 μM PRL-2903 increased venous histamine output from 41.7±7.3 nmol h−1 with gastrin alone to 95.2±9.8 nmol h−1; P=0.016. Further, 10 μM PRL-2903 increased acid output from baseline 8.5±1.8 to 37.4±11 μmol h−1; P=0.017. When combined with 10 μM ranitidine, PRL-2903 did not significantly stimulate acid secretion. Bombesin/PRL-2903 increased venous histamine concentration from 50.4±14.8 to 292±64.2 nM; P=0.008, and gastrin concentration from 38.6±13.1 to 95.8±20.3 pM; P=0.037.
Endogenous somatostatin exerts a continuous restraint on histamine and gastrin release from the gastric mucosa and significantly reduces baseline acid secretion.
Keywords: D-cell, somatostatin, G-cell, gastrin, ECL cell, histamine, acid secretion, somatostatin receptor 2-antagonist
Introduction
Somatostatin was first isolated and identified as an inhibitor of growth hormone release from anterior pituitary cells (Brazeau et al., 1973). Its inhibitory effects on pancreatic release of glucagon and insulin, and gastric release of gastrin, are well known. In the stomach, somatostatin is produced by the D cells, which are closely associated with the histamine-producing enterochromaffin-like (ECL) cells in the oxyntic mucosa and with the gastrin-producing G cells in the antrum (Larsson et al., 1979). The D cells constitute about 26%, and the ECL cells 35% of the neuroendocrine cells in the human oxyntic mucosa, compared to 10 and 65%, respectively, in the rat (Simonsson et al., 1988; Sundler & Håkanson, 1991). Somatostatin is a potent inhibitor of gastric acid secretion (Makhlouf & Schubert, 1990; Varga et al., 1997). This effect of somatostatin is probably mediated by several mechanisms, both directly on the parietal cell as evidenced by the inhibition of histamine-stimulated acid secretion (Hummelt et al., 1977), and indirectly by attenuating histamine release from the oxyntic mucosa (Sandvik & Waldum, 1988; Sandvik et al., 1989; 1997). Moreover, another mechanism of action of somatostatin may be inhibition of the release of the acid secretagogue hormone gastrin (Bloom et al., 1974). The effects of somatostatin are mediated through specific somatostatin receptors (ssts). Five different sst subtypes, designated sst1–5, have been identified by molecular cloning techniques and pharmacologically characterized (Patel et al., 1994). Studies of sst messenger RNA in the rat demonstrated a widespread expression of all five subtypes throughout the gastrointestinal tract (Krempels et al., 1997). It has been shown that both the ECL cells and the parietal cells express sst2 receptors (Prinz et al., 1994; Allen et al., 2002). Several studies show that the sst2 receptor has a central role in mediating the effects of somatostatin on acid secretion. Experiments with sst2 agonists indicate that this receptor mediates a direct inhibitory effect of somatostatin on the parietal cell (Wyatt et al., 1996). Moreover, somatostatin has been found to inhibit both gastric acid secretion and histamine release in rats, mediated through the sst2 receptor (Aurang et al., 1997). In studies on sst2 receptor knockout mice, a high basal gastric acid secretion was found (Martinez et al., 1998) and a bombesin-induced inhibition of gastric acid secretion was found to be sst2 receptor-mediated (Piqueras et al., 2003).
The availability of somatostatin receptor agonists and antagonists with high specificity has contributed significantly to the understanding of the role of somatostatin in the regulation of gastric secretion. The first somatostatin receptor antagonists were synthesized in 1996 (Bass et al., 1996). A large number of linear octapeptides, some of these containing a cyclic hexapeptide core, has been synthesized and biologically characterized (Hocart et al., 1998). Many of these compounds are characterized by a phenylalanine analogue at the N-terminus and a large hydrophobic amino acid, most often Nal, at the C-terminus. The analogue PRL-2903 (also known as DC-41-33) with the sequence Fpa-c[D-Cys-Pal-D-Trp-Tle-Cys]-Nal-NH2 and a molecular weight of 1160.5 Da was the first antagonist to show selectivity for sst2 (Hocart et al., 1999). The action of PRL-2903 on the sst2 receptor has been extensively studied in several species. In the rat gastric mucosa, the inhibition of atrial natriuretic peptide release by both endogenous and exogenous somatostatin was abolished by PRL-2903 (Gower et al., 2003). Further, in hamster pancreatic islet cell suspensions PRL-2903 abolished the effect of somatostatin on intracellular Ca2+ influx and insulin secretion in the presence of arginine vasopressin (Cheng et al., 2002). By studying binding affinities of PRL-2903 on transfected cells, a highly preferential binding to hsst2 was found, and in vivo experiments, using chronic gastric fistula equipped rats, showed that PRL-2903 dose-dependently reversed the inhibitory effect of somatostatin on pentagastrin-stimulated gastric acid secretion (Rossowski et al., 1998). Moreover, PRL-2903 reversed urethane-induced somatostatin-mediated inhibition of gastric acid secretion and gastrin release (Kawakubo et al., 1999).
The present study was carried out to examine whether endogenous somatostatin, acting by a paracrine mechanism on sst2 receptors, has a significant effect on acid secretion itself and on the release of the gastric acid secretagogues gastrin and histamine. PRL-2903 was used to characterize sst2 receptor-mediated effects on these factors. The acid-secreting, isolated vascularly perfused rat stomach is very well suited for these experiments. The model maintains full control over humoral factors since the vascular perfusate is not recirculated, and secretagogues or inhibitors can be added to the perfusate in exact concentrations. Moreover, all paracrine mechanisms are intact and the release of endogenous substances may be measured in the venous effluent immediately after the gastric vascular bed.
Methods
Animals
Male Wistar rats, mean body weight 232 g (range 192–271), were purchased from Møllegaard (Skensved, Denmark). The rats were housed in wire-mesh cages at 24°C with constant humidity and a 12 : 12 h light–dark cycle, and fed ad libitum with a commercial rat diet and tap water. The animal experiments were approved by the Animal Welfare Committee of the University Hospital of Trondheim.
Totally isolated vascularly perfused rat stomach
After a 36 h fast, the rats were anesthetized with 0.25–0.30 ml per 100 g body weight of a combination of (per ml) 2.5 mg fluanison, 0.05 mg fentanyl, and 1.25 mg midazolam. Totally isolated vascularly perfused rat stomachs were prepared as previously described (Kleveland et al., 1986a). The preparations were transferred to an organ bath filled with Krebs–Ringer buffer, and perfused vascularly with a Krebs–Ringer buffer with ionized calcium at a concentration of 1.12 mmol l−1 (pH 7.25), 40 g l−1 dextran T70, 5 mmol l−1 glucose, 5 mmol l−1 pyruvate, and gassed with 96% O2 and 4% CO2 using a membrane oxygenator. In the study recording acid secretion 10% washed ovine erythrocytes was added to the vascular perfusate. Vascular perfusion rate was 2 ml min−1 and the perfusate was not recirculated. The gastric lumen was perfused 1 ml min−1 with distilled water at pH 7.0 and gassed with 100% O2. All perfusates and the organ bath were kept at 37°C. Luminal effluents were collected in 10-min portions for subsequent measurement of acid output. Venous effluents were collected on ice, immediately centrifuged and kept at −20°C until analysis for histamine and gastrin.
Experimental protocols
Five or six stomach preparations were included in each group (basal, single drug or drug combination) and for each drug concentration. Drugs were administered intravascularly through the arterial catheter.
First, the effect of PRL-2903 on baseline venous histamine release was examined, designed as a dose–response study. The purpose of this experiment was to find the maximally effective PRL-2903 concentration and to use this for further secretion studies. After a stabilization period of 40 min, the histamine response to different concentrations of the drug (0.1,1.0, 5.0, 10.0 and 50.0 μM, respectively) was studied by collecting the venous effluent in 1-min portions after drug administration and measuring the peak histamine concentration within 4 min.
Maximal histamine releasing effect was obtained with the 50 μM PRL-2903 concentration. However, when administering PRL-2903 in this concentration for the prolonged period necessary to do acid secretion studies, edema ensued and the preparations lost the acid secretion capability. The 10 μM PRL-2903 concentration, on the other hand, had no such effect and was thus used in the further studies on acid secretion and histamine release.
For acid secretion studies, 3-isobutyl-1-methylxanthine (IBMX) was added to enhance the effect of the different secretagogues. Thus, after an initial 20 min stabilization period, IBMX 50 μM was administered (Kleveland et al., 1986b), and for 40 min the stomach preparations were stimulated with 10 μM PRL-2903 or 520 pM human amidated gastrin 1–17 (G-17) alone, or a combination of both. To study the effect of endogenous somatostatin directly on the parietal cell, 10 μM PRL-2903 was administered simultaneously with the histamine-2 receptor antagonist ranitidine in a concentration of 10 μM. The luminal and venous effluents were collected in 10-min fractions throughout the 100-min perfusion period.
In a separate series of experiments, the effect on venous histamine and gastrin release of bombesin 1 nM, alone or in combination with 10 μM PRL-2903, was studied. The experimental design was similar to that described for the initial dose–response study of the PRL-2903 effect on venous histamine concentration.
Acid, histamine and gastrin analysis
Acid output was measured by titration to pH 7.0 with 1 mM NaOH using a pH meter (Radiometer, Copenhagen, Denmark). Histamine was measured in the venous effluent by a radioimmunoassay (RIA)-method (Sandvik et al., 1987) using kits purchased from Beckman Coulter (Marseille, France) with a detection limit of 1 nM histamine, and an interassay variability of 6.1%. There was no crossreactivity to any of the substances added to the perfusate. Gastrin was measured with a RIA-method described previously (Kleveland et al., 1985). Gastric acid output was expressed as μmol h−1 and histamine and gastrin as nM and pM, respectively, except for the acid secretion studies where histamine release was expressed as accumulated 1-h output of histamine.
Drugs
G-17 and IBMX were purchased from Sigma (St Louis, MO, U.S.A.), Dextran T70 from Pharmacia (Uppsala, Sweden) and bombesin from Bachem (Bubendorf, Switzerland). PRL-2903 was synthetized at the Peptide Research Laboratories, Tulane University Health Sciences Center (New Orleans, LA, U.S.A.). Ranitidine (Zantac) was purchased from GlaxoSmithKline (Brentford, Middlesex, U.K.).
Statistics
The results are presented as mean±standard error of the mean (s.e.m.). For comparison of two groups the two-tailed Mann–Whitney U-test was used. Differences between multiple groups were evaluated using the one-way nonparametric analysis of variance (ANOVA) with Kruskall–Wallis test and Dunn's test for multiple comparisons. A P-value <0.05 was considered statistically significant.
Results
Histamine release
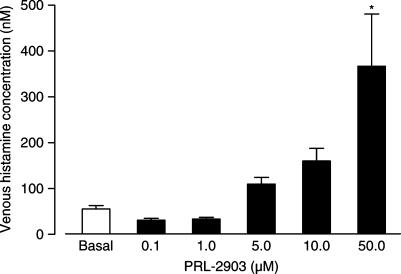
PRL-2903 induced a concentration-dependent change in venous histamine concentration from baseline 55.6±7.5 nM (mean±s.e.m.) to a maximum of 367±114 nM at 50 μM PRL-2903 (P<0.05). Although not significant at the P<0.05 level, there was a trend towards increase in venous histamine concentration also with the PRL-2903 concentrations 5.0 and 10.0 μM (Figure 1). On the other hand, for the 0.1 and 1.0 μM PRL-2903 concentrations, a slight decrease in venous histamine concentration from 55.6±7.5 to 31.2±4.0 nM, and 33.6±3.8 nM, respectively, was found (Figure 1). This decrease in venous histamine with the lower PRL-2903 concentrations was not significant.
Figure 1.
Venous histamine concentrations in isolated rat stomachs in response to increasing concentrations of PRL-2903. Results are given as mean±s.e.m., n=5–6. Significance of difference from basal is indicated by *P<0.05.
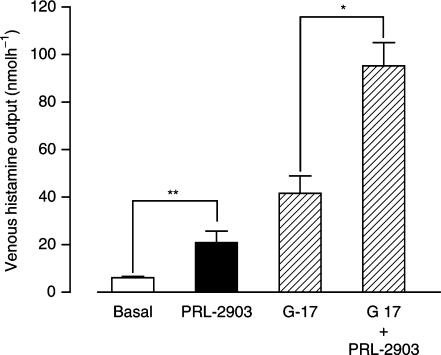
PRL-2903 in 10 μM concentration was used for the acid secretion studies. This increased the accumulated 1-h venous histamine output significantly from baseline 6.2±0.5 nmol h−1 (mean±s.e.m.) to 20.9±4.9 nmol h−1; P=0.008. Combined with 520 pM G-17, 10 μM PRL-2903 also gave a significant increase in histamine output from 41.7±7.3 nmol h−1 with gastrin alone, to 95.2±9.8 nmol h−1; P=0.016, with the combination (Figure 2).
Figure 2.
Venous histamine output from isolated rat stomachs in response to 10 μM PRL-2903, 520 pM gastrin (G-17) and combination of both. Results are expressed as mean±s.e.m., n=5. Significant differences are indicated by *P<0.05 and **P<0.01.
In this study, with 1 nM bombesin alone histamine release was only marginally lowered from basal. The combination of 1 nM bombesin and 10 μM PRL-2903, on the other hand, gave an increase in venous histamine concentration from 50.4±14.8 nM (mean±s.e.m.), with bombesin alone, to 292±64.2 nM; P=0.008, with the combination.
Acid output
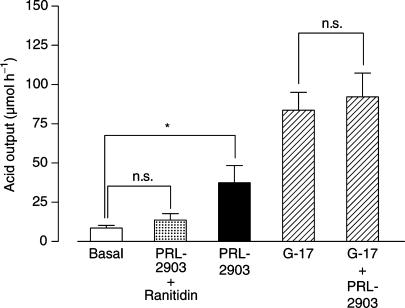
Infusion of 10 μM PRL-2903 increased acid output significantly from baseline 8.5±1.8 μmol h−1 (mean±s.e.m.) to 37.4±11 μmol h−1; P=0.017 (Figure 3). The combination of 520 pM G-17 and 10 μM PRL-2903 gave a minor and nonsignificant increase in acid output from 83.7±11.4 μmol h−1 with gastrin alone, to 92.2±15.2 μmol h−1 with the combination. The acid output with 10 μM PRL-2903 and 10 μM ranitidine given in combination was 13.6±4.1 μmol h−1, not significantly different from basal (Figure 3).
Figure 3.
Acid secretion in isolated rat stomachs in response to 10 μM PRL-2903 alone, 10 μM PRL-2903 combined with 10 μM ranitidine, 520 pM gastrin (G-17) and the combination of gastrin and PRL-2903. Results are given as mean±s.e.m., n=5–6. Significance of difference from basal is indicated by *P<0.05 and nonsignificant difference by n.s.
Gastrin release
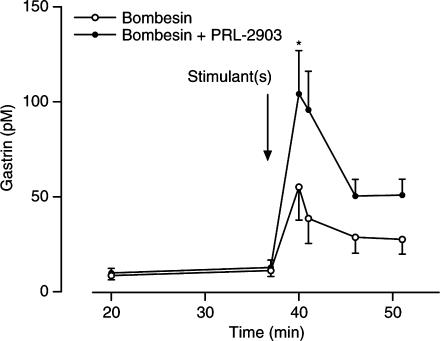
Bombesin alone increased venous gastrin concentration from basal 11.2±3.1 pM (mean±s.e.m.) to a peak concentration of 55.2±17.5 pM; P<0.05. The combination of 1 nM bombesin and 10 μM PRL-2903 at 40–41 min resulted in a significant increase in venous gastrin concentration from 38.6±13.1 pM with bombesin alone to 95.8±20.3 pM with the combination; P=0.037 (Figure 4). Throughout the rest of the stimulation period, a close to significant (P=0.056) difference in venous gastrin concentration and in gastrin output was maintained between the bombesin alone and the bombesin/PRL-2903 group. Gastrin output during the stimulation period was 0.3±0.1 pmol (mean±s.e.m.) for the bombesin group and 0.6±0.1 pmol for the bombesin/PRL-2903 group (P=0.056).
Figure 4.
Venous gastrin concentration in isolated rat stomachs in response to 1 nM bombesin, alone and in combination with 10 μM PRL-2903. Results are given as mean±s.e.m., n=5. Significant difference indicated by *P<0.05 was achieved at 40 min, during the rest of the stimulation period the difference was close to significant; P=0.056.
Discussion
Administration of PRL-2903 to the isolated, vascularly perfused, rat stomach induced a significant increase in acid secretion compared with basal. In this model, all paracrine mechanisms are preserved while there is no influence from humoral substances (like gastrin) other than those added to the perfusate. Thus, this effect on acid secretion is mediated either by an interaction with sst2 receptors on the parietal cell, on cells involved in paracrine functions that influence the local oxyntic mechanisms regulating acid secretion like, for instance, the release of ECL cell histamine, or on several of these mechanisms simultaneously. Previous studies have shown that sst2 receptors are present both on the ECL and the parietal cells (Allen et al., 2002), and sst2 receptor-specific agonists have been shown to inhibit acid secretion induced by gastric acid secretagogues acting directly on the parietal cell like the H2 receptor agonist dimaprit (Wyatt et al., 1996). In the present experiments, PRL-2903 had a slight but not significant stimulatory effect on acid secretion when given together with ranitidine. This suggests that, if any, the direct effect of endogenous somatostatin directly on the parietal cell is less important than that mediated via modulation of histamine release.
We found no significant additional effect on acid secretion when combining a maximally effective dose of gastrin with PRL-2903 even if the substance augmented histamine release. There are several possible explanations for this. Even if there was no apparent deterioration of the stomach preparations during the combined stimulation, histamine release with gastrin and 10 μM PRL-2903 in combination was massive and an effect similar, but less potent, than that of 50 μM PRL-2903 alone could have been present. Also, in this model gastrin-stimulated acid secretion exhibits a rather large interstomach variability, which could have obscured a low-grade effect of 10 μM PRL-2903 when given together with gastrin. Finally, since the histamine–-acid output dose–response curve is sigmoidal (Kleveland et al., 1987) an augmented histamine release will not necessarily produce a comparable increase in acid output.
To further clarify the effect of PRL-2903 on acid secretion, histamine was measured in the venous effluent. Numerous studies from our group and others have shown that ECL cell histamine has a pivotal role in gastrin-induced acid secretion. The results from the present study show a clear concentration–response relationship between PRL-2903 and venous histamine concentration, strongly suggesting that endogenous somatostatin exerts a continuous restraint on histamine release from the ECL cell. This is in accordance with previous results from our group using a somatostatin-neutralizing antibody. The simultaneous effect on acid secretion is a novel observation, suggesting that this restraint on histamine release may be part of an integrated acid regulatory mechanism operating at least in the fasting state.
In the intact organism, circulating gastrin is an important meal-induced acid secretagogue. In our studies on the effect of antral somatostatin on gastrin release, we chose to induce gastrin release with the gastrin-releasing peptide (GRP) analogue bombesin. GRP, originating from neurons in the antral and oxyntic parts of the stomach, is probably an important factor in the physiological regulation of gastrin release. In the present study, we found that bombesin-induced release of gastrin was potentiated by PRL-2903, suggesting that the G cells posess sst2 receptors and further indicating a sst2 receptor-mediated inhibition of gastrin release. This finding is in accordance with results from a previous study in intact animals, comparing peptide analogues relatively selective for sst2, sst3 and sst5 (Lloyd et al., 1997).
Thus, somatostatin through sst2 receptors seems to exert a restraint on the acid secretory process on several points in the stimulatory chain; that is, gastrin release from the antrum, histamine release from the oxyntic mucosa, and as inferred from other studies possibly directly on the parietal cell. In the present study, acid output was significantly augmented by the sst2 receptor antagonist PRL-2903. However, in this model, endogenous gastrin has no effect on acid secretion since gastrin acts on acid secretion solely as an humoral secretagogue, and all humoral effects are excluded by not recirculating the venous effluent. In an intact animal where the gastrin mechanism is functional, the effect of PRL-2903 on acid secretion would probably be further accentuated.
In this study a slight (although non-significant) decrease in histamine release with the 0.1 and 1.0 μM concentrations of PRL-2903 was observed. However, the compound was found to be devoid of any agonist activity when tested alone at concentrations up to 10 μM (Hocart et al., 1999).
Conclusion
Endogenous somatostatin, acting through the sst2 receptor, exerts a significant chronic restraint on gastric acid secretion. The present study shows that this effect on acid secretion is mediated through inhibition of histamine and gastrin release. Furthermore, a direct effect of endogenous somatostatin on the parietal cell may be present.
Acknowledgments
This work was financially supported by the Norwegian Research Council for Science and the Cancer Fund at the University Hospital of Trondheim. The technical assistance of Bjørn Munkvold, Anne Kristensen, Britt Schulze, Nils Nesjan and Knut Grøn is greatly appreciated.
Abbreviations
- ANOVA
analysis of variance
- Cys
cysteine
- ECL
enterochromaffine-like
- Fpa
4-fluorophenylalanine
- G-17
human amidated gastrin 1-17
- GRP
gastrin-releasing peptide
- IBMX
3-isobutyl-1-methylxanthine
- Lys
lysine
- Nal
3-(2-naphtyl) alanine
- Pal
3-pyridylalanine
- RIA
radioimmunoassay
- sst receptor
somatostatin receptor subtype
- Tlc
tert-leucine
- Trp
tryptophan
References
- ALLEN J.P., CANTY A.J., SCHULZ S., HUMPHREY P.P., EMSON P.C., YOUNG H.M. Identification of cells expressing somatostatin receptor 2 in the gastrointestinal tract of Sstr2 knockout/lacZ knockin mice. J. Comp. Neurol. 2002;454:329–340. doi: 10.1002/cne.10466. [DOI] [PubMed] [Google Scholar]
- AURANG K., WANG J., LLOYD K.C. Somatostatin inhibition of acid and histamine release by activation of somatostatin receptor subtype 2 receptors in rats. J. Pharmacol. Exp. Ther. 1997;281:245–252. [PubMed] [Google Scholar]
- BASS R.T., BUCKWALTER B.L., PATEL B.P., PAUSCH M.H., PRICE L.A., STRNAD J., HADCOCK J.R. Identification and characterization of novel somatostatin antagonists. Mol. Pharmacol. 1996;50:709–715. [PubMed] [Google Scholar]
- BLOOM S.R., MORTIMER C.H., THORNER M.O., BESSER G.M., HALL R., GOMEZ-PAN A., ROY V.M., RUSSELL R.C., COY D.H., KASTIN A.J., SCHALLY A.V. Inhibition of gastrin and gastric-acid secretion by growth-hormone release-inhibiting hormone. Lancet. 1974;2:1106–1119. doi: 10.1016/s0140-6736(74)90869-1. [DOI] [PubMed] [Google Scholar]
- BRAZEAU P., VALE W., BURGUS R., LING N., BUTCHER M., RIVIER J., GUILLEMIN R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- CHENG H., YIBCHOK-ANUN S., COY D.H., HSU W.H. SSTR2 mediates the somatostatin-induced increase in intracellular Ca(2+) concentration and insulin secretion in the presence of arginine vasopressin in clonal beta-cell HIT-T15. Life Sci. 2002;71:927–936. doi: 10.1016/s0024-3205(02)01774-5. [DOI] [PubMed] [Google Scholar]
- GOWER W.R., JR, MCCUEN R.W., ARIMURA A., COY D.H., DIETZ J.R., LANDON C.S., SCHUBERT M.L. Reciprocal paracrine pathways link atrial natriuretic peptide and somatostatin secretion in the antrum of the stomach. Regul. Pept. 2003;110:101–116. doi: 10.1016/s0167-0115(02)00206-9. [DOI] [PubMed] [Google Scholar]
- HOCART S.J., JAIN R., MURPHY W.A., TAYLOR J.E., COY D.H. Highly potent cyclic disulfide antagonists of somatostatin. J. Med. Chem. 1999;42:1863–1871. doi: 10.1021/jm9806289. [DOI] [PubMed] [Google Scholar]
- HOCART S.J., JAIN R., MURPHY W.A., TAYLOR J.E., MORGAN B., COY D.H. Potent antagonists of somatostatin: synthesis and biology. J. Med. Chem. 1998;41:1146–1154. doi: 10.1021/jm970730q. [DOI] [PubMed] [Google Scholar]
- HUMMELT H., JENNEWEIN H.M., TREICHEL R., WALDECK F. Somatostatin mode of action on gastric acid secretion in dogs. Digestion. 1977;15:151–155. doi: 10.1159/000197996. [DOI] [PubMed] [Google Scholar]
- KAWAKUBO K., COY D.H., WALSH J.H., TACHE Y. Urethane-induced somatostatin mediated inhibition of gastric acid: reversal by the somatostatin 2 receptor antagonist, PRL-2903. Life Sci. 1999;65:L115–L120. doi: 10.1016/s0024-3205(99)00340-9. [DOI] [PubMed] [Google Scholar]
- KLEVELAND P.M., HAUGEN S.E., SANDVIK A.K, WALDUM H.L. The effect of pentagastrin on the gastric secretion by the totally isolated vascularly perfused rat stomach. Scand. J. Gastroenterol. 1986a;21:379–384. doi: 10.3109/00365528609003091. [DOI] [PubMed] [Google Scholar]
- KLEVELAND P.M., HAUGEN S.E., WALDUM H.L. The preparation of bioactive 125I-gastrin, using Iodo-gen as oxidizing agent, and the use of this tracer in receptor studies. Scand. J. Gastroenterol. 1985;20:569–576. doi: 10.3109/00365528509089698. [DOI] [PubMed] [Google Scholar]
- KLEVELAND P.M., HAUGEN S.E., WALDUM H.L. Effect of pentagastrin on gastric acid secretion in the totally isolated vascularly perfused rat stomach stimulated with the phosphodiesterase inhibitor isobutyl methylxanthine. Scand. J. Gastroenterol. 1986b;21:577–584. doi: 10.3109/00365528609003103. [DOI] [PubMed] [Google Scholar]
- KLEVELAND P.M., WALDUM H.L., LARSSON H. Gastric acid secretion in the totally isolated, vascularly perfused rat stomach. Scand. J. Gastroenterol. 1987;22:705–713. doi: 10.3109/00365528709011147. [DOI] [PubMed] [Google Scholar]
- KREMPELS K., HUNYADY B., O'CARROLL A.M., MEZEY E. Distribution of somatostatin receptor messenger RNAs in the rat gastrointestinal tract. Gastroenterology. 1997;112:1948–1960. doi: 10.1053/gast.1997.v112.pm9178687. [DOI] [PubMed] [Google Scholar]
- LARSSON L.I., GOLTERMANN N., DE MAGISTRIS L., REHFELD J.F., SCHWARTZ T.W. Somatostatin cell processes as pathways for paracrine secretion. Science. 1979;205:1393–1395. doi: 10.1126/science.382360. [DOI] [PubMed] [Google Scholar]
- LLOYD K.C., AMIRMOAZZAMI S., FRIEDIK F., CHEW P., WALSH J.H. Somatostatin inhibits gastrin release and acid secretion by activating sst2 in dogs. Am. J Physiol. 1997;272:G1481–G1488. doi: 10.1152/ajpgi.1997.272.6.G1481. [DOI] [PubMed] [Google Scholar]
- MAKHLOUF G.M., SCHUBERT M.L. Gastric somatostatin: a paracrine regulator of acid secretion. Metabolism. 1990;39:138–142. doi: 10.1016/0026-0495(90)90232-2. [DOI] [PubMed] [Google Scholar]
- MARTINEZ V., CURI A.P., TORKIAN B., SCHAEFFER J.M., WILKINSON H.A., WALSH J.H., TACHE Y. High basal gastric acid secretion in somatostatin receptor subtype 2 knockout mice. Gastroenterology. 1998;114:1125–1132. doi: 10.1016/s0016-5085(98)70417-2. [DOI] [PubMed] [Google Scholar]
- PATEL Y.C., GREENWOOD M.T., WARSZYNSKA A., PANETTA R., SRIKANT C.B. All five cloned human somatostatin receptors (hSSTR1-5) are functionally coupled to adenylyl cyclase. Biochem. Biophys. Res. Commun. 1994;198:605–612. doi: 10.1006/bbrc.1994.1088. [DOI] [PubMed] [Google Scholar]
- PIQUERAS L., TACHE Y., MARTINEZ V.Somatostatin receptor type 2 mediates bombesin-induced inhibition of gastric acid secretion in mice J. Physiol. 2003549889–901.Epub 2003 Apr 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRINZ C., SACHS G., WALSH J.H., COY D.H., WU S.V. The somatostatin receptor subtype on rat enterochromaffinlike cells. Gastroenterology. 1994;107:1067–1074. doi: 10.1016/0016-5085(94)90231-3. [DOI] [PubMed] [Google Scholar]
- ROSSOWSKI W.J., CHENG B.L., JIANG N.Y., COY D.H. Examination of somatostatin involvement in the inhibitory action of GIP, GLP-1, amylin and adrenomedullin on gastric acid release using a new SRIF antagonist analogue. Br. J. Pharmacol. 1998;125:1081–1087. doi: 10.1038/sj.bjp.0702160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANDVIK A.K., BRENNA E., SUNDAN A., HOLST J.J., WALDUM H.I. Bombesin inhibits histamine release from the rat oxyntic mucosa by a somatostatin-dependent mechanism. Scand. J. Gastroenterol. 1997;32:427–432. doi: 10.3109/00365529709025076. [DOI] [PubMed] [Google Scholar]
- SANDVIK A.K., HOLST J.J., WALDUM H.L. The effect of gastrin-releasing peptide on acid secretion and the release of gastrin, somatostatin, and histamine in the totally isolated, vascularly perfused rat stomach. Scand. J. Gastroenterol. 1989;24:9–15. doi: 10.3109/00365528909092232. [DOI] [PubMed] [Google Scholar]
- SANDVIK A.K., WALDUM H.L. The effect of somatostatin on baseline and stimulated acid secretion and vascular histamine release from the totally isolated vascularly perfused rat stomach. Regul. Pept. 1988;20:233–239. doi: 10.1016/0167-0115(88)90079-1. [DOI] [PubMed] [Google Scholar]
- SANDVIK A.K., WALDUM H.L., KLEVELAND P.M., SCHULZE S.B. Gastrin produces an immediate and dose-dependent histamine release preceding acid secretion in the totally isolated, vascularly perfused rat stomach. Scand. J. Gastroenterol. 1987;22:803–808. doi: 10.3109/00365528708991918. [DOI] [PubMed] [Google Scholar]
- SIMONSSON M., ERIKSSON S., HÅKANSON R., LIND T., LØNROTH H., LUNDELL L., O'CONNOR D.T., SUNDLER F. Endocrine cells in the human oxyntic mucosa. A histochemical study. Scand. J. Gastroenterol. 1988;23:1089–1199. doi: 10.3109/00365528809090174. [DOI] [PubMed] [Google Scholar]
- SUNDLER F., HÅKANSON R.Gastric endocrine cell typing at the light microscopical level The Stomach as an Endocrine Organ 1991Amsterdam: Elsevier Science Publishers; 9–26.ed. Håkanson, R. & Sundler, F. pp. [Google Scholar]
- VARGA G., KISFALVI I., JR, KORDAS K., WONG H., WALSH J.H., SOLOMON T.E. Effect of somatostatin immunoneutralization on gastric acid and pancreatic enzyme secretion in anesthetized rats. J. Physiol. Paris. 1997;91:223–227. doi: 10.1016/s0928-4257(97)89489-2. [DOI] [PubMed] [Google Scholar]
- WYATT M.A., JARVIE E., FENIUK W., HUMPHREY P.P. Somatostatin sst2 receptor-mediated inhibition of parietal cell function in rat isolated gastric mucosa. Br. J. Pharmacol. 1996;119:905–910. doi: 10.1111/j.1476-5381.1996.tb15758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]