Abstract
This study was designed to determine whether the endothelium-dependent hyperpolarizations evoked by acetylcholine in guinea-pig carotid artery involve a cytochrome P450 metabolite and whether they are linked to the activation of two distinct populations of endothelial KCa channels, SKCa and IKCa.
The membrane potential was recorded in the vascular smooth muscle cells of the guinea-pig isolated carotid artery. All the experiments were performed in the presence of Nω-L-nitro arginine (100 μM) and indomethacin (5 μM).
Under control conditions (Ca2+: 2.5 mM), acetylcholine (10 nM to 10 μM) induced a concentration- and endothelium-dependent hyperpolarization of the vascular smooth muscle cells. Two structurally different specific blockers of SKCa, apamin (0.5 μM) or UCL 1684 (10 μM), produced a partial but significant inhibition of the hyperpolarization evoked by acetylcholine whereas charybdotoxin (0.1 μM) and TRAM-34 (10 μM), a nonpeptidic and specific blocker of IKCa, were ineffective. In contrast, the combinations of apamin plus charybdotoxin, apamin plus TRAM-34 (10 μM) or UCL 1684 (10 μM) plus TRAM-34 (10 μM) virtually abolished the acetylcholine-induced hyperpolarization.
In the presence of a combination of apamin and a subeffective dose of TRAM-34 (5 μM), the residual hyperpolarization produced by acetylcholine was not inhibited further by the addition of either an epoxyeicosatrienoic acid antagonist, 14,15-EEZE (10 μM) or the specific blocker of BKCa, iberiotoxin (0.1 μM).
In presence of 0.5 mM Ca2+, the hyperpolarization in response to acetylcholine (1 μM) was significantly lower than in 2.5 mM Ca2+. The EDHF-mediated responses became predominantly sensitive to charybdotoxin or TRAM-34 but resistant to apamin.
This investigation shows that the production of a cytochrome P450 metabolite, and the subsequent activation of BKCa, is unlikely to contribute to the EDHF-mediated responses in the guinea-pig carotid artery. Furthermore, the EDHF-mediated response involves the activation of both endothelial IKCa and SKCa channels, the activation of either one being able to produce a true hyperpolarization.
Keywords: TRAM-34; UCL 1684; 14,15-EEZE; endothelium; EDHF; Ca2+-activated potassium channel; cytochrome P450, smooth muscle
Introduction
Endothelium-dependent hyperpolarizations are blocked by the combination of two toxins, apamin, a selective inhibitor of the small-conductance Ca2+-activated K+ channel: (SKCa), and charybdotoxin, a nonselective inhibitor of intermediate-conductance Ca2+-activated K+ channel (IKCa), large-conductance Ca2+-activated K+ channel (BKCa) as well as some voltage-dependent K+ channels (KV) (Garcia et al., 1991; Garland & Plane, 1996; Corriu et al., 1996a; Chataigneau et al., 1998; Edwards et al., 1998; 2000). The effect of apamin can be mimicked by scyllatoxin, a structurally distinct SKCa inhibitor, indicating that SKCa are involved in endothelium-dependent hyperpolarizations (Corriu et al., 1996a). However, iberiotoxin cannot substitute for charybdotoxin (Zygmunt & Högestätt, 1996; Chataigneau et al., 1998) excluding a pivotal role for BKCa in many EDHF-mediated responses. Pharmacological proofs of the involvement of IKCa were obtained with maurotoxin, a blocker of both IKCa and KV, and more convincingly with recently developed specific and nonpeptidic blockers of IKCa, TRAM-34 and TRAM-39 (Wulff et al., 2000; 2001) as they fully mimic the effects of charybdotoxin, at least in rat arteries (Crane et al., 2003; Eichler et al., 2003; Hinton & Langton, 2003; Sandow et al., 2004). In most blood vessels, these two channels, SKCa and IKCa, are thought to be located on the endothelial cells and are activated by an initial increase in the endothelial intracellular calcium concentration (Busse et al., 2002).
However, many questions, concerning the effects of the inhibitors of endothelium-dependent hyperpolarizations, remain unanswered. For instance, as charybdotoxin blocks both IKCa and BKCa, it is difficult to determine whether or not this toxin prevents the hyperpolarization of the endothelial cell and/or that of the smooth muscle cells, which could have been evoked by the diffusion of an endothelium-derived diffusible factor such as an epoxyeicosatrienoic acid, an arachidonic acid metabolite generated via the cytochrome P450 monooxygenase pathway (Campbell et al., 1996; Fisslthaler et al., 1999; Edwards et al., 2001). In order to assess properly the involvement of a putative epoxyeicosatrienoic acid in endothelium-derived hyperpolarizing factor (EDHF)-mediated responses, the specific blockade of both IKCa and SKCa is required.
In addition, in many vessels, including in the guinea-pig carotid artery, each toxin alone produces no or minor inhibition of EDHF-mediated responses while the toxin combination abolishes the responses (Corriu et al., 1996a; Chataigneau et al., 1998). Two interpretations of these findings are possible. Endothelial SKCa and IKCa channels could be activated similarly with each system capable of generating a “full” response. Alternatively, and as previously suggested, the endothelial channel could be a novel channel, possibly a heteromultimer composed of IK1 and SK1-3 α-subunits, which requires the presence of both toxins in order to be inhibited (Zygmunt et al., 1997; Ding & Triggle, 2000; Ding et al., 2003). Indeed, in a heterologous expression system, the SK3 α-subunit, known to be expressed in endothelial cells and thought to be involved in EDHF-mediated responses (Burnham et al., 2002; Ding et al., 2003; Eichler et al., 2003; Taylor et al., 2003), can interact with SK1 and SK2 α-subunits to form heteromeric SKCa channels (Ishii et al., 1997; Monaghan et al., 2004). Similarly, IK1 α-subunit could form heteromultimers with corresponding SK1, SK2 or SK3 products (Castle, 1999), although it is not known whether or not these resulting heteromers are formed in native cells.
The purpose of the present work was to further clarify the respective roles of IKCa and SKCa in the endothelium-dependent hyperpolarizations evoked by acetylcholine in the guinea-pig carotid artery. This was made possible using the recently synthesized nonpeptide inhibitors of IKCa and SKCa, TRAM-34 (a clotrimazole derivative, Wulff et al., 2000) and UCL 1684 (a dequalinium-related compound, Campos-Rosa et al., 2000). Also, the possible involvement of an arachidonic acid metabolite generated via the cytochrome P450 monooxygenase pathway was investigated using the recently described epoxyeicosatrienoic acid antagonist, 14,15-EEZE (Gauthier et al., 2002). A preliminary account of these findings has been presented to the BPS (Gluais et al., 2003; Edwards et al., 2004).
Methods
Male Hartley guinea-pigs (250–300 g) were killed with an overdose of pentobarbitone (200 mg kg−1, i.p.) and the internal carotid arteries with their branches were dissected free. Segments of artery (1 cm in length) were cleaned of adherent connective tissues and pinned down to the bottom of an organ chamber (0.5 ml in volume) superfused at a constant flow (2 ml min−1 and 37°C) with modified Krebs–Ringer bicarbonate solution of the following composition (in mM): NaCl 118.3, KCl 4.7, CaCl2 2.5, MgSO4 1.2, KH2PO4 1.2, NaHCO3 25, glucose 11.1 and ethylenediamine tetra-acetic acid (EDTA) 0.026 (buffered with 95% O2 and 5% CO2, pH 7.4). In some experiments, referred to as low-calcium experiments, the concentration of CaCl2 was 0.5 mM. Transmembrane potentials were recorded from the adventitial side of the internal carotid arteries with glass capillary microelectrodes (tip resistance of 30 to 90 MΩ) filled with KCl (3 M) and connected to the headstage of a recording amplifier (World Precision Instrument (intra 767), New Haven, CT, U.S.A.); an Ag/AgCl pellet, in contact with the bathing solution and directly connected to the amplifier, served as the reference electrode. The signal was continuously monitored on an oscilloscope (Gould DSO 405, Valley view, OH, U.S.A.) and recorded using a pClamp software (Axon instrument, Foster City, CA, U.S.A.). Successful impalements were signalled by a sudden negative drop in potential from the baseline (zero potential reference) followed by a stable negative potential for at least 3 min. The acetylcholine-induced hyperpolarizations that, in this artery, are fully endothelium-dependent (Corriu et al., 1996a, 1996b) were analysed using pClampfit (Axon instrument, Foster City, CA, U.S.A.) and hyperpolarization values are expressed as the maximal amplitude between resting membrane potential and the membrane potential in the presence of the hyperpolarizing drugs (peak amplitude). Drugs were added by continuous superfusion via the Krebs solution reservoir. The preincubation time was at least 20 min with the various inhibitors studied.
Drugs
The following drugs were used: acetylcholine, indomethacin, Nω-nitro-L-arginine, 6,10-diaza-3(1,3),8(1,4)-dibenzena-1,5(1,4)-diquinolinacyclodecaphane (UCL 1684; Sigma, La Verpillère, France); synthetic charybdotoxin, apamin and iberiotoxin (Latoxan, Rosans, France); 1-[(2-chlorophenyl) diphenylmethyl]-1H-pyrazole (TRAM-34) and 1,3-dihydro-1-[2-hydroxy-5-(trifluoromethyl)phenyl]-5-(trifluoromethyl)-2H-benzimidazol-2-one (NS1619) were generous gifts from Dr H. Wulff (University of California Irvine, CA, U.S.A.) and Dr S.P. Olesen (Neurosearch Laboratory, Glostrup, Denmark), respectively; 14, 15-epoxyeicosa-5(Z)-enoic acid (14, 15 EEZE) was synthesized in the department of Biochemistry of the University of Texas Southwestern Medical School (Dallas, U.S.A.) and cromakalim by the Institut de Recherches Servier (Suresnes, France). The drugs were prepared as concentrated stock solutions and subsequently diluted with the Krebs solution. Indomethacin was dissolved in deionized water and an equimolar concentration of Na2CO3, 14, 15 EEZE and cromakalim were dissolved in ethanol (final concentration: 1%). UCL 1684, TRAM-34 and NS1619 were dissolved in DMSO (final concentrations: 1.1, 1 and 0.1%, respectively). All other drugs were dissolved in deionized water.
Statistics
Data are shown as mean±s.e.m.; n indicates the number of cells in which membrane potential was recorded. Comparisons versus control were performed statistically using an analysis of variance (ANOVA1 or ANOVA2 followed by the Dunnett's t-test or Bonferroni post-tests) or by use of Student's t-test for paired or unpaired observations, as appropriate. Differences were considered to be statistically significant when the P-value was less than 0.05.
Results
All the experiments were performed in the presence of Nω-nitro-L-arginine (100 μM) and indomethacin (5 μM) in order to inhibit nitric oxide synthases and cyclooxygenases, respectively.
Charybdotoxin and apamin
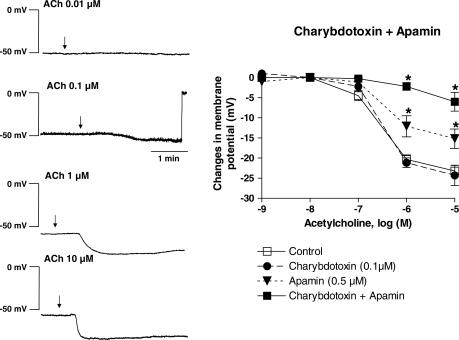
The resting membrane potential of the vascular smooth muscle cells was −51.0±0.9 mV (n=41). Noncumulative addition of acetylcholine (1 nM–10 μM) induced a concentration-dependent hyperpolarization, which was maximal at the highest concentration tested and reached 23.2±1.4 mV (Figure 1).
Figure 1.
Acetylcholine (ACh) concentration-dependently induced an endothelium dependent hyperpolarization in the guinea-pig isolated carotid artery. Effects of charybdotoxin and apamin. Left: Original traces; Right: Concentration–response curves to acetylcholine (1 nM to 10 μM) obtained in control conditions and in presence of charybdotoxin (0.1 μM), apamin (0.5 μM) and their combination. Data are shown as mean±s.e.m. (n=2–3 for acetylcholine 1 and 10 nM and 4 to 7 for acetylcholine 0.1 to 10 μM, and indicates the number of different animals from which the arteries were taken). The asterisks indicate a statistically significant difference versus the control values (P<0.05). Besides, for the two same concentrations of acetylcholine (1 and 10 μM), the charybdotoxin plus apamin group is significantly different from the two other groups (charybdotoxin alone and apamin alone) and the apamin group is significantly different from the charybdotoxin group.
The presence of apamin (0.5 μM) did not significantly affect the resting membrane potential whereas charybdotoxin (0.1 μM) alone or in combination with apamin produced a small but statistically significant depolarization (Table 1). The endothelium-dependent hyperpolarization was not modified by the presence of charybdotoxin at any of the acetylcholine concentrations tested. In contrast, apamin partially but significantly inhibited the hyperpolarization induced by 1 and 10 μM of acetylcholine. The combination of charybdotoxin plus apamin was significantly more effective than apamin alone and abolished the endothelium-dependent hyperpolarization to acetylcholine at virtually all the concentrations tested (Figure 1).
Table 1.
Resting membrane potential of guinea-pig carotid artery smooth muscle cells
| Drugs | Membrane potential in mV (cell number) | |
|---|---|---|
| Ca2+: 2.5 mM | Ca2+: 0.5 mM | |
| Control | −51.0±0.9 (41) | −44.9±1.1# (16) |
| Apamin (0.5 μM) | −50.7±0.9 (37) | −45.6±1.0# (15) |
| Charybdotoxin (0.1 μM) | −46.8±1.2* (35) | −40.9±1.1#,* (16) |
| Apamin+charybdotoxin | −45.7±0.9* (38) | −38.0±2.5#,* (4) |
| TRAM-34 (10 μM) | −50.1±1.5 (23) | −43.2±0.6# (16) |
| TRAM-34 (10 μM)+apamin | −45.1±1.1* (14) | |
| UCL 1684 (1 μM) | −54.2±1.3 (9) | |
| UCL 1684 (1 μM)+TRAM-34 (10 μM) | −51.9±1.6 (8) | |
| UCL 1684 (10 μM) | −48.6±1.6 (4) | |
| UCL 1684 (10 μM)+TRAM-34 (10 μM) | −46.2±1.2* (19) | |
| TRAM-34 (5 μM) | −50.2±1.2 (21) | |
| TRAM-34 (5 μM)+apamin | −50.4±1.0 (35) | |
| TRAM-34 (5 μM)+apamin+EEZE (10 μM) | −54.9±1.5§ (16) | |
| TRAM-34 (5 μM)+apamin+IbTX (0.1 μM) | −44.2±1.2§ (21) | |
Data are shown as mean±s.e.m. The asterisk indicates a statistically significant difference with controls. The # sign indicates a difference between 2.5 and 0.5 mM Ca2+ while the § sign indicates a statistically significant difference versus TRAM-34 (5 μM)+apamin (P<0.05). EEZE: 14,15-epoxyeicosa(Z)-enoic acid; IbTX: iberiotoxin.
TRAM-34
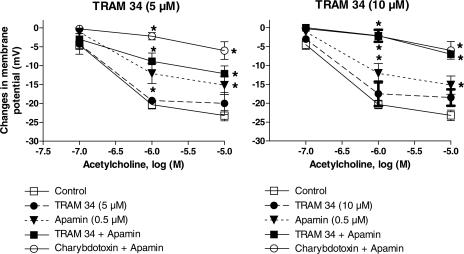
TRAM-34 (5 or 10 μM), a nonpeptidic and selective blocker of IKCa, did not significantly affect the resting membrane potential of the vascular smooth muscle cells whereas the highest concentration of TRAM-34 (10 μM) in combination with apamin produced a small but significant reduction of the resting membrane potential (Table 1).
TRAM-34 alone, at either 5 or 10 μM, did not affect the hyperpolarizations in response to acetylcholine (Figure 2). The combination of apamin plus TRAM-34 (5 μM) partially inhibited the hyperpolarizations but this inhibition was not significantly different from that evoked by acetylcholine in the presence of apamin alone (Figure 2). However, the combination of apamin plus TRAM-34 (10 μM) virtually abolished the hyperpolarization to acetycholine. The inhibition produced by this combination was significantly different from that induced by apamin alone and was undistinguishable from that produced by the combination of apamin plus charybdotoxin (Figure 2).
Figure 2.
Acetylcholine (0.1–10 μM)-induced endothelium-dependent hyperpolarization in the guinea-pig isolated carotid artery. Effects of TRAM-34 (5 and 10 μM) and apamin (0.5 μM). Left: TRAM-34 (5 μM). Data are shown as mean±s.e.m. (n=3 to 9 and indicates the number of different animals from which the blood vessels were taken). The asterisks indicate a statistically significant difference versus the control values (P<0.05). Besides, for the two same concentrations of acetylcholine (1 and 10 μM), the charybdotoxin plus apamin group is significantly different from the three other groups (TRAM-34 , apamin and TRAM-34+apamin) and the apamin group as well as the apamin+TRAM-34 groups are significantly different from the TRAM 34 group. Right: TRAM-34 (10 μM; n=3 to 7). The asterisks indicate a statistically significant difference versus the control values (P<0.05). Besides, for the two same concentrations of acetylcholine (1 and 10 μM), the charybdotoxin+apamin and the apamin+TRAM-34 groups are significantly different from the two other groups (TRAM-34 and apamin, alone) and the apamin group is significantly different from the TRAM 34 group.
UCL 1684
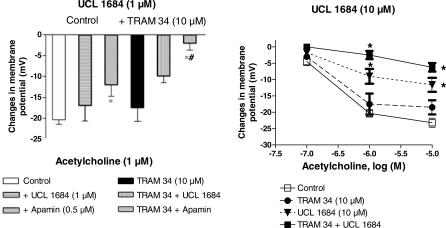
UCL 1684 (1 or 10 μM), a nonpeptidic and selective blocker of SKCa, did not affect the resting membrane potential of the vascular smooth muscle cells whereas in combination with TRAM-34 (10 μM), UCL 1684 (10 μM) produced a small but significant reduction of the resting membrane potential (Table 1).
UCL 1684 at 1 μM did not induce any significant changes in the concentration–response to acetylcholine (Figure 3). At 10 μM, UCL 1684 produced a partial but significant inhibition of the hyperpolarization, an effect which was similar to that evoked by apamin alone.
Figure 3.
Acetylcholine-induced endothelium-dependent hyperpolarization in the guinea-pig isolated carotid artery. Effects of UCL 1634 and TRAM-34 (10 μM). Left: UCL 1684 (1 μM) and acetylcholine (1μM); Data are shown as mean±s.e.m. (n=3 to 5 and indicates the number of different animals from which the blood vessels were taken). The asterisks indicate a statistically significant difference versus the control values (P<0.05). The sign # indicates a statistically significant difference between UCL 1684 and apamin. Right: UCL 1684 (10 μM) and acetylcholine (0.1–10 μM, n=3 to 7). The asterisks indicate a statistically significant difference versus the control values (P<0.05). Besides, for the two same concentrations of acetylcholine (1 and 10 μM), the TRAM-34+UCL 1684 group is significantly different from TRAM-34 group and, for acetylcholine 1 μM, this combination is significantly different from the UCL 1684 group. Additionally, the UCL 1684 group is significantly different from the TRAM 34 group.
The combination of UCL 1684 (1 μM) plus TRAM-34 (10 μM) significantly inhibited the hyperpolarization induced by acetylcholine (Figure 3) but this effect was significantly less than the inhibition evoked by the combination of apamin plus charybdotoxin. The combination of 10 μM UCL 1684 plus 10 μM of TRAM-34 further inhibited the endothelium-dependent hyperpolarization to acetylcholine. The inhibition achieved was then indistinguishable from that produced by either the combination of apamin plus charybdotoxin or that of TRAM-34 (10 μM) plus apamin (Figure 3).
Selectivity of TRAM-34 and UCL 1684
Cromakalim (10 μM), the opener of K-ATP channel, produced a large and sustained hyperpolarization of resting membrane potential of the smooth muscle cells (25.7±2.9 mV, n=3). In contrast, NS1619 (10 μM), an activator of BKCa (Olesen et al., 1994), produced a small but measurable hyperpolarization of the vascular smooth muscle cells (2.2±0.6 mV, n=3). TRAM-34 (10 μM), UCL 1684 (10 μM) and their combination did not affect the hyperpolarization produced by either cromakalim (26.0±1.0, 20.7±6.7 and 28.0±4.0 mV, n=3, respectively), or NS1619 (1.8±0.2, 2.0±0.6, 1.3±0.7 mV, n=3, respectively).
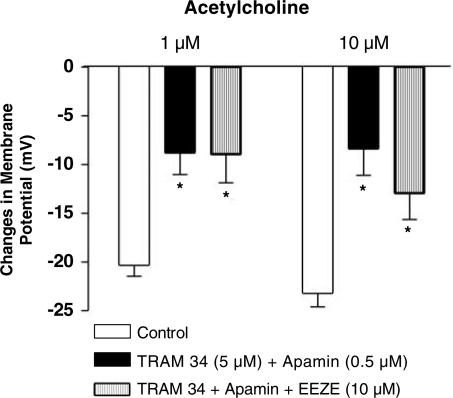
In the presence of the combination of TRAM-34 (5 μM) and apamin, the addition of 14,15-EEZE (10 μM), an antagonist of epoxyeicosatrienoic acid (Gauthier et al., 2002), produced a small but significant hyperpolarization of the smooth muscle cells while the addition of iberiotoxin (0.1 μM) produced a significant depolarization (Table 1). The residual endothelium-dependent hyperpolarization to acetylcholine (1 and 10 μM) observed in the presence of the combination of TRAM-34 (5 μM) and apamin was not significantly affected by the addition 14,15-EEZE (Figure 4). Similarly, the remaining hyperpolarization observed in presence of TRAM-34 (5 μM) plus apamin was not inhibited and was even significantly increased in the presence of iberiotoxin (acetylcholine 1 μM: 8.8±2.2 and 21.2±4.0 mV, n=6 and 4, in presence of TRAM-34 plus apamin and TRAM-34 plus apamin+iberiotoxin, respectively, P<0.05).
Figure 4.
Acetylcholine (1 and 10 μM)-induced endothelium-dependent hyperpolarization in the presence of the combination of TRAM-34 (5 μM) plus apamin (0.5 μM). Effect of 14,15-EEZE (10 μM). Data are shown as mean±s.e.m. (n=3 and indicates the number of different animals from which the blood vessels were taken). The asterisks indicate a statistically significant difference versus the control values (P<0.05).
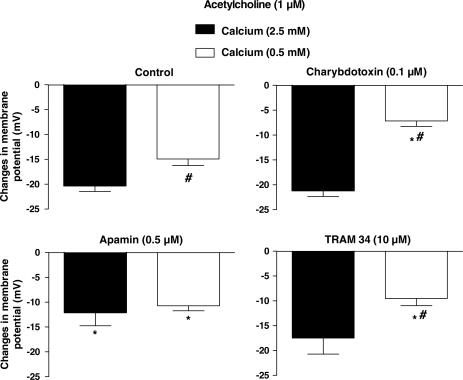
Low calcium
In physiological salt solution containing 0.5 mM Ca2+, the smooth muscle was significantly depolarized when compared to that observed in control solution (2.5 mM Ca2+; Table 1). Furthermore, the hyperpolarization produced by acetylcholine (1 μM) was significantly smaller than that observed in presence of 2.5 mM Ca2+.
In the presence of 0.5 mM Ca2+, charybdotoxin (0.1 μM) or TRAM-34 (10 μM) produced a significant inhibition of the acetylcholine (1 μM)-induced hyperpolarization (Figure 5) while in 2.5 mM Ca2+ neither charybdotoxin nor TRAM-34 affected the cholinergic responses (Figure 1). In the presence of charybdotoxin or TRAM-34, the amplitude of the hyperpolarizations evoked by acetylcholine was significantly smaller in 0.5 mM than in 2.5 mM Ca2+ (Figure 5).
Figure 5.
Acetylcholine (1 μM)-induced endothelium-dependent hyperpolarization in the presence of either 2.5 or 0.5 mM Ca2+. Effects of apamin (0.5 μM), charybdotoxin (0.1 μM) or TRAM-34 (5 μM). Data are shown as mean±s.e.m. (n=5–6 and indicates the number of different animals from which the vessels were taken). The asterisk indicates a statistically significant difference between control and the presence of a potassium channel blocker while the # sign indicates a difference between 2.5 and 0.5 mM Ca2+ (P<0.05).
In the presence of either 2.5 or 0.5 mM of Ca2+, apamin (0.5 μM) produced a significant inhibition of the hyperpolarization evoked by acetylcholine (1 μM). However, in the presence of apamin, the amplitude of the acetylcholine-induced hyperpolarizations, as observed in 0.5 and 2.5 mM Ca2+, were not significantly different (Figures 1 and 5).
In the presence of the combination of charybdotoxin plus apamin, the acetylcholine-induced hyperpolarization was abolished under both Ca2+ conditions (acetylcholine 1 μM: 2.2±1.0 and −0.3±0.9 mV, n=5 and 4, in 2.5 and 0.5 mM Ca2+, respectively).
Discussion
This study confirms that, in the guinea-pig carotid artery, SKCa and IKCa independently but simultaneously play a key role in the concentration-dependent and endothelium-dependent hyperpolarization in response to acetylcholine.
UCL 1684 and TRAM-34, two nonpeptide blockers of SKCa and IKCa, respectively (Campos-Rosa et al., 2000; Wulff et al., 2000), fully mimicked the effects of apamin and charybdotoxin. However, the micromolar concentrations of TRAM-34 and UCL 1684, required in the present study to block the endothelium-dependent hyperpolarizations fully, were significantly higher than the concentrations reported to be effective in the early publications demonstrating the activity of these agents (TRAM-34 and the cloned IK1 channel: Kd of 20 to 25 nM; UCL 1684 and SKCa channel: IC50 of 4 to 10 nM, in rat chromaffin cells or in rabbit blood cells; Dunn, 1999; Campos-Rosa et al., 2000; Malik-Hall et al., 2000; Wulff et al., 2000; 2001). In functional work dedicated to the study of endothelium-dependent hyperpolarizations, the range of concentration used, if higher than that reported in the cellular assays, was still lower than that required in the present study (Andersson et al., 2000; Eichler et al., 2003; Hinton & Langton, 2003; Crane et al., 2003).
In the guinea-pig carotid artery, a high concentration of TRAM-34 may have been required to inhibit the endothelium-dependent hyperpolarization because, besides the activation of SKCa and IKCa, an additional mechanism is involved. It is well established that epoxyeicosatrienoic acids, products of cytochrome P450 monooxygenases, are EDHFs in coronary arteries (see Busse et al., 2002). TRAM-34 is a derivative of clotrimazole, a potent inhibitor of both IKCa and cytochrome P450, which has been designed to show minimum inhibitory activity toward cytochrome P450 monooxygenase (inactive up to 10 μM; Alvarez et al., 1992; Castle, 1999; Wulff et al., 2000; 2001). Nevertheless, the high concentration used in the present study may have jeopardized the selectivity of TRAM-34. In order to test this hypothesis, a subthreshold concentration of TRAM-34 (5 μM, in combination with apamin) was studied in the presence or absence of either the epoxyeicosatrienoic acid inhibitor, 14,15-EEZE, a selective blocker of 11,12 epoxyeicosatrienoic acid (Gauthier et al., 2002), or iberiotoxin, the selective blocker of BKCa, the potassium channel target of epoxyeicosatrienoic acids on smooth muscle cells (Campbell et al., 1996; Fisslthaler et al., 1999; Quilley & McGiff, 2000; Edwards et al., 2001). However, neither the presence of 14,15-EEZE or iberiotoxin produced any additional inhibitory effects. Paradoxically, in the presence of iberiotoxin, the hyperpolarization was enhanced. This enhanced hyperpolarization observed in presence of iberiotoxin could be linked, at least in part, to the 6 mV depolarization produced by the toxin. In the guinea-pig carotid artery, the absolute value of the endothelium-dependent hyperpolarization is correlated with the value of the resting membrane potential (Chataigneau et al., 1998).
It could still be argued firstly that, since epoxyeicosatrienoic acids activate smooth muscle BKCa, they could induce relaxation and repolarization of contracted arteries but not the true hyperpolarization of the smooth muscle cells, and secondly that, in guinea-pig arteries, TRAM-34, at the concentration of 5 μM, could have already fully blocked the cytochrome P450 monooxygenase, but not IKCa, hence explaining the absence of any further inhibitory effects of either 14,15-EEZE or iberiotoxin. As this work involves the measurement of membrane potential and includes neither functional studies of vessel tone nor the measurement of cytochrome P450 activity, these two points cannot be refuted with the data shown in the present paper. However, earlier studies have shown that, in guinea-pig carotid, coronary and basilar arteries various inhibitors of cytochrome P450 monooxygenase did not affect endothelium-dependent relaxation and/or hyperpolarizations and that epoxyeicosatrienoic acids, up to 3 μM, failed to produce relaxation and/or hyperpolarizations (Corriu et al., 1996b, Chataigneau et al., 1998; Eckman et al., 1998; Petersson et al., 1998; Yamanaka et al., 1998). Taken into conjunction, all these studies strongly suggest that, in guinea-pig arteries, the endothelial production of epoxyeicosatrienoic acids does not play a major role in the endothelium-dependent hyperpolarization elicited by acetylcholine.
Furthermore, as TRAM-34 and UCL 1684 (either alone or combination) did not affect the robust hyperpolarization in response to the KATP channel opener cromakalim, nor the small hyperpolarization to the BKCa channel opener, NS 1619 (Olesen et al., 1994), the two compounds can be considered, under the present experimental conditions, to act solely as specific blockers of IKCa and SKCa, respectively. The differences in potency could be attributed to species differences since previous functional studies were performed in rat while the present work was performed in guinea-pig.
The two structurally different specific inhibitors of SKCa, the bee venom peptide apamin and the nonpeptide dequalinium derivative, UCL 1684 (Campos-Rosa et al., 2000), per se produced a partial inhibition of the hyperpolarization. In contrast, the inhibitors of IKCa, the scorpion venom peptide charybdotoxin and the nonpeptide clotrimazole derivative TRAM-34 (Wulff et al., 2000) were each completely ineffective alone. The inhibitory effects of the two structurally different SKCa blockers confirm a predominant role for SKCa in the resting guinea-pig carotid artery (Chataigneau et al., 1998) and are in agreement with previous studies performed in other arteries such as the rabbit and rat mesenteric arteries (Murphy & Brayden, 1995; Chen & Cheung, 1997, Crane et al., 2003) as well as the bovine coronary artery (Drummond et al., 2000).
In the rat brain cortex, the binding of [125I]charybdotoxin is increased in a dose-dependent manner by apamin (Zygmunt et al., 1997) suggesting that a novel channel with the two binding sites could be expressed. The endothelial potassium channel, responsible for EDHF-mediated responses, could be a heteromultimer composed of both SK and IK1 α-subunits since these subunits can theoretically coassemble (Castle, 1999). Both IKCa and SKCa are activated by a rise in intracellular Ca2+, but IK1, when first cloned and characterized, was described as a unique KCa channel with a very high affinity for Ca2+ (Joiner et al., 1997). Even if this report has been tempered by more recent results, IK1 still appears slightly more sensitive to Ca2+ (Kd=0.1–0.3 μM; Ishii et al., 1997; Joiner et al., 1997; Logsdon et al., 1997; Ghanshani et al., 2000) than SK1 (Kd=0.7 μM; Xia et al., 1998), SK2 (Kd=0.6 μM; Hirschberg et al., 1998) or SK3 (Kd=0.6 μM; Barfod et al., 2001) channels. The principal mechanism that sustains the opening of endothelial KCa channels, following agonist stimulation, is the capacitive Ca2+ entry elicited by the depletion of Ca2+ stores (Marchenko & Sage, 1993; Sedova et al., 2000; Nilius & Droogmans, 2001). Earlier studies as well as the present one have shown that endothelium-dependent hyperpolarizations are dependent on the extracellular calcium concentration (Chen & Suzuki, 1990). It was further hypothesized that if, in the guinea-pig carotid artery, an heteromultimer (constituted of SK and IK1 α-subunits) is involved in the acetylcholine-induced hyperpolarization, the endothelium-dependent hyperpolarization observed in 0.5 mM Ca2+ should still be similarly sensitive to the combination of apamin and charybdotoxin whereas if homotetramers of IK1 and SK channels are expressed, IKCa should become preferentially activated.
In presence of 0.5 mM Ca2+, charybdotoxin and TRAM-34, which were ineffective in control solution (2.5 mM Ca2+), both produced a marked inhibition of the acetylcholine-induced endothelium-dependent hyperpolarization (% inhibition of the hyperpolarization: −52 and −36% for charybdotoxin and TRAM-34, respectively) while, in contrast, apamin was less effective in presence of 0.5 mM Ca2+ than in control condition (% inhibition of the hyperpolarization: −41 and −28% in 2.5 and 0.5 mM Ca2+, respectively). Charybdotoxin and TRAM-34 are structurally different IKCa blockers, which do not share the same binding site (Wulff et al., 2000; 2001). In presence of 0.5 mM Ca2+, the inhibition produced by each of these two blockers was revealed and was larger than that produced by apamin, which strongly suggests that, under these experimental conditions, IKCa became preferentially activated. These data are consistent with the hypothesis that, in the guinea-pig carotid artery, the targets of charybdotoxin (or TRAM-34) and apamin are homomeric IKCa and SKCa and do not support the existence of SK and IK heterotetramers. These results are in agreement with a recent report demonstrating that IK1 does not form heteromeric entity with SK1, although the latter coassemble with either SK2 or SK3 (Monaghan et al., 2004).
The present study demonstrates that, in the guinea-pig carotid artery, the activation of either IKCa or SKCa produces a true hyperpolarization (i.e. driving the membrane potential below the resting membrane potential toward the equilibrium value for potassium ions). In contrast, a recent work involving the mesenteric artery of the rat has shown that the activation of SKCa produced hyperpolarization whereas IKCa could only evoke repolarization (Crane et al., 2003). Since the activation of either SKCa or IKCa is virtually voltage-independent (Burnham et al., 2002; Bychkov et al., 2002), Crane et al. (2003) have suggested that the [Ca2+]i in the microdomains surrounding SKCa and IKCa must be differently regulated. In the guinea-pig carotid artery, such a differential regulation of [Ca2+]i may also explain the predominant role of SKCa observed in 2.5 mM Ca2+ although a higher level of expression of this channel when compared to IKCa can also be a plausible explanation. Additionally, the many discrepancies reported in the literature, concerning the relative contribution of SKCa and IKCa in endothelium-dependent hyperpolarizations, could be related to the differential cell- or species-dependent expression of endogenously expressed dominant-negative isoform of SK3, such as the recently described SK3-1C, a dominant-negative suppressor of SKCa and IKCa (Kolski-Andreaco et al., 2004).
In conclusion, these results suggest that the EDHF response is triggered via the combined opening of endothelial IKCa and SKCa channels. Furthermore, in this artery the production of a cytochrome P450 metabolite, and the subsequent activation of BKCa, is unlikely to contribute in the EDHF-mediated responses. The two selective, nonpeptide blockers of IKCa and SKCa, TRAM-34 and UCL 1684, respectively, should allow the design of in vivo experimental protocols that could help to clarify the potential physiological and pathophysiological role of EDHF-mediated responses.
Abbreviations
- BKCa
large-conductance calcium-activated potassium channels
- EDHF
endothelium-derived hyperpolarizing factor
- IKCa
intermediate-conductance calcium-activated potassium channels
- SKCa
small-conductance calcium-activated potassium channels
References
- ALVAREZ J., MONTERO M., GARCIA-SANCHO J. High affinity inhibition of Ca(2+)-dependent K+ channels by cytochrome P-450 inhibitors. J. Biol. Chem. 1992;267:11789–11793. [PubMed] [Google Scholar]
- ANDERSSON D.A., ZYGMUNT P.M., MOVAHED P., ANDERSSON T.L., HOGESTATT E.D. Effects of inhibitors of calcium-activated potassium channels, inwardly-rectifying potassium channels and Na(+)/K(+)-ATPase on EDHF relaxation in the rat hepatic artery. Br. J. Pharmacol. 2000;129:1490–1496. doi: 10.1038/sj.bjp.0703226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARFOD E.T., MOORE A.L., LIDOFSKY S.D. Cloning and functional expression of a liver isoform of the small conductance Ca2+-activated K+ channel SK3. Am. J. Physiol. 2001;280:C836–C842. doi: 10.1152/ajpcell.2001.280.4.C836. [DOI] [PubMed] [Google Scholar]
- BURNHAM M.P., BYCHKOV R., FELETOU M., RICHARDS GR VANHOUTTE P.M., WESTON A.H., EDWARDS G. Characterization of an apamin-sensitive small conductance Ca2+-activated K+ channel in porcine coronary artery endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;135:1133–1143. doi: 10.1038/sj.bjp.0704551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUSSE R., EDWARDS G., FELETOU M., FLEMING I., VANHOUTTE P.M., WESTON A.H. Endothelium-dependent hyperpolarization, bringing the concepts together. Trends Pharmacol. Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- BYCHKOV R., BURNHAM M.P., RICHARDS G.R., EDWARDS G., WESTON A.H., FÉLÉTOU M., VANHOUTTE P.M. Characterization of a charybdotoxin-sensitive intermediate conductance Ca2+-activated K+ channel in porcine coronary endothelium: relevance to EDHF. Br. J. Pharmacol. 2002;138:1346–1354. doi: 10.1038/sj.bjp.0705057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL W.B., GEBREMEDHIN D., PRATT P.F., HARDER D.R. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factor. Circ. Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- CAMPOS-ROSA J., GALANAKIS D., PIERGENTILI A., BHANDARI K., GANELLIN C.R., DUNN P.M., JENKINSON D.H. Synthesis, molecular modeling, and pharmacological testing of bis-quinolinium cyclophanes: potent, non-peptidic blockers of the apamin-sensitive Ca(2+)-activated K(+) channel. J. Med. Chem. 2000;43:420–431. doi: 10.1021/jm9902537. [DOI] [PubMed] [Google Scholar]
- CASTLE N.A. Recent advances in the biology of small conductance calcium-activated potassium channels. Perspect. Drug. Discov. 1999;15/16:131–154. [Google Scholar]
- CHATAIGNEAU T., FÉLÉTOU M., DUHAULT J., VANHOUTTE P.M. Epoxyeicosatrienoic acids, potassium channel blockers and endothelium-dependent hyperpolarisation in the guinea-pig carotid artery. Br. J. Pharmacol. 1998;123:574–580. doi: 10.1038/sj.bjp.0701629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHEN G., CHEUNG D.W. Effects of K+ channel blockers on ACh-induced hyperpolarization and relaxation in mesenteric arteries. Am. J. Physiol. 1997;41:H2306–H2312. doi: 10.1152/ajpheart.1997.272.5.H2306. [DOI] [PubMed] [Google Scholar]
- CHEN G., SUZUKI H. Calcium dependency of the endothelium-dependent hyperpolarization in smooth muscle of the rabbit carotid artery. J. Physiol. 1990;421:521–534. doi: 10.1113/jphysiol.1990.sp017959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRIU C., FÉLÉTOU M., CANET E., VANHOUTTE P.M. Endothelium-derived factors and hyperpolarisations of the isolated carotid artery of the guinea-pig, Br. J. Pharmacol. 1996a;119:959–964. doi: 10.1111/j.1476-5381.1996.tb15765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORRIU C., FÉLÉTOU M., CANET E., VANHOUTTE P.M. Inhibitors of the cytochrome P450-monooxygenase and endothelium-dependent hyperpolarisations in the guinea-pig isolated carotid artery. Br. J. Pharmacol. 1996b;117:607–610. doi: 10.1111/j.1476-5381.1996.tb15233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRANE G.J., GALLAGHER N., DORA K.A., GARLAND C.J. Small- and intermediate-conductance calcium-activated K+ channels provide different facets of endothelium-dependent hyperpolarization in rat mesenteric artery. J. Physiol. 2003;553:183–189. doi: 10.1113/jphysiol.2003.051896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DING H., TRIGGLE C.R. Novel endothelium-derived relaxing factors. Identification of factors and cellular targets. J. Pharmacol. Toxicol. Methods. 2000;44:441–452. doi: 10.1016/s1056-8719(00)00127-1. [DOI] [PubMed] [Google Scholar]
- DING H., JIANG Y., TRIGGLE C.R.The contribution of D-tubocurarine and apamin-sensitive potassium channels to endothelium-derived hyperpolarizing factor-mediated relaxation of small arteries from e-NOS-/- mice EDHF 2002 2003London: Taylor & Francis; 283–296.ed. Vanhoutte, P.M. pp [Google Scholar]
- DRUMMOND G.R., SELEMIDIS S., COCKS T.M. Apamin-sensitive non-nitric oxide (NO) endothelium-dependent relaxations to bradykinin in the bovine isolated coronary artery: no role for cytochrome P450 and K+ Br. J. Pharmacol. 2000;129:811–819. doi: 10.1038/sj.bjp.0703107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUNN P.M. UCL 1684: a potent blocker of Ca2+-activated K+ channel in rat adrenal chromaffin cells in culture. Eur. J. Pharmacol. 1999;368:119–123. doi: 10.1016/s0014-2999(99)00029-1. [DOI] [PubMed] [Google Scholar]
- ECKMAN D.M., HOPKINS N.O., MCBRIDE C., KEEF K.D. Endothelium-dependent relaxation and hyperpolarization in guinea-pig coronary artery: role of epoxyeicosatrienoic acid, Br. J. Pharmacol. 1998;124:181–189. doi: 10.1038/sj.bjp.0701778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., DORA K.A., GARDENER M.J., GARLAND C.J., WESTON A.H. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- EDWARDS G., FÉLÉTOU M., GARDENER M.J., GLEN C.D., RICHARDS G.R., VANHOUTTE P.M., WESTON A.H. Further investigations into the endothelium-dependent hyperpolarizing effects of bradykinin and substance P in porcine coronary artery. Br. J. Pharmacol. 2001;133:1145–1153. doi: 10.1038/sj.bjp.0704157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDWARDS G., GLUAIS P., WESTON A.H., VANHOUTTE P.M., FELETOU M. Low calcium unmasks a preferential involvement of IKCa in endothelium-dependent hyperpolarization of the guinea-pig carotid artery to acetylcholine. 2004.
- EDWARDS G., THOLLON C., GARDENER M.J., FÉLÉTOU M., VILAINE J.P., VANHOUTTE P.M., WESTON A.H. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br. J. Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EICHLER I., WIBAWA J., GRGIC I., KNORR A., BRAKEMEIER S., PRIES A.R., HOYER J., KOHLER R. Selective blockade of endothelial Ca2+-activated small and intermediate-conductance K+-channels suppresses EDHF-mediated vasodilatation. Br. J. Pharmacol. 2003;138:594–601. doi: 10.1038/sj.bjp.0705075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FISSLTHALER B., POPP R., KISS L., POTENTE M., HARDER D.R., FLEMING I., BUSSE R. Cytochrome P4540 2C is an EDHF synthase in coronary arteries. Nature. 1999;401:493–497. doi: 10.1038/46816. [DOI] [PubMed] [Google Scholar]
- GARCIA M.L., GALVEZ A., GARCIA-CALVO M., KING V.F., VAZQUEZ J., KACZOROWSKI G.J. Use of toxins to study potassium channels. J. Bioenerg. Biomembr. 1991;23:615–646. doi: 10.1007/BF00785814. [DOI] [PubMed] [Google Scholar]
- GARLAND C.J., PLANE F.Relative importance of endothelium-derived hyperpolarizing factor for the relaxation of vascular smooth muscle in different arterial beds Endothelium-Derived Hyperpolarizing Factor 1996Amsterdam: Harwood Academic Publishers; 173–179.ed. Vanhoutte P.M. Vol. 1, pp [Google Scholar]
- GAUTHIER K.M., DEETER C., KRISHNA U.M., REDDY Y.K., BONDLELA M., FALCK J.R., CAMPBELL W.B. 14,15-Epoxyeicosa-5(Z)-enoic acid: a selective epoxyeicosatrienoic acid antagonist that inhibits endothelium-dependent hyperpolarization and relaxation in coronary arteries. Circ. Res. 2002;90:1028–1036. doi: 10.1161/01.res.0000018162.87285.f8. [DOI] [PubMed] [Google Scholar]
- GHANSHANI S., WULFF H., MILLER M.J., ROHM H., NEBEN A., GUTMAN G.A., CAHALAN M.D., CHANDY K.G. Up-regulation of the IKCa1 potassium channel during T-cell activation. Molecular mechanism and functional consequences. J. Biol. Chem. 2000;275:37137–37149. doi: 10.1074/jbc.M003941200. [DOI] [PubMed] [Google Scholar]
- GLUAIS P., EDWARDS G., WESTON A.H., FALCK J.R., VANHOUTTE P.M., FELETOU M. Reassessment of EDHF-mediated responses in guinea-pig carotid artery using new non-peptidic blockers of SKCa and IKCa: a role for a cytochrome P450 metabolite. 2003.
- HINTON J.M., LANGTON P.D. Inhibition of EDHF by two new combinations of K+-channel inhibitors in rat isolated mesenteric arteries. Br. J. Pharmacol. 2003;138:1031–1035. doi: 10.1038/sj.bjp.0705171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRSCHBERG B., MAYLIE J., ADEMAN J.P., MARRION N.V. Gating of recombinant small-conductance Ca-activated K+ channel by calcium. J. Gen. Physiol. 1998;111:565–581. doi: 10.1085/jgp.111.4.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISHII T.M., SILVIA C., HIRSCHBERG B., BOND C.T., ADELMAN J.P, MAYLIE J. A human intermediate conductance calcium-activated potassium channel. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11651–11656. doi: 10.1073/pnas.94.21.11651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOINER W.J., WANG L.Y., TANG M.D., KACZMAREK L.K. hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc. Natl. Acad. Sci. U.S.A. 1997;94:11013–11018. doi: 10.1073/pnas.94.20.11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLSKI-ANDREACO A., TOMITA H., SHAKKOTAI V.G., GUTMAN G.A., CAHALAN M.D., GARGUS J.J., CHANDY K.G. SK3-1C, a dominant-negative suppressor of SKCa and IKCa channel. J. Biol. Chem. 2004;279:6893–6904. doi: 10.1074/jbc.M311725200. [DOI] [PubMed] [Google Scholar]
- LOGSDON N.J., KANG J., TOGO J.A., CHRISTIAN E.P., AIYAR J. A novel gene, hKCa4, encodes the calcium-activated potassium channel in human T lymphocytes. J. Biol. Chem. 1997;272:32723–32726. doi: 10.1074/jbc.272.52.32723. [DOI] [PubMed] [Google Scholar]
- MALIK-HALL M., GANELLIN C.R., GALANAKIS D., JENKINSON D.H. Compounds that blocks both intermediate-conductance (IK(Ca)) and small conductance (SK(Ca)) calcium-activated potassium channels. Br. J. Pharmacol. 2000;129:1431–1438. doi: 10.1038/sj.bjp.0703233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARCHENKO S.M., SAGE S.O. Electrical properties of resting and acetylcholine-stimulated endothelium in intact rat aorta. J. Physiol. 1993;462:735–751. doi: 10.1113/jphysiol.1993.sp019579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONAGHAN A.S., BENTON D.C., BAHIA P.K., HOSSEINI R., SHAH Y.A., HAYLETT D.G., MOSS G.W. The SK3 subunit of small conductance Ca2+-activated K+ channels interacts with both SK1 and SK2 subunits in a heterologous expression system. J. Biol. Chem. 2004;279:1003–1009. doi: 10.1074/jbc.M308070200. [DOI] [PubMed] [Google Scholar]
- MURPHY M.E., BRAYDEN J.E. Apamin-sensitive K+ channels mediate an endothelium-dependent hyperpolarization in rabbit mesenteric arteries. J. Physiol. 1995;489:723–734. doi: 10.1113/jphysiol.1995.sp021086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILIUS B., DROOGMANS G. Ion channels and their functional role in vascular endothelium. Physiol. Rev. 2001;81:1416–1459. doi: 10.1152/physrev.2001.81.4.1415. [DOI] [PubMed] [Google Scholar]
- OLESEN S.P., MUNCH E., MOLDT P., DREJER J. Selective activation of Ca2+-dependent K+ channels by novel benzimidazolone. Eur. J. Pharmacol. 1994;251:53–59. doi: 10.1016/0014-2999(94)90442-1. [DOI] [PubMed] [Google Scholar]
- PETERSSON J., ZYGMUNT P.M., JONSSON P., HÖGESTÄTT E.D. Characterization of endothelium-dependent relaxation in guinea-pig basilar artery – effect of hypoxia and role of cytochrome P450 monooxygenase. J. Vasc. Res. 1998;35:285–294. doi: 10.1159/000025595. [DOI] [PubMed] [Google Scholar]
- QUILLEY J., MCGIFF J.C. Is EDHF an epoxyeicosatrienoic acid. Trends Pharmacol. Sci. 2000;21:121–124. doi: 10.1016/s0165-6147(00)01445-0. [DOI] [PubMed] [Google Scholar]
- SANDOW S.L., GOTO K., Rummery N.M., Hill C.E. Developmental changes in myoendothelial gap junction mediated vasodilator activity in the rat saphenous artery. J. Physiol. 2004;556:875–886. doi: 10.1113/jphysiol.2003.058669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEDOVA M., KLISHIN A., HUSER J., BLATER L.A. Capacitive Ca2+ entry is graded with degree of intracellular Ca2+ store depletion in bovine vascular cells. J. Physiol. 2000;523:549–559. doi: 10.1111/j.1469-7793.2000.t01-3-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR M.S., BONEV A.D., GROSS T.P., ECKMAN D.M., BRAYDEN J.E., BOND C.T., ADELMAN J.P., NELSON M.T. Altered expression of small-conductance Ca2+-activated K+ (SK3) channels modulate arterial tone and blood pressure. Circ. Res. 2003;93:124–131. doi: 10.1161/01.RES.0000081980.63146.69. [DOI] [PubMed] [Google Scholar]
- WULFF H., GUTMAN G.A., CAHALAN M.D., CHANDY K.G. Delineation of the clotrimazole/TRAM-34 binding site on the intermediate conductance calcium-activated potassium channel, IKCa1. J. Biol. Chem. 2001;276:32040–32045. doi: 10.1074/jbc.M105231200. [DOI] [PubMed] [Google Scholar]
- WULFF H., MILLER M.J., HAENSEL W., GRISSNER S., CAHALAN M.D., CHANDY K.G. Design of potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc. Natl. Acad. Sci. U.S.A. 2000;97:8151–8156. doi: 10.1073/pnas.97.14.8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XIA X.M., FAKLER B., RIVARD A., WAYMAN G., JOHNSON6PAIS T., KEEN J.E., ISHII T., HIRSCHBERG B., BOND C.T., LUTSENKO S., MAYLIE J., ADELMAN J.P. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- YAMANAKA A., ISHIKAWA K., GOTO K. Characterization of endothelium-dependent relaxation independent of NO and prostaglandins in guinea-pig coronary artery. J. Pharmacol. Exp. Ther. 1998;285:480–489. [PubMed] [Google Scholar]
- ZYGMUNT P.M., EDWARDS G., WESTON A.H., LARSSON B., HÖGESTÄTT E.D. Involvement of voltage-dependent potassium channels in the EDHF-mediated relaxation of rat hepatic artery. Br. J. Pharmacol. 1997;121:141–149. doi: 10.1038/sj.bjp.0701108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZYGMUNT P.M., HÖGESTÄTT E.D.Endothelium-dependent hyperpolarization and relaxation in the hepatic artery of the rat Endothelium-Derived Hyperpolarizing Factor 1996Amsterdam: Harwood Academic Publishers; 191–202.ed. Vanhoutte P.M. Vol. 1, pp [Google Scholar]